Abstract
This study aimed to evaluate the effects of 4-hydroxyphenylacetic acid (4-HPAA), a phenolic acid compound from Yucca schidigera extract, in Caco-2 cells and artificial rearing of pigeons. In vitro experiments showed that 4-HPAA at different levels exhibited higher cell viability than shikimic acid and 3,4-dihydroxybenzoic acid, and with the highest viability observed at 600 μM. Additionally, 600 μM 4-HPAA alleviated LPS-induced upregulation of IL-1β and CASPASE1 expressions (P < 0.05). In vivo results demonstrated that supplemental 4-HPAA at 500, 1000, and 2000 mg/kg levels did not affect the body weight of 25-day-old pigeons (P > 0.05), but all levels increased immunoglobulin G (IgG) and IgM levels (P < 0.05). Compared with control, 2000 mg/kg 4-HPAA significantly reduced serum L-lactic acid, D-lactic acid and lactate dehydrogenase (LDH) activity (P < 0.05); increased spleen and bursa of Fabricius indices and intestinal total antioxidant capacity activity of 25-day-old pigeons (P < 0.05); and elevated ileal barrier-related genes (OCCLUDIN, CLAUDIN1 and MUC2) in 7-day-old pigeons, as well as jejunal MUC2 and ileal OCCLUDIN in 25-day-old meat pigeons (P < 0.05). It also inhibited glycolytic gene hexokinase 2 expression, while upregulating the signaling pathway genes CAMK2G and MKNK1 (P < 0.05). Supplemental 500 and 1000 mg/kg 4-HPAA significantly increased serum total cholesterol levels (P < 0.05). Notably, 500 mg/kg 4-HPAA had an adverse effect on intestinal integrity, as indicated by the highest serum diamine oxidase content, and increased glycolytic gene LDH-B expression; However, it enhanced microbial richness and diversity in the intestines of 7-day-old pigeon (P < 0.05). Besides, 1000 mg/kg 4-HPAA impaired intestinal morphology, as evidenced by a lower ileal villus height-to-crypt depth ratio (P < 0.05), but resulted in the highest abundance of Lactococcus in 25-day-old pigeons. In conclusion, 600 μM 4-HPAA exhibits anti-inflammatory activity in Caco-2 cells, and 2000 mg/kg 4-HPAA promotes immune function and intestinal barrier integrity while inhibiting glycolytic gene expression and the production of related serum metabolites in meat pigeons.
Keywords: Yucca schidigera extract, 4-hydroxyphenylacetic acid, Meat pigeon, Gut health
Introduction
Against the backdrop of the rapid global development of the meat pigeon industry, meat pigeons have garnered widespread attention due to their unique nutritional value and economic significance (Jin et al., 2023). Rich in high-quality protein, low in fat, and containing essential amino acids required by the human body, meat pigeons are considered a premium source of meat (Lan et al., 2025). As consumer demand for healthy and green food continues to rise, meat pigeon products show great market potential. However, the meat pigeon industry is facing several challenges. On one hand, the frequent occurrence of intestinal diseases significantly hampers the growth performance of meat pigeons and reduces farming efficiency, becoming a major bottleneck in the industry's development (Santos et al., 2020). On the other hand, restrictions on the use of traditional antibiotics have driven the need for safer and more effective alternatives that align with the market’s demand for environmentally friendly and sustainable farming practices (Du et al., 2022; Dai et al., 2025). Natural plants and their extracts-due to their abundant resources, wide availability, broad functionality, high safety, low side effects, minimal risk of resistance, and low residue-have demonstrated strong application potential under systematic scientific evaluation methods.
Yucca schidigera is a plant native to the Mojave Desert, Chihuahuan Desert, and Sonoran Desert of southeastern California, Baja California, New Mexico, southern Nevada, and Arizona (Kozłowski et al., 2022). Yucca schidigera extract (YSE) is a complex mixture obtained from plants through physical or chemical methods, typically containing a variety of bioactive components such as polysaccharides, polyphenols, alkaloids, saponins, and terpenoids. These components possess antibacterial, antioxidant, immunomodulatory, digestive enzyme-promoting, and gut health-enhancing properties. In recent years, YSE has been widely applied in the livestock and poultry industry. It was reported that YSE can reduce the diarrhea rate by improving intestinal health and increasing the diversity and abundance of cecal microflora in weaned piglets (Yang et al., 2021). Dietary supplemented with YSE can improve productive performance, immune functions, antioxidant enzyme activities and gut health in laying hens (Alagawany et al., 2016; Mao et al., 2023) and even in laying Japanese quails (Alagawany et al., 2018). Our previous studies showed that oral supplementation of YSE in meat pigeons can alter gut microbial diversity, increase the abundance of beneficial bacteria, improve serum biochemical parameters and intestinal tissue morphology, and reduce fecal ammonia-nitrogen levels (Sun et al., 2023). Although it has been reported to have many benefits as a feed additive, there are also some potential risks associated with YSE application. In cats treated with YSE, blood urea levels significantly increased, which may be attributed to the saponins in YSE affecting intestinal wall permeability (Lowe et al., 1997). Saponins were the main component of YSE, which have generally been considered as deleterious compounds in domestic animals, influencing metabolism such as feed intake (Francis et al., 2002). It also possess harmful hemolytic toxicity, leading to the lysis of erythrocytes and thereby hamper their applications in medicine (Zheng et al., 2019). Therefore, we need to search for other key functional components in YSE and verify their functions. Polyphenolic compounds are known for their diverse biological activities, including anti-inflammatory, antioxidant, and anti-stress effects (Hu et al., 2019; Ávila-Gálvez et al., 2024). As a metabolic derivative of polyphenols in Yucca genus (Culhuac et al., 2023), 4-hydroxyphenylacetic acid (4-HPAA), may inherit some of these beneficial properties. It has been demonstrated to attenuate inflammation and edema in seawater aspiration-induced lung injury in rats (Liu et al., 2014). Moreover, we have demonstrated that the content of 4-HPAA in yucca extract was approximately 0.048% by liquid chromatography analysis previously. Whether it can be used in meat pigeons to improve immune function and gut health remains unclear. Therefore, in the present study, the effects of 4-hydroxyphenylacetic acid on immune function and intestinal health of meat pigeons, as well as its underlying mechanisms, were systematically investigated, so as to provide a novel green farming strategy for the meat pigeon industry. First, Caco-2 cells were used as an in vitro model to evaluate the toxicity and anti-inflammatory activity of 4-HPAA. Subsequently, 4-HPAA was added to traditional artificial pigeon milk, and its effects on intestinal health of meat pigeons from 1 to 25 days of age were investigated in vivo.
Materials and methods
Ethical approval
All experimental procedures were approved by the Animal Welfare Committee of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (IHVM11-2409-78).
Cell culture
Human Caco-2 cells were kindly provided by Professor Bingkun Zhang from China Agricultural University and cultured in DMEM supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin-Amphotericin B at 37 °C in a humidified atmosphere with 5% CO2. The cells were subcultured every 2 days using a 0.25% trypsin-EDTA solution. Cells at passages 4-5 after thawing were used in this experiment.
Toxicity analysis of Yucca phytochemicals on Caco-2 cells
Through literature review, a total of 93 compounds from Yucca genus plants were identified (Culhuac et al., 2023). Among them, 13 economical compounds were ordered from Aladdin Biochemical Technology Co., Ltd., (Shanghai, China). Subsequently, three compounds with good water solubility and relatively simple structures-4-HPAA, shikimic acid, and 3,4-dihydroxybenzoic acid-were selected for Caco-2 cytotoxicity testing. The Caco-2 cell line is derived from human colon cancer cells and is widely used in intestinal injury model research. It is commonly employed for drug absorption studies, intestinal barrier function assessment, cytotoxicity evaluation, and research on intestinal-related diseases (Martínez et al., 2023).
The CCK-8 assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used to assess the mitochondrial dehydrogenase activity of Caco-2 cells treated with different concentrations of 4-hydroxyphenylacetic acid, shikimic acid, and 3,4-dihydroxybenzoic acid, reflecting cell number and viability. Caco-2 cells were seeded into a 96-well plate at a density of 1 × 104 cells/mL with 100 μL per well and incubated for 24 hours. After aspirating the culture medium and rinsing with PBS, drug-containing culture media with different concentrations (0, 50, 100, 200, 300, 400, 600, 800, and 1,000 μM) of 4-hydroxyphenylacetic acid, shikimic acid, and 3,4-dihydroxybenzoic acid were added, followed by incubation for 24 hours. After aspirating the culture medium and rinsing with PBS, 100 μL of 10% CCK-8 solution was added to each well, and the absorbance at 450 nm was measured using a microplate reader within 4 hours (n = 6).
Cell viability (%) = (OD of the experimental group - OD of the blank group) / (OD of the control group - OD of the blank group) × 100%.
Protective effect of 4-hydroxyphenylacetic acid on LPS-induced inflammatory injury in Caco-2 cells
Caco-2 cells were seeded into 6-well plates at a density of 2.5 × 105 cells/mL with 2 mL per well, and cultured until a confluent monolayer was formed, followed by the following experimental treatments. Control group (CON), in which cells were treated with complete culture medium for 24 hours followed by a 4-hour blank treatment; LPS group, in which cells were treated with complete medium for 24 hours and then exposed to 10 μg/mL LPS for 4 hours (The concentration and duration of LPS treatment were based on our preliminary experimental results); 4-HPAA+ LPS group, in which cells were pretreated with 4-HPAA for 24 hours followed by 4-hour stimulation with 10 μg/mL LPS; and 4-HPAA group, in which cells were pretreated with 4-HPAA for 24 hours followed by a 4-hour blank treatment (The concentration of 4-HPAA used in this experiment was determined based on the cell viability assay). Each group included three replicate wells (n = 3). The expression levels of intestinal barrier function-related genes-including IL-8, IL-1β, TNF-α, PCNA, and CASPASE1-were analyzed. The primer sequences were shown in Supplemental Table 1.
Animals, treatments, and management
A total of 144 one-day-old health Silver King meat pigeons (Hebei Wanxiang Squab Breeding Co., Ltd., Hebei, China) with similar body weights were randomly divided into four treatment groups, with 36 meat pigeons in each group. The four groups of meat pigeons were fed the control diet (CON), and control diet supplemented with 500 mg/kg (4-HPAA 500), 1,000 mg/kg (4-HPAA 1000), and 2,000 mg/kg 4-HPAA (4-HPAA 2000). The duration of the experiment was 25 days. 4-HPAA was purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). The compositions and nutrient levels of the basal diet are shown in Table 1.
Table 1.
Ingredients and nutrient composition of basal squab milk replacer for meat pigeons (as dry-basis).
| Ingredients, | % | Nutrient composition, | % unless noted |
|---|---|---|---|
| Maize | 53.60 | ME, Kcal/kg | 3003 |
| Soybean meal | 37.78 | Crude Protein3 | 21.60 |
| Soybean oil | 4.60 | Lysine | 1.10 |
| CaHPO4·2H2O1 | 1.94 | Methionine | 0.56 |
| Limestone1 | 1.16 | Methionine+Cysteine | 0.91 |
| NaCl1 | 0.29 | Calcium3 | 1.05 |
| DL-Methionine1 | 0.31 | Nonphytate P | 0.46 |
| Micronutrients2 | 0.32 |
Feed grade.
Supplied per kilogram of diet: vitamin A 12,500 IU, vitamin D3 3,750 IU, vitamin E 20 U, vitamin K3 2.5 mg, vitamin B1 2.5 mg, vitamin B2 8 mg, vitamin B6 2.5 mg, vitamin B12 0.015 mg, Pantothenic acid calcium 12.5 mg, Niacin 32.5 mg, Folic acid 1.25 mg, Biotin 0.125 mg, Choline 700 mg, Zn (ZnSO4.7H2O) 60 mg, Cu (CuSO4.5H2O) 8 mg, Mn (MnSO4.H2O) 110 mg, Fe (FeSO4•7H2O) 40 mg, I (KI) 0.35 mg, Se (Na3SeO3) 0.15 mg, Chlortetracycline 50 mg. Day 22-42: vitamin A 10,000 IU, vitamin D3 3,400 IU, vitamin E 12.8 U, vitamin K3 1.6 mg, vitamin B1 0.8 mg, vitamin B2 6.8 mg, vitamin B6 1.6 mg, vitamin B12 0.008 mg, Pantothenic acid calcium 8 mg, Niacin 26 mg, Folic acid 0.8 mg, Biotin 0.158 mg, Choline 500 mg, Zn (ZnSO4.7H2O) 40 mg, Mn (MnSO4.H2O) 80 mg, Fe (FeSO4•7H2O) 30 mg, I (KI) 0.35 mg, Se (Na3SeO3) 0.15 mg.
Analyzed values and each value based on triplicate determinations.
The meat pigeons were housed in cages within a brooding house from day 1 to 14 (length × width × height, 60 × 60 × 10 cm), with the ambient temperature maintained at 42 ± 1°C and a lighting schedule of 6 hours per day. At 14 days of age, when their feather development was nearly complete and they were able to move freely, they were transferred to a fattening house with cages measuring 60 × 60 × 20 cm (length × width × height). During this stage, the ambient temperature was maintained at 28 ± 1 °C, with the lighting duration increased to 8 hours per day. Throughout the experimental period, the meat pigeons did not receive any vaccinations or antibiotic treatments. The milk replacer used for feeding was prepared by mixing milk replacer powder with drinking water at specific ratios according to age: 1:4.5 for days 1-8, 1:4 for day 9, and 1:3.5 for days 10-25. 4-HPAA was first dissolved in drinking water and then mixed with the milk replacer powder for administration. Feed and water were artificially gavaged twice daily, at 4 am and 4 pm. During the experimental period, the health status of the meat pigeons was observed and recorded daily. Sick or deceased meat pigeons were promptly recorded and disposed of in a harmless manner.
Sample collection
At 7 days of age, a total of 24 meat pigeons (six pigeons from each treatment) were randomly selected from four treatments. After a 6-hour fasting period (10 am), their live body weights were measured, and they were euthanized by carotid bleeding. The spleen and bursa of Fabricius were collected and weighed. Segments (approximately 1 cm) of the jejunum and ileum were isolated and placed in 1.5 mL centrifuge tubes for storage at -80°C. A portion of ileal content was also collected and stored at -80°C. At 25 days of age, upon completion of the feeding trial, 24 meat pigeons (six pigeons close to the average body weights were selected from each treatment) were selected from four treatment groups. After a 12-hour fasting period (4 am), 5 mL of blood was collected from the brachial vein into blood collection tubes and left to stand at room temperature until serum separation. The samples were centrifuged at 3,000 rpm for 15 minutes, and the serum was aliquoted into 1.5 mL centrifuge tubes and stored at -80°C. After blood collection, the meat pigeons were euthanized by carotid bleeding. The spleen and bursa of Fabricius were collected and weighed. Two segments (approximately 1 cm) each of the jejunum, and ileum were isolated. One segment was fixed in 4% paraformaldehyde at room temperature, and the fixative was replaced after 24 hours. The other segment was placed in 1.5 mL centrifuge tubes and stored at -80°C. A portion of ileal content was also collected and stored at -80°C.
Body weight and survival rate
Initial Body Weight (IBW) = Fasting body weight of individual meat pigeons in all four groups measured at 5:00 a.m. on the first day of the trial.
Final Body Weight (FBW) = Fasting body weight of individual meat pigeons in all four groups measured at 5:00 a.m. on the day following the end of the trial.
Survival rate on day 25 (%) = Survival rate of the previous day × (number of meat pigeons observed that day - number of deaths that day) / number of meat pigeons observed that day. Survival rate on day 1 is considered 100%.
Serum biochemical and immune indices
According to the instructions provided with the assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), the following serum biochemical parameters were measured in 25-day-old meat pigeons: total protein (TP), albumin (ALB), triglycerides (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and L-lactic acid (L-LA).
Similarly, the serum levels of immunoglobulin A, G, M (IgA, IgG, IgM) were determined in 25-day-old meat pigeons following the assay kit instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China).
The organ indices of the spleen and bursa of Fabricius were measured in meat pigeons at 7 and 25 days of age.
Immune organ index (g/kg) = Immune organ weight (g) / Live body weight (kg).
Serum and intestinal antioxidant indices
According to the instructions provided with the assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), the following antioxidant parameters were measured in the serum, duodenum, jejunum, and ileum of 25-day-old meat pigeons: total antioxidant capacity (T-AOC), catalase (CAT) activity, total superoxide dismutase (T-SOD) activity, glutathione peroxidase (GSH-PX) activity, and malondialdehyde (MDA) content.
Intestinal morphology and barrier integrity
The jejunum, and ileum of 25-day-old meat pigeons were fixed, dehydrated, embedded in paraffin, sectioned, mounted, and stained with hematoxylin and eosin (HE). Villus height and crypt depth were observed and measured under a microscope, and the villus height-to-crypt depth ratio was calculated. Ten villi were selected per field of each intestinal section for measurement, and the average value was used.
According to the instructions provided with the assay kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), the serum levels of diamine oxidase (DAO), D-lactic acid (D-LA), and the activity of lactate dehydrogenase (LDH) were measured in 25-day-old meat pigeons.
Gene expression
Total RNA was extracted from the jejunum and ileum of 7- and 25-day-old meat pigeons using Trizol reagent. RNA purity and concentration were measured using a microplate reader. The extracted RNA was rapidly reverse transcribed into cDNA using the HiScript III RT SuperMix for qPCR (+gDNA wiper). Quantitative real-time PCR (qRT-PCR) was performed on a real-time fluorescence PCR system using 2 × M5 HiPer SYBR Premix EsTaq Plus (with Tli RNaseH). The PCR conditions were as follows: initial denaturation at 95°C for 30 seconds; 40 cycles of denaturation at 95°C for 10 seconds and annealing/extension at 60°C for 30 seconds. The melting curve program was: 95°C for 15 seconds, 60°C for 60 seconds, and 95°C for 15 seconds. The total reaction volume was 20 μL. Each sample was run in duplicate. The target genes include the following: intestinal barrier integrity genes CLAUDIN1, OCCLUDIN, ZO1, and MUC2; glycolysis-related genes hexokinase-2 (HK2) and L-lactate dehydrogenase B chain-like (LDH-B); mitochondrial function gene PPARGC1A; signaling regulation genes CAMK2G, PIK3CB, and MKNK1. GAPDH was used as the internal reference gene, and the relative expression levels of target genes were calculated using the 2–ΔΔCt method. The sequences of the target genes are listed in Supplemental Table 2.
Intestinal microbiota
Total DNA of the ileal microbial community from 7 and 25-day-old meat pigeons was extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA) according to the manufacturer's instructions. The integrity and quality of the extracted DNA were assessed by 1% agarose gel electrophoresis (voltage: 5 V/cm; duration: 20 minutes). DNA concentration and purity were measured using a NanoDrop 2000 micro-spectrophotometer. The V3-V4 hypervariable region of the 16S rRNA gene was amplified using primers 338F (5′-ACTCCTACGGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The amplified PCR products were verified by 2% agarose gel electrophoresis and purified using the AxyPrep DNA Gel Extraction Kit (Axygen, Silicon Valley, CA). The purified products were quantified with a Quantus™ fluorometer according to the manufacturer’s protocol. Purified amplicons were pooled in equimolar amounts and subjected to paired-end sequencing on the Illumina MiSeq PE300 platform at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The paired-end reads obtained from sequencing were assembled based on their overlapping regions, and quality control and filtering were performed. After distinguishing the samples, OTU clustering and taxonomic analysis were conducted.
Statistical analysis
All data were expressed as mean ± standard deviation (mean ± SD). Statistical analyses were performed using SPSS version 20.0. For data with normal distribution and homogeneity of variance, one-way analysis of variance (ANOVA) followed by Duncan’s post hoc test was used to determine differences among groups (Sun et al., 2023). Alpha diversity (including Shannon, ACE, and Chao1 estimators), and the relative abundance of intestinal microbiota at the phylum or genus level among the groups were tested with Kruskal-Wallis H test. A P-value of less than 0.05 was considered statistically significant, while a P-value greater than 0.05 is considered not statistically significant.
Results
Effect of 4-hydroxyphenylacetic acid on cell viability and LPS-induced inflammatory injury in Caco-2 Cells
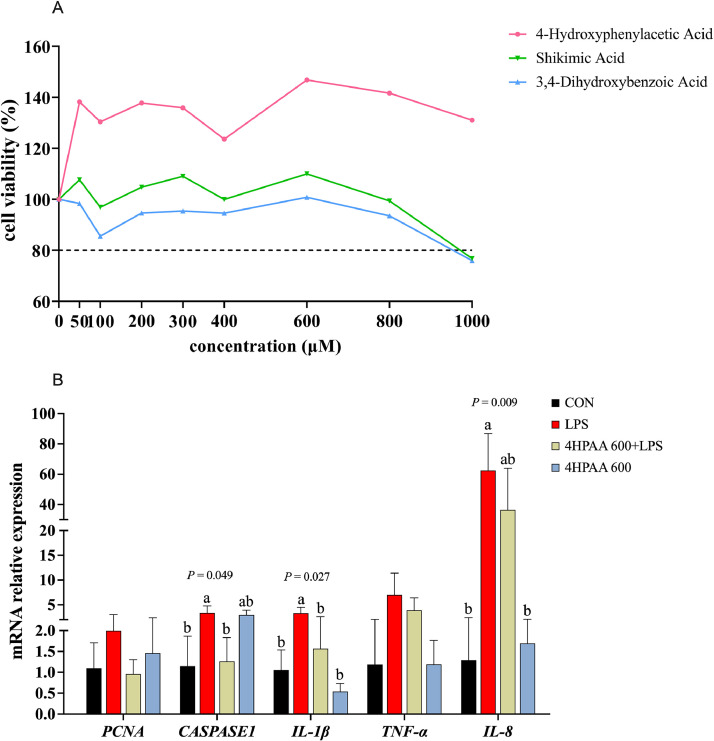
As shown in Fig. 1; 4-HPAA exhibited higher cell viability at all tested concentrations than shikimic acid and 3,4-dihydroxybenzoic acid, and the cell viability was the highest when the concentration of 4-HPAA was 600 μM. After that, cell viability decreased as the drug concentration increased (Fig. 1A). LPS significantly increased CASPASE1 (P = 0.049), IL-1β (P = 0.027) and IL-8 (P = 0.009) gene expressions, while pretreatment with 600 μM 4-hydroxyphenylacetic acid reduced the expression of IL-1β and CASPASE1 genes (P < 0.05, Fig. 1B) thereby suppressing the LPS-induced inflammatory response in Caco-2 cells. However, it did not significantly alleviate LPS-induced the high mRNA level of IL-8 (P > 0.05).
Fig. 1.
Effects of Yucca genus plant chemicals on the cell viability and LPS-induced inflammatory injury of Caco-2 cells. A. Cell viability (n = 6). B. Cell proliferation, apoptosis and inflammation-related gene expression (n = 3). Data are presented as mean ± SD. CON, control. 4-HPAA 600+LPS, 600 μmol hydroxyphenylacetic acid pretreatment for 24 hour followed by 4-hour stimulation with 10 μg/mL LPS. 4-HPAA 600, 600 μmol hydroxyphenylacetic acid pretreatment for 24 hour.
Fig. 4.
Effects of 4-hydroxyphenylacetic acid on jejunal glycolysis and signaling regulation-related gene expressions in 25-day-old meat pigeons. A-B. glycolytic gene expressions. C-F. Gene expressions involved in signaling pathway. Data are presented as mean ± SD; n = 6. control. 4-HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. LDH-B, lactate dehydrogenase B. HK2, hexokinase 2.
Effects of 4-hydroxyphenylacetic acid on body weight and survival rate of meat pigeons
As shown in Table 2, there were no significant differences in the initial body weight (IBW) of 1-day-old pigeons among the groups (P > 0.05). Supplementation with different levels of 4-hydroxyphenylacetic acid had no significant effect on the average body weight on day 25 (FBW) (P > 0.05). The survival rates of pigeons during the trial period (1-25 days of age) were 78.63% in the 4-HPAA 500 group, 73.75% in the 4-HPAA 1000 group, and 73.75% in the 4-HPAA 2000 group, compared to 67.46% in the CON group.
Table 2.
The effect of 4-hydroxyphenylacetic acid on the body weight and survival rate of meat pigeons.
| Items | Treatments |
P-value | |||
|---|---|---|---|---|---|
| CON | 4HPAA 500 | 4HPAA 1000 | 4HPAA 2000 | ||
| IBW (g) | 16.29±0.65 | 16.30±0.29 | 16.78±0.27 | 16.52±0.39 | 0.189 |
| FBW (g) | 437.42±36.22 | 421.59±26.49 | 426.30±30.76 | 430.54±23.09 | 0.385 |
| Survival rate on day 25 (%) | 67.46 | 78.63 | 73.75 | 73.75 | - |
Values of IBM (n = 36) and FBW (n = 30) are presented as the mean ± SD. CON, control. 4HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. IBW, initial body weight. FBW, final body weight. -, no P value.
Effects of 4-hydroxyphenylacetic acid on serum biochemical and immune parameters in meat pigeons
As shown in Table 3, compared with the CON group, the 4-HPAA 500 group significantly increased the serum total protein (P = 0.023) and total cholesterol levels (P = 0.047) and decreased the L-lactic acid content in 25-day-old pigeons (P < 0.001). The 4-HPAA 1000 group significantly increased the total cholesterol content in serum (P = 0.047), while 4-HPAA 2000 group significantly reduced the serum L-lactic acid content (P < 0.001). Moreover, the 4-HPAA 2000 group showed a significantly lower L-lactic acid level than the 4-HPAA 500 group (P < 0.001). Supplementation with different levels of 4-hydroxyphenylacetic acid had no significant effect on albumin, triglycerides, ALT, AST, HDL-C or LDL-C levels in 25-day-old meat pigeons (P > 0.05).
Table 3.
The effect of 4-hydroxyphenylacetic acid on serum biochemical parameters in 25-day-old meat pigeons.
| Items | Treatments |
P-value | |||
|---|---|---|---|---|---|
| CON | 4HPAA 500 | 4HPAA 1000 | 4HPAA 2000 | ||
| TP (g/L) | 20.49±0.87b | 23.02±0.37a | 21.10±1.01ab | 21.64±0.11ab | 0.023 |
| ALB (g/L) | 11.46±1.11 | 11.13±0.60 | 11.54±0.78 | 11.33±0.92 | 0.859 |
| TG (mmol/L) | 1.83±0.43 | 1.90±0.36 | 1.80±0.44 | 1.75±0.30 | 0.928 |
| TC (mmol/L) | 8.71±1.21b | 10.84±1.50a | 10.96±1.53a | 10.08±1.00ab | 0.047 |
| ALT (U/L) | 7.12±3.72 | 11.35±7.81 | 9.70±4.00 | 10.42±5.81 | 0.558 |
| AST (U/L) | 8.88±2.79 | 13.13±7.23 | 10.96±4.72 | 9.85±5.18 | 0.754 |
| HDL-c (mmol/L) | 3.35±0.19 | 3.49±0.40 | 3.49±0.47 | 3.01±0.26 | 0.097 |
| LDL-c (mmol/L) | 5.11±1.01 | 6.52±1.25 | 6.25±1.51 | 5.42±0.54 | 0.114 |
| L-LA (μmol/g) | 11.04±0.76a | 7.00±0.87b | 10.29±0.74a | 4.10±0.91c | < 0.001 |
Values are presented as the mean ± SD; n = 6. In the same row, values with different letter superscripts indicate significant differences (P < 0.05). CON, control. 4HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. TP, total protein. ALB, albumin. TG, triglycerides. TC, total cholesterol. ALT, alanine aminotransferase. AST, aspartate aminotransferase. HDL-c, high-density lipoprotein cholesterol. LDL-c, low-density lipoprotein cholesterol. L-LA, L-lactic acid.
As shown in Table 4, compared with the CON group, the 4-HPAA 500 and 1000 groups significantly increased the levels of IgA, IgG, and IgM in the serum of 25-day-old pigeons (P < 0.001), and the 4-HPAA 2000 group significantly increased IgG and IgM levels (P < 0.001). The 4-HPAA 500 and 4-HPAA 1000 groups had higher levels of IgA and IgM than the 4-HPAA 2000 group (P < 0.001), and the 4-HPAA 500 group produced more IgG levels than the 4-HPAA 2000 group (P < 0.001).
Table 4.
The effect of 4-hydroxyphenylacetic acid on serum immunoglobulins in 25-day-old meat pigeons.
| Items | Treatments |
P-value | |||
|---|---|---|---|---|---|
| CON | 4HPAA 500 | 4HPAA 1000 | 4HPAA 2000 | ||
| IgA (ug/ml) | 156.74±28.74b | 251.82±20.31a | 243.20±38.05a | 189.13±22.38b | < 0.001 |
| IgG (g/l) | 13.79±1.83c | 20.90±1.66a | 18.85±1.47ab | 17.58±1.99b | < 0.001 |
| IgM (ug/ml) | 1579.48±171.42c | 2370.82±233.55a | 2365.72±178.71a | 2044.98±171.79b | < 0.001 |
Values are presented as the mean ± SD; n = 6. In the same row, values with different letter superscripts indicate significant differences (P < 0.05). CON, control. 4HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. IgA, G, M: immunoglobulin A, G, M.
As shown in Table 5, compared with the CON group, the 4-HPAA 500 and 1000 groups significantly elevated the bursa of Fabricius index in 7-day-old meat pigeons (P = 0.003), and the 4-HPAA 2000 group significantly increased the spleen (P = 0.017) and bursa of Fabricius (P < 0.001) indices in 25-day-old pigeons. Supplementation with different levels of 4-hydroxyphenylacetic acid had no effect on the spleen index of 7-day-old meat pigeons (P > 0.05).
Table 5.
The effect of 4-hydroxyphenylacetic acid on the immune organ index of meat pigeons (g/kg).
| Items | Treatments |
P-value | |||
|---|---|---|---|---|---|
| CON | 4HPAA 500 | 4HPAA 1000 | 4HPAA 2000 | ||
| Spleen index on 7 d | 0.83±0.36 | 0.81±0.16 | 1.14±0.65 | 0.77±0.10 | 0.859 |
| Bursa of Fabricius index on 7 d | 0.92±0.12b | 1.13±0.10a | 1.16±0.13a | 0.76±0.13b | 0.003 |
| Spleen index on 25 d | 0.67±0.09b | 0.76±0.05ab | 0.90±0.15ab | 1.13±0.23a | 0.017 |
| Bursa of Fabricius index on 25 d | 0.78±0.16b | 0.73±0.06b | 0.75±0.20b | 1.21±0.13a | <0.001 |
Values are presented as the mean ± SD; n = 6. In the same row, values with different letter superscripts indicate significant differences (P < 0.05). CON, control. 4HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively.
Effects of 4-hydroxyphenylacetic acid on serum and intestinal antioxidant parameters in meat pigeons
As shown in Table 6, compared with the CON group, the 4-HPAA 500 and 4-HPAA 2000 groups significantly increased T-AOC activity in the duodenum (P = 0.034), jejunum (P = 0.001) and ileum (P = 0.037), and the 4-HPAA 1000 group significantly promoted serum T-SOD (P = 0.037) and duodenal GSH-PX activity (P = 0.038). However, serum GSH-PX activity was obviously decreased in the 4-HPAA 1000 and 4-HPAA 2000 groups compared to the CON group (P =0.013), while the 4-HPAA 500 group showed higher serum GSH-PX activity than the 4-HPAA 1000 group (P < 0.05) and lower jejunal MDA content than the 4-HPAA 2000 group (P = 0.009).
Table 6.
The effect of 4-hydroxyphenylacetic acid on antioxidant indices in the serum and intestines of 25-day-old meat pigeons.
| Items | Treatments |
P-value | ||||
|---|---|---|---|---|---|---|
| CON | 4HPAA 500 | 4HPAA 1000 | 4HPAA 2000 | |||
| Serum | T-AOC (mmol/L) | 0.90±0.06b | 0.98±0.06a | 0.88±0.05b | 0.90±0.03b | 0.018 |
| CAT (U/mL) | 0.61±0.21 | 0.88±0.34 | 0.68±0.29 | 0.88±0.24 | 0.288 | |
| MDA (nmol/mL) | 5.37±0.59 | 5.37±0.72 | 5.45±0.56 | 5.98±0.57 | 0.290 | |
| T-SOD (U/mL) | 66.82±2.02b | 68.06±4.57b | 74.54±4.10a | 72.37±2.64ab | 0.037 | |
| GSH-PX (U/mL) | 651.73±31.64a | 634.67±24.32ab | 591.11±21.18c | 608.00±38.61bc | 0.013 | |
| Duodenum | T-AOC (mmol/L) | 0.13±0.01b | 0.15±0.01a | 0.12±0.01b | 0.14±0.00ab | 0.034 |
| CAT (U/mL) | 3.60±0.47 | 4.00±0.44 | 4.09±0.44 | 4.27±0.17 | 0.170 | |
| MDA (nmol/mL) | 1.05±0.20 | 1.09±0.33 | 1.27±0.27 | 1.02±0.32 | 0.445 | |
| T-SOD (U/mL) | 5.60±0.25 | 6.84±0.26 | 6.15±0.33 | 5.93±1.20 | 0.200 | |
| GSH-PX (U/mL) | 5.36±0.56b | 4.98±0.63b | 6.78±0.87a | 5.73±0.94ab | 0.038 | |
| Jejunum | T-AOC (mmol/L) | 0.18±0.03c | 0.22±0.03b | 0.18±0.01bc | 0.27±0.01a | 0.001 |
| CAT (U/mL) | 5.53±1.53 | 4.32±1.57 | 4.48±1.30 | 3.51±1.80 | 0.199 | |
| MDA (nmol/mL) | 0.24±0.06ab | 0.23±0.05b | 0.36±0.13ab | 0.37±0.04a | 0.009 | |
| T-SOD (U/mL) | 95.36±4.39 | 104.05±6.34 | 99.17±3.97 | 95.41±4.43 | 0.082 | |
| GSH-PX (U/mL) | 5.76±1.27 | 5.03±0.64 | 4.26±0.75 | 4.88±0.88 | 0.073 | |
| Ileum | T-AOC (mmol/L) | 0.17±0.01b | 0.20±0.03a | 0.20±0.02ab | 0.22±0.02a | 0.037 |
| CAT (U/mL) | 3.74±0.85 | 2.55±0.81 | 3.02±0.76 | 2.80±0.42 | 0.056 | |
| MDA (nmol/mL) | 0.27±0.04 | 0.25±0.05 | 0.30±0.06 | 0.25±0.04 | 0.269 | |
| T-SOD (U/mL) | 102.27±6.65 | 101.30±9.33 | 106.30±6.24 | 98.25±9.80 | 0.415 | |
| GSH-PX (U/mL) | 5.39±1.14 | 4.92±1.06 | 4.53±1.17 | 4.03±1.05 | 0.213 | |
Values are presented as the mean ± SD; n = 6. In the same row, values with different letter superscripts indicate significant differences (P < 0.05). CON, control. 4HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. T-AOC, total antioxidant capacity. CAT, catalase. T-SOD, total superoxide dismutase. GSH-PX, glutathione peroxidase. MDA, malondialdehyde.
Effects of 4-hydroxyphenylacetic acid on intestinal morphology and barrier integrity in meat pigeons
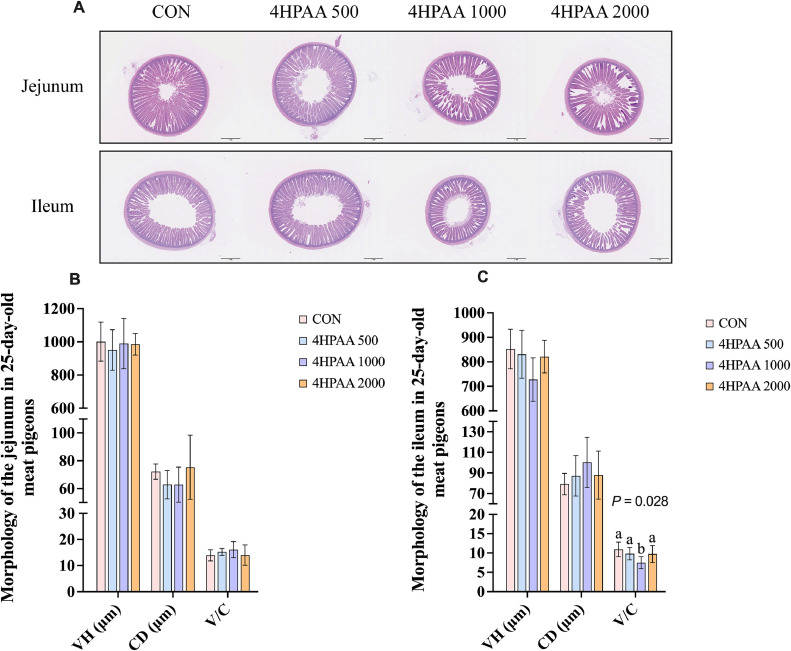
As shown in Fig. 2A-B, the villus height-to-crypt depth ratio in the ileum of the 4-HPAA 1000 group was significantly lower than that in the CON, 4-HPAA 500, and 4-HPAA 2000 groups (P = 0.028). Supplementation with different levels of 4-hydroxyphenylacetic acid had no significant effect on jejunal intestinal morphology in 25-day-old meat pigeons (P > 0.05, Fig. 2A; 2C).
Fig. 2.
Intestinal tissue morphology of 25-day-old meat pigeons. A. The morphology of jejunum and ileum with hematoxylin and eosin staining under a microscope. B. The statistically analysis of jejunal morphology. C. The statistically analysis of ileal morphology. Data are presented as mean ± SD; n = 6. CON, control. 4-HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. VH, villus height. CD, crypt depth. V/C, villus height to crypt depth ratio.
The effects of 4-hydroxyphenylacetic acid on intestinal barrier integrity in 25-day-old meat pigeons are shown in Table 7. Compared with the CON group, all different levels of 4-HPAA significantly increased the serum DAO content, with the 4-HPAA 500 group showing the highest content (P < 0.001). The 4-HPAA 1000 and 2000 group significantly decreased D-LA levels (P < 0.001), and the LDH activity was also reduced in 4-HPAA 2000 group compared to the CON group (P < 0.001).
Table 7.
The effect of 4-hydroxyphenylacetic acid on the intestinal barrier integrity of 25-day-old meat pigeons.
| Items | Treatments |
P-value | |||
|---|---|---|---|---|---|
| CON | 4HPAA 500 | 4HPAA 1000 | 4HPAA 2000 | ||
| DAO (pg/mL) | 102.38±8.51c | 191.80±13.71a | 161.77±13.34b | 146.62±16.48b | < 0.001 |
| D-LA (μmol/g) | 3.63±0.11a | 2.50±0.06ab | 1.98±0.15bc | 1.80±0.09c | < 0.001 |
| LDH (nmol/min/g) | 875.42±29.78a | 904.46±50.89a | 1304.96±55.96a | 721.34±27.82b | < 0.001 |
Values are presented as the mean ± SD; n = 6. In the same row, values with different letter superscripts indicate significant differences (P < 0.05). 4HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. DAO, diamine oxidase. D-LA, D-lactic acid. LDH, lactate dehydrogenase.
Effects of 4-hydroxyphenylacetic acid on gene expressions of intestinal barrier, glycolysis and signaling regulation in meat pigeons
The effects of 4-hydroxyphenylacetic acid on intestinal barrier gene expressions are shown in Fig. 3. The results indicated that the 4-HPAA 2000 group significantly increased ileal OCCLUDIN (P = 0.026), CLAUDIN1 (P < 0.001) and MUC2 (P = 0.032) gene expression (Fig. 3B), but decreased jejunal and ileal ZO1 (P < 0.001; P = 0.006) gene expression in 7-day-old meat pigeons compared to the CON (Fig. 3A-B). In addition, the 4-HPAA 2000 group also significantly promoted jejunal MUC2 (P = 0.021) and ileal OCCLUDIN (P = 0.033) gene expressions in 25-day-old meat pigeons compared to the CON (Fig. 3C-D).
Fig. 3.
Effects of 4-hydroxyphenylacetic acid on intestinal barrier gene expression in 7 and 25-day-old meat pigeons. A. Jejunal mRNA expression in 7-day-old pigeons. B. Ileal mRNA expression in 7-day-old pigeons. C. Jejunal mRNA expression in 25-day-old pigeons. D. Ileal mRNA expression in 25-day-old pigeons. Data are presented as mean ± SD; n = 6. CON, control. 4-HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively. ZO-1, zonula occludens-1. MUC2, mucin 2.
The effects of 4-hydroxyphenylacetic acid on jejunal glycolysis and signaling regulation-related gene expressions in 25-day-old meat pigeons are shown in Fig. 4. The results indicated that the glycolytic gene LDH-B expression was highest in the 4-HPAA 500 group (P = 0.046, Fig. 4A). The 4-HPAA 2000 group significantly inhibited glycolytic gene HK2 mRNA expression (P = 0.018, Fig. 4B), while up-regulating CAMK2G and MKNK1 signaling pathway gene expressions compared to the CON group (P < 0.05, Fig. 4D-E). Besides, the 4-HPAA 1000 group significantly promoted MKNK1 gene expression (P = 0.015), and there was no significant difference with the 4-HPAA 2000 group. Different levels 4-HPAA had no effects on PPARGC1A and PI3KCB gene expressions (P > 0.05, Fig. 4C; 4F).
Intestinal microbiota
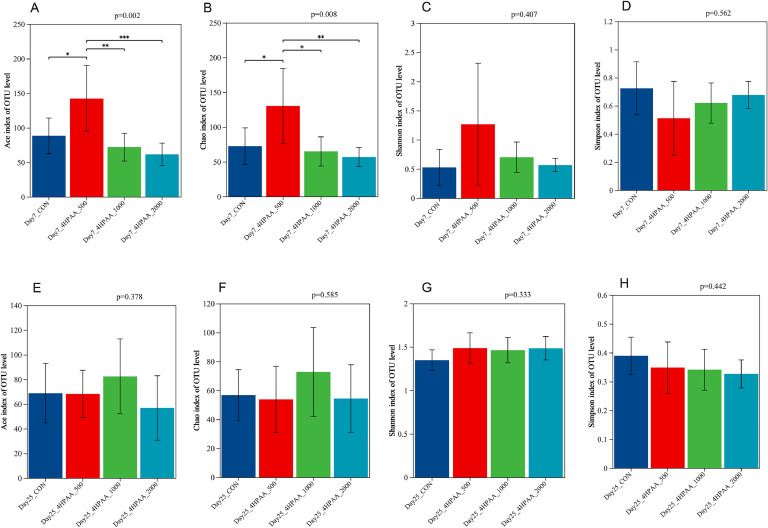
Alpha diversity analysis of the intestinal microbiota was performed for each group of 7- and 25-day-old meat pigeons, including Ace, Chao, Shannon and Simpson indices (Fig. 5). The Ace and Chao indices reflect the richness of the community within a sample (or group), measuring the number of species without considering their abundance. The Shannon and Simpson indices reflect community diversity, taking into account both species richness and evenness. The results showed that in 7-day-old meat pigeons, the 4-HPAA 500 group exhibited significantly higher Ace (P = 0.002) and Chao indices (P = 0.008, Fig. 5A-B), but no significant differences in Shannon and Simpson indices (P > 0.05, Fig. 5C-D), indicating an increase in intestinal microbiota richness. In contrast, for 25-day-old meat pigeons, there were no significant changes in microbiota richness and diversity among the groups overall (Fig. 5E-H).
Fig. 5.
Analysis of α diversity in each group. A-D, Ace, Chao, Shannon and Simpson indices in 7-day-old pigeons. E-H, Ace, Chao, Shannon and Simpson indices in 25-day-old pigeons. CON, control. 4-HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively.
Analysis of the intestinal microbial composition revealed notable differences at the genus level (Fig. 6). In 7-day-old meat pigeons (Fig. 6A, B), Lactobacillus dominated across all groups, with particularly high abundance in the CON, 4-HPAA 1000, and 4-HPAA 2000 groups. In comparison, the 4-HPAA 500 group exhibited a highly diverse microbial composition, with a higher levels (P < 0.05) of Weissella and Leuconostoc, Glutamicibacter, Allorhizobium, Stenotrophomonas, Luteimonas, Klebsiella, Sphingobacterium, and Brevundimonas. In 25-day-old meat pigeons (Fig. 6C, D), Lactobacillus remained dominant across all groups. Staphylococcus was also present in all groups at a certain abundance. Other genera were detected at relatively low levels. Lactococcus emerged as a specific genus associated with 4-HPAA supplementation, showing the highest abundance in the 4-HPAA 1000 group.
Fig. 6.
Analysis of differences in ileal microbiota relative abundance at the genus level among groups. A. Percent of community abundance on 7 days. B. Kruskal-Wallis H test bar plot on 7 days. C. Percent of community abundance on 25 days. D. Kruskal-Wallis H test bar plot on 25 days. CON, control. 4-HPAA 500, 1000, 2000: dietary supplemental 500, 1000 and 2000 mg/kg 4-hydroxyphenylacetic acid, respectively.
Discussion
This study preliminarily revealed the biosafety and potential functional properties of 4-HPAA through cell line assays and in vivo trials. In the Caco-2 cell study, different levels of 4-HPAA (0-1,000 μM) did not exhibit a significant toxicity to Caco-2 cells. This finding is consistent with Armand et al. (2019), who found 350-1000 μM 4-HPAA displayed no cytotoxic effect on HT-29 human colonocytes. In addition, pretreatment with 600 μM 4-HPAA significantly inhibited the LPS-induced upregulation of IL-1β and CASPASE1 gene expression in Caco-2 cells. Inflammasomes are intracellular multiprotein complexes that recognize pathogen-associated molecular patterns and activate inflammatory responses (Chang et al., 2020; Corpetti et al., 2021). The assembly of inflammasomes leads to the activation of CASPASE1, which subsequently promotes the maturation and secretion of inflammatory factors such as IL-1β (Chen et al., 2023; Xu et al., 2023). Therefore, the in vitro experiments confirm that 4-HPAA possesses low cytotoxicity and potential functions in preventing cell apoptosis and inflammatory injury of the intestinal cells, which could be a potential novel green feed additive for meat pigeon industry.
Generally, meat pigeons reach market weight between 25 to 28 days of age, after which their body weight shows little further increase. Therefore, this study selected meat pigeons aged 1 to 25 days as experimental subjects. The results showed that dietary supplementation with different levels of 4-HPAA did not show a statistically significant effect on body weight of 25-day-old pigeons and survival rate, but they increased the survival rate of meat pigeons by 9.3-16.6%. It was reported that orally intake of 4-HPAA reduced the body weight of obese mice, but 4-HPAA did not alter body weight gain when injected intraperitoneally into the mice (Jiang et al., 2025). In meat pigeon farming, improving survival rate is crucial for economic efficiency, and 4-HPAA may contribute positively to pigeon health to a certain extent.
Serum biochemical indices can reflect changes in nutrient metabolism and organ functions, such as TP and TC levels, which reflect the body’s protein and fat metabolism (Ghasemi et al., 2020). In our present, 500 mg/kg 4-HPAA supplementation significantly increased serum TP and TC levels, indicating low level of 4-HPAA could promote the utilization of proteins and fats, and reduce their degradation and catabolism. Besides, 2,000 mg/kg 4-HPAA supplementation significantly reduced L-LA level and LDH activity, suggesting high level of 4-HPAA enhanced aerobic metabolism and reduced the glycolysis, which was further evidenced by a decrease in HK2 gene expression induced by 2,000 mg/kg 4-HPAA. Previous studies found that a decrease in LDH activity can reduce lactate production, promote the entry of pyruvate into the tricarboxylic acid cycle, thereby increasing the proportion of aerobic metabolism and ultimately achieving a steady-state balance of energy metabolism (Sola-Penna, 2008).
Furthermore, polyphenols in food can help limit oxidative damage by acting directly on ROS or stimulating endogenous defense systems (Förstermann, 2008). 4-HPAA, an important metabolite of polyphenols in the gastrointestinal tract, also has antioxidant effects. In the current study, dietary supplementation with different levels of 4-HPAA improved the activities of various antioxidant enzymes to varying degrees, including an increase of intestinal T-AOC activity by 4-HPAA 500 and 4-HPAA 2000 and serum T-SOD by 4-HPAA 1000. A previous study has shown that 4-HPAA inhibits acute liver injury through increasing the antioxidant enzymes CAT and SOD levels in mice (Zhao et al., 2018).
Additionally, we found that different levels of 4-HPAA significantly elevated serum IgG and IgM levels, and 200 mg/kg 4-HPAA increased immune organ index in 25-day-old pigeons, further supporting its potential role in enhancing immune function. These findings align with the observation that Yucca schidigera extract addition increased serum IgM content in 42-day-old pigeons (Sun et al., 2023). B cell-mediated immunoglobulin production constrains the outgrowth of certain microorganisms and diversifies the microbiome, which helps to reduce intestinal inflammation and epithelial leakage (Kawamoto et al., 2014). The high fat diet-fed mice treated with 4-HPAA mice demonstrated improved epithelial integrity and reduced inflammation by upregulating B cell-related the immune response (Jiang et al., 2025). Our research also demonstrated 2000 mg/kg 4-HPAA supplementation enhanced the intestinal integrity by decreasing serum D-LA levels and LDH activity, and promoted barrier functions and mucin secretion by up-regulating intestinal OCCLUDIN and MUC2 gene expression. Simultaneously, we has confirmed that high level of 4-HPAA enhance the signaling pathways related genes CAMK2G and MKNK1 transcription. The stability of tight junction proteins may be associated with calcium signaling pathways. Research has found that CAMK2γ, encoded by CAMK2G, plays a protective role in mouse intestinal epithelial cells (IECs) by enhancing STAT3 activation, which promotes epithelial cell survival and proliferation while reducing tissue damage caused by colitis (Ma et al., 2017). However, 500 mg/kg 4-HPAA had an adverse effect of intestinal integrity, with the highest level of the serum DAO. It is well known that an increase in DAO typically indicates intestinal mucosal damage and increased gut permeability. Nevertheless, low level of 4-HPAA exhibited a higher diverse microbial richness and compositions in 7-day-old pigeon, and the medium level of 4-HPAA was shown to alter the jejunal microbiota structure in 25-day-old meat pigeons by specifically increasing the abundance of the beneficial genus Lactococcus. It is possible that the high levels of serum TP and TC produced by low and medium 4-HPAA enhanced the intestinal microbial diversity and Lactococcus growth. In contrast to our findings, Jiang et al. (2025) revealed that the mice had no overt microbiome differences before and after treatment with drinking water containing 1.5 mg/ml 4-HPAA, indicating that the direct effects of 4-HPAA on the gut microbiome are minimal.
Conclusions
In vitro studies demonstrated that 600 μM 4-HPAA possesses anti-inflammatory function in Caco-2 cells, and in vivo studies showed that 2000 mg/kg 4-HPAA promotes antioxidant function, immune response and intestinal barrier integrity, while inhibiting intestinal glycolytic gene expression and the production of serum metabolites in 1-25 day-old meat pigeons. Our results provide a theoretical basis for the application of 4-HPAA in poultry nutrition and gut health, as well as valuable references for the use of natural phenolic compounds as feed additives.
Disclosures
The authors declare no conflicts of interest.
Acknowlegments
This work was supported by the Innovation capacity building project of the Beijing Academy of Agriculture and Forestry Science (KJCX20251207); the Heibei Agriculture Research System (HBCT2024270202).
Footnotes
4-HPAA IMPROVES INTESTINAL HEALTH OF PIGEONS
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.105543.
Appendix. Supplementary materials
References
- Alagawany M., El-Hack M.E.A., El-Kholy M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. 2016;23(7):6774–6782. doi: 10.1007/s11356-015-5919-z. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Hack M.E.A., Farag M.R., Elnesr S.S., El-Kholy M.S., Saadeldin I.M., Swelum A.A. Dietary supplementation of Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult. Sci. 2018;97(9):3126–3137. doi: 10.3382/ps/pey186. [DOI] [PubMed] [Google Scholar]
- Armand L., Andriamihaja M., Gellenoncourt S., Bitane V., Lan A., Blachier F. In vitro impact of amino acid-derived bacterial metabolites on colonocyte mitochondrial activity, oxidative stress response and DNA integrity. Biochim. Biophys. Acta, Gen. Subj. 2019;1863(8):1292–1301. doi: 10.1016/j.bbagen.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Ávila-Gálvez M.Á., Giménez-Bastida J.A., Karadeniz B., Romero-Reyes S., Espín J.C., Pelvan E., González-Sarrías A. Polyphenolic characterization and anti-inflammatory effect of in vitro digested extracts of Echinacea purpurea L. plant parts in an inflammatory model of human colon cells. Int. J. Mol. Sci. 2024;25(3):1744. doi: 10.3390/ijms25031744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Yuan L., Liu J., Muhammad I., Cao C., Shi C., Zhang Y., Li R., Li C., Liu F. Dihydromyricetin attenuates Escherichia coli lipopolysaccharide-induced ileum injury in chickens by inhibiting NLRP3 inflammasome and TLR4/NF-κb signalling pathway. Vet. Res. 2020;51(1):72. doi: 10.1186/s13567-020-00796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ye X., Escames G., Lei W., Zhang X., Li M., Jing T., Yao Y., Qiu Z., Wang Z., Acuña-Castroviejo D., Yang Y. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023;28(1):51. doi: 10.1186/s11658-023-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpetti C., Re A.D., Seguella L., Palenca I., Rurgo S., Conno B.D., Pesce M., Sarnelli G., Esposito G. Cannabidiol inhibits SARS-Cov-2 spike (S) protein-induced cytotoxicity and inflammation through a pparγ-dependent TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line. Phytother. Res. 2021;35(12):6893–6903. doi: 10.1002/ptr.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhuac E.B., Maggiolino A., Elghandour M.M.M.Y., Palo P.D., Salem A.Z.M. Antioxidant and anti-inflammatory properties of phytochemicals found in the Yucca genus. Antioxidants. 2023;12(3):574. doi: 10.3390/antiox12030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Zhu H., Chen J., Chen H., Dai D., Wu J. Metagenomic insights into pigeon gut microbiota characteristics and antibiotic-resistant genes. Biology. 2025;14(1):25. doi: 10.3390/biology14010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Bao T., Wang Z., Sun J. A combination of garlic oil and cooked chilli oil could be effective and efficient for pigeon production. J. Anim. Physiol. Anim. Nutr. 2022;106(5):1097–1106. doi: 10.1111/jpn.13646. [DOI] [PubMed] [Google Scholar]
- Francis G., Kerem Z., Makkar H.P.S., Becker K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88(6):587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(6):338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- Ghasemi H.A., Nari N. Effect of supplementary betaine on growth performance, blood biochemical profile, and immune response in heat-stressed broilers fed different dietary protein levels. J. Appl. Poult. Res. 2020;29:301–313. [Google Scholar]
- Hu R., He Y., Arowolo M.A., Wu S., He J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants. 2019;8(3):67. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., He L., Li D., Zhuo L., Chen L., Shi R.Q., Luo J., Feng Y., Liang Y., Li D., Congmei X., Fu Y., Chen Y.M., Zheng J.S., Tao L. Human gut microbial aromatic amino acid and related metabolites prevent obesity through intestinal immune control. Nat. Metab. 2025;7(4):808–822. doi: 10.1038/s42255-025-01246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C.L., He Y.A., Jiang S.G., Wang X.Q., Yan H.C., Tan H.Z., Gao C.Q. Chemical composition of pigeon crop milk and factors affecting its production: a review. Poult. Sci. 2023;102(6) doi: 10.1016/j.psj.2023.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Maruya M., Kato L.M., Suda W., Atarashi K., Doi Y., Tsutsui Y., Qin H., Honda K., Okada T., Hattori M., Fagarasan S. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41(1):152–165. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Kozłowski K., Vervenne-Zetteler P., Konieczka P., Szymański Ł., Vilsteren A.V. Yucca schidigera improves performance and lowers oocyst counts in Eimeria challenged broilers. Animals. 2022;12(13):1668. doi: 10.3390/ani12131668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., He Q., Gibril B.A.A., Xu J., Shang H., Xiong X. Influencing factors and quality traits of pigeon meat: a systematic review. Poult. Sci. 2025;104(4) doi: 10.1016/j.psj.2025.105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xi R., Zhang Z., Li W., Liu Y., Jin F., Wang X. 4-hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1α in seawater aspiration-induced lung injury in rats. Int. J. Mol. Sci. 2014;15(7):12861–12884. doi: 10.3390/ijms150712861. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J.A., Kershaw S.J. The ameliorating effect of Yucca schidigera extract on canine and feline faecal aroma. Res. Vet. Sci. 1997;63(1):61–66. doi: 10.1016/s0034-5288(97)90159-4. [DOI] [PubMed] [Google Scholar]
- Ma X., Meng Z., Jin L., Xiao Z., Wang X., Tsark W.M., Ding L., Gu Y., Zhang J., Kim B., He M., Gan X., Shively J.E., Yu H., Xu R., Huang W. CAMK2γ in intestinal epithelial cells modulates colitis-associated colorectal carcinogenesis via enhancing STAT3 activation. Oncogene. 2017;36(28):4060–4071. doi: 10.1038/onc.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Dou Y., Fan X., Yu B., He J., Zheng P., Yu J., Luo J., Luo Y., Yan H., Wang J., Wang H., Wang Q. The effect of dietary Yucca schidigera extract supplementation on productive performance, egg quality, and gut health in laying hens with Clostridium perfringens and coccidia challenge. Poult. Sci. 2023;102(8) doi: 10.1016/j.psj.2023.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M.A., Aedo H., Lopez-Torres B., Maximiliano J.E., Martínez-Larrañaga M.R., Anadón A., Martínez M., Peteiro C., Cueto M., Rubiño S., Hortos M., Ares I. Bifurcaria bifurcata extract exerts antioxidant effects on human Caco-2 cells. Environ. Res. 2023;231(Pt 1) doi: 10.1016/j.envres.2023.116141. [DOI] [PubMed] [Google Scholar]
- Santos H.M., Tsai C.Y., Catulin G.E.M., Trangia K.C.G., Tayo L.L., Liu H.J., Chuang K.P. Common bacterial, viral, and parasitic diseases in pigeons (Columba livia): a review of diagnostic and treatment strategies. Vet. Microbiol. 2020;247 doi: 10.1016/j.vetmic.2020.108779. [DOI] [PubMed] [Google Scholar]
- Sola-Penna M. Metabolic regulation by lactate. IUBMB Life. 2008;60(9):605–608. doi: 10.1002/iub.97. [DOI] [PubMed] [Google Scholar]
- Sun X., Wang Z., Li X., Du S., Lin D., Shao Y. Effects of Yucca schidigera extract on serum biochemical parameters, humoral immune response, and intestinal health in young pigeons. Front. Vet. Sci. 2023;9 doi: 10.3389/fvets.2022.1077555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem. Sci. 2023;48(4):331–344. doi: 10.1016/j.tibs.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang Y., He T., Bumbie G.Z., Wu L., Sun Z., Sun W., Tang Z. Effects of dietary Yucca schidigera extract and oral Candida utilis on growth performance and intestinal health of weaned piglets. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.685540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Jiang Z., Chang X., Xue H., Yahefu W., Zhang X. 4-Hydroxyphenylacetic acid prevents acute APAP-induced liver injury by increasing phase II and antioxidant enzymes in mice. Front. Pharmacol. 2018;9:653. doi: 10.3389/fphar.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Wang Y., Liu H., Chang W., Xu Y., Lin F. Prediction of hemolytic toxicity for saponins by machine-learning methods. Chem. Res. Toxicol. 2019;32(6):1014–1026. doi: 10.1021/acs.chemrestox.8b00347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.