Abstract
Molecular graph theory provides a powerful mathematical framework for representing chemical structures, where atoms and bonds are modeled as vertices and edges of a graph. Topological indices, derived from these graphs, serve as numerical descriptors capturing the structural features of molecules. These indices are widely applied in Quantitative Structure–Property Relationship (QSPR) analysis to predict the physicochemical behavior of chemical compounds. In this study, we investigate a novel class of bioactive polyphenols—namely ferulic acid, syringic acid, p-hydroxybenzoic acid, benzoic acid, vanillic acid, and sinapic acid—well known for their antioxidant, anti-inflammatory, antibacterial, anticancer, and antiviral properties. Using several widely recognized degree-based topological indices, we construct molecular graph models of these polyphenols and establish linear regression models correlating the computed indices with essential physicochemical properties. Our QSPR analysis demonstrates strong predictive correlations, highlighting the potential of graph-theoretical descriptors in rational drug design and bioactivity prediction. The results validate the utility of topological indices as efficient computational tools in cheminformatics, offering valuable insights for future applications in pharmaceutical chemistry and material sciences.
Keywords: Polyphenols, Molecular graph, Topological index, QSPR analysis, Cheminformatics
Subject terms: Theoretical chemistry, Computational chemistry, Structure prediction, Cheminformatics, Applied mathematics
Introduction
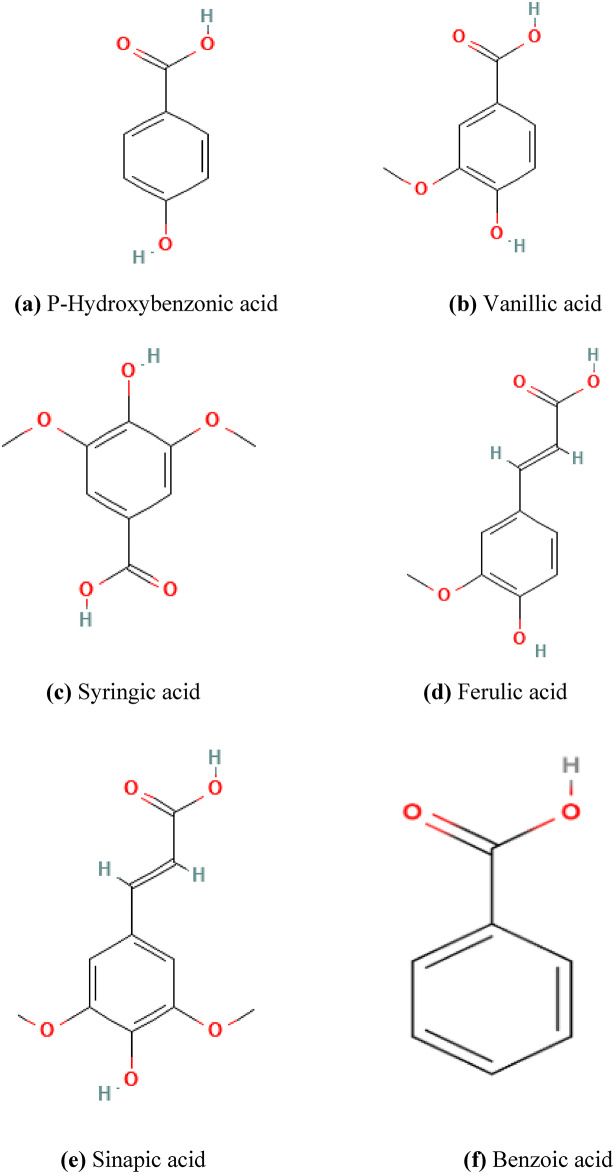
Polyphenols are a diverse group of natural compounds found in plants. They are well-known for having antioxidant qualities that help shield the body from damaging free radicals. Fruits, vegetables, tea, coffee, cocoa, and certain spices are some of the known sources of polyphenols. However, it is important to note that the specific health effects of polyphenols depend on its type and concentration present in different foods. Research suggests that polyphenols have several health benefits. They can reduce the risk of various chronic diseases like heart disease, certain cancers, and neurodegenerative disorders. Polyphenols are secondary bioactive naturally occurring chemicals produced by plants. They have a broad spectrum of bioactivities that support health promotion1,2. Polyphenols can be described as phenolic rings connected to various functional groups. These compounds have gained significant attention and interest due to their multiple applications, ranging from food processing and preservation, to the pharmaceutical industry3–5. The past investigations revealed that, numerous phenols have been used for preparing traditional medicines6. Many deaths worldwide have been attributed to factors like oxidative stress, hypertension, weak immune system, microbial infections, and the development of resistance to antibiotics7. It enables them to assist in treating various illnesses and other medical conditions8. Dietary polyphenols are a diverse class of naturally occurring compounds with two phenyl rings and one or more hydroxyl (O H) groups which belongs to the kingdom Plantae9. Around 4000–8000 currently known polyphenolic substances exclusively includes flavonoids10. A heterogeneous group of phenolic chemicals are called polyphenols11. Flavonoids and phenolic acids are the two main groups of polyphenols. Hydroxycinnamic and Hydroxybenzonic acids are the two subcategories of Phenolic acids12. They are either non-conjugated (as an aglycone) or conjugated with substances, such as glucose, amines, lipids, organic acids, and carboxylic acids1. The structures of some notable polyphenols are shown in the Fig. 1. Polyphenols are also known as secondary metabolites, which are mostly found in the kingdom of plants. Due to the anti-bacterial, anti-oxidant, anti-cancer, anti-hypertensive, immunomodulatory, and anti-inflammatory properties, polyphenols have considerable health-promoting benefits. Therefore, it is the prime objective of this paper to model the molecular topology of these important polyphenols and perform a QSPR analysis to predict the physicochemical properties.
Fig. 1.
Molecular structures of considered Polyphenols.
Chemical graph theory is the branch of graph theory that applies to the mathematical modelling of chemical substances. A molecular graph/ chemical graph is a graph representation of structural interrelation of atoms and chemical bonds among them in a molecule. Chemical graph theory applies mathematical methods to predictions of the properties of chemicals. This approach relates molecular chemical structure to its chemical reactivity, physical behavior, and physicochemical properties. Chemical graph theory is widely applicable to chemical reaction analysis, material design, drug design etc1,13–25. A molecular graph G represents the unsaturated hydrocarbon skeletons of molecules/compounds. The vertex set denoted by V (G) correspond to non-hydrogen atoms. The edge set E(G) of a molecular graph represent covalent bonds between atoms26–30. Omar et al. developed eight derivatives based on the main structure of hydroxychloroquine to treat COVID-19 and used QSAR investigation to calculate the biological activity of the designed compounds. These compounds were evaluated for their biological activity using a method called QSAR investigation31. Havare generated curvilinear regression models for the boiling point of prospective medicines against COVID-19 using multiple topological criteria32.
Gutman, in 197233, defined and formulated The first and second Zagreb indices as
 |
 |
Shirdel et al.34 formulated The Hyper Zagreb index as
 |
The second and third Zagreb index was redefined by Ranjini et al.35 as
 |
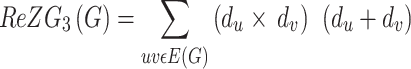 |
Vukičević et al.36 suggested the Symmetric division degree index as
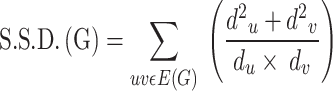 |
Similarly, many other indices can be used in QSPR/QSAR analysis14,26,37–52. Recently, many scientists have shown an increased interest in mathematical chemistry. Since 1988, numerous academic articles on mathematical chemistry are being released annually. Chemical graph theory connects graph theory with chemistry, and produces useful results that chemists can use. The chemical applications of graph theory have been thoroughly discussed in a wide range of works53–68.
Materials and methods
Simple polyphenol graphs are considered for molecular topological modeling. Edge partitioning, Vertex partitioning, and computational techniques of graph theory are applied to compute the topological indices of the six structures under consideration. Regression models are then formulated to compare the computed topological indices with the properties of the considered molecules. The regression analysis was performed using MS Excel software.
Results and discussion
Regression model
Four physical properties (Complexity, Boiling Point (BP), Molecular Weight (MW), and Polar Surface Area (PSA)) are studied for each of the six Polyphenols. Regression analysis is performed for the six polyphenols based on the below model
 |
1 |
where , → constants, → Physical property of the drug, → topological descriptor.
The regression model for the topological indices in question is defined using this linear regression equation. Six polyphenols’ molecular networks’ topological indices are regarded as independent variables. On the other hand, the physical attributes are considered as dependent variables. Models for linear regression are created in MS Excel package. The constants A and B in the regression Eq. (1) can be found by the data in Tables 1 and 2.
Table 1.
Experimental properties.
| Polyphenols | Complexity | Molecular weight (g/mol) | Polar surface area (Ų) | Boling point °C |
|---|---|---|---|---|
| p-Hydroxybenzonic acid | 125 | 138.12 | 57.5 | 335 |
| Vanillic acid | 168 | 168.15 | 66.8 | 353.43 |
| Syringic acid | 191 | 198.17 | 76 | 440 |
| Serulic acid | 224 | 194.18 | 68.8 | 373 |
| Sinapic acid | 249 | 224.21 | 76 | 403.41 |
| Benzoic acid | 104 | 122.12 | 37.3 | 249.2 |
Table 2.
Topological indices values.
| Polyphenols | M1 | M2 | HM | ReZG2 | ReZG3 | SSD |
|---|---|---|---|---|---|---|
| p-Hydroxybenzonic acid | 46 | 50 | 216 | 10.55 | 242 | 24.6667 |
| Vanillic acid | 56 | 63 | 270 | 12.91667 | 316 | 29.3333 |
| Syringic acid | 66 | 76 | 324 | 15.28333 | 390 | 34 |
| Serulic acid | 64 | 70 | 300 | 14.81667 | 338 | 33.6667 |
| Sinapic acid | 74 | 83 | 354 | 17.18333 | 412 | 38.3333 |
| Benzoic acid | 40 | 43 | 182 | 9.4 | 202 | 21 |
For first Zagreb index M1 (G)
 |
For second Zagreb index M2 (G)
 |
For hyper Zagreb index HM (G)
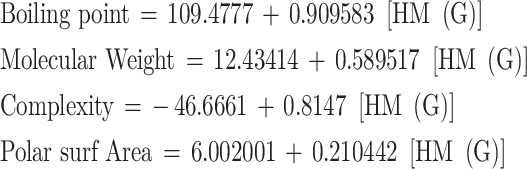 |
For Redefined second Zagreb index ReZG2 (G)
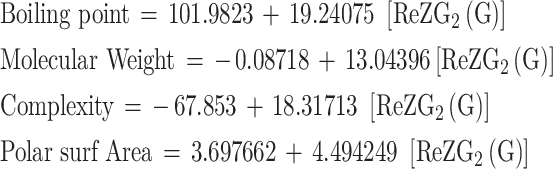 |
For Redefined third Zagreb index RezG3 (G)
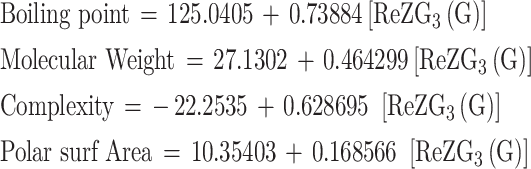 |
For symmetric division degree index SSD(G)
 |
The correlation coefficients
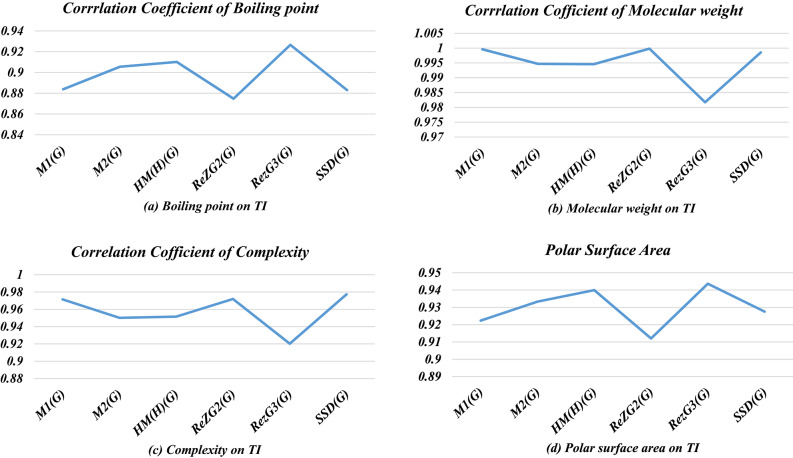
Table 1 lists the four physical parameters of the Polyphenols used in this study, these properties have been taken from Pubchem database. Table 2 shows the six topological indices values, which have been obtained via edge partitioning, vertex partitioning, and computational techniques of graph theory. Table 3 shows the correlation coefficients of six physical attributes and topological indices. From Table 3, it can be observed that, the first Zagreb index shows a strong correlation value (r = 0.992706) for molecular weight. Figure 2 is a graphic depiction of the correlation coefficients of TIs and physical properties. Tables 4, 5, 6, 7, 8 and 9 depict the statistical parameters. The parameter N shows sample size, b is slope, A is a constant and r shows the correlation coefficient. The null hypothesis is tested when each term’s coefficient is equal to zero; the greater the p-value, the more probable it is that changes in the predictor have nothing to do with changes in the responder. In this case, the null hypothesis’s regression coefficients are all zero, yet the test yields a F value. This kind of scenario cannot be predicted by the model. This test can be used to assess whether the coefficients in a model are superior to those without predictor variables. Table 10 gives the standard error of estimation for physical properties of polyphenols under study. Tables 11, 12, 13 and 14 is a comparison of computed and actual values of all physical attributes of polyphenols.
Table 3.
Correlation Coefficients.
| Topological indices | Complexity | Molecular weight | Polar Surface Area | Boiling point |
|---|---|---|---|---|
| M1(G) | 0.971506 | 0.999604 | 0.922344 | 0.883799 |
| M2(G) | 0.950196 | 0.994674 | 0.933317 | 0.905492 |
| HM(H)(G) | 0.951506 | 0.994565 | 0.939974 | 0.910158 |
| ReZG2(G) | 0.971951 | 0.999813 | 0.912038 | 0.87472 |
| RezG3(G) | 0.920267 | 0.981735 | 0.943655 | 0.926583 |
| SSD(G) | 0.977303 | 0.99853 | 0.927559 | 0.882992 |
Fig. 2.
Physical properties on T1.
Table 4.
The statistical parameters for M1.
| Properties | N | A | B | r | r 2 | F | P | Indicator |
|---|---|---|---|---|---|---|---|---|
| BP | 6 | 99.84728 | 4.494093 | 0.883799 | 0.781101 | 14.27324 | 0.01947 | Significant |
| MW | 6 | 0.306809 | 3.014766 | 0.999604 | 0.999207 | 5041.65 | 2.36E-07 | Significant |
| Complexity | 6 | -67.2393 | 4.232474 | 0.971506 | 0.943824 | 67.20491 | 0.001206 | Significant |
| PSA | 6 | 3.143836 | 1.050685 | 0.922344 | 0.850718 | 22.79496 | 0.008812 | Significant |
Table 5.
The statistical parameters for M2.
| Properties | N | A | B | r | r 2 | F | P | Indicator |
|---|---|---|---|---|---|---|---|---|
| BP | 6 | 111.4944 | 3.857334 | 0.905492 | 0.819915 | 18.21178 | 0.012976 | Significant |
| MW | 6 | 12.89697 | 2.513164 | 0.994674 | 0.989377 | 372.5453 | 4.25E-05 | Significant |
| Complexity | 6 | -45.6952 | 3.467977 | 0.950196 | 0.902873 | 37.18327 | 0.003659 | Significant |
| PSA | 6 | 6.581182 | 0.890683 | 0.933317 | 0.871082 | 27.02736 | 0.006522 | Significant |
Table 6.
The statistical parameters for HM.
| Properties | N | A | B | r | r 2 | F | P | Indicator |
|---|---|---|---|---|---|---|---|---|
| BP | 6 | 109.4777 | 0.909583 | 0.910158 | 0.828387 | 19.30822 | 0.011745 | Significant |
| MW | 6 | 12.43414 | 0.589517 | 0.994565 | 0.98916 | 365.0007 | 4.42E-05 | Significant |
| Complexity | 6 | -46.6661 | 0.8147 | 0.951506 | 0.905365 | 38.26746 | 0.00347 | Significant |
| PSA | 6 | 6.002001 | 0.210442 | 0.939974 | 0.883552 | 30.35011 | 0.005296 | Significant |
Table 7.
The statistical parameters for ReZG2.
| Properties | N | A | B | r | r 2 | F | P | Indicator |
|---|---|---|---|---|---|---|---|---|
| BP | 6 | 101.9823 | 19.24075 | 0.87472 | 0.765135 | 13.03106 | 0.02256 | Significant |
| MW | 6 | -0.08718 | 13.04396 | 0.999813 | 0.999627 | 10719.48 | 5.22E-08 | Significant |
| Complexity | 6 | -67.853 | 18.31713 | 0.971951 | 0.944688 | 68.31685 | 0.001169 | Significant |
| PSA | 6 | 3.697662 | 4.494249 | 0.912038 | 0.831813 | 19.78312 | 0.011266 | Significant |
Table 8.
The statistical parameters for ReZG3.
| Properties | N | A | B | r | r 2 | F | P | Indicator |
|---|---|---|---|---|---|---|---|---|
| BP | 6 | 125.0405 | 0.73884 | 0.926583 | 0.858556 | 24.27966 | 0.007887 | Significant |
| MW | 6 | 27.1302 | 0.464299 | 0.981735 | 0.963804 | 106.5096 | 0.000497 | Significant |
| Complexity | 6 | -22.2535 | 0.628695 | 0.920267 | 0.846891 | 22.12514 | 0.009283 | Significant |
| PSA | 6 | 10.35403 | 0.168566 | 0.943655 | 0.890484 | 32.52435 | 0.004673 | Significant |
Table 9.
The statistical parameters for SSD.
| Properties | N | A | B | r | r 2 | F | P | Indicator |
|---|---|---|---|---|---|---|---|---|
| BP | 6 | 89.26778 | 8.941635 | 0.882992 | 0.779675 | 14.15496 | 0.019735 | Significant |
| MW | 6 | -6.76119 | 5.997342 | 0.99853 | 0.997063 | 1357.899 | 3.24E-06 | Significant |
| Complexity | 6 | -78.9525 | 8.479101 | 0.977303 | 0.955122 | 85.13038 | 0.000767 | Significant |
| PSA | 6 | 0.255878 | 2.104229 | 0.927559 | 0.860366 | 24.6463 | 0.007681 | Significant |
Table 10.
Standard error of estimate.
| Topological indices | Complexity | Molecular weight | Polar Surface Area | Boiling point |
|---|---|---|---|---|
| M1(G) | 14.85026 | 1.221258 | 6.329849 | 34.21536 |
| M2(G) | 19.52669 | 4.470514 | 5.882304 | 31.03396 |
| HM(G) | 19.27463 | 4.515985 | 5.590567 | 30.29524 |
| ReZG2(G) | 14.73565 | 0.837719 | 6.718704 | 35.44117 |
| RezG3(G) | 24.51657 | 8.252133 | 5.421615 | 27.50374 |
| SSD(G) | 13.27322 | 2.350681 | 6.121892 | 34.32663 |
Table 11.
Comparison of the computed values generated by regression model of T1 with actual values of boiling points.
| Name | BP | M1 | M2 | HM | ReZG2 | ReZG3 | SSD |
|---|---|---|---|---|---|---|---|
| p- Hydr .acid | 335 | 306.575558 | 304.3611 | 119.0738007 | 304.9722125 | 303.83978 | 309.8284081 |
| Vanillic acid | 353.43 | 351.516488 | 354.506442 | 121.2264834 | 350.5087183 | 358.51394 | 351.5527595 |
| Syringic acid | 440 | 396.457418 | 404.651784 | 123.3791572 | 396.0450317 | 413.1881 | 393.28337 |
| Ferulic acid | 373 | 387.469232 | 381.50778 | 122.9546911 | 387.0661433 | 374.76842 | 390.3031231 |
| Sinapic acid | 403.41 | 432.410162 | 431.653122 | 125.1073649 | 432.6024567 | 429.44258 | 432.0301569 |
| Benzoic acid | 249.2 | 279.611 | 277.359762 | 118.0277802 | 282.84535 | 274.28618 | 277.042115 |
Table 12.
Comparison of the computed values generated by regression model of T1 with actual values of molecular weight.
| Name | MW | M1 | M2 | HM | ReZG2 | ReZG3 | SSD |
|---|---|---|---|---|---|---|---|
| p- Hydr .acid | 138.12 | 138.986045 | 138.55517 | 18.65354435 | 137.526598 | 139.490558 | 141.1734459 |
| Vanillic acid | 168.15 | 169.133705 | 171.226302 | 20.04873655 | 168.3973468 | 173.848684 | 169.1588429 |
| Syringic acid | 198.17 | 199.281365 | 203.897434 | 21.44392285 | 199.2679652 | 208.20681 | 197.148438 |
| Ferulic acid | 194.18 | 193.251833 | 188.81845 | 21.16881885 | 193.1808708 | 184.063262 | 195.1495239 |
| Sinapic acid | 224.21 | 223.399493 | 221.489582 | 22.56400515 | 224.0514892 | 218.421388 | 223.1367201 |
| Benzoic acid | 122.12 | 120.897449 | 120.963022 | 17.9755998 | 122.526044 | 120.918598 | 119.182992 |
Table 13.
Comparison of the computed values generated by regression model of T1 with actual values of complexity.
| Name | Complexity | M1 | M2 | HM | ReZG2 | ReZG3 | SSD |
|---|---|---|---|---|---|---|---|
| p- Hydr .acid | 125 | 127.454504 | 127.70365 | 129.3091 | 125.3927215 | 129.89069 | 130.1989406 |
| Vanillic acid | 168 | 169.779244 | 172.787351 | 173.3029 | 168.7433236 | 176.41412 | 169.7649696 |
| Syringic acid | 191 | 212.103984 | 217.871052 | 217.2967 | 212.0937424 | 222.93755 | 209.336934 |
| Ferulic acid | 224 | 203.639036 | 197.06319 | 197.7439 | 203.5458706 | 190.24541 | 206.5108496 |
| Sinapic acid | 249 | 245.963776 | 242.146891 | 241.7377 | 246.8962894 | 236.76884 | 246.0794224 |
| Benzoic acid | 104 | 102.05966 | 103.427811 | 101.6093 | 104.328022 | 104.74289 | 99.108621 |
Table 14.
Comparison of the computed values generated by regression model of T1 with actual values of Polar surface area.
| Name | PSA | M1 | M2 | HM | ReZG2 | ReZG3 | SSD |
|---|---|---|---|---|---|---|---|
| p-Hydro acid | 57.5 | 51.475346 | 51.115332 | 51.457473 | 51.11198895 | 51.147002 | 52.16026347 |
| Vanillic acid | 66.8 | 61.982196 | 62.694211 | 62.821341 | 61.74839323 | 63.620886 | 61.97922726 |
| Syringic acid | 76 | 72.489046 | 74.27309 | 74.185209 | 72.38475257 | 76.09477 | 71.799664 |
| Ferulic acid | 68.8 | 70.387676 | 68.928992 | 69.134601 | 70.28746633 | 67.329338 | 71.09832447 |
| Sinapic acid | 76 | 80.894526 | 80.507871 | 80.498469 | 80.92382567 | 79.803222 | 80.91791953 |
| Benzoic acid | 37.3 | 45.171236 | 44.880551 | 44.302445 | 45.9436026 | 44.404362 | 44.444687 |
Table 3; Fig. 2 demonstrate that all the topological indices show a good correlation with the appropriate physical characteristic. By examining correlation coefficients, we see that M1(G) index gives the highest correlation value (r = 0.992706) for molecular weight. The second Zagreb index has a high correlation (r = 0.999204) with Complexity, the atom bond connectivity index has a high correlation (r = 0.99985) with polar surface area, the geometric arithmetic index has the highest correlation coefficient (r = 0.999883) for molecular weight, the symmetric division degree gives the best correlated value (r = 0.999484) for polar surface area, and the harmonic index shows good correlation (r = 0.999483) with molecular weight. These results indicate that, the considered topological indices have the potential to predict the properties efficiently and can replace the laborious laboratory experimentations as alternative theoretical tools.
Conclusions
Degree-based topological indices can be used to quantify and analyze the structural features of polyphenolic compounds. By incorporating these indices into QSPR models, we can establish relationships between the structural characteristics of polyphenols and their physical properties. The results demonstrate that all the topological indices show a good correlation with the appropriate physical characteristic. By examining correlation coefficients, we see that M1(G) index gives the highest correlation value (r = 0.992706) for molecular weight. The second Zagreb index has a high correlation (r = 0.999204) with Complexity, the atom bond connectivity index has a high correlation (r = 0.99985) with polar surface area, the geometric arithmetic index has the highest correlation coefficient (r = 0.999883) for molecular weight, the symmetric division degree gives the best correlation value (r = 0.999484) for polar surface area, and the harmonic index shows good correlation (r = 0.999483) with molecular weight. Degree-based topological indices also provide insights into the number of bonds, connectivity patterns, and branching characteristics in polyphenolic compounds. QSPR analysis utilizes these indices and corresponding experimental data on the physical characteristics of polyphenols to develop predictive models.
In future work, the integration of machine learning techniques, such as random forests, support vector machines, and gradient boosting regressors, can enhance the predictive power of QSPR models by capturing complex, non-linear relationships between topological indices and physicochemical properties. Ensemble learning methods, in particular, offer robustness against overfitting and can aggregate predictions from multiple base models to improve generalization. These data-driven approaches can complement traditional linear regression by identifying subtle structural patterns and interactions that may be overlooked in linear models. As more polyphenolic data becomes available, combining degree-based descriptors with advanced regression frameworks could lead to more accurate, scalable, and interpretable models for screening and evaluating polyphenolic compounds in drug discovery, nutraceuticals, and materials chemistry.
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-94).
Author contributions
All the authors Abdul Hakeem, Asad Ullah, Shahid Zaman, Emad E. Mahmoud, Hijaz Ahmad, Parvez Ali and Melaku Berhe Belay have equally contributed to this manuscript in all stages, from conceptualization to the write-up of final draft.
Funding
This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2024-94).
Data availability
All data generated or analyzed during this study are included within this article.
Declarations
Competing interests
The authors declare no competing interests.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT 3.5 in order to improve readability and language of the manuscript. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Asad Ullah, Email: dr.asadullah@kiu.edu.pk.
Shahid Zaman, Email: zaman.ravian@gmail.com.
Melaku Berhe Belay, Email: melaku.berhe@aastu.edu.et.
References
- 1.Abbas, M. et al. Natural polyphenols: an overview. Int. J. Food Prop.20, 1689–1699 (2017). [Google Scholar]
- 2.Rathod, N. B. et al. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf.20, 4182–4210 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Kammerer, D. R., Kammerer, J., Valet, R. & Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int.65, 2–12 (2014). [Google Scholar]
- 4.Sajadimajd, S. et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res.151, 104584 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Inanli, A. G., Tümerkan, E. T. A., Abed, N. E., Regenstein, J. M. & Özogul, F. The impact of Chitosan on seafood quality and human health: A review. Trends Food Sci. Technol.97, 404–416 (2020). [Google Scholar]
- 6.Liu, J. & Henkel, T. Traditional Chinese medicine (TCM): are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem.9, 1483–1485 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Gupta, S. C. et al. Downregulation of tumor necrosis factor and other Proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys.559, 91–99 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Kim, Y. H. et al. Green tea Catechin metabolites exert immunoregulatory effects on CD4 + T cell and natural killer cell activities. J. Agric. Food Chem.64, 3591–3597 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Hanhineva, K. et al. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci.11, 1365–1402 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr.81, 223S–229S (2005). [DOI] [PubMed] [Google Scholar]
- 11.Pandey, K. B. & Rizvi, S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev.2, 270–278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias, R., Pereira, C. B., Pérez-Gregorio, R., Mateus, N. & Freitas, V. Recent advances on dietary polyphenol’s potential roles in Celiac disease. Trends Food Sci. Technol.107, 213–225 (2021). [Google Scholar]
- 13.Havare, Ö. Ç. Topological indices and QSPR modeling of some novel drugs used in the cancer treatment. Int. J. Quantum Chem.121, e26813 (2021). [Google Scholar]
- 14.Zaman, S., Jalani, M., Ullah, A., Ali, M. & Shahzadi, T. On the topological descriptors and structural analysis of cerium oxide nanostructures. Chem. Pap.77, 2917–2922 (2023). [Google Scholar]
- 15.Mondal, S. & Das, K. C. Zagreb connection indices in structure property modelling. J. Appl. Math. Comput. 69, 3005–3020 (2023).
- 16.Mondal, S., Dey, A., De, N. & Pal, A. QSPR analysis of some novel neighbourhood degree-based topological descriptors. Complex. Intell. Syst.7, 977–996 (2021). [Google Scholar]
- 17.Mondal, S., De, N. & Pal, A. On neighborhood Zagreb index of product graphs. J. Mol. Struct.1223, 129210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan, A. R. et al. Computation of differential and integral operators using M-polynomials of gold crystal. Heliyon 10, e34419 (2024). [DOI] [PMC free article] [PubMed]
- 19.Sharma, K., Bhat, V. K. & Liu, J. B. Second leap hyper-Zagreb coindex of certain benzenoid structures and their polynomials. Comput. Theor. Chem.1223, 114088 (2023). [Google Scholar]
- 20.Sharma, K., Bhat, V. K. & Sharma, S. K. On Degree-Based Topological Indices of Carbon Nanocones, ACS Omegapp. 45562–45573 (American Chemical Society, 2022). [DOI] [PMC free article] [PubMed]
- 21.Radhakrishnan, M., Prabhu, S., Arockiaraj, M. & Arulperumjothi, M. Molecular structural characterization of superphenalene and supertriphenylene. Int. J. Quantum Chem.122, e26818 (2022). [Google Scholar]
- 22.Zhang, Q. et al. Mathematical study of silicate and oxide networks through Revan topological descriptors for exploring molecular complexity and connectivity. Sci. Rep.15, 8116 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, Q., Zaman, S., Ullah, A., Ali, P. & Mahmoud, E. E. The Sharp lower bound of tricyclic graphs with respect to the ISI index: applications in octane isomers and benzenoid hydrocarbons. Eur. Phys. J. E. 48, 10 (2025). [DOI] [PubMed] [Google Scholar]
- 24.Tang, J. H. et al. Chemical applicability and predictive potential of certain graphical indices for determining structure-property relationships in polycrystalline acid magenta (C20H17N3Na2O9S3). Sci. Rep.15, 13886 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kara, Y., Özkan, Y. S., Ullah, A., Hamed, Y. S. & Belay, M. B. QSPR modeling of some COVID-19 drugs using neighborhood eccentricity-based topological indices: A comparative analysis. PLoS ONE. 20, e0321359 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah, A., Qasim, M., Zaman, S. & Khan, A. Computational and comparative aspects of two carbon nanosheets with respect to some novel topological indices. Ain Shams Eng. J.13, 101672 (2022). [Google Scholar]
- 27.Ö & Çolakoğlu QSPR modeling with topological indices of some potential drug candidates against COVID-19, Journal of Mathematics, (2022) 1–9. (2022).
- 28.Prabhu, S. et al. Computational Analysis of Some More Rectangular Tessellations of Kekulenes and Their Molecular Characterizations, Molecules, (2023). [DOI] [PMC free article] [PubMed]
- 29.Arulperumjothi, M., Prabhu, S., Liu, J. B., Rajasankar, P. Y. & Gayathri, V. On counting polynomials of certain classes of polycyclic aromatic hydrocarbons. Polycycl. Aromat. Compd.43, 4768–4786 (2023). [Google Scholar]
- 30.Prabhu, S., Arulperumjothi, M., Manimozhi, V. & Balasubramanian, K. Topological characterizations on hexagonal and rectangular tessellations of Antikekulenes and its computed spectral, nuclear magnetic resonance and electron spin resonance characterizations. Int. J. Quantum Chem.124, e27365 (2024). [Google Scholar]
- 31.Wazzan, S. & Ozalan, N. U. Exploring the symmetry of curvilinear regression models for enhancing the analysis of fibrates drug activity through molecular descriptors. Symmetry15, 1160 (2023). [Google Scholar]
- 32.Omar, R. M. K., Najar, A. M., Bobtaina, E. & Elsheikh, A. F. Pryazolylpyridine and Triazolylpyridine Derivative of Hydroxychloroquine as Potential Therapeutic against COVID-19 (Theoretical Evaluation, 2020).
- 33.Gutman, I. & Trinajstić, N. Graph theory and molecular orbitals. Total φ-electron energy of alternant hydrocarbons. Chem. Phys. Lett.17, 535–538 (1972). [Google Scholar]
- 34.Shirdel, G., Rezapour, H. & Sayadi, A. The hyper-Zagreb index of graph operations, DOI (2013).
- 35.Ranjini, P., Lokesha, V. & Usha, A. Relation between phenylene and hexagonal squeeze using harmonic index. Int. J. Graph Theory. 1, 116–121 (2013). [Google Scholar]
- 36.Vukicevic, D. & Gasperov, M. Bond additive modeling 1. Adriatic indices. Croat Chem. Acta. 83, 243 (2010). [Google Scholar]
- 37.Iqbal, Z., Aslam, A., Ishaq, M. & Gao, W. The edge versions of degree-based topological descriptors of dendrimers. J. Cluster Sci.31, 445–452 (2020). [Google Scholar]
- 38.Aslam, A., Ahmad, S., Binyamin, M. A. & Gao, W. Calculating topological indices of certain OTIS interconnection networks. Open. Chem.17, 220–228 (2019). [Google Scholar]
- 39.Khabyah, A. A., Zaman, S., Koam, A. N., Ahmad, A. & Ullah, A. Minimum Zagreb eccentricity indices of two-mode network with applications in boiling point and benzenoid hydrocarbons. Mathematics10, 1393 (2022). [Google Scholar]
- 40.Zaman, S., Yaqoob, H. S. A., Ullah, A. & Sheikh, M. QSPR analysis of some novel drugs used in blood Cancer treatment via degree based topological indices and regression models, polycyclic aromatic compounds, (2023). 10.1080/10406638.2023.2217990 1–17 .
- 41.Zaman, S. et al. Three-Dimensional Structural Modelling and Characterization of Sodalite Material Network concerning the Irregularity Topological Indices, Journal of Mathematics, (2023) 1–9. (2023).
- 42.Zaman, S., Jalani, M., Ullah, A., Saeedi, G. & Guardo, E. Structural Analysis and Topological Characterization of Sudoku Nanosheet, Journal of Mathematics, (2022) 1–10. (2022).
- 43.Zaman, S., Jalani, M., Ullah, A., Ahmad, W. & Saeedi, G. Mathematical analysis and molecular descriptors of two novel metal–organic models with chemical applications. Sci. Rep.13, 5314 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullah, A., Zaman, S., Hussain, A., Jabeen, A. & Belay, M. B. Derivation of mathematical closed form expressions for certain irregular topological indices of 2D nanotubes. Sci. Rep.13, 11187 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ullah, A., Zaman, S., Hamraz, A. & Muzammal, M. On the construction of some bioconjugate networks and their structural modeling via irregularity topological indices. Eur. Phys. J. E. 46, 72 (2023). [DOI] [PubMed] [Google Scholar]
- 46.Ullah, A. et al. Network-Based Modeling of the Molecular Topology of Fuchsine Acid Dye with Respect to Some Irregular Molecular Descriptors, Journal of Chemistry, (2022) 1–8. (2022).
- 47.Ullah, A., Shamsudin, S., Zaman, A. & Hamraz Zagreb connection topological descriptors and structural property of the triangular chain structures. Phys. Scr.98, 025009 (2023). [Google Scholar]
- 48.Ullah, A., Bano, Z. & Zaman, S. Computational aspects of two important biochemical networks with respect to some novel molecular descriptors. J. Biomol. Struct. Dynamics. 1–15. 10.1080/07391102.2023.2195944 (2023). [DOI] [PubMed]
- 49.Hayat, S. & Asmat, F. Sharp Bounds on the Generalized Multiplicative First Zagreb Index of Graphs with Application to QSPR Modeling, Mathematics, (2023).
- 50.Khan, A. et al. Computational and topological properties of neural networks by means of graph-theoretic parameters. Alexandria Eng. J.66, 957–977 (2023). [Google Scholar]
- 51.Hayat, S., Mahadi, H., Alanazi, S. J. F. & Wang, S. Predictive potential of eigenvalues-based graphical indices for determining thermodynamic properties of polycyclic aromatic hydrocarbons with applications to polyacenes. Comput. Mater. Sci.238, 112944 (2024). [Google Scholar]
- 52.Saravanan, B., Prabhu, S., Arulperumjothi, M., Julietraja, K. & Siddiqui, M. K. Molecular structural characterization of supercorenene and Triangle-Shaped discotic graphene. Polycycl. Aromat. Compd.43, 2080–2103 (2023). [Google Scholar]
- 53.Golbraikh, A., Bonchev, D. & Tropsha, A. Novel ZE-isomerism descriptors derived from molecular topology and their application to QSAR analysis. J. Chem. Inf. Comput. Sci.42, 769–787 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Das, K. C., Gutman, I. & Furtula, B. On atom-bond connectivity index. Chem. Phys. Lett.511, 452–454 (2011). [Google Scholar]
- 55.Hakeem, A., Ullah, A. & Zaman, S. Computation of some important degree-based topological indices for γ-graphyne and zigzag Graphyne nanoribbon. Mol. Phys. 121, e2211403 (2023).
- 56.Liu, J. B., Zheng, Y. Q. & Peng, X. B. The statistical analysis for Sombor indices in a random polygonal chain networks. Discrete Appl Math.338, 218–233 (2023). [Google Scholar]
- 57.Liu, J. B., Zheng, Q., Cai, Z. Q. & Hayat, S. On the laplacians and normalized laplacians for graph transformation with respect to the Dicyclobutadieno derivative of [n]Phenylenes. Polycycl. Aromat. Compd.42, 1413–1434 (2022). [Google Scholar]
- 58.Liu, J. B., Xie, Q., Gu, J. J. & Wu, S. Statistical Analyses of a Class of Random Pentagonal Chain Networks with respect to Several Topological Properties, Journal of Function Spaces, (2023) 1–17. (2023).
- 59.Das, K. C., Mondal, S. & Raza, Z. On Zagreb connection indices. Eur. Phys. J. Plus. 137, 1242 (2022). [Google Scholar]
- 60.Balasubramaniyan, D. & Chidambaram, N. On some neighbourhood degree-based topological indices with QSPR analysis of asthma drugs. Eur. Phys. J. Plus. 138, 823 (2023). [Google Scholar]
- 61.Zhao, D. et al. Topological analysis of entropy measure using regression models for silver iodide. Eur. Phys. J. Plus. 138, 805 (2023). [Google Scholar]
- 62.Arockiaraj, M. et al. QSPR analysis of distance-based structural indices for drug compounds in tuberculosis treatment. Heliyon10, e23981 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raza, Z., Arockiaraj, M., Maaran, A., Kavitha, S. R. J. & Balasubramanian, K. Topological entropy characterization, NMR and ESR spectral patterns of Coronene-Based transition metal organic frameworks. ACS Omega. 8, 13371–13383 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arockiaraj, M., Paul, D., Ghani, M. U., Tigga, S. & Chu, Y. M. Entropy structural characterization of zeolites BCT and DFT with bond-wise scaled comparison. Sci. Rep.13, 10874 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shanmukha, M. C., Gowtham, K. J., Usha, A. & Julietraja, K. Expected values of Sombor indices and their entropy measures for graphene. Mol. Phys.122, e2276905 (2024). [Google Scholar]
- 66.Kirana, B., Shanmukha, M. C. & Usha, A. Comparative study of Sombor index and its various versions using regression models for top priority polycyclic aromatic hydrocarbons. Sci. Rep.14, 19841 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shanmukha, M. C. et al. Chemical applicability and computation of K-Banhatti indices for benzenoid hydrocarbons and triazine-based covalent organic frameworks. Sci. Rep.13, 17743 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Govardhan, S., Roy, S., Prabhu, S. & Arulperumjothi, M. Topological characterization of cove-edged graphene nanoribbons with applications to NMR spectroscopies. J. Mol. Struct.1303, 137492 (2024). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included within this article.