Abstract
Telomerase is up-regulated in the vast majority of human cancers and serves to halt the progressive telomere shortening that ultimately blocks would-be cancer cells from achieving a full malignant phenotype. In contrast to humans, the laboratory mouse possesses long telomeres and, even in early generation telomerase-deficient mice, the level of telomere reserve is sufficient to avert telomere-based checkpoint responses and to permit full malignant progression. These features in the mouse provide an opportunity to determine whether enforced high-level telomerase activity can serve functions that extend beyond its ability to sustain telomere length and function. Here, we report the generation and characterization of transgenic mice that express the catalytic subunit of telomerase (mTERT) at high levels in a broad variety of tissues. Expression of mTERT conferred increased telomerase enzymatic activity in several tissues, including mammary gland, splenocytes, and cultured mouse embryonic fibroblasts. In mouse embryonic fibroblasts, mTERT overexpression extended telomere lengths but did not prevent culture-induced replicative arrest, thus reinforcing the view that this phenomenon is not related to occult telomere shortening. Robust telomerase activity, however, was associated with the spontaneous development of mammary intraepithelial neoplasia and invasive mammary carcinomas in a significant proportion of aged females. These data indicate that enforced mTERT expression can promote the development of spontaneous cancers even in the setting of ample telomere reserve.
Telomerase—the reverse transcriptase that synthesizes telomeric repeats—is present at low levels in human tissue stem cells, progenitor cells, and germ cells, and is undetectable in the vast majority of adult somatic tissues. Insufficient telomerase activity and the inability of DNA polymerase to replicate the extreme ends of chromosomes lead to telomere attrition with each round of cell division in the setting of organ renewal with advancing age (1), high-turnover disease states (2), and passage in culture (3). During human tumorigenesis, telomerase becomes reactivated by transcriptional up-regulation of TERT, the catalytic subunit of telomerase (4). One critical function served by TERT reactivation during cancer progression is to avert the adverse consequences of telomere shortening and loss of chromosomal capping function (5). Less clear, however, is whether the prooncogenic activity of telomerase extends beyond its role in maintaining telomere function.
In human cells, progressive shortening of telomeres precipitates replicative senescence after 60–80 divisions of primary human fibroblasts (3, 6) and crisis after extended division of cells expressing viral oncoproteins (7, 8). Introduction of telomerase into primary human cells stabilizes telomeres, prevents both senescence and crisis, and endows cells with unlimited proliferative potential (9). The ability of telomerase to rescue cells from the adverse consequences of telomere dysfunction is likely critical for its role in facilitating malignant transformation of primary human cells (10) and in maintaining the viability of established cancer cells (11–14).
Similarly, in telomerase-deficient mice, telomere shortening can limit tumorigenesis by activating checkpoints and by compromising chromosomal stability (15–17). However, impaired telomere function and associated genomic instability can also fuel the initiation of neoplastic transformation, particularly in p53-deficient cells (17–19). These complex telomere dynamics do not seem to play a role in wild-type laboratory mice where long telomeres and baseline telomerase expression preclude the development of critical telomere shortening.
Despite these ample telomere reserves, telomerase is up-regulated during tumorigenesis (15, 20, 21) and after mitogen stimulation of lymphocytes in laboratory mice (22, 23). These data raise the possibility that telomerase activation might influence proliferation or survival pathways even in the absence of critically short telomeres. The long telomeres of mice and the lack of critical telomere shortening in mouse tissues provide an in vivo model system in which to evaluate the impact of enforced TERT expression in a setting where telomere length is not limiting. Thus, to uncover unanticipated biological roles of telomerase distinct from its well established role in averting senescence and crisis responses, we have generated a transgenic (Tg) mouse that possesses high level, constitutive mTERT expression in a broad variety of somatic tissues throughout embryonic and postnatal life.
Materials and Methods
Generation of Tg Mice.
An EcoRI fragment comprising the mTERT ORF was cloned into the EcoRI site of pCAGS (24), placing it under control of β-actin promoter and cytomegalovirus (CMV) enhancer elements. Plasmid sequences were excised and the DNA was injected into FVB/N oocytes. Transgene-positive mice were mated with FVB/N mice to generate mTERT Tg and non-Tg mice on the same inbred FVB/N background for all subsequent analyses.
Northern Blots and Telomerase Repeat Amplification Protocol (TRAP) Assays.
RNA was isolated from organs or cells by means of homogenization in Trizol. Ten to 20 μg of total RNA was fractionated on a 1% agarose gel containing 0.7% formaldehyde, transferred to nylon membrane, and hybridized with mTERT or mTERC 32P-labeled probes. For TRAP assays, S-100 extracts were prepared from 50–100 mg of tissue, and telomerase extension and PCR amplification of telomerase addition products were performed as described (25). Reaction products were fractionated on an 8% acrylamide/0.5X Tris-borate-EDTA gel, dried, and exposed to film.
Telomere Length Analysis.
Telomere lengths in mouse embryonic fibroblasts (MEFs) were analyzed by immobilizing 5 × 104 cells in low-melt agarose, lysing the cells, and digesting the genomic DNA in the agarose plug with RsaI and HinFI. Agarose plugs were loaded on 1% agarose gels, and the DNA was fractionated by field inversion gel electrophoresis. The gel was dried and hybridized directly to a (TTAGGG)3 radiolabeled oligonucleotide. Terminal restriction fragments were visualized by autoradiography.
Proliferation Assays.
Proliferation of mTERT Tg and non-Tg littermate MEFs were compared by using the 3T9 protocol. At each passage, 9 × 105 cells were plated in 6-cm tissue culture dishes, grown in DMEM plus 10% FBS and 1% penicillin/streptomycin, and harvested 3 days later. Population doublings were calculated as log2 (number harvested/number plated) and the experiment was continued for 19 passages. Single cell suspensions from thymus, spleen, and bone marrow were prepared by disaggregating organs in complete media (RPMI 1640/10% FBS/0.1% penicillin/streptomycin), followed by hypotonic lysis of red blood cells. Cells were plated in triplicate (2 × 105 thymocytes and splenocytes or 7.5 × 104 bone marrow cells) in 96-well plates and cultured for 48 h in the presence or absence of mitogen. Cells were pulsed with [3H]thymidine for the final 16 h of growth and incorporated [3H]thymidine was measured.
Tumor Incidence and Analysis.
For carcinogen experiments, 6-day-old mice were treated with a single dose of 0.25 μg of 7,12-dimethylbenz[a]anthracene (DMBA) in acetone to their dorsal surface. To generate compound mTERT Tg INK4A−/− mice, mTERT Tg mice were mated with INK4A+/− mice that had been backcrossed to the FVB/N strain (N7). All mice were examined closely for evidence of ill health or overt tumor growth. Mice were killed if profoundly ill or if external tumors exceeded 2 cm in diameter. Statistical significance was measured with Kaplan–Meier analysis and the log-rank test. Five micrometer paraffin sections were stained with hematoxylin and eosin.
Results
Broad Expression of mTERT mRNA and Increased Telomerase Activity in mTERT Tg Mice.
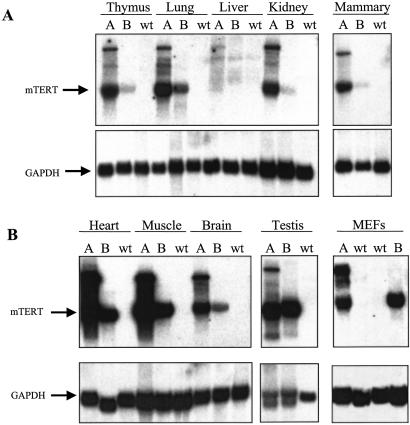
Three founder lines (A, B, and C) were generated in which expression of the mTERT ORF was controlled by cis-regulatory elements that direct high-level transgene expression in most adult tissues (24). In the C line, the mTERT transgene segregated only with male mice, consistent with an integration event on the Y chromosome. To allow comparable analyses of both genders in the carcinogenesis experiments, most subsequent experiments were performed on founder lines A and B. Robust mTERT mRNA expression was evident by Northern blot in thymus, lung, kidney, mammary gland, heart, skeletal muscle, spleen, and skin (Fig. 1 A and B and data not shown). Levels of mTERT mRNA expression were significantly greater in line A than in line B, although tissue-specific distribution patterns were similar in both lines. Endogenous mTERT mRNA was not detected by Northern blot analysis of organs and MEFs from non-Tg littermate controls (Fig. 1 A and B), consistent with low levels of mTERT in wild-type mice.
Figure 1.
Northern blot showing TERT mRNA expression in tissues of mTERT Tg mice. Twenty micrograms of total RNA were analyzed for each tissue except mammary gland, for which 10 μg was used. Samples included thymus, lung, liver, kidney, mammary gland (A), heart, skeletal muscle, brain, testis, and MEFs (B). For each organ, RNA was isolated from founder line A mTERT Tg mice, founder line B mTERT Tg mice, and from non-Tg controls (wt, wild type). The membranes were first probed for mTERT then stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control.
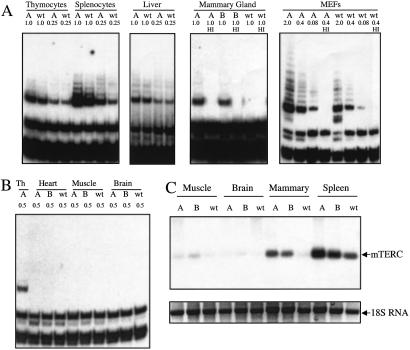
In mTERT Tg mice, telomerase activity was elevated but only in a subset of the organs in which abundant transgene-encoded mTERT mRNA was documented. Increased telomerase activity was detected by the TRAP in mTERT Tg extracts from splenocytes, mammary gland, and MEFs, whereas extracts from thymocytes and liver showed comparable TRAP activity in mTERT Tg mice and in non-Tg controls (Fig. 2A). High-level mTERT transgene expression was insufficient to confer detectable telomerase activity on some organs such as heart, skeletal muscle, and brain, which lack basal telomerase in wild-type mice (Fig. 2B). This dissociation between mTERT transgene mRNA levels and telomerase activity in a subset of organs suggests posttranscriptional regulation of mTERT or insufficient levels of mTERC, the RNA component of telomerase, in those particular tissues. Steady-state mTERC levels were much lower in organs that lacked telomerase activity, such as skeletal muscle and brain, compared with organs with telomerase activity, such as spleen (Fig. 2C). These limiting levels of mTERC in skeletal muscle and brain explain in part the inability of mTERT Tg expression to confer detectable telomerase activity on these organs. Notably, mTERC levels increased significantly in the spleen and mammary glands of mTERT Tg mice (Fig. 2C), indicating that in certain tissue compartments, such as the mammary gland and spleen, mTERT overexpression can lead to increased mTERC steady-state levels and increased telomerase activity.
Figure 2.
Telomerase activity and mTERC RNA levels in tissues and cells from mTERT Tg mice. (A) TRAP assays on extracts from thymocytes, splenocytes, and liver from A line mTERT Tg mice and non-Tg littermate controls. Amount of extract is indicated in micrograms. Untreated or heat-inactivated (HI) extracts (1 μg) from virgin A and B line mTERT Tg and wild-type (wt) control mammary glands were assayed for TRAP activity. Five-fold serial dilutions and HI extract were assayed for mTERT Tg or wt littermate MEFs. (B) Lack of telomerase activity in Tg heart, skeletal muscle, and brain. Amount of extract is indicated. Thymocytes were used as a positive control. (C) Northern blot analysis of steady-state mTERC levels. Twenty micrograms of total RNA was analyzed from skeletal muscle, brain, mammary gland, and spleen from A and B line mTERT Tg mice and non-Tg controls. A photograph of ethidium bromide-stained 18S RNA is shown as a loading control.
Increased Telomerase and Longer Telomeres Are Not Sufficient to Bypass Senescence in Primary MEFs.
To determine whether elevated mTERT mRNA levels and increased telomerase activity altered the length of telomeres, field inversion gel electrophoresis was used to measure the telomere lengths of MEFs from founder lines A and B. In wild-type MEFs, mean telomere lengths were approximately 50 kb, consistent with previous measurements (26). However, in mTERT Tg littermate MEFs, telomeres were elongated significantly to a mean length of approximately 70 kb in both Tg lines examined (P = 0.018, Fig. 3 A and B). These data indicate that the level of telomerase expression can be a determinant of telomere length in vivo.
Figure 3.
Telomere lengths and proliferation assays for MEFs and hematopoietic cells. (A) Terminal restriction fragments from mTERT Tg MEFs or non-Tg littermate MEFs were resolved by field inversion gel electrophoresis. MEF cultures were from littermate embryos from the A mTERT Tg line (lanes 1–3) and the B Tg line (lanes 4–7). (B) Mean telomere lengths are shown in adjacent bar graph, 50 kb non-Tg mice and 70 kb for TERT Tg littermates (P = 0.018 by Student's t test). (C) 3T9 assay on independent MEF cultures from wild-type embryos (gray circles) and littermate TERT Tg embryos (black triangles). Each point represents the mean of 13 independent cultures from mTERT Tg MEFs (7 from line A and 6 from line B) or the mean of 12 independent cultures from littermate non-Tg controls. Mitogen proliferation assays from thymocytes and splenocytes (D) and bone marrow cells (E). Cells were stimulated with the indicated mitogens and proliferation was assayed by measuring incorporation of [3H]thymidine. Each bar represents the mean of triplicates. Error bars show SD.
MEFs enter a culture-induced slow-growth phase that occurs in the setting of long telomeres, detectable levels of telomerase activity, and after relatively few cell divisions. It is therefore unlikely that telomere shortening contributes to this replicative arrest; however, it remains formally possible that a single or limited subset of very short telomeres become uncapped with limited passage. To test this possibility, mTERT Tg and non-Tg MEF cultures were assayed by means of the 3T9 protocol that measures proliferation as a function of passage number (27). Thirteen MEF cultures from mTERT Tg embryos (7 from line A and 6 from line B) and 12 MEF cultures from non-Tg littermates were analyzed. Ten of 13 mTERT Tg MEF cultures (76%) and 8 of 12 non-TG littermate MEF cultures (67%) exhibited dramatic slowing of growth between passages 7 and 10, consistent with a period of senescence (Fig. 3C). There was no significant difference in the rate or timing of growth arrest in each group (P = 0.4). These data indicate that mTERT overexpression and concomitant increased telomerase activity and telomere elongation do not prevent passage-induced growth arrest of MEF cultures, further supporting the view that this “senescence” response is not a result of critical telomere shortening.
mTERT Overexpression Does Not Alter Sensitivity of Hematopoietic Cells to Mitogen Stimulation.
The observation that telomerase is up-regulated during mitogenic stimulation of lymphocytes in humans and in mice prompted us to evaluate the effect of constitutive mTERT expression on the hematopoietic compartment. There were no significant differences between mTERT Tg mice and non-Tg controls in percentages of red blood cell precursors or granulocyte precursors in the bone marrow, in CD4/CD8 ratios in the thymus, in T cell/B cell ratios in spleen or in peripheral lymph nodes, or in histology of bone marrow, spleen, and thymus (data not shown). To determine whether telomerase overexpression altered proliferation rates of hematopoietic cells, we measured rates of [3H]thymidine incorporation in thymocytes, splenocytes, and bone marrow cells from 6-week-old mTERT Tg and non-Tg littermate controls in response to a panel of mitogens. There were no significant differences in proliferation between the two groups (Fig. 3 D and E), indicating that mTERT overexpression does not alter the responsiveness of hematopoietic cells to mitogenic signals.
mTERT Transgene Expression Modifies Neither Sensitivity to DMBA Nor the Cancer-Prone Phenotype of Ink4A/Arf-Deficient Mice.
To assess whether TERT overexpression influences the rate of tumor formation in vivo, mTERT Tg pups and wild-type littermates were treated with the mutagen DMBA. Forty-seven mTERT Tg mice and 36 wild-type littermates were studied, approximately half from the A founder line and half from the B founder line. Mice in both groups began to develop tumors at approximately 15 weeks of age and showed no difference in median tumor incidence (45.4 weeks, mTERT Tg and 44.7 weeks, wild type; P = 0.52) (see Fig. 6A, which is published as supporting information on the PNAS web site, www.pnas.org). Histological analysis revealed no difference in the type of tumors derived from each group: one-third were lymphomas and the remaining two-thirds were adenocarcinomas of the lung (Fig. 6A). The large numbers of lung adenocarcinomas in this study are attributed to an identified susceptibility of the FVB strain to this tumor type (28).
The importance of Ink4a/Arf and telomere dynamics in processes of senescence and cancer prompted an assessment of their genetic interactions in vivo. Eighty-three mTERT Tg Ink4a/Arf−/− mice and 75 non-Tg Ink4a/Arf−/− littermates were studied. Median tumor incidence was 35.1 weeks in the TERT Tg Ink4a/Arf−/− cohort and 33.1 weeks in the non-Tg Ink4a/Arf−/− controls (P = 0.42), indicating that enforced mTERT expression in many tissues does not alter the high rate of spontaneous tumor formation in Ink4a/Arf−/− mice (Fig. 6B). Histological analysis of tumors derived from these mice revealed a majority of lymphomas and fewer sarcomas, a tumor spectrum typical of Ink4a/Arf-null mice (ref. 29; Fig. 6B).
TERT Transgenesis Promotes Spontaneous Breast Cancer and Mammary Intraepithelial Neoplasia (MIN) in Aging Female Mice.
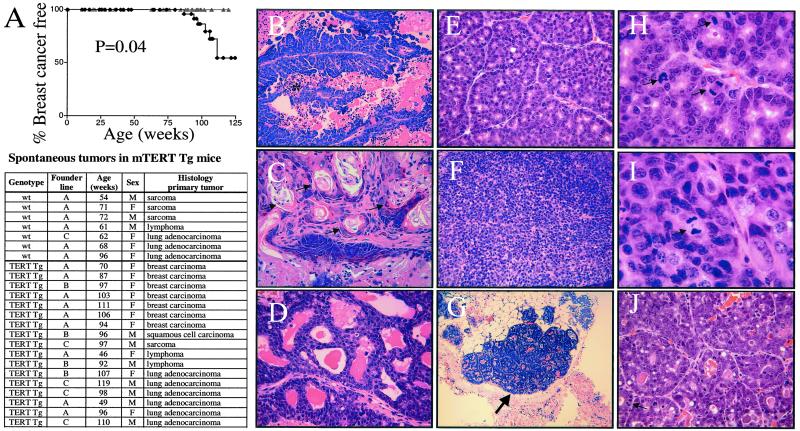
The rapid onset of tumors in the model systems discussed previously may mask a more subtle effect of mTERT overexpression on tumorigenesis over the lifetime of the animal. To address this issue, 116 mTERT Tg mice (49 females and 67 males) from all three founder lines and 96 non-Tg littermate controls (53 females and 43 males) were monitored for signs of ill health and overt tumor development. Histological analysis of the 7 tumors in 96 wild-type mice revealed sarcoma, lymphoma, and lung adenocarcinoma, a spectrum of tumors expected from previous analyses of FVB mice. Seventeen mice presented with 20 tumors in the 116 mTERT Tg mice examined (Fig. 4A). Significantly, in contrast to the non-Tg littermates, mTERT Tg mice developed spontaneous breast carcinomas in addition to the same background of lung adenocarcinomas, lymphomas, and sarcomas observed in the non-Tg cohort. No breast cancers were detected in wild-type littermate mice, consistent with the reported resistance of the FVB strain to spontaneous breast cancer (28). Kaplan–Meier analysis of breast cancer incidence in female mTERT Tg mice and non-Tg controls showed a statistically significant susceptibility to breast cancer in the mTERT Tg cohort (Fig. 4A). These breast cancers were invasive, exhibited diverse histological patterns, and showed malignant characteristics including nuclear pleiomorphism, anisocytosis, increased nuclear/cytoplasmic ratios, and high mitotic activity (Fig. 4 B–J).
Figure 4.
TERT transgene expression promotes breast cancer in aging mice. (A) Kaplan–Meier analysis of breast cancer incidence in female mice, mTERT Tg (black diamonds), and non-Tg littermate controls (gray triangles). Tumor spectrum in aging mice (Table). (B–J) Histopathology of breast cancers in mTERT Tg mice. Representative histologic patterns of papillary (B), adenosquamous (C), cribiform (D), acinar (E and H), and solid (F and I) invasive breast cancers and preinvasive MIN lesions (G and J) are shown. In B, * indicates necrosis. In C, arrows indicate areas of squamous differentiation. In G, the large arrow indicates an in situ lesion. In H–J, the arrows indicate mitotic figures. Magnification: ×25 (G), ×100 (B–F and J), and ×250 (H and I).
Adjacent to the major tumor masses, and in anatomically separate mammary glands of breast tumor-bearing mice, foci of alveolar epithelial hyperplasia were also present (Fig. 4 G and J). These lesions thus exhibited features of MIN, a preinvasive precursor to breast carcinoma in the mouse (30). The presence of these in situ neoplastic lesions in anatomically distinct mammary glands indicated that mTERT overexpression may promote formation of MIN lesions at high rates.
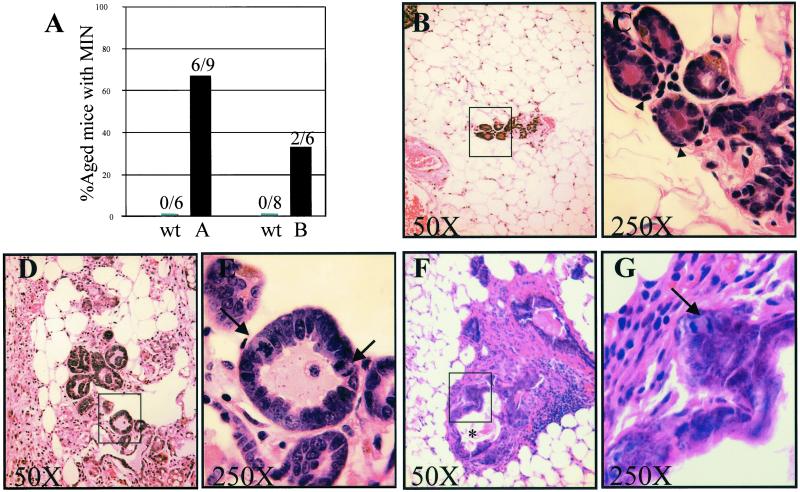
To determine the frequency of MIN lesions in the population, an extensive histological survey was conducted on the mammary glands of tumor-free virgin females from A and B founder lines along with age-matched non-Tg littermate controls. Nine healthy mTERT Tg mice from the A founder line and 6 non-Tg controls were analyzed at ages ranging from 92 to 114 weeks. Whole-mount analysis indicated that mTERT Tg expression did not alter the normal pattern of mammary ductal and lobular development (data not shown). In contrast, histological analysis of 30–40 sections obtained through a single pair of inguinal mammary glands from each mouse revealed neoplastic MIN foci in 6 of 9 (67%) mTERT Tg A line mice compared with 0 of 6 age-matched non-Tg controls (Fig. 5). Similar analysis of mTERT Tg mice from the B founder line with a mean age of 60 weeks revealed MIN lesions in 2 of 6 (33%) mTERT Tg mice, and in 0 of 8 age-matched non-Tg controls (Fig. 5). The lower penetrance of the MIN phenotype in the B line compared with the A line is consistent with the younger ages of the B line mTERT Tg cohort and the lower levels of mTERT transgene in the B line mammary gland. Together, these data indicate that enforced expression of mTERT promotes formation of early neoplastic lesions in the mammary gland with high penetrance and that these incipient lesions can progress to invasive breast adenocarcinoma in aged females.
Figure 5.
High penetrance of MIN in mTERT Tg mammary glands. (A) Bar graph indicating penetrance of MIN in virgin female mTERT Tg (black bars) and non-Tg age-matched littermate control mice (gray bars). MIN lesions were seen in 6/9 A line mTERT Tg mice compared with 0/6 non-Tg control mice (left bars, age range 92–114 weeks). MIN lesions were found in 2/6 B line mTERT Tg mice compared with 0/8 non-Tg mice (right bars, mean age 60 weeks). (B and C) Normal mammary tissue from aged non-Tg mouse. (D and E) Histology of MIN lesion in aged A line mTERT Tg mouse. Note that the cells are enlarged and pleiomorphic with hyperchromatic nuclei, increased N/C ratios, and elevated mitotic activity (arrows in E). The glands in the MIN lesion lack the normal basal cell layer seen in the non-Tg sample (arrowheads in C). (F and G) Histology of MIN lesion in B line mTERT Tg mouse. Note the thickened epithelial layers, stromal proliferation, necrosis (asterisk, F), and mitotic figures (arrow, G). Magnification: ×25 (B, D, and F) and ×100 (C, E, and G).
Discussion
mTERT Overexpression Promotes Breast Cancer in the Setting of Long Telomeres.
In this study, abundant mTERT expression was without discernable effects in most tissues, even those with documented increased telomerase activity and telomere lengths. In contrast, enforced mTERT expression in mammary tissue was associated with highly penetrant MIN lesions and increased incidence of invasive breast cancer in aging females. Thus, whereas mTERT overexpression is well tolerated in the vast majority of cell types, elevated mTERT levels encourage the neoplastic transformation of mammary epithelium in the mouse. Two aspects of these results were unexpected: first, that TERT should promote cancer in the setting of ample telomere reserve, and second, that a cancer-prone phenotype should be limited to the mammary gland despite broad transgene expression.
The expectation for a role for telomerase only in the setting of short telomeres derives in part from characterization of telomerase-deficient mice that lack mTERC. First-generation mTERC−/− mice are viable and do not exhibit the severe defects in organ renewal and homeostasis seen in late-generation mTERC−/− mice (31). Similarly, first-generation mTERC−/− mice do not exhibit the significant resistance to spontaneous cancer typical of later generation mTERC−/− mice with short, dysfunctional telomeres (15–17). These studies clearly established that critical telomere shortening, rather than loss of telomerase enzymatic activity, impairs proliferation of normal cells and limits tumor formation in vivo. The view that the role of telomerase is restricted to telomere maintenance also stems from two comprehensive studies in primary human cells showing the ability of hTERT transduction to promote immortal growth while apparently maintaining normal growth regulatory and checkpoint circuits (32, 33).
Notwithstanding these observations, emerging evidence has pointed to additional activities for TERT beyond those involved in telomere maintenance. In mice with ample telomere reserves, overexpression of mTERT under control of a keratin-5 promoter rendered these Tg mice more susceptible to papilloma formation when treated with chemical carcinogens that included phorbol esters and DMBA. In our study, we observed no susceptibility to spontaneous or DMBA-induced papillomas in mTERT Tg mice. These differences may relate to the absence of phorbol esters in our study and/or to differences in the levels or keratinocyte compartments of mTERT transgene expression in the two studies (34). More pertinent to our study is the recent observation that hTERT overexpression increases transforming growth factor-β resistance in primary human mammary cells that have epigenetically silenced p16INK4a (35). Together, these data suggest that robust TERT expression can influence key cell signaling pathways, at least in mammary epithelial cells. These findings should encourage a more comprehensive analysis of such pathways in a full spectrum of cell types.
Increased mTERC and Telomerase Activity in mTERT Tg Mammary Glands.
The tissue-restricted effect of mTERT may relate to at least two factors: efficiency of holoenzyme assembly and tissue-specific sensitivity to mTERT overexpression. Increased telomerase activity was seen in mTERT Tg mammary gland, splenocytes, and MEFs, whereas telomerase activity was comparable in thymocytes and liver from Tg and non-Tg controls. We showed that mTERT Tg mRNA levels in heart, skeletal muscle, and brain were among the highest of all tissues in the mTERT Tg mouse, yet these levels were insufficient to confer significant telomerase activity, perhaps because of low levels of mTERC. Baseline mTERC levels were comparably low in mammary gland; however, in marked contrast to skeletal muscle and brain, mTERT Tg expression in mammary gland conferred significant telomerase activity. The efficient assembly of the telomerase holoenzyme in the mammary gland does not adequately explain the increased susceptibility to breast cancer, because splenocytes and MEFs showed similar increases in telomerase activity without a corresponding increase in rates of spontaneous lymphoma or mesenchymal cancers. Together, these data suggest that the sensitivity to mTERT overexpression is tissue-specific and that the oncogenic activity of mTERT is especially prominent in the mammary gland.
Two Functions for Telomerase.
Finally, our data indicate that telomerase activation during tumorigenesis may serve two purposes: one in stabilizing short telomeres and averting the deleterious consequences of telomere dysfunction and another that does not depend on a setting of limited telomere reserve. Although the former activity may be important in the majority of human cancers, the latter activity may promote carcinogenesis in only a subset of tissues, including the mammary gland and skin. Notably, whereas telomerase is up-regulated in most human solid tumors at the invasive stage, in breast cancers, telomerase is reactivated in 80–90% of cases at the preinvasive ductal carcinoma in situ (DCIS) stage (36), and hTERT mRNA is similarly up-regulated in nearly 100% cases of DCIS (37). Reactivation of telomerase at this stage may both stabilize telomeres and promote growth and maturation of these incipient lesions. Our data also question the notion that telomerase expression can be safely used to immortalize human cells for therapeutic purposes without concomitant increased risk for malignancy. A better understanding of the latter mechanism may guide the appropriate use of somatic telomerase therapy for chronic high-turnover disease states and enhance the effectiveness of telomerase inhibition therapy in human cancers.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01CA84628 and U01CA84313) and by the American Cancer Society (to R.A.D.). S.E.A., D.H.C., and R.D.C. are supported by Mentored Clinician Scientist Awards (K08CA82176, K08CA84044, and K08AG0103). S.E.A. is a V Foundation Scholar and a Rita Allen Foundation Scholar. N.E.S. is a Howard Hughes Medical Institute Physician Scientist Postdoctoral Fellow. R.A.D. is an American Cancer Society Research Professor and a Steven and Michele Kirsch Foundation Investigator.
Abbreviations
- Tg
transgenic
- TRAP
telomerase repeat amplification protocol
- MEF
mouse embryonic fibroblast
- DMBA
7,12-dimethylbenz[a]anthracene
- MIN
mammary intraepithelial neoplasia
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hastie N D, Dempster M, Dunlop M G, Thompson A M, Green D K, Allshire R C. Nature (London) 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph K L, Chang S, Millard M, Schreiber-Agus N, DePinho R A. Science. 2000;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 3.Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 4.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn E H. Nature (London) 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L, Moorhead P S. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Shay J W, Pereira-Smith O M, Wright W E. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 8.Counter C M, Avilion A A, LeFeuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart S A, Weinberg R A. Oncogene. 2002;21:627–630. doi: 10.1038/sj.onc.1205062. [DOI] [PubMed] [Google Scholar]
- 10.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Nature (London) 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Funk W D, Wang S S, Weinrich S L, Avilion A A, Chiu C P, Adams R R, Chang E, Allsopp R C, Yu J, et al. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 12.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H, Meyerson M, Weinberg R A. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert B, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg R A, Chin L, Femino A, Lee K H, Gottlieb G J, Singer R H, Greider C W, DePinho R A. Cell. 1999;97:515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Suarez E, Samper E, Flores J M, Blasco M A. Nat Genet. 2000;26:114–117. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- 17.Rudolph K L, Millard M, Bosenberg M W, DePinho R A. Nat Genet. 2001;28:155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- 18.Chin L, Artandi S E, Shen Q, Tam A, Lee S L, Gottlieb G J, Greider C W, DePinho R A. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 19.Artandi S E, Chang S, Lee S L, Alson S, Gottlieb G J, Chin L, DePinho R A. Nature (London) 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 20.Broccoli D, Godley L A, Donehower L A, Varmus H E, de Lange T. Mol Cell Biol. 1996;16:3765–3772. doi: 10.1128/mcb.16.7.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasco M A, Rizen M, Greider C W, Hanahan D. Nat Genet. 1996;12:200–204. doi: 10.1038/ng0296-200. [DOI] [PubMed] [Google Scholar]
- 22.Ogoshi M, Takashima A, Taylor R S. J Immunol. 1997;158:622–628. [PubMed] [Google Scholar]
- 23.Hathcock K S, Weng N P, Merica R, Jenkins M K, Hodes R. J Immunol. 1998;160:5702–5706. [PubMed] [Google Scholar]
- 24.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 25.Kim N W, Wu F. Nucleic Acids Res. 1997;25:2595–2597. doi: 10.1093/nar/25.13.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipling D, Cooke H J. Nature (London) 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 27.Todaro G J, Green H. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahler J F, Stokes W, Mann P C, Takaoka M, Maronpot R R. Toxicol Pathol. 1996;24:710–716. doi: 10.1177/019262339602400606. [DOI] [PubMed] [Google Scholar]
- 29.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho R A. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 30.Cardiff R D, Anver M R, Gusterson B A, Hennighausen L, Jensen R A, Merino M J, Rehm S, Russo J, Tavassoli F A, Wakefield L M, et al. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 31.Lee H W, Blasco M A, Gottlieb G J, Horner J W, Greider C W, DePinho R A. Nature (London) 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 32.Morales C P, Holt S E, Ouellette M, Kaur K J, Yan Y, Wilson K S, White M A, Wright W E, Shay J W. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 33.Jiang X R, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar A G, Wahl G M, Tlsty T D, Chiu C P. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Suarez E, Samper E, Ramirez A, Flores J M, Martin-Caballero J, Jorcano J L, Blasco M A. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stampfer M R, Garbe J, Levine G, Lichtsteiner S, Vasserot A P, Yaswen P. Proc Natl Acad Sci USA. 2001;98:4498–4503. doi: 10.1073/pnas.071483998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shpitz B, Zimlichman S, Zemer R, Bomstein Y, Zehavi T, Liverant S, Bernehim J, Kaufman Z, Klein E, Shapira Y, Klein A. Breast Cancer Res Treat. 1999;58:65–69. doi: 10.1023/a:1006394209922. [DOI] [PubMed] [Google Scholar]
- 37.Kolquist K A, Ellisen L W, Counter C M, Meyerson M, Tan L K, Weinberg R A, Haber D A, Gerald W L. Nat Genet. 1998;19:182–186. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.