Abstract
Food consumption will rise rapidly as the global population grows over the next several decades. The current agricultural production system cannot solve this challenge, forcing crop growth to experience more adverse conditions. To promote the long-term sustainability of crop production and reduce reliance on excessive agrochemical use, the implementation of integrated nutrient management systems that involve the combination of chemical and biological fertilizers represents an enormous challenge. The experiment aimed to improve tomato plants (Lycopersicon esculentum Mill.) germination, agronomic, and physiological characteristics through seed priming and foliar spraying with resorcinol (0.1 µM/L), biochar (30 mg/L), and nanobiochar (30 mg/L) and inoculation with or without a mixture of arbuscular mycorrhizal fungi (AMF). Physico-chemical characterization of nano-biochar revealed the presence of elements like carbon, oxygen, calcium, and silicon. Spectroscopic analyses confirmed the presence of functional groups and a mix of crystalline and amorphous structures. The surface showed a moderate negative zeta potential with particles averaging hydrodynamic size of around 77 nm.Notably, either alone or in combination with nanobiochar, resorcinol-primed seeds significantly improved tomato seed germination parameters, such as the germination rate index (GRI), emergence energy (EE), coefficient velocity of germination (CVG), final germination percentage (FGP), and seed vigor index (SVI), resulting in a decrease in the mean germination time (MGT) in both the Saaho and Lerica varieties. AMF inoculation and foliar application of biochar and nanobiochar considerably improved shoot (109.57 ± 0.88, 103.00 ± 0.93 cm), and root length (21.89 ± 0.21, 21.40 ± 0.20cm) and leaf area. Furthermore, increases in the biomass of shoots and fruits under fresh and dry conditions were also investigated. Treatment T13 notably boosted the levels of flavonoids (3.54 ± 0.01, 3.36 ± 0.01 mg/g), total phenol (21.23 ± 0.08, 20.31 ± 0.06 mg/g), total protein contents (44.97 ± 0.45, 42.55 ± 0.41 µg/g), total soluble sugar contents (47.97 ± 0.49, 44.88 ± 0.31 µg/g), and anthocyanin contents (0.70 ± 0.00, 0.68 ± 0.00 mg/g) in both Saaho and Lerica tomato varieties compared to the control. The activity of catalase (CAT) and ascorbate peroxidase (APX) exhibited significant increases in response to treatment T13, showing enhancements of (6.93 ± 0.02, 6.84 ± 0.01 units/g) for CAT, and (6.14 ± 0.02, 5.87 ± 0.04 units/g) for APX, respectively. In contrast, proline levels (3.55 ± 0.02, 3.02 ± 0.00 mg/g) declined in both tomato varieties. The present research showed that resorcinol-functionalized nanobiochar has a beneficial influence on germination parameters and that nanobiofertilizer has a synergistic influence on the morphophysiological properties of tomato plants.
Graphical abstract
Keywords: Sustainable, Chemical fertilizers, Antioxidant, Arbuscular mycorrhizal fungi, Osmolytes
Introduction
Tomato is a major vegetable that belongs to the Solanaceae family and is the third most significant commercial crop family in terms of economic importance [1]. As living standards continue to improve, the demand for high-quality fruits is steadily increasing. Enhancing tomato fruit quality requires a comprehensive understanding and thorough evaluation of the fundamental processes governing fruit growth [2]. However, a fundamental problem of today's plant production system is the sustainable production of food for a rising global population. Germination is crucial to crop quality and plant establishment in agriculture [3, 4]. The rapid growth of seedlings allows leaves and roots to expand and elongate, which aids in nutrient intake, transpiration, and biomass production [5]. Seedlings faced slow germination under environmental stressors and pathogen attacks, resulting in weakened growth, lower agricultural productivity, and significant financial losses for farmers [6]. Researchers are discovering new methods to improve crop yield by addressing seed germination, and abiotic stressors [7]. Seed priming improves germination and crop production leads to a physiological shift that speeds up germination [8]. Growth regulators are chemical compounds that influence plant growth and development. In field crops, they enhance physiological efficiency, photosynthetic ability, and partitioning from source to sink [9]. Resorcinol, a phenolic chemical (1, 3-isomer of benzenediol) with the formula C6H4 (OH)2, features an orthogonal two-hydroxyl functional group (-OH) [10]. This chemical and its derivatives have anti-inflammatory, antitumor, anticonvulsant, and antioxidant effects [11, 12]. It is present in various derivative forms in plants (e.g., fruits, vegetables, and tobacco) and, at lesser concentrations, in microorganisms [13]. The effects of other phenolic benzenediol compounds, such as catechol (1, 2-benzenediol) and hydroquinone (1, 4-benzenediol), on the growth, osmolytes, and antioxidant activity of lemongrass, soybean, and alfalfa plants have been studied by various authors [14–16]. Research on the effects of resorcinol on vegetative and reproductive parameters in soybean, tobacco, and sunflower plants is scarce [17–19].
Biochar is a carbon-rich solid produced through the pyrolysis of biomass feedstocks like agricultural and animal wastes [20]. It is a highly porous, amorphous material with a good surface area and various functional groups [21], rich in nutrients [22] that enhance soil fertility, leading to improved plant growth and crop yields. The nutrient content of biochar is influenced by factors such as pyrolysis temperature, duration, and feedstock type. Depending on the pyrolysis process, biochar particles can range in size from micrometers to centimeters [23]. Further reduction to the nanoscale, up to 100 nm or smaller, significantly enhances its physical, chemical, and structural properties. The elemental composition, crystalline form, aromatic/polar nature, specific surface area, pore size, cation exchange capacity, zeta potential, graphitic nature, pH, temperature-dependent dispersibility, and stability of nano-biochar differ from those of bulk biochar [24, 25]. Incorporating nanobiochar into plant nutrition offers an alternative to chemical fertilizers for nutrient provision. Additionally, nano-biochar-containing fertilizers can increase crop output by 10–20% while reducing fertilizer use by 30–50% [26]. In light of the increased nutrient accumulation by plants, the use of nanoparticles (NPs) for foliar and/or seed priming has garnered considerable interest in recent years [27, 28]. Additionally, research has been conducted on the production of nanobiochar for agricultural and environmental purposes [29, 30]. Arbuscular mycorrhizal fungi (AMF) play a vital role as soil microorganisms, forming symbiotic associations with approximately 90% of plant species [31]. Arbuscules facilitate the exchange of nutrients and water in plant roots in return for carbon derived from photosynthesis [32]. AMF strengthens plant resistance to biotic and abiotic stressors [33, 34] and soil stability and fertility [35]. The conventional objective of studying AMFs as biofertilizers is to increase crop yield without increasing the amount of fertilizer used to cultivate organic foods [36, 37]. Sustainable crop production that does not compromise environmental integrity requires the consideration of AMF [38]. Tomato being a vital crop in global agriculture, yet challenges such as slow seed germination and poor plant growth under stressors, including soil degradation and nutrient scarcity, continue to hinder its productivity. While individual interventions, such as seed priming with biochar and the use of arbuscular mycorrhizal fungi (AMF), have shown promise in enhancing plant growth and stress resistance, the combined application of these methods, especially with additional biochemicals like resorcinol, remains underexplored. Resorcinol has demonstrated potential in seed priming, improving germination and antioxidant activity in other crops [39], but its specific effect on tomato plants is yet to be fully understood. Furthermore, nanobiochar, a more advanced form of biochar, has gained attention for its ability to enhance soil properties and increase plant growth while reducing the reliance on synthetic fertilizers [40]. Recent studies suggest that combining biochar with AMF can synergistically promote plant health and productivity [17], yet limited research exists on how nanobiochar and AMF work together, especially in conjunction with resorcinol, to improve tomato growth under both normal and stress conditions.
The lack of comprehensive studies addressing the combined effects of resorcinol, nanobiochar, and AMF in tomato cultivation presents a significant research gap. Filling this gap could not only provide insights into optimizing sustainable agricultural practices but also contribute to the development of more eco-friendly plant growth promoters for tomato production. This research aims to explore the synergistic effects of resorcinol foliar application, nanobiochar amendment, and AMF inoculation on tomato seed germination, plant growth, and yield under controlled and field conditions.
Methodology
Experimental site
The experiment was conducted in a greenhouse at the Department of Botany at the University of Peshawar. The department lies 345 m above sea level, at 34.04° N latitude and 71.5° E longitude. Tomato (Lycopersicon esculentum Mill.) seeds were procured from the National Agriculture and Research Center (NARC), Islamabad, Pakistan, following standard protocols and obtaining the necessary permissions.
Preparation of AMF inoculum
Before being processed in a lab, three soil samples (weighing 200–300 g each) were gathered from the grass rhizospheric soil and kept at 4–8 °C to maintain the survival of AMF spores.
Isolation of AMF spores
Wet sieving and decanting techniques were used to separate AMF spores from the collected rhizospheric soil [41]. Spores were isolated at a rate of 230 spores/ 100 g of soil and analyzed using a compound microscope. The morphological categorization was carried out using spore recognition keys [42, 43] from INVAM (International Culture Collection of Arbuscular and Vesiculo Arbuscular Endomycorrhizal Fungi) and BEG (International Bank for the Glomales, formerly known as La Banque Europeene de Glomales). The most common genera were Glomus (80%), Sclerocystis (13%), Acaulospora (5%) and Gigaspora (2%).
Mass production of AMF spores
To mass-produce AMF spores, the National Agricultural Research Centre (NARC) provided the Zea mays L. cultivar Islamabad Gold, which was cultivated in a greenhouse. Maize seedlings were sterilized and pre-germinated [44]. The soil and manure were sterilized separately. Viable AMF spores were then placed into pots containing sterilized soil and manure mixtures. To encourage AMF inoculum production, isolated spores were further inoculated with maize in pots for around two months in a controlled greenhouse situation. The inoculum used in pot experiments was a combination of AMF-colonized roots and spores (40 AMF spores), applied at a rate of 30 g/ 5 kg of soil for mycorrhizal treatments [45]. AMF intensity levels were calculated using the methods used by Richard et al. [46].
Synthesis and characterization of biochar and nanobiochar
Biochar was generated from the biomass obtained from sawmill shavings of Cedrus deodara (Roxb. ex D. Don) G. Don. Temperature of 340 °C for 2 h were used for the pyrolysis process [47]. According to Liu et al. [48], nanobiochar was produced by first ultrasonically treating bulk biochar (B-BC) for 15 min at 25 °C, with the pH carefully maintained at 6.9 ± 0.2. After sonication, the biochar suspension was centrifuge first for 24 min at 4000 rpm to remove larger biochar particles, and then for an additional 10 min at 10,000 rpm to isolate the nanobiochar fraction. The resulting nanobiochar was characterized using a range of analytical techniques, including UV–visible spectrophotometry, Fourier-transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), and microscopic methods such as scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX). Additionally, zeta potential (ZP) analysis and dynamic light scattering (DLS) were employed to assess particle stability and size distribution [49]. The nanobiochar particles with an average size of 77.01 nm was separated.
Sterilization and priming of seeds
Seeds of both varieties were carefully prepared by first soaking in a 2% sodium hypochlorite solution for 20 min after being cleaned with tap water for 25 min [50]. Following a sterilizing procedure, the tomato seeds were primed for a full day with resorcinol (0.1 μM/L), biochar (30 mg/L), nanobiochar (30 mg/L), and AMF inoculum (40 spores) [49]. The primed seeds (10 seeds/pot) were placed on filter paper in Petri dishes and incubated at 25 °C. Resorcinol, CAS number 108–46-3, and a molecular weight of 110.11 g/mol were used in the investigation by Sigma-Aldrich.
Plant preparation
A pot experiment was conducted in a botanical garden under realistic environmental conditions, with an initial temperature of 22 °C/17 °C (day/night) and a wind velocity of 8 km/h. The average daytime temperature and humidity ranged from 28 °C and 58% at the beginning to 39 °C and 68% at the end of the study. Earthen pots (18 cm diameter, 20 cm height, 2 cm thickness) were filled with 5 kg of a sterilized mixture of manure, soil, and sand (3:1) [51]. Each pot was supplemented with 30 g of AMF inoculum containing approximately 40 spores. Ten seeds were sown per pot, and germination parameters were recorded during the first week. The physicochemical properties of the soil used in this study were determined following [52] AOAC (2005) standard procedures. The soil had an electrical conductivity of 3.5, a pH of 7.23, and a moisture content ranging from 11 to 14% [53] (Table 1).
Table 1.
Experimental design (complete randomised complete block design)
| Treatments | Descriptions |
|---|---|
| T1 | Control |
| T2 | AMF |
| T3 | Biochar |
| T4 | Resorcinol |
| T5 | Nanobiochar |
| T6 | AMF + biochar |
| T7 | AMF + resorcinol |
| T8 | AMF + nanobiochar |
| T9 | Biochar + resorcinol |
| T10 | Biochar + nanobiochar |
| T11 | Resorcinol + nanobiochar |
| T12 | AMF + biochar + resorcinol |
| T13 | AMF + biochar + nanobiochar |
| T14 | Biochar + resorcinol + nanobiochar |
| T15 | AMF + biochar + resorcinol + nanobiochar |
Germination parameters
After 1 week data related to germination were examined. Germination parameters, including the germination rate index [54], final germination percentage [55], mean germination time [56], emergence energy [57], Timson germination index [58], and seed vigor index [59], were determined by using the following formulas:
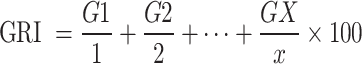 |
G1 represents the percentage of seeds germinated on the first day, G2 on the second day, and Gx on the final day.
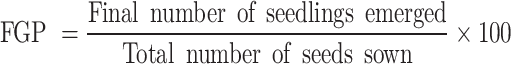 |
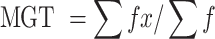 |
where "f" is the number of seeds that grew on day x.
 |
Xn represents the final germinant counted, while Yn denotes the number of days from seed planting to the final day of counting.
 |
The proportion of seeds that sprouted each day was G, and the germination period was T.
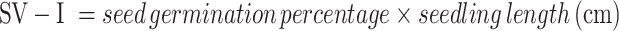 |
Foliar application
Hussain et al. [60] techniques were modified slightly to obtain solutions of exact concentrations for the foliar application of each treatment. These solutions were prepared by rotating corresponding solute concentrations in 1 L of distilled water in separate flasks for about 30 min before application. The foliar spray was generated at three concentrations: biochar (30 mg/L), nano-biochar (30 mg/L), and resorcinol (0.1 μM/L). After one week, germinated seedlings were trimmed down to five plants per pot. According to the experiment's method, the first foliar spray was applied 7 days after germination using a hand-held sprayer bottle (small hand spray), with the soil mainly covered to avoid entry into the soil. The remaining foliar sprays were administered weekly till harvest (total spray 07). Each spray resulted in a new solution. Sprays on the leaves were done using 1 L of solution for each of the three treatments. An equivalent amount of distilled water was also sprayed on the controls.
Plant harvesting
After 56 days of growth in the pot, the plants were harvested (after 8 weeks). To analyze agronomic parameters, half of the plant samples were dried in an air-blown oven at 65 ± 05 °C for three days and weighed. To investigate several physiological processes, the extra uprooted half were kept in a refrigerator at − 20 °C.
Vegetative parameters
The standard protocol of Basra et al. [57] has been used to examine vegetative characteristics, such as shoot (length, fresh weight, and dry weight), root (length, root-to-shoot ratio), leaf (length, width, fresh and wilted leaves per plant), and percentage moisture contents (shoot). The root-to-shoot ratio (RSR) and percent moisture content (PMC) were calculated using the following formulas:
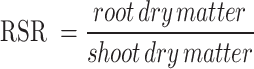 |
 |
Determination of yield parameters
The crop yield was ascertained by gathering the fruit for fresh weight analysis after 56th days of the germination period. Among the yield parameters were.
Fresh weight of fruit
Dry weight of fruit
Fruit PMC (percentage moisture contents)
Osmolyte attributes
Dry shoot samples (0.5 g) were soaked in 0.5 mL of methanol (1:10 g/mL) to determine the total flavonoid content. After adding a mixture of 1 M potassium acetate (0.1 mL), methanol (1.5 mL), clean water (2.8 mL), and 10% aluminum chloride (0.1 mL), spectrophotometry was used to determine the absorbance at 415 nm [61]. Dried shoot samples (0.25 g) were combined with 10 mL of 90% methanol and agitated for an hour to determine the phenolic contents. Following the centrifugation process, 1 mL of Folin–Ciocalteu reagent (4:1) was added. The final density was measured at 760 nm after the addition of 1 mL of 10% Na2CO3 [62]. A total of 250 µL of Bradford reagent and 5 µL of plant extract were mixed to quantify total soluble proteins using bovine serum albumin (BSA) as the protein standard. Following a 30-min incubation period at room temperature, the optical density was measured at a wavelength of 595 nm [63].
After crushing 0.5 g of fresh plant shoot in 10 mL of distilled water, the mixture was centrifuged to determine the total amount of soluble sugars. Using 0.1 mL of filtrate and 1 mL of 80% phenol (w/v), the absorbance of each sample was measured at 420 nm [64]. Fresh shoot material (0.25 g) was ground in 5 mL of methanol-HCL solution (1% HCL, v/v) and centrifuged at 1500 rpm for 15 min to assess the anthocyanin content. The wavelength at which the optical density was measured for the first time was 530 nm, and the last measurement was 657 nm [65]. A sample of 0.5 g of leaf material was pulverized in 10 mL of 3% sulfosalicylic acid to analyze the proline content. A filtered solution of 10 mL was combined with 2 mL of glacial acetic acid and acid ninhydrin, and 4 mL of toluene was added. The absorption was measured at 520 nm using a spectrophotometer [66].
Antioxidant enzyme analysis
A buffer solution was mixed with 0.5 g of shoot material to measure the catalase (CAT) activity. Following centrifugation, 1 mL of phosphate buffer and 1.9 mL of H2O2 were added. At a wavelength of 240 nm, the optical density was measured, with the first and last measurements taken 60 s apart [67]. Shoot samples (0.5 g) were crushed in 5 mL of phosphate water and centrifuged to determine the activity of ascorbic acid (APX). Ascorbic acid (0.6 mM), H2O2 (0.1 mM), and EDTA (0.1 mM) made up the final filtrate. The optical density was measured at 290 nm, with a 60-s interval between the first and last observations [68].
Statistical analysis
The data were analyzed through 2-way ANOVA using SPSS Statistics v. 22 (IBM). The relationship between various parameters was determined using Pearson's coefficient and principal component analysis (PCA). The quantitative data presented in this study represent the Mean ± SE of three independent biological replicates, and the differences between means were determined using Duncan's multiple range test (DMRT) at a significance level of p ≤ 0.05.
Results
Characterization of nano-biochar
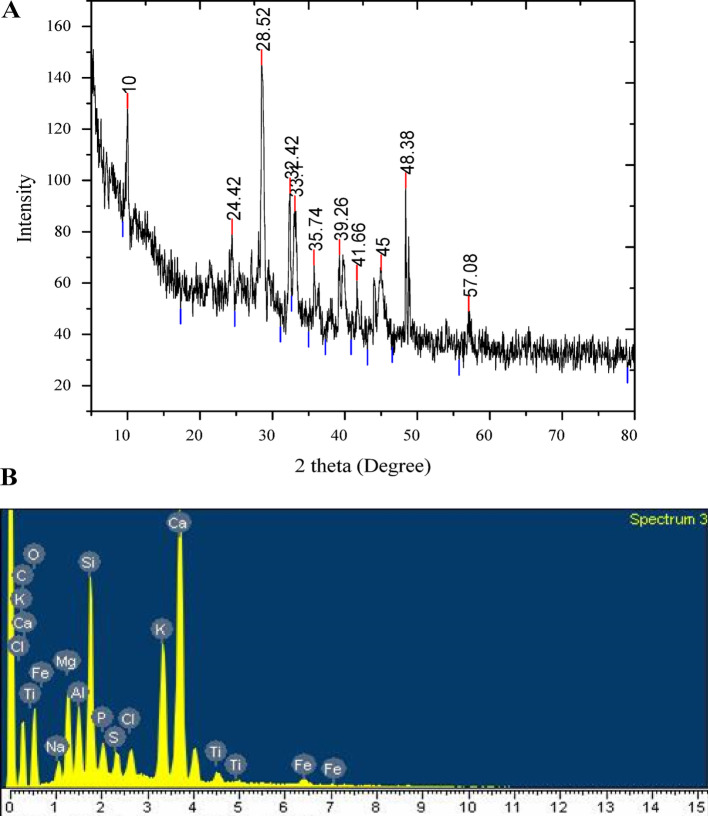
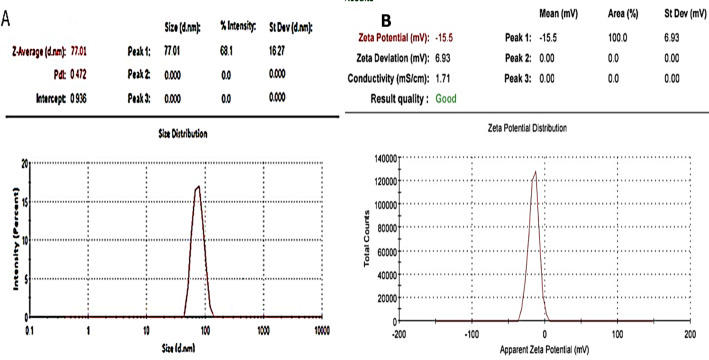
The purity and composition of the nano-biochar was assessed using Energy-Dispersive X-ray Spectroscopy (EDX). The EDX spectrum (Fig. 1B) revealed strong peaks for Carbon (weight%: 18.79), Oxygen (weight%: 30.57), Silicon (weight%: 5.33), and Calcium (weight%: 15.26). The results also showed other elements like Potassium (7.23%), Iron (0.73%), Magnesium (3.47%), Chlorine (1.48%), Sodium (1.07%), and Sulphur content (0.22%) as shown in Table 2. The particle size distribution of the investigated nano-biochar is shown in Fig. 2A. The average hydro-dynamic size of nano-biochar particles was 77.01 nm. The surface functional groups present in all carbon-based samples were analyzed using FT-IR spectroscopy (Fig. 3A). FTIR spectra was obtained in transmission mode within the wavenumber range of 500–4000 cm−1. Prominent peaks were observed, particularly at the wavenumber ranges of 3350 cm−1 (OH group), 2937 cm−1 (CH/CH2), 2127 cm−1 (C= C), 1622 cm−1 (C= O), 1377 cm−1 (CH), 1160 cm−1 (C–O–C), 1047 cm−1 (C–O), and 875 cm−1 (–C= O). These peaks correspond to the out-of-plane bending of ring C–H bonds in heteroatomic and aromatic compounds. Surface characteristics of nano biochar, notably discernible at a wavelength of 420 nm, was further investigated using UV–visible spectroscopy (Fig. 3B). The XRD analysis revealed distinct peaks at specific 2θ values (10, 24.42, 28.52, 32.4, 33.2, 35.74, 39.26, 41.66, 45.0, 48.38, 57.08 degrees), which corresponded to crystal planes within the face-centered cubic structure of the nano-biochar. Notably, (Fig. 1A) in the XRD pattern, a peak at 28.52 and 48.38 degrees was observed, indicating the presence of both crystalline and amorphous features within the organic phase of the nano-biochar extracted. The zeta potential measurement of the nano-biochar (40 nm) by the citrate method was − 15.5 mV (Fig. 2B), confirming the negative charges. Negative charges result from citrate, which plays the role of both a reducing and a stabilizing agent, creating repulsion between nano-biochar that forestalls the aggregation of nano-biochar. The particles have low zeta potential (< − 25 values), reflects that there was no hindrance technique for particles flocculating.
Fig. 1.
Characterization and physio-chemical properties of nano-biochar. XRD patterns of nano-biochar (A) and EDX spectrum of nano-biochar (B)
Table 2.
Elemental composition by EDX analysis of nano-biochar
| Elements | Weight% | Atomic% |
|---|---|---|
| C | 18.79 | 31.40 |
| O | 30.57 | 38.36 |
| Na | 1.01 | 1.21 |
| Mg | 3.47 | 3.69 |
| Si | 5.33 | 5.95 |
| P | 1.25 | 1.07 |
| S | 0.22 | 0.77 |
| Cl | 1.48 | 0.84 |
| K | 7.23 | 4.23 |
| Ca | 15.26 | 9.60 |
| Ti | 1.05 | 0.44 |
| Fe | 0.73 | 0.26 |
Fig. 2.
Nano biochar particle size and zeta potential analysis
Fig. 3.
Characterization and physico-chemical properties of nano-biochar. A Fourier-Transforms Infrared Spectroscopy (FTIR), B UV–vis spectra of nano-biochar
Germination parameters
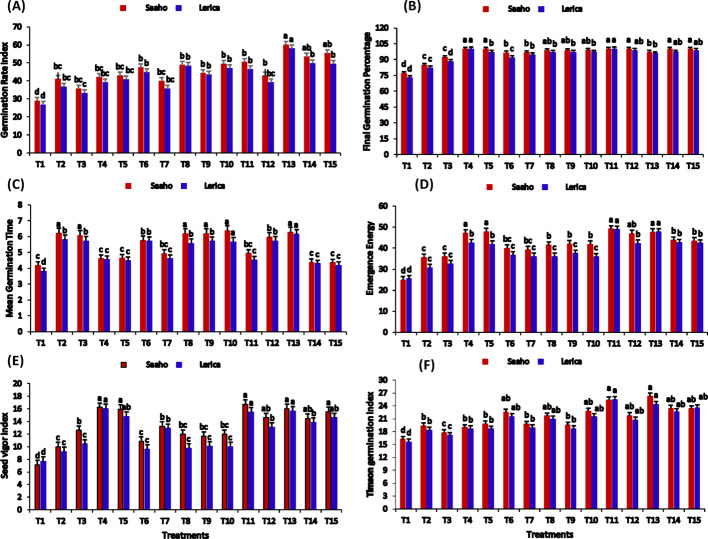
In the present study, the Saaho and Lerica tomato varieties exhibited the most significant increases in GRI values under treatments T13 (59.93 ± 0.41, 57.95 ± 0.36), T15 (55.24 ± 0.50, 49.36 ± 0.49), T14 (53.31 ± 0.60, 49.68 ± 0.34), T11 (50.33 ± 0.65, 46.26 ± 0.57), and T4 (31.92 ± 0.73, 29.04 ± 0.94), compared to the control (Fig. 4A). The Saaho variety achieved a peak final germination percentage (FGP) of 100% in treatments T4, T5, T11, T13, and T15, whereas the Lerica variety reached its maximum FGP in treatments T4 and T11 (Fig. 4B). A notable decrease in mean germination time (MGT) was observed with resorcinol and nanobiochar treatments, specifically in T4, T5, T7, T11, T14, and T15 (Fig. 4C). A reduction in MGT was also evident in T1 for both varieties. The highest emergence energy (EE) for both varieties was recorded in treatment T11, followed by T4 (Fig. 4D). Furthermore, the seedling vigor index (SVI) showed the highest values in treatments T4 and T11 for both varieties (Fig. 4E). Treatment T13 yielded the highest total germination index (TGI) values for Saaho and Lerica (64.93 ± 0.41, 62.61 ± 0.30), followed by T15 (60.24 ± 0.50, 54.36 ± 0.49), T14 (58.31 ± 0.60, 54.68 ± 0.34), and T11 (55.33 ± 0.65, 52.26 ± 0.55), relative to the control T1 (33.50 ± 0.62, 31.73 ± 0.33) (Fig. 4F). Overall, the results indicate that the treatments incorporating resorcinol and nanobiochar, either individually or in combination, led to the highest germination parameters for both tomato varieties. Two-way ANOVA revealed that treatment and variety had significant influences (p ≤ 0.05) on the MGT, TGI, FGP, and GRI.
Fig. 4.
Germination parameters of tomato plants (Saaho and Lerica varieties) upon inoculation with AMF or foliar spray of biochar, nanobiochar, or resorcinol, individually or in combination. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = nanobiochar, T6 = AMF + biochar, T7 = AMF + resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = biochar + nanobiochar, T11 = resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
Vegetative parameters
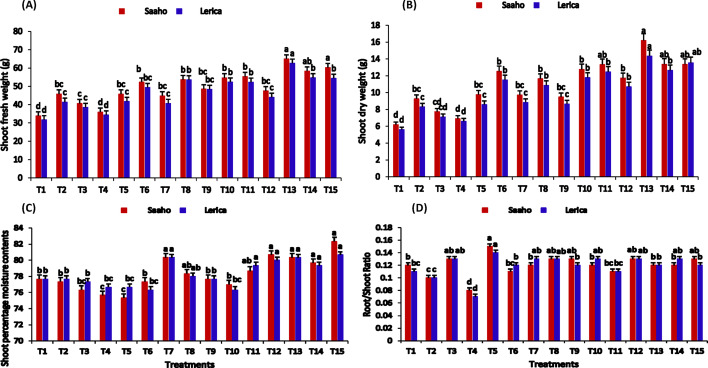
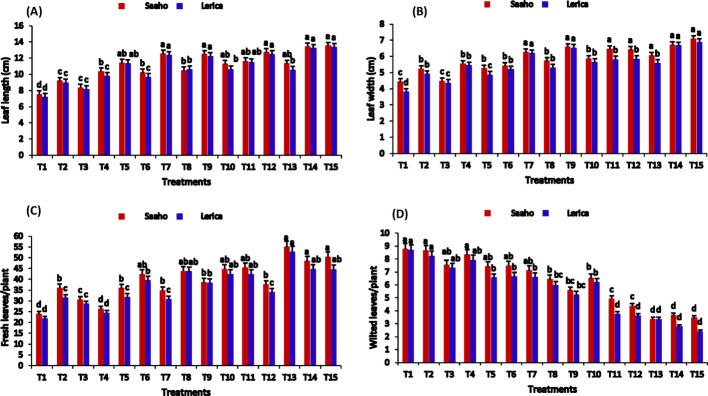
The T13 treatment significantly enhanced shoot length in both Saaho and Lerica varieties compared to the control (T1) (Fig. 5A, 8A). Root length significantly increased under T13, with increments of (21.89 ± 0.21 cm) in Saaho and (21.40 ± 0.20 cm) in Lerica compared to T1 (10.93 ± 0.29 cm) (Fig. 5B). Furthermore, T13 demonstrated the greatest cumulative impact, achieving the highest shoot fresh weight and dry weight (Fig. 6A-B). Conversely, shoot percentage moisture content (PMC) showed varied responses, with the lowest concentrations observed in T11 (75.33 ± 0.33, 75.66 ± 0.23%) relative to the control (82.33 ± 0.33, 80.66 ± 0.76%) (Fig. 6C). Treatments incorporating resorcinol (T4, T7, T9, T11) exhibited a negative impact on root length. The lowest root/shoot ratios were recorded in T2 and T4 (Fig. 6D). The greatest leaf length and width were observed in T15, followed by T14 (Fig. 7A-B). T13 also demonstrated the greatest improvement in fresh leaf count per plant while reducing the number of wilted leaves (Fig. 7C-D). A two-way ANOVA revealed that both treatment and plant variety had significant effects (p < 0.05) on all measured shoot and root parameters, except for shoot percentage moisture content.
Fig. 5.
The impact of AMF, biochar, nanobiochar, and resorcinol, individually and in combination, on tomato Saaho and Lerica varieties. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = nanobiochar, T6 = AMF + biochar, T7 = AMF + Resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = biochar + nanobiochar, T11 = resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
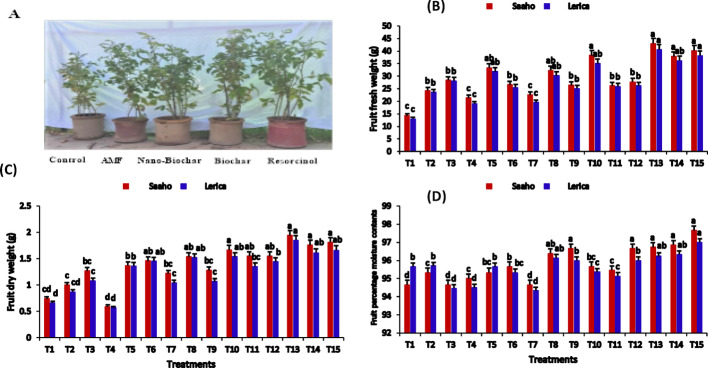
Fig. 8.
The impact of AMF, biochar, nanobiochar, and resorcinol on tomato Saaho and Lerica plants. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = nanobiochar, T6 = AMF + biochar, T7 = AMF + resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = Biochar + nanobiochar, T11 = resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
Fig. 6.
The impact of AMF, biochar, nanobiochar, and resorcinol, individually and in combination, on tomato Saaho and Lerica varieties. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = nanobiochar, T6 = AMF + biochar, T7 = AMF + resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = biochar + nanobiochar, T11 = resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
Fig. 7.
The impact of AMF, biochar, nanobiochar, and resorcinol, individually and in combination, on tomato Saaho and Lerica varieties. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = Nanobiochar, T6 = AMF + biochar, T7 = AMF + resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = biochar + nanobiochar, T11 = resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
Reproductive parameters
Additionally, compared to the control T1, the fresh weight of the tomato fruit (Fig. 8B) was significantly enhanced by (42.97 ± 1.42g), (40.19 ± 1.05g) and (35.05 ± 0.40 g) in T13, T15, and T10 for the Saaho variety. Similarly, the Lerica variety showed increases of (40.55 ± 1.41 g), (38.15 ± 0.64 g) and (38.26 ± 0.85 g) in T13, T15, and T10. Furthermore, the fruit dry weight was significantly enhanced in T13, T15, and T14 (Fig. 8C). Among the 15 treatments, the fruit percentage moisture content (PMC) was significantly higher in both Saaho and Lerica varieties in T10 (97.66 ± 0.66, 97.33 ± 0.64%), T15 (96.66 ± 0.66, 96.33 ± 0.33%) and T14 (96.21 ± 0.86, 95.33 ± 0.10%) (Fig. 8D). A two-way ANOVA revealed that treatment and variety had significant effects (p < 0.05) on fruit dry weight.
Osmolyte contents
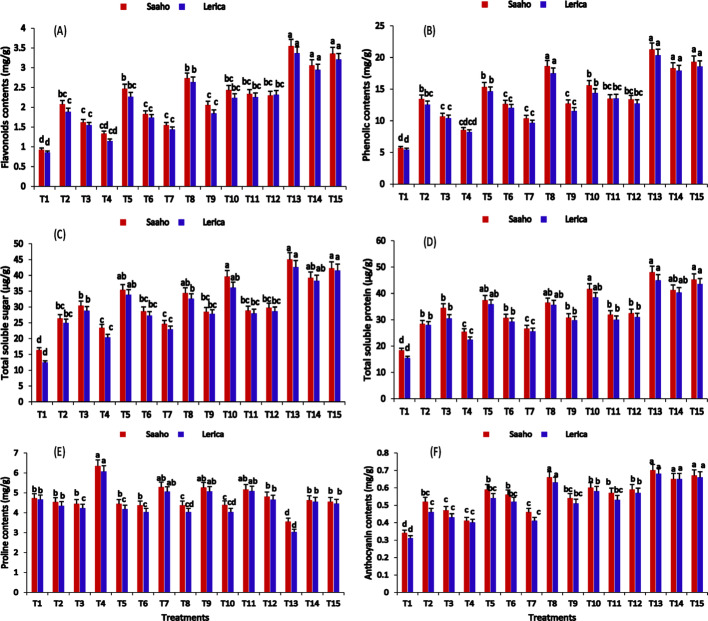
Treatment T13 (Fig. 9A–F) significantly enhanced the accumulation of flavonoids (3.54 ± 0.01, 3.36 ± 0.01 mg/g), total phenol (21.23 ± 0.08, 20.31 ± 0.06 mg/g), total protein contents (44.97 ± 0.45, 42.55 ± 0.41 µg/g), total soluble sugar contents (47.97 ± 0.49, 44.88 ± 0.31 µg/g), and anthocyanin contents (0.70 ± 0.00, 0.68 ± 0.00 mg/g) in the Saaho and Lerica tomato varieties compared to the control. For proline content, the highest values were observed in T4, followed by T7, T9, and T11, indicating that resorcinol application induced stress conditions in the tomato varieties. A two-way ANOVA revealed that treatment, plant variety, and their interaction had significant (p < 0.05) effects on the osmolytic activity of the tomato plants.
Fig. 9.
The impacts of AMF, biochar, nanobiochar, and resorcinol individually and in combination on the enhancement of osmolyte activity in tomato plants were evaluated. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = Control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = nanobiochar, T6 = AMF + biochar, T7 = AMF + resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = biochar + nanobiochar, T11 = Resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
Antioxidant enzyme analysis
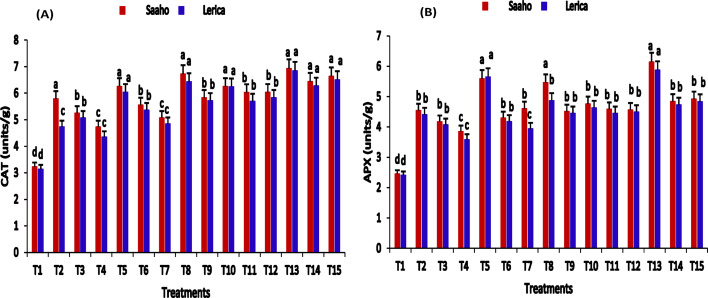
The levels of catalase (CAT) and ascorbate peroxidase (APX) exhibited significant increases in response to treatments with AMF, biochar, or nanobiochar, either alone or in combination, compared to the control tomato plants. Notably, the highest CAT contents (Fig. 10A) were recorded in T13, followed by T8, T15, and T14. APX activity (Fig. 10B) also showed a significant increase in T13 (6.14 ± 0.02, 5.87 ± 0.04 units/mg), followed by T5 (5.59 ± 0.05, 5.65 ± 0.00 units/mg) and T8 (5.46 ± 0.10, 4.87 ± 0.06 units/mg) relative to the control T1 (2.45 ± 0.00, 2.41 ± 0.03 units/mg). The application of AMF, biochar, resorcinol, and nanobiochar notably enhanced CAT and APX contents, indicating an improved antioxidant defense mechanism in the Saaho and Lerica varieties. A two-way ANOVA revealed that treatment, plant variety, and their interaction had significant effects (p < 0.05) on antioxidant activity.
Fig. 10.
Impact of AMF, biochar, nanobiochar, and resorcinol. Bars show means of three replicates. Different letters indicate significant difference at p ≤ 0.05. T1 = Control, T2 = AMF, T3 = biochar, T4 = resorcinol, T5 = nanobiochar, T6 = AMF + biochar, T7 = AMF + resorcinol, T8 = AMF + nanobiochar, T9 = biochar + resorcinol, T10 = biochar + nanobiochar, T11 = resorcinol + nanobiochar, T12 = AMF + biochar + resorcinol, T13 = AMF + biochar + nanobiochar, T14 = biochar + resorcinol + nanobiochar, T15 = AMF + biochar + resorcinol + nanobiochar
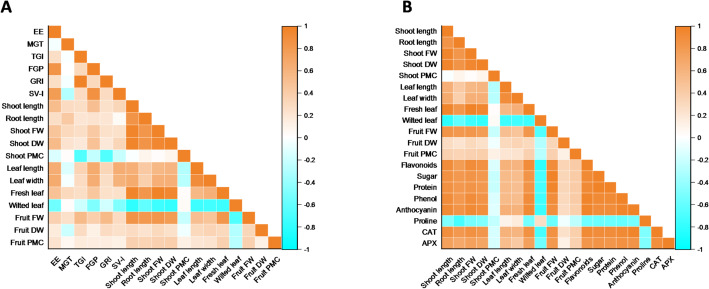
Pearson correlation and principal component analysis (PCA)
The Pearson correlation graph (Fig. 11A) indicates a positive correlation between the seed vigor index (SVI) and shoot length for all the parameters except for shoot PMC (r = − 0.20, r = − 0.80) and wilted leaves (r = − 0.48, r = − 0.01). The fresh and dry weights of the fruits were positively associated with the fresh weight of the leaves, with correlation coefficients of 0.83 and 0.31, respectively. The shoot dry weight (Fig. 11B) was strongly positively correlated with osmolytes such as flavonoids (r = 0.87), sugars (r = 0.81), proteins (r = 0.81), phenols (r = 0.85), and anthocyanins (r = 0.89). However, proline was significantly negatively correlated with CAT (r = -0.49) and APX (r = − 0.50) at the p < 0.05 level of significance. PCA was used to analyze the experimental dataset, which included a control, treatment, six germinations, twelve morphological, and eight physical-biochemical variables (Fig. 12). The results demonstrated that, over the whole dataset, the effective treatments were all properly dispersed. The first two principal components explained 70.7% of the total variation in the dataset. PC2 explained 14.1% of the total variance and was significantly correlated with FGP, EE, TGI, GRI, SVI-I, and leaf length and width, which are specifically related to germination, whereas PC1 explained 56.6% of the total variance and was specifically correlated with shoot length; biomass, phenols, protein, sugar, flavonoids, CAT, and APX, which are related to plant growth, osmolytes, and antioxidant enzymes.
Fig. 11.
Pearson correlation between A germination and agronomic parameters and B agronomic and physiological parameters of tomato (Lycopersicon esculentum Mill.) varieties Saaho (V1) and Lerica (V2) under different treatments. Key: emergence energy (EE); mean germination time (MGT); Timson germination index (TGI); final germination percentage (FGP); germination rate index (GRI); seed vigor index (SVI); percentage moisture contents (PMC); fresh weight (FW); dry weight (DW); catalase (CAT); ascorbic peroxidase (APX)
Fig. 12.
Analysis of the principal components of the tomato (Lycopersicon esculentum Mill.) varieties (Saaho and Lerica). Key: emergence energy (EE); mean germination time (MGT); Timson germination index (TGI); final germination percentage (FGP); germination rate index (GRI); seed vigor index (SVI); percentage moisture contents (PMC); fresh weight (FW); dry weight (DW); catalase (CAT); ascorbic peroxidase (APX)
Discussion
This study showed that among the tested treatments, T11, which involved the application of resorcinol in combination with nano-biochar, significantly enhanced germination metrics relative to the control. Resorcinol and nanobiochar treatments, particularly T11, T13, T14, and T15, significantly enhanced germination performance in Saaho and Lerica tomato varieties. Treatment T13 produced the highest germination rate index (GRI) with values of 59.93 for Saaho and 57.95 for Lerica. It also yielded the highest total germination index (TGI) values 64.93 (Saaho) and 62.61 (Lerica). Saaho achieved a 100% final germination percentage (FGP) in treatments T4, T5, T11, T13, and T15, while Lerica reached 100% in T4 and T11. Two-way ANOVA revealed that treatment and variety had significant effects (p ≤ 0.05) on GRI, TGI, FGP, and MGT, confirming the effectiveness of these treatments in enhancing tomato seed germination and vigor. Notably, resorcinol-primed seeds consistently displayed exceptional performance across all treatments for both varieties. The findings of the present study are consistent with those of Li et al. [69], who demonstrated that various phenolic acids used as priming agents enhanced root length and biomass in rice cultivars and wheat plants. Previous research [70–72] has indicated that the process of seed imbibition generates reactive oxygen species (ROS), leading to oxidative stress. Polyphenols are instrumental in mitigating ROS through multiple mechanisms, including direct scavenging, inhibition of ROS production, and activation of antioxidant enzymes such as catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPX). These enzymes decompose superoxide anions, hydrogen peroxide, and hydroperoxides, thereby preventing the activation of xanthine oxidase [73]. Antioxidant enzyme activities, CAT and APX, increased significantly in tomato plants treated with AMF, biochar, or nanobiochar. The highest CAT activity was recorded in T13, with APX peaking in the same treatment at 6.14 (Saaho) and 5.87 units/mg (Lerica), compared to the control T1 (2.45, 2.41 units/mg). Treatments T5 and T8 also showed elevated APX levels. These results indicate enhanced antioxidant defense, with significant effects (p < 0.05) confirmed by two-way ANOVA for treatment, variety, and their interaction. The antioxidant efficacy of polyphenols is influenced by several factors, including the number of hydroxyl groups, the degree of methoxylation, and the specific molecular structure [74, 75]. The germination parameters of the tomato varieties Saaho and Lerica were significantly enhanced by nano-biochar treatments. Zhang et al. [76] demonstrated that nano-biochar positively influenced the growth of rice and reed plants, although no effects were observed on tomato seed germination or seedling growth. Nano-priming enhances seed germination by generating nanopores and increasing the expression of aquaporin genes, facilitating better water uptake. Multiple studies have confirmed the penetration of nanoparticles into seeds [77, 78], leading to ROS buildup and enhanced antioxidant enzyme activity, which in turn improves plant growth. The impacts of nanoparticles on seed germination are influenced by their concentration and the duration of priming, with variations observed across different plant species [8, 27, 79].
The current investigation elucidated the positive effects of treatment T13, which involved the combined application of AMF inoculation and foliar spraying of nanobiochar and biochar, on various vegetative and reproductive parameters. This enhancement can be attributed to the synergistic action of these biostimulants. Notably, nanobiochar at a concentration of 30 mg/L significantly augmented the expression of tomato plants. These findings resonate with those of Li et al. [80], underscoring the capacity of nanoparticles (NPs) to enhance rice growth at appropriate concentrations, although higher levels may exhibit adverse effects. Moreover, Li et al. [81] proposed that lower NP concentrations can positively influence vegetative parameters in Zea mays. The efficacy of foliar-applied nano-biochar stems from its more negative zeta potential, smaller size, low-dose [82] and increased functional group density, which collectively bolster its adsorption capability relative to macro-biochar counterparts [83]. Upon foliar application, nanoparticles translocate to diverse plant regions through apoplastic and symplastic routes [84]. It is pertinent to consider that factors such as plant species and environmental conditions can influence the entry of nanoparticles into stomatal pores [85]. The findings of the present study demonstrate that inoculation with AMF results in diverse enhancements in plant growth parameters. These results are consistent with those of Sané et al. [86], who observed that tomato cultivars treated with AMF exhibited the greatest shoot and root dry weights, measuring 2.58 g and 0.79 g, respectively. Ziane et al. [87] demonstrated that the addition of mycorrhizal fungi to tomato plants (Solanum lycopersicum L. var. Fahla F1) increased plant height, shoot dry biomass, root dry biomass, total yield, and fruit number by 8.18%, 21.5%, 34.45%, 8.07%, and 19.05%, respectively. AMF may alter the structure of plant roots, resulting in enhancements in root biomass, length, root-to-shoot ratio, and the number of root tips [88]. Mycorrhizal plants possess a highly efficient root system, enabling extraradical hyphae to penetrate the depletion zone of the plant rhizosphere. This process facilitates the absorption of water and immobile mineral nutrients by plants [89].
Highly dispersed hyphal networks in plant roots treated with biochar and nano-biochar (T8 and T13) indicate a positive influence on AMF. Luo et al. [90] reported that biochar boosts fungal diversity more than bacterial diversity. Combining biochar with AMFs enhances plant development, reduces disease severity, and increases production [91]. Nano-biochar significantly increased AMF colonization. Aleksandrowicz-Trzcinska et al. [92] demonstrated that foliar nanoparticle treatments at 25 ppm significantly improve pine seedling development and mycorrhizal colonization, increasing root dry mass. The results of the study demonstrated the positive impact of biochar application on various growth parameters. These findings are consistent with prior research by Arshad et al. [93], who observed significant enhancements in multiple growth parameters of the tomato cultivar "Money Maker" compared to control groups. Parameters including plant height, leaf count, fresh and dry shoot weight, fresh and dry root weight, and root length were notably improved with biochar application. The utilization of biochar presents a promising avenue for enhancing plant growth, attributed to its ability to augment the availability of essential nutrients such as phosphorus, potassium, calcium, sodium, magnesium, iron, manganese, and zinc [94, 95]. Chemical analysis of the biochar utilized in our study revealed substantial concentrations of phosphorus (2.11%), calcium (18.74%), potassium (8.79%), magnesium (4.54%), carbon (20.09%), and other essential elements [49]. These nutrient profiles hold significant potential for stimulating seedling growth [96, 97].
In this study, secondary metabolites including total soluble sugars and proteins, phenolics, anthocyanins, and flavonoids were quantified in tomato plants. Treatment T13 notably improved the biochemical composition of both Saaho and Lerica tomato varieties. It led to the highest accumulation of flavonoids (3.54, 3.36 mg/g), total phenols (21.23, 20.31 mg/g), proteins (44.97, 42.55 roteins (44.97, 42.553mprove, 44.88 µg/g), and anthocyanins (0.70, 0.68 mg/g), indicating enhanced metabolic activity and stress tolerance. Conversely, the highest proline content was observed in T4, followed by T7, T9, and T11, suggesting resorcinol-induced stress. Tomato plants inoculated with AMF exhibited higher contents of flavonoids, phenolics, sugars, and proteins, corroborating findings from previous studies that reported increased soluble protein levels in AMF-inoculated wheat compared to untreated controls [98, 99]. Several studies have demonstrated that AMF treatment enhances sugar content and photosynthetic activity [100, 101]. Soussani et al. [102] documented that AMF-treated tomato plants showed significant increases in carotenoid, lycopene, polyphenol, and flavonoid levels by 73%, 53%, 310%, and 158%, respectively. Similarly, Khaliq et al. [103] reported that nano-biochar positively influences carrot growth, pigment content, and nutritional levels. Both soil integration and foliar application of nano-biochar resulted in the highest growth, pigmentation, and metabolite accumulation, including sugars, free amino acids, phenols, flavonoids, and nutrients. In a related study, Mazhar et al. [104] found that seed priming with 75 ppm nanoparticles increased the levels of osmolytes, specifically total soluble sugars and proteins, by 14% and 81%, respectively. The highest proline content was observed in treatments with resorcinol application. These findings are consistent with other studies that reported significant increases in proline levels following the application of phenolic substances [105, 106]. This increase is attributed to the activation of enzymes in response to oxidative stress conditions [107].
Plants can activate antioxidant systems, wherein enzymes such as ascorbate peroxidase (APX) and catalase (CAT) play crucial roles in mitigating oxidative stress induced by reactive oxygen species (ROS) [107]. These enzymatic biomarkers are essential components of the plant's defense mechanism [108]. In the present study, a mixed AMF inoculum was utilized, resulting in increased antioxidant activity. This observation aligns with findings by Duc et al. [109], who reported that a mixed inoculum of AMF species was more effective than a single AMF species in enhancing the activities of catalase, peroxidase, proline, and total phenolic compounds. Additionally, Soussani et al. [110] demonstrated that inoculating tomato plants with AMF significantly elevated their levels of antioxidant compounds. In the present study, the application of nano-biochar significantly enhanced the activities of ascorbate peroxidase and catalase. Waqas Mazhar et al. [111] examined rice plants primed with 25 ppm nanoparticles (NPs) before seed germination and reported notable increases in the activities of superoxide dismutase (SOD), CAT, and peroxidase (POD), by 11%, 13%, and 38%, respectively. Additionally, the application of resorcinol also enhanced antioxidant activity. These findings are consistent with those of Sapakhova et al. [104], who observed increased APX, CAT, glutathione peroxidase (GPX), and SOD activities in salicylic acid-treated wheat leaves. Azmat et al. [112] similarly reported substantial increases in APX and CAT activities in wheat plants treated with a combination of salicylic acid and biofertilizer, with activities increasing by 156% and 169% in leaves and by 116% and 200% in roots, respectively. The antioxidant activity of phenolic acids is primarily due to their ability to neutralize free radicals through the donation of hydrogen atoms or electrons, or by chelating metal ions. This mechanism enhances the stability of these ions and reduces their availability, thereby inhibiting autoxidation processes [113, 114].
Conclusions
This study highlights the substantial potential of utilizing resorcinol, biochar, nanobiochar, and AMF to enhance growth, secondary metabolite production, and antioxidant activities in tomato plants. The Saaho variety responded more positively to the treatments than the Lerica variety. Specifically, resorcinol at a concentration of 0.1 µM/L significantly improved seed germination parameters, particularly when applied alone or in conjunction with nanobiochar. In both tomato varieties examined, the synergistic application of nanobiochar, AMF, and biochar resulted in marked improvements in shoot length, root weight, and leaf characteristics. Furthermore, the incorporation of AMF, biochar, and nanobiochar positively influenced the osmolytic activity and the activities of antioxidant enzymes in tomato plants. These results indicate that the integration of these components can enhance plant growth and increase resilience to stressful conditions, laying a strong foundation for future research and practical applications in sustainable tomato cultivation.
Author contributions
S.B, R.U, T.B, and Z.U, designed and conceived the study idea. R.U completed the experiments. Z.U, J.U, M.N.A, and M.K. reviewed the manuscript and provided funds. R.U provided the resources and supervision. All authors made valuable revisions and edited the manuscript and approved the last version.
Funding
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (ORF-2025-301), King Saud University, Riyadh, Saudi Arabia.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study does not include human or animal subjects.
Consent for publication
Not applicable.
Statement on guidelines
All experimental studies and experimental materials involved in this research are in full compliance with relevant institutional, national and international guidelines and legislation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rehman Ullah, Email: rehmanbotany@uop.edu.pk.
Zakir Ullah, Email: zakirullah@bs.qau.edu.pk.
References
- 1.Naz F, Hamayun M, Rauf M, Arif M, Afzal Khan S, Ud-Din J, Gul H, Hussain A, Iqbal A, Kim HY, Lee IJ. Molecular mechanism of Cu metal and drought stress resistance triggered by Porostereum spadiceum AGH786 in Solanum lycopersicum L. Front Plant Sci. 2022;13:1029836. 10.3389/fpls.2022.1029836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullah S, Shah W, Hafeez A, Ali B, Khan S, Ercisli S, Al-Ghamdi AA, Elshikh MS. Biochar and seed priming technique with gallic acid: An approach toward improving morpho-anatomical and physiological features of Solanum melongena L. under induced NaCl and boron stresses. ACS Omega. 2023;8(31):28207–32. 10.1021/acsomega.3c01720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya P, Jayaprakasha GK, Crosby KM, Jifon JL, Patil BS. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci Rep. 2020;10:5037. 10.1038/s41598-020-61696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi Khalaki M, Moameri M, Asgari Lajayer B, Astatkie T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021;93:13–28. 10.1007/s10725-020-00670-9. [Google Scholar]
- 5.Adhikari B, Adhikari M, Ghimire B, Adhikari BC, Park G, Choi EH. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radical Biol Med. 2020;156:57–69. 10.1016/j.freeradbiomed.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Rahman MS, Kibria MG, Hoque A. Climate change and its adverse impacts on plant growth in South Asia: current status and upcoming challenges. Phyton. 2022;91(4):1–13. 10.32604/phyton.2022.018898. [Google Scholar]
- 7.Farooq M, Usman M, Nadeem F, Ur Rehman H, Wahid A, Basra SM, Siddique KH. Seed priming in field crops: potential benefits, adoption and challenges. Crop Pasture Sci. 2019;70(9):731–71. 10.1071/CP18604. [Google Scholar]
- 8.Rhaman MS, Tania SS, Imran S, Rauf F, Kibria MG, Ye W, Murata Y. Seed priming with nanoparticles: An emerging technique for improving plant growth, development, and abiotic stress tolerance. J Soil Sci Plant Nutr. 2022;22(4):4047–62. 10.1007/s42729-022-01007-3. [Google Scholar]
- 9.Hemavathy RV, Kumar PS, Kanmani K, Jahnavi N. Adsorptive separation of Cu (II) ions from aqueous medium using thermally/chemically treated Cassia fistula based biochar. J Clean Prod. 2020;249: 119390. 10.1016/j.jclepro.2019.119390. [Google Scholar]
- 10.Topal F. Antidiabetic potential: effect of resorcinol on α-glycosidase and α-amylase enzymes. Cumhuriyet Sci J. 2018;39(4):828–32. 10.17776/csj.452514. [Google Scholar]
- 11.Enache TA, Oliveira-Brett AM. Phenol and para-substituted phenols electrochemical oxidation pathways. J Electroanal Chem. 2011;655(1):9–16. 10.1016/j.jelechem.2011.02.022. [Google Scholar]
- 12.Salazar R, Vidal J, Martínez-Cifuentes M, Araya-Maturana R, Ramírez-Rodríguez O. Electrochemical characterization of hydroquinone derivatives with different substituents in acetonitrile. New J Chem. 2015;39(2):1237–46. 10.1039/C4NJ01657B. [Google Scholar]
- 13.Sun YG, Cui H, Li YH, Lin XQ. Determination of some catechol derivatives by a flow injection electrochemiluminescent inhibition method. Talanta. 2000;53(3):661–6. 10.1016/S0039-9140(00)00550-6. [DOI] [PubMed] [Google Scholar]
- 14.Khan MMA, Afreen R, Quasar N, Khanam N, Uddin M. Steam-mediated foliar application of catechol and plant growth regulators enhances the growth attributes, photosynthesis, and essential oil production of lemongrass [Cymbopogon flexuosus (Steud) Wats]. Biocatal Agric Biotechnol. 2023;48:102638. 10.1016/j.bcab.2023.102638. [Google Scholar]
- 15.Elblasy SA, Shehata HS, Ebrahiem AM, Hewait HM. Effectiveness of some bio-control agents and chemical resistance inducers against brown stem rot in soybean (Glycine max (L.) Merrill). Egypt J Phytopathol. 2023;51(1):103–21. 10.21608/ejp.2023.196335.1087. [Google Scholar]
- 16.Khaleda L, Kim MG, Jeon JR, Cha JY, Kim WY. Foliar application of humic acid or a mixture of catechol and vanillic acid enhanced growth and productivity of alfalfa. J Korean Soc Grassl Forage Sci. 2017;37(3):248–53. 10.5333/KGFS.2017.37.3.248. [Google Scholar]
- 17.Jagetiya BL, Kaur MJ. Effect of foliar application of resorcinol on certain biochemical parameters and yield of soybean. Asian J Bio Sci. 2006;1(2):129–32. [Google Scholar]
- 18.Wang M, Schoettner M, Xu S, Paetz C, Wilde J, Baldwin IT, Groten K. Catechol, a major component of smoke, influences primary root growth and root hair elongation through reactive oxygen species-mediated redox signaling. New Phytol. 2017;213(4):1755–70. 10.1111/nph.14317. [DOI] [PubMed] [Google Scholar]
- 19.Noel R, Benoit M, Wilder SL, Waller S, Schueller M, Ferrieri RA. Treatments with liquid smoke and certain chemical constituents prevalent in smoke reduce phloem vascular sectoriality in the sunflower with improvement to growth. Int J Mol Sci. 2022;23(20):12468. 10.3390/ijms232012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama DM, Abd El-Aziz ME, El-Naggar ME, Shaaban EA, Abd El-Wahed MS. Synthesis of an eco-friendly nanocomposite fertilizer for common bean based on carbon nanoparticles from agricultural waste biochar. Pedosphere. 2021;31(6):923–33. 10.1016/S1002-0160(21)60024-3. [Google Scholar]
- 21.Goswami L, Kushwaha A, Goswami S, Sharma YC, Kim T, Tripathi KM. Nanocarbon-based-ZnO nanocomposites for supercapacitor application. In: Nanostructured zinc oxide. Netheralands: Elsevier; 2021. p. 553–73. 10.1016/B978-0-12-818900-9.00008-5. [Google Scholar]
- 22.Xiu L, Zhang W, Sun Y, Wu D, Meng J, Chen W. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. CATENA. 2019;173:481–93. 10.1016/j.catena.2018.10.041. [Google Scholar]
- 23.Mansoor S, Kour N, Manhas S, Zahid S, Wani OA, Sharma V, Ahmad P. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere. 2021;271: 129458. 10.1016/j.chemosphere.2020.129458. [DOI] [PubMed] [Google Scholar]
- 24.Ramanayaka S, Vithanage M, Alessi DS, Liu WJ, Jayasundera AC, Ok YS. Nanobiochar: production, properties, and multifunctional applications. Environ Sci Nano. 2020;7(11):3279–302. 10.1039/D0EN00486C. [Google Scholar]
- 25.Chausali N, Saxena J, Prasad R. Nanobiochar and biochar based nanocomposites: Advances and applications. J Agric Food Res. 2021;5: 100191. 10.1016/j.jafr.2021.100191. [Google Scholar]
- 26.Wang Y, Wei Y, Sun J. Biochar application promotes growth parameters of soybean and reduces the growth difference. Commun Soil Sci Plant Anal. 2016;47(12):1493–502. 10.1080/00103624.2016.1194988. [Google Scholar]
- 27.Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Waris AA. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–77. 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 28.Seleiman MF. Use of plant nutrients in improving abiotic stress tolerance in wheat. In: Wheat Production in Changing Environments: Responses, Adaptation and Tolerance. Singapore: Springer; 2019. p. 481–95. 10.1007/978-981-13-6883-7_19. [Google Scholar]
- 29.Nath BK, Chaliha C, Kalita E. Iron oxide permeated mesoporous rice-husk nanobiochar (IPMN) mediated removal of dissolved arsenic (As): chemometric modelling and adsorption dynamics. J Environ Manag. 2019;246:397–409. 10.1016/j.jenvman.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Rajput VD, Minkina T, Ahmed B, Singh VK, Mandzhieva S, Sushkova S, Wang B. Nano-biochar: A novel solution for sustainable agriculture and environmental remediation. Environ Res. 2022;210: 112891. 10.1016/j.envres.2022.112891. [DOI] [PubMed] [Google Scholar]
- 31.Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Zhang L. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci. 2019;10:1068. 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keymer A, Pimprikar P, Wewer V, Huber C, Brands M, Bucerius SL, Gutjahr C. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife. 2017;6:e29107. 10.7554/eLife.29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comby M, Mustafa G, Magnin-Robert M, Randoux B, Fontaine J, Reignault P, Lounès-Hadj Sahraoui A. Arbuscular mycorrhizal fungi as potential bioprotectants against aerial phytopathogens and pests. Springer, Singapore: Arbuscular mycorrhizas and stress tolerance of plants; 2017. p. 195–223. 10.1007/978-981-10-4115-0_9. [Google Scholar]
- 34.Hamza N, Ziane H, Meddad-Hamza A, Gianinazzi S. Mycorrhizal contribution to water economy in agriculture under hot and dry climate: the case study of water melon in Algeria. Biosci Res. 2019;16:3781–91. [Google Scholar]
- 35.Garbaye J. La symbiose mycorhizienne: une association entre les plantes et les champignons. 2013.
- 36.Berruti A, Lumini E, Balestrini R, Bianciotto V. Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front Microbiol. 2016;6:1559. 10.3389/fmicb.2015.01559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igiehon NO, Babalola OO. Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Appl Microbiol Biotechnol. 2017;101:4871–81. 10.1007/s00253-017-8344-z. [DOI] [PubMed] [Google Scholar]
- 38.Baslam M, Garmendia I, Goicoechea N. The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. Sci Hortic. 2013;164:145–54. 10.1016/j.scienta.2013.09.021. [Google Scholar]
- 39.Shahzadi J, Ali N, Iftikhar M, Shah AA, Ashraf MY, Chao C, Gatasheh MK. Foliar application of nano biochar solution elevates tomato productivity by counteracting the effect of salt stress insights into morphological physiological and biochemical indices. Sci Rep. 2025;15(1):3205. 10.1038/s41598-025-87399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jatuwong K, Aiduang W, Kiatsiriroat T, Kamopas W, Lumyong S. Effects of biochar and arbuscular mycorrhizal fungi on soil health in Chinese Kale (Brassica oleracea var. alboglabra L.) cultivation. Microbiol Res. 2024;15:404–21. 10.3390/microbiolres15010027. [Google Scholar]
- 41.Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving & decanting. Trans Br Mycol Soc. 1963. 10.1016/S0007-1536(63)80079-0. [Google Scholar]
- 42.Walker, C. Taxonomic concepts in the Endogonaceae: spore wall characteristics in species descriptions. 1983.
- 43.Schenck, N. C. (1990). Manual for the identification of VA mycorrhizal fungi. Synergistic Publications Gainesville, 286.
- 44.Colombo RP, Recchi M, Silvani VA, Pérgola M, Martínez A, Godeas AM. Detection of arbuscular mycorrhizal fungi associated with pecan (Caryaillinoinensis) trees by molecular and morphological approaches. MycoKeys. 2018;42:73. 10.3897/mycokeys.42.26118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrol N, Calvente R, Cano C, Barea JM, Azcón-Aguilar C. Analysing arbuscular mycorrhizal fungal diversity in shrub-associated resource islands from a desertification-threatened semiarid Mediterranean ecosystem. Appl Soil Ecol. 2004;25(2):123–33. 10.1016/j.apsoil.2003.08.006. [Google Scholar]
- 46.Richard R, Smith SE, Verma ME. Effect of fertilizer and VAM inoculation on growth of Hard wood seedlings. Soil Sci Soc Am J. 1981;45:961–5. [Google Scholar]
- 47.Nisar J, Nasir U, Ali G, Shah A, Farooqi ZH, Iqbal M, Shah MR. Kinetics of pyrolysis of sugarcane bagasse: effect of catalyst on activation energy and yield of pyrolysis products. Cellulose. 2021;28:7593–607. 10.1007/s10570-021-04015-1. [Google Scholar]
- 48.Liu G, Zheng H, Jiang Z, Zhao J, Wang Z, Pan B, Xing B. Formation and physicochemical characteristics of nano biochar: insight into chemical and colloidal stability. Environ Sci Technol. 2018;52(18):10369–79. 10.1021/acs.est.8b01481. [DOI] [PubMed] [Google Scholar]
- 49.Bibi S, Ullah R, Burni T, Ullah Z, Kazi M. Impact of resorcinol and biochar application on the growth attributes, metabolite contents, and antioxidant systems of tomato (Lycopersicon esculentum Mill.). ACS Omega. 2023;8(48):45750–62. 10.1021/acsomega.3c06233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barampuram S, Allen G, Krasnyanski S. Effect of various sterilization procedures on the in vitro germination of cotton seeds. Plant Cell Tissue Organ Cult (PCTOC). 2014;118:179–85. 10.1007/s11240-014-0472-x. [Google Scholar]
- 51.Yang A, Akhtar SS, Li L, Fu Q, Li Q, Naeem MA, Jacobsen SE. Biochar mitigates combined effects of drought and salinity stress in quinoa. Agronomy. 2020;10(6):912. 10.3390/agronomy10060912. [Google Scholar]
- 52.AOAC. Official Methods of Analysis. 18th ed. Arlington, VA, USA: Association of Official Analytical Chemists; 2005. [Google Scholar]
- 53.Horwitz W. Official methods of analysis of AOAC international. In: Horwitz W, editor. Volume I, agricultural chemicals, contaminants, drugs. Maryland: AOAC International, Gaithersburg; 2010. [Google Scholar]
- 54.Kader MA. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J Proc R Soc New South Wales. 2005;138:65–75. [Google Scholar]
- 55.Babar S, Siddiqi EH, Hussain I, Hayat Bhatti K, Rasheed R. Mitigating the effects of salinity by foliar application of salicylic acid in fenugreek. Physiol J. 2014;2014(1): 869058. 10.1155/2014/869058. [Google Scholar]
- 56.Soltani N, Dille JA, Burke IC, Everman WJ, VanGessel MJ, Davis VM, Sikkema PH. Potential corn yield losses from weeds in North America. Weed Technol. 2016;30(4):979–84. 10.1614/WT-D-16-00046.1. [Google Scholar]
- 57.Basra SMA, Farooq M, Tabassam R, Ahmad N. Physiological and biochemical aspects of pre-sowing seed treatments in fine rice (Oryza sativa L.). Seed Science and Technology. 2005;33(3):623–8. 10.15258/sst.2005.33.3.09. [Google Scholar]
- 58.Al-Ansari F, Ksiksi T. A quantitative assessment of germination parameters: The case of Crotalaria persica and Tephrosia apollinea. Open Ecol J. 2016;9(1):13–21. 10.2174/1874213001609010013. [Google Scholar]
- 59.Bina F, Bostani A. Effect of Salinity (NaCl) stress on germination and early seedling growth of three medicinal plant species. Adv Life Sci. 2017;4(3):77–83. [Google Scholar]
- 60.Hussain A, Ali S, Rizwan M, Ur Rehman MZ, Javed MR, Imran M, Nazir R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut. 2018;242:1518–26. 10.1016/j.envpol.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–82. [Google Scholar]
- 62.Baydar NG, Özkan G, Sağdiç O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control. 2004;15(5):335–9. 10.1016/S0956-7135(03)00083-5. [Google Scholar]
- 63.Wang W, Vignani R, Scali M, Cresti M. A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis. 2006;27(13):2782–6. 10.1002/elps.200500722. [DOI] [PubMed] [Google Scholar]
- 64.Marcińska I, Czyczyło-Mysza I, Skrzypek E, Filek M, Grzesiak S, Grzesiak MT, Quarrie SA. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol Plant. 2013;35:451–61. 10.1007/s11738-012-1088-6. [Google Scholar]
- 65.Eryılmaz F. The relationships between salt stress and anthocyanin content in higher plants. Biotechnol Biotechnol Equip. 2006;20(1):47–52. 10.1080/13102818.2006.10817303. [Google Scholar]
- 66.Płażek A, Tatrzańska M, Maciejewski M, Kościelniak J, Gondek K, Bojarczuk J, Dubert F. Investigation of the salt tolerance of new Polish bread and durum wheat cultivars. Acta Physiol Plant. 2013;35:2513–23. 10.1007/s11738-013-1287-9. [Google Scholar]
- 67.Tyburski J, Dunajska K, Mazurek P, Piotrowska B, Tretyn A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol Plant. 2009;31:249–60. 10.1007/s11738-008-0225-8. [Google Scholar]
- 68.Salimi F, Shekari F, Hamzei J. Methyl jasmonate improves salinity resistance in German chamomile (Matricaria chamomilla L.) by increasing activity of antioxidant enzymes. Acta Physiol Plant. 2016;38:1–14. 10.1007/s11738-015-2023-4. [Google Scholar]
- 69.Li Y, Liang L, Huang S, Li W, Ashraf U, Ma L, Mo Z. Exogenous melatonin and catechol application modulate physio-biochemical attributes and early growth of fragrant rice under Cd toxicity. J Soil Sci Plant Nutr. 2021;21(3):2285–96. 10.1007/s42729-021-00521-0. [Google Scholar]
- 70.Bhardwaj RD, Kaur L, Srivastava P. Comparative evaluation of different phenolic acids as priming agents for mitigating drought stress in wheat seedlings. Proc Natl Acad Sci, India Sect B: Biol Sci. 2017;87:1133–42. 10.1007/s40011-015-0690-y. [Google Scholar]
- 71.Demiray S, Pintado ME, Castro PML. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad Sci, Eng Technol. 2009;54:312–7. [Google Scholar]
- 72.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016(1):7432797. 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belščak-Cvitanović A, Durgo K, Huđek A, Bačun-Družina V, Komes D. Overview of polyphenols and their properties. In: Polyphenols: Properties, recovery, and applications. Cambridge: Woodhead Publishing; 2018. p. 3–44. 10.1016/B978-0-12-813572-3.00001-4. [Google Scholar]
- 74.Van Acker SA, Tromp MN, Griffioen DH, Van Bennekom WP, Van Der Vijgh WJ, Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radical Biol Med. 1996;20(3):331–42. 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 75.Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84(4):551–62. 10.1016/S0308-8146(03)00278-4. [Google Scholar]
- 76.Zhang K, Wang Y, Mao J, Chen B. Effects of biochar nanoparticles on seed germination and seedling growth. Environ Pollut. 2020;256: 113409. 10.1016/j.envpol.2019.113409. [DOI] [PubMed] [Google Scholar]
- 77.Ye J, Wang X, Wang W, Yu H, Ai G, Li C, Ye Z. Genome-wide association study reveals the genetic architecture of 27 agronomic traits in tomato. Plant Physiol. 2021;186(4):2078–92. 10.1093/plphys/kiab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naseer M, Zhu Y, Li FM, Yang YM, Wang S, Xiong YC. Nano-enabled improvements of growth and colonization rate in wheat inoculated with arbuscular mycorrhizalfungi. Environ Pollut. 2022;295: 118724. 10.1016/j.envpol.2021.118724. [DOI] [PubMed] [Google Scholar]
- 79.Gross MS, Bean TG, Hladik ML, Rattner BA, Kuivila KM. Uptake, metabolism, and elimination of fungicides from coated wheat seeds in Japanese quail (Coturnix japonica). J Agric Food Chem. 2020;68(6):1514–24. 10.1021/acs.jafc.9b05668. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Liang L, Li W, Ashraf U, Ma L, Tang X, Mo Z. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J Nanobiotechnol. 2021;19:1–19. 10.1186/s12951-021-00820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Hu J, Ma C, Wang Y, Wu C, Huang J, Xing B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere. 2016;159:326–34. 10.1016/j.chemosphere.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 82.Sani MNH, Amin M, Siddique AB, Nasif SO, Ghaley BB, Ge L, Yong JWH. Waste-derived nanobiochar: A new avenue towards sustainable agriculture, environment, and circular bioeconomy. Sci Total Environ. 2023;905: 166881. 10.1016/j.scitotenv.2023.166881. [DOI] [PubMed] [Google Scholar]
- 83.Noreen S, Abd-Elsalam KA. Biochar-based nanocomposites: A sustainable tool in wastewater bioremediation. In: Aquananotechnology. Netherlands: Elsevier; 2021. p. 185–200. 10.1016/B978-0-12-821141-0.00023-9. [Google Scholar]
- 84.Hong J, Wang C, Wagner DC, Gardea-Torresdey JL, He F, Rico CM. Foliar application of nanoparticles: mechanisms of absorption, transfer, and multiple impacts. Environ Sci: Nano. 2021;8(5):1196–210. 10.1039/D0EN01129K. [Google Scholar]
- 85.Su Y, Ashworth V, Kim C, Adeleye AS, Rolshausen P, Roper C, Jassby D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environ Sci: Nano. 2019;6(8):2311–31. [Google Scholar]
- 86.Sané AK, Diallo B, Kane A, Ngom M, Cissoko M, Sy MO. Response to inoculation with arbuscular mycorrhizal fungi of two tomato (Solanum lycopersicum L) varieties subjected to water stress under semi-controlled conditions. Agric Sci. 2022;13(6):790–819. 10.4236/as.2022.136051. [Google Scholar]
- 87.Ziane H, Hamza N, Meddad-Hamza A. Arbuscular mycorrhizal fungi and fertilization rates optimize tomato (Solanum lycopersicum L) growth and yield in a Mediterranean agroecosystem. J Saudi Soc Agric Sci. 2021;20(7):454–8. 10.1016/j.jssas.2021.05.009. [Google Scholar]
- 88.Liu M, Lin Z, Ke X, Fan X, Joseph S, Taherymoosavi S, Pan G. Rice seedling growth promotion by biochar varies with genotypes and application dosages. Front Plant Sci. 2021;12: 580462. 10.3389/fpls.2021.580462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jabborova D, Annapurna K, Azimov A, Tyagi S, Pengani KR, Sharma P, Sayyed RZ. Co-inoculation of biochar and arbuscular mycorrhizae for growth promotion and nutrient fortification in soybean under drought conditions. Front Plant Sci. 2022;13: 947547. 10.3389/fpls.2022.947547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo S, Wang S, Tian L, Li S, Li X, Shen Y, Tian C. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl Soil Ecol. 2017;117:10–5. 10.1016/j.apsoil.2017.04.024. [Google Scholar]
- 91.Yang Q, Ravnskov S, Pullens JWM, Andersen MN. Interactions between biochar, arbuscular mycorrhizal fungi and photosynthetic processes in potato (Solanum tuberosum L.). Sci Total Environ. 2022;816:151649. 10.1016/j.scitotenv.2021.151649. [DOI] [PubMed] [Google Scholar]
- 92.Aleksandrowicz-Trzcinska M, Szaniawski A, Studnicki M, Bederska-Blaszczyk M, Olchowik J, Urban A. The effect of silver and copper nanoparticles on the growth and mycorrhizal colonisation of Scots pine (Pinus sylvestris L.) in a container nursery experiment. iFor-Biogeosci For. 2018;11(5):690. 10.3832/ifor2855-011. [Google Scholar]
- 93.Arshad U, Raheel M, Ashraf W, Ur Rehman A, Zahid MS, Moustafa M, Ali MA. Influence of Biochar Application on morpho-physiological attributes of tomato (Lycopersicon esculentum Mill) and soil Properties. Commun Soil Sci Plant Anal. 2023;54(4):515–25. 10.1080/00103624.2022.2118295. [Google Scholar]
- 94.Solaiman ZM, Murphy DV, Abbott LK. Biochars influence seed germination and early growth of seedlings. Plant Soil. 2012;353:273–87. 10.1007/s11104-011-1031-4. [Google Scholar]
- 95.Rees F, Germain C, Sterckeman T, Morel JL. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd-Zn hyperaccumulator (Noccaea caerulescens) in contaminated soils amended with biochar. Plant Soil. 2015;395:57–73. [Google Scholar]
- 96.Zhang Y, Wang J, Feng Y. The effects of biochar addition on soil physicochemical properties: A review. CATENA. 2021;202: 105284. 10.1016/j.catena.2021.105284. [Google Scholar]
- 97.Sun J, Jia Q, Li Y, Zhang T, Chen J, Ren Y, Fu S. Effects of arbuscular mycorrhizal fungi and biochar on growth, nutrient absorption, and physiological properties of maize (Zea mays L.). J Fungi. 2022;8(12):1275. 10.3390/jof8121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ait-El-Mokhtar M, Baslam M, Ben-Laouane R, Anli M, Boutasknit A, Mitsui T, Meddich A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front Sustain Food Syst. 2020;4:131. 10.3389/fsufs.2020.00131. [Google Scholar]
- 99.Anli M, Baslam M, Tahiri A, Raklami A, Symanczik S, Boutasknit A, Meddich A. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci. 2020;11: 516818. 10.3389/fpls.2020.516818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hadian-Deljou M, Esna-Ashari M, Mirzaie-Asl A. Alleviation of salt stress and expression of stress-responsive gene through the symbiosis of arbuscular mycorrhizal fungi with sour orange seedlings. Sci Hortic. 2020;268: 109373. 10.1016/j.scienta.2020.109373. [Google Scholar]
- 101.Gupta B, Mishra A, Singh R, Thakur IS. Fabrication of calcite based biocomposites for catalytic removal of heavy metals from electroplating industrial effluent. Environ Technol Innov. 2021;21: 101278. 10.1016/j.eti.2020.101278. [Google Scholar]
- 102.Soussani FE, Boutasknit A, Ben-Laouane R, Benkirane R, Baslam M, Meddich A. Arbuscular mycorrhizal fungi and compost-based biostimulants enhance fitness, physiological responses, yield, and quality traits of drought-stressed tomato plants. Plants. 2023;12(9):1856. 10.3390/plants12091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khaliq H, Anwar S, Shafiq F, Ashraf M, Zhang L, Haider I, Khan S. Interactive effects of soil and foliar-applied nanobiochar on growth, metabolites, and nutrient composition in Daucus carota. J Plant Growth Regul. 2023;42(6):3715–29. 10.1007/s00344-022-10832-w. [Google Scholar]
- 104.Mazhar MW, Ishtiaq M, Maqbool M, Akram R. Seed priming with zinc oxide nanoparticles improves growth, osmolyte accumulation, antioxidant defence and yield quality of water-stressed mung bean plants. Arid Land Res Manag. 2023;37(2):222–46. 10.1080/15324982.2022.2132547. [Google Scholar]
- 105.Munsif F, Shah T, Arif M, Jehangir M, Afridi MZ, Ahmad I, Alansi S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in wheat. Saudi J Biol Sci. 2022;29(6): 103294. 10.1016/j.sjbs.2022.103294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh S, Prakash P, Singh AK. Salicylic acid and hydrogen peroxide improve antioxidant response and compatible osmolytes in wheat (Triticum aestivum L) under water deficit. Agric Res. 2021;10:175–86. 10.1007/s40003-020-00490-3. [Google Scholar]
- 107.Ullah R, Ullah Z, Iqbal J, Chalgham W, Ahmad A. Aspartic acid-based nano-copper induces resilience in Zea mays to applied lead stress via conserving photosynthetic pigments and triggering the antioxidant biosystem. Sustainability. 2023;15(16):12186. 10.3390/su151612186. [Google Scholar]
- 108.Pirzadah TB, Malik B, Tahir I, Rehman RU, Hakeem KR, Alharby HF. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol Biochem. 2019;144:178–86. 10.1016/j.plaphy.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 109.Duc NH, Vo AT, Haddidi I, Daood H, Posta K. Arbuscular mycorrhizal fungi improve tolerance of the medicinal plant Eclipta prostrata (L.) and induce major changes in polyphenol profiles under salt stresses. Front Plant Sci. 2021;11: 612299. 10.3389/fpls.2020.612299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waqas Mazhar M, Ishtiaq M, Hussain I, Parveen A, Hayat Bhatti K, Azeem M, Nasir N. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE. 2022;17(3): e0264967. 10.1371/journal.pone.0264967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sapakhova Z, Irkitbay A, Madenova A, Suleimanova G. Mitigation effect of salicylic acid on wheat (Triticum aestivum L.) under drought stress. Res Crops. 2022;23(2):267–75. 10.31830/2348-7542.2022.037. [Google Scholar]
- 112.Azmat A, Yasmin H, Hassan MN, Nosheen A, Naz R, Sajjad M, Akhtar MN. Co-application of bio-fertilizer and salicylic acid improves growth, photosynthetic pigments and stress tolerance in wheat under drought stress. PeerJ. 2020;8: e9960. 10.7717/peerj.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054(1–2):95–111. 10.1016/j.chroma.2004.08.059. [PubMed] [Google Scholar]
- 114.Kiokias S, Varzakas T, Oreopoulou V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit Rev Food Sci Nutr. 2008;48(1):78–93. 10.1080/10408390601079975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.