Abstract
CD25+4+ regulatory T cells (Treg) play an indispensable role in preventing autoimmunity. Little is known, however, about the antigen specificities required for their development and effector functions. Mice transgenic for an anti-myelin basic protein (MBP) T cell antigen receptor (TCR) spontaneously develop experimental autoimmune encephalomyelitis (EAE) when deficient for the RAG-1 gene (T/R−), whereas RAG-1-competent transgenic animals (T/R+) remain healthy, protected by CD4+ Treg-expressing endogenous TCRs. We have now investigated the role and specificity of CD25+4+ Treg in this system. The results show that T/R+ animals contain MBP-specific suppressive CD25+4+ cells, whereas T/R− do not. Adoptive transfer of CD25+4+ cells from nontransgenic or T/R+ donors into T/R− mice prevented the development of EAE. Surprisingly, transfer of nontransgenic CD25+4+ cells purified from T/R+ donors conferred only a limited protection, possibly because of their restricted repertoire diversity that we demonstrate here. Absence of transgenic CD25+4+ cells in animals deficient for endogenous TCRα chains and analyses of endogenous TCR gene expression in subsets of CD4+ cells from T/R+ mice demonstrate that development of transgenic MBP-specific CD25+4+ Treg depends on the coexpression of endogenous TCRα chains. Taken together, these results indicate that specificity to MBP is required for effector functions but is not sufficient for thymic selection/commitment of CD25+4+ Treg preventing EAE.
Compelling evidence shows that immune activities are regulated by a subpopulation of CD4+ T cells that display antiinflammatory and antiproliferative functions. Such regulatory T cells (Treg) are believed to mediate tolerance and generally inhibit immune responses potentially deleterious to the host. CD4+ Treg have been implicated in the induction and maintenance of transplantation tolerance (1–3), in the control of pathogenic inflammatory responses to non-self-antigens (4, 5), and in the prevention of “spontaneous” autoimmunity (6, 7). Treg are enriched in the naturally activated subset of CD4+ T cells, which constitutively express the CD25 (IL-2Rα) molecule (8–16) and are also detected in the CD25−45RB/Clow compartment (12, 15). In vitro CD25+4+ T cells are nonproliferative to antigenic stimulation, and upon T cell antigen receptor (TCR) triggering, inhibit the activation/proliferation of other T cells in an antigen-nonspecific manner (17, 18). Little is known, however, about antigens that control the development and effector functions of CD4+ Treg.
Treg are generated in the thymus through high-avidity interactions with self-peptides presented by thymic epithelium (12, 19–25). However, CD4+ Treg preventing organ-specific autoimmunity recognize tissue-specific self-antigens in the periphery, a necessary requirement for their function and maintenance (23, 26–28). Two alternative models aim at explaining this apparent paradox: one postulates that all relevant “tissue-specific” peptides are also expressed intrathymically, such that Treg can be locally selected and committed (23, 29); the other suggests that intrathymically selected CD4+ Treg, once in the periphery, induce tissue-specific naïve T cells to differentiate into Treg through a process of infectious tolerance (3, 30) or “peripheral education” (2).
The anti-myelin basic protein (MBP) TCR transgenic (Tg) mice generated by Lafaille et al. (31) provide a powerful tool to investigate the repertoire requirements for Treg development and function. These animals, transgenic for the α- and β-chains of a TCR specific for the NH2-terminal Ac1–17 peptide of MBP associated with the I-Au molecule (31), present a peripheral T cell repertoire largely dominated by the MBP-specific CD4+ T cells. Although these cells are not anergic, and the original TCR had been isolated from a pathogenic T cell clone, the mice (designated T/R+) never spontaneously develop experimental autoimmune encephalomyelitis (EAE). However, when the transgenes are introduced into a recombination-activating gene (RAG)-1-deficient genetic background, the T cell repertoire is strictly monoclonal, and all mice (T/R−) develop EAE spontaneously at an early age (31). Extensive analyses of transgenic animals deficient for various lymphocyte populations established that prevention of EAE in T/R+ mice is due to CD4+ Treg expressing endogenous TCRs (32, 33). In addition, a single injection of as few as 2 × 105 CD4+ single-positive thymocytes or peripheral CD4+ cells obtained from nontransgenic wild-type (R+) mice prevents the development of EAE in T/R− mice (32, 33). Finally, a monoclonal CD4+ T cell population specific to a foreign antigen failed to prevent EAE, indicating that TCR specificity is critical for Treg generation and/or effector function (34). However, the nature of the ligand(s) necessary for Treg to prevent EAE remains to be identified.
In this study, we investigated the role of CD25+4+ cells in preventing autoimmune disease in this model. We further assessed the TCR specificities required for generation and effector functions of CD25+4+ Treg cells. We show that MBP-specific CD25+4+ T cells prevent spontaneous EAE in T/R+ mice, and that their development requires additional, endogenously encoded TCRα chains. Thus the effector function of Treg is MBP-specific, but their development and/or commitment requires interactions with other antigenic ligands in the thymus.
Materials and Methods
Mice.
All animals were bred and maintained under specific pathogen-free conditions in our animal facilities. Anti-MBP TCR Tg mice on a wild-type (T/R+), RAG-1−/− (T/R−), or TCR Cα−/− (T/α−) C57BL-H2u genetic background have been described (31–33) and were kindly provided by S. Tonegawa (Massachusetts Institute of Technology, Cambridge, MA).
Preparation of Lymphocytes.
Single-cell suspension from thymus, spleen, and inguinal, axillar, brachial, cervical, and mesenteric LN cells were obtained by forcing the organs through a 100-μm nylon mesh in Hanks' balanced salt solution/2% FCS (Life Technologies, Gaithersburg, MD). Erythrocytes were lysed with a buffer containing 154 mM NH4Cl, 14.2 mM NaHCO3, 0.1 mM EDTA, pH 7.2.
Antibodies, Flow Cytometry, and Cell Sorting.
The anti-mouse CD25 (PC61), anti-MBP TCR clonotype (3H12) (33), and anti-FcγR (2.4G2) mAbs were produced in the laboratory. The PC61 and 3H12 hybridoma lines were kind gifts from S. Sakaguchi (Kyoto University, Kyoto, Japan), and J. Lafaille (New York University School of Medicine), respectively. The PC61 and 3H12 mAbs were conjugated with Alexa Fluor 488, R-PE (Molecular Probes) or biotin (Sigma) according to the manufacturer's instructions. FITC-, phycoerythrin-, or allophycocyanin-labeled anti-CD4 (RM4–5) and biotinylated anti-CD8α (53–6.7) were purchased from PharMingen.
All stainings were performed on cells preincubated for 10 min at 4°C with anti-FcγR mAb. Biotinylated Abs were revealed with FITC-, phycoerythrin-, or allophycocyanin-labeled Streptavidin (PharMingen). Samples were suspended in 2%FCS/PBS containing 1 μg/ml propidium iodide to gate out dead cells, and analyzed on a FACSCalibur instrument (Becton Dickinson). To assess expression of CD25 and Tg-TCR in the thymus, thymocytes were first depleted of CD8+ cells by magnetic separation with biotinylated anti-CD8α and Streptavidin-conjugated Dynabeads M280 (Dynal, Oslo, Norway). The resulting population contained <0.1% CD8+ cells.
For fluorescence-activated cell-sorter purification, pooled LN and spleen cells were first depleted of IgM+ lymphocytes by panning with anti-IgM Ab (Sigma) before staining. Cell sorting was performed on a MoFlo High Speed Cellsorter (Cytomation, Fort Collins, CO). The purity of all cell preparations was >96%.
In Vitro Proliferation Assay.
Sorted subpopulations of CD4+ lymphocytes (2.5 × 104 per well, U-bottomed 96-well plate) together with the same number of γ-irradiated (30 Gy) antigen-presenting cells (RBC-lysed H2u, RAG-1−/− splenocytes) were cultured for 3 days in the presence of 1 μg/ml of anti-CD3 mAb (2C11, PharMingen) or 3.0 μM mouse MBP Ac1–17 peptide (MedProbe, Oslo, Norway) in RPMI medium 1640 supplemented with 10% FCS/100 units/ml of penicillin/100 μg/ml of streptomycin/50 μM 2-mercaptoethanol/10 mM Hepes/1 mM sodium pyruvate (all purchased from Life Technologies). 3H-thymidine (1 μCi per well; Amersham Pharmacia) was added to the culture during the last 6 h.
Adoptive Transfer Experiments and Disease Evaluation.
Sorted subpopulations of CD4+ cells were suspended in PBS and injected i.v. into 30- to 32-day-old T/R− mice. EAE was scored daily as described (31): level 1, limp tail; level 2, weak or partial hind leg paralysis; level 3, total hind leg paralysis; level 4, hind leg paralysis and weak or partial front leg paralysis; level 5, moribund.
Analysis of Endogenous TCRα and TCRβ Chains Expression by Semiquantitative Reverse Transcription–PCR.
Total cellular RNA was extracted from 1 × 105 sorted CD4+ cell subpopulations supplemented with 10 μg of glycogen by using Trizol reagent and reverse transcribed by using Superscript II RT and oligo(dT)12–18 primer (Life Technologies). The amount of cDNA in each sample was first normalized after semiquantitative PCR for CD3ɛ. Reaction mixture (40 μl) contained 1/50 of the cDNA, 1.5 mM MgCl2, 0.2 mM dNTP, 0.5 μM sense and antisense primer, and 1.0 units of Taq DNA polymerase (Life Technologies) in the supplier's buffer. PCRs were performed on a PTC-100 Programmable Thermal Controller (MJ Research, Watertown, MA) and consisted of a 2.5-min denaturation step at 94°C followed by 27 cycles of 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C, terminated by a 5-min extension step at 72°C. CD3ɛ primers were 5′-GCC TCA GAA GCA TGA TAA GC-3′ (sense) and 5′-CCC AGA GTG ATA CAG ATG TC-3′ (antisense). Amplification of specific Vβ and Vα cDNA was performed by pairing each Vβ (or Vα)-specific primer with a Cβ (or Cα)-specific primer as described (35, 36).
Immunoscope Analysis of TCR Vβ and Vα Repertoires.
Amplicon from each Vβ-Cβ (or Vα-Cα) PCRs was used as a template for an elongation (runoff) reaction with carboxyfluorescein-labeled Cβ- (or Cα-) specific nested primers as described (35, 36). The runoff products were subjected to electrophoresis in an automated DNA sequencer (377; PE Applied Biosystems). Size and fluorescent intensity of each peak were determined with immunoscope software (35)
Results
MBP-Specific CD25+4+ T Cells Are Present in T/R+ but Not in T/R− Mice.
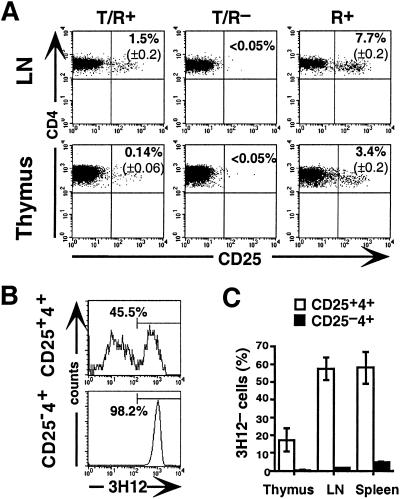
We initially examined expression of CD25 by flow cytometry on LN and splenic CD4+ cells and CD4 single-positive thymocytes from 6- to 7-week-old T/R+, T/R−, and R+ litters (Fig. 1A). In LN and spleen (not shown), CD25 was expressed by 1.5% CD4+ cells in T/R+ mice and 7.7% in R+ mice. In the thymus, the percentage of CD25+ cells was 2-fold lower in R+ mice and 10-fold lower in T/R+ mice. In young T/R− mice, which had not yet developed EAE, the CD25+4+ cells were undetectable. These analyses reveal a correlation between the generation of CD25+4+ cells and resistance to EAE.
Figure 1.
A CD25+4+ T cell population is generated in T/R+ but not in T/R− mice. (A) Expression of CD25 on CD4+ LN cells and CD4+8− thymocytes from 6- to 7-week-old T/R+, T/R−, and R+ litters, determined by flow cytometry. The number in the upper right quadrant of each profile refers to the mean (±SD.) percentage of CD4+ cells expressing CD25 (n = 3 in each group). (B) Expression of Tg-TCR on CD25+ and CD25−4+ LN cells, determined by flow cytometry with anti-clonotype mAb, 3H12. (C) Representation of 3H12− cells in each lymphoid organ. Mean ± SD (n = 3).
The peripheral CD25+4+ population of both T/R+ and R+ mice constitutively expressed intracellular CD152 (CTLA-4), whereas the CD25− subset did not (not shown). In contrast to a previous report (8), CD122 (IL-2Rβ) was expressed by CD25+ cells but poorly expressed by the CD25− cells (not shown).
We next examined the expression of the Tg-TCR in the CD25+ and CD25− compartments (Fig. 1 B and C) by using the anti-clonotype mAb 3H12 (33). As shown (31–33), almost all peripheral, and nearly all thymic, CD25−4+ cells expressed the Tg-TCR. In contrast, in the CD25+CD4+ subset the Tg-TCR (Tg cells) is reduced to 40% in LN and spleen, and to 80% in the thymus. The CD25+4+ subset is therefore enriched for cells expressing endogenous TCRs, and a preferential selection is in the periphery for nontransgenic (NTg) cells in both the CD25+ and the CD25−4+ subsets.
Tg CD25+4+ Cells from T/R+ Mice Inhibit MBP-Specific Proliferation of CD25−4+ Cells in Vitro.
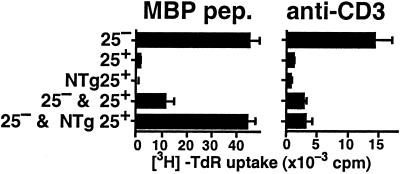
We next investigated the functional abilities of isolated CD25+4+ cells from T/R+ mice by in vitro assays. Peripheral CD25+4+ or CD25−4+ cells were purified from spleen and LN of T/R+ mice and stimulated in culture with MBP Ac1–17 peptide or anti-CD3 in the presence of appropriate antigen-presenting cells. As shown in Fig. 2, whereas CD25−4+ proliferated well in response to both stimuli, CD25+4+ did not respond to either. On coculture, CD25+4+ cells equally suppressed the proliferation of the CD25−4+ cells stimulated either with the MBP peptide or anti-CD3. Moreover, the results demonstrate that the MBP-specific suppressive activity is due to the Tg CD25+4+ cells, because CD25+4+ cells depleted of 3H12+ cells inhibited only the anti-CD3 response of the CD25−4+ cells and had no effect on the MBP-specific response. CD25+4+ cells from R+ mice also consistently inhibited only the anti-CD3 response (not shown). Finally, NTg CD25−4+ cells purified from T/R+ mice proliferated as much as total CD25−4+ cells when stimulated with anti-CD3, and did not inhibit either the anti-CD3 or the MBP-specific proliferation of total CD25−4+ cells (not shown). Thus, MBP-specific suppressor cells are found in Tg CD25+4+ cells from T/R+ mice, but neither in NTg CD25+4+ cells from T/R+ mice nor in CD25+4+ cells from R+ mice, which nevertheless suppress responses to anti-CD3 stimulation.
Figure 2.
Tg CD25+4+ cells from T/R+ mice exhibit MBP Ac1–17 peptide-specific suppressive activity in vitro. CD25−4+ cells (2.5 × 104) sorted from T/R+ mice were cultured for 3 days with 2.5 × 104 antigen-presenting cells and 3 μM MBP Ac1–17 peptide (pep) (Left) or 1 μg/ml of anti-CD3 mAb (Right) in the presence or absence of 2.5 × 104 3H12−CD25+4+ or total CD25+4+ cells sorted from T/R+ cells.
MBP-Specific CD25+4+ Prevent Spontaneous EAE in T/R+ Mice.
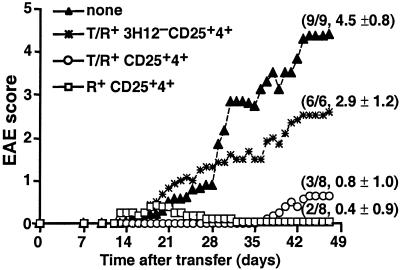
We next investigated whether CD25+4+ cells are capable of inhibiting EAE in vivo, and if so, whether the MBP specificity of the T/R+ CD25+4+ population is required for the protection from EAE. As shown in Fig. 3, adoptive transfer of CD25+4+ cells (2 × 105 per mouse) purified from R+ or T/R+ mice into T/R− mice protected the recipients from EAE, only a few of which developed mild EAE (levels 1–2). Strikingly, transfer of the same number of NTg CD25+4+ cells purified from T/R+ mice was less protective, because all six recipients developed more severe EAE (levels 2–5). This poor protective activity could not be attributed to an impaired expansion capacity, because no significant difference occurred in the representation of donor cells in CD4+ PBL from T/R− recipients that had received either NTg CD25+4+ cells from T/R+ donors or CD25+4+ cells from R+ animals (1.3 vs. 1.5%, respectively, not shown). We conclude that CD25+4+ cells from T/R+ mice contain Treg capable of inhibiting EAE, and that expression of the transgenic TCR is indispensable for their protective activity. In contrast, MBP specificity does not seem to be needed for the protective activity of R+ CD25+4+ cells because they do prevent EAE but fail to inhibit the MBP-specific response of CD25−4+ cells in vitro (Fig. 2).
Figure 3.
Adoptive transfer of CD25+4+ cells from T/R+ or R+ mice protects T/R− mice from EAE; the protection by T/R+ CD25+4+ cells depends on the Tg subset. Sort-purified CD4+ populations were transferred into 30- to 32-day-old T/R− mice (2 × 105 cells per mouse). EAE level was scored for 7 weeks after transfer as described in Materials and Methods. The mean score of each group is plotted over time. The numbers in parentheses indicate the final number of mice that developed EAE per total number of animals analyzed, and individual maximum EAE score (mean ± SD). Group comparisons for the maximum EAE score during the observation period were statistically significant by the Mann-Whitney test: [None vs. R+CD25+], P < 0.01; [None vs. T/R+ CD25+], P < 0.01; [T/R+CD25+ vs. T/R+3H12−CD25+], P < 0.02.
To assess whether CD25−4+ cells also contained Treg that control EAE, peripheral CD25−4+ cells purified from R+ mice and NTg CD25−4+ cells isolated from T/R+ animals were transferred into T/R− mice (not shown). As expected from previous work (32, 33), the four control animals that received total CD4+ cells purified from R+ animals remained healthy. In contrast, transfer of either population of CD25−4+ cells caused acute inflammatory bowel disease, and all animals died about 3 weeks after transfer. This result is consistent with studies showing the essential role for CD25+4+ cells in the prevention of inflammatory bowel disease induced by CD25−45RBhigh4+ T cells (14, 15), but made it impossible to conclude whether CD25−4+ cells contained Treg which control EAE.
The NTg CD25+4+ T Cell Population in T/R+ Mice Exhibits an Oligoclonal Repertoire.
We showed above that transfer into T/R− mice of 2 × 105 NTg CD25+4+ cells purified from T/R+ mice does not prevent EAE, whereas the same number of CD25+4+ cells isolated from R+ mice does prevent EAE. Analyses of BrdUrd incorporation in various CD4+ subsets in vivo indicated that NTg peripheral CD25+4+ cells from T/R+ animals proliferate about 3-fold more than the equivalent population in R+ animal (28 and 10% BrdUrd-positive cells, respectively, after a 3 days of BrdUrd treatment, not shown).The same range of extensive proliferation was found in both NTg CD25−4+ and Tg CD25+4+ populations (29 and 22%, respectively) and not in the Tg CD25−4+ pool (0.7%). These results, together with the very low representation of CD25+ cells in T/R+ CD4 single-positive thymocytes and their markedly higher representation in the periphery, suggest that the peripheral pool of CD25+4+ T cells in T/R+ animals is mostly generated by expansion of rare thymic emigrants. We therefore hypothesized that the NTg CD25+4+ pool from T/R+ mice may express a restricted TCR repertoire, a potential cause for their limited protective activity.
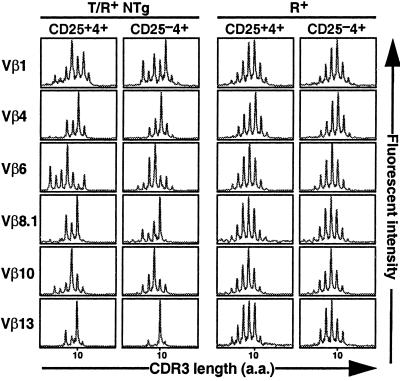
Analyses by flow cytometry of the Vβ gene segment usage in NTg CD25+4+ or NTg CD25−4+ cells from T/R+ mice, compared with CD25+4+ cells from R+ mice, revealed a similar distribution (not shown). The repertoires of the endogenous Vβ and Vα genes expressed in each cell population were then assessed by the immunoscope technique (35), which measures the size heterogeneity of the TCR complementarity determining region 3 (CDR3) (Fig. 4; not shown for Vα). The collective CDR3 lengths of the Vβ and Vα genes expressed in both CD25+4+ and CD25−4+ cells from R+ mice displayed Gaussian-like distributions, demonstrating that the respective repertoires are diverse (35). In contrast, for all of the five Vα and eight Vβ gene rearrangements analyzed, both NTg CD25+ and NTg CD25−4+ cells from T/R+ animals showed non-Gaussian, irregular patterns that are typical of oligoclonal repertoires (37). These restricted profiles were similar between the NTg CD25+4+ and CD25−4+ compartments in each individual, suggesting that they may share identical TCR sequences, whereas they were highly variable among the three animals examined (not shown), suggesting that the repertoires of NTg T cells in T/R+ animals emerge in a stochastic manner.
Figure 4.
Immunoscope analysis of TCRβ rearrangements reveals oligoclonality of the NTg populations of CD25+ and CD25−4+ cells from T/R+ mice. cDNA made from total RNA extracted from the sorted CD4+ T cell subpopulations (1 × 105) was subjected to PCR amplification with the indicated Vβ- and a Cβ-specific primer. The PCR products were subjected to run-off reactions with a nested fluorescent Cβ-specific primer and their CDR3 length profiles determined. Results are representative of 3 T/R+ and 2 R+ mice.
These results suggest that the limited ability of the NTg CD25+4+ cell pool from T/R+ mice in preventing EAE is due to its restricted TCR repertoire.
The Development of MBP-Specific CD25+4+ Cells in T/R+ Mice Depends on the Expression of Endogenous TCRα Genes.
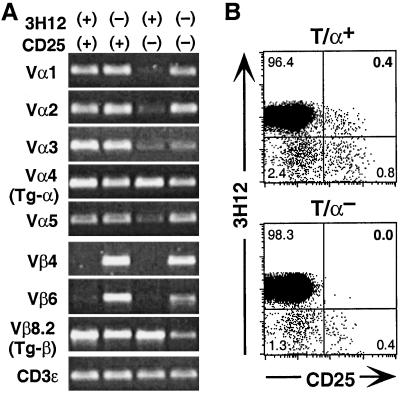
T/R− mice are entirely devoid of CD25+4+ cells being Tg or NTg (Fig. 1A), indicating that the generation of these populations relies on the production of endogenous TCR-expressing cells. Because allelic exclusion at the endogenous TCRα locus operates at low stringency (33), Tg CD25+4+ cells in TR+ animals may express dual (or triple) TCRs: one composed of the Tg-α coupled with the Tg-β chain and the other(s) by endogenous α-chains paired with the Tg-β-chain. We used semiquantitative PCR to assess the expression of transgenic and endogenous TCR chains in four subsets of peripheral CD4+ T cells from T/R+ mice (3H12+CD25+, 3H12−CD25+, 3H12+CD25−, and 3H12−CD25−) (Fig. 5A). In CD25− cells, expression of endogenous TCRα chains (Vα1, 2, 3, and 5) was markedly higher in the 3H12− subset when compared with the 3H12+ subset. Among CD25+ cells both the 3H12+ and the 3H12− subsets expressed high levels of endogenous Vα transcripts. In contrast, transcripts for endogenous Vβ4 and Vβ6 were undetectable in both 3H12+ subsets, but abundant in 3H12− cells, confirming a stringent allelic exclusion at the endogenous TCRβ locus (33). Finally, the levels of Tg-encoded transcripts (Vα4 and Vβ8.2) were comparable between CD25+ and CD25− 3H12+ subsets, indicating that expression of endogenous Vα chains in CD25+ cells does not impair Tg-α expression. The 3H12+CD25+ subset is thus enriched for cells coexpressing two TCRs: an endogenous TCRα chain paired with the Tg-TCRβ, and the complete Tg-TCR. It is this subset of double-TCR-expressing CD25+ cells that protects against EAE, because transfer of CD25+ cells from T/R+ mice that are depleted of 3H12+ cells (and thus enriched for cells expressing a TCR composed of endogenous TCR α- and β-chains) do not protect T/R− mice from EAE (Fig. 3).
Figure 5.
Tg CD25+4+ cells require expression of endogenous TCRα chains for their development. (A) Tg CD4+ cells coexpressing endogenous TCRα chains are enriched in the CD25+ subset. cDNA was synthesized from the indicated sorted T/R+ CD4+ subpopulations (1 × 105 with ≥97% purity), and semiquantitative PCR analyses were conducted with a Cα (or Cβ-)-specific and a Vα (or Vβ-)-specific primer, or with CD3ɛ-specific primers as a control. (B) Absence of the Tg CD25+4+ T cell population in TCR Cα−/− background (T/α−) but not in Cα+/− (T/α+) littermates. LN cells were stained for CD25, CD4, and Tg-TCR (3H12) and gated on the CD4+ population. The results are representative of four animals in each group.
Because 3H12+CD25+ T cells are generated in T/R+ mice but not in T/R− mice (Fig. 1A), their development is likely to depend on the expression of endogenous TCR chains. If this dependence were the case, 3H12+CD25+ cells would not be expected to be generated in TCR transgenic mice with a Cα-deficient background (T/α−) (32). Indeed, as can be seen in Fig. 5B, T/α− mice entirely lack the 3H12+CD25+ population among peripheral CD4+ T cells. 3H12−CD25+ and CD25− cells likely expressing endogenous TCRβ− were detectable, although their frequencies were reduced when compared with T/α+ littermates. The absence of 3H12+CD25+ cells in T/α− explains the previous finding that nearly all of these mice spontaneously develop EAE (32, 33).
Discussion
In this study, we have investigated the specificity requirements for development and effector functions of CD25+4+ regulatory T cells in the anti-MBP TCR Tg mouse model. We report that T/R+ mice contain MBP-specific “anergic”/suppressive CD25+4+ T cells expressing the Tg-TCR, which play an indispensable role in inhibiting the MBP-specific response of CD25−4+ cells in vitro, and in preventing EAE in vivo. Furthermore, we show that the development of this Tg Treg population requires the coexpression of endogenously encoded TCRα chains.
CD25+4+ T Cells Protect T/R− Mice from Spontaneous EAE.
Previous studies have shown that CD4+ Treg expressing endogenously encoded TCRs ensure tolerance to MBP in transgenic mice (32, 33). We now report that CD25+4+ T cells, coexpressing CTLA-4 and IL-2Rβ chains, obtained from either T/R+ or R+ donors, suffice to prevent the development of spontaneous EAE on transfer to T/R− recipients. These markers are also expressed by Treg that are protective in other disease models (8–13). However, a previous study described the transfer of tolerance to T/R− recipients by R+ splenocytes that were depleted of CD25+ cells (34). We were unable to confirm this finding, because in our hands TR− recipients of CD25− cells developed fatal bowel disease, presumably because our mice had more intestinal bacteria than the recipients used in the previous study. In other systems, peripheral CD4+ Treg have been detected in a compartment of activated/memory cells defined by markers other than CD25 (reviewed in refs. 5 and 6), including the CD25−45RB/Clow population (12, 15). We found that all cells expressing endogenous encoded TCR in T/R+ mice display an activated/memory phenotype (CD45RBlow, CD44high, CD62Llow) (not shown) and present the same oligoclonal repertoire, irrespective of CD25 expression (Fig. 4), an indication that they may also share the same function. Not withstanding the fact that some Treg may lack CD25 expression, the present study deals exclusively with CD25 expressing Treg.
Specificity to MBP Is Required for T/R+ CD25+4+ Cells to Prevent EAE.
The expression of the Tg-TCR is required for CD25+4+ cells purified from T/R+ mice to inhibit MBP-specific proliferation of Tg CD25−4+ cells in vitro. Most importantly, the regulatory function of the CD25+4+ cells in vivo also seems to require MBP specificity, because the elimination of the MBP-specific population (expressing the transgenic clonotype) from the T/R+ CD25+4+ cells results in loss of their protective activity against EAE in T/R− recipients. In addition, a strict correlation has been observed between the selective absence of this Tg CD25+4+ population and susceptibility to spontaneous EAE. Thus, both T/R− and T/α− mice, which spontaneously develop EAE (32, 33), do not contain Tg CD25+4+ cells.
Our reverse transcription–PCR and genetic analyses revealed that MBP-specific CD25+4+ cells also express TCRs containing endogenous TCRα chains (TCRαend/βTg). This raises the possibility that Treg protective functions may depend on the recognition of an antigen other than the nominal MBP peptide. However, this possibility is unlikely. First, NTg CD25+4+ cells express αend/βend, αTg/βend, or αend/βTg but show little protection upon adoptive transfer, strongly suggesting that expression of the MBP-specific αTg/βTg receptor is required for efficient protection. Second, immunoscope analyses of the endogenous TCRα chain repertoires expressed by the Tg CD25+4+ population revealed oligoclonal CDR3 size distributions that were highly variable in different individuals (not shown), suggesting that the specificities encoded by αend/βTg TCR are very restricted and generated in a stochastic manner.
Our experiments also show that specificity to the nominal peptide does not seem to be strictly necessary for CD25+4+ cells to exert their protective activity. Thus, CD25+4+ T cells purified from R+ mice were protective upon transfer in T/R− mice, although they did not inhibit the proliferative response of Tg CD25− T cells to the MBP peptide. Presumably these Treg recognize other peptides which may be coexpressed with the MBP peptide by the same antigen-presenting cells in vivo. Such peptides are unlikely to be recognized by Treg from T/R+ cells because they display, in contrast to Treg from R+ mice, a highly limited TCR repertoire.
Selection of MBP-Specific CD25+4+ Regulatory T Cells Is Determined by Endogenous α-Chains.
Our finding that T/R+, but not T/R− mice contain CD25+4+ cells is consistent with previous studies of transgenic mice carrying TCRs for foreign antigens. In these models, antigen-specific “anergic” and suppressive CD25+4+ cells are also found in conventional, but not in a RAG-deficient or severe combined immunodeficient background (22, 38). It has been proposed that TCRs composed of endogenous α-chains and transgenic β-chains are required for the development and/or function of Treg (22, 38). Acquisition of regulatory functions (and CD25 expression) by CD4+ T cells expressing an ovalbumin-specific Tg-TCR has been shown to require exposure to specific antigen (39). A more recent study demonstrated that thymocytes expressing self-antigen-specific transgenic TCR differentiate into CD25+4+ Treg only, if they recognize with high avidity the agonist peptide on the surface of thymic epithelium (24). It is likely, therefore, that in the transgenic TCR specific for non-self-antigens (22, 38), no high-affinity ligands are available in the thymus for Tg-TCR-dependent selection/commitment to regulatory functions. Commitment to regulatory functions (and CD25 expression) in TCR Tg mice would thus have to be ensured by novel TCR reactivities provided by endogenous TCRα chain expression. In agreement with this model, we show here that Tg CD25+4+ cells indeed expressed endogenous TCRα chains, but not endogenous TCRβ chains.
On the other hand, in contrast with systems of anti-nonself-TCRs, we are dealing here with an anti-self (MBP) TCR, ensuring the potential exposure of MBP-specific Tg cells to their nominal peptide during all phases of development, and making it difficult to interpret the requirement for additional (endogenous) specificities in Treg development. Thus, expression and presentation of both Golli-MBP and classic MBP in the thymus and in LN has been reported repeatedly (40–42). As T/R− mice spontaneously develop EAE, it is clear that Tg T cells and their effector progenies can productively interact with the respective peptide/MHC complexes in the periphery. On the other hand, because the same T/R− mice contain undetectable Tg CD25+4+ cells either in the thymus or in the periphery, we must conclude that TCR Tg cells are either not exposed to specific peptide/MHC complexes in the thymus, or else that the T cell avidity requirements for commitment to regulatory functions are more stringent that those required for activation of other effector functions. Because, at present, no specific information exists on the intrathymic availability of the nominal peptide, we would prefer the former alternative. Even if the MBP Ac1–17 peptide is expressed intrathymically, because it forms short-lived complexes with I-Au (43), this expression may be incompatible with the threshold required for induction of CD25+4+ Treg, but be sufficient for activation of Tg cells in the concentrations and conditions of presentation that are available in the periphery. Yet another alternative is that Treg commitment would generally require signaling through two distinct TCRs, a hypothesis we find unlikely because anti-MBP and anti-ovalbumin double transgenic mice on a RAG-1-deficient background display CD4+ cells expressing various ratios of each Tg-TCR and systematically develop EAE (5). The present results are therefore in agreement with the current notion that CD4+ Treg are committed in the thymus, possibly through selective interactions with thymic epithelium (12, 19–25).
Our analyses open up the possibility that the establishment of peripheral tolerance largely depends on Treg which express more than one TCR. One TCR, specific for antigens present exclusively in the periphery, mediates specific regulatory functions, whereas the other allows thymic selection/commitment. Indeed, the relaxed allelic exclusion at the α locus may have developed to allow for the generation of double-TCR expressing Treg. Quantitative and functional analyses of double Vα expressors inside CD25+4+ populations will help clarify this point.
Acknowledgments
We are most grateful to Dr. S. Tonegawa for providing the transgenic mice and to Dr. Juan Lafaille and Dr. S. Sakaguchi for offering the 3H12 and the PC61 hybridoma, respectively. We also acknowledge Bruce Lenhart for rederivation of the mice, Magda Carlos for assistance in cell sorting, Rosa Maria Santos for mAb production and labeling, and Rute Marques for help with the immunoscope analyses and follow-up of the experimental animals. We thank Jorge Carneiro, Werner Haas, Thiago Carvalho, and Iris Caramalho for persistent intellectual and emotional support. Finally, we are grateful to Juan Lafaille for kindly sharing unpublished data and, together with Werner Haas, for critical reading of the manuscript. This work was conducted in the framework of the Laboratoire Européen Associé “Génétique et developpement de la tolérance naturelle”, Centre National de la Recherche Scientifique (France). S.H. received fellowships from The Cell Science Research Foundation (Osaka, Japan) and the Yamanouchi Foundation for Research on Metabolic Disorders (Tsukuba, Japan). J.D. was supported by Fundaçao para a Ciencia e a Tecnologia PRAXIS XXI (Portugal).
Abbreviations
- Treg
regulatory T cells
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- Tg
transgenic
- NTg
nontransgenic
- TCR
T cell antigen receptor
- RAG
recombination-activating gene
- T/R−
anti-MBP TCR Tg mice homozygote for a null mutation of the RAG-1 gene
- T/R+
anti-MBP TCR Tg mice with a wild-type RAG-1 gene
- R+
NTg mice with a wild-type RAG-1 gene
- T/α−
anti-MBP TCR Tg mice with disrupted TCR Cα genes
- T/α+
anti-MBP TCR Tg mice with a wild-type TCR Cα gene
- LN
lymph node
- CDR3
complementarity determining region 3
References
- 1.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, Coutinho A, Salaun J. Immunol Rev. 1996;149:35–53. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 2.Modigliani Y, Bandeira A, Coutinho A. Immunol Rev. 1996;149:155–120. doi: 10.1111/j.1600-065x.1996.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann H, Cobbold S. Annu Rev Immunol. 1998;16:619–644. doi: 10.1146/annurev.immunol.16.1.619. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho A, Hori S, Carvalho T, Caramalho I, Demengeot J. Immunol Rev. 2001;181:89–98. doi: 10.1034/j.1600-065x.2001.1820107.x. [DOI] [PubMed] [Google Scholar]
- 5.Mason D, Powrie F. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 7.Shevach E M. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 9.Asano M, Toda M, Sakaguchi N, Sakaguchi S. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suri-Payer E, Amar A Z, Thornton A M, Shevach E M. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 11.Alard P, Thompson C, Agersborg S S, Thatte J, Setiady Y, Samy E, Tung K S. J Immunol. 2001;166:4363–4369. doi: 10.4049/jimmunol.166.7.4363. [DOI] [PubMed] [Google Scholar]
- 12.Stephens L A, Mason D. J Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow D J, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone J A. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Read S, Malmstrom V, Powrie F. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa T C, Cumano A, Bandeira A. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 16.Hara M, Kingsley C I, Niimi M, Read S, Turvey S E, Bushell A R, Morris P J, Powrie F, Wood K J. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 18.Thornton A M, Shevach E M. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salaun J, Bandeira A, Khazaal I, Calman F, Coltey M, Coutinho A, Le Douarin N M. Science. 1990;247:1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- 20.Modigliani Y, Thomas-Vaslin V, Bandeira A, Coltey M, Le Douarin N M, Coutinho A, Salaun J. Proc Natl Acad Sci USA. 1995;92:7555–7559. doi: 10.1073/pnas.92.16.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saoudi A, Seddon B, Fowell D, Mason D. J Exp Med. 1996;184:2393–2398. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 23.Seddon B, Mason D. J Exp Med. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan M S, Boesteanu A, Reed A J, Petrone A L, Holenbeck A E, Lerman M A, Naji A, Caton A J. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 25.Bensinger S J, Bandeira A, Jordan M S, Caton A J, Laufer T M. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Takahashi T, Nishizuka Y. J Exp Med. 1982;156:1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi O, Nishizuka Y. J Exp Med. 1987;165:146–156. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taguchi O, Kontani K, Ikeda H, Kezuka T, Takeuchi M, Takahashi T. Immunology. 1994;82:365–369. [PMC free article] [PubMed] [Google Scholar]
- 29.Seddon B, Mason D. Immunol Today. 2000;21:95–99. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- 30.Cobbold S, Waldmann H. Curr Opin Immunol. 1998;10:518–524. doi: 10.1016/s0952-7915(98)80217-3. [DOI] [PubMed] [Google Scholar]
- 31.Lafaille J J, Nagashima K, Katsuki M, Tonegawa S. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 32.Van de Keere F, Tonegawa S. J Exp Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivares-Villagomez D, Wang Y, Lafaille J J. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivares-Villagomez D, Wensky A K, Wang Y, Lafaille J J. J Immunol. 2000;164:5499–5507. doi: 10.4049/jimmunol.164.10.5499. [DOI] [PubMed] [Google Scholar]
- 35.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casanova J L, Romero P, Widmann C, Kourilsky P, Maryanski J L. J Exp Med. 1991;174:1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regnault A, Cumano A, Vassalli P, Guy-Grand D, Kourilsky P. J Exp Med. 1994;180:1345–1358. doi: 10.1084/jem.180.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornton A M, Shevach E M. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 39.Thorstenson K M, Khoruts A. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 40.Voskuhl R R. Immunol Rev. 1998;164:81–92. doi: 10.1111/j.1600-065x.1998.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 41.Harrington C J, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, Beeson C, Goverman J. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- 42.Huseby E S, Sather B, Huseby P G, Goverman J. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 43.Fairchild P J, Wildgoose R, Atherton E, Webb S, Wraith D C. Int Immunol. 1993;5:1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]