Abstract
Here, the  compound has been investigated as a novel candidate material for supercapacitor electrodes. Moreover, its alloy with Na in replacement of Cr atoms (
compound has been investigated as a novel candidate material for supercapacitor electrodes. Moreover, its alloy with Na in replacement of Cr atoms ( ) has been structured in order to prove Na-ion battery applicability and supercapacitance behavior as a hybrid energy material. The density functional theory with the general gradient approximation has been used for the present work calculations. The structural, thermodynamic, and dynamic stability of the compounds have been tested using the cohesive energy, enthalpy of formation energy, and phonon calculation, respectively. The diffusion coefficient for the
) has been structured in order to prove Na-ion battery applicability and supercapacitance behavior as a hybrid energy material. The density functional theory with the general gradient approximation has been used for the present work calculations. The structural, thermodynamic, and dynamic stability of the compounds have been tested using the cohesive energy, enthalpy of formation energy, and phonon calculation, respectively. The diffusion coefficient for the  is 9
is 9  10
10
 /s which shows an improvement of 1
/s which shows an improvement of 1 10
10
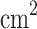 /s in compare with the pure compound. While its largest value at 300 K for electrical conductivity per relaxation time is 2.64
/s in compare with the pure compound. While its largest value at 300 K for electrical conductivity per relaxation time is 2.64  10
10 and lower than that for
and lower than that for  . Furthermore, the largest peaks of areal quantum capacitance are 511.06
. Furthermore, the largest peaks of areal quantum capacitance are 511.06  F/
F/ (at 1.41 V) and 414.03
(at 1.41 V) and 414.03  F/
F/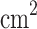 (at – 0.63 V) for
(at – 0.63 V) for  and
and  , respectively. These results introduce new candidates for supercapacitance and Na-ion battery industries.
, respectively. These results introduce new candidates for supercapacitance and Na-ion battery industries.
Keywords: Quantum capacitance, Na-ion batteries, Supercapacitors, Hybrid energy materials
Subject terms: Physics; Condensed-matter physics; Surfaces, interfaces and thin films
Introduction
In recent years, the need for efficient, cost-effective, and environmentally friendly energy storage systems has become increasingly urgent due to the rapid growth in portable electronics, electric vehicles, and renewable energy integration. Supercapacitors and sodium-ion batteries (SIBs) are among the most promising alternatives to conventional lithium-ion batteries, owing to their fast charge/discharge rates, long cycle life, and the abundance of sodium resources1–12. However, the development of high-performance electrode materials remains a critical challenge for realizing the full potential of these technologies. Transition metal-based intermetallic compounds have gained considerable attention due to their unique electronic structures, mechanical robustness, and tunable physicochemical properties8,13–18. Particularly, Laves phase compounds, such as  , exhibit favorable thermodynamic and structural stability, making them suitable for electrochemical applications19,20.
, exhibit favorable thermodynamic and structural stability, making them suitable for electrochemical applications19,20.  among the Laves phase compounds has a stable cubic C15 structure, mechanical robustness, and high electronic density of states (DOS) near the Fermi level, which is beneficial for charge transfer, potentially enhancement of capacitance behavior, and electrochemical activity. The partial substitution of Cr with Na is motivated by the abundance, low cost, and lightweight nature of sodium compared to lithium or heavier alkali metals. Although Na doping slightly reduces the quantum capacitance due to changes in the electronic structure, the calculated values remain significantly high, making the Na-alloyed compound a promising candidate for energy storage applications. Furthermore, our calculations reveal that Na substitution leads to a trade-off between ion transport and electronic conduction: the diffusion coefficient for Na-ion mobility increases, which is beneficial for battery performance, while the overall electrical conductivity slightly decreases due to reduced electronic states at the Fermi level. Nevertheless, the Na-alloyed
among the Laves phase compounds has a stable cubic C15 structure, mechanical robustness, and high electronic density of states (DOS) near the Fermi level, which is beneficial for charge transfer, potentially enhancement of capacitance behavior, and electrochemical activity. The partial substitution of Cr with Na is motivated by the abundance, low cost, and lightweight nature of sodium compared to lithium or heavier alkali metals. Although Na doping slightly reduces the quantum capacitance due to changes in the electronic structure, the calculated values remain significantly high, making the Na-alloyed compound a promising candidate for energy storage applications. Furthermore, our calculations reveal that Na substitution leads to a trade-off between ion transport and electronic conduction: the diffusion coefficient for Na-ion mobility increases, which is beneficial for battery performance, while the overall electrical conductivity slightly decreases due to reduced electronic states at the Fermi level. Nevertheless, the Na-alloyed  compound maintains sufficient electronic conductivity and exhibits enhanced ionic transport, making it a promising multifunctional electrode material for hybrid energy storage systems.
compound maintains sufficient electronic conductivity and exhibits enhanced ionic transport, making it a promising multifunctional electrode material for hybrid energy storage systems.
Pysarenko et al.20 studied properties of  by experimental methods. Their results showed that the
by experimental methods. Their results showed that the  intermetallic compound exhibits brittle behavior at temperatures below 1100 °C, where fracture primarily occurs along grain boundaries. However, at elevated temperatures of 1200–1300 °C, the material undergoes noticeable plastic deformation. Specifically, it shows a plastic strain of about 5–7
intermetallic compound exhibits brittle behavior at temperatures below 1100 °C, where fracture primarily occurs along grain boundaries. However, at elevated temperatures of 1200–1300 °C, the material undergoes noticeable plastic deformation. Specifically, it shows a plastic strain of about 5–7 before crack initiation, and up to nearly 30
before crack initiation, and up to nearly 30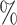 total strain before complete fracture.
total strain before complete fracture.
Cui et al.19 investigated the heat capacities of both phases of C14 and C15 over a temperature range from 2 to 1063 K and fitted using a modified Einstein model. They determined the room temperature entropy and the enthalpy of formation for the first time by drop solution calorimetry in liquid aluminum at 1173 K, showing good agreement with theoretical calculations based on density functional theory (DFT).
According to the results reported by van Midden and co-workers21, repulsion occurs between closely positioned hydrogen atoms in the C15 structure, which aligns with their DFT-based findings on C14 and C15 polymorphs.
Moreover, Abel and Craig22 reported that the hexagonal Laves phases  ,
,  , and
, and  exhibit Pauli paramagnetism with nearly identical magnetic susceptibility values of approximately 4
exhibit Pauli paramagnetism with nearly identical magnetic susceptibility values of approximately 4  10
10 e.m.u/mol, while the cubic modification of
e.m.u/mol, while the cubic modification of  shows a slightly higher value of 5
shows a slightly higher value of 5  10
10 e.m.u/mol.
e.m.u/mol.
Li et al.23 employed density functional theory to assess the potential of the  intermetallic compound as an anode for Mg-ion batteries. They demonstrated that its intrinsic metallic conductivity, combined with excellent thermal, mechanical, and dynamic stability, makes it suitable for fast and stable cycling. Their calculations revealed a moderate volume expansion (
intermetallic compound as an anode for Mg-ion batteries. They demonstrated that its intrinsic metallic conductivity, combined with excellent thermal, mechanical, and dynamic stability, makes it suitable for fast and stable cycling. Their calculations revealed a moderate volume expansion (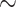 107%) upon conversion to
107%) upon conversion to  Cu, a high theoretical capacity of 1688 mAh/g, a low open-circuit voltage (0.221 V), and low Mg-ion diffusion barriers (
Cu, a high theoretical capacity of 1688 mAh/g, a low open-circuit voltage (0.221 V), and low Mg-ion diffusion barriers ( 0.31 eV), all of which highlight its promise as a high-rate anode material.
0.31 eV), all of which highlight its promise as a high-rate anode material.
In addition, Xiao et al.24 conducted a first-principles investigation on metal-doped  monolayers and demonstrated that substitutional doping with Pd, Ti, and V significantly improves their electrochemical performance as anode materials for alkali-ion batteries. Their results revealed enhanced structural stability, superior electronic conductivity, and mechanical flexibility. Additionally, the doped structures exhibit lower diffusion barriers for alkali ions (Li and Na), a reduced open-circuit voltage (down to 0.17 V), and a high theoretical capacity of up to 1470.87 mAh/g, indicating their strong potential for next-generation high-rate energy storage systems.
monolayers and demonstrated that substitutional doping with Pd, Ti, and V significantly improves their electrochemical performance as anode materials for alkali-ion batteries. Their results revealed enhanced structural stability, superior electronic conductivity, and mechanical flexibility. Additionally, the doped structures exhibit lower diffusion barriers for alkali ions (Li and Na), a reduced open-circuit voltage (down to 0.17 V), and a high theoretical capacity of up to 1470.87 mAh/g, indicating their strong potential for next-generation high-rate energy storage systems.
In this study, we explore the potential of  , as a novel electrode material for supercapacitor applications using first-principles density functional theory (DFT) calculations. Additionally, to evaluate the compound’s applicability in sodium-ion batteries, we investigate the structural and electronic properties of its Na-alloyed counterpart,
, as a novel electrode material for supercapacitor applications using first-principles density functional theory (DFT) calculations. Additionally, to evaluate the compound’s applicability in sodium-ion batteries, we investigate the structural and electronic properties of its Na-alloyed counterpart,  , where Cr atoms are partially substituted by Na. A comprehensive computational analysis is performed to assess the structural, thermodynamic, and dynamic stability through cohesive energy, formation enthalpy, and phonon dispersion calculations. Moreover, we compute the electronic transport properties, including electrical conductivity and Na-ion diffusion coefficients, as well as the quantum capacitance behavior under different electrochemical potentials.
, where Cr atoms are partially substituted by Na. A comprehensive computational analysis is performed to assess the structural, thermodynamic, and dynamic stability through cohesive energy, formation enthalpy, and phonon dispersion calculations. Moreover, we compute the electronic transport properties, including electrical conductivity and Na-ion diffusion coefficients, as well as the quantum capacitance behavior under different electrochemical potentials.
The results reveal that both  , and
, and  possess characteristics suitable for hybrid energy storage systems, with enhanced diffusion and capacitance performance in the alloyed structure. This study provides valuable insights into the design and optimization of intermetallic compounds for multifunctional energy storage applications and smooth the way for their experimental realization in future supercapacitor and sodium-ion battery devices.
possess characteristics suitable for hybrid energy storage systems, with enhanced diffusion and capacitance performance in the alloyed structure. This study provides valuable insights into the design and optimization of intermetallic compounds for multifunctional energy storage applications and smooth the way for their experimental realization in future supercapacitor and sodium-ion battery devices.
Computational details
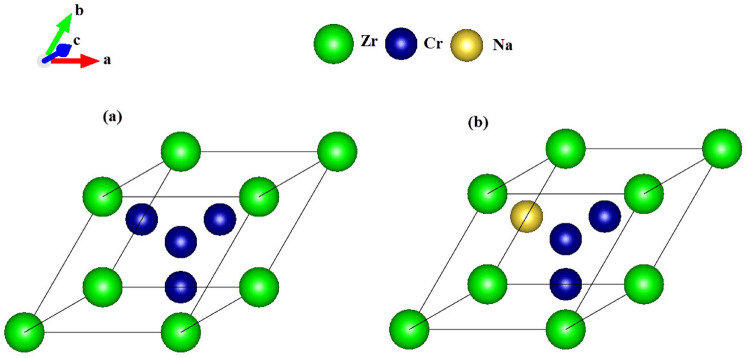
All calculations were performed within the framework of density functional theory (DFT) using the generalized gradient approximation (GGA) for the exchange-correlation functional. Norm-conserving pseudopotentials of the Perdew-Burke-Ernzerhof (PBE) type were employed25,26. The study focuses on the intermetallic compound ZrCr2 crystallizing in the Fd m space group, as well as a sodium-substituted alloy where one chromium atom is replaced by a sodium atom within a six-atom supercell (i.e.,
m space group, as well as a sodium-substituted alloy where one chromium atom is replaced by a sodium atom within a six-atom supercell (i.e.,  ). The corresponding crystal structure is illustrated in Fig. 1a, b. The reason of the replacement of the Cr with Na atom was because of the higher chemical activity of Cr atoms and the structural role of Zr in maintaining the C15 Laves phase framework. Interstitial doping was avoided due to the dense atomic packing of the cubic structure, which limits available interstitial space and would likely lead to high formation energy and lattice distortion. Moreover, our calculations showed that partial substitution of Cr with Na preserves the dynamic stability of the system, as evidenced by the absence of imaginary modes in phonon dispersion spectra. A doping level of
). The corresponding crystal structure is illustrated in Fig. 1a, b. The reason of the replacement of the Cr with Na atom was because of the higher chemical activity of Cr atoms and the structural role of Zr in maintaining the C15 Laves phase framework. Interstitial doping was avoided due to the dense atomic packing of the cubic structure, which limits available interstitial space and would likely lead to high formation energy and lattice distortion. Moreover, our calculations showed that partial substitution of Cr with Na preserves the dynamic stability of the system, as evidenced by the absence of imaginary modes in phonon dispersion spectra. A doping level of  in
in 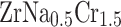 was selected to achieve a balance between modifying the material’s electronic and ionic transport properties while preserving its structural and dynamic integrity.
was selected to achieve a balance between modifying the material’s electronic and ionic transport properties while preserving its structural and dynamic integrity.
Fig. 1.
The crystal structure of (a):  and (b):
and (b): 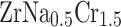 .
.
Moreover, Self-consistent field (SCF) calculations were carried out using the Quantum ESPRESSO package27. The optimized kinetic energy cutoff was set to 40 Ry, and the Brillouin zone was sampled using k-point mesh of 8  8
8  8 Monkhorst–Pack. Structural relaxation was performed via energy and force minimization, with convergence thresholds of
8 Monkhorst–Pack. Structural relaxation was performed via energy and force minimization, with convergence thresholds of  eV for total energy and
eV for total energy and  eV/Å for atomic forces.
eV/Å for atomic forces.
In addition, electronic density of states (DOS) calculations were conducted using a denser 20  20
20  20 k-point grid. Phonon properties were investigated within the framework of density functional perturbation theory (DFPT)28. All simulations were performed for both the pristine compound and the sodium alloy. Furthermore, spin-polarized calculations were performed to assess potential magnetic contributions from Cr atoms, but the results showed negligible magnetic moments, confirming the non-magnetic nature of the systems.
20 k-point grid. Phonon properties were investigated within the framework of density functional perturbation theory (DFPT)28. All simulations were performed for both the pristine compound and the sodium alloy. Furthermore, spin-polarized calculations were performed to assess potential magnetic contributions from Cr atoms, but the results showed negligible magnetic moments, confirming the non-magnetic nature of the systems.
Table 1 presents the optimized lattice constants, diffusion coefficients (D) along with the ratio of electrical conductivity to relaxation time ( ) at 300 K. The diffusion coefficients were obtained from molecular dynamics simulations and yielded values of
) at 300 K. The diffusion coefficients were obtained from molecular dynamics simulations and yielded values of 
 /s and
/s and 
 /s for the pure and alloyed systems, respectively. The introduction of Na weakens the local electrostatic binding environment and increases the spacing between active sites, leading to lower diffusion energy barriers and enhanced Na-ion mobility. This enhanced diffusivity is beneficial for high-rate battery operation.
/s for the pure and alloyed systems, respectively. The introduction of Na weakens the local electrostatic binding environment and increases the spacing between active sites, leading to lower diffusion energy barriers and enhanced Na-ion mobility. This enhanced diffusivity is beneficial for high-rate battery operation.
Table 1.
the lattice constant (a), diffusion coefficient, D, (at 300K), and electrical conductivity per relaxation time,  , (at 300 K) for
, (at 300 K) for  and
and 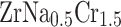 .
.
| a (Å ) | D ( /s) /s) |
 (1/ (1/ .m.s) .m.s) |
|
|---|---|---|---|

|
3.49 | 8  10 10
|
4.81  10 10
|

|
3.61 | 9  10 10
|
2.64  10 10
|
In comparison with other results reported for Na-ion batteries, for instance, the experimental work by Liu et al.29 on Na-ion diffusion in  /
/ -reduced graphene oxide, which reported a diffusion coefficient of
-reduced graphene oxide, which reported a diffusion coefficient of 
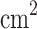
 , the present results are compatible.
, the present results are compatible.
The calculated  values using the Boltztrap package30 were 4.81
values using the Boltztrap package30 were 4.81  10
10 (
( m s)
m s) for the pure compound and 2.64
for the pure compound and 2.64  10
10 (
( .m.s)
.m.s) for the alloy, both indicative of excellent electrical transport characteristics, making these materials promising candidates for sodium-ion battery applications.
for the alloy, both indicative of excellent electrical transport characteristics, making these materials promising candidates for sodium-ion battery applications.
Compared to our previous work where the highest electrical conductivity of pristine  was calculated as 4.14
was calculated as 4.14  10
10 (
( m s)
m s)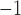 , the current study shows a slightly higher conductivity for pristine
, the current study shows a slightly higher conductivity for pristine  , while the Na-alloyed
, while the Na-alloyed  exhibits somewhat lower conductivity than both pristine and alloyed
exhibits somewhat lower conductivity than both pristine and alloyed  systems8.
systems8.
Furthermore, Fig. 2 displays the cohesive energy and bond length analysis for both systems. The cohesive energies were found to be  eV and
eV and  eV for the pure and alloy compounds, respectively, confirming their structural stability. The Zr–Cr bond length was 2.89 Å in the pure compound and increased slightly to 2.90 Å upon Na substitution. Additionally, the Na–Cr and Na–Zr bond lengths were calculated as 2.89 Å and 2.55 Å, respectively.
eV for the pure and alloy compounds, respectively, confirming their structural stability. The Zr–Cr bond length was 2.89 Å in the pure compound and increased slightly to 2.90 Å upon Na substitution. Additionally, the Na–Cr and Na–Zr bond lengths were calculated as 2.89 Å and 2.55 Å, respectively.
Fig. 2.
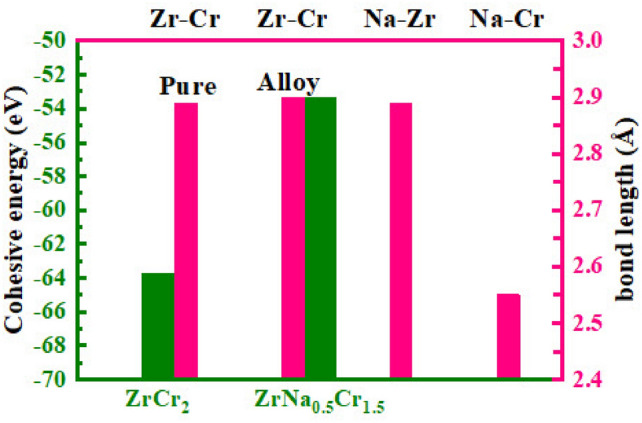
The cohesive energy and bondlength for  and
and  .
.
The formation enthalpies were negative for both structures, amounting to  kJ/mol for the pure compound and
kJ/mol for the pure compound and  kJ/mol for the alloy, confirming their thermodynamic stability. Moreover, the quantum capacitance (
kJ/mol for the alloy, confirming their thermodynamic stability. Moreover, the quantum capacitance ( ) and the surface charge storage capacity (Q) were also evaluated using the relation31–34:
) and the surface charge storage capacity (Q) were also evaluated using the relation31–34:
 |
1 |
 |
2 |
where D(E) is the electronic density of states, 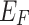 is the Fermi level, and e is the elementary charge. The applied bias voltage is introduced by mapping the energy shift relative to the Fermi level, i.e.,
is the Fermi level, and e is the elementary charge. The applied bias voltage is introduced by mapping the energy shift relative to the Fermi level, i.e.,  . These quantities further support the suitability of these materials in energy storage applications.
. These quantities further support the suitability of these materials in energy storage applications.
Results and discussions
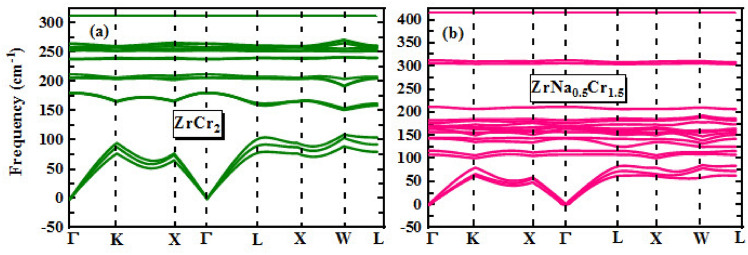
Figure 3a, b illustrate the phonon density of states (PhDOS) as a function of frequency for  and
and  . Notably, the absence of imaginary (negative) phonon modes across the Brillouin zone confirms the dynamical stability of both the pristine and Na-alloy compounds, indicating that no structural instabilities exist at 0 K. In the low-frequency region (below 50
. Notably, the absence of imaginary (negative) phonon modes across the Brillouin zone confirms the dynamical stability of both the pristine and Na-alloy compounds, indicating that no structural instabilities exist at 0 K. In the low-frequency region (below 50  ), the PhDOS remains nearly zero, suggesting the lack of low-energy acoustic-like vibrations or any soft modes. This behavior could be attributed to the relatively high atomic masses of constituent elements and strong interatomic bonding, which suppress the emergence of low-frequency phonon modes. The main PhDOS peaks are located at 252.13
), the PhDOS remains nearly zero, suggesting the lack of low-energy acoustic-like vibrations or any soft modes. This behavior could be attributed to the relatively high atomic masses of constituent elements and strong interatomic bonding, which suppress the emergence of low-frequency phonon modes. The main PhDOS peaks are located at 252.13  and 416
and 416  , with intensities of 1.27 and 1.13 for
, with intensities of 1.27 and 1.13 for 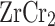 and
and  , respectively. The shift in peak position and intensity upon Na substitution indicates a modification of the local vibrational environment, possibly due to mass difference and bond softening introduced by the Na atoms. Moreover, Fig. 4a, b present the phonon dispersion curves of
, respectively. The shift in peak position and intensity upon Na substitution indicates a modification of the local vibrational environment, possibly due to mass difference and bond softening introduced by the Na atoms. Moreover, Fig. 4a, b present the phonon dispersion curves of  and its Na-alloyed along high-symmetry directions in the Brillouin zone. The absence of imaginary modes confirms the dynamic stability of both compounds and is consistent with the Phonon DOS results.
and its Na-alloyed along high-symmetry directions in the Brillouin zone. The absence of imaginary modes confirms the dynamic stability of both compounds and is consistent with the Phonon DOS results.
Fig. 3.
The Phonon density of states ad function of frequency for (a):  and (b):
and (b):  .
.
Fig. 4.
The phonon dispersion curves of  and its Na-alloyed along high-symmetry directions in the Brillouin zone for (a):
and its Na-alloyed along high-symmetry directions in the Brillouin zone for (a):  and (b):
and (b):  .
.
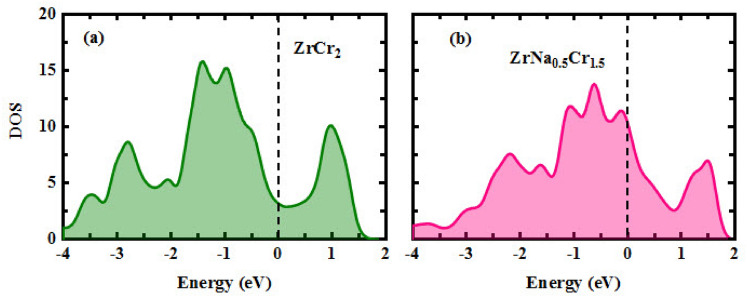
In addition, Fig. 5a, b depict the total electronic density of states (DOS) for  and
and  , respectively. The Fermi level is aligned to 0 eV. In both cases, a non-zero DOS is observed at the Fermi level, clearly indicating the metallic nature of these compounds, consistent with their expected electronic configurations. The dominant DOS peaks are located in the valence band region, with maximum values of 15.8 and 13.8 states/eV at binding energies of –1.42 eV and –0.63 eV for
, respectively. The Fermi level is aligned to 0 eV. In both cases, a non-zero DOS is observed at the Fermi level, clearly indicating the metallic nature of these compounds, consistent with their expected electronic configurations. The dominant DOS peaks are located in the valence band region, with maximum values of 15.8 and 13.8 states/eV at binding energies of –1.42 eV and –0.63 eV for 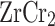 and
and  , respectively. The shift of the main peak toward the Fermi level in the Na alloy structure suggests an electronic redistribution due to Na incorporation, possibly resulting from charge transfer effects and modified hybridization between the Cr-3d and Zr-4d orbitals. Furthermore, the presence of pronounced valleys near the Fermi level (in the conduction band for the doped compound and slightly in the valence band for the pristine one) may indicate regions of reduced electronic states, which could affect transport properties such as electrical conductivity.
, respectively. The shift of the main peak toward the Fermi level in the Na alloy structure suggests an electronic redistribution due to Na incorporation, possibly resulting from charge transfer effects and modified hybridization between the Cr-3d and Zr-4d orbitals. Furthermore, the presence of pronounced valleys near the Fermi level (in the conduction band for the doped compound and slightly in the valence band for the pristine one) may indicate regions of reduced electronic states, which could affect transport properties such as electrical conductivity.
Fig. 5.
The total electronic density of states as a function of energy for (a):  and (b):
and (b):  .
.
Furthermore, Fig. 6a, b present the partial density of states (PDOS) for  and
and 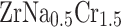 as a function of energy. The Fermi level is set at 0 eV for reference. In both systems, the dominant electronic contributions near the Fermi level arise from the Cr-3d and Zr-4d orbitals, reflecting the key role of transition-metal d states in defining the electronic structure. For the pristine
as a function of energy. The Fermi level is set at 0 eV for reference. In both systems, the dominant electronic contributions near the Fermi level arise from the Cr-3d and Zr-4d orbitals, reflecting the key role of transition-metal d states in defining the electronic structure. For the pristine  compound, the most intense peak in the PDOS originates from the Cr-3d states, reaching a maximum of 3.27 states/eV at − 1.41 eV in the valence band. The second-largest contribution corresponds to the Zr-4d states with a peak value of 1.93 states/eV at − 0.99 eV. These features indicate strong d–d hybridization, typical of intermetallic compounds involving early transition metals. In the Na-doped alloy,
compound, the most intense peak in the PDOS originates from the Cr-3d states, reaching a maximum of 3.27 states/eV at − 1.41 eV in the valence band. The second-largest contribution corresponds to the Zr-4d states with a peak value of 1.93 states/eV at − 0.99 eV. These features indicate strong d–d hybridization, typical of intermetallic compounds involving early transition metals. In the Na-doped alloy,  , the Cr-3d and Zr-4d states remain predominant, with peak values of 2.84 and 1.53 states/eV at − 0.62 eV and − 0.63 eV, respectively. The noticeable shift of these peaks toward the Fermi level in the alloyed structure implies a modification in the local electronic environment, likely due to the perturbation of the crystal field and the redistribution of electronic charge caused by Na insertion. The s-orbital contributions (Cr-4s, Zr-5s, Na-3s) are relatively minor throughout the energy range, consistent with their delocalized character and lower density of states in transition-metal-based systems. Overall, the PDOS analysis reveals that Na alloy does not alter the metallic character of the material, but subtly modifies the orbital-resolved electronic structure, which could influence the bonding characteristics and possibly affect the electron–phonon coupling strength in future phonon-related investigations
, the Cr-3d and Zr-4d states remain predominant, with peak values of 2.84 and 1.53 states/eV at − 0.62 eV and − 0.63 eV, respectively. The noticeable shift of these peaks toward the Fermi level in the alloyed structure implies a modification in the local electronic environment, likely due to the perturbation of the crystal field and the redistribution of electronic charge caused by Na insertion. The s-orbital contributions (Cr-4s, Zr-5s, Na-3s) are relatively minor throughout the energy range, consistent with their delocalized character and lower density of states in transition-metal-based systems. Overall, the PDOS analysis reveals that Na alloy does not alter the metallic character of the material, but subtly modifies the orbital-resolved electronic structure, which could influence the bonding characteristics and possibly affect the electron–phonon coupling strength in future phonon-related investigations
Fig. 6.
The partial electronic density of states as a function of energy for (a):  and (b):
and (b):  .
.
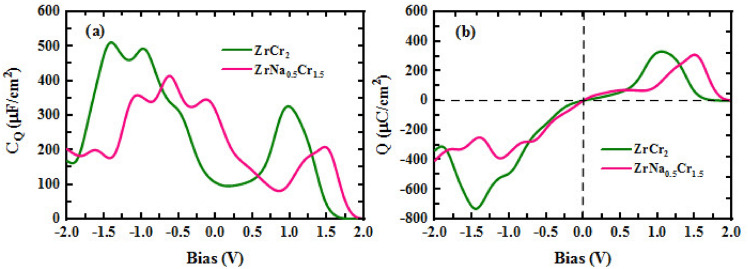
Moreover, Fig. 7a, b illustrate the variation of areal quantum capacitance (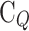 ) and charge storage capacity as functions of applied bias for
) and charge storage capacity as functions of applied bias for  and
and  . As observed in Fig. 7a, the maximum values of
. As observed in Fig. 7a, the maximum values of  are achieved under negative bias, reaching 511.06
are achieved under negative bias, reaching 511.06 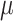 F/
F/ at – 1.41 V for the pristine compound and 414.03
at – 1.41 V for the pristine compound and 414.03  F/
F/ at – 0.63 V for the Na alloy system. These prominent peaks in the negative bias region suggest a strong electronic response and efficient charge accumulation, which make these materials promising anode candidates for supercapacitor applications. Additionally, at positive bias, both compounds maintain considerable capacitance values, with
at – 0.63 V for the Na alloy system. These prominent peaks in the negative bias region suggest a strong electronic response and efficient charge accumulation, which make these materials promising anode candidates for supercapacitor applications. Additionally, at positive bias, both compounds maintain considerable capacitance values, with  and
and  exhibiting peaks of 326.24
exhibiting peaks of 326.24 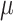 F/
F/ at 0.98 V and 311.21
at 0.98 V and 311.21  F/
F/ at 0.01 V, respectively. The relatively symmetric capacitance response in both positive and negative voltage windows reflects the metallic nature of the compounds and the delocalized nature of electronic states near the Fermi level. Upon Na substitution, the electronic DOS near the Fermi level decreases slightly, leading to a moderate reduction in quantum capacitance. This is attributed to the alteration of the local electronic environment and partial disruption of Cr-dominated states. However, this change simultaneously results in enhanced ionic transport properties. Specifically, the diffusion coefficient of Na ions increases due to reduced electrostatic binding and local structural distortion, which lowers the diffusion energy barrier. Therefore, Na alloying introduces a trade-off between quantum capacitance and ionic mobility: while the capacitance slightly decreases, the diffusion kinetics improve. This balance ultimately enhances the overall rate capability and makes the Na-alloyed compound a promising candidate for high-performance hybrid energy storage devices.
at 0.01 V, respectively. The relatively symmetric capacitance response in both positive and negative voltage windows reflects the metallic nature of the compounds and the delocalized nature of electronic states near the Fermi level. Upon Na substitution, the electronic DOS near the Fermi level decreases slightly, leading to a moderate reduction in quantum capacitance. This is attributed to the alteration of the local electronic environment and partial disruption of Cr-dominated states. However, this change simultaneously results in enhanced ionic transport properties. Specifically, the diffusion coefficient of Na ions increases due to reduced electrostatic binding and local structural distortion, which lowers the diffusion energy barrier. Therefore, Na alloying introduces a trade-off between quantum capacitance and ionic mobility: while the capacitance slightly decreases, the diffusion kinetics improve. This balance ultimately enhances the overall rate capability and makes the Na-alloyed compound a promising candidate for high-performance hybrid energy storage devices.
Fig. 7.
(a): The areal quantum capacitance and (b): the areal charge storage as function of bias for  and
and  .
.
To benchmark our findings, we compared them with our previous DFT study on  and its Li- and Mg-doped derivatives, which were investigated using consistent computational parameters8. The quantum capacitance of pristine
and its Li- and Mg-doped derivatives, which were investigated using consistent computational parameters8. The quantum capacitance of pristine  was calculated to be 676.77
was calculated to be 676.77  F/
F/ , while it decreased to 381.75
, while it decreased to 381.75  F/
F/ and 344.02
and 344.02  F/
F/ upon Li and Mg substitution, respectively. In contrast, the Na-alloyed
upon Li and Mg substitution, respectively. In contrast, the Na-alloyed  compound examined in this study retains a higher quantum capacitance than both of those doped
compound examined in this study retains a higher quantum capacitance than both of those doped  systems, despite a moderate reduction compared to the pristine
systems, despite a moderate reduction compared to the pristine  . As shown in Fig. 7b, the maximum areal charge storage, Q, occurs at negative potentials, with values of − 780.84
. As shown in Fig. 7b, the maximum areal charge storage, Q, occurs at negative potentials, with values of − 780.84  C/
C/ at − 0.84 V for
at − 0.84 V for  and − 404.96
and − 404.96  C/
C/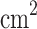 at − 2.00 V for
at − 2.00 V for  . The enhanced charge storage in the pristine compound can be attributed to its higher quantum capacitance and stronger electronic localization at the relevant energy levels. Overall, the electronic structure analysis, large values of diffusion coefficient and electrical conductivity, high quantum capacitance values, and considerable areal charge storage indicate that
. The enhanced charge storage in the pristine compound can be attributed to its higher quantum capacitance and stronger electronic localization at the relevant energy levels. Overall, the electronic structure analysis, large values of diffusion coefficient and electrical conductivity, high quantum capacitance values, and considerable areal charge storage indicate that  and ZrNa0.5Cr1.5 exhibit favorable characteristics for integration in hybrid energy storage systems.
and ZrNa0.5Cr1.5 exhibit favorable characteristics for integration in hybrid energy storage systems.
Conclusions
In this study, a comprehensive first-principles investigation was carried out on the structural, dynamical, electronic, and energy storage properties of  and its sodium-alloyed counterpart,
and its sodium-alloyed counterpart,  . Phonon calculations confirmed the dynamical stability of both compounds through the absence of imaginary modes in the phonon spectra. The substitution of Na introduced noticeable shifts in phonon density of states, reflecting modifications in local bonding environments and mass-induced softening effects. Electronic structure analysis revealed that both systems exhibit metallic behavior, with finite electronic density of states at the Fermi level. The Na substitution led to a slight redistribution of DOS peaks toward the Fermi energy, without disrupting the overall metallic character. Partial DOS analysis confirmed that the dominant electronic contributions near the Fermi level arise from Cr-3d and Zr-4d orbitals in both compounds, with the Na orbitals contributing minimally. From a transport perspective, both compounds demonstrated excellent electrical conductivity, with
. Phonon calculations confirmed the dynamical stability of both compounds through the absence of imaginary modes in the phonon spectra. The substitution of Na introduced noticeable shifts in phonon density of states, reflecting modifications in local bonding environments and mass-induced softening effects. Electronic structure analysis revealed that both systems exhibit metallic behavior, with finite electronic density of states at the Fermi level. The Na substitution led to a slight redistribution of DOS peaks toward the Fermi energy, without disrupting the overall metallic character. Partial DOS analysis confirmed that the dominant electronic contributions near the Fermi level arise from Cr-3d and Zr-4d orbitals in both compounds, with the Na orbitals contributing minimally. From a transport perspective, both compounds demonstrated excellent electrical conductivity, with  values of
values of  and
and  (
( m·s)
m·s) for
for  and
and  , respectively. The diffusion coefficients were also found to be relatively high, amounting to
, respectively. The diffusion coefficients were also found to be relatively high, amounting to 
 /s for the pristine and
/s for the pristine and 
 /s for the alloyed system, indicating favorable ionic mobility. In terms of electrochemical properties, both compounds showed significant quantum capacitance, with maximum values of 511.06 and 414.03
/s for the alloyed system, indicating favorable ionic mobility. In terms of electrochemical properties, both compounds showed significant quantum capacitance, with maximum values of 511.06 and 414.03 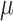 F/
F/ under negative bias for
under negative bias for  and
and  , respectively. The alloyed system also retained appreciable capacitance in the positive bias range, reflecting a stable and symmetric electrochemical response. Taken together, these findings indicate that
, respectively. The alloyed system also retained appreciable capacitance in the positive bias range, reflecting a stable and symmetric electrochemical response. Taken together, these findings indicate that  and
and  offer a promising balance between electronic conductivity, ion diffusion capability, and electrochemical performance, making them suitable candidates for hybrid energy storage applications, including supercapacitor battery systems.
offer a promising balance between electronic conductivity, ion diffusion capability, and electrochemical performance, making them suitable candidates for hybrid energy storage applications, including supercapacitor battery systems.
Acknowledgements
The authors acknowledge the use of computational facilities provided by the High Performance Computing Center (HPC) of Alzahra University, Tehran, Iran, which significantly contributed to the simulations presented in this work.
Author contributions
F. Shirvani: Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Roles/Writing - original draft. M.Jafari: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing - review and editing. A. Shokri: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing - review and editing.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Shirvani, Email: F.Shirvani@alzahra.ac.ir.
Mohammad Reza Jafari, Email: mo.jafari@alzahra.ac.ir.
Aliasghar Shokri, Email: aashokri@alzahra.ac.ir.
References
-
1.Adamczyk, E. & Pralong, V. Na
 Mn
Mn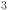 O
O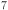 : a suitable electrode material for Na-Ion batteries?. Chem. Mater.29, 4645–4648 (2017). [Google Scholar]
: a suitable electrode material for Na-Ion batteries?. Chem. Mater.29, 4645–4648 (2017). [Google Scholar] - 2.Ahmad, F. et al. Advances in graphene-based electrode materials for high-performance supercapacitors: a review. J. Energy Storage.72, 108731 (2023). [Google Scholar]
- 3.Buchholz, D. et al. Toward Na-ion batteries-synthesis and characterization of a novel high capacity Na Ion intercalation material. Chem. Mater.25, 142–148 (2013). [Google Scholar]
- 4.Luo, W. et al. Na-ion battery anodes: materials and electrochemistry. Acc. Chem. Res.49, 231–240 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Shirvani, F., Jafari, M. & Shokri, A. A density functional theory study on supercapacitor electrode applicability of ZnO/TiC heterostructure along with its vacancies, covacancies, codoped, alloys and coalloys. Eur. Phys. J. Plus.138, 43 (2023). [Google Scholar]
- 6.Shirvani, F., Jafari, M. & Shokri, A. Effect of alloying Li on lithium-ion batteries applicability of two-dimensional TiN and TiC as novel electrode materials: A first principle study. Sci. Rep.13, 15680 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirvani, F., Jafari, M. & Shokri, A. Effect of vacancy, doping and alloying on supercapacitance properties of monolayer CrN/CuN heterostructure as an electrode material: A first principle study. Phys. B: Condens. Matter689, 416168 (2024). [Google Scholar]
-
8.Shirvani, F., Jafari, M. & Shokri, A. Exploring superconductivity, supercapacitance and thermoelectric properties of NbCr
 and its alloys with Li and Mg: A first-principles investigation. J. Energy Storage.99, 113298 (2024). [Google Scholar]
and its alloys with Li and Mg: A first-principles investigation. J. Energy Storage.99, 113298 (2024). [Google Scholar] -
9.Shirvani, F., Jafari, M. & Shokri, A. Studying supercapacitance and thermoelectric performance of semiconductor BaTiO
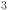 along with its halfmetal alloy of BaTiFO
along with its halfmetal alloy of BaTiFO : A DFT+U investigation. J. Phys. Chem. Solids.193, 112173 (2024). [Google Scholar]
: A DFT+U investigation. J. Phys. Chem. Solids.193, 112173 (2024). [Google Scholar] -
10.Shirvani, F. & Shokri, A. Studying supercapacitance properties of Mg
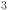 Si and Ti
Si and Ti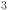 Si as novel electrode materials: A first-principles study. Chem. Phys.596, 112740 (2025). [Google Scholar]
Si as novel electrode materials: A first-principles study. Chem. Phys.596, 112740 (2025). [Google Scholar] - 11.Zhang, H., Hu, M., Huang, Z., Kang, F. & Lv, R. Sodium-ion capacitors with superior energy-power performance by using carbon-based materials in both electrodes. Prog. Nat. Sci. Mater. Int.30, 13–19 (2020). [Google Scholar]
- 12.Zhu, J. & Schwingenschlogl, U. Silicene for Na-ion battery applications. 2D Materials3, 035012 (2016). [Google Scholar]
-
13.Bai, H. & Qin, X. First-principles study of the structural phase transition, elastic and thermodynamic properties of hfcr
 . Eur. Phys. J. B96, 25 (2023). [Google Scholar]
. Eur. Phys. J. B96, 25 (2023). [Google Scholar] -
14.Robina-Merlino, A., Luna, C.R., Juan, A., & Pronsato. M.E. A DFT study of hydrogen storage in Zr(Cr
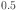 Ni
Ni 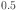 )
)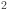 Laves phase. Int. J. Hydrogen Energy41, 2700–2710 (2016).
Laves phase. Int. J. Hydrogen Energy41, 2700–2710 (2016).
-
15.Peng, L., Takizawa, S., Ikeda, K., Horiuchi, T. & Miura, S. Effect of Si on the stability ofNbCr
 laves phase in Cr-Mo-Nb system. Intermetallics110, 106457 (2019). [Google Scholar]
laves phase in Cr-Mo-Nb system. Intermetallics110, 106457 (2019). [Google Scholar] -
16.Sato, R., Tajima, I., Nakagawa, T. & Uchida, H. Kinetics of hydrogen absorption by TiCr
 and Ti-Cr based alloys at low temperatures. J. Alloys Compd.580, S21–S24 (2013). [Google Scholar]
and Ti-Cr based alloys at low temperatures. J. Alloys Compd.580, S21–S24 (2013). [Google Scholar] -
17.Li, B. et al. Mitigating ZrCr
 formation at the Cr/Zr interface through trace doping of Zn, Mg and Sn into Cr coatings: a combined first-principles computational and experimental investigation. J. Nucl. Mater.603, 155375 (2025). [Google Scholar]
formation at the Cr/Zr interface through trace doping of Zn, Mg and Sn into Cr coatings: a combined first-principles computational and experimental investigation. J. Nucl. Mater.603, 155375 (2025). [Google Scholar] - 18.Li, B. et al. Interfacial evolution in Cr/Mo-coated Zr alloys near the Mo-Zr eutectoid temperature. J. Nucl. Mater.603, 155406 (2025). [Google Scholar]
-
19.Cui, J. et al. Revisiting the thermodynamic properties of the ZrCr
 laves phases by combined approach using experimental and simulation methods. J. Alloys Compd.1002, 175186 (2024). [Google Scholar]
laves phases by combined approach using experimental and simulation methods. J. Alloys Compd.1002, 175186 (2024). [Google Scholar] -
20.Pysarenko, V. O., Kuznetsova, T. L. & Samelyuk, A. V. Structure and mechanical properties of ZrCr
 intermetallic compound within the temperature range 20–1300
intermetallic compound within the temperature range 20–1300  C. Mater. Sci.40, 83–88 (2024). [Google Scholar]
C. Mater. Sci.40, 83–88 (2024). [Google Scholar] -
21.van Midden, H. J. P. et al. Structural and electronic properties of the hydrogenated ZrCr
 Laves phases. Phys. Chem. C.114, 4221–4227 (2010). [Google Scholar]
Laves phases. Phys. Chem. C.114, 4221–4227 (2010). [Google Scholar] -
22.Abel, A. W. & Craig, R. S. Magnetic and structural characteristics of TiCr
 , ZrCr
, ZrCr , HfCr
, HfCr and the TiCo
and the TiCo ZrCo
ZrCo and YFe
and YFe YCo
YCo alloy systems. J. Less-Common Met.16, 77–83 (1986). [Google Scholar]
alloy systems. J. Less-Common Met.16, 77–83 (1986). [Google Scholar] -
23.Li, R. et al. Potential application of Mg Cu
 alloy as a high-performance electrode material for Mg-Ion batteries: a computational study. J. Phys. Chem. C.127, 23618–23627 (2023). [Google Scholar]
alloy as a high-performance electrode material for Mg-Ion batteries: a computational study. J. Phys. Chem. C.127, 23618–23627 (2023). [Google Scholar] -
24.Xiao, M. et al. Insight into the potential of M-NbS
 (M = Pd, Ti and V) monolayers as anode materials for alkali ion (Li/Na/K) batteries. Phys. Chem. Chem. Phys.26, 28554–28564 (2024).
[DOI] [PubMed] [Google Scholar]
(M = Pd, Ti and V) monolayers as anode materials for alkali ion (Li/Na/K) batteries. Phys. Chem. Chem. Phys.26, 28554–28564 (2024).
[DOI] [PubMed] [Google Scholar] - 25.Perdew, H. & Yue, J. Accurate and simple density functional for the electronic exchange energy: generalized gradient approximation. Phys. Rev. B33, 8800–8802 (1986). [DOI] [PubMed] [Google Scholar]
- 26.Perdew, B. & Ernzerhof, K. Generalized gradient approximation made simple. Phys. Rev. Lett.77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Giannozzi, P. et al. Quantum espresso: a modular and open-source software project for quantum simulations of materials. J. Condens. Matter Phys.21, 395502 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Giannozzi, P. et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Condens. Matter Phys.29, 465901 (2017). [DOI] [PubMed] [Google Scholar]
-
29.Liu, J., Xu, Y. & Kong, L. High-capacity and fast Na-ion diffusion rate three-dimensional MoS
 /SnS
/SnS -RGO anode for advanced sodium-ion batteries and sodium-ion capacitors. Solid State Ion.355, 115416 (2020). [Google Scholar]
-RGO anode for advanced sodium-ion batteries and sodium-ion capacitors. Solid State Ion.355, 115416 (2020). [Google Scholar] - 30.Madsen, G. K. H., Singh, D. J. & Boltztrap, G. A code for calculating band-structure dependent quantities. Comput. Phys. Commun.175, 67–71 (2006). [Google Scholar]
- 31.Zhou, Q. et al. Effect of the N/P/S and transition-metal co-doping on the quantum capacitance of supercapacitor electrodes based on mono- and multilayer graphene. Carbon170, 368–379 (2020). [Google Scholar]
- 32.John, D. L., Castro, L. C. & Pulfrey, D. L. Quantum capacitance in nanoscale device modeling. J. Appl. Phys.96, 5180–5184 (2004). [Google Scholar]
- 33.Hirunsit, P., Liangruksa, M. & Khanchaitit, P. Electronic structures and quantum capacitance of monolayer and multilayer graphenes influenced by Al, B, N and P doping, and monovacancy: Theoretical study. Carbon108, 7–20 (2016). [Google Scholar]
- 34.Xu, Q., Yang, G., Fan, X. & Zheng, W. Improving the quantum capacitance of graphene-based supercapacitors by the doping and co-Doping: first-principles calculations. ACS omega4, 13209–13217 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.