Summary
Dissemination of ovarian cancer cells during metastasis is heavily influenced by omental cells from the adipose tumor microenvironment. We previously found that omental adipocyte precursors, or preadipocytes, promote stress tolerance and tumorigenesis of ovarian cancer cells and this was correlated with upregulation of insulin-like growth factor-binding protein 5 (IGFBP5). Here, we tested whether IGFBP5 mediates stress tolerance and tumorigenesis. Using in vitro and in vivo knockdown and overexpression models we found that IGFBP5 drives tumor-initiating cell phenotypes with increased adhesion, clonogenic potential, stress tolerance, and tumor initiation. Reciprocally, knocking down IGFBP5 reduced these features. Mechanistically, we found that preadipocyte-secreted factors increase expression of IGFBP5 in cancer cells which activates CREB to enhance cell survival. Targeting CREB using a pharmacological inhibitor reversed the IGFBP5 induced phenotypes. These findings further support the role of preadipocytes in ovarian cancer progression and suggest therapeutically targeting IGFBP5 or CREB may reduce ovarian cancer metastasis.
Subject areas: Cancer, Molecular biology
Graphical abstract

Highlights
-
•
IGFBP5 is upregulated in ovarian cancer cells in response to omental preadipocytes
-
•
IGFBP5 knockdown decreases preadipocyte-induced tumor growth
-
•
IGFBP5 overexpression enables survival and tumor initiation during stress
-
•
CREB signaling is partially responsible for IGFBP5 mediated phenotypes in ovarian cancer
Cancer; Molecular biology
Introduction
Ovarian cancer is the most lethal gynecologic malignancy due to a lack of targeted therapies and a complex tumor environment.1 There are five main types of epithelial ovarian cancer including high-grade serous, low-grade serous, endometrioid, clear cell, and mucinous. Recent studies indicate many of these likely originate in the fallopian tube, especially for high-grade serous, however, there is also evidence that high-grade serous ovarian cancers can originate in the ovary and metastasize to the fallopian tube.2 The unknown origins of many ovarian cancers, and of the cell lines used to study its biology, likely contribute to our poor understanding of this heterogeneous disease and the lack of therapeutic targets.
Metastatic progression in ovarian cancer is heavily influenced by the omentum, an adipose organ that lines the abdominal wall and is the primary site for metastasis. The omentum is composed primarily of lipid-storing mature adipocytes which provide an energy-rich niche for invading ovarian cancer cells.3 These adipocytes are supported by stromal cells including preadipocytes (adipocyte-precursor cells) fibroblasts, and immune cells, which localize to immunogenic milky spots and regulate wound healing within the abdomen.4,5,6 Preadipocyte differentiation into mature adipocytes is regulated in part by insulin-like growth factor (IGF) signaling, which is a driver of ovarian cancer progression in the adipose tumor microenvironment (TME).7,8,9
IGF signaling canonically regulates cell differentiation, survival, and proliferation, and is tightly controlled by IGF binding proteins, or IGFBPs, which bind IGF to control its bioavailability.10 The IGFBP family contains 6 classically defined isoforms, IGFBP-1 through −6, all of which are highly conserved in eukaryotes.11 Importantly, IGF signaling and the IGFBPs have been implicated in numerous types of cancer, including glioblastoma, osteosarcoma, melanoma, breast, and ovarian cancers.10 Of the IGFBPs, IGFBP5 is perhaps the most versatile with several functions that are IGF independent, such as regulating ECM composition, cell adhesion and cell migration.12
Previous studies in our lab indicate that preadipocyte-induced IGF signaling in tumor cells supports ovarian cancer cell survival and metastatic properties.9 In this study, we sought to understand the role of IGFBP5 in mediating the oncogenic properties of ovarian cancer cells by leveraging both CRISPR-mediated gene knockdown and overexpression to study gain- and loss-of-function of IGFBP5. Using these cellular models, we show that IGFBP5 loss-of-function delays tumor initiation and growth in vivo, while IGFBP5 overexpression promotes tumor initiation. In vitro assays further demonstrate that cells overexpressing IGFBP5 have improved viability, adhesion, and proliferation, which is specific to nutrient deprivation-induced stress. IGFBP5 overexpression induces CREB activity by increasing phosphorylation at serine 133, and we show that inhibiting CREB signaling blunts the in vitro phenotypes associated with elevated IGFBP5, suggesting that the IGFBP5-CREB signaling axis is potentially a therapeutic target for ovarian cancer.
Results
IGFBP family genes are upregulated in response to omental preadipocytes
Our previous work showed that omental preadipocyte-induced IGF signaling enabled ovarian cancer cells to overcome isolation stress for early tumor initiation. Here, we further examined our previous RNA sequencing (GSE262869) for changes in IGFBPs by ACI-23 ovarian cancer cells co-cultured with omental preadipocytes or differentiated adipocytes. A majority of the IGFBP genes were elevated in response to preadipocyte co-culture, with the exception of IGFBP6 (Figure 1A). IGFBP gene expression in preadipocytes or differentiated adipocytes was unchanged after co-culture with ACI-23 cells (Figure S1A), indicating that cancer cells drive fluctuations of IGFBPs in the microenvironment. While IGFBP3 and IGFBP4 were elevated in ACI-23 cells in response to co-culture with either preadipocytes or differentiated adipocytes, IGFBP2 and IGFBP5 were only upregulated in cells co-cultured with preadipocytes. IGFBP1 was not detected under any conditions (Figure 1A).
Figure 1.
IGFBP family genes are upregulated in response to omental preadipocytes
(A) RNA sequencing of the IGFBP family members in response to preadipocyte or differentiated adipocyte co-culture (n = 3–4). Statistical significance level is indicated using the adjusted p value of multiple t tests.
(B) RT-qPCR for IGFBP5 mRNA expression in multiple ovarian cancer cell lines in response to preadipocyte co-culture measured via RT-qPCR (multiple unpaired t tests, n = 3).
(C) GEPIA plot of IGFBP5 mRNA expression in tumor (T, red) and normal (N, gray) in OV, ovarian samples (unpaired t test).
(D) Forest plot demonstrating the hazard ratio of a meta-analysis of datasets containing ovarian cancer overall survival patient outcomes.
Data are represented as individual replicates with mean ± SD. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
The most significantly changed IGFBP after preadipocyte co-culture was IGFBP5, with a fold increase of 128.5 relative to monocultured cells, which is striking given that IGFBP5 was not detected after differentiated adipocyte co-culture. Co-culturing ACI-23, CAOV4, OVCAR8, and SKOV3 cell lines with omental preadipocytes universally induced expression of IGFBP5, indicating that both low- and high-grade serous ovarian cancer are responsive to preadipocytes (Figure 1B). Furthermore, treating ACI-23 cells with preadipocyte-conditioned media confirms that IGFBP5 is upregulated in ACI-23 cells specifically in response to secreted factors from preadipocytes (Figure S1B).
In healthy tissue, IGFBP5 expression is highest in gynecologic organs, particularly the ovaries and cervix (Figure S1C). Interestingly, when evaluating IGFBP5 mRNA in normal versus tumor tissues of the gynecologic tract using the gene expression profiling interactive analysis (GEPIA), we found that primary tumor tissue from gynecologic cancers (ovarian, uterine and cervical) all had decreased IGFBP5 expression compared to normal tissue (Figure 1C and S1D). The low endogenous expression in primary tumors is mirrored in our transcriptomic assessment of unstimulated ACI-23 cells, where IGFBP5 had the lowest transcript abundance of all the IGFBP family members with an average of 4.4 fragments per million reads (Figure S1E).
Although IGFBP5 expression is decreased in tumors relative to normal ovarian tissue and poorly expressed by ovarian cancer cells, both endometrioid and serous ovarian cancer patients expressing higher levels of IGFBP5 have significantly decreased overall and progression-free survival, as do patients with high IGFBP6.13,14 Conversely, patients with high IGFBP1 have better overall and progression-free survival. The correlation with IGFBP5 was confirmed via meta-analysis of 2970 epithelial ovarian cancer patient datasets which showed a strong association between increased IGFBP5 expression and decreased overall survival (hazard ratio = 1.14, Figure 1D). While overall IGFBP5 expression is low in tumor relative to normal tissue, suggesting a tumor suppressor function, elevated expression is correlated with reduced overall survival, indicating that low expression is not necessarily tumor-suppressive and it’s pro- or anti-tumor effects may be context specific, such as in metastatic sites.
IGFBP5 is expressed in patient tissues from serous ovarian cancers and is enriched in metastases.
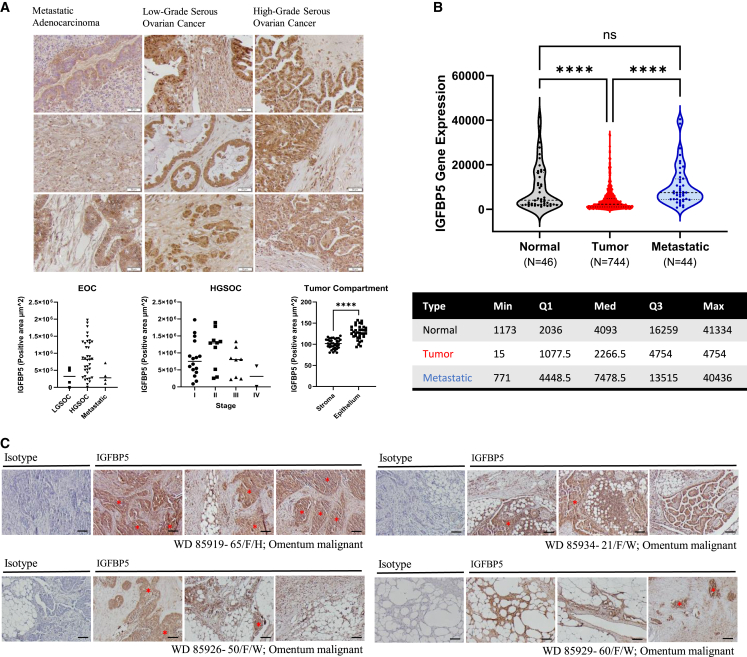
To observe the spatial distribution of IGFBP5 in patient tissues, we performed IHC staining for IGFBP5 on a tissue array containing cores derived from 40 patients with serous ovarian cancer as well as several samples from normal adjacent ovarian tissue. Notably, IGFBP5 was expressed in all tissues, with more staining observed in the leading edge of malignant areas in LGSOC and HGSOC (Figure 2A, S2A, and S2B). Expression was broadly dispersed in normal ovarian tissue, with no areas of notable concentrated signal. No significant differences were observed that correlate with disease type or stage (Figure 2A and S2C).
Figure 2.
IGFBP5 is expressed in patient tissues from serous ovarian cancers and is enriched in metastases
(A) IGFBP5 expression via IHC in an ovarian cancer tumor microarray. EOC, epithelial ovarian cancer; LGSOC, low-grade serous ovarian cancer; HGSOC, high-grade serous ovarian cancer; ROI, region of interest. Scale bar, 50 μm. EOC and HGSOC, one-way ANOVA with Tukey’s multiple comparisons; tumor compartment, unpaired t test, n = 73 regions, data are represented as individual tumor cores with the mean.
(B) IGFBP5 gene expression in normal, primary tumor and metastatic tumor patient samples from TMN plot, one-way ANOVA with Dunn’s multiple comparisons, data are presented as individual points with the mean and standard deviation.
(C) Representative images of IGFBP5 protein expression in malignant omental tumors from four patients with metastatic ovarian cancer. Scale bar, 100 μm. ∗∗∗∗p < 0.0001.
IGFBP5 has been associated with a migratory phenotype and epithelial-to-mesenchymal (EMT) transition in glioblastoma.15 Given the increase in IGFBP5 expression observed at the leading edge of numerous tumors, we next asked whether IGFBP5 might be specifically elevated in metastatic disease. Indeed, when we use TMNplot to assess IGFBP5 expression in normal, primary tumor and metastatic tissues in ovarian cancer,16 we observed an increase in IGFBP5 expression between primary tumors and metastatic tumors (Figure 2B). Additional analysis of gene expression using GEPIA for the other IGFBPs and IGF signaling components showed that IGFBP4 and IGF1R were also elevated in metastatic tumors when compared to primary tumors (Figure S3A), and that expression of IGFBP2, -3 and -4 were positively correlated with expression of IGFBP5 in a tumor-specific manner (Figure S3B). Furthermore, staining fixed tissue derived from patients with omental metastases shows tumor compartment-specific expression of IGFBP5 in omental tumors (Figure 2C).
IGFBP5 expression was further characterized in a panel of ovarian cancer cell lines using RT-qPCR to measure mRNA expression and immunoblot to measure protein expression. All cell lines assessed contained measurable levels of IGFBP5, however, expression was variable and was therefore grouped into low, moderate, and high expressing cell lines. ACI-23, OVCAR8 and CAOV4 expressed low levels of IGFBP5; OV90 and OVCAR5 expressed moderate levels; and SKOV3, OVCAR4,- and CAOV3 expressed the highest levels (Figure S4A). Protein expression levels followed similar patterns, with SKOV3 and OVCAR4 expressing higher amounts of IGFBP5 than the other cell lines (Figure S4B). Using ELISA to quantitate IGFBP5 in cell culture supernatants of a subset of the lines showed no significant difference (Figure S4C).
IGFBP5 knockdown decreases preadipocyte-induced tumor growth
Given that (1) preadipocyte-secreted factors modulate tumor cell behavior, (2) IGFBP5 is the most significantly upregulated gene in response to preadipocyte co-culture,9 and (3) IGFBP5 expression is correlated with reduced overall survival in ovarian cancer, we proceeded to analyze the functional significance of IGFBP5 in our previously established subcutaneous model of preadipocyte-mediated tumorigenesis (Figure 3A). IGFBP5 knockdown (BP5-KD) in ACI-23 cell lines was established via CRISPR-Cas9 editing as ACI-23 cells exhibit the strongest upregulation of IGFBP5 after preadipocyte co-culture (Figure 1B). BP5-KD in ACI-23 was confirmed via immunoblot and RT-qPCR, and on-target CRISPR editing was sequence-verified via Sanger sequencing (Figure 3B, S5A and S5D). Clonal expansion of IGFBP5-KD cells did not have any effects on cell morphology or cell viability (Figure S5E). While a cell line with complete excision of IGFBP5 in both alleles was intended, screening of 48 isolated clonal lines did not produce any lines that were confirmed to have full gene ablation after sequencing.
Figure 3.
IGFBP5 knockdown decreases preadipocyte-induced tumor growth
(A) Schematic depicting study design for preadipocyte-stimulated tumor initiation model with ACI-23 sgNeg and ACI-23 sgIGFBP5.
(B) Representative immunoblot of ACI-23 sgNeg and ACI-23 sgIGFBP5 cell lysates (n = 3).
(C) ACI-23 sgNeg (WT) or ACI-23 sgIGFBP5 (BP5-KD) ovarian cancer cells were subcutaneously injected subcutaneously into the left flank of 8-week-old female athymic Nu/J mice in the presence or absence of human omental preadipocytes (pAdip) and tumor initiation (left) and growth (right) was measured out to 90 days, two-way ANOVA with Tukey’s multiple comparisons (n = 8).
Data are represented as mean ± SEM. ∗∗∗∗p < 0.0001, ∗p < 0.05.
Despite this limitation, we proceeded to assess changes in tumor growth with partial loss of IGFBP5. Mice were injected with ACI-23 wild type (WT) or ACI-23 BP5-KD cells in the presence or absence of preadipocytes. We injected a heterogeneous cell mixture at a ratio of 500 ACI-23 cells to 20,000 preadipocytes (1:40), which we have demonstrated functions as a model for preadipocyte-induced tumorigenesis.9 In mice that received BP5-KD cells with or without preadipocytes, initial tumor formation was slightly delayed, and the growth rate of preadipocyte-induced tumors was significantly reduced relative to mice that received WT cells (Figure 3C). Tumor development in mice receiving only tumor cells was minimal, replicating the data from our previous study showing that ACI-23 cells at this concentration retain a low tumor forming ability unless co-injected with preadipocytes.9 Specifically, 2/10 mice formed tumors when injected with ACI-23 WT cells only, and 1/10 mice formed a tumor when injected with ACI-23 BP5-KD cells only (Figure 3C). Importantly, significant changes in tumor volume from BP5-KD cells relative to WT cells were only evident when co-injected with preadipocytes (Figure 3C).
Overexpression of IGFBP5 enables survival during cellular stress
We next sought to model the effects of increased cancer cell-specific expression of IGFBP5 by overexpressing IGFBP5 in our cancer cell lines. This was first attempted in ACI-23, however, this cell line proved intractable to G418 selection. We therefore utilized SKOV3 cells, as they exhibited the second highest increase (after ACI-23) in IGFBP5 expression after preadipocyte co-culture (Figure 1B). Transfected cells were positively selected with G418 (Figures S6A and S6B) and increased IGFBP5 expression was confirmed via immunoblot of whole cell lysate and ELISA of supernatant (Figure 4A) and RT-qPCR (Figure S6C). Interestingly, we found that growth in serum-free medium (SFM) increases IGFBP5 transcript levels. Furthermore, IGFBP5 overexpression increases not only basal levels of IGFBP5 in cells grown in complete media, but also significantly increases IGFBP5 expression over the higher baseline observed in SFM (Figure S6C).
Figure 4.
Overexpression of IGFBP5 enables survival during cellular stress
(A) Immunoblot demonstrating ectopic expression of IGFBP5 in SKOV3 transfected with pcDNA3-IGFBP5-V5 compared to parental cells (left) and ELISA quantification of IGFBP5 in supernatants, unpaired t test (right, n = 3).
(B) Cell viability via CellTiter-GLO in SKOV3 and SKOV3 overexpressing IGFBP5 (SKOV3-BP5) in complete media or serum free media after 3 and 6 days, two-way ANOVA with Sidak’s multiple comparisons (n = 3).
(C) Cell viability via CellTiter-GLO in wild-type (WT) vs. IGFBP5-knockdown (BP5-KD) in ACI-23 after 3 and 7 days of growth in complete media, two-way ANOVA with Sidak’s multiple comparisons (n = 3).
(D) Colony formation assay using SKOV3 and SKOV3-BP5 cells stained with crystal violet after 7 days of growth in complete media, unpaired t test (n = 4). Scale bars are 5 mm.
(E) Representative images of SKOV3 and SKOV3-BP5 grown in SFM, 24 and 48 h after plating. Scale bars are 100 μm.
(F) Adhesion assay showing a time course (24, 48, and 72 hr) for attachment to TC treated plastic in complete media (10% FBS) or serum free media (SFM). Adherent cells are stained with Hoechst (nuclei, blue) and phalloidin (cytoskeleton, red). SKOV3-BP5 grown in either medium is relativized to separate media controls, two-way ANOVA with Sidak’s multiple comparisons (n = 3). Scale bars are 40 μm.
(G) Proliferation assay after 72 h via EdU incorporation (pink) in complete media. Scale bars are 40 μm (10% FBS) or serum free media (SFM). All groups are relativized to SKOV3-10% FBS, two-way ANOVA with Sidak’s multiple comparisons, (n = 3).
(H) qRT-PCR for mRNA expression of IGFBP and metastasis-associated genes CDH1 (E-cadherin), CDH2 (N-cadherin), VIM (vimentin), and PTK2 (focal adhesion kinase/FAK), multiple t tests (n = 3).
Data are represented as individual replicates with mean ± SD. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
We previously showed that preadipocytes cf. enhanced cell viability in serum-free conditions that provoke nutrient stress,9 and here, we show that overexpression of IGFBP5 (SKOV3-BP5) is sufficient to recapitulate this phenotype (Figure 4B). Given the increase in serum-independent survival with IGFBP5 overexpression, we performed the reciprocal experiment using our ACI-23 BP5-KD cells and found that, relative to cells with intact IGFBP5 expression, BP5-KD cells had reduced cell viability under serum-free conditions at day 3 and 7 days in culture (Figure 4C). Furthermore, when we performed a crystal violet colony formation assay, we similarly found that SKOV3-BP5 cells were better able to form colonies during isolation stress (Figure 4D).
During these experiments we observed visually that the BP5-overexpressing cells appeared to have increased adhesion in vitro and were better able to form colonies in SFM, while cells expressing endogenous levels of IGFBP5 failed to settle (Figure 4E). To quantitatively characterize this pro-adhesion phenotype, we performed an adhesion assay by staining SKOV3 and SKOV3-BP5 with phalloidin and Hoechst after 24, 48, and 72 h in culture. There was a notable, but not significant, increase in adherent SKOV3-BP5 cells in complete media at 24 and 48 h, however, the confluency between SKOV3 and SKOV3-BP5 was equivalent by 72 h (Figure 4F). Strikingly, at all time points cells grown in SFM showed a significant increase in adherent SKOV3-BP5 cells relative to WT with enhanced cell-spreading at 48 h and colony formation by 72 h (Figure 4F). These findings suggest IGFBP5 supports an adaptive cell response which enables survival during both isolation and nutrient stress.
IGFBP5 regulates proliferation and apoptosis through the IGF signaling pathway10,17; therefore, we measured proliferation by EdU incorporation and apoptosis by caspase 3/7 activity in SKOV3 and SKOV3-BP5 cells. While proliferation was equivalent between SKOV3 and SKOV3-BP5 in complete media after 72 h, SKOV3-BP5 were significantly more proliferative than SKOV3 in SFM (Figure 4G). There was no difference in apoptosis between the two groups after a 7-day time course in either SFM or complete media (Figure S6D).
As increased adhesion is often concordant with an increase in focal adhesion kinase (FAK) activity, which promotes proliferation, we also probed for total and phosphorylated FAK in lysates from SKOV3 and SKOV3-BP5 cells, however, we did not observe a significant increase in FAK activation (Figure S6E). In fact, when we measured PTK2 gene expression, which encodes the FAK protein, in SKOV3 and SKOV3-BP5 cells grown in SFM, we observed a significant decrease in PTK2 with IGFBP5 overexpression (Figure 4H). We also measured several traditional markers of epithelial-to-mesenchymal transition (EMT) and found that SKOV3-BP5 cells have decreased E-cadherin (CDH1) and N-cadherin (CDH2), as well as decreased vimentin (VIM) (Figure 4H). These results suggest that while IGFBP5 is important for adhesion, survival, and colony formation, it is not critical for driving cells through traditional EMT.
IGFBP5 enables tumor initiation at low dilutions
Decreased CDH1 along with CDH2 and VIM indicates that SKOV3-BP5 cells may be losing their epithelial phenotype without specifically gaining a mesenchymal phenotype, which may be important for early tumor-initiation processes.18 We therefore proceeded to test our SKOV3-BP5 cells in an in-vitro extreme limiting dilution assay (ELDA). SKOV3-BP5 cells displayed an increased ability to form spheroids in low attachment settings (Figure 5A), and when we repeated this experiment using flat-bottom adherent plates which do not synthetically force cell-cell contact, there was a similar increase in colony formation which approached significance (Figure S6F, p = 0.06). We measured several markers of tumor-initiating cells (TICs) via qRT-PCR, specifically SOX2, OCT4, and NANOG, in ACI-23 cells after treatment with recombinant IGFBP5 or CRISPR-knockdown and confirmed that IGFBP5 treatment increases expression of TIC markers. Conversely, in BP5-KD cells we found a reduction in the expression of these transcription factors (Figures S7A and S7B).
Figure 5.
IGFBP5 enables tumor initiation at low dilutions
(A) ELDA analysis of an in vitro limiting dilution assay comparing spheroid forming ability in round bottom plates between SKOV3 (black) and SKOV3-BP5 (red) cells (n = 3).
(B) Graphic showing experimental design for a limiting dilution assay comparing SKOV3 and SKOV3-BP5, n = 4 mice per condition.
(C) Time to tumor initiation in a limiting dilution of SKOV3 and SKOV3-BP5 using 1 × 105, 1 × 104, or 1 × 103 cells, two-way ANOVA (n = 4).
(D) Time course of tumor growth in a limiting dilution of SKOV3 and SKOV3-BP5 using 1 × 105, 1 × 104, or 1 × 103 cells, two-way ANOVA (n = 4).
(E) Table of results for tumor initiation with the time to initiation in days averaged per group. Data are represented as mean ± SEM.
Given the pro-survival and tumor-initiation phenotype observed in vitro with IGFBP5 overexpression, we next tested whether increased cell-intrinsic IGFBP5 influences tumor initiation in vivo. To do this, we subcutaneously injected limiting dilutions of tumor cells in immune compromised mice using cell doses of 1 × 105, 1 × 104, and 1 × 103 SKOV3 or SKOV3-BP5 cells (Figure 5B). Indeed, we found that while there was a slight decrease in tumor initiation (p = 0.08) by SKOV3-BP5 cells at the highest dose, there was a significant increase in tumor initiation at the middle and lowest doses (Figures 5C–5E). At the lowest dose 100% of mice injected with SKOV3-BP5 formed tumors while none of the mice injected with SKOV3 parental cells formed tumors out to 100 days (Figure 5C). Moreover, SKOV3-BP5 tumors exhibited an increased growth rate relative to control SKOV3 cells in all dilutions (Figure 5D). RNA harvested from the tumors confirmed elevated expression of IGFBP5 in the SKOV3-BP5 derived xenografts (Figure S7C), however when we assessed expression of SOX2, OCT4, and NANOG, we found no consistent differences between SKOV3 and SKOV3-BP5 tumors (Figure S7D). This discrepancy could be due to the late stage at which the tumors were harvested, at which point TICs are a minor subpopulation compared to the proliferative cells which comprise the bulk tumor.
IGFBP5 stimulates CREB signaling to regulate tumorigenic phenotypes
We next sought to elucidate the intracellular mechanisms of IGFBP5-mediated tumorigenesis. First, whole cell lysates derived from SKOV3 and SKOV3-BP5 cells were applied to a phospho-kinase array to determine which signaling pathways are activated with IGFBP5 upregulation. We found that GSK-3 and CREB were activated in SKOV3-BP5 cells, while downregulated kinases included Chk-2, PYK2, and STAT6. CREB, or cAMP response element-binding protein, is a transcription factor that controls cell proliferation and survival, both of which are phenotypically altered in our BP5 cells (Figure 6A). We therefore evaluated the correlation between IGFBP5 and CREB activation via immunoblot using both overexpression and knockdown of IGFBP5 in SKOV3 cells. Indeed, we found that pCREB increased with IGFBP5 overexpression and decreased with IGFBP5 knockdown (Figure S8A), although the decrease is more subtle, possibly due to knocking down an already less-abundant protein. Using TCGA, we further interrogated the correlations between IGFBP5 expression and members of the CREB signaling axis and found significant positive correlations with 13/18 of the genes queried (Figure 6B). As all the tumors in that dataset are primary ovarian tumors, we also used a publicly available dataset of omental metastases (GSE218939) and found a similarly strong and significant correlation between IGFBP5 and CREB (Figure 6C).
Figure 6.
IGFBP5 stimulates CREB signaling to regulate tumorigenic phenotypes
(A) Human phospho-kinase array comparing whole cell lysates from SKOV3 and SKOV3-BP5 cells grown in SFM. Boxes 1–3 are identifying increased kinase phosphorylation in SKOV3-BP5, boxes 4–6 are identifying decreased kinase phosphorylation in SKOV3-BP5 compared to parental SKOV3.
(B) Correlation matrix comparing mRNA expression of IGFBP5 with CREB pathway genes in TCGA ovarian cancer samples.
(C) Pearson correlation between IGFBP5 and CREB in omental metastases from GSE218939, a publicly available dataset.
(D) Representative immunoblot on SKOV3 and SKOV3-BP5 lysates frown in complete or serum-free media with quantifications relative to GAPDH then relativized to SKOV3 in complete media, one-way ANOVA with Tukey’s multiple comparisons, data are represented as mean ± SEM (n = 3).
(E) Cell viability via CellTiter-GLO in SKOV3 and SKOV3-BP5 treated with 666-15 in serum-free media after 3 and 7 days, two-way ANOVA with Sidak’s multiple comparisons (n = 3).
(F) Adhesion assay after 48 h for attachment to TC treated plastic in serum-free media (SFM). Adherent cells are stained with Hoechst (nuclei, blue) and phalloidin (cytoskeleton, red). Scale bar 200 μm. SKOV3-BP5 grown in either medium is relativized to separate media controls, two-way ANOVA with Tukey’s multiple comparisons (n = 3).
Data are presented as individual replicates with mean ± SD (E, F). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
Given that the phenotypes observed with IGFBP5 overexpression occur in the context of nutrient19 deprivation in SFM, we next measured CREB activity in SKOV3 and SKOV3-BP5 cells in both complete media and SFM via immunoblot. We found that the strongest expression of IGFBP5, phosphorylated CREB, and total CREB occurs in SKOV3-BP5 cells grown in SFM, indicating that this signaling pathway is likely a component of the adaptive pro-survival response in SKOV3-BP5 cells (Figure 6D). However, IHC analysis of CREB expression and phosphorylation within the tumors harvested from the limiting dilution found no difference in nuclear translocation of phospho-CREB or expression of CREB between SKOV3 and SKOV3-BP5 xenografts (Figures S8B and S8C). We postulate that this could again be due to the late stage at which the tumors were harvested, at which point the initial pro-survival response is no longer required and more proliferative phenotypes constitute the bulk of the tumor.
To demonstrate the functional importance of CREB signaling in tumorigenic properties of cells with elevated expression of IGFBP5, we utilized a selective CREB inhibitor, 666-15. We found that the lowest concentration of 666-15 tested caused a 25% loss in cell viability in SKOV3-BP5 while the same dose caused only a 7.5% decrease in SKOV3 cells, indicating that IGFBP5 expressing cells are more sensitive to CREB inhibition (Figure S8D). We next treated SKOV3 and SKOV3-BP5 cells with 666-15 and subjected them to our cell viability and adhesion assays in serum-free conditions. Indeed, when SKOV3-BP5 cells are treated with 250 nM 666-15, the increase in cell viability observed after a week of nutrient deprivation in SFM (relative to SKOV3 parental cells) is lost (Figure 6E). We also observed a decrease in cell viability in parental SKOV3 cells treated with 666-15, indicating that CREB signaling is important for cell survival during nutrient deprivation even when IGFBP5 is low. Finally, we repeated our adhesion assay in SFM in the presence of 666-15 or a vehicle control and found that inhibiting CREB in SKOV3-BP5 cells eliminated their adhesion phenotype (Figure 6F), strongly indicating that CREB activity drives IGFBP5-mediated survival under cellular stress (Figure 7).
Figure 7.
Preadipocyte-stimulated IGFBP5 expression activates CREB signaling and pro-survival phenotypes
Discussion
In this study, we provide evidence that IGFBP5, a preadipocyte-induced gene, is sufficient to activate an adaptive response enabling cancer cells to overcome stress experienced during tumor initiation and/or metastatic growth, specifically isolation stress and nutrient deprivation. Overexpression of IGFBP5 increased cell adhesion, proliferation, and viability at low densities in serum-free conditions in a CREB-dependent manner, with the highest activation of CREB occurring in conditions that combine nutrient stress and IGFBP5 overexpression. Increasing expression of IGFBP5 in cancer cell lines was also sufficient to facilitate tumor formation in the lowest cell dose of a limiting dilution assay.
IGFBP5 is reportedly pro-tumorigenic in breast and pancreatic cancers as well as glioblastoma,15,20,21,22 however, its actions in cancer appear to be highly context or tumor type specific. In Hs578 T breast cancer cells, IGFBP5 enhanced cell attachment in the presence of fibronectin, and this attachment was necessary for IGFBP5 to influence cell survival.23 Additionally, in patients with breast carcinomas, cytoplasmic IGFBP5 is increased in tumors with lymph node involvement.24 Other studies describe IGFBP5 as a tumor suppressor, including studies in melanoma and ovarian cancer.25,26 Some of this activity is restricted to specific regions of IGFBP5, and it was shown that overexpression of the truncated C-terminal region of IGFBP5 inhibited tumor growth by 2774 ovarian cancer cells in a subcutaneous xenograft by decreasing VEGF expression and reducing angiogenesis.27
While IGFBP5 can be tumor suppressive in certain contexts, we have yet to reconcile why increased expression of IGFBP5, a potential tumor suppressor, is associated with decreased overall survival and disease-free survival, a phenomenon which may be related to primary versus metastatic disease.14 Of particular relevance, a recent study in glioblastoma reported that IGFBP5 was enriched in invasive subtypes relative to non-invasive subtypes in vivo.22 Furthermore, invasiveness was decreased with IGFBP5 knockdown and enhanced by overexpression using both in vitro and in vivo models, including patient-derived xenografts. While this indicates a tumor supportive role in glioblastoma, and other reports in ovarian cancer indicate a tumor suppressive role, these findings nevertheless highlight a need to better understand the implications of increased expression of IGFBP5 during tumor cell dissemination. Importantly, using an unbiased phospho-kinase array, we found that our IGFBP5 cells are increasing CREB signaling, as was shown in more invasive BP5-high tumors in glioblastoma. Furthermore, our data showing that IGFBP5 overexpression had a slight tumor-suppressive effect on the highest dose of the limiting dilution while being starkly tumor-promoting in the lowest dilution suggests that the pro-tumorigenic features of IGFBP5 might be limited to periods of stress adaptation such as dissemination.
While our phospho-kinase array strongly implicated CREB signaling, there were several notable differences in other kinases for future investigation. Glycogen synthase kinase 3 (GSK3) phosphorylation was increased at both serine 9 and 21, suggesting that IGFBP5 may also affect glucose metabolism, perhaps due to the overlapping actions of insulin and IGF signaling in mitochondrial membrane potential.28 As our study was initially driven by an interest in understanding why IGFBP5 transcripts are elevated in metastatic ovarian cancer, it was encouraging to find that IGFBP5 activates CREB in glioblastoma to drive an aggressive and invasive metastatic phenotype.22 Importantly, that study used nanocapsules to silence IGFBP5 in vivo in an orthotopic model to improve overall survival, thus providing evidence that IGFBP5 could be a viable therapeutic target. Given our findings, IGFBP5 may offer a novel therapeutic strategy in ovarian cancer.
Though IGFBP5 upregulation is clearly sufficient to allow tumor initiation in otherwise impermissible conditions, it is not yet known whether this mechanism is occurring in an IGF-dependent or -independent manner. Expression of the IGFBP family is regulated in a coordinated manner to respond to fluctuating levels of IGF ligands,29 and it remains unclear how IGFBP5 upregulation impacts other members of the IGFBP family, as well as members of the signaling cascade downstream of the IGF-1R. Canonically, when IGF binds to a dimerized IGF1R, it activates PI3K/AKT. However, CREB is also a putative downstream target of the IGF-1R via alternative activation of MAPK and p38 MAPK.30 Further investigation is needed to better understand the mechanism(s) by which IGF-mediated IGFBP5 activity increases tumorigenic potential.
Alternatively, IGF-independent activity of IGFBP5 has been tied to both expression of extracellular matrix proteins and an adhesive phenotype in breast cancer, suggesting the possibility of an IGF-independent role for IGFBP5 in ovarian cancer.20 Cell adhesion is highly contextual during dissemination of metastatic cells and requires plasticity between epithelial and mesenchymal phenotypes during EMT and mesenchymal to epithelial transition (MET).31 Surface proteins that encourage cell-cell interactions in epithelia are downregulated to enable cells to shed from the primary tumor. A subsequent mesenchymal phenotype enables motility and formation of multicellular spheroids that can survive dissemination routes. Re-expression of surface proteins during MET allows metastatic cells to latch onto and colonize distant tissues.32
IGFBP5 was highly correlated with EMT, angiogenesis and hypoxia gene signatures in a pan-cancer analysis of the IGFBPs, with greater significance observed for ovarian cancer in EMT over the other two biological pathways.13 The same study found that exogenously administering IGFBP5 to SKOV3 cells decreased E-cadherin while increasing N-cadherin, and increased migration and invasion in conditions with limited serum, supporting a pro-metastatic role for IGFBP5 in nutrient deprived cells. Our results showing a decrease in both CHD1 and CHD2 gene expression in SKOV3-BP5 cells suggest differences in phenotypes resulting from IGFBP5 activity in cancer cells, which may be IGF-independent, relative to paracrine effects of IGFBP5 stimulation, which may be IGF-dependent. Alternatively, IGFBP5 activity in cancer cells may be induced by IGF stimulation, which could be inhibited by the presence of exogenous IGFBP5. These questions remain unclear and require further investigation. Either way, these findings suggest a role for IGFBP5 in metastatic properties of ovarian cancer cells.
Limiting dilution assays are employed to test long-term self-renewal and tumor initiation, typical of CSCs. Our limiting dilution and colony forming assays, combined with increased expression of stem cell transcription factors suggests that IGFBP5 supports a tumorigenic phenotype. The increase in adhesion may indicate the cells are moving from EMT to MET. Recent discussions examining the nuances of shifting from EMT to MET have described the possibility of a condition termed “partial MET” or “MET-independent metastasis,” in which cells enter a post-EMT state fixed in a proliferationhigh/invasionhigh phenotype, which is more aggressive and STEM-like.33 We found that IGFBP5 is important for ovarian cancer cell viability in conditions of isolation or nutrient stress, and while it downregulates most markers of EMT, it upregulates markers of CSCs as well as adhesion potential. These findings suggest IGFBP5 supports a transitory phenotype potentially critical at the end of the dissemination route where colonization of distant tissue begins, and nutrient and isolation stress are common. Given the propensity for ovarian cancer cells to metastasize to adipose tissue and the higher expression of IGFBP5 In metastatic sites relative to primary sites, it is plausible that preadipocyte-derived factors, such as IGF, enhance IGFBP5 expression in these disseminating cells to ensure successful metastatic colonization.
To our knowledge, this study is the first to report a direct link between IGFBP5 and signal transducer and activator of transcriptions 6 (STAT6) in cancer. The primary function of STAT6 is in the polarization of M2 macrophages via activation of JAK/STAT signaling by IL-4 and IL-13,34 a process which our lab and others have shown is a critical determinant of CSC maintenance, disease progression, and chemotherapeutic response.35 STAT6 also drives Th2 polarization,36 further supporting a potential role for IGFBP5 in immune-regulation in the tumor microenvironment. However, these properties are intrinsic to expression by immune cells and are thus difficult to interpret in the context of cancer cells with decreased STAT6 activity. As of 2006 there were approximately 35 loci classified as “STAT6 regulated,” which included the adhesion molecules E-selectin, P-selectin, and β3 integrin.37,38 Integrin β3 is a robust driver of the TIC/cancer-stem-like cell phenotype across cancer types,39 and thus further investigation could explore whether IGFBP5 and STAT6 cooperatively manage expression of ITGB3.
In conclusion, we describe a pro-metastatic role for IGFBP5 in ovarian cancer which is induced in response to secreted factors from preadipocytes, an intermediate resident adipose stem cell of the omentum. Overexpression of IGFBP5 models the increased IGFBP5 observed in TMN plotter and promotes numerous phenotypes associated with TICs, increases phosphorylation of CREB, and is sufficient to drive tumor initiation in low dilutions of SKOV3 cells. Targeting CREB signaling with the small molecular inhibitor 666-15 or through downregulation of IGFBP5 reverses several of these pro-survival phenotypes. Taken together, our findings demonstrate a critical role for IGFBP5 in ovarian cancer, highlighting a tumorigenic function induced by adipose tissue.
Resource availability
Lead contact
Requests for further information and resources or reagents should be directed to the lead contact, Carrie D. House, at cdhouse@sdsu.edu.
Materials availability
This study did not generate new unique reagents.
Data and code availability
Limitations of the study
A limitation of our study is the use of subcutaneous xenografting for in vivo studies, given that ovarian cancer is primarily confined to the peritoneum. This model, however, is ideal for measuring tumor-initiation capacity of low tumor cell dilutions, as in this study, and permitted investigation of preadipocytes in this process. Given our data suggesting IGFBP5 drives metastatic colonization, we will investigate IGFBP5 in orthotopic models in future studies.
Acknowledgments
This work was funded by the National Institute on Minority Health and Health Disparities of the NIH under award number U54MD012397. Additional funding was provided by the National Cancer Institute of the NIH under award number R01CA260281 and the Conrad Prebys Foundation. Fellowship support to J.A. Waters was provided by the Rees-Stealy Research Foundation and the Achievement Rewards for College Scientists. During the development of this study, C.D.H. was a Transdisciplinary Research in Energetics and Cancer (TREC) fellow, a program supported by the National Cancer Institute under award number R25CA203650. R.M.C. was supported by grants U54CA285115 and U54CA285117 from the NIH to SDSU and UCSD. We thank Larkin Slater of the SDSU vivarium, Lexi Pero and Dishant Vandra for their assistance with mouse studies, the SDSU FACS core facility for their excellent assistance with flow cytometry experiments, Natalie Gude for her assistance with histology studies, and Steven Johnson for the pcDNA-V5-IGFBP5 construct.
Author contributions
Conceptualization, J.A.W., R.M.C., M.R., and C.D.H.; methodology J.A.W., R.M.C., M.R., and C.D.H.; formal analysis J.A.W., C.L., S.H., G.J.J., I.U., S.F.G., M.R., and C.D.H.; investigation J.A.W., C.L., S.H., G.J.J., I.U., S.F.G., M.R., and C.D.H.; writing—original draft J.A.W. and C.D.H.; writing—review and editing J.A.W., C.L., S.H., G.J.J., I.U., S.F.G., M.R., and C.D.H.; visualization, J.A.W. and M.R., supervision, C.D.H.
Declaration of interests
Senior author, C.D.H., has received research funds from Vaxiion Therapeutics for studies unrelated to the submitted work.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| IGFBP5, polyclonal rabbit | Proteintech | Cat# 55205-1-AP; RRID: AB_2736835; IHC 1:500 |
| IGFBP5, polyclonal rabbit | Cell Signaling | Cat# 10941; RRID: AB_3698836; Immunoblot 1:1000 |
| GAPDH, monoclonal mouse | Millipore | Cat# MAB374; RRID: AB_2107445; Immunoblot 1:10000 |
| Lamin A/C, monoclonal mouse | Millipore | Cat# MABT538; RRID: AB_3698837; Immunoblot 1:1000 |
| α-Tubulin, monoclonal mouse | Santa Cruz Biotechnology | Cat# sc-5286; RRID: AB_628411; Immunoblot 1:1000 |
| Phospho-CREB (Ser133), monoclonal rabbit | Cell Signaling | Cat# 9198; RRID: AB_2561044; Immunoblot 1:1000 |
| CREB, monoclonal rabbit | Cell Signaling | Cat# 9197; RRID: AB_331277; Immunoblot 1:1000 |
| Phospho-FAK (Y576), polyclonal rabbit | Abcam | Cat# ab226847; RRID: AB_3698838; Immunoblot 1:1000 |
| FAK, monoclonal mouse | Millipore | Cat# 05-537; RRID: AB_2173817; Immunoblot 1:1000 |
| Anti-Rb-IgG-HRP | Cell Signaling | Cat# 7074; RRID: AB_2099233; Immunoblot 1:2000 |
| Anti-Ms-IgG-HRP | Cell Signaling | Cat# 7076; RRID: AB_330924; Immunoblot 1:2000 |
| Cell Signaling Technology SignalStain Boost IHC Detection Reagent (HRP, Rabbit) | VECTOR | 50-195-930, IHC |
| Integrin αVΒ3 Mouse anti-Human, FITC | Millipore | Cat# FCMAB282FMI; RRID: AB_10807959; Flow Cytometry |
| Bacterial and virus strains | ||
| JM109 Competent Cells | Promega | L2005 |
| Lentiviral sgIGFBP5 #1, DNA Target Sequence: GTGTTTGGGCCGGAAGATCT | Horizon Discovery | VSGH10143-246471744 |
| Lentiviral sgIGFBP5 #2, DNA Target Sequence: GCAGGGCTCGCAGTGCACGA | Horizon Discovery | VSGH10143-246471746 |
| Lentiviral sgIGFBP5 #3, DNA Target Sequence: GGTGGGCTCCTCGTGCTCAC | Horizon Discovery | VSGH10143-246471749 |
| Lentiviral Non-targeting Control #1 | Horizon Discovery | VSGC10215 |
| Lentiviral Non-targeting Control #2 | Horizon Discovery | VSGC10216 |
| Lentiviral Non-targeting Control #3 | Horizon Discovery | VSGC10217 |
| Lentiviral hEF1aBlast-Cas9 Nuclease Particles | Horizon Discovery | VCAS10126 |
| Biological samples | ||
| Ovary Tumor Array | Tissue Array LLC | OV722 |
| Deidentified patient samples | NCI Cooperative Human Tissue Network | 85919, 85934, 85926, 85929 |
| Chemicals, peptides, and recombinant proteins | ||
| Phalloidin-iFluor 647 Reagent | Abcam | Ab176759, 1:1000 |
| Hoechst 33342 | Cell Signaling | #4082, 5 μg/mL |
| Crystal Violet | Sigma | C0775, 0.5% solution in 25% methanol |
| Recombinant human IGFBP5 | R&D Systems | 875-BP5 |
| 666-15 (CREB inhibitor) | MedChemExpress | HY-101120 |
| Critical commercial assays | ||
| CellTiter-Glo® 2.0 Cell Viability Assay | Promega | G9242 |
| High-Capacity cDNA Reverse Transcription Kit | ThermoFisher | 4368814 |
| TaqMan™ Fast Advanced Master Mix for qPCR | ThermoFisher | 4444556 |
| Caspase-Glo 3/7 Assay | Promega | G8090 |
| Proteome Profiler Human Phospho-Kinase Array Kit | R&D Systems | ARY003C |
| Super Signal West Pico Chemiluminescent Substrate kit | ThermoFisher | PI34080 |
| NucleoSpin RNA, Mini kit for RNA purification | Macherey-Nagel | 740955.50 |
| Direct-zol RNA Miniprep Kits | Zymo Research | R2050 |
| Human IGFBP-5 DuoSet ELISA | R&D Systems | DY875 |
| Pierce™ Rapid Gold BCA Protein Assay Kit | ThermoFisher | A53225 |
| Vector Laboratories DAB Peroxidase (HRP) Substrate Kit (With Nickel), 3,3′-Diaminobenzidine 300 ml | VECTOR | SK4100 |
| Click-iT EdU Alexa Fluor 647 Imaging Kit | ThermoFisher | C10340 |
| DharmaFECT 1 Transfection Reagent | Horizon Discovery | T-2001-01 |
| Opti-MEM™ I Reduced Serum Medium, 100 mL | ThermoFisher | 31985062 |
| Lipofectamine™ 3000 Transfection Reagent | ThermoFisher | L3000015 |
| TaqMan Gene Expression Assay: GAPDH | ThermoFisher | 402869 |
| TaqMan Gene Expression Assay: IGFBP5 | ThermoFisher | Hs00181213_m1 |
| TaqMan Gene Expression Assay: CDH1 | ThermoFisher | Hs01023895_m1 |
| TaqMan Gene Expression Assay: CDH2 | ThermoFisher | Hs00983056_m1 |
| TaqMan Gene Expression Assay: VIM | ThermoFisher | Hs00185584_m1 |
| TaqMan Gene Expression Assay: PTK2 | ThermoFisher | Hs01056457_m1 |
| TaqMan Gene Expression Assay: SOX2 | ThermoFisher | Hs01053049_s1 |
| TaqMan Gene Expression Assay: OCT4 | ThermoFisher | Hs04260367_gH |
| TaqMan Gene Expression Assay: NANOG | ThermoFisher | Hs02387400_g1 |
| Deposited data | ||
| RNA sequencing of co-cultures between ACI-23 and preadipocytes/adipocytes | Waters et al.9 | GSE262869 |
| WashU Primary and Metastatic Tumors with Survival in High-Grade Serous Ovarian Cancer RNA-seq | Kotnik et al. | GSE218939 |
| Experimental models: Cell lines | ||
| Human: ACI-23 | NCI, Annunziata lab | CVCL_N830 |
| Human: CAOV4 | NCI, Annunziata lab | CVCL_0202 |
| Human: OVCAR8 | NCI, Annunziata lab | CVCL_1629 |
| Human: SKOV3 | NCI, Annunziata lab | CVCL_0532 |
| Cryopreserved, Omental (1 million cells/vial), BMI 25.0-29.9 | ZenBio | OP-F-2 |
| Cryopreserved, Omental (1 million cells/vial), (POOLED Donor Lot) | ZenBio | OP-F-SL |
| Experimental models: Organisms/strains | ||
| Nu/J | JAX | 002019 |
| Oligonucleotides | ||
| ON-TARGETplus Non-targeting Pool: UGGUUUACAUGUCGACUAA, UGGUUUACAUGUUGUGUGA, UGGUUUACAUGUUUUCUGA, UGGUUUACAUGUUUUCCUA | Horizon Discovery | D-001819-10-20 |
| ON-TARGETplus Human IGFBP5: CGCAAAGGAUUCUACAAGA, GAAAGAAGCUGACCCAGUC, GAUCAUCUCUGCACCUGAG, GCAGGGAACGCAUGAUUCA | Horizon Discovery | 3488 |
| IGFBP5 Exon 1 Fwd: 5’ – CAG CCC TCC ACC TCT CTC TAC – 3’ | IDT | 100 nmole DNA Oligo |
| IGFBP5 Exon 1 Rev: 5’ – GCT CAC CGA TCT TGA CTT GCT C – 3’ | IDT | 100 nmole DNA Oligo |
| IGFBP5 Exon 2 Fwd: 5’ – TCT GCT TCC TGG TTC TTA TCC C – 3’ | IDT | 100 nmole DNA Oligo |
| IGFBP5 Exon 2 Rev: 5’ – GCT CTG GAA ACT CTA CTC CCA C – 3’ | IDT | 100 nmole DNA Oligo |
| Recombinant DNA | ||
| pcDNA3-IGFBP5-V5 | Steven Johnson, Addgene | 11608 |
| pGEM®-T Easy Vector | Promega | A1360 |
| Software and algorithms | ||
| R | CRAN | https://cran.r-project.org/ |
| Bioconductor | N/A | https://www.bioconductor.org/ |
| Corrplot | CRAN | https://cran.r-project.org/web/packages/corrplot/index.html |
| ImageJ | NIH | https://imagej.nih.gov/ij/download.html |
| HALO | Indica labs | N/A |
| BioRender | BioRender | https://biorender.com/ |
| TNMplot | Bartha et al.16 | https://tnmplot.com/analysis/ |
| ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays | Hu et al.19 | https://pubmed.ncbi.nlm.nih.gov/19567251/ |
| CellSens | Evident Scientific | https://evidentscientific.com/en/products/software/cellsens |
| Quantstudio 3/5 Design and Analysis Software | ThermoFisher | DA2 |
| iBright Analysis Software | ThermoFisher | https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-assays-analysis/western-blotting/detect-proteins-western-blot/western-blot-imaging-analysis/ibright-systems/software.html |
| Other | ||
| BD FACSMelody Cell Sorter | BD Biosciences | https://www.bdbiosciences.com/en-us/products/instruments/flow-cytometers/research-cell-sorters/bd-facsmelody |
| iBright CL1000 Imaging System | ThermoFisher | https://www.thermofisher.com/us/en/home/references/newsletters-and-journals/bioprobes-journal-of-cell-biology-applications/bioprobes-76/ibright-imaging-systems-western-blot-analysis.html |
| QuantStudio 3 Real-Time PCR System | ThermoFisher | https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-instruments/quantstudio-systems/models/quantstudio-3-5.html |
| ImageXpress Pico Automated Cell Imaging System | Molecular Devices | https://www.moleculardevices.com/products/cellular-imaging-systems/high-content-imaging/imagexpress-pico |
| Zeiss Primo Star HAL/LED Microscope | Zeiss | https://mms.mckesson.com/product/971523/FISHER-HEALTHCAR-12171480 |
| SpectraMax iD3 | Molecular Devices | https://info.moleculardevices.com/ppc/br/one-spectramax-endless-discoveries-na-bofu |
Experimental model and study participant details
CAOV4 (CVCL_0202), OVCAR8 (CVCL_1629), SKOV3 (CVCL_0532), and ACI-23 (CVCL_N830) ovarian cancer cell lines were provided by the National Cancer Institute (NCI) via Materials Transfer Agreement (MTA). Cells were authenticated via Short Tandem Repeat Analysis prior to distribution and are tested for mycoplasma quarterly. Primary human female omental preadipocytes were purchased from ZenBio, Inc. and were tested for mycoplasma before use. Female Nu/J immunocompromised mice were purchased from Jackson Laboratories at 5 weeks of age and used for studies at 6-8 weeks of age.
Method details
Cell lines, culture conditions and reagents
CAOV4 (HTB-76), OVCAR8 (CVCL_1629), SKOV3 (HTB-77) and ACI-23 were obtained from the NCI via MTA. and maintained in RPMI medium supplemented with 10% FBS and 1% penicillin–streptomycin (Gibco) at 37°C and 5% CO2. The ACI-23 cell line was maintained in Dulbecco Modified Eagle Nutrient Mixture F-12 (DMEM/F12) medium (Gibco) supplemented with 10% FBS and 1% penicillin–streptomycin (Gibco) at 37°C/5%CO2. Human omental preadipocytes were purchased from ZenBio Inc. and maintained in omental preadipocyte media (OM-PM, ZenBio). Cells were maintained and differentiated into mature adipocytes as instructed by the suppliers. Briefly, cells were differentiated by culturing in Omental Adipocyte Differentiation Medium (OM-DM, ZenBio) for 7 days, after which half the volume was removed and replaced with Omental Adipocyte Maintenance Medium (OM-AM, ZenBio). Differentiated cells were used immediately for experiments after the 2-week differentiation protocol was complete. Preadipocyte co-cultures were performed by seeding 2x104 preadipocytes in OM-PM into 0.4 μM polycarbonate inserts (Corning) in a 6-well plate and subsequently seeding 500 cancer cells in the bottom compartment in complete DMEM, after which cells were co-cultured for 72hr. Preadipocyte-conditioned media (CM) was made by washing preadipocytes with PBS to remove residual serum and replenishing with low-volume SFM for 48hr. CM was spun at 3000 rpm for 10 minutes and aliquots were stored at -20°C. When applicable, cells were cultured in a serum-free media (SFM) consisting of DMEM/F12, 0.1% bovine serum albumin (BSA), and 1% penicillin–streptomycin. A selective CREB inhibitor, 666-15 (MedChemExpress), was purchased and used at a concentration of 250 nM. All cells were routinely tested for mycoplasma.
In vivo xenograft studies and ethical approval
All procedures and animal protocols were approved by the IACUC committee at San Diego State University on protocol 21-05-003H or IACUC-24-054 and performed in the AALAC accredited SDSU vivarium. Nu/J female mice were purchased from the Jackson Laboratory and were housed in HEPA ventilated cages on a 12-hour light-dark cycle. In vivo work was performed by trained technicians.
For the preadipocyte-stimulated tumors, 500 ACI-23 cells (sgNEG and sgBP5) were injected subcutaneously with or without 2x105 omental preadipocytes (1:40 cancer cell to preadipocyte ratio) in 200 μL 1:1 Matrigel and PBS as previously described.9 The limiting dilution with SKOV3 and SKOV3-BP5 was performed by subcutaneously injecting 1x105, 1x104, or 1x103 cells in 200 μL of 1:1 Matrigel and PBS into the left flank of 8-week-old female athymic Nu/J mice. Mice were weighed and tumors measured twice weekly. Mice were sacrificed once tumors reached 20 mm length or after 100 days without tumor formation. Tumors were divided into portions for RNA and protein which were snap-frozen in LN2, and a portion was fixed in formalin for immunohistochemistry.
RNA extraction and qRT-PCR
RNA was isolated using the NucleoSpin RNA mini kit for cultured cells (Macherey-Nagel) and the Direct-zol kit for tumors (Zymo). RNA generally comprising of 200–1000 ng was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher). qRT-PCR was performed using TaqMan Assays (ThermoFisher) for IGFBP5 (Hs00181213_m1), SOX2 (Hs01053049_s1), OCT4 (Hs04260367_gH), NANOG (Hs02387400_g1), CDH1 (Hs01023895_m1), CDH2 (Hs00983056_m1), VIM (Hs00185584_m1), and PTK2 (Hs01056457_m1), and GAPDH (402869) using TaqMan Fast Advanced Master Mix. Relative expression is reported as ddCT and calculated by first relativizing target genes to GAPDH as an endogenous control gene and then normalizing to a control sample.
Enzyme-linked immunosorbent assay
IGFBP5 was measured using human IGFBP5 DuoSet sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems) per the manufacturer’s instruction using media harvested from specified conditions at a 1:2 dilution in reagent diluent.
Immunoblotting
Cells were washed twice with cold PBS and lysed in RIPA with 1X Halt protease/phosphatase inhibitor cocktail (ThermoFisher). Protein concentration was determined using Rapid Gold BCA Protein Assay (ThermoFisher). Samples generally comprising 10–30 μg of protein were mixed with 1X bond-breaker TCEP solution (ThermoFisher) and 1X NuPAGE LDS sample buffer (ThermoFisher), boiled for 5 minutes at 95°C, then subjected to SDS-PAGE using 4-12% Bis-Tris gels with MOPS running buffer followed by transfer to a PVDF membrane for immunoblotting analysis. Membranes were blocked in 5% milk or 1% BSA for 1 hour at RT, and primary antibodies were incubated overnight at 4°C as described in the Key resources table. Secondary antibodies were incubated for 1 hour at RT. Blots were imaged using an iBright CL1000 Imaging System. Densitometry analysis was performed using iBright Analysis Software. All blots shown as a single figure were stripped with OneMinute® Advance WB Stripping Buffer (GM Biosciences) and re-probed for additional proteins up to 4 times with phosphorylated proteins probed first and the loading control performed last.
Spheroid formation
Spheroid formation was measured by plating cells in complete media at a density ranging from 0-20 cells/well in 96-well non-treated flat-bottom plates. Cells were grown for 5 days prior staining with Hoechst (5ug/ml) and imaging on a Pico ImageXpress (Molecular Devices). Spheroids were defined as having a size between 30-400 mm. Spheroid frequency was defined as (# of spheroids formed)/(# of cells plated per well).
Extreme Limiting Dilution Assay (ELDA)
The ELDA was completed stepwise from 20 cells/well serially diluted down to 1 cell/well in ultra-low attachment round bottom 96-well plates in SCM (Corning).40 SCM is DMEM:F12 with L-glutamine and 15 mmol/L HEPES (Gibco) supplemented with 1% penicillin/streptomycin, 1% KnockOut serum replacement (Gibco), 0.4% BSA, and 0.1% insulin transferrin-selenium (Gibco), and supplemented with fresh human recombinant epidermal growth factor (EGF, Sigma) and basic fibroblast growth factor (FGF, Sigma) every 2 to 3 days for a final concentration of 20 and 10 ng/mL, respectively). Spheroids were allowed to grow for 5 days before being stained with Hoechst 33342 (ThermoFisher). Spheroids were then imaged using the ImageXpress Pico (Molecular Devices). CSC frequency was calculated by counting how many cells plated formed a spheroid and statistically assessed by using the ELDA software developed by Hu et al.19, which calculates and compares the active stem cell frequency between treatment groups. Spheroids were defined as having a size between 30 and 400 mm.
Colony formation
Colony formation by 500 cells was measured after 7 days in complete media (RPMI-1640 or DMEM-F12) in a 6-well plate. Cells were washed with PBS and stained with 0.5% crystal violet for 10 minutes at RT, then washed until all residual crystal violet was removed. Plates were dried overnight, imaged with a high-content scanner and quantified by solubilizing the crystal violet with 300ul of 10% acetic acid/40% DI water/50% ethanol. 100ul was transferred in duplicate to a 96 well black plate and absorbance was measured at 590 nm.
Cell viability assay
Thousand cells were seeded into 96-well, white-wall clear-bottom plates and grown under specified conditions. Viability was assessed by adding 100 μl CellTiter-Glo 2.0 (Promega) followed by 10 min on a rotary shaker and reading luminescence on a SpectraMax iD3 plate reader (Molecular Devices).
Caspase 3/7 apoptosis assay
1000 cells were seeded into 96-well, white-wall clear-bottom plates and allowed to adhere overnight in complete media. Cells were then starved in SFM for 4-6 hours. SFM was aspirated and replaced with either fresh SFM or CM. Cells were cultured for 3/7 days and apoptosis was measured using the Caspase-Glo 3/7 Assay (Promega) per manufacturer’s instructions. Plates designated for a 7-day experiment had their media replenished on day 3. Luminescence was measured on a SpectraMax iD3 plate reader.
Phospho-kinase array
The Proteome Profiler Human Phospho-Kinase Array Kit (R&D Systems) was used according to the manufacturer’s instructions, and blots were imaged using an iBright CL1000 Imaging System.
Immunohistochemistry
Tumors were resected, fixed in 10% neutral buffered formalin, and stored in 70% ethanol. Tumors were embedded in paraffin and sectioned at 5 μm, air dried at room temperature overnight, and baked at 60°C for 1 hour. Antigen retrieval was performed in citrate buffer and quenched with hydrogen peroxide. Slides were incubated with primary antibodies overnight at 4°C followed by an HRP-linked secondary for 1 hour at room temperature and processed using DAB (Vector, SK-4100). Slides were scanned using an Aperio Scanscope FL (Leica Biosystems). Image analysis was done using semiautomated image analysis software to quantify digital H-scores for nuclear phospho-CREB and cellular total CREB (HALO, Indica Labs).
CRISPR
Cells were transduced with Edit-R Lentiviral hEF1aBlast-Cas9 Nuclease Particles (Horizon) at an MOI = 0.3 for 48 hr according to the manufacturer’s instructions and selected with 20ug/mL blasticidin. sgIGFBP5 cells were then transduced with three Edit-R Human Lentiviral sgRNAs targeting IGFBP5 (Horizon) in the first and second exons at an MOI = 0.3 for 48 hours and selected with 2 μg/mL puromycin. Control cells were transduced with three non-targeting sgRNAs (Horizon) at an MOI = 0.3 for 48 hours. sgIGFBP5 and sgNEG cells were selected with 2ug/mL puromycin. On-target editing was confirmed via western blotting and individual allelic mutations were profiled by cloning DNA isolated from sgIGFBP5 cells into a pGEM-T easy backbone (Promega), miniprepping individual colonies (Qiagen) and performing sanger sequencing.
IGFBP5 overexpression
pcDNA3-IGFBP5-V5 was a gift from Steven Johnson (Addgene plasmid #11608). SKOV3 cells were transfected with pcDNA3-IGFBP5-V5 using Lipofectamine 3000 (ThermoFisher) and positively selected with 0.5 mg/mL G418 for 2 weeks.
Proliferation assay
Proliferation was measured via Click-iT EdU Assay (ThermoFisher) in cells grown either in SFM or complete media. Cells were grown in 0.3umol/L EdU prior to fixing in 10% neutral buffered formalin for 10 minutes at RT and blocking using 3% BSA with 0.2% Triton-X in PBS. The Click-iT Assay with Alexa Fluor 647 was performed according to the manufacturer’s instructions. Cells were washed and counterstained with 5ug/mL Hoechst, then imaged using a Pico ImageXpress (Molecular Devices).
siRNA transfections
Cells were cultured to a confluency of 60% to 70%; 30-nmol/L pools of four siRNAs (Horizon) targeting IGFBP5 (siIGFBP5) or a nontargeting control (siNEG) were transfected at a concentration of 30 nM using Lipofectamine RNAiMAX (ThermoFisher) for 24hr per the manufacturer’s instructions. Gene silencing was confirmed via qRT-PCR.
Adhesion assay
Single cell suspensions were generated using Cellstripper (Corning) and plated at a density of 1 × 103 cells/well in serum free media for 24, 48 and 72 h at 37°C. Non-attached cells were aspirated, followed by two washes in PBS and adherent cells were then fixed with 4% PFA (methanol free) for 10 minutes at room temperature and washed twice with PBS. For quantification of cell attachment, fixed cells were permeabilized and blocked with 0.2% Triton X-100 and 3% BSA in PBS for 30 minutes at room temperature then washed twice with PBS. Permeabilized cells were incubated with 5 ug/ml Hoechst and 10 ug/ml Phalloidin-AF647 in 0.02% Triton X-100 and 0.3% BSA in PBS at 4°C overnight. The following day, plates were washed twice with PBS before imaging in an ImageXpress.
Public databases
All analyses of TCGA data were performed in R, version 4.4.2 (https://www.r-project.org/). TCGA data from GDC was downloaded using Bioconductor package TCGAbiolinks,41 the package SummarizedExperiment was used to store ovarian tumor data. For consistency, we converted all FPKM gene expression data to TPM data using the equation described by Collins et al.42 IGFBP5 expression was correlated with CREB signaling genes contained in BIOCARTA_CREB_PATHWAY (https://www.gsea-msigdb.org) using corrplot. The Bioconductor package curatedOvarianData (n=2970) was utilized to generate a forest plot of hazard ratio and IGFBP5 expression, and the P-value was calculated according to a fixed-effects model. Primary data from ovarian and omental tumors was downloaded from GEO (GSE218939) as normalized TPM and compared to IGFBP5 via Pearson correlation. Tumor, normal and metastasis differential gene expression analysis was performed by accessing and analyzing IGFBP5 chip data in TNMplot (https://tnmplot.com/analysis/) and results were visualized in GraphPad. Correlations between IGFBP5 and other IGF signaling components were performed using GEPIA2 using OV Tumor and GTEx Ovary.
Quantification and statistical analysis
Data are expressed as mean ± S.D. unless otherwise noted. Exact numbers of biological replicates representing at least two technical replicates for each experiment are reported in the Figure Legends. p-values less than 0.05 were considered statistically significant based on the appropriate statistical test for the experiment in question. For all data, ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001, ∗∗∗∗p<0.0001. Data were analyzed using Prism Software 10.0 (GraphPad).
Published: June 28, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113034.
Supplemental information
References
- 1.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., Gaudet M.M., Jemal A., Siegel R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert M.A., Pan S., Hernandez K.M., Loth R.M., Andrade J., Volchenboum S.L., Faber P., Montag A., Lastra R., Peter M.E., et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6:1342–1351. doi: 10.1158/2159-8290.CD-16-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meza-Perez S., Randall T.D. Immunological functions of the omentum. Trends Immunol. 2017;38:526–536. doi: 10.1016/j.it.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai J., Tang H., Xu L., Wang X., Yang C., Ruan S., Guo J., Hu S., Wang Z. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis. 2012;33:20–29. doi: 10.1093/carcin/bgr230. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 7.Baxter R.C., Twigg S.M. Actions of IGF binding proteins and related proteins in adipose tissue. Trends Endocrinol. Metab. 2009;20:499–505. doi: 10.1016/j.tem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Smith P.J., Wise L.S., Berkowitz R., Wan C., Rubin C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988;263:9402–9408. [PubMed] [Google Scholar]
- 9.Waters J.A., Robinson M., Lujano-Olazaba O., Lucht C., Gilbert S.F., House C.D. Omental preadipocytes stimulate matrix remodeling and IGF signaling to support ovarian cancer metastasis. Cancer Res. 2024;84:2073–2089. doi: 10.1158/0008-5472.CAN-23-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters J.A., Urbano I., Robinson M., House C.D. Insulin-like growth factor binding protein 5: diverse roles in cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1052457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firth S.M., Baxter R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 12.Akkiprik M., Hu L., Sahin A., Hao X., Zhang W. The subcellular localization of IGFBP5 affects its cell growth and migration functions in breast cancer. BMC Cancer. 2009;9:103. doi: 10.1186/1471-2407-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan W., Zhang J., Deng Z., Dai F., Tang L., Hu W., Liu H. Pan-cancer analysis of oncogenic role of insulin-like growth factor-binding proteins and validation in ovarian cancer. Cancer Med. 2023;12:14833–14850. doi: 10.1002/cam4.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng R., Chen W., Xia W., Zheng J., Zhou Q. The prognostic values of the insulin-like growth factor binding protein family in ovarian cancer. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/7658782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong C., Zhang J., Fang S., Liu F. IGFBP5 increases cell invasion and inhibits cell proliferation by EMT and Akt signaling pathway in Glioblastoma multiforme cells. Cell Div. 2020;15 doi: 10.1186/s13008-020-00061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartha Á., Győrffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int. J. Mol. Sci. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butt A.J., Dickson K.A., McDougall F., Baxter R.C. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J. Biol. Chem. 2003;278:29676–29685. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- 18.Rapanotti M.C., Cugini E., Campione E., Di Raimondo C., Costanza G., Rossi P., Ferlosio A., Bernardini S., Orlandi A., De Luca A., Bianchi L. Epithelial-to-mesenchymal transition gene signature in circulating melanoma cells: biological and clinical relevance. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241411792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth G.K., Hu Y. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Sureshbabu A., Okajima H., Yamanaka D., Tonner E., Shastri S., Maycock J., Szymanowska M., Shand J., Takahashi S.I., Beattie J., et al. IGFBP5 induces cell adhesion, increases cell survival and inhibits cell migration in MCF-7 human breast cancer cells. J. Cell Sci. 2012;125:1693–1705. doi: 10.1242/jcs.092882. [DOI] [PubMed] [Google Scholar]
- 21.Ting D.T., Wittner B.S., Ligorio M., Vincent Jordan N., Shah A.M., Miyamoto D.T., Aceto N., Bersani F., Brannigan B.W., Xega K., et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W., Niu R., Park S.M., Zou Y., Kim S.S., Xia X., Xing S., Yang Q., Sun X., Yuan Z., et al. IGFBP5 is an ROR1 ligand promoting glioblastoma invasion via ROR1/HER2-CREB signaling axis. Nat. Commun. 2023;14:1578. doi: 10.1038/s41467-023-37306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaig C., Perks C.M., Holly J.M.P. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J. Cell Sci. 2002;115:4293–4303. doi: 10.1242/jcs.00097. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Arun B.K., Wang H., Fuller G.N., Zhang W., Middleton L.P., Sahin A.A. IGFBP2 and IGFBP5 overexpression correlates with the lymph node metastasis in T1 breast carcinomas. Breast J. 2008;14:261–267. doi: 10.1111/j.1524-4741.2008.00572.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Ding N., Li Y., Cheng H., Wang D., Yang Q., Deng Y., Yang Y., Li Y., Ruan X., et al. Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells. Oncotarget. 2015;6:20636–20649. doi: 10.18632/oncotarget.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rho S.B., Dong S.M., Kang S., Seo S.S., Yoo C.W., Lee D.O., Woo J.S., Park S.Y. Insulin-like growth factor-binding protein-5 (IGFBP-5) acts as a tumor suppressor by inhibiting angiogenesis. Carcinogenesis. 2008;29:2106–2111. doi: 10.1093/carcin/bgn206. [DOI] [PubMed] [Google Scholar]
- 27.Hwang J.R., Cho Y.J., Lee Y., Park Y., Han H.D., Ahn H.J., Lee J.H., Lee J.W. The C-terminus of IGFBP-5 suppresses tumor growth by inhibiting angiogenesis. Sci. Rep. 2016;6 doi: 10.1038/srep39334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiernan K., Alwarawrah Y., Nichols A.G., Danzaki K., MacIver N.J. Insulin and IGF-1 have both overlapping and distinct effects on CD4. Sci. Rep. 2024;14:4331. doi: 10.1038/s41598-024-54836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemmons D.R. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol. Metab. 2016;27:375–391. doi: 10.1016/j.tem.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W.H., Quirion R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006;7:51. doi: 10.1186/1471-2202-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jolly M.K., Ware K.E., Gilja S., Somarelli J.A., Levine H. EMT and MET: necessary or permissive for metastasis? Mol. Oncol. 2017;11:755–769. doi: 10.1002/1878-0261.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yousefi M., Dehghani S., Nosrati R., Ghanei M., Salmaninejad A., Rajaie S., Hasanzadeh M., Pasdar A. Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles. Cell. Oncol. 2020;43:515–538. doi: 10.1007/s13402-020-00513-9. [DOI] [PubMed] [Google Scholar]
- 33.Jolly M.K., Huang B., Lu M., Mani S.A., Levine H., Ben-Jacob E. Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. J. R. Soc. Interface. 2014;11 doi: 10.1098/rsif.2014.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong M., Zhuo X., Ma A. STAT6 upregulation promotes M2 macrophage polarization to suppress atherosclerosis. Med. Sci. Monit. Basic Res. 2017;23:240–249. doi: 10.12659/msmbr.904014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruz L.S., Robinson M., Stevenson D., Amador I.C., Jordan G.J., Valencia S., Navarrete C., House C.D. Chemotherapy enriches for proinflammatory macrophage phenotypes that support cancer stem-like cells and disease progression in ovarian cancer. Cancer Res. Commun. 2024;4:2638–2652. doi: 10.1158/2767-9764.CRC-24-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goenka S., Kaplan M.H. Transcriptional regulation by STAT6. Immunol. Res. 2011;50:87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHugh K.P., Kitazawa S., Teitelbaum S.L., Ross F.P. Cloning and characterization of the murine beta(3) integrin gene promoter: identification of an interleukin-4 responsive element and regulation by STAT-6. J. Cell. Biochem. 2001;81:320–332. doi: 10.1002/1097-4644(20010501)81:2<320::aid-jcb1047>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Hebenstreit D., Wirnsberger G., Horejs-Hoeck J., Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Seguin L., Kato S., Franovic A., Camargo M.F., Lesperance J., Elliott K.C., Yebra M., Mielgo A., Lowy A.M., Husain H., et al. An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson M., Gilbert S.F., Waters J.A., Lujano-Olazaba O., Lara J., Alexander L.J., Green S.E., Burkeen G.A., Patrus O., Sarwar Z., et al. Characterization of SOX2, OCT4 and NANOG in ovarian cancer tumor-initiating cells. Cancers (Basel) 2021;13 doi: 10.3390/cancers13020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colaprico A., Silva T.C., Olsen C., Garofano L., Cava C., Garolini D., Sabedot T.S., Malta T.M., Pagnotta S.M., Castiglioni I., et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44 doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.