Abstract
Intracerebral hemorrhage (ICH) is a critical medical condition associated with a high mortality rate. Surgical evacuation of extensive hemorrhage can improve survival and functional outcomes, but deep brain location poses a challenge. Our study seeks to provide robust evidence highlighting the efficacy, safety, and contribution of Robotic Stereotactic Assistance (ROSA) in advancing neurosurgical practices in the evacuation of ICH compared to conventional treatment methods. We searched for relevant papers comparing ROSA with conventional treatments until October 2024, using four electronic databases: PubMed, Scopus, the Cochrane Library, and Web of Science. The analysis was done using R Software. Continuous data was pooled as mean difference (MD), and dichotomous data was pooled as odds ratio (OR) in a random effect model with a relative 95% CI. A sub-group analysis was conducted according to the type of conventional therapy and the site of the hemorrhage. Eleven studies, comprising 968 patients (478 in the ROSA group and 490 in the control group “non-ROSA”), were included in the systematic review and meta-analysis. The analysis of primary outcomes revealed significantly higher postoperative Glasgow Coma Scale (GCS) scores, MD of 1.80 (95% CI: 0.68 to 2.92; p < 0.01), and lower rebleeding rates, OR of 0.26 (95% CI: 0.10 to 0.66; p < 0.01). However, no significant difference in mortality was found between the two groups, with an OR of 0.38 (95% CI: 0.11 to 1.38; p = 0.14). Regarding secondary outcomes, the ROSA group significantly reduced surgery duration and decreased intracranial infections and pneumonia. No significant difference was observed between the groups concerning central hypothermia. Using the ROSA robotic system for ICH evacuation is associated with improved neurological outcomes. These findings highlight the potential benefits of ROSA in advancing neurological practices for the management of ICH.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10143-025-03735-3.
Keywords: Intracerebral haemorrhage, ROSA, GCS, Rebleeding
Introduction
Intracerebral hemorrhage (ICH) represents a critical medical and often a surgical emergency, carrying a one-month mortality rate approaching 50% [1]. Extensive hemorrhages necessitate urgent decompression and evacuation to improve the chances of survival and good functional recovery [2]. However, most hemorrhages occur in deep brain structures, which poses challenges for safe and rapid surgical access to these areas [3]. Multiple studies have demonstrated that hematoma size and expansion are essential for poor survival rates [4, 5]. Hematoma expansion (HE) following intracerebral hemorrhage is a significant contributor to early neurological deterioration that is strongly associated with poor outcomes [6]. Furthermore, the basal ganglia exhibit a higher risk of HE [7]. Consequently, removing or reducing clots in patients with ICH is theoretically advantageous, as it reduces hematoma volume, decreases intracranial pressure, and diminishes subsequent perifocal edema [8]. Considering the significant mortality and morbidity associated with massive and life-threatening ICH (hematoma volume ≥ 30 mL; Glasgow Coma Scale ≤ 8), patients require urgent and immediate intervention because neurological deterioration usually occurs within the first few hours [5, 9, 10].
In general, craniotomy remains the standard method for hematoma evacuation. However, this procedure can lead to some degree of iatrogenic injury, various risks, and complications, resulting in high rates of mortality and morbidity following the procedure [11]. Recently, endoscopic surgery has emerged as a technique that can precisely locate hematomas and is gradually taking the place of craniotomy to reduce brain injury [12]. Minimally invasive puncture and drainage, along with endoscopic surgery, are currently regarded as less invasive techniques and have demonstrated considerable advantages over craniotomy in treating ICH [13, 14].
With the advancements in precision machinery, surgical robotics for treating ICH marks a significant breakthrough in neurosurgery [15, 16]. The Robotic Stereotactic Assistance (ROSA) system is a highly accurate surgical positioning tool that personalizes the surgical plan for each patient [17]. Recently, the ROSA One® Brain system has emerged as a comprehensive platform that offers advanced image-guided neurosurgical planning, accurate navigation, and highly stable robotic arms. The ROSA platform consists of a compact robotic arm, a robotic stand, and a touchscreen mounted on a telescopic support arm. More than just a global positioning system (GPS) for the brain, ROSA’s stereotactic and navigation system features a stable robotic arm equipped with precise GPS technology, enabling it to accurately and steadily position surgical instruments anywhere in the brain. This technology significantly aids neurosurgeons in performing minimally invasive brain surgeries with exceptional precision and accuracy [15]. Neurosurgical robots such as ROSA one®, SR1®, Remebot®, SR1-3D®, and CAS-R-2®, hence referred to as ROSA for simplicity.
As robotic surgery becomes increasingly adopted worldwide, this meta-analysis evaluates whether the ROSA robotic platform improves the evacuation of ICH relative to conventional techniques. Building on the most recent review of the topic [18], we (i) incorporated all studies published since that analysis, thereby enlarging the evidence base, (ii) performed two new subgroup analyses – one stratified by the type of conventional treatment and the other by hemorrhage location – and (iii) examined two additional outcomes, postoperative Glasgow Coma Scale (GCS) score and the incidence of pneumonia, which were not considered previously, along with performing GRADE assessment. These extensions provide a more comprehensive and up-to-date assessment of ROSA’s efficacy and safety.
Methods
This systematic review and meta-analysis were conducted and reported following the PRISMA statement guidelines [19]. The Cochrane Handbook of Systematic Reviews and Meta-analysis of Interventions has been followed in every step [20]. The protocol for this study was registered on PROSPERO (CRD42024604231), and every step was pre-specified.
Eligibility criteria
We included studies in our review if they satisfied the following criteria:
Population: patients with ICH.
Intervention: ROSA.
Comparator: conventional treatments (conservative medical treatment, endoscopic surgery, stereotactic drilling and drainage surgery, or craniotomy).
-
Outcome:
-
(i)Primary outcomes: Postoperative GCS, rebleeding and mortality
-
(ii)Secondary outcomes: surgery duration, intracranial infection, pneumonia, and central hyperthermia.
-
(i)
Study design: we included all full-text comparative studies, single-arm studies, and randomized controlled trials (RCTs).
We excluded reviews, case reports, case series, conference abstracts, and studies that reported outcomes after any neurosurgery not specific to intracerebral hemorrhage. Moreover, studies that assessed different outcomes were excluded.
Search strategy and selection process
Using MeSH terms, we comprehensively searched four electronic databases (Scopus, Cochrane, PubMed, and Web of Science) from the inception to October 2024. Additionally, a manual search was conducted through the references of the included papers to find any potentially relevant studies. Endnote (Clarivate Analytics, PA, USA) eliminated duplicates. Two writers independently searched the remaining entries in two steps: (1) title and abstract screening to ascertain relevance to this meta-analysis, and (2) full-text screening for ultimate eligibility to meta-analysis. Two authors independently reviewed the titles and abstracts of all citations considered for inclusion. Then, we retrieved the full text of selected studies to evaluate their applicability and validate them according to our systematic review. The differences were resolved through discussions with a third author.
Data extraction
Two authors independently extracted data using a uniform data extraction sheet. The following data extracted from each included study: 1) Summary of the included studies, 2) baseline characteristics of the included population, 3) risk of bias domains, 4) study outcomes; mortality, rebleeding, surgery duration, postoperative GCS scores, intracranial infection, pneumonia, and central hyperthermia. Disagreements were solved by consensus.
Quality assessment
We independently evaluated the quality of each included study by two authors. The Cochrane ROB-2 assessment tool [21] was used for RCTs, and the Newcastle Ottawa scale (NOS) [22] for observational studies. A third author solved disagreements.
Data synthesis and sensitivity analysis
The analysis was done using R Software. Continuous data pooled as mean difference (MD) in a random effect model with a relative 95% CI. Dichotomous data pooled as odds ratio (OR) in a random effect model with a relative 95% CI. P-value < 0.05 will be considered statistically significant.
A sub-group analysis was conducted according to the type of conventional therapy in the control arm: either conservative medical treatment, endoscopic surgery, stereotactic drilling and drainage surgery, or craniotomy. In addition, a subgroup analysis, according to the site of the hemorrhage, was conducted in either infra-tentorial or supra-tentorial.
Heterogeneity among studies was assessed by visual inspection and using the I2 and χ2 tests. A p-value of < 0.1 indicated significant heterogeneity. We used sensitivity analysis, commonly known as a leave-one-out meta-analysis, to perform a certainty assessment to evaluate the strength of the evidence. One study was excluded from the cache scenario to ensure that the aggregate effect magnitude was independent of any one study.
Publication bias
We employed the Trim and Fill method to assess publication bias due to the limited number of included studies in the quantitative analysis (fewer than 10). This approach helps to estimate the potential impact of publication bias on our results by adjusting for any missing studies that could affect the overall findings.
Results
Literature search
Our search strategy yielded a total of 552 records. After the title and abstract screening and removing the duplicates, 32 full-text articles were evaluated for eligibility. Eleven studies met our criteria and were included in our systematic review, of which eight were included in the meta-analysis. We also performed a manual reference search and found no extra studies. Figure 1 shows the PRISMA flow diagram of the present study. One RCT [23] and ten observational studies [8, 16, 24–31] were included (eight were used in the meta-analysis [16, 23, 25–30], and three were reported in the systematic review [8, 24, 31]).
Fig. 1.
PRISMA flow diagram
Characteristics and quality of the included studies
Eleven studies [8, 16, 23–31], comprising 968 patients (478 in the ROSA group and 490 in the control group"non-ROSA"), were included in the systematic review and meta-analysis. These studies were conducted across various geographical locations: nine in China, one in the USA, and one in Taiwan. The characteristics of the included studies are detailed in Table 1, while the baseline characteristics of the patients are presented in Table 2. The studies’ Quality assessment revealed good quality for the RCT according to the ROB-2 tool. Seven observational studies revealed good quality, and three showed moderate quality, according to NOS. The details of each domain are present in Online Resource 1.
Table 1.
Summary of the included studies
| Study ID | Country | Study design | Population | Groups | Main Findings | ||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion criteria | Exclusion criteria | Intervention | Number of Population | Control | Number of Population | ||||
| Alan 2021 [31] | USA | Retrospective observational study | Patients with large-volume intraparenchymal hemorrhages requiring emergent evacuation are managed using ROSA for catheter placement | NR | ROSA-assisted catheter placement in the intraparenchymal hemorrhage and intraventricular spaces | 4 | _ | _ | - ROSA-assisted catheter placement significantly reduced hematoma volume by up to 95% with no reported intraoperative complications |
| Luh 2024 [8] | Taiwan | Retrospective observational study | Patients with spontaneous ICH and HV greater than 30 mL, or as the surgeon and evaluating physician agreed |
1. Traumatic ICH or brain tumor 2. Underlying vascular pathology or suspected cause of secondary ICH (ruled out via CT angiography) |
ROSA-guided stereotactic ICH aspiration | 7 | _ | _ | - ROSA-guided ICH aspiration reduced hematoma volume by over 50% in most cases, with minimal perioperative blood loss and no complications |
| Wang 2019 [24] | China | Retrospective observational study | Patients diagnosed with HICH confirmed by cranial CT and Consciousness disorder and focal neurological signs | NR | Hematoma evacuation and tube drainage using Remebot frameless stereotaxic techniques | 17 | _ | _ |
- Robot‐assisted stereotactic techniques can accurately guide hematoma punctures, and 70%−90% of the hematoma was successfully removed in most patients - There were no deaths, and the 3-month follow-up showed improved neurological function and quality of life for all patients |
| Wang 2019 [16] | China | Retrospective observational study |
1. Thalamic hemorrhage with HV between 5–15 mL confirmed by CT 2. First-time onset with no previous history of brain disease 3. Age ≥ 18 years 4. GCS ≥ 8 |
1. aneurysms, arteriovenous malformations, tumors, trauma, or other cranial diseases cause ICH 2. Patients with severe cardiovascular, liver, kidney, hematopoietic disorders, mental illness, or cognitive impairment 3. Long-term use of anticoagulants 4. Loss to follow-up 5. HV < 5 mL or > 15 mL |
Robot-assisted minimally invasive surgery for thalamic hemorrhage drainage, guided by the ROSA system | 35 | Standard medical treatment (non-surgical management) | 49 | - Robot-assisted drainage of thalamic hemorrhage can improve prognosis and reduce the incidence of pneumonia and renal dysfunction |
| Zou 2024 [25] | China | Retrospective observational study |
1. Patients diagnosed with basal ganglia SICH with surgical indications 2. Age < 80 years 3. GCS score > 5 4. HV > 30 mL on brain CT |
1. ICH due to tumor, trauma, aneurysm, arteriovenous malformation, or infarction 2. Thalamic hemorrhage is mainly located on the medial side of the internal capsule and extends to the brain stem 3. Intraventricular hematoma causing hydrocephalus 4. Incomplete medical data |
Basal ganglia HICH robot CAS-R-2 assisted stereotactic drainage | 26 | Endoscopic surgery for hematoma evacuation | 68 |
- The ES group had a significantly higher rate of good prognosis (67.65%) than the robot CAS-R-2 group (42.31%) - The hematoma evacuation rate was also significantly higher in the ES group (82.35%) than in the robot CAS-R-2 group (19.23%) |
| Tang 2024 [26] | China | Retrospective observational study | Patients with a GCS score ≤ 8 on admission and spontaneous HBSH |
1. Suspected cavernous hemangioma hemorrhage 2. Hemorrhage caused by aneurysm rupture or arteriovenous malformation 3. Respiratory or circulatory failure requiring curative care 4. History of brainstem trauma or tumor 5. Brainstem hemorrhage combined with significant thalamic or basal ganglia hemorrhage |
Robot-assisted minimally invasive drainage surgery using the Sinovation SR1 robot | 51 | Conservative management | 74 |
- At 6 months, mortality was significantly lower in the surgical group (17.6%) compared to the conservative group (79.7%) - Additionally, 47.1% of patients in the surgical group achieved favorable outcomes, compared to only 5.4% in the conservative group - The surgical group also showed better neurological outcomes based on GCS and NIHSS scores at 6 months |
| Tan 2023 [27] | China | Retrospective observational study |
1. Supratentorial intracerebral hemorrhage ≥ 20 ml 2. GCS score ≤ 12 3. No significant coagulation disorders |
1. Secondary causes of hemorrhage (e.g., aneurysms, brain tumors, trauma) 2. Brainstem hemorrhage 3. Lack of 3-month or 1-year follow-up data |
Robot-assisted stereotactic hematoma drainage | 77 | Neuro-endoscopic surgery | 65 | - The Remebot surgery group demonstrated lower total hospital costs and a shorter surgery time |
| Liang 2022 [23] | China | RCT |
1. Supratentorial hematoma volume ≥ 20 mL or infratentorial hematoma volume ≥ 10 mL on imaging 2. History of hypertension and meeting surgical indications 3. GCS score ≥ 5 |
1. Cerebral hemorrhage caused by intracranial aneurysm rupture or trauma 2. Brainstem hemorrhage 3. Dysfunction of vital organs such as the liver or kidneys |
Robot-assisted stereotactic surgery | 71 | Frame-assisted stereotactic drilling and drainage surgery | 71 |
- The ROSA group had significantly shorter surgical duration (28.62 vs. 41.05 min), postoperative extubation time (1.36 vs. 3.52 days), and lower complication rates, including infection (0% vs. 12.68%) and postoperative re-hemorrhage (1.41% vs. 15.49%) compared to the frame group - Additionally, the ROSA group showed lower inflammatory markers (TNF-α, hs-CRP, IL-6) and better neurological recovery (higher NGF and BDNF, lower NSE levels) than the frame group |
| Jin 2024 [28] | China | Retrospective observational study |
1. GCS score > 8 2. HV > 10 mL or diameter > 3 cm |
1. SCH is caused by intracranial aneurysm, arteriovenous malformation, trauma, or tumor stroke accompanied by severe primary disease 3. Long-term use of anticoagulants 4. Patients who abandoned treatment |
Robotic-assisted stereotactic hematoma drainage | 77 | Suboccipital craniotomy for hematoma evacuation | 61 |
- The ROSA group had a shorter operation time (60.54 vs. 287.53 min) and lower incidences of pneumonia (5.4% vs. 20.5%) and stress ulcers (3.9% vs. 14.8%) compared to the control group - The evacuation rate was higher in the control group (93.20%) compared to the ROSA group (89.13%) - However, at 90 days, the ROSA group had better functional outcomes, including lower mRS scores (1.26 vs. 2.20), higher GCS scores (4.48 vs. 3.96), and lower incidence of balance disorders (40.3% vs. 67.3%) |
| Han 2024 [29] | China | Retrospective observational study |
1. History of HTN 2. Supratentorial HV 20–80 mL 3. Age 30–70 years 4. GCS score ≥ 5 5. Hemorrhage onset within 24 h of presentation |
1. Intraventricular hemorrhage 2. Coagulopathy or platelet abnormality 3. Cerebral herniation 4. Systemic or intracranial infection 5. Other causes of intracerebral hemorrhage (aneurysm, trauma, tumor) 6. Pregnancy or multiple hemorrhages 7. expected survival < 6 months |
Robot-assisted minimally invasive stereotactic puncture therapy using the ROSA system | 77 | Conventional craniotomy for hematoma removal | 56 | - The ROSA group had a significantly shorter operation time (40.3 vs 143.1 min), lower complication rates (pneumonia: 35.1% vs. 64.3%; gastrointestinal bleeding: 9.1% vs. 35.7%; intracranial infection: 0% vs. 8.9%), and better neurological outcomes at discharge (GCS score: 13.5 vs. 11.6, p < 0.001) |
| Bao 2024 [30] | China | Case–control study |
1. Diagnosis of PBSH 2. HV > 5 mL, transverse diameter > 2 cm 3. acute progressive symptoms, neurological dysfunction, hydrocephalus, or space-occupying effects |
1. Brainstem hemorrhage caused by traumatic brain injury or brain tumor 2. Patients who died within 24 h of admission |
Robot-assisted minimally invasive hematoma puncture and drainage | 36 | Conventional symptomatic and supportive treatments | 46 |
- The surgical group had significantly lower mortality (19.44% vs. 50.00%) and better neurological recovery compared to the conservative group. The surgical group also showed higher postoperative GCS and GOS scores and lower incidence of hydrocephalus - At 60 days, the surgical group showed a significantly higher survival rate than the conservative group |
NR; non-reported, ROSA; Robotic Stereotactic Assistance, HV; hematoma volume, ICH; intra-cerebral hemorrhage, HICH; hypertensive intra-cerebral hemorrhage, CT; Computed tomography, SICH; spontaneous intra-cerebral hemorrhage, GCS; Glasgow Coma Scale, GOS; Glasgow Outcome Score, HBSH; hypertensive brainstem hemorrhage, HTN; hypertension, mRS; modified Rankin Scale, PBSH; primary brainstem hemorrhage, SCH; spontaneous cerebellar hemorrhage
Table 2.
Baseline characteristics of the included population
| Study ID | Population number | Age | Gender; female (%) | GCS score at admission; mean (SD) | Hematoma volume (mL); mean (SD) | Hematoma side, right (%) | DM; n (%) | HTN; n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROSA | Control | ROSA | Control | ROSA | Control | ROSA | Control | ROSA | Control | ROSA | Control | ROSA | Control | ROSA | Control | |
| Alan 2021 [31] | 4 | - | 64 ¥ | - | 2 (50) | - | (10—14) ǂ | - | 51.8 (19.1) | - | NR | - | NR | - | NR | - |
| Luh 2024 [8] | 7 | - | 59 ¥ | - | 1 (14.3) | - | 10.4 ¥ | - | 45.5 ¥ | - | NR | - | NR | - | NR | - |
| Wang 2019 [24] | 17 | - | 64.7 ǁ | - | 6 (35.3) | - | NR | - | 34.2 ¥ | - | NR | - | 8 (47.1) | - | 17 (100) | - |
| Wang 2019 [16] | 35 | 49 | 60.1(11.4) | 58.6(10.2) | 22(62.86) | 36(73.47) | 12.6(2.5) | 12.4(2) | 10.7(2.3) | 9.6(3) | 19(54.29) | 25(51.02) | NR | NR | NR | NR |
| Zou 2024 [25] | 26 | 68 | 56.5(12.91) | 51.26(9.18) | 11(42.31) | 17(25) | NR | NR | 32.9(14.27) | 42.3(16.21) | NR | NR | 4(15.38) | 6(8.82) | NR | NR |
| Bao 2024 [30] | 36 | 46 | 48.98(9.89) | 50.26(10.68) | 12(33.33) | 14(30.43) | NR | NR | 10.79(5.31) | 7.17(1.78) | NR | NR | NR | NR | NR | NR |
| Han 2024 [29] | 77 | 56 | 52.8(9.6) | 55.3(7.8) | 23(29.87) | 18(32.14) | 10.7(2.2) | 9.8(2.8) | 38.4(10.4) | 41.1(11) | 39(50.6) | 20(35.7) | NR | NR | NR | NR |
| Jin 2024 [28] | 77 | 61 | 63.02(11.56) | 62.77(7.51) | 41(53.2) | 39(63.9) | 12.96(2.09) | 12.1(2.26) | 14.34(4.48) | 15.46(4.61) | 33 | 23 | NR | NR | 42(72.7) | 50(82) |
| Liang 2022 [23] | 71 | 71 | 57.24(10.23) | 59.41(10.01) | 13(18.31) | 11(15.49) | 11.62(3.25) | 11.45(3.4) | 34.26(10.62) | 32.96(10.26) | NR | NR | NR | NR | NR | NR |
| Tan 2023 [27] | 37 | 37 | 57.29(12.74) | 57.27(11.12) | 10(27.3) | 10(27.3) | 8.92(0.61) | 8.86(0.47) | 44.54(10.49) | 44.7(10.86) | NR | NR | 9(24.32) | 11(29.7) | 33(89.18) | 32(86.49) |
| Tang 2024 [26] | 51 | 74 | 55.67(10.68) | 53.27(12.25) | 16(31.4) | 15(2.3) | 4(1.53) | 4(1.51) | 7.93(2.44) | 7.53(3.02) | NR | NR | 7(13.7) | 10(13.5) | NR | NR |
¥; mean, ǁ; median, ǂ; range
GCS; Glasgow Coma Scale, DM; diabetes mellitus, HTN; hypertension, SD; standard deviation, NR; non-reported
Meta-analysis
Primary outcomes
Overall analysis
Postoperative Glasgow coma scale (GCS)
Three studies reported Postoperative GCS scores [27, 29, 30] involving 340 patients—164 in the ROSA group and 176 in the control group. Our analysis revealed a significant difference favoring the ROSA group (MD 1.80; 95% CI: 0.68 to 2.92; p < 0.01) Fig. 2A. Heterogeneity was moderate (I2 = 58%), but it was resolved after removing Tang et al. (2024) [26], resulting in (I2 = 0%) Fig. 2B.
Fig. 2.
Forest plot of GCS
Rebleeding
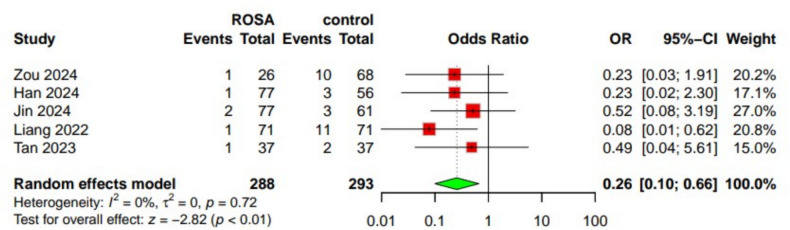
Five studies [23, 25, 27–29] reported data on the rebleeding outcome, including 581 patients—288 in the ROSA group and 293 in the control group. Our analysis found that the incidence of rebleeding was significantly lower in the ROSA group(OR 0.26; 95% CI: 0.10 to 0.66; p < 0.01). No heterogeneity was present. Observed (I2 = 0%) Fig. 3.
Fig. 3.
Forest plot of rebleeding incidence
Mortality
Mortality was reported in six studies [16, 25, 26, 28–30] involving 656 patients—302 in the ROSA group and 354 in the control group. The analysis showed no significant difference between the two groups regarding mortality (OR 0.38; 95% CI:0.11 to 1.38; p = 0.14) Fig. 4A. Significant heterogeneity was found (I2 = 76%), which was reduced after removing Tang et al. (2024) [26], resulting in (I2 = 40%) Fig. 4B.
Fig. 4.
Forest plot of mortality
Subgroup analysis
By the site of hemorrhage
Postoperative GCS
Han et al. (2024) [29] reported data on supratentorial hemorrhages, including 133 patients (77 in the ROSA group and 56 in the control group), showing no difference between ROSA and control groups (MD 1.00; 95% CI:−0.29 to 2.29). Two studies [26, 30] focused on infratentorial hemorrhages, including 207 patients (87 in the ROSA group and 120 in the control group). The analysis showed a significant difference favoring the ROSA group (MD 2.37; 95% CI: 0.39 to 4.35) Online Resource 2 Fig. 1.
Rebleeding
Four studies [23, 25, 27, 29] focused on supratentorial hemorrhages with 443 patients (211 in the ROSA group and 232 in the control group). The analysis showed a significant reduction in rebleeding favoring the ROSA group (OR 0.20; 95% CI: 0.07 to 0.60). Jin et al. (2024) [28] reported on infratentorial hemorrhages in 138 patients (77 in the ROSA group and 61 in the control group). There was no difference between the two groups regarding the incidence of rebleeding (OR 0.52; 95% CI: 0.08 to 3.19) Online Resource 2 Fig. 2.
Mortality
Three studies [16, 25, 29] focused on supratentorial hemorrhages with 311 patients (138 in the ROSA group and 173 in the control group). The analysis showed no significant difference (OR 1.38; 95% CI:0.46 to 4.20). Three studies [26, 28, 30] reported infratentorial hemorrhages in 345 patients (164 in the ROSA group and 181 in the control group). The analysis revealed a significant reduction in mortality in the ROSA group (OR 0.12; 95% CI: 0.04 to 0.42) Online Resource 2 Fig. 3.
By the type of conventional treatment
Postoperative GCS
Two studies [26, 30] involved patients receiving conservative management with 207 patients (87 in the ROSA group and 120 in the control group). The analysis showed a significant difference favoring the ROSA group (MD 2.37; 95% CI: 0.39 to 4.35). Han et al. (2024) [29] reported on patients undergoing craniotomy with 133 patients (77 in the ROSA group and 56 in the control group), showing no difference between ROSA and control group in postoperative GCS (MD 1.00; 95% CI: −0.29 to 2.29) Online Resource 2 Fig. 4.
Rebleeding
Two studies [28, 29] involved patients undergoing craniotomy with 271 patients (154 in the ROSA group and 117 in the control group). The analysis showed no significant difference between the groups (OR 0.38; 95% CI: 0.09 to 1.58). Two studies [25, 27] reported endoscopic surgery with 168 patients (63 in the ROSA group and 105 in the control group). The analysis showed no significant difference (OR 0.32; 95% CI: 0.06 to 1.57). Liang et al. (2022) [23] reported on stereotactic drilling and drainage with 142 patients (71 in each group). There was a reduction in rebleeding in the ROSA group (OR 0.08; 95% CI: 0.01 to 0.62) Online Resource 2 Fig. 5.
Mortality
Zou et al. (2024) [25] reported on endoscopic surgery with 94 patients (26 in the ROSA group and 68 in the control group). There was no difference between the ROSA and the control group regarding the incidence of mortality (OR 0.86; 95% CI: 0.16 to 4.57). Two studies [28, 29] involved craniotomy and hematoma evacuation with 271 patients (154 in the ROSA group and 117 in the control group). The analysis showed no significant difference (OR 0.47; 95% CI: 0.06 to 3.84). Three studies [16, 26, 30] reported conservative treatment with 291 patients (122 in the ROSA group and 169 in the control group). The analysis showed no significant difference (OR 0.31; 95% CI: 0.03 to 2.84) Online Resource 2 Fig. 6.
Robustness of the evidence
We conducted the leave-one-out test to see if the results depended on a single study. We found that, for the postoperative GCS, The survey of Bao et al. (2024) [30] seemed to have notably influenced the results, as omitting it led to the loss of statistical significance (p = 0.10)—online Resource 2 Fig. 7. The findings for the rebleeding outcome were statistically affected (p = 0.05) when omitting Liang et al. (2022) [23], indicating that this study contributes notably to the precision of the results Online Resourcse 2 Fig. 8. For the mortality outcome, Wang 2019 [16] appears to have the most influence, as omitting it yields a significant effect (p = 0.01), making it an essential study in the overall analysis Online Resourcse 2 Fig. 9.
Secondary outcomes
Overall analysis
Surgery duration (minutes)
Four studies [23, 27–29] reported surgery duration with 487 patients—262 in the ROSA group and 225 in the control group. Our analysis revealed a significant reduction in surgery duration favoring the ROSA group (MD −114.06 min; 95% CI: −200.17 to −27.95; p < 0.01). Significant heterogeneity (I2 = 100%) could not be resolved through sensitivity analysis Online Resourcse 2 Fig. 10.
Intracranial infection
Four studies [25, 27–29] with 439 patients—217 in the ROSA group and 222 in the control group- reported intracranial infection. Our analysis revealed a significant reduction in intracranial infections in the ROSA group (OR 0.36; 95% CI: 0.13 to 0.98; p = 0.05). No heterogeneity was observed (I2 = 0%) Online Resource 2 Fig. 11.
Pneumonia
Pneumonia was reported in four studies [16, 25, 28, 29] involving 449 patients—215 in the ROSA group and 234 in the control group. The analysis showed a significant reduction in pneumonia incidence in the ROSA group (OR 0.35; 95% CI: 0.19 to 0.66; p < 0.01). Heterogeneity was low (I2 = 37%) Online Resource 2 Fig. 12.
Central hyperthermia
Three studies [16, 26, 30] reported on central hyperthermia involving 291 patients—122 in the ROSA group and 169 in the control group. Our analysis revealed no significant difference between the groups (OR 0.91; 95% CI: 0.53 to 1.58; p = 0.74). Heterogeneity was low (I2 = 0%) Online Resource 2 Fig. 13.
Subgroup analysis
By the site of hemorrhage
Surgery duration (minutes)
Three studies [23, 26, 27] focused on supratentorial hemorrhages with 349 patients (185 in the ROSA group and 164 in the control group). The analysis showed a significant reduction in surgery duration, favoring the ROSA group (MD −76.81 min; 95% CI: −140.72 to −12.50). Jin et al. (2024) [28] reported on infratentorial hemorrhages in 138 patients (77 in the ROSA group and 61 in the control group). The ROSA group showed reduced surgery duration compared to the control group (MD −226.99 min; 95% CI: −249.42 to −204.56) Online Resource 2 Fig. 14.
Intracranial infection
Three studies [25, 27, 29] focused on supratentorial hemorrhages with 301 patients (140 in the ROSA group and 161 in the control group). The analysis showed a significant reduction in intracranial infections in the ROSA group (OR 0.19; 95% CI: 0.04 to 0.84). Jin et al. (2024) [28] reported on infratentorial hemorrhages in 138 patients (77 in the ROSA group and 61 in the control group). No difference existed between ROSA and the control group in intracranial infection incidence (OR 0.61; 95% CI: 0.16 to 2.39) Online Resource 2 Fig. 15.
Pneumonia
Three studies [16, 25, 29] focused on supratentorial hemorrhages with 311 patients (138 in the ROSA group and 173 in the control group). The analysis showed a significant reduction in pneumonia in the ROSA group (OR 0.40; 95% CI: 0.19 to 0.85). Jin et al. (2024) [28] reported on infratentorial hemorrhages in 138 patients (77 in the ROSA group and 61 in the control group). The ROSA group showed a reduced incidence of pneumonia compared to the control group (OR 0.20; 95% CI: 0.06 to 0.66) Online Resource 2 Fig. 16.
Central hyperthermia
Wang et al. (2019) [16] reported on supratentorial hemorrhages in 84 patients (35 in the ROSA group and 49 in the control group). The analysis showed no difference between ROSA and the control group in the incidence of central hyperthermia (OR 1.42; 95% CI: 0.19 to 10.63). Two studies [26, 30] focused on infratentorial hemorrhages with 207 patients (87 in the ROSA group and 120 in the control group). The analysis showed no significant difference (OR 0.88; 95% CI: 0.50 to 1.55) Online Resource 2 Fig. 17.
By the type of conventional treatment
Surgery duration (minutes)
Liang et al. (2022) [23] reported on stereotactic drilling and drainage with 142 patients (71 in each group). A reduction in surgery duration was revealed in the ROSA group (MD −12.43 min; 95% CI:−15.30 to −9.56). Two studies [28, 29] involved craniotomy with 271 patients (154 in the ROSA group and 117 in the control group). The analysis showed a significant reduction in surgery duration, favoring the ROSA group (MD −164.64 min; 95% CI: −286.34 to −42.94). Tan et al. (2023) [27] reported on endoscopic surgery with 74 patients (37 in each group). The ROSA group reduced surgery duration compared to the control group (MD −115.80 min; 95% CI: −130.62 to −100.98) Online Resource 2 Fig. 18.
Intracranial infection
Two studies [25, 27] reported endoscopic surgery with 168 patients (63 in the ROSA group and 105 in the control group). The analysis showed no significant difference (OR 0.28; 95% CI: 0.05 to 1.62). Two studies [28, 29] involved craniotomy and hematoma evacuation with 271 patients (154 in the ROSA group and 117 in the control group). The analysis showed no significant difference (OR 0.28; 95% CI: 0.03 to 2.40) Online Resource 2 Fig. 19.
Pneumonia
Wang et al. (2019) [16] reported on conservative treatment with 84 patients (35 in the ROSA group and 49 in the control group). The ROSA group showed a reduced incidence of pneumonia compared to the control group (OR 0.23; 95% CI: 0.06 to 0.89). Two studies [28, 29] involved craniotomy and hematoma evacuation with 271 patients (154 in the ROSA group and 117 in the control group). The analysis showed a significant reduction in pneumonia in the ROSA group (OR 0.27; 95% CI: 0.15 to 0.50). Zou et al. (2024) [25] reported on endoscopic surgery with 94 patients (26 in the ROSA group and 68 in the control group). There was no difference between the ROSA and the control group regarding the incidence of pneumonia (OR 0.82;95% CI: 0.33 to 2.05) Online Resource 2 Fig. 20.
Robustness of the evidence
The overall result remained significant for surgery duration outcome when omitting Jin et al. [28] or Liang et al. [23]. However, when Han et al. (2024) [29] were omitted, the result lost statistical significance (p = 0.06). Similarly, omitting Tan et al. (2023) [27] led to the loss of statistical significance (p = 0.07), indicating that Han et al. (2024) [29] and Tan et al. (2023) [27] have a notable influence on the results. The overall result of intracranial infection remained significant when omitting Jin et al. [28], but lost statistical significance when Zou et al. (2024) [25] was omitted (p = 0.09). Similarly, omitting Han et al. (2024) [29] (p = 0.15) or Tan et al. (2023) [27] (p = 0.10) also led to the loss of statistical significance, indicating the influence of these studies on the overall result. The results of pneumonia and hyperthermia were not dependent on any single study Online Resources 2 Fig. 21, 22, 23, 24.
Publication bias
The Trim and Fill method assessed potential publication bias in the included studies. No additional studies were identified, indicating that the funnel plots showed no significant asymmetry. The random-effects model for postoperative GCS yielded a mean difference of 1.80 (p = 0.0017). For rebleeding, the random-effects model yielded a p-value of 0.0076. The random-effects model estimated a p-value of 0.1049 for mortality Online Resource 4.
GRADE assessment
The overall quality of evidence was high for rebleeding, intra-cranial infection, and pneumonia. On the other hand, low-quality evidence was reported for the rest of the outcomes, primarily due to inconsistency and imprecision. Details of each domain in the GRADE assessment are reported in Online Resource 3.
Qualitative synthesis for studies included only in the systematic review
Our search yielded three studies that met our criteria for systematic review inclusion but did not have relevant data for analysis. These studies were retrospective observational single-arm conducted in three different countries. Alan et al. 2021 [31] conducted in the USA and included four patients with large volume intraparenchymal hemorrhages requiring emergent evacuation, managed between 2017 and 2019 using ROSA for catheter placement. It revealed that utilization of ROSA-assisted catheter placement significantly reduced hematoma volume by up to 95%, with a mean decrease from 51.8 to 13.0 cc (p < 0.01). The mean placement error was 3.48 mm, with no reported intraoperative complications. Seven patients older than 18 with more than 30 mL hematoma, or as determined by the surgeon and reviewing physician, were included in the Taiwan Luh et al. 2024 [25] study. According to the trial, ROSA-guided ICH aspiration is often reduced by more than 50% hematoma volume. On average, there was little perioperative blood loss and no problems throughout the 1.3-h procedure. In 2019, Wang et al. [24] conducted a study in China involving 17 patients diagnosed with hypertensive intracerebral hemorrhage (HICH), confirmed through cranial CT. The patients also exhibited consciousness disorders and focal neurological signs. The study demonstrated that robot-assisted stereotactic techniques can accurately guide hematoma punctures. The average positioning error was measured at 1.28 ± 0.49 mm, and 70% to 90% of the hematoma was successfully removed in most patients. There were no reported deaths, and a 3-month follow-up indicated improvements in neurological function and quality of life for all participants.
Discussion
Summary of findings
We found that ROSA improved postoperative GCS scores and reduced surgery duration. It was also associated with lower incidences of rebleeding, intracranial infection, and pneumonia. Comparable results were found between the ROSA and control groups for mortality and the incidence of central hyperthermia.
Although the infra-tentorial site of ICH is known to be a poor prognostic factor of the outcomes [32], our hemorrhage-site-based sub-group analysis revealed that patients with supra-tentorial and infra-tentorial hemorrhages favored the ROSA technique in reducing the incidence of pneumonia, intracranial infection, rebleeding, and surgery duration. However, we might not be able to generalize the results regarding surgery duration due to the high heterogeneity among the included studies. Surprisingly, reduced mortality rates and improved GCS scores were noted only in infra-tentorial hemorrhage patients in the ROSA group, compared to controls from the same group. It is worth mentioning that for both outcomes, the study of Tang et al. was the source of heterogeneity in the overall analysis and the infra-tentorial hemorrhage sub-group, which means that the results can not be generalized. This could be attributed to follow-up period variation. For example, Jin et al.’s study reported short- and long-term follow-up results at 30 and 90 days, respectively. Similarly, Bao et al. set the follow-up time to 60 days. However, Tang et al. reported the 6-month mortality outcome and compared the results of the remaining surviving patients. Unfortunately, the outcome of central hyperthermia did not seem to be reduced in the overall or sub-group analysis.
When analyzing subgroups according to conventional treatment methods, ROSA significantly reduced surgery duration compared to Endoscopic surgery and craniotomy. In addition, the incidence of pneumonia was decreased in the ROSA group compared to craniotomy and conservative treatment. Patients in the ROSA group experienced less rebleeding than those undergoing Stereotactic drilling and drainage surgery and improved postoperative GCS compared to those undergoing conservative treatment. On the other hand, the differences between the ROSA and control groups were generally insignificant in patients receiving endoscopic surgery or craniotomy regarding intracranial infection, mortality, and rebleeding.
Interpretation of the findings
Our findings align with those reported in the previous meta-analysis by Luo et al. [18], except for mortality rates. While Luo et al. did not perform a meta-analysis on GCS scores, they reported improvements in two studies [29, 30]. Our study demonstrated that ROSA significantly enhances postoperative GCS scores compared to control groups, suggesting better neurological recovery in patients treated with ROSA. Traditional open craniotomy, although effective in evacuating hypertensive intracerebral hematomas by providing excellent visualization and complete clearance [33, 34], has not consistently led to neurological improvement over conservative medical treatment in several RCTs [35–37]. Recent advancements in endoscopic surgery and minimally invasive puncture and drainage techniques aim to minimize brain injury and have shown promising results in early studies [14, 38, 39], making surgical treatment of ICH easier and safer and leading to better prognoses than craniotomy [14, 40].
The precision and minimal invasiveness of robotic assistance, such as ROSA, may contribute to reduced brain tissue damage during hematoma evacuation. Minimally invasive approaches can preserve surrounding neural structures, leading to better functional outcomes [41]. The enhanced accuracy of ROSA in targeting hematomas minimizes collateral damage, thereby improving postoperative neurological status [41]. This precision likely contributes to the observed reduction in surgery duration with ROSA. The efficiency of robotic systems in planning and executing surgical trajectories streamlines the surgical workflow, reducing the time required for complex neurosurgical procedures [42, 43]. For example, in the study by Jin and Yang [28], the surgery time was reduced from 287.5 ± 87.6 min to only 60.5 ± 20 min—an approximately 80% reduction. Similarly, Han et al. [29] found that ROSA involved smaller incisions, shorter surgery durations, and minimized brain tissue damage compared to traditional craniotomy.
The lower incidence of rebleeding observed in the ROSA group may be attributed to precise hematoma evacuation and reduced manipulation of brain tissue. Minimally invasive techniques decrease rebleeding rates by ensuring complete hematoma removal while preserving hemostasis [44]. ROSA’s planning capabilities allow for precise surgical design based on the hematoma’s morphology, enabling surgeons to avoid critical brain areas and blood vessels, thereby minimizing the risk of bleeding postoperatively [45]. ROSA’s advanced surgical planning and navigation technologies improve surgical safety and effectiveness, mainly by lowering the risk of postoperative complications.
Furthermore, ROSA was associated with lower incidences of intracranial infections and pneumonia. The precision of ROSA reduces surgical trauma, reduces brain tissue exposure time during the operation, and lowers surgical pressure, resulting in a milder inflammatory response. Liang et al. [23] reported that patients treated with ROSA had significantly lower serum levels of tumor necrosis factor-α, hs-CRP, and IL-6 after surgery than those who underwent conventional treatment. This reduction in inflammatory markers could account for the reduced risk of postoperative intracranial infections, accelerated postoperative recovery, and fewer complications. Additionally, ROSA offers several registration modes, enabling the selection of the most suitable approach depending on individual circumstances during the operation [46]. ROSA’s personalized surgical planning can effectively prevent unnecessary damage to crucial functional areas and visible blood vessels, reduce the hematoma’s mass effect, enhance local blood circulation, and minimize inflammation and cerebral edema following nerve tissue injury [47, 48].
Lastly, no significant differences were observed between the ROSA and control groups regarding mortality rates and the incidence of central hyperthermia, which contradicts previous meta-findings [18]. However, the authors also observed high heterogeneity in their analysis, and after its resolution, mortality rates became insignificant. The same situation occurred during our analysis, and removing Tang et al. [26] study resolved the heterogeneity, but the mortality was negligible regardless of heterogeneity. Notably, Han et al. also reported a few mortalities, including one related to frailty in the conventional craniotomy group and another due to lung infection in the ROSA group, possibly associated with pre-existing cardiac disease. Some studies did not report the cause or whether a death event occurred; therefore, we needed to perform the subgroup analysis, as we observed that ROSA significantly reduced mortality in patients with infratentorial hemorrhages. However, with this said, it is critical to emphasize that sub-group analysis according to follow-up duration was not applicable; hence, it would have been more valuable to identify if the sole cause of death was the hemorrhage or the post-surgical complications that appeared during the follow-ups.
Comparison with previous meta-analysis
We conducted a comparison table to summarise our results head-to-head with the most recently published systematic review [18] that reported the efficacy of ROSA on ICH. All data are presented in Table 3. Luo et al. [18] included eight studies with 844 patients. However, we have included 11 studies (all of them met the inclusion criteria in our methods), of which three were included only in our qualitative synthesis, with 968 patients in our quantitative synthesis. Regarding the analyzed outcomes and the quality of evidence, we rKeywordseported that the postoperative GCS score and the incidence of pneumonia favored the ROSA group with a low and high quality of evidence, respectively. Although Luo et al. did not report them, we have done a subgroup analysis based on the place of hemorrhage and the type of conventional treatment. This is especially crucial since the consequences and prognosis of supratentorial and infratentorial hemorrhages vary greatly. Because of brainstem involvement, increased risk of herniation, and limited surgical access, infratentorial hemorrhages are sometimes linked with a worse prognosis. Understanding these differences in subgroup analysis helps one to have a more exact knowledge of treatment results and prognostic relevance.
Table 3.
Comparable table with the previous recent SR&MA
| Luo 2024 [18] | Our study | |
|---|---|---|
| Databases searched | PubMed, CNKI, Embase, and Google Scholar | Pubmed, Scopus, Web of Science, Cochrane Library |
| Number of Studies | 8 | 11 |
| Study Designs | RCTs and Cohort studies | RCTs and Cohort studies |
| Number of Patients | 844 | 968 |
| Quality of included studies | Most of the studies were judged as"Moderate." | Most of the studies were judged as"Good." |
| Publication bias assessment | NR | Present |
| Grade assessment | NR | Present |
| Outcomes | ||
| Postoperative GCS score | NR | Favored ROSA |
| Surgery duration | Favored ROSA | Favored ROSA |
| Postoperative rebleeding | Favored ROSA | Favored ROSA |
| Mortality | There is no difference between ROSA and control | There is no difference between ROSA and control |
| Intracranial infection | Favored ROSA | Favored ROSA |
| Pneumonia | NR | Favored ROSA |
| Central hyperthermia | There is no difference between ROSA and control | There is no difference between ROSA and control |
| Subgroup analysis | NR | Regarding the site of hemorrhage (supra–infra–tentorial) |
| Regarding the conventional treatment (craniotomy- endoscopic surgery- stereotactic drilling and drainage- conservative treatment) | ||
NR; non-reported
Clinical implications
Despite its technical advantages, as evidenced by improved postoperative neurological outcomes, reduced surgery duration, and lower incidence of certain postoperative complications, economics still holds ROSA back. Comparative work in general and colorectal surgery shows that a robotic case typically raises hospital expenditure by 40%−100% once operating room time, consumables, and servicing are included [49–51]. Skull-base and intracranial reviews reach the same conclusion, noting that the capital price of the platform and the absence of neurosurgery-specific instruments remain the most frequently cited barriers to broader use [42]. Higher institutional costs also translate into larger patient charges in systems with cost-sharing, so affordability also becomes a clinical access issue. Cost-utility modeling suggests that these front-loaded expenses can be recouped in services that already own a robot and can keep it busy. In a 557-case spine series, Menger et al. estimated that fewer revisions, infections, and bed days would save about US $ 600,000 annually, enough to cover maintenance contracts and disposables [52]. A worldwide survey of 406 neurosurgeons confirmed that financing the purchase and training of the team, rather than physical infrastructure, are the main obstacles, even though ROSA was already the second most common platform [53]. Reviews focused on low- and middle-income countries outline practical mitigations: lease or shared-ownership agreements, cross-specialty scheduling to lift case volumes, remote proctoring to shorten learning curves, and using public or philanthropic innovation funds to cover start-up costs [54]. Where such measures are in place, and outcome data (revisions, infections, length of stay) are tracked transparently, ROSA can move from an aspirational purchase to a financially sustainable part of neurosurgical care.
Limitations
Our meta-analysis has several important constraints. First, with the exception of a single randomized controlled trial, all included studies were observational. Such designs are prone to residual confounding and cannot establish definitive causal links between the intervention and clinical outcomes. Second, all studies were conducted in China, limiting our findings’ external validity: practice patterns, patient characteristics, and healthcare infrastructures elsewhere may differ substantially.
We also detected appreciable heterogeneity in Glasgow Coma Scale scores, mortality, and operative time. Excluding Tang et al. (2024) attenuated the heterogeneity for GCS and mortality, but variability in operative time persisted. Our ability to explore effect modifiers was restricted to subgroup analyses by type of conventional therapy and hemorrhage location. Larger primary studies would allow future reviews to stratify results more finely, such as hematoma volume categories and differing follow-up intervals.
Finally, the overall sample size was modest, reflecting the limited number of published investigations on ROSA, a relatively new surgical platform, which also could be influenced by surgical expertise – which we could not assess. The resulting wide confidence intervals, along with the overall GRADE low-quality evidence reported, temper our conclusions’ robustness. Additional multicentre studies with larger cohorts and longer follow-ups are needed to confirm and extend these observations.
Conclusion
Our study indicates that ROSA improves postoperative neurological outcomes, reduces surgery duration, and lowers the incidence of certain postoperative complications in patients undergoing surgery for cerebral hemorrhage. ROSA’s precision, efficiency, and minimally invasive nature contribute to these improved outcomes, highlighting its potential as a valuable tool in neurosurgical interventions. Due to the lack of included RCTs, further are required to validate these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Nada Mostafa Al-dardery & Mohamed Diaa Elfakhrany: conceptualized and designed the manuscript. Nada Mostafa Al-dardery, Mohamed Abouzid & Mohamed El-Samahy: participated in drafting the article and acquiring data. Nada Mostafa Al-dardery: analyzed and interpreted data. Shahd Alqato, Sadil Mohammad Bani Khaled, Ahmed Taha Abdelsattar & Suhel.F.Batarseh: prepared the figures and tables. Nada Mostafa Al-dardery, Ahmed Taha Abdelsattar & Mohamed Abouzid: wrote, edited, and revised the manuscript critically. Mohamed Abouzid: Supervision. All authors critically revised the manuscript concerning intellectual content and approved the final manuscript.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Authors declare that no new data generated during the work.
Declarations
Ethics declaration
Not applicable.
Consent for publication
Not applicable.
Human ethics and consent to participate declarations
Not applicable.
Conflict of interest
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohamed Diaa Elfakhrany and Nada Mostafa Al-dardery have the same contribution to this work as the first author.
Contributor Information
Nada Mostafa Al-dardery, Email: Ndardery449@gmail.com.
Mohamed Abouzid, Email: mmahmoud@ump.edu.pl.
References
- 1.Sacco S, Marini C, Toni D, Olivieri L, Carolei A (2009) Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 40:394–399 [DOI] [PubMed] [Google Scholar]
- 2.Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G et al (1989) Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg 70:530–535 [DOI] [PubMed] [Google Scholar]
- 3.Sprügel MI, Kuramatsu JB, Volbers B, Gerner ST, Sembill JA, Madžar D et al (2019) Perihemorrhagic edema: Revisiting hematoma volume, location, and surface. Neurology 93:e1159–e1170 [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Sorimachi T, Osada T, Baba T, Inoue G, Atsumi H et al (2014) Risks and benefits of CT angiography in spontaneous intracerebral hemorrhage. Acta Neurochir (Wien) 156:911–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang GY (2007) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 68:471–2 [DOI] [PubMed] [Google Scholar]
- 6.Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M et al (2018) Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet 391:2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng L, Chen K, Yang L, Deng Z, Zheng H (2021) Different effects of hematoma expansion on short-term functional outcome in basal ganglia and thalamic hemorrhages. Biomed Res Int 2021:9233559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luh H-T, Zhu C, Kuo L-T, Lo W-L, Liu H-W, Su Y-K, et al. Application of Robotic Stereotactic Assistance (ROSA) for spontaneous intracerebral hematoma aspiration and thrombolytic catheter placement. J Formosan Med Assoc. 2024; S0929664624002547. [DOI] [PubMed]
- 9.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L et al (1997) Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28:1–5 [DOI] [PubMed] [Google Scholar]
- 10.Mayer SA, Rincon F (2005) Treatment of intracerebral haemorrhage. Lancet Neurol 4:662–672 [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Chen J, Zhao G, Xie Z, Huang N, Yang Q et al (2020) Decompressive hemicraniectomy associated with ultrasound-guided minimally invasive puncture and drainage has better feasibility than the traditional hematoma evacuation for deteriorating spontaneous intracranial hemorrhage in the Basal Ganglia Region: A retrospective observational cohort study. Front Neurol 11:561781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Z, Ai X, Hu X, Fang F, You C (2017) Comparison of neuroendoscopic surgery and craniotomy for supratentorial hypertensive intracerebral hemorrhage: a meta-analysis. Medicine (Baltimore) 96:e7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T (2020) The dataset on the characteristics of the intracerebral hemorrhage patients treated by endoscopic hematoma removal or craniotomy. Data Brief 33:106387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Wu X, Tan Z, Guo H, Bai H, Wang B et al (2020) Long-term effect of endoscopic evacuation for large Basal Ganglia Hemorrhage With GCS Scores ≦ 8. Front Neurol 11:848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Chen D, Pan C, Zhang G, Chen S, Shi J et al (2023) Surgical robotics for intracerebral hemorrhage treatment: State of the art and future directions. Ann Biomed Eng 51:1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Jin H, Gong S, Yang X, Sun X, Xu M et al (2019) Efficacy analysis of robot-assisted minimally invasive surgery for small-volume spontaneous thalamic hemorrhage. World Neurosurgery 131:e543–e549 [DOI] [PubMed] [Google Scholar]
- 17.Xu F, Jin H, Yang X, Sun X, Wang Y, Xu M et al (2018) Improved accuracy using a modified registration method of ROSA in deep brain stimulation surgery. Neurosurg Focus 45:E18 [DOI] [PubMed] [Google Scholar]
- 18.Luo L, He C, Li W, Tang X (2024) Systematic review and meta-analysis of ROSA vs. conventional therapy for intracerebral hemorrhage. J Robotic Surg 18:326 [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (2019). Cochrane handbook of systematic reviews of interventions. [Internet]. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Chichester (UK): John Wiley & Sons, Ltd; [cited 2023 Oct 11]. Available from: https://training.cochrane.org/handbook
- 21.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898 [DOI] [PubMed] [Google Scholar]
- 22.Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605 [DOI] [PubMed] [Google Scholar]
- 23.Liang L, Li X, Dong H, Gong X, Wang G. (2022). A comparative study on the efficacy of robot of stereotactic assistant and frame-assisted stereotactic drilling, drainage for intracerebral hematoma in patients with hypertensive intracerebral hemorrhage. Pak J Med Sci [Internet]. [cited 2024 Nov 25]; 38. Available from: https://www.pjms.org.pk/index.php/pjms/article/view/5481 [DOI] [PMC free article] [PubMed]
- 24.Wang T, Zhao Q, Gu J, Shi T, Yuan X, Wang J et al (2019) Neurosurgery medical robot Remebot for the treatment of 17 patients with hypertensive intracerebral hemorrhage. Robotics Computer Surgery 15:e2024 [DOI] [PubMed] [Google Scholar]
- 25.Zou D, Chen X, Chen S, Zhang P, Lu Y (2024) Impact of endoscopic surgery versus robot CAS-R-2 assisted with stereotactic drainage on prognosis of basal ganglia hypertensive intracerebral hemorrhage. World Neurosurg 181:e589–e596 [DOI] [PubMed] [Google Scholar]
- 26.Tang Z, Huang W, Chen Q, Guo C, Zheng K, Wei W et al (2024) Curative effect analysis of robot-assisted drainage surgery in treatment of spontaneous hypertensive brainstem hemorrhage. Front Neurol 15:1352949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan K, Peng Y, Li J, Liu C, Tao L (2023) Long-term outcomes and cost-effectiveness evaluation of robot-assisted stereotactic hematoma drainage for spontaneous intracerebral hemorrhage. Front Neurol 14:1291634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin C, Yang Y (2024) Surgical evacuation of spontaneous cerebellar hemorrhage: Comparison of safety and efficacy of suboccipital craniotomy and robotic-assisted stereotactic hematoma drainage. Clin Neurol Neurosurg 239:108192 [DOI] [PubMed] [Google Scholar]
- 29.Han W, Xie A, Chen T, Sun X, Liu X (2024) Efficacy of robot-assisted minimally invasive stereotactic puncture therapy for supratentorial hypertensive intracerebral hemorrhage. Brain and Behavior 14:e3402 [Google Scholar]
- 30.Bao D, Ni S, Chang B, Zhang W, Zhang H, Niu C (2024) Short-term outcomes of robot-assisted minimally invasive surgery for brainstem hemorrhage: A case-control study. Heliyon 10:e25912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alan N, Patel A, Abou-Al-Shaar H, Agarwal N, Zenonos GA, Jankowitz BT et al (2021) Intraparenchymal hematoma and intraventricular catheter placement using robotic stereotactic assistance (ROSA): A single center preliminary experience. J Clin Neurosci 91:391–395 [DOI] [PubMed] [Google Scholar]
- 32.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32:891–897 [DOI] [PubMed] [Google Scholar]
- 33.Wang W (2016) Minimally invasive surgical treatment of acute epidural hematoma: Case series. Biomed Res Int 2016:6507350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Su Y-C, Chen C-C, Guo J-H, Wu C, Wei S-T et al (2019) Long-term follow-up in patients with spontaneous intracerebral hemorrhage treated with or without surgical intervention: a large-scale retrospective study. Neurotherapeutics 16:891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT et al (2005) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 365:387–397 [DOI] [PubMed] [Google Scholar]
- 36.Bösel J, Zweckberger K, Hacke W (2015) Haemorrhage and hemicraniectomy: refining surgery for stroke. Curr Opin Neurol 28:16–22 [DOI] [PubMed] [Google Scholar]
- 37.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM et al (2013) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 382:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K-Y, Kung W-M, Kuo L-T, Huang AP-H (2021) Ultrarapid endoscopic-aided hematoma evacuation in patients with thalamic hemorrhage. Behav Neurol. 2021:8886004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowman KM, Ahmed AS (2022) Surgical indications and options for hypertensive hemorrhages. Neurol Clin 40:337–353 [DOI] [PubMed] [Google Scholar]
- 40.Sun G, Li X, Chen X, Zhang Y, Xu Z (2019) Comparison of keyhole endoscopy and craniotomy for the treatment of patients with hypertensive cerebral hemorrhage. Medicine 98:e14123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M et al (2012) Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke 43:2923–2930 [DOI] [PubMed] [Google Scholar]
- 42.Pangal DJ, Cote DJ, Ruzevick J, Yarovinsky B, Kugener G, Wrobel B et al (2022) Robotic and robot-assisted skull base neurosurgery: systematic review of current applications and future directions. Neurosurg Focus 52:E15 [DOI] [PubMed] [Google Scholar]
- 43.Faria C, Erlhagen W, Rito M, De Momi E, Ferrigno G, Bicho E (2015) Review of robotic technology for stereotactic neurosurgery. IEEE Rev Biomed Eng 8:125–137 [DOI] [PubMed] [Google Scholar]
- 44.Hannah TC, Kellner R, Kellner CP (2021) Minimally invasive intracerebral hemorrhage evacuation techniques: A review. Diagnostics 11:576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han WY, Tao YQ, Xu F, Zhang YQ, Li ZY, Liang GB (2017) The short- and long-term efficacy analysis of stereotactic surgery combined external ventricular drainage in the treatment of the secondary intraventricular hemorrhage. Brain Behav 7:e00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vadera S, Chan A, Lo T, Gill A, Morenkova A, Phielipp NM et al (2017) Frameless stereotactic robot-assisted subthalamic nucleus deep brain stimulation: case report. World Neurosurg 97:762.e11-762.e14 [DOI] [PubMed] [Google Scholar]
- 47.Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S et al (2002) Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 33:2631–2635 [DOI] [PubMed] [Google Scholar]
- 48.Kim H, Edwards NJ, Choi HA, Chang TR, Jo KW, Lee K (2016) Treatment strategies to attenuate perihematomal edema in patients with intracerebral hemorrhage. World Neurosurgery 94:32–41 [DOI] [PubMed] [Google Scholar]
- 49.Barbash GI, Glied SA (2010) New technology and health care costs–the case of robot-assisted surgery. N Engl J Med 363:701–704 [DOI] [PubMed] [Google Scholar]
- 50.Alshowaikh K, Karpinska-Leydier K, Amirthalingam J, Paidi G, IroshaniJayarathna AI, Salibindla DBAMR et al (2021) Surgical and patient outcomes of robotic versus conventional laparoscopic hysterectomy: a systematic review. Cureus. 13:e16828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quilici PJ, Wolberg H, McConnell N (2022) Operating costs, fiscal impact, value analysis and guidance for the routine use of robotic technology in abdominal surgical procedures. Surg Endosc 36:1433–1443 [DOI] [PubMed] [Google Scholar]
- 52.Menger RP, Savardekar AR, Farokhi F, Sin A (2018) A cost-effectiveness analysis of the integration of robotic spine technology in spine surgery. Neurospine 15:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stumpo V, Staartjes VE, Klukowska AM, Golahmadi AK, Gadjradj PS, Schröder ML et al (2021) Global adoption of robotic technology into neurosurgical practice and research. Neurosurg Rev 44:2675–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta A, Ng JC, Awuah WA, Huang H, Kalmanovich J, Agrawal A, et al. (2022). Embracing robotic surgery in low- and middle-income countries: potential benefits, challenges, and scope in the future. Ann Med Surg [Internet]. [cited 2025 Jun 9];84. Available from: https://journals.lww.com/annals-of-medicine-and-surgery/fulltext/2022/12000/embracing_robotic_surgery_in_low__and.7.aspx [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Authors declare that no new data generated during the work.