Abstract
Allogeneic MHC-incompatible organ or cell grafts are usually promptly rejected by immunocompetent hosts. Here we tested allogeneic β-islet cell graft acceptance by immune or naïve C57BL/6 mice rendered diabetic with streptozotocin (STZ). Fully MHC-mismatched insulin-producing growth-regulated β-islet cells were transplanted under the kidney capsule or s.c. Although previously or simultaneously primed mice rejected grafts, STZ-treated diabetic mice accepted islet cell grafts, and hyperglycemia was corrected within 2–4 weeks in absence of conventional immunosuppression. Allogeneic grafts that controlled hyperglycemia expressed MHC antigens, were not rejected for >100 days, and resisted a challenge by allogeneic skin grafts or multiple injections of allogeneic cells. Importantly, the skin grafts were rejected in a primary fashion by the grafted and corrected host, indicating neither tolerization nor priming. Such strictly extralymphatic cell grafts that are immunologically largely ignored should be applicable clinically.
Successful transplantation of histoincompatible (allogeneic) organs or cells is usually difficult because they are rejected by cellular and humoral immune responses (1–5). The following general methods have been applied to reduce or inhibit immunological rejection: general or selective immunosuppression, induction and maintenance of tolerance by lymphohemopoietic chimerism causing deletion of specific T cells (1, 6–9), transplantation at immunologically privileged sites that lack lymph drainage (5), rendering antigenic organs nonimmunogeneic through depletion of passenger leukocytes (2, 10), and prevention of emigration to draining lymph nodes of transplanted parenchymal cells by encapsulation (11–13).
The concept that allogeneic grafts lacking passenger leukocytes may be nonimmunogeneic has been examined (2, 10, 14, 15). One major problem has been elimination of passenger leukocytes and of endothelial cells from endocrine organs, e.g., pancreatic islets or thyroids while preserving the viability and function of the transplant (4). Transplantation of transformed permanently growing β-islet cells as an alternative approach has not been feasible because growth arrest was uncontrollable. This problem has now been solved by the introduction of the simian virus 40 (SV40)-large T antigen under the control of a tandem array of tet-operator sequences and a minimal promotor into C3H (H-2k)-derived β-islet cells (16). When transplanted into syngeneic diabetic mice, the cells grow and regulate blood glucose within 2–4 weeks. Addition of tetracycline to the drinking water then prevents further growth and the potential of hypoglycemic complications.
Because this regulated transformed endocrine cell line can be transplanted without contaminating passenger leukocytes, we had a unique opportunity to evaluate whether completely allogeneic islet cells can be transplanted under the kidney capsule or s.c. to correct streptozotocin (STZ)-induced diabetes (17) in mice without additional conventional immunosuppression and how resistant such grafts are against forced immune rejection (2, 6, 8–10).
Materials and Methods
Mice.
C57BL/6 mice were obtained from the Institut für Labortierkunde (University of Zürich, Switzerland), C3H mice were purchased from Iffacredo (Lyon, France) or from Harlan Olac (Amsterdam).
Cell Lines.
The βTC-tet cell line (16) (H-2k, C3H origin) was cultured in Dulbecco's modified Eagles medium (Life Technologies) containing 25 mM glucose and supplemented with 15% horse serum (Amimed), 2.5% FBS (Life Technologies), 10 mM Hepes, 1 mM sodium pyruvat, 2 mM glutamine, 100 international units (IU) of penicillin per ml, and 100 μg of streptomycin per ml. The fibroblast L929 cell line (ATCC-CCL-1, H-2k, C3H origin) was cultured in Dulbecco's modified Eagle medium (Life Technologies) supplemented with 10% FBS (Life Technologies), 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 1 mM sodium pyruvate. All cell lines were controlled for mycoplasma contamination.
Engraftment of βTC-tet Cells.
βTC-tet cells were either directly injected as cell suspension s.c., mixed with recipient's blood to form a coagulum for transplantation as cell aggregate under the left kidney capsule, or transplanted as solid graft of 2 × 2 × 2 mm (obtained from established βTC-tet grafts) under the left kidney capsule or s.c. into the right flank. Mice to be transplanted were anesthetized by means of ether inhalation.
Reagents and Glucose Measurements.
STZ (Upjohn) was dissolved in 0.9% sodium chloride and injected iv at a dose of 180 mg/kg body weight. Glucose measurements were performed with the glucometer Elite (Bayer, Leverkusen, Germany). When glucose levels had dropped to physiological levels, mice were given tetracycline in the drinking water (1 mg/ml in 2.5% glucose to reduce or stop growth of the grafts. The bottles were wrapped with aluminium foils to block light).
Skin Grafting.
Skin grafting was done by using the method of Billingham and Medawar (1, 5). Full-thickness skin (1.5 × 1.5 cm) from the belly of a donor mouse was engrafted onto the right side of the thorax of a recipient mouse. The graft was covered with gauze and plaster that were removed on day 8. Grafts were scored daily until rejection (defined as loss of >80% of the grafted tissue).
Immunohistology.
Freshly removed organs were immersed in Hank's balanced salt solution (HBSS) and snap-frozen in liquid nitrogen (18, 19). Tissue sections of 5-μm thickness were cut in a cryostat and fixed in acetone for 10 min. Sections were incubated with polyclonal guinea pig antibodies against insulin (Dakopatts, Glostrup, Denmark).
Alkaline phosphatase-labeled, species-specific goat antibodies (Tago, Burlingame, CA) were used as secondary reagents. The substrate for the red color reaction was AS-BI phosphate/New Fuchsin. Sections were counterstained with hemalaun.
Allo-CTL Assay.
Spleen cell suspensions (4 × 106 cells) were prepared from naïve or H-2k allosensitized or βTC-tet cell-treated C57BL/6 mice, restimulated for 5 days with titrated numbers (106, 3 × 105, 105, 3 × 104) of irradiated C3H splenocytes and then tested in vitro for 5 h against 51Cr-labeled L929 target cells (20).
Results
Histoincompatible βTC-tet Cells Grow In STZ-Diabetic Mice, Form Functional Islet Cell Grafts, and Correct Hyperglycemia.
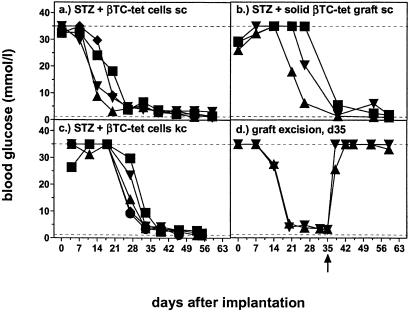
Diabetes was induced in C57BL/6 mice by a single injection of STZ iv (180 mg/kg). When hyperglycemia developed 2–7 days later, histoincompatible βTC-tet cells were implanted as cell suspension, cell aggregate, or solid graft. Grafts grew locally at the implantation site (Fig. 1) and corrected hyperglycemia within 2–7 weeks in 78–91% of treated mice (Table 1, Fig. 2). Mice were treated with tetracycline in the drinking water from the time when blood glucose was corrected. After local excision of βTC-tet grafts growing s.c. (Fig. 2d) or correspondingly after nephrectomy (data not shown), mice became hyperglycemic within 1 day, and then remained diabetic. Thus, grafts grew strictly locally and no recipient islet cell regeneration occurred.
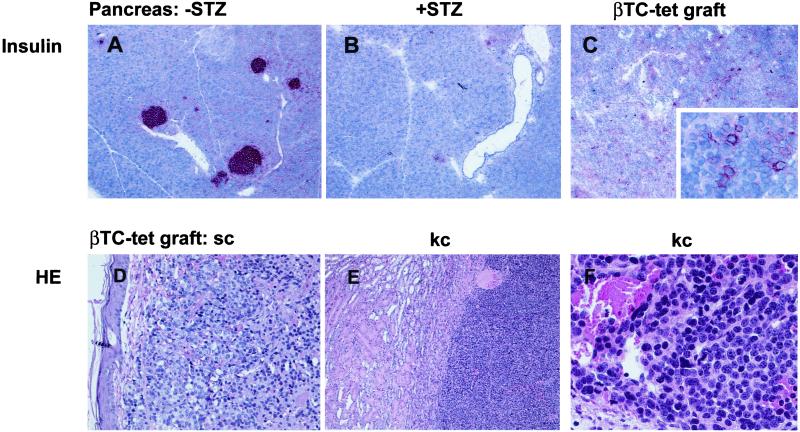
Figure 1.
Histologic analysis of pancreas and βTC-tet grafts. Frozen sections of pancreas from naïve C57BL/6 mice (A) or from C57BL/6 mice treated 9 days earlier with STZ iv (B) or sections from established βTC-tet grafts (C Inset) were stained for insulin. Tissue-sections from established βTC-tet grafts implanted s.c. (D) or under the kidney capsule (E and F, higher magnification) were fixed in formalin and examined by using hematoxilin and eosin (HE).
Table 1.
Functional histo-incompatible βTC-tet cell grafts in C57BL/6 mice with and without STZ treatment
| Route of implantation | βTC-tet cell preparation | Number of mice with a functional βTC-tet graft/number of treated mice (%)
|
|
|---|---|---|---|
| −STZ | +STZ | ||
| s.c. | cell aggregate | 0/14 (0%) | 21/23 (91%) |
| s.c. | solid graft* | 0/19 (0%) | 4/5 (80%) |
| k.c. | cell aggregate | 0/4 (0%) | 85/109 (78%) |
| k.c. | solid graft* | ND | 4/5 (80%) |
s.c., subcutaneously; k.c., under the kidney capsule; ND, not determined.
A 2×2×2 mm piece prepared from a βTC-tet graft of a STZ treated and reconstituted C57Bl/6 mouse.
Figure 2.
Histoincompatible βTC-tet cells grow in STZ-diabetic mice, form functional islet cell grafts, and correct hyperglycemia. STZ-diabetic C57BL/6 mice were treated with histoincompatible βTC-tet cells and monitored for blood glucose levels. βTC-tet cells were either injected s.c. as cell suspension (107 cells) (a), implanted as solid graft of 2 × 2 × 2 mm s.c. (b), implanted as cell aggregate under the left kidney capsule (2–3 × 106 cells) (c), or injected s.c. with subsequent excision of the established grafts on day 35 after implantation (d). Different symbols indicate the values of individual mice. Dashed lines represent upper and lower detection limits.
Immunization with H-2k Cells Prevents Establishments of Freshly Transplanted Allogeneic βTC-tet Cells in STZ-Diabetic Mice but Does Not Induce Rejection of Established βTC-tet Grafts.
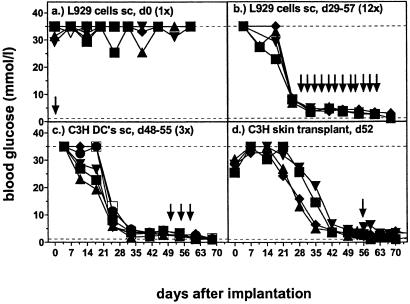
STZ-diabetic C57BL/6 mice treated s.c. with 107 histoincompatible βTC-tet cells and immunized simultaneously or 2 days (not shown) later with a single s.c. injection of 107 fibroblast L929 cells (H-2k) were unable to correct hyperglycemia (Fig. 3a). In contrast, after successful reconstitution of STZ-diabetic mice with βTC-tet cells (H-2k), single or multiple immunizations with L929 cells (Fig. 3b), C3H spleen cells (not shown), or C3H dendritic cells (H-2k) did not induce rejection of established βTC-tet grafts (Fig. 3c). Furthermore, allogeneic C3H skin grafts were rejected with similar kinetics by control C57BL/6 mice (11.3 ± 0.7 days; mean ± SD) and STZ-diabetic C57BL/6 mice corrected with βTC-tet cells (11.4 ± 0.7 days); however, this skin rejection did not cause rejection of the βTC-tet grafts (Fig. 3d). Such strong resistance to rejection of established grafts in parallel with proven normal primary rejection of new grafts may reflect differential susceptibility associated with inflammation and the healing-in process. Suppressive mechanisms mediated by primed host T cells seemed unlikely because rejection of a skin graft was neither accelerated nor delayed.
Figure 3.
Immunisation with H-2k cells prevents the growth of βTC-tet cells in STZ-diabetic mice but does not induce rejection of established βTC-tet grafts. STZ-diabetic C57BL/6 mice were treated with histoincompatible βTC-tet cells (day 0) as cell suspension s.c. (a and b) or as cell aggregate under the left kidney capsule (c and d) and subsequently blood glucose levels were monitored. Different symbols indicate the values of individual mice. They were either immunized simultaneously (day 0) with a single injection of 107 L929 cells s.c. (a), or multiple times after correction of hyperglycemia (3 times per week during the 4 weeks between day 29–57) (b). Additionally, mice with established βTC-tet grafts were challenged 3 times with 4 × 105 C3H DC's s.c. on day 48, 50, and 55 (c) or with a C3H skin transplant on day 52 after βTC-tet cell implantation (d).
Allo-CTL Responses of the Host in Vitro.
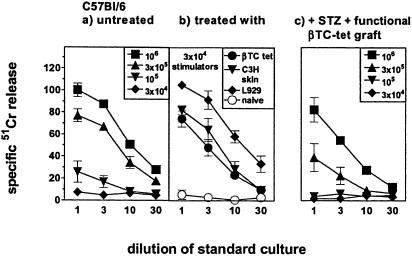
Splenocytes from STZ-diabetic C57BL/6 mice with established βTC-tet grafts were restimulated for 5 days with irradiated C3H (H-2k) splenocytes and then analyzed in vitro for their H-2k specific allo-CTL activity. Because the H-2k specific allo-CTL response is normally already detectable in naïve C57BL/6 mice the numbers of stimulators were titrated from 106 to 3 × 104 per standard culture (20). At <105 cells per culture, H-2k-specific allo-CTL activity was no longer detected in naïve mice (Fig. 4a), whereas spleen cells from H-2k allo-sensitized C57BL/6 mice primed with L929 cells, βTC-tet cells (treated i.v. or i.p.), or C3H skin generated allo-CTLs against 3 × 104 stimulators (Fig. 4b). Thus immunization of mice with βTC-tet cells i.v. or i.p. that reach secondary lymphatic organs at sufficient levels primes anti-H-2k CTL responses. In contrast, STZ-diabetic C57BL/6 mice with established βTC-tet grafts exhibited no primed status by titration (Fig. 4c). Presumably, the peripheral grafts have been ignored, or only very small numbers of transplanted cells reached lymphatic organs insufficiently to prime allospecific CTLs (19). However, the same mice were not tolerant to H-2k because they rejected skin grafts in a primary fashion and responded in vitro comparably to control mice. Thus, in βTC-tet cells treated and corrected STZ-diabetic mice alloreactive CD8+ T cells were neither obviously primed nor anergized/tolerized or deleted. The allogeneic islet cells seemed to be largely ignored by CD8+ T cells.
Figure 4.
Allo-CTL response in vitro. The H-2k specific allo-CTL response was measured in naïve C57BL/6 mice (H-2b) (a), in C57BL/6 mice primed either with 107 L929 cells (H-2k) or βTC-tet cells (H-2k), both given as single cell suspension (but where βTC-tet cell recipients diabetes was not corrected) s.c. (or i.v. not shown) 10 days earlier, or with C3H skin (H-2k) transplanted 98 days earlier (b), and in STZ-diabetic C57BL/6 mice corrected by βTC-tet cells transplanted as aggregates s.c. or under the kidney capsule (see Materials and Methods) (c). The effectors (4 × 106) were restimulated for 5 days with titrated numbers (106, 3 × 105, 105, 3 × 104) of irradiated C3H spleen cells (a and c) or used at the concentration of 3 × 104 cells per ml (b). Cultures were then tested on 51Cr labeled L929 target cells.
MHC Class I Expression of βTC-tet Cells Is Unchanged After Successful Implantation.
This resistance to immune rejection was not caused by the possibility that βTC-tet cells lost MHC class I expression in vivo. Staining with anti-H-2Kk antibody and FACS analysis of cells before implantation and after isolation from grown grafts were comparable (data not shown). Furthermore, when cells isolated from grafts were infected with lymphocytic choriomeningitis virus (LCMV) and then used as targets in a virus specific MHC-class I restricted CTL assay, the targets were lysed to similar extents by effector cytotoxic T cells as were infected in vitro cultured βTC-tet cells (data not shown). Together, these results suggest that MHC class I antigens were expressed and there was no significant change as compared with the original βTC-tet cell line cultured in vitro.
Discussion
We report here that a pure islet cell transplant that differs for the entire MHC but expresses MHC class I antigen only can be transplanted successfully into transiently (17) STZ-immunosuppressed diabetic recipients. These allogeneic grafts are not rejected despite the absence of continued immunosuppression or T cell deletion by lympho-hemopoietic chimerism.
Usually, MHC incompatible organs or cells are rejected by immunocompetent hosts, and general long-term immunosuppression is necessary to allow successful transplantation. This result is influenced by several factors, including antigenic differences between graft and host (number of allelic differences, major versus minor histocompatiblity differences), the frequency and functional capacity of T and B cells on the one hand and the capacity of rejected cells and organs to regenerate on the other.
Induction of an immune response leading to rejection is mainly dependent on either grafted cells (19) or passenger leukocytes reaching draining lymph nodes where they induce specific CTLs. In STZ-diabetic mice, allogeneic cells that were transplanted strictly peripherally did not induce an allo-response that prevented graft acceptance or that promoted graft rejection. These results indicate that a healed-in, strictly peripheral allogeneic cell graft is largely ignored by the immune system for a long time. The present experiments cannot exclude a small number of cells reaching the draining lymph nodes or spleen to prime an allo-response; by day 30–150 after transplantation, the consequences of such a low-level leakage are, however, indistinguishable from a naïve unprimed situation.
This model situation should be compared with vascularized organ grafts or when islet or other parenchymal cells are injected i.p. or into the portal vein (4, 21, 22). In these cases, substantially greater numbers of parenchymal allogeneic cells or passenger leukocytes can reach lymphatic organs to induce an immune response resulting in cell and graft rejection. Also in vascularized organ grafts, alloantigens on endothelial cells are more readily accessible to both antibodies and T cells. Comparable with these conditions, simultaneous immunization of STZ-treated mice with allogeneic fibroblasts prevented graft acceptance in the presented experiments (Fig. 3a).
Our findings here illustrate that completely allogeneic well-healed-in islet cell grafts are immunologically largely ignored at least by CD8+ T cells, are not rejected and are resistant to allo-responses. They parallel studies on the role of autoimmunity and immunopathology in diabetes in various model situations, particularly in transgenic mice expressing the LCMV glycoprotein in islet cells (23, 24). These mice did not develop diabetes spontaneously because the (syngeneic!) transgenic neo-self antigen was also ignored. After infection of the mice with a vaccinia recombinant virus expressing GPLCMV, CTL responses were efficiently primed but did not reject the transgenic (syngeneic) islet cells to an extent causing diabetes (23, 24). This condition corresponds roughly to the resistance to allogeneic skin grafts tested here. In both cases, CTL responses induced are relatively weak and about a hundred times less potent than the exceptionally strong CTL responses against LCMV which induce diabetes in the LCMV GP transgenic mice within 9–12 days.
Nevertheless, it is remarkable that once the graft has healed-in, even strong allogeneic stimuli (i.e., skin grafts) are not capable of rejecting the allogeneic islet cell graft. Interestingly, healed-in MHC-class I-disparate renal allografts stay functional under conditions where a second graft is rejected and alloreactive CD8+ T cells are induced (25). This indicates that perhaps even vascularized grafts may, with time, become lymphocyte-depleted so as to behave similar to our pure MHC class I-disparate cell grafts.
Two conclusions seem medically important. First, our results indicate that injection of islet cells into the portal vein (2–4, 22, 26) perhaps is better avoided to keep islet cells outside of secondary lymphatic organs and prevent induction of an early rejecting allo-response. Second, the simple rule that antigens (including even alloantigenic cells) that largely stay outside organized lymphatic tissues are immunologically ignored should be exploitable by reconstitutional surgery, particularly of endocrine, bone, cartilage, muscle, fibroblastic, epithelial, or neuronal cells (4, 21).
Acknowledgments
This study was supported by Swiss National Science Foundation Grant 24.6362 and by the Canton of Zurich.
Abbreviations
- STZ
streptozotocin
- LCMV
lymphocytic choriomeningitis virus
References
- 1.Brent L. A History of Transplantation Immunology. New York: Academic; 1996. [Google Scholar]
- 2.Lafferty K J, Prowse S J, Simeonovic C J, Warren H S. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- 3.Rogers N J, Lechler R I. Am J Transplant. 2001;1:97–102. [PubMed] [Google Scholar]
- 4.Lacy P E, Scharp D W. Annu Rev Med. 1986;37:33–40. doi: 10.1146/annurev.me.37.020186.000341. [DOI] [PubMed] [Google Scholar]
- 5.Medawar P B. Proc R Soc London B. 1958;149:145–166. doi: 10.1098/rspb.1958.0058. [DOI] [PubMed] [Google Scholar]
- 6.Lubaroff D M, Silvers W K. J Immunol. 1973;111:65–71. [PubMed] [Google Scholar]
- 7.Lechler R I, Batchelor J R. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ildstad S T, Sachs D H. Nature (London) 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 9.Starzl T E, Demetris A J, Murase N, Ildstad S, Ricordi C, Trucco M. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone H B, Owings J C, Gey G O. Calif West Med. 1933;38:409–411. [PMC free article] [PubMed] [Google Scholar]
- 11.O'Shea G M, Sun A M. Diabetes. 1986;35:943–946. doi: 10.2337/diab.35.8.943. [DOI] [PubMed] [Google Scholar]
- 12.Aebischer P, Winn S R, Galletti P M. Brain Res. 1988;448:364–368. doi: 10.1016/0006-8993(88)91278-4. [DOI] [PubMed] [Google Scholar]
- 13.Hasse C, Klock G, Schlosser A, Zimmermann U, Rothmund M. Lancet. 1997;350:1296–1297. doi: 10.1016/S0140-6736(05)62473-7. [DOI] [PubMed] [Google Scholar]
- 14.Summerlin W T, Broutbar C, Foanes R B, Payne R, Stutman O, Hayflick L, Good R A. Transplant Proc. 1973;5:707–710. [PubMed] [Google Scholar]
- 15.Talmage D W, Dart G, Radovich J, Lafferty K J. Science. 1976;191:385–388. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- 16.Efrat S, Fusco-DeMane D, Lemberg H, al Emran O, Wang X. Proc Natl Acad Sci USA. 1995;92:3576–3580. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols W K, Spellman J B, Vann L L, Daynes R A. Diabetologia. 1979;16:51–57. doi: 10.1007/BF00423151. [DOI] [PubMed] [Google Scholar]
- 18.Karrer U, Althage A, Odermatt B, Roberts C W M, Korsmeyer S J, Miyawaki S, Hengartner H, Zinkernagel R M. J Exp Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochsenbein A F, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel R M. Nature (London) 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 20.Engers H D, Thomas K, Cerottini J C, Brunner K T. J Immunol. 1975;115:356–360. [PubMed] [Google Scholar]
- 21.Shapiro A M, Lakey J R, Ryan E A, Korbutt G S, Toth E, Warnock G L, Kneteman N M, Rajotte R V. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 22.Bumgardner G L, Li J, Heininger M, Ferguson R M, Orosz C G. Transplantation. 1998;65:47–52. doi: 10.1097/00007890-199801150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi P S, Oehen S, Aichele P, Pircher H P, Odermatt B, Herrera P, Higuchi Y, Buerki K, Hengartner H, Zinkernagel R M. J Immunol. 1993;150:5185–5194. [PubMed] [Google Scholar]
- 24.Speiser D E, Miranda R, Zakarian A, Bachmann M F, McKall-Faienza K J, Odermatt B, Hanahan D, Zinkernagel R M, Ohashi P S. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianello P R, Fishbein J M, Rosengard B R, Lorf T, Vitiello D M, Arn J S, Sachs D H. Transplantation. 1995;59:772–777. doi: 10.1097/00007890-199503150-00023. [DOI] [PubMed] [Google Scholar]
- 26.Shoskes D A, Wood K J. Immunol Today. 1994;15:32–38. doi: 10.1016/0167-5699(94)90023-X. [DOI] [PubMed] [Google Scholar]