Summary
Developing standardized bioengineered constructs that accurately replicate human skin is a largely sought-after goal. Pathways initiated at the nurturing interface with the dermal compartment have the potential to modulate the developing epidermal architecture. Here, we identified ascorbic acid, a dermis-donated metabolite, as key in modulating the phenotypical identity of immortalized keratinocytes. Priming monolayers with 2 μg/mL of the culture-stable derivative L-ascorbic acid 2-phosphate (A2P) led to the emergence of a basal-like phenotype within the cells, which showed increased clonogenicity, nuclear/cytoplasmic ratio, and upregulation of progenitor markers. Instead, surpassing this dose induced intracellular ascorbic acid accumulation and promoted a motile status. In organotypic cultures, pre-incubation of founding keratinocytes with 2 μg/mL of A2P improved epithelial layering, whereas higher pretreatments resulted in poor stratification. These findings suggest that ascorbic acid levels in the self-renewing epithelium have a fundamental role in determining whether cells initially commit to differentiation, ultimately influencing regenerative outcomes.
Subject areas: Biological sciences, Biochemistry, Cell biology
Graphical abstract

Highlights
-
•
Culturing HaCaT cells in 0.09 mM Ca2+ ameliorates spontaneous differentiation
-
•
A 2 μg/mL bolus of ascorbic acid 2-phosphate fosters HaCaT progenitor-like features
-
•
Increasing the dose onsets cell differentiation, revealing a molecular switch
-
•
Priming initial HaCaT cultures with A2P improves organotypic skin layering
Biological sciences; Biochemistry; Cell biology
Introduction
The demand to find alternatives to animal models in regenerative medicine increasingly drives the need to improve the design of biomimetic structures that replicate human tissue complexity.1 This holds particular significance for studies focusing on skin diseases or aiming to uncover the mechanisms promoting skin auto-regeneration.2,3 On one side, skin affections are leading contributors to non-fatal disease burden, with estimations of over 1 in 4 individuals affected worldwide.4 On the other, defects in tissue repair underlie the most life-threatening skin disorders treated in hospitals.5 Either way, developing reliable tissue equivalents that can be used for research on skin physiology or to test the efficacy of pharmaceutical compounds is of utmost concern.
To establish a standardized epidermal compartment, the HaCaT cell line stands out from other available cell models.6,7,8 Derived from spontaneously immortalized keratinocytes, HaCaT cells can be used in long-term studies while maintaining a large differentiation potential.9,10,11,12 Additionally, this cell line reforms a structured tissue when transplanted onto nude mice. In stark contrast, when used to engineer skin 3D substitutes, many authors have noticed abnormalities in cellular organization within the construct,13,14,15,16,17,18,19 leading to the convincement that they are intrinsically inefficient epithelium formers. Remarkably, most of the aberrations reported would be compatible with the presence of a mixed population heterogeneously constituted by basaloid and squamoid cells at the beginning of the 3D culture, suggesting that differentiation has early arisen during monoculturing. Here, we explored whether optimizing HaCaT cell culture conditions prior to generating skin substitutes improves the quality of the equivalents produced. To this end, we first limited Ca2+ content in culture media to permit signaling while avoiding ion-promoted terminal differentiation.20,21 Then, we pretreated cells with defined concentrations of L-ascorbic acid 2-phosphate (A2P), a stable derivative of the pro-stemness factor ascorbic acid,22,23 which would be normally delivered by fibroblasts to keratinocytes under physiological conditions. Our results show that low concentrations of A2P boost the emergence of progenitor-like phenotypes, enhancing the expression of basal keratinocytes features, whereas when increasing the dose, the metabolic factor accumulates intracellularly and induces less-proliferative, motile states. The behavioral switch can be further recognized in the organotypic constructs founded by differently treated monocultures, as low A2P-cultured HaCaT showed improved epithelial reconstitution compared to non-treated cells, while pretreatments with higher doses lead to poor tissue stratification. Altogether, our data suggest the existence of a phenotypic switch that impacts the timed release of crucial basal compartment-building molecules based on vitamin C levels in the self-renewing epithelium, while substantially improving the generation of skin substitutes with immortalized cell lines.
Results
Balancing Ca2+ content in media for optimal HaCaT cell culture
It is widely known that keratinocytes differentiate in culture upon increasing Ca2+ concentration. Remarkably, many studies conducted in HaCaT have been performed while raising the cells in the presence of a large proportion of these ions. For instance, standard DMEM contains 1.8 mM of Ca2+24 and fetal bovine serum (FBS) ranges between 3.5 and 4 mM,25 substantially surpassing together the differentiating threshold (above 0.1 mM,21,26). Therefore, we first explored whether cell amplification in DMEM 10% FBS is associated with features of mature phenotypes that could be ameliorated by modulating Ca2+ content. To this end, HaCaT cells that had been maintained for three passages in DMEM 10% FBS were plated in a commercially available Ca2+-free medium (Figure S1). Lower Ca2+ conditions were achieved by complementing this medium either with normal FBS (that would reduce Ca2+ to around 0.35 mM) or with a Ca2+-chelated FBS supplemented with 0.09 mM of CaCl2. As control, parental cells were also replated, and we included a totally Ca2+-free culture in our analyses.
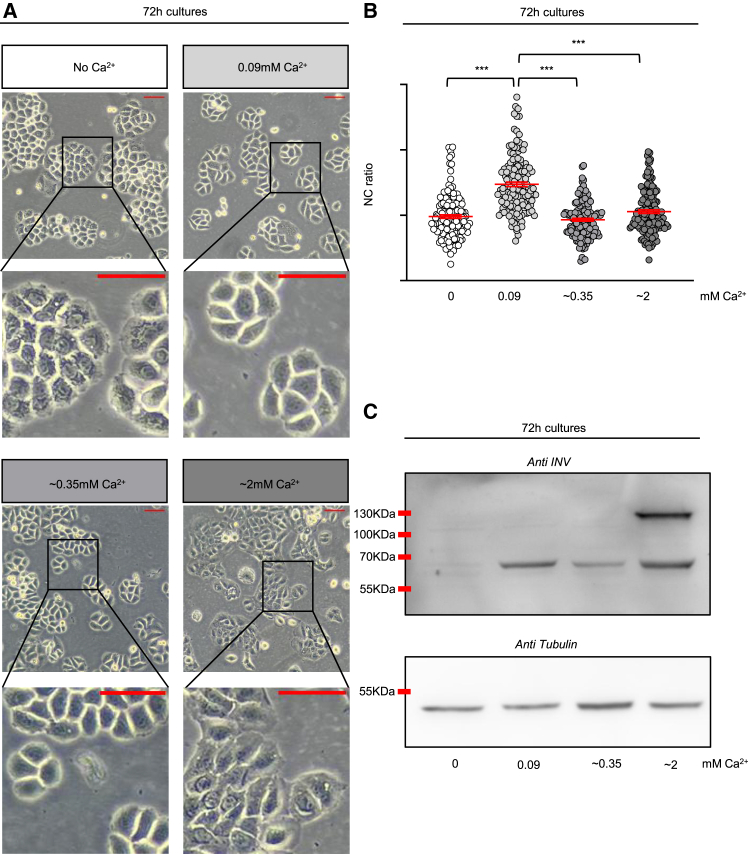
Cell morphology examination after 72 h (Figure 1A) revealed that cells grown in DMEM 10% FBS (lower right panels) had adopted a full squamoid profile. In contrast, HaCaT cells cultured in Ca2+-limited media were less packed and polygonal, reflecting the typical shape of poorly differentiated cells,26 with no major differences between the ∼0.35 (lower left panels) and 0.09 mM Ca2+ (upper right panels) supplemented cultures. Remarkably, the cytoplasm of cells cultured without Ca2+ (upper left panels) appeared filled with dense granules, possibly due to failure of ion-mediated exocytic pathways.27,28 By measuring the respective cross-sectional areas in the images, we quantified the nucleus-to-cytoplasm ratio (NC), plotting the values obtained for single cells in each of the cultures (Figure 1B). High values define less differentiated keratinocytes.29,30,31 Accordingly, only HaCaT cells maintained below the differentiating 0.1 mM limit (0.09 mM cells, lightest gray color) exhibited an increase in NC ratio, whereas cells grown without Ca2+ (0 mM cells, white color) or bred at higher Ca2+ concentrations (∼0.35 mM and ∼2 mM cells, darkest gray colors) showed lower NC mean levels. Taken together, these results corroborate the role of Ca2+ as a promoter of structural changes associated to differentiation12,26 and emphasize the importance of controlled supply of this ion for proper physiology.
Figure 1.
Optimization of calcium content in media for optimal HaCaT cell culture
(A) Representative morphologies adopted by HaCaT cells after 72 h of culture under different calcium concentrations. No Ca2+ (white label) corresponds to cells cultured in Ca2+-free DMEM with 10% chelated FBS, 0.09 mM Ca2+ (light gray label) are cells grown in Ca2+-free DMEM with 10% chelated FBS supplemented with 0.09 mM of CaCl2, ∼0.35 mM (intermediate gray label) denotes HaCaT cultured in Ca2+-free DMEM with 10% FBS, while ∼2 mM (darkest gray label) are cells grown in the standard medium DMEM 10% FBS. Scale bar = 50 μm.
(B) The plot shows the nucleus-to-cytoplasm (NC) ratio of individual HaCaT cells cultured for 72 h under the different calcium-containing media assayed. The values were calculated from the phase contrast images obtained in 3 independent experiments. Each dot in the plot corresponds to a single cell, while the red bars represent the mean NC ratio ±standard error of the mean (SEM). The color code is the same as in the labels of panel A. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗∗∗, p < 0.001.
(C) The image shows a representative western blot analyzing involucrin (INV) expression (upper panel) and tubulin expression as loading control (lower panel) in whole lysates of HaCaT cells grown under the indicated calcium concentrations for 72 h. The low and high molecular weight bands correspond to the soluble and the crosslinked forms of the protein, respectively. See also Figure S2 for band quantification across experiments.
Morphological transitions in keratinocytes upon differentiation are frequently monitored using the expression of the cornifying protein involucrin (INV) as a reporter.20,32 When monocultures thrive in low Ca2+ (<0.1 mM) few cells synthesize INV, but envelopes are not assembled.20 At higher concentrations, most cells express the protein and INV becomes cross-linked by Ca2+-coupled transglutaminases to start cornification.33,34 From this perspective, we analyzed INV expression in our four culture conditions (Figures 1C and S2). Under no Ca2+ addition (0 mM, lane 1), the INV band was hardly discernible, indicating that growing the cells for 72 h under these conditions is enough time to empty any internal stores that could activate INV expression in the absence of an exogenous Ca2+ source, while the protein was abundantly crosslinked at the highest Ca2+ concentration (∼2 mM, lane 4, corresponding to DMEM 10% FBS grown cells). Conversely, in HaCaT cells cultured in intermediate concentrations (0.09 mM, lane 2, and ∼0.35 mM, lane 3), no apparent crosslinking was present and only the soluble form of the protein clearly appeared, albeit at marginally different levels. Since ∼0.35 mM-cultured cells also showed a barely visible faint band when quantifying the area at the molecular weight of the crosslinked form in 2 out of 10 experiments performed (Figure S2, lower panel), we interpreted its slightly lower levels as indicative that this concentration may elicit crosslinked intermediates that were not effectively detected by the antibody employed.
As overall the cells cultured in Ca2+-free medium supplemented with chelated 10% FBS and 0.09 mM CaCl2 matched the less differentiated profile for all the parameters analyzed, we selected this setting for subsequent experiments.
Addition of low doses of ascorbic A2P enhances clonal growth of HaCaT cells
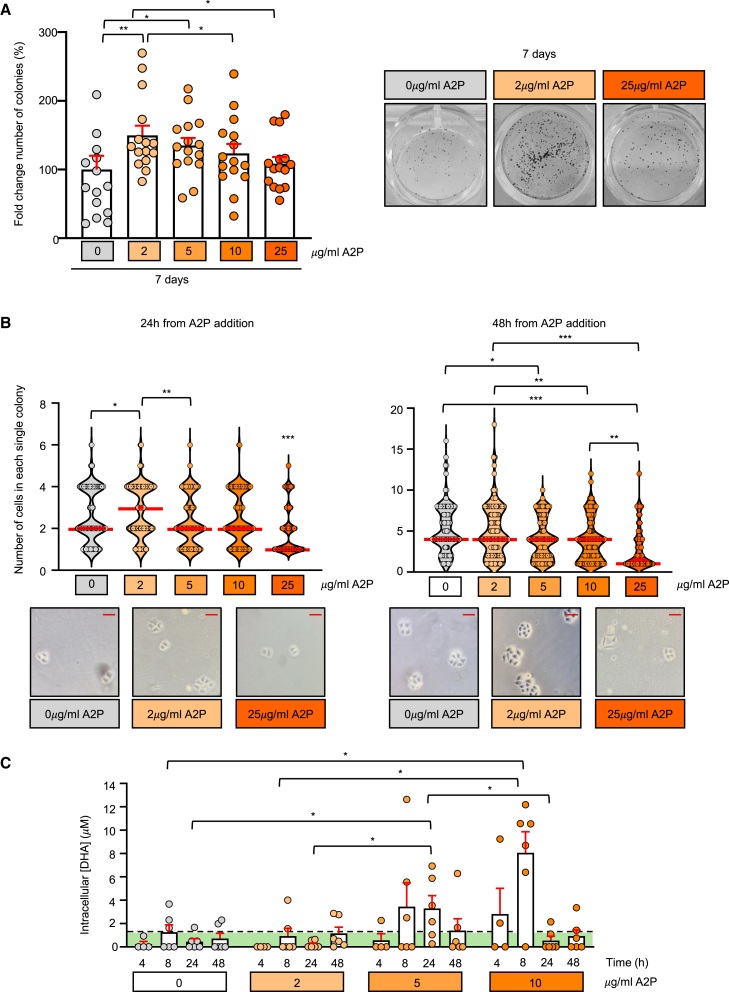
Though indisputably Ca2+ is determinant to drive terminal differentiation, transitional pathways presumably involve additional factors.35,36 Considering that epidermal profiles are influenced by the underlying dermis,37 we decided to explore whether HaCaT cells can be prompted to adopt a more proliferative phenotype when cultured in the presence of a soluble metabolite physiologically delivered by dermal cells. Among such metabolites, ascorbic acid emerges as a promising candidate to modulate keratinocyte fate due to its capacity to induce cell reprogramming and self-renewal.23 However, spontaneous oxidation of this vitamin in cultures38,39 results in abundant cytotoxicity.40 Hence, we employed a stable derivative, in which the sensitive moiety of ascorbic acid is protected by a phosphate.22,41,42 Thus, HaCaT cells were seeded at clonal density, let adhere for 24 h and then cultured in continuous presence of scaled concentrations of L-ascorbic A2P or in its absence; after 7 days, we scored the number of colonies per well (Figure 2A). The treatment with the modified vitamin (left panel, columns with orange dots) produced an overall increase in the number of clones present at the end of the experiment, peaking at 2 μg/mL A2P (column with lighter orange dots) and then gradually declining to reach non-treated cells values (column with gray dots) at the largest concentration used (25 μg/mL). We concomitantly counted the number of cells that formed each colony in the first 48 h following A2P addition (Figure 2B). After 24 h (left panel), 2 μg/mL A2P-resulting clones contained more cells than the rest of its counterparts. At 48 h (right panel) a second upshot appeared: in the presence of 5 μg/mL A2P and beyond, the number of exponentially expanding clones was markedly reduced, suggesting that early colonies had lost their self-renewing capacity. The phenomenon was prevalent at the highest concentration used, indicating that most of its colonies corresponded to abortive clones, a fact further reinforced by the appearance of squamoid cell morphologies (see lower right panel in Figure 2B).
Figure 2.
Addition of A2P enhances survival and clonogenicity of HaCaT cells
(A) The percentage of the number of colonies obtained after 7 days of culturing cells in Ca2+-free DMEM with 10% chelated FBS and 0.09 mM of CaCl2 in the continuous presence of scaled concentrations of L-ascorbic acid 2-phosphate (A2P, orange columns) or in its absence (gray column) is shown (left panel). Data correspond to ≥10 independent experiments ±SEM and is presented as the fold change of the number of colonies respect to non-treated cells. Statistics were performed with the non-parametric Wilcoxon test for paired data; ∗, p < 0.05; ∗∗, p < 0.01. All significative results showed a large effect (|r| = 0.5–1). Representative images of the clonal density quantified in A of HaCaT cells cultured in continuous presence of either 0, 2, or 25 μg/mL of A2P (right panel).
(B) Quantification of the number of cells per single colony after 24 h (upper left panel) and 48 h (upper right panel) of A2P supplementation. The red line in each violin plot represents the median for each condition in ≥5 independent experiments. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001. On the lower panels representative images show colonies formed after 24 h (left panel) or 48 h (right panel) of culturing the cells with either 0, 2, or 25 μg/mL of A2P. Scale bar = 50 μm.
(C) Intracellular content of dehydroascorbic (DHA) in HaCaT cells grown in Ca2+-free DMEM with 10% chelated FBS and 0.09mM of CaCl2 in the continuous presence of the indicated A2P concentrations. The green area under the dashed line represents the maximum background signal as obtained from cells cultured in the absence of the modified vitamin (A2P). The average of ≥10 independent experiments ±SEM is shown. Statistics were performed with the non-parametric Wilcoxon test for paired data; ∗, p < 0.05. All significative results showed a large effect (|r| = 0.5–1).
The hormetic impact on clonal growth of the A2P treatment led us to determine whether a correlation exists between the amount of ascorbic acid internalized and its opposing (either promoting or halting) outcomes. To measure the intracellular content before and after the A2P treatment, we used a validated method based on the use of the radical tempol to oxidize all the ascorbic acid contained in cell lysates to dehydroascorbic acid (DHA), so it can react with o-phenylenediamine to form a fluorescent product.43 As shown in Figure 2C, a significant increase in DHA was soon detected in 10 μg/mL A2P-treated HaCaT cells (darker orange columns), peaking at 8 h from addition and disappearing thereafter. Accumulation was also evident in 5 μg/mL-supplemented cells at later time points (24 h, intermediate orange columns), as it is expected for a lower input. In contrast, the 2 μg/mL A2P treatment (lighter orange columns) maintained ascorbic acid levels below significant ranges, suggesting that, to produce the enhancing effect, the vitamin should be limited to a quantity that can be effectively derived to consuming intracellular pathways. Notably, a modest increase in ascorbic acid content was observed at 48 h in this sample, a fact that may reflect incipient accumulation. Because at 48 h, the number of cells cultured in 2 μg/mL A2P also started to return to control values (Figure 2B, right panel), we resolved to further investigate the phenotype derived from the treatment after a single 24 h-incubation bolus instead of after continuous exposure to the compound.
A proliferation-differentiation switch controlled by ascorbic acid?
The limited division capacities of 5 μg/mL A2P-grown clones, together with the gain in self-renewal promoted by the 2 μg/mL treatment, may recall for the existence of a transitional switch mediated by ascorbic acid internalization. If this is the case and HaCaT cells can be either rewound to basaloid phenotypes or induced to differentiate depending on the quantity of A2P supplied to cultures, the cell NC ratio would also vary accordingly. Therefore, we seeded HaCaT cells onto coverslips, let adhere for 24 h and incubated them with or without the indicated A2P concentrations for further 24 h before fixing. Then, we fluorescently labeled the plasma membrane of non-permeabilized cells using an anti-integrin β1 antibody (Lia1/244) and stained the nucleus with Hoescht. Figure 3A depicts the results obtained after computing the ratio between the cytoplasmic and the nuclear areas. Following our predictions, the 2 μg/mL bolus clearly induced an increase in the NC ratio with respect to non-treated cells (light orange and gray plots, respectively). The NC ratio of 5 μg/mL A2P cultures (dark orange plot) did not significantly differ from that of cells cultured without the vitamin, though it appeared slightly sloped toward the base of the plot. High integrin β1 expression is a marker for epidermal basal-like phenotypes.45 Thus, the anti-β1 antibody also enabled assessing integrin surface levels by quantifying the mean fluorescence intensity (MFI, Figure 3B). An evident increase in the number of reacting integrin β1 subunits was detected in cells incubated with the 2 μg/mL A2P dose (light orange column), indicating an important upregulation of the basaloid marker under these conditions. Remarkably, 5 μg/mL-treated cells (dark orange column) showed a decrease in their β1 surface levels. Yet, it was non-significant in the frame of the number of experiments performed.
Figure 3.
Low doses of A2P enhance proliferative progenitor features of HaCaT cells
(A) The plot shows the individual nucleus-to-cytoplasm (NC) ratio of HaCaT cells cultured in Ca2+-free DMEM with 10% chelated FBS and 0.09 mM CaCl2 and supplemented for 24 h with the indicated L-ascorbic acid 2-phosphate (A2P) concentrations. The values were obtained from the fluorescently-labeled images corresponding to ≥5 independent experiments. Each dot corresponds to a single cell, while the red bars represent the mean NC ratios ±SEM. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
(B) Quantification of the mean fluorescence intensity (MFI) of the surface expression of total integrin β1 on non-permeabilized HaCaT cells grown under the same conditions as in A, as detected with the Lia1/2 specific antibody. Each dot corresponds with a single cell, while bars reflect the average of ≥5 independent experiments ±SEM. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗∗∗, p < 0.001.
(C) The graphs represent the distribution frequency of HaCaT cell populations in the phenotypic compartments defined as basal (blue label), intermediate (green label), and differentiated (red label) after the supplementation with A2P for 24 h, expressed as a percentage. The graphs show the mean of ≥50 experiments ±SEM. Statistics were performed with the non-parametric Wilcoxon test for paired data; ∗, p < 0.05; ∗∗, p < 0.01; p < 0.001. All significative results showed a medium effect (|r| = 0.3–0.5).
(D) Left graphs show the β1-activated cluster frequency distribution after staining HaCaT cells grown and primed as in A with the integrin β1 activation reporter HUTS4 antibody. The MFI of each cluster is plotted against its respective area. Two categories, an early-formed group (0.5-1 μm2, in blue) and a more mature subclass (>1 μm2, in red), were defined among clusters and are highlighted in the figure by dashed boxes. To comparatively represent cluster maturation transitions among treatments, the MFI of the foci lying in each of these boxes was extracted and plotted in the right graph. The red lines are the mean MFI ±SEM obtained when representing ≥10 independent experiments. Statistics to parallel data obtained for the early-formed clusters among treatments were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test, which showed no significances; statistics for the mature cluster category and the internal differences between the two subclasses of clusters under each culture condition were performed with the non-parametric Mann-Whitney U test for independent measurements; ∗, p < 0.05; ∗∗∗, p < 0.001; ns, non-significative. With the latter tests, significance showed a small (|r| = 0.1–0.3) and medium effect (|r| = 0.3–0.5), respectively. See also Figure S4 for representative images of the recorded foci and distribution quantifications.
As our measurements may be partially influenced by the restricted sample size of the field of view, we performed flow cytometry assays on whole populations. We first analyzed the size/complexity distribution of 0.09 mM Ca2+-supplemented cultures in the absence of any A2P treatment to define phenotypical identities based on β1 staining (Figures S3A and S3C). The highest β1-expressing subpopulation was interpreted as the most basal-like, while the lowest was qualified as the most differentiated. An intermediate compartment was also delineated, presumably corresponding to partially committed phenotypes. Parallel labeling of DMEM 10% FBS-grown cells served as a control (Figures S3B and S3C). In agreement with our previous observations (see Figure 1), basal-like cells were less abundant in DMEM 10% FBS cultures than in the Ca2+-controlled medium (Figures S3A and S3B), and most of the cells were detected in the intermediate area. In contrast, fewer cells were recorded in the differentiated region, likely because terminally differentiated HaCaT were shed during the analysis. As expected, lower levels of the integrin were detected in all compartments in the presence of high Ca2+ concentrations (Figure S3C), confirming that the marker is downregulated when differentiation is triggered.45 Building on this basis, we characterized the distribution of 24 h-treated 2 μg/mL and 5 μg/mL A2P populations and represented the population percentages in each compartment in comparison to non-treated cultures (Figure 3C). In accordance with their enhanced NC ratio, cells incubated with 2 μg/mL A2P (light orange columns) were mainly detected in the basaloid region, in detriment of the other subpopulations. Opposite was the behavior of non-treated cells (gray columns), which were more abundant in the differentiated compartment. Also, 5 μg/mL A2P cultures experienced an increase in their basal-like subpopulation and a decrease in the number of cells recorded in the differentiated region with respect to the controls (dark orange columns). However, their intermediate subpopulation was significantly enriched in comparison with the 2 μg/mL A2P bolus, indicating that a higher number of cells were transitioning to committed phenotypes. Instead, when monitoring the β1 integrin profile in each compartment (Figure S3D) we were surprised, as we were only able to record a non-significative higher surface expression of the marker for the 2 μg/mL A2P-treated cells (light orange columns) in the basal compartment. Nevertheless, 5 μg/mL A2P cultures (dark orange columns) scored less β1 positive cells in all subpopulations both relative to the lower dose and to non-treated cultures. Since non-adhered keratinocytes promptly mature and change their marker repertoire,45,46 we interpreted that these results stemmed from the differentiation-inducing effects of plate detachment required for flow cytometry sample preparation.
In light of this, we returned to the adhesive setup to analyze integrin activation. In line with their total β1 expression levels, detection of integrin open/active conformation (characterized by higher ligand-binding affinity) using the monoclonal antibody HUTS447,48,49 identified a greater number of cells containing a larger quantity of β1-active foci in 2 μg/mL-treated cultures (Figures S4A and S4B), while these were greatly reduced in cells incubated with 5 μg/mL A2P with respect to controls. To visualize whether the treatments affected cluster size and integrin density, we plotted the area and mean fluorescence intensity of each of the foci under the three conditions (Figure 3D, left panels). Quantification was performed separately for two cluster populations: an early-formed group with sizes ranging between 0.5 and 1 μm2 and a population representing mature β1-active complexes with sizes up to 1.5 μm2. Then, we computed the percentage of foci that laid in each size class relative to total cluster numbers (Figure S4C). Cells treated with the 2 μg/mL dose of A2P and non-treated cells reached similar frequency values in the smaller category (0.5–1 μm2, gray and light orange columns, upper panel), while 5 μg/mL-incubated cells formed fewer of these structures (dark orange column). Among the largest class (>1.5 μm2, lower panel), the prevalence of foci in 2 μg/mL A2P-treated monolayers was significantly higher when compared to the other cultures, suggesting enhanced transitions from early to mature foci. To better monitor progression among the heterogeneous dense foci, we established a threshold based on HUTS4 staining. Small, bright foci (blue box in Figure 3D, left panels) will either disperse, showing a less intense but still comparable area, or grow into mature, larger clusters of diverse brightness. The most stable conformation will correspond to the largest and brightest clusters (red box). Hence, if the 2 μg/mL A2P bolus stimulates stable complex formation, the frequency and intensity of their mature, denser population should be greater than the rest. Indeed, the percentage of large clusters in the highest β1-positive region was increased in 2 μg/mL-treated cultures when compared to non-treated cells (light orange column, Figure S4D). No foci over the brightness threshold were recorded upon incubation with 5 μg/mL A2P. Interestingly, the largest integrin complexes in 2 μg/mL A2P cells were not only brighter than those in control cultures (Figure 3D, red dots in the right panel), but on average, were denser than their smaller counterparts (blue dots), denoting the progression of cluster maturation. Instead, non-treated cells slightly tended to deplete active integrins when increasing in size, suggesting that the stable small clusters previously formed contained integrins that undergo a relatively rapid turnover. Due to their absence in the largest and brightest region, foci disassembly would be even faster in 5 μg/mL cultures. As it has been shown that bigger, denser integrin-mediated adhesions hinder cell migration,50 this specific outcome predicts that 5 μg/mL may more easily disengage their adhesive structures to acquire a motile state.
Effects of the ascorbic acid-based switch in 2D cultures
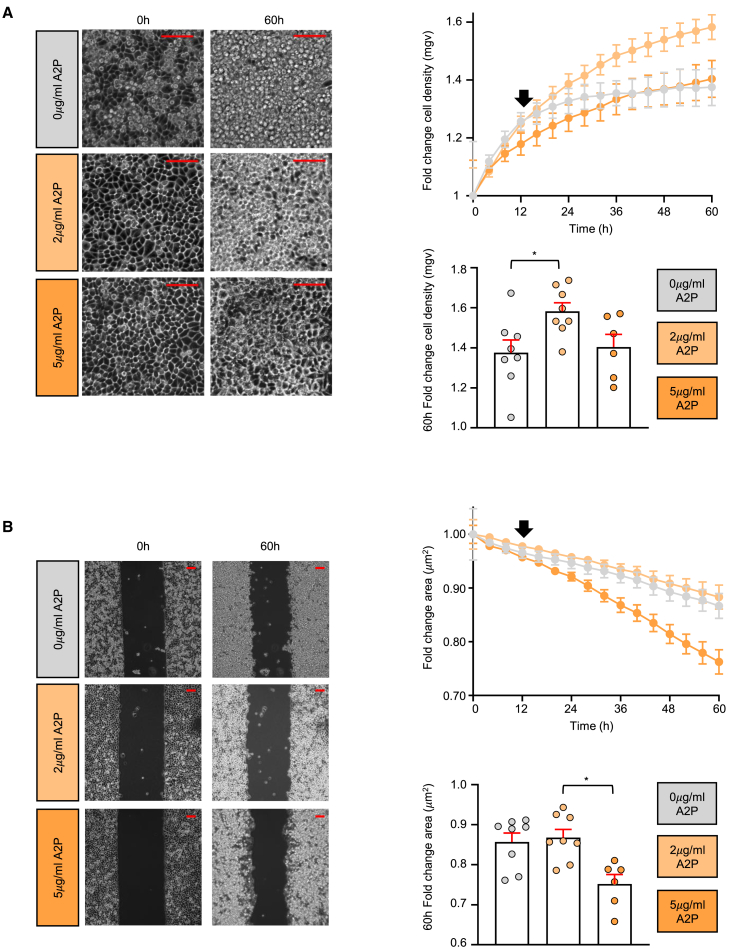
We next characterized the behavior of our cultures in an experimental setting that assesses both proliferation and migration.51,52 Thus, we conducted a gap filling assay in which we seeded either non-treated cells or 24 h-treated with either 2 or 5 μg/mL A2P HaCaT in a plate with two chambers separated by a silicon dam. After dam removal, we followed their fate by live imaging. Two parameters were monitored: the mean gray value of a fixed region of interest, denoting monolayer density (Figure 4A, left panel), and the refilling of the space previously occupied by the dam (Figure 4B, left panel). Regarding the former, all treatments displayed similar increasing cell densities in the first 8 h (Figure 4A, right upper panel), indicating that all of them equally sensed the proliferative need. However, after 12 h, non-treated and 5 μg/mL A2P-incubated cells (gray and dark orange lines, respectively) reached a growing plateau. In contrast, the density curve corresponding to the 2 μg/mL A2P-treated monolayer (light orange line) increased throughout the length of the experiment (Figure 4A, right lower panel), in consonance with the greater capacity of self-renewal observed for these cells. When gap closure efficiency was evaluated, all cultures performed similarly initially (Figure 4B, right upper panel). Surprisingly, after 12 h of imaging, the cells incubated with 5 μg/mL A2P experienced a remarkable acceleration in their rate of closure compared to the 2 μg/mL and the non-treated conditions. Since the proliferative plateau is reached at the same time in which gap refilling is enhanced (compare upper right panels of Figures 4A and 4B), these results suggest that in the frame of a regenerative process, differential ascorbic acid accumulation may timely regulate the acquisition of either a proliferative or a motile phenotype.
Figure 4.
Monitoring the effects of the distinct doses of A2P on 2D HaCaT cultures
Live imaging gap filling assays were performed using HaCaT cells grown in Ca2+-free DMEM with 10% chelated FBS and 0.09 mM of CaCl2 and primed for 24 h with the indicated L-ascorbic acid 2-phosphate (A2P) concentrations.
(A) Left panels correspond to representative fixed regions of interest showing monolayer cell density at the beginning (0 h) and end (60 h) of the time course analyses. Scale bars = 100 μm. The results of ≥5 independent experiments are averaged on the upper right panel and represented as fold change of cell density as measured from the mean gray value plotted against time ±SEM. On the lower right panel, the mean values for the 60 h time point are shown using bars for clarity. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗, p < 0.05.
(B) Left panels show representative whole frames of the same experiments described in A. Scale bars = 100 μm. The closure capacity of the cells is represented in the upper right panel and expressed as the fold change of the empty space area separating both cell monolayer borders against time in ≥5 independent experiments ±SEM. Again, the lower right panel highlights the results for the last time point acquired (60h). Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗, p < 0.05. In both A and B, the black arrow in the graphs denotes the 12 h time point in which differences between conditions start to become evident.
From 2D to 3D: Construction of lab-grown skin substitutes starting from monolayers pretreated with A2P
In organotypic collagen-based cultures, HaCaT cells have been shown either to poorly stratify13,14,17,18,19,53,54,55,56 or to require a number of supporting fibroblasts higher than primary keratinocyte-based constructs to do so.53,57,58 However, in surface transplants on nude mice, HaCaT cells reform a rather structurally normal epidermis conserving its functional features,9,11 suggesting that epidermal tissue organization strongly relies on a molecular defect under the control of a mesenchymal-derived factor. Furthermore, in most reports DMEM 10% FBS-grown monocultures were directly transferred to the 3D setup. To assess whether our preconditioning treatment improves the quality of the constructs produced by the cell line, we established 3D cultures starting from monolayers grown under our carefully controlled Ca2+ conditions and treated or not with either 2 μg/mL or 5 μg/mL A2P for 24 h. As collagen matrices have been shown to hinder fibroblast activity,59,60 we substituted the scaffolding collagen by plasma-derived fibrin gels,61 which are also the most advanced implanted matrix currently used in clinics.62 Notwithstanding, for strict comparison with the culturing conditions performed in previous analyses, we engineered in parallel fibrin-based constructs founded by DMEM 10% FBS-cultured cells.
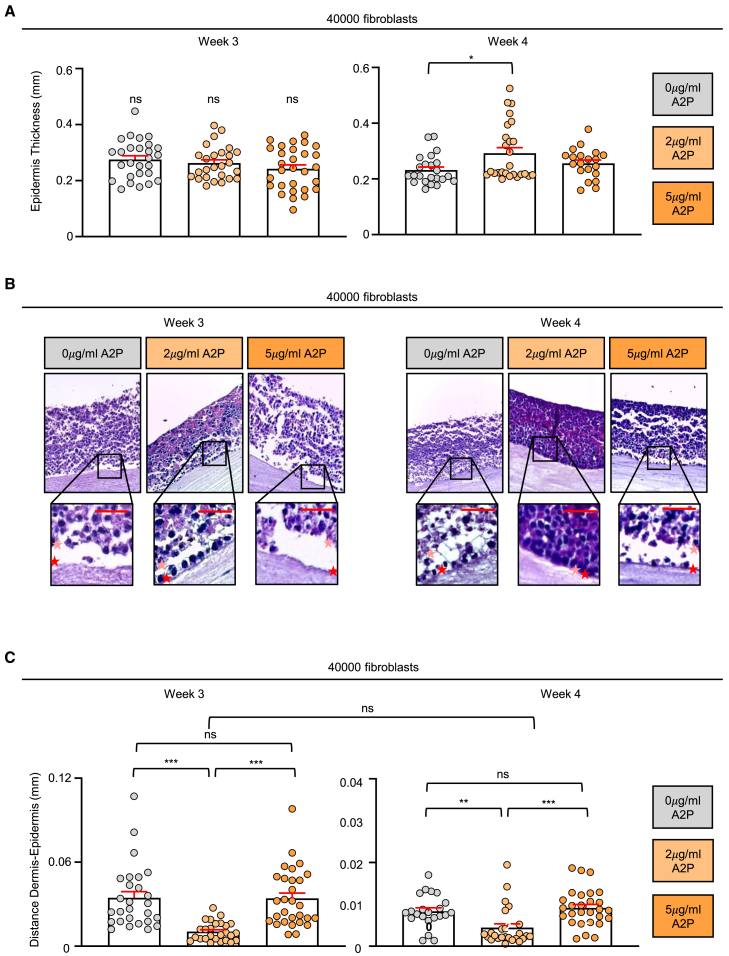
We thus generated fibrin dermal equivalents populated by 4 × 104 primary fibroblasts (Figure S5), a number within the range determined for primary keratinocytes63,64 and below the optimal defined for HaCaT.53 When embedded in this kind of matrix, fibroblasts maximize keratinocyte expansion,65,66 allowing for seeding a number of epidermal cells tightly fitted to the insert dimensions. This strategy greatly diminishes focal cell accumulation due to an excess of cells, a fact that results in abnormal epidermal layering in the final construct. As the media for gel-included fibroblasts includes L-ascorbic acid,67 we replaced the canonical vitamin with A2P to maintain cohesiveness between the nutrients supplied to the whole bilayered equivalent. For comparative purposes, the precaution was not taken with the DMEM 10% FBS destined dermis that was regularly fed with L-ascorbic acid. Then, we seeded on top of each hydrogel 7.5 × 105 HaCaT cells that had been previously grown as monolayers under the four culture conditions under testing (DMEM 10% FBS cultured cells, and non-treated and treated for 24 h with 2 or 5 μg/mL A2P HaCaT in Ca2+-free DMEM 10% chelated FBS supplemented with 0.09 mM Ca2+) and allowed them to differentiate by air-lifting. After 3 weeks of exposing its surface, gel contraction was remarkably pronounced in the constructs founded by DMEM 10% FBS-cultured cells, which consistently appeared macroscopically thinner than the rest of their counterparts (Figure S6A). When revealing epidermal structure in paraffined histological sections stained with hematoxylin/eosin, these cultures also showed deficient epithelia composed by only two to three layers of flattened cells (Figure S6B, left panels), reproducing the previous results observed by others.13,14,17,18,19,53,54,55,56 Dosing Ca2+ in the medium of HaCaT pre-monolayers was sufficient to improve epithelium thickness of the organotypic cultures (Figure S6B, right panel), with no significant differences between cells pretreated with A2P and non-treated HaCaT (Figure 5A, left panel). However, distinct epidermal organization was evident in the histological analysis (Figure 5B left panel). While the constructs built with cells that had been pre-incubated with 2 μg/mL of A2P were clearly endowed with a basal-like layer formed by firmly attached cuboidal cells, this structure was absent in the 3D cultures corresponding to either non-treated or 5 μg/mL-treated monolayers. Accordingly, shear forces induced during histological sectioning greatly affected the latter constructs, frequently promoting the detachment of the epidermis from the dermis (Figure 5C, left panel). In the organotypic cultures founded by 2 μg/mL A2P pre-treated cells, disruption of the dermo-epithelial junction was minimal, suggesting that the basal-like cells in this localization were more tightly anchored to the underlying compartment. At 4 weeks since air-lifting, gel contraction increased in the 5 μg/mL A2P-primed ensembles, reaching the levels recorded before for DMEM-10%-founded cultures (Figure S6C), likely due to increased keratinocyte pulling. Epidermal maturation progressed only in the 2 μg/mL A2P monolayer-derived cultures (Figure 5A, right panel), not only in terms of thickness but also at the level of tissue organization. Their basal layer appeared more aligned and compact, while the epidermal surface was evenly smooth (Figure 5B, right panel). In contrast, the constructs initiated by non-treated or 5 μg/mL-incubated HaCaT presented few cells attached to the dermis, mostly showing an abnormal morphology. Remarkably, the surface of the organotypic cultures established by non-treated HaCaT appeared mostly degraded, while the outermost layer of the epidermis built by 5 μg/mL A2P-treated monolayers was composed by viable cells that tended to accumulate in this location. Similarly to the earlier time point, enhanced resistance to shear forces was solely a feature of the 2 μg/mL A2P-primed constructs (Figure 5C, right panel), suggesting that only cells precultured with this dose of the modified vitamin were able to establish tight contacts with the basement membrane.
Figure 5.
Monitoring the effects of priming HaCaT monolayers with A2P before building 3D organotypic cultures
Fibrin-based hydrogels containing 4 × 104 embedded fibroblasts were populated with 7.5 × 105 HaCaT cells grown in Ca2+-free DMEM with 10% chelated FBS and 0.09 mM of CaCl2, pretreated for 24 h with the indicated L-ascorbic acid 2-phosphate (A2P) concentrations and allowed to differentiate. See also Figure S5 for a detailed scheme showing the features of each type of construct tested.
(A) The graphs show the epidermal thickness of the constructs as measured from the internal border near the dermis to the external border. The results obtained after either 3 (left panel) or 4 weeks (right panel) since air lifting are shown. Error bars are the mean of 6–8 slices, belonging to ≥4 independent experiments ±SEM. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗, p < 0.05.
(B) Representative morphological features of the organotypic constructs after culture sectioning and processing with hematoxylin/eosin at either 3 (left images) or 4 weeks (right images) since air lifting are shown; an enlarged view of the dermo-epidermal region exhibits the organization of the compartments and the orientation of the basal layers. Scale bars = 50 μm.
(C) The distance between the dermis and epidermis was determined by measuring the distance between the dermal border (highlighted by a red star in the enlarged view showed in (B) and the first line of keratinocytes (pink star in B) along three different sections of each image, and represented in separated graphs for either 3 (left panel) or 4 weeks (right panel) since air lifting. Error bars result from 6 to 8 slices obtained from ≥4 independent experiments ±SEM. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test when comparing either week values, while differences between weeks for the 2 μg/mL samples were evaluated with the non-parametric Mann-Whitney U test for independent measurements; ∗∗, p < 0.01; ∗∗∗<0.001; ns, non-significative. With the latter test, significance showed a very small effect (|r| < 0.1).
Altered cell-matrix interactions could be a direct consequence of the recognized role of ascorbic acid in promoting collagen synthesis and folding.68 Keratinocytes are relevant contributors secreting in part the collagen IV network that guarantees the mechanical stability of the basement membrane.69 At the same time, the anchorage of the epidermis to this structural scaffold is mediated by hemidesmosomes, in which a transmembrane keratinocyte collagen-collagen XVII- plays an integral function securing attachment in conjunction with α6β4 integrins.70 Therefore, we analyzed the expression pattern of collagen IV and collagen XVII in the keratinocytes of our differently-generated constructs while the epithelium is still in progress at week 3 since air-lifting (Figure 6A). For both proteins and all treatments, staining with specific antibodies revealed that the most basally located cells were the preferential collagen producers, as shown by the ratio of signal distribution when comparing the mean fluorescence intensity of innermost against mid epithelium situated layers (Figure S7A). However, clear differences could also be appreciated. Preincubation of founding cells with the 2 μg/mL bolus resulted in an upregulation of collagen IV expression in the first row of cells lining the basement membrane (upper central frame in the left panel of Figure 6A and light orange-dotted columns in the upper right panel) respect to its counterparts, in line with other studies that have reported a stimulating effect on the synthesis of this type of collagen in other cell models71. Conversely, collagen XVII exhibited a significant restriction of its signal in the low A2P dose-treated cultures to the cells near the dermal interface (lower central frame in the left panel and light orange-dotted columns in the lower right panel), which appeared expanded to mid-layers in the other constructs. In light of a recent paper in which a leading role for keratinocytes on collagen I production has been uncovered,72 we also quantified the levels of this isoform in the dermal area proximal to the epidermis (Figure S7B). However, probably because collagen I generation by fibroblasts dilutes most of the effects, we were not able to record any significant variation in this case, though the 5 μg/mL A2P-founded constructs largely tended to accumulate less fibrils.
Figure 6.
The expression of relevant markers in the 3D cultures reveal that low doses of A2P impact endosomal-trafficking
(A) Representative immunofluorescence of paraffin-embedded fibrin-based hydrogels containing 4 × 104 fibroblasts, populated with 7.5 × 105 HaCaT cells grown in Ca2+-free DMEM with 10% chelated FBS and 0.09 mM of CaCl2, and pretreated for 24 h with the indicated L-ascorbic acid 2-phosphate (A2P) concentrations before differentiating for 3 weeks. The left panels show representative images after staining collagen IV (Col IV) and XVII (Col XVII) with specific antibodies. Scale bars = 100 μm. The right panels show signal quantification of the mean fluorescence intensity (MFI), evaluated in regions of interest at the innermost epidermal layers (dark purple) or at mid layers (light purple). Error bars are the average of 6–8 independently labeled slices, belonging to ≥2 separated experiments ±SEM. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗, p < 0.05; ∗∗, p < 0.01. See also Figure S7A for the relative quantification of the signal distribution between layers.
(B) Representative immunofluorescence of the organotypic constructs built as in A, fluorescently labeled with Integrin β1 (Int β1) and involucrin (INV) antibodies (left panels). Scale bars = 100 μm. The right panels show the quantification of the MFI±SEM, evaluated using regions of interest positioned at the innermost layers (dark purple) or outermost layers (faint purple) of the epithelium. Statistics were performed with the non-parametric Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test; ∗, p < 0.05; ∗∗, p < 0.01. See also Figure S7C for the relative quantification of the signal distribution between layers.
(C) On the upper panel, a representative analysis of XBP1 splicing, as described in the study by Calfon et al.,73 is shown. HaCaT cells pretreated with 5 μg/mL of the ER stressor tunicamycin (TUN) serve as control for the uprising of the spliced mRNA. XBP1U, unspliced form; XBP1S, spliced form. ≥4 independent experiments were performed. The lower panel represents the fold change in the levels of Integrin β1 mRNA expression relative to non-treated cells values. GAPDH was used as internal control and plotted data correspond to the mean ± SEM of 8 independent experiments. Statistics were performed with the non-parametric Wilcoxon test for paired data; ∗, p < 0.05. All significative results showed a large effect (|r| = 0.5–1).
(D) Quantification of IL1α secretion after 24 h pretreatment with the indicated A2P concentrations. The results are represented as the fold change of the values recorded ±SEM of 8 independent experiments. Statistics were performed with the non-parametric Wilcoxon test for paired data; ∗, p < 0.05. All significative results showed a large effect (|r| = 0.5–1).
Importantly, conditional deletion of the integrin β1 subunit in mouse skin produces severe blistering accompanied by massive failure of basement membrane assembly and α6β4/hemidesmosome mislocalization.74,75 Considering that 2 μg/mL-cultured cells harbored higher surface levels of β1 integrin (Figure 3B) that forms increased stable interactions (Figure 3D), we reasoned that the deficiency in productive adhesion receptors should be situated upstream the defects in collagen distribution observed in the 3D cultures built by non-treated and 5 μg/mL A2P-grown cells. If this is the case, then integrin β1-staining in the 2 μg/mL A2P-derived constructs should overlap with collagen IV and XVII allocations. As expected, β1-labelling of the epidermis produced by cells treated with the low dose of A2P was finely limited to the contact interface with the dermal compartment (Figure 6C, upper central frame in the left panel and light orange-dotted columns in the upper right panel), showing a marked decrease while progressing to suprabasal locations (Figure S7C, upper panel). Layer-restrained distribution of the basal marker likely reflects integrin downregulation as a result of differentiation.45 Accordingly, 2 μg/mL A2P-founded constructs showed an opposite gradient of INV staining (middle central frame in the left panel and light orange-dotted column in the central right panel in Figure 6C, and lower panel in Figure S7C) with higher intensities peaking at the outermost layer of the epithelium. In alignment with a more mature initial state of its founding cells, β1 signals were barely detectable in cultures established using 5 μg/mL A2P-incubated HaCaT (Figure 6C, upper right frame in the left panel and dark orange-dotted column in the upper right panel), while these constructs had abundant INV expression across all layers (middle right frame in the left panel and dark orange-dotted column in the middle right panel). A similar INV staining pattern was observed in the 2D cultures built by non-treated cells (middle left frame in the left panel and gray-dotted column in the middle right panel). In contrast, when examining the expression of integrin β1 in these constructs we gathered a major surprise. Not only was the adhesion receptor uniformly distributed across the entire epidermis but also showed a remarkable increase in intensity considering its poor surface expression (see the results in Figure 3B and compare with the upper left frame in the left panel and gray-dotted column in the upper right panel). As only properly folded, balanced expressed proteins are allowed to exit the secretory pathway and aberrant proteins trapped in its composing organelles frequently to increased fluorescent read-outs,76,77 misplaced integrin subunits may in part represent inactive forms prone to be turned over. Indeed, it has been demonstrated that the intracellular accumulation of misfolded collagens observed in the absence of ascorbic acid can create a burden on the entire secretory pathway, leading to a decreased secretion of many different collagen-unrelated secretory proteins.78 Therefore, we next investigated whether non-treated cells manifest a non-resolved activated unfolded protein response which may be alleviated by the low A2P treatment. In response to accumulation of misfolded proteins, the mRNA of the transcription factor Xbp1 is early spliced to promote cell survival.79 Analyses of XBP1 splicing, however, showed no differences among the samples (Figure 6C, upper panel). Yet, the protein might be retained either because an increase in its load overwhelms the secretory machinery or by lack of its major binding partner α3. Notwithstanding, non-treated 3D cultures still expressed significant levels of the α3 expression controller TP63 (Figure S7D, compare the upper and middle frames and the gray and light orange-dotted columns in the right panel), the transcription factor both responsible for anchoring basal cells to the niche and for regulating progenitor cell survival and self-renewal programs,80,81,82 even if tended to be less abundant at the basal layers than in 2 μg/mL A2P-derived constructs. Correspondingly, the mRNA of the β1 chain of the integrin was also reduced in the epidermis built by untreated HaCaT (Figure 6C, right panel), suggesting that 2 μg/mL A2P founding cells might have higher levels of the integrin, if any. Since apparently folded, sufficiently expressed β1 is retained intracellularly in the constructs produced by cells grown in absence of the A2P and our previous results suggested an impact in its recycling kinetics (Figure 3D), we presumed that the adhesion receptor could be sequestered in plasma membrane-proximal vesicles belonging to the late endosome secretory pathway, prompt to be delivered to its functional location in presence of the metabolite. To check for this possibility, we focused on a keratinocyte-relevant molecule that is released through a process independent from the conventional secretory route. Interleukin-1α (IL1α) is a cytokine secreted by keratinocytes during re-epithelialization to engage in a dual paracrine mechanism leading to the synthesis of keratinocyte-destined growth factors by fibroblasts.83 Importantly, it is also a leaderless protein that reaches the extracellular space through unconventional fusion of endo/lysosomes with the plasma membrane.84,85 To measure whether incubation with A2P has an impact on the membrane fusion of the late vesicles containing IL1α, we used a specific enzyme-linked immunosorbent assay (ELISA) that quantifies the extracellular levels of IL1α. Collected supernatants of cells grown either in absence or presence of 2 or 5 μg/mL of A2P showed remarkable differences in their ability to externalize the cytokine: when compared, 2 μg/mL A2P-incubated cells were efficient secretors, while non-treated cells and HaCaT exposed to 5 μg/mL A2P showed diminished rates, more pronounced in the latter case (Figure 6D). Considering that the constructs established by 5 μg/mL A2P-cultured HaCaT were originated by cells in a presumably more differentiated state that actively downregulate β1 subunits, the reduction of IL1α extracellular levels in 5 μg/mL A2P-grown cells may be due to a similar process, which would transcriptionally repress the proliferation promoter axis. In contrast, in absence of the vitamin, HaCaT are not able to culminate the process of plasma membrane fusion of vesicles containing β1 and, concomitantly, IL1α is less secreted, suggesting that fundamental basal compartment-building molecules just barely reach the cell surface when there is no ascorbic acid.
Taken together, our data indicates that HaCaT cells have not intrinsically lost their capacity for regenerating the epidermis but rather are not competent to do so due to impairment of the communication route with the attachment surface and the dermal cellular supporters. Rescuing vesicle externalization by prior supplementation of the cells with A2P is sufficient to promote the formation of a closely physiological basal lining which then timely organizes differentiation to reconstitute the epithelium. Nonetheless, the modified vitamin must be carefully dosed to achieve normalized skin structures, as when in excess, cells mature. On that basis it can be hypothesized that ascorbic acid in the lower epidermis has a dual switch function which stimulates contact with the extracellular matrix below at low concentrations while marking the onset of differentiation above critical internalized thresholds, thus constituting a crucial element for establishment and maintenance of the proliferating progenitor area.
Discussion
In the past, pioneering work demonstrated that closer control of medium components is a crucial requirement to understand all aspects of keratinocyte physiology.26,86 While many authors have explored the role of different growth factors on the fate of these cells, less attention has been paid to metabolites supplied through the tight connections established between the dermal and the epidermal compartments. In this sense, our results indicate that keratinocyte preconditioning with L-ascorbic A2P has a direct impact on the quality of organotypic cultures founded by these cells. As the protective phosphate is removed only when accessing the cell surface,22,41,42 most likely the species responsible for the effect is the non-radical form of the vitamin. In vivo, fibroblasts assimilate ascorbic acid and donate it to nearby keratinocytes residing in the basal lamina through a not yet completely elucidated mechanism.87 In contrast, most keratinocyte cell lines are routinely grown in a scurvy-like environment, possibly because the unrestrained division of cells hinders noticing prejudicial consequences. Specifically concerning immortalized keratinocytes, something similar happens with Ca2+. Although few manuscripts have warned against the possibility of raising heterogeneous phenotypes in cultures,12,88 the use of high Ca2+ medium for cell expansion is generalized. HaCaT cells grow well in DMEM 10% FBS with rough doubling times of 24 h. Fully differentiated phenotypes are not often perceived, as they are shed from cultures during maintenance passages. However, partially differentiated cells persist (see Figure 1). Indeed, balancing Ca2+ supplementation to achieve complete homeostasis during cell culture is not a banal issue. As an example, even if we selected to work with a fixed Ca2+ concentration below the differentiating limit, we were not able to completely abrogate involucrin expression (Figure 1C). A careful titration of the cation in the medium, considering renewal times and the control of internal stores will be something highly desirable to investigate.
Equally exciting would be to ascertain whether the signals that impel differentiation elicited by ascorbic acid and Ca2+ converge. While DMEM 10% FBS-grown cells change their morphology and initiate envelope assembling (Figure 1), this signs of terminal differentiation are never observed in 5 μg/mL A2P-treated cells (not shown). Interestingly, the complete picture of signaling in which Ca2+ intervenes is still poorly understood.36,89,90 Some authors have proposed that terminal differentiation may well be a two-step process: the first one will be a reversible primed state in which proliferation ceases, preparing the cell for a stronger commitment signal such as Ca2+.35,91 Most likely, several pathways will be functioning both in sequential and parallel fashions. At this regard, it is remarkable that a Ca2+ gradient is organized vertically in the epidermis -the lowest levels near the basal membrane, the highest toward the exterior92,93- and that ascorbic acid also distributes in a gradient, whose levels run in the opposite direction.94 As due to environmental exposure the lowest amounts of ascorbic acid correspond to surface layers, it is tempting to speculate that keratinocytes are loaded with this metabolite at the basal membrane in sufficient quantity to confront oxidative damage, and that its internal levels would be the discharger that “signals” that they are prepared for the outer world. Our data would support this concept because concomitantly with ascorbic acid intracellular accumulation, proliferation does actually decline (Figure 2). At the same time, cells treated with the highest dose of A2P (5 μg/mL) gain increased migratory capacities (Figure 4B), in accordance with the notion that early differentiated cells lose their attachments and move toward suprabasal cell layers. Along these lines, the positional control of cell fate existing in the multilayered epidermis could acquire a wider dimension. Active cell division is mainly restricted to the dermal-epidermal interface,95 which is as a matter of fact the preferential vitamin C internalization site.23,37 In this location, two daughter cells coming from the same progenitor can both stay proliferative, both exit the cell cycle and differentiate, or one cell may commit to differentiation while the other resumes proliferation.96 Since the decision is made in the restrained bidimensional area in communication with the nourishing surface and ascorbic acid facilitates the exchange of messages (Figures 6B and 6D), we propose that fate choice is not random and that ascorbic acid acts as a physiological timer that sets a limited number of divisions. Thus, while contact with vitamin sources would be necessary to maintain progenitor features (as seen in Figures 2, 3, and 4A), long residences in the proliferative compartment would increase the chances of differentiation. Remarkably, studies focusing on the stem niche of human epidermis came up with similar hypotheses suggesting that diligent keratinocyte proliferation may enhance the likelihood of committing to terminal phenotypes,97 and that cycling progenitors would have an internal “memory,” which specifies a fixed number of cell divisions prior to the onset of differentiation.98 In fact, indications that a switch based on ascorbic acid levels may exist at the edge between proliferation and differentiation can be found throughout scientific literature. In the presence of ascorbate, a wide variety of human cells show bi-phasic growth curves, with stimulating profiles at low concentrations that reverse at higher levels.99,100,101,102 Reports denoting the impact of this vitamin in only one of these processes are even more abundant, retrieving countless hits in any quick bibliography search. As an example, in HaCaT cells, high doses of ascorbic acid induce the latest phases of terminal differentiation.103 The range in which the compound triggers one or the opposite behavior differs between cell types, probably influenced either by differential expression of membrane transporters or by the respective pool of targeted effectors.104,105 Significantly, an analogous boost in late endosome trafficking, akin to our findings, has been documented to influence the surface expression of the high-affinity otherwise intracellular ascorbic acid transporter SVCT2 in neuronal and embryonic kidney cells.106 In conjunction with our data, this may hold considerable importance in the growing epithelium. When building the construct, 2 μg/mL A2P-primed keratinocytes would readily express β1 integrins on their surface, forming productive focal adhesions that efficiently promote initial migration to initiate epithelialization (Figures 3A, 3D, and 6B). IL1α-containing vesicles will be also extruded (Figure 6D), stimulating fibroblasts to produce the necessary growth factors that will maintain proliferation. Substrate-dependent SVCT2 plasma membrane insertion might then substitute or outcompete the keratinocyte surface constitutive low-capacity transporter SVCT1. Whether the cell accumulates enough vitamins would determine that it initiates a committed pathway toward differentiation. These advantages would be lost or delayed in the 3D epitheliums founded by non-treated or 5 μg/mL A2P monolayers, which being formed by immortalized keratinocytes are still able to grow though resulting in unstable, unstructured epitheliums (Figures 5, 6A, and 6B). In support of this, the molecular defect present in epidermis built by scorbutic cells could be rescued, as done by others18,53 by increasing the number of ascorbic acid-donating fibroblasts in the supporting dermis, or by stimulation of IL1α release by the pro-inflammatory molecule TGFα. Noteworthy, the addition of a relatively costless and easily available metabolite in the 2D set-up allows for engineering the hydrogels in the optimal range for primary cell-based cultures, minimizing the risks of generating denser matrices or not expected unbalanced epithelial-stromal interactions. The treatment yields closely physiological skin equivalents (Figures 5 and 6) that can be used as enhanced standardized models, for instance to elucidate in more detail the complex sequence of epidermal-dermal interactions promoting each keratinocyte phenotype, avoiding the intrinsic genetic variability of donors. Additionally, our organotypic cultures also constitute a valuable animal-free platform for the initial testing of pharmacological compounds. Furthermore, the most advanced skin engraftments ever implanted to full-thickness wounded patients are engineered expanding autologous keratinocytes in standard media formulations that, even if often depleted from Ca2+, do not consider supplementation with ascorbic acid.62,107,108 These therapeutical approaches may surely take advantage of the benefits for the formation of the basement membrane ascribed to the effects of priming cells with an optimized dose of A2P prior to building skin implants for clinical practice, which would potentially favor a faster re-epithelialization of the future implant. Indeed, comprehending each cellular component, its interactions, and its behavior within a 3D tissue context is essential for developing more precise, effective, and biologically relevant solutions that address the existing limitations of bioartificial skin grafts, which require improvements in mechanical integrity and the incorporation of immune and vascular elements.109,110 In this sense, our organotypic cultures represent a promising platform from which expand our knowledge in keratinocyte physiology and represent an exemplification of the importance of building organotypic tissues with a molecular biology perspective.
Limitations of the study
It is important to note that there are significant limitations inherent to working with immortalized cell lines. In particular, the exact doses and timing of L-ascorbic A2P supplementation may vary in other models. Thus, we have preferred to highlight that the molecular switch promoted by ascorbic acid is based on low versus high concentrations. This needs to be considered in other cellular or tissular contexts, which can surely benefit from our findings. Also, the dose may vary in immortalized keratinocytes originated by female donors, as HaCaT are a male-derived cell line.9
Additionally, though we extensively documented the outcomes of different dosages of ascorbic acid on cell phenotypes and revealed a significant impact on the wealth of the late secretory pathway in deprived cells, other putative effects of the metabolite that induce timed physiology remain undetermined. As an example, 2 μg/mL A2P-treated cells showed an upregulation of β1 transcripts when compared to non-treated HaCaT (Figure 6C), and the corresponding protein is downregulated in the constructs founded by 5 μg/mL A2P-incubated cells (Figure 6B). However, we firmly established the necessity of dosing the metabolite for building close-to-physiological 3D organotypic epitheliums and its importance for keratinocyte fate beyond its known role in collagen folding. In this sense, our study actually lays the rationality for exploring with advanced high-throughput methods the systemic cell remodeling programs triggered in each case by ascorbic acid supplementation.
Resource availability
Lead contact
Further information and requests for resources and additional data should be directed to and will be fulfilled by the lead contact, Iria Medraño-Fernandez (imedrano@salud.uc3m.es).
Materials availability
This study did not generate new unique reagents or specific biological materials. Further information on the commercially available reagents used can be found in the STAR Methods section. The antibodies Lia1/2 and HUTS4 were purified from the original hybridomas, which are available in our laboratories and can also be purchased from commercial sources.
Data and code availability
-
•
Raw images and cytometry data have been deposited at the e-cienciaDatos repository of the Madroño consortium and are publicly available as of the date of publication. The accession number is listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
This paper does not report any original code.
-
•
The software used to analyze the reported data are disclosed in the key resources table.
Acknowledgments
We thank R. Sitia (San Raffaele Scientific Institute, Milan, Italy) for useful suggestions, exciting discussions, and constructive criticisms, and Dariusz Lachowski for providing the immunohistochemical protocols. In addition, we are grateful to J.L. Jorcano and other members of the Tissue Engineering and Regenerative Medicine (TERMEG) Unit at Universidad Carlos III de Madrid for supporting us while entering the fascinating field of bioengineering. We also acknowledge the technical staff of the CleanRooms of Bioengineering facility core of Universidad Carlos III de Madrid, which housed most of our experimental activities. This work was supported through grants from the Madrid Government (Comunidad de Madrid) under the multiannual agreement with UC3M in the line of “Research Funds for Beatriz Galindo Fellowships” (REDOXSKIN-CM-UC3M to I.M.-F.), and in the context of the V PRICIT (Regional Programme of Research and Technological Innovation), and by “Proyectos de I + D + I” (PID2020-114230 GA-I00 to I.M.-F.) funded by MICIU/AEI/10.13039/501100011033/. A.K.M.-O. was supported by a UC3M-PhD research training scholarship (PIPF), and I.S. has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Action Individual postdoctoral fellowship (grant agreement No [101111496]). C.C. is supported by “Proyectos de I + D + I” (PID2021-123199OB-I00 to C.C.), funded by the Spanish Ministry of Science, Innovation and Universities (MICIU/AEI/10.13039/501100011033) and by the European Union. I.C.-P.’s salary is funded by the Spanish Ministry of Science, Innovation and Universities (MICIU/AEI/10.13039/501100011033) and by the European Union through its program “Ayudas para Contratos Predoctorales para la Formación de Doctores” (Predoctoral Fellowship: PRE2022-101403). The CBMSO, in whose installations we performed part of this work, is a “Severo Ochoa Center of Excellence” (grant CEX2021-001154-S) funded by MICIU/AEI/10.13039/501100011033 and also receives an institutional grant from the Fundación Ramón Areces. Because of space limitations, we apologize to all those colleagues and researchers whose work is not directly cited here.
Author contributions
I.M.-F., A.K.M.-O., and I.S. designed the strategy of the study. A.K.M.-O. performed the experiments depicted in Figures 1, 2, 5, and 6A and 6B. I.S. was responsible for the experiments in Figures 3, 4, and 6C and 6D. I.C.-P. and C.C. assisted in the flow cytometry assays and provided critical reagents, while C.S.-G. performed in part the gap filling experiments. E.A.-N.A. helped to design the integrin clustering analyses, and M.S.M. optimized our histology protocols. I.M.-F. supervised the project and the analyses. A.K.M.-O., I.S., and I.M.-F. wrote the manuscript with input from all authors.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Involucrin | Invitrogen | Cat#MA5-11803; Clone SY5; RRID: AB_10982738; Lot WK3207493 |
| HUTS4, active Integrin β1 monoclonal antibody | Donated by Prof. Carlos Cabañas Gutierrez (CBM-SO, Madrid, Spain); Luque et al.,47 Su et al.,48 Arimori et al.49 | N/A |
| Lia1/2, total Integrin β1 monoclonal antibody | Donated by Prof. Carlos Cabañas Gutierrez (CBM-SO, Madrid, Spain); Campanero et al.44 | N/A |
| Tubulin | Sigma-Aldrich | Cat#T6074; Clone B-5-1-2; RRID:AB_477582; Lot 0000127282 |
| Collagen I | Invitrogen | Cat#PA5-95137; RRID:AB_2806942; Lot XE3589821 |
| Collagen IV | Invitrogen | Cat#14987182; clone 1042; RRID:AB_10870985; Lot 4329568 |
| Collagen XVII | Donated by Prof. Marcela del Rio (UC3M, Madrid, Spain); Yuen et al.111 | N/A |
| Integrin β1 | Abcam | Cat#Ab30394; clone 12G10; RRID:AB_775726 |
| TP63 | Invitrogen | Cat#703809; clone 10H7L17; RRID: AB_2809251; Lot 3005440 |
| Polyclonal goat anti-mouse HRP | Dako | Cat#P0447; RRID:AB_2617137; Lot 41424131 |
| Goat anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 488 | Invitrogen | Cat#A32723; RRID: AB_2633275; Lot TF266577 |
| Donkey anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 |
Invitrogen | Cat#A31571; RRID: AB_162542; Lot 2720365 |
| Goat anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 647 | Invitrogen | Cat#A21235; RIDD: AB_2535804; Lot 2930840 |
| Goat anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 647 | Invitrogen | Cat#A21244; RIDD: AB_2535812; Lot 2433883 |
| Biological samples | ||
| Frozen human apheresis plasma (aPlasma) | Banco de Transfusiones de la Comunidad de Madrid; | Cat#E 03.1.6.1.06.2 https://www.comunidad.madrid/hospital/centrodetransfusion/ |
| Chemicals, peptides, and recombinant proteins | ||
| Chelex® 100 Resin | BioRad | Cat#142-2842 |
| Benzonase® Nuclease | Merck | Cat#E1014 |
| L-Ascorbic Acid-2-phosphate | Sigma-Aldrich | Cat#A8960; CAS 1713265-25-8 |
| L- ascorbic acid | Sigma-Aldrich | Cat#A92902; CAS 50-81-7 |
| Diethylenetriaminepentaacetic acid (DETAPAC) | Sigma-Aldrich | Cat#D6518; CAS 67-43-6 |
| 4-Hydroxy-TEMPO (Tempol) | Sigma-Aldrich | Cat#176141; CAS 2226-96-2 |
| O-phenylenediamine (OPDA) | Sigma-Aldrich | Cat#P1526; CAS 615-28-1 |
| Tranexamic acid (Amchafibrin 500mg injectable solution) | Mylan | Cat#08470007005063; Lot A21006 |
| Deposited data | ||
| Raw data | This paper; e-cienciaDatos repository of the Madroño consortium. | e-cienciaDatos UC3M: https://doi.org/10.21950/BUUT5Q |
| Experimental models: Cell lines | ||
| Human: HaCaT cell line | Donated by Prof. José Luis Jorcano (Dept. Bioengineering, UC3M, Madrid, Spain); Montero et al.,61 Boukamp et al.9,10 | N/A |
| Human: Spontaneously immortalized dermal fibroblasts | Donated by Prof. José Luis Jorcano (Dept. Bioengineering, UC3M, Madrid, Spain); Montero et al.61 | N/A |
| Oligonucleotides | ||
| PCR XBP1 fw: AAACAGAGTAGCAGCGCAGACTGC |
Calfon et al.73 | Metabion |
| PCR XBP1 rv: GGATCTCTAAAACTAGAGGCTTGGTG |
Calfon et al.73 | Metabion |
| qPCR Integrin β1 fw: CAAGCAGGGCCAAATTGTGG |
Yao et al.112 | Metabion |
| qPCR Integrin β1 rv: CCTTTGCTACGGTTGGTTACATT |
Yao et al.112 | Metabion |
| qPCR GAPDH fw:TGAAGGTCGGAGTCAACGGATTT |
Anelli et al.78 | Metabion |
| qPCR GAPDH rv: CATGTAAACCATGTAGTTGAGGT |
Anelli et al.78 | Metabion |
| Software and algorithms | ||
| ImageJ (version 2.14.0/1.54f) | National Institutes of Health (NIH) | https://imagej.net/ij/download.html |
| GraphPad PRISM (version 10.3.1) | GraphPad | https://www.graphpad.com/ |
| Leica LAS X software (version 3.7.5.24914) | Leica Microsystems CMS GmbH | https://www.leica-microsystems.com/ |
| FlowJo software (version v10) | BD Biosciences | https://www.bdbiosciences.com/en-eu/products/software/flowjo-v10-software |
| Other | ||
| μ-dish, 35-mm, low culture insert two-well plate | Ibidi | Cat#80206 |
| Transwell (Permeable Support for 6-well plate, 1.0um pore) | Falcon® | Cat#353102 |
| 6-well Deep Well Plate | Falcon® | Cat#355467 |
| Human IL-1α ELISA Kit | Abcam | Cat# ab178008; RRID:AB_2814805 |
| Dark 96-well plate | Corning | Cat#cls4580 |
Experimental model and study participant details
HaCaT cell cultures
Initial non-authenticated HaCaT cultures routinely maintained in standard DMEM 10%FBS (a male-derived cell line9) were generously provided by Prof. José Luis Jorcano (Department of Bioengineering, University Carlos III of Madrid).
For analyzing the influence of Ca2+ on cell phenotype, HaCaT cells were maintained for at least 3 passages in the same medium (DMEM GlutaMAX-I from Gibco (61965-026), 10%FBS Good, EU-approved regions 0.2μm sterile filtered from PanBiotech (P40-37500)), below 70–80% confluency to avoid unwanted cell differentiation.12 After that period, the cells were transferred to medium containing different concentrations of Ca2+: i. complete DMEM 10%FBS (∼2mM Ca2+, the starting medium); ii. DMEM, high glucose, no glutamine, no calcium (Gibco, 21068-028) plus GlutaMAX (Gibco 35050-038) and 10%FBS (∼0.35mM Ca2+); iii. DMEM, high glucose, no glutamine, no calcium plus GlutaMAX and supplemented with 10% Ca2+-chelated FBS (prepared using Chelex 100 Resin from BioRad, 142–2842), following the manufacturer instructions) and 0.09mM CaCl2 (Calbiochem cat #208291); iv. Free Ca2+ medium, composed only by DMEM, high glucose, no glutamine, no calcium plus GlutaMAX and 10% Ca2+-chelated FBS. All culture media were complemented with 100U/mL penicillin and 0.1mg/mL streptomycin (PanBiotech, P06-07100).
Once the Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii)-bred population was selected as optimal, cell stocks were generated and continuously cultured under those conditions.
In all cases, the cells were incubated in a Napco 6000 incubator under 37°C, 5%CO2, 80–90% humidity.
Fibroblast cell cultures
Spontaneously non-authenticated immortalized human fibroblasts from male foreskin pools destined to support organotypic 3D cultures were donated by Prof. José Luis Jorcano (Department of Bioengineering, University Carlos III of Madrid) and maintained in standard DMEM 10%FBS (medium i), for at least 3 passages before being embedded in fibrin hydrogels. The cells were routinely grown in the incubator under 37°C, 5%CO2, 80–90% humidity.
Organotypic skin 3D cultures
HaCaT cells were cultured for 3 passages, below 70–80% confluency, either in the Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii) or in DMEM 10%FBS (medium i). 24h before seeding the cells on top of the fibrin hydrogels, fresh medium containing either 0, 2, or 5μg/mL of L-Ascorbic Acid 2-phosphate (A2P, Sigma-Aldrich, A8960) was added to the cells growing in the 0.09mM Ca2+ defined medium (medium iii). The medium was also freshly replaced in DMEM 10%FBS (medium i)-thriving cells that were used as control samples.
Fibrin hydrogels were prepared as described by Montero et al.61 Frozen human plasma from apheresis-subjected anonymous donors (aPlasma) that served as a source of fibrin for our gels was supplied by the blood bank of the Madrid Government (Banco de Transfusiones de la Comunidad de Madrid, Av. de la Democracia, s/n, Vicálvaro, 28032 Madrid, Spain). All procedures were approved both by the biobank Ethical Commission and by the Biosecurity and Safety Committee of the University Carlos III of Madrid. Each fibrin gel was formulated to contain 2.4mg/mL of fibrinogen, starting from the known initial concentrations provided by the bank. Before launching gelification, we added to aPlasma 0.8mg/mL tranexamic acid (Amchafibrin 500mg injectable solution, Mylan, 08470007005063) as an antifibrinolytic agent, 61.6mM NaCl (Thermo scientific, 207790050) to adjust ionicity, and 4 × 104 spontaneously immortalized fibroblasts per hydrogel to be constructed, in strict order. After delicately homogenizing the mixture by pipetting, 7.2mM CaCl2 was included as the clotting agent. From this solution, we add 3.5mL to each transwell (Falcon Permeable Support for 6-well plate, 1.0μm pore, 353102), avoiding the formation of bubbles. The resulting fibrin gel was incubated at 37°C, 5%CO2, 80–90% humidity in the cell incubator for 1h until total gelification. Afterward, we filled the upper and lower chambers of the transwells with DMEM 10%FBS (medium i) and kept them inside the incubator for further 24h for equilibration. After this period, the hydrogel was ready to host the re-epithelizing cells. The medium was extracted from both chambers of the transwell, and HaCaT cells previously detached using 0.25% Trypsin-EDTA solution (Gibco, 25200-072) were seeded on top of the fibrin gels at a density of 7.5 × 105 cells per transwell. Then, fresh DMEM 10%FBS (medium i) was added to the lower chamber of the transwell in order to feed fibroblasts. To enable the development of a keratinocyte monolayer, the constructs were incubated for 48h at 37°C, 5%CO2, 80–90% humidity in the incubator. Subsequently, the medium was removed from both the upper and lower compartments, and the whole was allowed to dry under the sterile hood. The transwells were then transferred to Falcon 6-well Deep Well Plates (355467), and DMEM 0.5%FBS (DMEM GlutaMAX-I, Gibco; FBS Good, EU approved regions 0.2μm sterile filtered, PanBiotech) containing either 50μg/mL of A2P or 50μg/mL L-ascorbic acid (Sigma-Aldrich, A92902) depending on the sample of destination (medium iii-grown cells or medium i-cultured cells, respectively) was introduced beneath the transwell, maintaining the surface of the construct at the air-liquid interface to promote HaCaT differentiation. The organotypic constructs were incubated for 3–4 weeks depending on the experiment at 37°C, 5%CO2, 80–90% humidity, replacing each specific hydrogel bathing medium every 2–3 days. At least n ≥ 4 constructs were developed for each condition assessed.
Method details
Analyses of cell morphology and western blot
Images of sub-confluent cultures were acquired at a 10× magnification after 72h from seeding cells in each Ca2+-conditioned media (∼2mM, ∼0.35mM, 0.09mM or without Ca2+, see i., ii., iii., and iv under the subheading HaCaT cell cultures) using a Leica DM IL LED microscope (Leica Microsystems CMS GmbH) coupled to the camera of an iPhone 13 device (Apple). Regions of interest (ROIs) of constant dimensions were selected and enlarged with ImageJ (version 2.14.0/1.54f). The nuclear-to-cytoplasm (NC) ratio was computed; briefly, the area of each cell (ROI cell) and its respective nucleus (ROI nucleus) were selected with the freehand tool, the area of each was measured, and the single NC ratio of the nuclear and cytoplasmic areas of 50 cells per condition were represented using GraphPad PRISM (version 10.3.1, GraphPad software).
Subsequently, cells were washed once with D-PBS without calcium and without magnesium (Thermo Scientific, J67802.AP) and scraped in a lysis buffer containing 2%SDS (Sigma-Aldrich, L3771), 62.5mM Tris pH 6.8 (Sigma-Aldrich, T1503), 10% glycerol (Sigma-Aldrich, G5516), freshly supplemented with complete protease inhibitors (Merck, 11873580001), 10mM sodium fluoride (Sigma-Aldrich, S7920), 10mM N-ethylmaleimide (Sigma-Aldrich, E3876), and 0.4mM sodium orthovanadate (Sigma-Aldrich, S6508). The resulting lysates were further treated with Benzonase® Nuclease (Merck, E1014) in the presence of 2mM MgCl2 (Sigma-Aldrich, 63068) to eliminate DNA from the sample. Afterwards, aliquots were run in a standard reducing SDS-PAGE and blotted with anti-involucrin (Invitrogen, MA5-11803) and anti-tubulin (Sigma-Aldrich, T6074) antibodies, both detected with anti-mouse coupled to HRP secondaries (Dako, P0447) at room temperature. In between, the membranes were stripped using a buffer composed of 100mM glycine (Sigma-Aldrich, G7126), 1%SDS, and 10%NP40 (Sigma-Aldrich, 74385) adjusted to pH 2.2. Quantification of 10 blots showing involucrin levels was performed using ImageJ and represented as relative band density ±standard error of the mean (SEM).
Clonogenic assays
HaCaT cells were cultured in the Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 defined medium (medium iii), detached with 0.25% Trypsin-EDTA, and re-plated in 24-well plates at serial two-fold dilutions starting from 5000 cells per well to achieve clonal density. After 24h, the medium was replaced by a fresh one containing scaled concentrations of A2P from 0 to 25μg/mL. Cells were incubated in constant presence of the modified vitamin for a further 7 days. At the end of this period, the wells were fixed with 3.7% formaldehyde (AppliChem, 252931.1211), permeabilized with 2% cold methanol (Labkem, MTOL-0IA-5K0), and stained with 0.5% crystal violet (Alfa Aesar, B21932) prepared in 20% methanol. The resulting colonies were scanned using an iPhone 13 device (Apple) and scored manually using ImageJ. The results are presented as the mean percentage of colonies formed under each concentration using the non-treated cells (0μg/mL A2P) as a reference value ±SEM. At least 10 experiments were analyzed.
The number of cells in each colony was counted in each well both after 24h and 48h from adding A2P in ≥5 independent experiments. Violin plots were constructed using GraphPad PRISM.
Intracellular ascorbic acid content
Intracellular ascorbic acid content quantification was performed as described by Vislisel et al.43 Briefly, the method consists in initially oxidizing the total pool of ascorbic acid inside cells with hydroxylamine (Tempol) to produce DHA. The resulting DHA is then reacted with o-phenylenediamine (OPDA), resulting in the condensation fluorescent product, 3-(dihydroxyethyl) furo[3,4-b] quinoxaline-1-one (DHA-OPDA). The formation of DHA-OPDA follows an exponential function, where the slope has a direct linear relationship to ascorbate concentration. To generate a reference slope for our measurements, we used a standard curve prepared from a stock solution of 100mM L-ascorbic acid. Serial dilutions were prepared to obtain 8 standard concentrations (0, 2.5, 5, 10, 20, 40, 80, and 160μM) in a solution of MeOH/H2O (60/40, v/v) containing diethylenetriaminepentaacetic acid (DETAPAC, Sigma-Aldrich, D6518).
To measure the intracellular content of ascorbic acid in our cells, Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii)- cultured HaCaT cells were seeded in 60mm dishes. After 24h, the medium was replaced and supplemented with concentrations of A2P ranging between 0 and 10μg/mL, for either 4, 8, 24, or 48h. Then, cells were detached using 0.25% Trypsin-EDTA, washed twice with D-PBS, and centrifuged at 300g for 5min to recover the cell pellets, which were subsequently frozen at −80°C to break cellular membranes for at least 30min. Ascorbate from the pellets was extracted and stabilized using MeOH/H2O (60/40, v/v) with DETAPAC on ice. The resulting solution was centrifuged at 4°C, 12,000g for 10min and supernatants containing intracellular ascorbate were recovered. Next, 100μL of each sample supernatant and each ascorbate standard was transferred into dark 96-well plates (Corning, CLS4580), 100μL of 2.32mM Tempol (Sigma-Aldrich, 176141) in 2M acetate buffer (Sigma-Aldrich, 236500) was added to each well and incubated for 10min at RT. In the dark, 42μL of a solution of 5.5mM OPDA (Sigma-Aldrich, P1526) prepared in acetate buffer was added and the resulting fluorescence was measured using a 345nm bandpass filter for excitation and recorded at 425nm for emission using the plate reader Synergy HTX (BioTeK, Santa Clara, United States). Ascorbate concentration in the samples was determined by extrapolation with the fluorescence values obtained for the standard curve. The mean results of ≥10 independent experiments ±SEM was represented using GraphPad PRISM.
Total integrin β1 surface expression
HaCaT cells growing in Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii) were detached using 0.25% Trypsin-EDTA and plated at a density of 8 × 104 cells on 13mm coverslips placed in 24-well plates. 24h after seeding, cells were incubated for a further 24h with different concentrations of A2P (0, 2, and 5μg/mL). Then, the coverslips were fixed in 4%PFA (Scharlau, PA00951000) for 15min at RT and blocked with 10%FBS in a buffer containing 20mM Tris-HCl pH 7.4, 154mM NaCl, 20mM EDTA (Sigma-Aldrich, ED2SS), 2mM MgCl2. No cell permeabilization was performed. Integrin β1 surface expression was detected at room temperature using the anti-total integrin β1 monoclonal antibody (Lia1/244, kindly donated by Carlos Cabañas), and the nuclear dye Hoechst (Thermo scientific, 62249) was employed for decorating the nuclei. Suitable anti-mouse secondary antibodies AlexaFluor-488 (Invitrogen, USA, A32723) were used for visualization. The fluorescent images were collected using a Leica DMi8 microscope (Leica Microsystems CMS GmbH) equipped with a Hamamatsu digital camera C11440 and a 63× objective (1.4N HC PL APO Oil, Leica Microsystems CMS GmbH). The resulting images were processed with ImageJ. For the NC ratio analyses, the area of each cell (ROI cell) and its respective nucleus (ROI nucleus) was manually drawn with the freehand selection tool of ImageJ, using the signals provided by β1 integrin labeling for delimiting cell borders and the Hoechst staining for the nucleus. The single NC ratio of at least 30 cells for each condition in 4 images belonging to ≥5 independent experiments ±SEM was computed and represented using GraphPad PRISM. From each ROI cell collected for the area analyses, we also extracted the values of the mean fluorescent intensity (MFI) of integrin β1 staining. The mean results of ≥5 independent experiments ±SEM were represented using GraphPad PRISM.
Flow cytometry assays
HaCaT cells thriving in Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii) from the same number of cell passage were treated for 24h with either 0, 2, or 5μg/mL A2P. Then, they were detached using 0.25% Trypsin-EDTA and stained with Lia 1/2 for 30min on ice. The samples were then incubated with secondary antibodies anti-mouse AlexaFluor™-647 (Invitrogen, A31571) for 30min at 4°C. The cells were analyzed by flow cytometry using a FACScan™ flow cytometer (Beckton-Dickinson). SSC-A/FSC-A values and integrin β1 staining were both obtained from the clouds gated as shown in Figures S3A and S3B using the FlowJo software (v10 version, BD Biosciences). Graphs generated with GraphPad PRISM represent the mean values of ≥50 independent experiments for the population distribution and 5 (for the confrontation of DMEM-cultured cells with 0.09mM Ca2+-grown cells) or ≥25 (for the comparison of the distinct A2P treatments) for the analyses of the expression of β1 integrin ±SEM.
Analyses of β1-active foci
HaCaT cells growing in Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii) were detached using 0.25% Trypsin-EDTA and plated at a density of 8 × 104 cells on 13mm coverslips placed in 24-well plates. After 24h, cells were supplemented with A2P at the different concentrations assayed (0, 2, and 5μg/mL) for 24h. Then, the coverslips were fixed for 15min at RT in 4%PFA and blocked with 10%FBS in a 20mM Tris-HCl pH 7.4, 154mM NaCl, 20mM EDTA, 2mM MgCl2 solution. No cell permeabilization was performed. Integrin clusters containing β1-active subunits were labeled with the anti-active integrin β1 monoclonal antibody HUTS447,48,49 (kindly donated by Carlos Cabañas), for 30min at 37°C. The binding of these primary antibodies was detected using anti-mouse Alexa Fluor-488 secondary antibodies (Invitrogen, A32723). Images were acquired using a Leica DMi8 microscope equipped with a Hamamatsu digital camera C11440 with a 63× objective. For highlighting the integrin β1 active foci, 2000 gray levels were removed using the background function of the Leica LAS X software (version 3.7.5.24914, Leica Microsystems CMS GmbH). Further processing was achieved using the following protocol in ImageJ: first, the images were converted to 8-bits; then, we produced a mask of the Integrin β1 dots, by adjusting the threshold using the Huang mode.113 Then we converted the result in a binary mask to obtain all the particles as single ROIs, each representing an integrin β1 cluster. The ROIs were superimposed on the cells to obtain the number of clusters per cell. For each, we also collected the MFI and the area values. In each experiment, 4 independent images corresponding to different fields of view were acquired. Structures smaller than 0.3μm2 were excluded from the analysis because they corresponded to signals emitted by less than two pixels plus the average diameter of their airy disk. We also eliminated the values of structures with areas higher than 1.5μm2 to quantify only isolated clusters. Dispersion plots representing each cluster MFI against its respective area and frequency distribution of the brightest clusters were constructed using GraphPad PRISM. This procedure quantified the features of ≥300 β1-active foci for each condition assayed in ≥10 independent experiments ±SEM.
Gap filling experiments
To monitor HaCaT cells migration and proliferation over time, we performed time-lapse imaging analyses onto cells plated in μ-dish, 35mm, low culture insert two-well plates (Ibidi, 80206). These culturing devices are mounted as two chambers separated by a silicone dam in the middle of a 35mm plate. Inside each chamber, we seeded 2.5 × 104 cells. Outside, we plated 2 × 105 cells to avoid monolayer movements after the removal of the support toward empty spaces out from the field of view. The cells were let adhere for 24h, and then supplemented with either 0, 2, and 5μg/mL of A2P for further 24h. Before acquisition, the medium was replaced by Ca2+-free DMEM 2%FBS for 4h, to synchronize cell responses. After this period, we removed the dam, washed twice with PBS, added fresh Ca2+-free DMEM 10% chelated FBS supplemented with 0.09mM CaCl2 (medium iii)) and started the phase contrast time-lapse recording. The cells were followed for 60h using a Leica DMi8 microscope equipped with a Hamamatsu digital camera C11440 and a 10× objective (HC PL Fluotar 0.32 PH1, Leica, Germany). A CO2/O2 manual mixer (Okolab, Italy) controlled environmental conditions (37°C, 5%CO2, 80–90% humidity). For analyzing the wound-like closure, the gap area was assessed using ImageJ by manually drawing a polygon covering the empty space between both monolayer borders in each of the images. The results were represented as the fold change in area against time with GraphPad PRISM. To quantify cell density, a rectangular ROI was drawn using ImageJ and equally applied to all images and conditions. The mean gray value (mgv) of these areas was calculated and represented as the fold change in cell density against time with GraphPad PRISM. Time-course plots show the mean value obtained for each time point in n ≥ 5 independent experiments ±SEM. For clarity, the last time point (60h) has also been represented using a column graph.
Contraction measurements and histology
For analyzing contraction, the initial height of the gel was labeled with a fine permanent marker just before seeding the HaCaT cells on top. After either 3 or 4 weeks since airlifting the construct to allow for epidermal differentiation, another mark was introduced reflecting the final height. At that moment, images were acquired from the side and the top of the transwell using a Samsung S22+ device (Samsung). The percentage of contraction was calculated from the data obtained for height variation and represented using GraphPad PRISM. At least n ≥ 4 constructs were analyzed for each condition assessed. Then, the 3D organotypic cultures were extracted from the transwell by making an incision following the transwell borders with a scalpel. Each sample was placed on histology cassettes, and images of the macromolecular morphological aspect were captured from above using the Samsung S22+ device. The cassettes were closed and immersed in 3.7% formaldehyde (PanReac AppliChem, 252931) during 24h for fixation and processed by standard paraffin-based histological procedures; briefly, increasing concentrations of ethanol and xylene (Roth, 2CR-9713.5) were used, followed by paraffin inclusion. After embedding, the paraffined blocks were sectioned at 6μm with a microtome (Histo-line laboratories, MR3000). Afterwards, the resulting tissue sections were stained with hematoxylin/eosin (QCA, 997750/Bio-Optica, 05-11007) and mounted on poly-L-Lysine-coated imaging slides (Epredia, J2800AMNZ) using the Roti histokitt II (Roth, T160.1). Four independent images corresponding to different sections of the same sample were acquired using a Leica DM750 microscope equipped with an ICC50 HD camera and a 10× objective (all from Leica Microsystems CMS GmbH). Epidermal thickness was analyzed in three different regions of each image by measuring the distance between the first line of cells situated in proximity to the dermal compartment and the surface with ImageJ. From these images, we also extracted the mean values for the width of the shearing-induced space between dermis and epidermis. The graphs in the correspondent figures show the mean of n ≥ 4 constructs for each condition ±SEM and were constructed using GraphPad PRISM.
Immunostaining of organotypic constructs
3D organotypic cultures were extracted at 3 weeks since air-lifting from the transwell using a scalpel. Samples were fixed by immersion in 3.7% formaldehyde (PanReac AppliChem, 252931) for 24, and then embedded in 1% low-melting point agarose (Roche, 11685660001),114 followed by the standard paraffin-based histological procedures described above Paraffined blocks were sectioned at 6μm with a microtome (Histo-line laboratories, MR3000). The resulting tissue sections were dehydrated, and antigen retrieval was completed by immersion in a 95°C-heated 10mM sodium citrate (Sigma, W302600) 0.05% Tween 20 (Merck, 655204) buffer pH 6 for 30min and subsequently cooled to RT for 30min. The samples were blocked with a 20mM Tris 154mM NaCl 20mM EDTA 2mM MgCl2 pH 7.4 solution containing 10%FBS 1% BSA for 2h at room temperature. Collagen IV (Col IV, Invitrogen, 14987182), collagen XVII111 (VK4 clone, kindly donated by Marcela del Río), integrin β1 (Int β1, abcam, ab30394) and Involucrin (INV, Invitrogen, MA511803) primary antibodies were incubated in 1%BSA at 4°C O/N, washed with 0.025% Triton X-100 and detected using anti-mouse Alexa Fluor™- 647 secondary antibodies (Invitrogen,mouse:A21235, rabbit: A22244) for 1h at RT. Images were acquired using a Leica DMi8 microscope equipped with a Hamamatsu digital camera C11440 with either a 20X or a 40× objective. Further processing was achieved using ImageJ. Shortly, background signals were subtracted for all images acquired using the sliding paraboloid feature at 50.0 pixels, and 2 regions of interest across the field of view, located either in the innermost or in the mid epithelium or the innermost and the outermost layers, depending of the experiment, were selected. The resulting computed mean fluorescence intensity was plotted or represented as the ratio of signal distribution between the two sections in which the fluorescence was measured. The graphs in the corresponding figures show the mean of 6–8 slices obtained by analyzing n ≥ 2 constructs for each condition ±SEM and were constructed using GraphPad PRISM.
Xbp1 splicing and qPCR of integrin β1
Total RNA was extracted from HaCaT cells using the TRI Reagent from ZymoResearch (R2050-1). Complementary DNA (cDNA) was synthesized using the ImProm-II Reverse Transcriptase (Promega, M314A) according to the manufacturer’s protocol. XBP1 mRNA splicing was analyzed by PCR with the forward primer 5′-AAACAGAGTAGCAGCGCAGACTGC-3′ and the reverse primer 5′-GGATCTCTAAAACTAGAGGCTTGGTG-3′ (as described in112). A positive splicing control was obtained by treating HaCaT cells cultured Ca2+-free DMEM supplemented with Ca2+-chelated FBS plus 0.09mM CaCl2 in absence of any A2P treatment with 5μg/mL tunicamycin (Sigma-Aldrich, T7765) for 4 h at 37°C in the incubator. PCR products were resolved on 3% agarose gels and visualized using a gel reader (E-BOX-VX2/20M, Vilber Lourmat, France). n ≥ 4 independent experiments were performed and a representative image is shown.
Quantitative real-time PCR (qPCR) for Integrin β1 expression was performed using the iTaq Universal SYBR® Green Supermix (Bio-Rad, 1725120) on a CFX96 Real-Time PCR Detection System (Bio-Rad). Briefly, 0.2μL of primer couples (25μM each, to a final concentration of 0.5μM; forward primer 5′-CAAGCAGGGCCAAATTGTGG-3′, reverse primer 5′-CCTTTGCTACGGTTGGTTACATT-3’112), 5μL of SYBR mix, and 4μL of cDNA and nuclease-free water (corresponding to 8 ng of template DNA) were added to each reaction well, in a total volume of 10μL. All the samples were loaded in triplicate. The amplification was performed with a denaturation step of 10 min at 95°C, followed by 40 cycles of PCR amplification (15 s at 95°C, 1 min at 60°C), and by a step-by-step temperature increase from 60°C to 95°C, to calculate the melting curve. For each sample, the Ct (cycle number at which the fluorescence signal crosses the threshold) was measured, and the value of the replicates was averaged. To compare different conditions, the Ct of the reference gene GAPDH78 (amplified with the forward primer 5′- TGAAGGTCGGAGTCAACGGATTT-3′ and the reverse prime 5′- CATGTAAACCATGTAGTTGAGGT-3′) was subtracted from the Ct of the gene of interest in the same condition (ΔCt); then, each ΔCt was expressed in relation to the corresponding value in the control condition (by subtraction, giving the ΔΔCt), and expressed as fold change of gene expression (2-ΔΔCt). The graph represents the fold change of the mean ± SEM of 8 independent experiments.
ELISA test of IL1α secretion
IL1α secretion was measured using the commercial Human IL-1α ELISA Kit (ab178008, Abcam). 9×104 HaCaT cells grown in Ca2+-free DMEM supplemented with Ca2+-chelated FBS plus 0.09mM CaCl2 were seeded in 24-well plates. After 24h, the culture medium was replaced with fresh medium supplemented with the indicated concentrations of A2P. After an additional 24h, supernatants were collected, and the ELISA was performed following the manufacturer’s instructions. Absorbance was measured using a Synergy HTX plate reader (BioTek). Data represent the fold change of the mean ± SEM from 8 independent experiments.
Quantification and statistical analyses
Every set of data was examined for normal distribution using different normality tests (Kolmogorov-Smirnov, Shapiro-Wilk and Anderson-Darling) available at https://statisty.app/. Calculations were accompanied with visual inspection of the data represented in histograms and Q-Q Plots. As result of the analyses, most of the datasets showed a non-normal distribution or, less frequently, should be compared for significant differences with non-normally distributed datasets. Therefore, we used non-parametric hypothesis tests. The Kruskal-Wallis test was used when confronting more than two non-correlated groups, complemented with the Dunn-Bonferroni post-hoc test. Correlated data were analyzed with the Wilcoxon test for dependent data. When testing two independent variables, we performed the Mann-Whitney U test. In the two latter cases we also indicated the effect size as determined by Cohen (Cohen, J. (1988), Statistical Power Analysis for the Behavioral Sciences, 2nd Edition. Hillsdale: Lawrence Erlbaum).
In each figure, the employed test is described in the figure legends. Statistical significance was defined as p < 0.05 (∗), p < 0.01 (∗∗) or p < 0.001 (∗∗∗).
Published: July 4, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113066.
Supplemental information
References
- 1.Omstead D.R., Baird L.G., Christenson L., Moulin G.D., Tubo R., Maxted D.D., Davis J., Gentile F.T. Voluntary Guidance for the Development of Tissue-Engineered Products. Tissue Eng. 1998;4:239–266. doi: 10.1089/ten.1998.4.239. [DOI] [PubMed] [Google Scholar]
- 2.Germain L., Goulet F., Moulin V., Berthod F., Auger F.A. Engineering Human Tissues for in Vivo Applications. Ann. N. Y. Acad. Sci. 2002;961:268–270. doi: 10.1111/j.1749-6632.2002.tb03099.x. [DOI] [PubMed] [Google Scholar]
- 3.Supp D.M., Boyce S.T. Engineered skin substitutes: practices and potentials. Clin. Dermatol. 2005;23:403–412. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Hay R.J., Johns N.E., Williams H.C., Bolliger I.W., Dellavalle R.P., Margolis D.J., Marks R., Naldi L., Weinstock M.A., Wulf S.K., et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Invest. Dermatol. 2014;134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 5.Segre J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker C.L., McHale M.T., Gillies A.K., Waller J., Pearce D.M., Osborne J., Hutchinson P.E., Smith G.M., Pringle J.H. The Development and Characterization of an In Vitro Model of Psoriasis. J. Invest. Dermatol. 2004;123:892–901. doi: 10.1111/j.0022-202X.2004.23435.x. [DOI] [PubMed] [Google Scholar]
- 7.Stark H.-J., Baur M., Breitkreutz D., Mirancea N., Fusenig N.E. Organotypic Keratinocyte Cocultures in Defined Medium with Regular Epidermal Morphogenesis and Differentiation. J. Invest. Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 8.Rikken G., Niehues H., van den Bogaard E.H. Organotypic 3D Skin Models: Human Epidermal Equivalent Cultures from Primary Keratinocytes and Immortalized Keratinocyte Cell Lines. Methods Mol. Biol. 2020:45–61. doi: 10.1007/978-1-0716-0648-3_5. [DOI] [PubMed] [Google Scholar]
- 9.Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boukamp P., Popp S., Altmeyer S., Hülsen A., Fasching C., Cremer T., Fusenig N.E. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer. 1997;19:201–214. doi: 10.1002/(SICI)1098-2264(199708)19:4<201::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Breitkreutz D., Schoop V.M., Mirancea N., Baur M., Stark H.-J., Fusenig N.E. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur. J. Cell Biol. 1998;75:273–286. doi: 10.1016/S0171-9335(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 12.Wilson V.G. In: Epidermal Cells: Methods and Protocols. Turksen K., editor. Springer; 2014. Growth and Differentiation of HaCaT Keratinocytes; pp. 33–41. [DOI] [Google Scholar]
- 13.Haake A.R., Polakowska R.R. Cell Death by Apoptosis in Epidermal Biology. J. Invest. Dermatol. 1993;101:107–112. doi: 10.1111/1523-1747.ep12363594. [DOI] [PubMed] [Google Scholar]
- 14.Schürer N., Köhne A., Schliep V., Barlag K., Goerz G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993;2:179–185. doi: 10.1111/j.1600-0625.1993.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 15.Syrjänen S., Mikola H., Nykänen M., Hukkanen V. In vitro establishment of lytic and nonproductive infection by herpes simplex virus type 1 in three-dimensional keratinocyte culture. J. Virol. 1996;70:6524–6528. doi: 10.1128/jvi.70.9.6524-6528.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinsträsser I., Koopmann K., Merkle H.P. Epidermal Aminopeptidase Activity and Metabolism as Observed in an Organized HaCaT Cell Sheet Model. J. Pharmaceut. Sci. 1997;86:378–383. doi: 10.1021/js960201u. [DOI] [PubMed] [Google Scholar]
- 17.Boelsma E., Verhoeven M.C.H., Ponec M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT) J. Invest. Dermatol. 1999;112:489–498. doi: 10.1046/j.1523-1747.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 18.Maas-Szabowski N., Stärker A., Fusenig N.E. Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-α. J. Cell Sci. 2003;116:2937–2948. doi: 10.1242/jcs.00474. [DOI] [PubMed] [Google Scholar]
- 19.Houghton P.J., Hylands P.J., Mensah A.Y., Hensel A., Deters A.M. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005;100:100–107. doi: 10.1016/j.jep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Pillai S., Bikle D.D., Mancianti M.-L., Cline P., Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: Modulation of differentiation competence by stages of growth and extracellular calcium. J. Cell. Physiol. 1990;143:294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- 21.Bikle D.D., Xie Z., Tu C.-L. Calcium regulation of keratinocyte differentiation. Expet Rev. Endocrinol. Metabol. 2012;7:461–472. doi: 10.1586/eem.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hata R.-I., Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell. Physiol. 1989;138:8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- 23.D’Aniello C., Cermola F., Patriarca E.J., Minchiotti G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cell. Int. 2017;2017:1–16. doi: 10.1155/2017/8936156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulbecco R., Elkington J. Induction of growth in resting fibroblastic cell cultures by Ca++ Proc. Natl. Acad. Sci. USA. 1975;72:1584–1588. doi: 10.1073/pnas.72.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R B.J., Joseph H. Calcineurin Is Required for Candida albicans To Survive Calcium Stress in Serum. Infect. Immun. 2005;73:5767–5774. doi: 10.1128/iai.73.9.5767-5774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 27.Jans R., Sartor M., Jadot M., Poumay Y. Calcium entry into keratinocytes induces exocytosis of lysosomes. Arch. Dermatol. Res. 2004;296:30–41. doi: 10.1007/s00403-004-0469-0. [DOI] [PubMed] [Google Scholar]
- 28.Pang Z.P., Südhof T.C. Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowden G. Ultrastructural Studies of the Keratinized Epithelia of the Mouse III. Determination of the Volumes of Nuclei and Cytoplasm of Cells in Murine Epidermis. J. Invest. Dermatol. 1975;64:1–3. doi: 10.1111/1523-1747.ep12540840. [DOI] [PubMed] [Google Scholar]
- 30.Sun T.-T., Green H. Differentiation of the epidermal keratinocyte in cell culture: Formation of the cornified envelope. Cell. 1976;9:511–521. doi: 10.1016/0092-8674(76)90033-7. https://api.semanticscholar.org/CorpusID:15007195 [DOI] [PubMed] [Google Scholar]
- 31.Barrandon Y., Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckert R.L., Green H. Structure and evolution of the human involucrin gene. Cell. 1986;46:583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- 33.Rice R.H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: Activation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 34.Nemes Z., Marekov L.N., Steinert P.M. Involucrin Cross-linking by Transglutaminase 1: BINDING TO MEMBRANES DIRECTS RESIDUE SPECIFICITY. J. Biol. Chem. 1999;274:11013–11021. doi: 10.1074/jbc.274.16.11013. [DOI] [PubMed] [Google Scholar]
- 35.Wilke M.S., Hsu B.M., Wille J.J., Pittelkow M.R., Scott R.E. Biologic mechanisms for the regulation of normal human keratinocyte proliferation and differentiation. Am. J. Pathol. 1988;131:171–181. [PMC free article] [PubMed] [Google Scholar]
- 36.Bikle D.D., Ng D., Tu C.-L., Oda Y., Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell. Endocrinol. 2001;177:161–171. doi: 10.1016/S0303-7207(01)00452-X. [DOI] [PubMed] [Google Scholar]
- 37.Bohnert A., Hornung J., Mackenzie I., Fusenig N. Epithelial-mesenchymal interactions control basement membrane production and differentiation in cultured and transplanted mouse keratinocytes. Cell Tissue Res. 1986;244:413–429. doi: 10.1007/BF00219217. [DOI] [PubMed] [Google Scholar]
- 38.Deutsch J.C. Spontaneous Hydrolysis and Dehydration of Dehydroascorbic Acid in Aqueous Solution. Anal. Biochem. 1998;260:223–229. doi: 10.1006/abio.1998.2700. [DOI] [PubMed] [Google Scholar]
- 39.Golubitskii G.B., Budko E.V., Basova E.M., Kostarnoi A.V., Ivanov V.M. Stability of ascorbic acid in aqueous and aqueous-organic solutions for quantitative determination. J. Anal. Chem. 2007;62:742–747. doi: 10.1134/S1061934807080096. [DOI] [Google Scholar]
- 40.Clément M.-V., Ramalingam J., Long L.H., Halliwell B. The In Vitro Cytotoxicity of Ascorbate Depends on the Culture Medium Used to Perform the Assay and Involves Hydrogen Peroxide. Antioxidants Redox Signal. 2001;3:157–163. doi: 10.1089/152308601750100687. [DOI] [PubMed] [Google Scholar]
- 41.Kanatate T., Nagao N., Sugimoto M., Kageyama K., Fujimoto T., Miwa N. Differential susceptibility of epidermal keratinocytes and neuroblastoma cells to cytotoxicity of ultraviolet-B light irradiation prevented by the oxygen radical-scavenger ascorbate-2-phosphate but not by ascorbate. Cell. Mol. Biol. Res. 1995;41:561–567. http://europepmc.org/abstract/MED/8777435 [PubMed] [Google Scholar]
- 42.Sugimoto M., Okugawa Y., Miwa N. Preventive effects of phosphorylated ascorbate on ultraviolet-B induced apoptotic cell death and DNA strand cleavage through enrichment of intracellular vitamin C in skin epidermal keratinocytes. Free Radic. Res. 2006;40:213–221. doi: 10.1080/10715760500417005. [DOI] [PubMed] [Google Scholar]
- 43.Vislisel J.M., Schafer F.Q., Buettner G.R. A simple and sensitive assay for ascorbate using a plate reader. Anal. Biochem. 2007;365:31–39. doi: 10.1016/j.ab.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campanero M.R., Arroyo A.G., Pulido R., Ursa A., de Matías M.S., Sánchez-Mateos P., Kassner P.D., Chan B.M.C., Hemler M.E., Corbí A.L., et al. Functional role of α2/β1 and α4/β1 integrins in leukocyte intercellular adhesion induced through the common β1 subunit. Eur. J. Immunol. 1992;22:3111–3119. doi: 10.1002/eji.1830221213. [DOI] [PubMed] [Google Scholar]
- 45.Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-K. [DOI] [PubMed] [Google Scholar]
- 46.Watt F.M., Boukamp P., Hornung J., Fusenig N.E. Effect of growth environment on spatial expression of involucrin by human epidermal keratinocytes. Arch. Dermatol. Res. 1987;279:335–340. doi: 10.1007/BF00431227. [DOI] [PubMed] [Google Scholar]
- 47.Luque A., Gómez M., Puzon W., Takada Y., Sánchez-Madrid F., Cabañas C. Activated Conformations of Very Late Activation Integrins Detected by a Group of Antibodies (HUTS) Specific for a Novel Regulatory Region(355-425) of the Common β1 Chain. J. Biol. Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 48.Su Y., Xia W., Li J., Walz T., Humphries M.J., Vestweber D., Cabañas C., Lu C., Springer T.A. Relating conformation to function in integrin α 5 β 1. Proc. Natl. Acad. Sci. USA. 2016;113:E3872–E3881. doi: 10.1073/pnas.1605074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arimori T., Miyazaki N., Mihara E., Takizawa M., Taniguchi Y., Cabañas C., Sekiguchi K., Takagi J. Structural mechanism of laminin recognition by integrin. Nat. Commun. 2021;12:4012. doi: 10.1038/s41467-021-24184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z., Lock J.G., Olofsson H., Kowalewski J.M., Teller S., Liu Y., Zhang H., Strömblad S. Integrin-mediated Cell Attachment Induces a PAK4-dependent Feedback Loop Regulating Cell Adhesion through Modified Integrin αvβ5 Clustering and Turnover. Mol. Biol. Cell. 2010;21:3317–3329. doi: 10.1091/mbc.e10-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medraño-Fernandez I., Bestetti S., Bertolotti M., Bienert G.P., Bottino C., Laforenza U., Rubartelli A., Sitia R. Stress Regulates Aquaporin-8 Permeability to Impact Cell Growth and Survival. Antioxidants Redox Signal. 2016;24:1031–1044. doi: 10.1089/ars.2016.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balza E., Castellani P., Moreno P.S., Piccioli P., Medraño-Fernandez I., Semino C., Rubartelli A. Restoring microenvironmental redox and pH homeostasis inhibits neoplastic cell growth and migration: therapeutic efficacy of esomeprazole plus sulfasalazine on 3-MCA-induced sarcoma. Oncotarget. 2017;8:67482–67496. doi: 10.18632/oncotarget.18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoop V.M., Fusenig N.E., Mirancea N. Epidermal Organization and Differentiation of HaCaT Keratinocytes in Organotypic Coculture with Human Dermal Fibroblasts. J. Invest. Dermatol. 1999;112:343–353. doi: 10.1046/j.1523-1747.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 54.Kehe K., Abend M., Kehe K., Ridi R., Peter R.U., van Beuningen D. Tissue engineering with HaCaT cells and a fibroblast cell line. Arch. Dermatol. Res. 1999;291:600–605. doi: 10.1007/s004030050461. [DOI] [PubMed] [Google Scholar]
- 55.Cho K.H., Lee D.Y., Park K.C., Cho K.H. Organotypic Culture of HaCaT cells: Use of Dermal Substrate that Combines de-epidermized Dermis with Fibroblast-populated Collagen Matrix. Ann. Dermatol. 2002;14:137. doi: 10.5021/ad.2002.14.3.137. [DOI] [PubMed] [Google Scholar]
- 56.Zinn M., Aumailley M., Krieg T., Smola H. Expression of laminin 5 by parental and c-Ha-ras-transformed HaCaT keratinocytes in organotypic cultures. Eur. J. Cell Biol. 2006;85:333–343. doi: 10.1016/j.ejcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Smola H., Thiekötter G., Fusenig N. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J. Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smola H., Stark H.-J., Thiekötter G., Mirancea N., Krieg T., Fusenig N.E. Dynamics of Basement Membrane Formation by Keratinocyte–Fibroblast Interactions in Organotypic Skin Culture. Exp. Cell Res. 1998;239:399–410. doi: 10.1006/excr.1997.3910. [DOI] [PubMed] [Google Scholar]
- 59.Clark R.A.F., Nielsen L.D., Welch M.P., McPherson J.M. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-β. J. Cell Sci. 1995;108:1251–1261. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- 60.Xu J., Clark R.A. Extracellular matrix alters PDGF regulation of fibroblast integrins. J. Cell Biol. 1996;132:239–249. doi: 10.1083/jcb.132.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montero A., Quílez C., Valencia L., Girón P., Jorcano J.L., Velasco D. Effect of Fibrin Concentration on the In Vitro Production of Dermo-Epidermal Equivalents. Int. J. Mol. Sci. 2021;22:6746. doi: 10.3390/ijms22136746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin-Piedra M.A., Carmona G., Campos F., Carriel V., Fernández-González A., Campos A., Cuende N., Garzón I., Gacto P., Alaminos M. Histological assessment of nanostructured fibrin-agarose skin substitutes grafted in burnt patients. A time-course study. Bioeng. Transl. Med. 2023;8 doi: 10.1002/btm2.10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth-Carter Q.R., Koetsier J.L., Broussard J.A., Green K.J. Organotypic Human Skin Cultures Incorporating Primary Melanocytes. Current Protocols. 2022;2 doi: 10.1002/cpz1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumbach C.-M., Anantama N.A., Savkovic V., Mülling C.K.W., Schinköthe J., Michler J.K. 3D Approaches to Culturing Bovine Skin: Explant Culture versus Organotypic Skin Model. Cells Tissues Organs. 2024;213:424–438. doi: 10.1159/000538438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meana A., Iglesias J., Del Rio M., Larcher F., Madrigal B., Fresno M.F., Martin C., San Roman F., Tevar F. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns. 1998;24:621–630. doi: 10.1016/S0305-4179(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 66.Llames S.G., Del Rio M., Larcher F., García E., García M., José Escamez M., Jorcano J.L., Holguín P., Meana A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation. 2004;77:350–355. doi: 10.1097/01.TP.0000112381.80964.85. [DOI] [PubMed] [Google Scholar]
- 67.Boyce S.T., Supp A.P., Swope V.B., Warden G.D. Vitamin C Regulates Keratinocyte Viability, Epidermal Barrier, and Basement Membrane In Vitro, and Reduces Wound Contraction After Grafting of Cultured Skin Substitutes. J. Invest. Dermatol. 2002;118:565–572. doi: 10.1046/j.1523-1747.2002.01717.x. [DOI] [PubMed] [Google Scholar]
- 68.Pinnell S.R. Regulation of collagen biosynthesis by ascorbic acid: a review. Yale J. Biol. Med. 1985;58:553–559. [PMC free article] [PubMed] [Google Scholar]
- 69.Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development (Cambridge, England) 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 70.Qiao H., Shibaki A., Long H.A., Wang G., Li Q., Nishie W., Abe R., Akiyama M., Shimizu H., McMillan J.R. Collagen XVII Participates in Keratinocyte Adhesion to Collagen IV, and in p38MAPK-Dependent Migration and Cell Signaling. J. Invest. Dermatol. 2009;129:2288–2295. doi: 10.1038/jid.2009.20. [DOI] [PubMed] [Google Scholar]
- 71.Kishimoto Y., Saito N., Kurita K., Shimokado K., Maruyama N., Ishigami A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013;430:579–584. doi: 10.1016/j.bbrc.2012.11.110. [DOI] [PubMed] [Google Scholar]
- 72.Ohashi A., Sakamoto H., Kuroda J., Kondo Y., Kamei Y., Nonaka S., Furukawa S., Yamamoto S., Satoh A. Keratinocyte-driven dermal collagen formation in the axolotl skin. Nat. Commun. 2025;16:1757. doi: 10.1038/s41467-025-57055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calfon M., Zeng H., Urano F., Till J.H., Hubbard S.R., Harding H.P., Clark S.G., Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 74.Brakebusch C., Grose R., Quondamatteo F., Ramirez A., Jorcano J.L., Pirro A., Svensson M., Herken R., Sasaki T., Timpl R., et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raghavan S., Bauer C., Mundschau G., Li Q., Fuchs E. Conditional Ablation of β1 Integrin in Skin: Severe Defects in Epidermal Proliferation, Basement Membrane Formation, and Hair Follicle Invagination. J. Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Anken E., Nixon-Abell J., Sitia R. Encyclopedia of Cell Biology. Elsevier; 2023. The Endoplasmic Reticulum; pp. 193–207. [DOI] [Google Scholar]
- 77.Beriault D.R., Werstuck G.H. Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound, Thioflavin T. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:2293–2301. doi: 10.1016/j.bbamcr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 78.Anelli T., Dalla Torre M., Borini E., Mangini E., Ulisse A., Semino C., Sitia R., Panina-Bordignon P. Profound architectural and functional readjustments of the secretory pathway in decidualization of endometrial stromal cells. Traffic. 2022;23:4–20. doi: 10.1111/tra.12822. [DOI] [PubMed] [Google Scholar]
- 79.Walter F., Schmid J., Düssmann H., Concannon C.G., Prehn J. Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival. Cell Death Differ. 2015;22:1502–1516. doi: 10.1038/cdd.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y., Giovannini S., Wang T., Fang J., Li P., Shao C., Wang Y., Agostini M., Bove P., Mauriello A., et al. p63: a crucial player in epithelial stemness regulation. Oncogene. 2023;42:3371–3384. doi: 10.1038/s41388-023-02859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurata S.i., Okuyama T., Osada M., Watanabe T., Tomimori Y., Sato S., Iwai A., Tsuji T., Ikawa Y., Katoh I. p51/p63 Controls Subunit α3 of the Major Epidermis Integrin Anchoring the Stem Cells to the Niche. J. Biol. Chem. 2004;279:50069–50077. doi: 10.1074/jbc.M406322200. [DOI] [PubMed] [Google Scholar]
- 82.Carroll D.K., Carroll J.S., Leong C.-O., Cheng F., Brown M., Mills A.A., Brugge J.S., Ellisen L.W. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 83.Maas-Szabowski N., Stark H.-J., Fusenig N.E. Keratinocyte Growth Regulation in Defined Organotypic Cultures Through IL-1-Induced Keratinocyte Growth Factor Expression in Resting Fibroblasts. J. Invest. Dermatol. 2000;114:1075–1084. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 84.Rider P., Kaplanov I., Romzova M., Bernardis L., Braiman A., Voronov E., Apte R.N. The transcription of the alarmin cytokine interleukin-1 alpha is controlled by hypoxia inducible factors 1 and 2 alpha in hypoxic cells. Front. Immunol. 2012;3 doi: 10.3389/fimmu.2012.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabouille C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017;27:230–240. doi: 10.1016/j.tcb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Cook P.W., Pittelkow M.R., Shipley G.D. Growth factor-independent proliferation of normal human neonatal keratinocytes: Production of autocrine- and paracrine-acting mitogenic factors. J. Cell. Physiol. 1991;146:277–289. doi: 10.1002/jcp.1041460213. [DOI] [PubMed] [Google Scholar]
- 87.Wang K., Jiang H., Li W., Qiang M., Dong T., Li H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deyrieux A.F., Wilson V.G. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007;54:77–83. doi: 10.1007/s10616-007-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lansdown A.B.G. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–285. doi: 10.1046/j.1524-475X.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 90.Tu C.-L., Oda Y., Komuves L., Bikle D.D. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 91.Wilke M.S., Hsu B.M., Scott R.E. Two subtypes of reversible cell cycle restriction points exist in cultured normal human keratinocyte progenitor cells. Lab. Invest. 1988;58:660–666. [PubMed] [Google Scholar]
- 92.Menon G.K., Grayson S., Elias P.M. Ionic Calcium Reservoirs in Mammalian Epidermis: Ultrastructural Localization by Ion-Capture Cytochemistry. J. Invest. Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 93.Vičanová J., Boelsma E., Mommaas A.M., Kempenaar J.A., Forslind B., Pallon J., Egelrud T., Koerten H.K., Ponec M. Normalization of Epidermal Calcium Distribution Profile in Reconstructed Human Epidermis Is Related to Improvement of Terminal Differentiation and Stratum Corneum Barrier Formation. J. Invest. Dermatol. 1998;111:97–106. doi: 10.1046/j.1523-1747.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- 94.Weber S.U., Thiele J.J., Packer L., Cross C.E. Vitamin C, Uric Acid, and Glutathione Gradients in Murine Stratum Corneum and their Susceptibility to Ozone Exposure. J. Invest. Dermatol. 1999;113:1128–1132. doi: 10.1046/j.1523-1747.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- 95.Smart I.H.M. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br. J. Dermatol. 1970;82:276–282. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- 96.Clayton E., Doupé D.P., Klein A.M., Winton D.J., Simons B.D., Jones P.H. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 97.Jensen K.B., Watt F.M. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc. Natl. Acad. Sci. USA. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Potten C.S. Cell Replacement in Epidermis (Keratopoiesis) via Discrete Units of Proliferation. Int. Rev. Cytol. 1981;69:271–318. doi: 10.1016/S0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- 99.Ivanov V.O., Ivanova S.V., Niedzwiecki A. Ascorbate Affects Proliferation of Guinea-pig Vascular Smooth Muscle Cells by Direct and Extracellular Matrix-mediated Effects. J. Mol. Cell Cardiol. 1997;29:3293–3303. doi: 10.1006/jmcc.1997.0555. [DOI] [PubMed] [Google Scholar]
- 100.Böhmer J.A., Sellhaus B., Schrage N.F. Effects of ascorbic acid on retinal pigment epithelial cells. Curr. Eye Res. 2001;23:206–214. doi: 10.1076/ceyr.23.3.206.5464. [DOI] [PubMed] [Google Scholar]
- 101.Sommer F., Kobuch K., Brandl F., Wild B., Framme C., Weiser B., Tessmar J., Gabel V.-P., Blunk T., Goepferich A. Ascorbic Acid Modulates Proliferation and Extracellular Matrix Accumulation of Hyalocytes. Tissue Eng. 2007;13:1281–1289. doi: 10.1089/ten.2006.0274. [DOI] [PubMed] [Google Scholar]
- 102.Choi K.-M., Seo Y.-K., Yoon H.-H., Song K.-Y., Kwon S.-Y., Lee H.-S., Park J.-K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008;105:586–594. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- 103.Savini I., Rossi A., Duranti G., Avigliano L., Catani M.V., Melino G. Characterization of keratinocyte differentiation induced by ascorbic acid: protein kinase C involvement and vitamin C homeostasis. J. Invest. Dermatol. 2002;118:372–379. doi: 10.1046/j.0022-202x.2001.01624.x. [DOI] [PubMed] [Google Scholar]
- 104.Koh W.S., Lee S.J., Lee H., Park C., Park M.H., Kim W.S., Yoon S.S., Park K., Hong S.I., Chung M.H., Park C.H. Differential effects and transport kinetics of ascorbate derivatives in leukemic cell lines. Anticancer Res. 1998;18:2487–2493. [PubMed] [Google Scholar]
- 105.Head K.A. Ascorbic acid in the prevention and treatment of cancer. Alternative Med. Rev. 1998;3:174–186. [PubMed] [Google Scholar]
- 106.Covarrubias-Pinto A., Acuña A.I., Boncompain G., Papic E., Burgos P.V., Perez F., Castro M.A. Ascorbic acid increases SVCT2 localization at the plasma membrane by accelerating its trafficking from early secretory compartments and through the endocytic-recycling pathway. Free Radic. Biol. Med. 2018;120:181–191. doi: 10.1016/j.freeradbiomed.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 107.Boyce S.T., Simpson P.S., Rieman M.T., Warner P.M., Yakuboff K.P., Bailey J.K., Nelson J.K., Fowler L.A., Kagan R.J. Randomized, Paired-Site Comparison of Autologous Engineered Skin Substitutes and Split-Thickness Skin Graft for Closure of Extensive, Full-Thickness Burns. J. Burn Care Res. 2017;38:61–70. doi: 10.1097/BCR.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meuli M., Hartmann-Fritsch F., Hüging M., Marino D., Saglini M., Hynes S., Neuhaus K., Manuel E., Middelkoop E., Reichmann E., Schiestl C. A Cultured Autologous Dermo-epidermal Skin Substitute for Full-Thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children. Plast. Reconstr. Surg. 2019;144:188–198. doi: 10.1097/PRS.0000000000005746. [DOI] [PubMed] [Google Scholar]
- 109.Shahin H., Elmasry M., Steinvall I., Söberg F., El-Serafi A. Vascularization is the next challenge for skin tissue engineering as a solution for burn management. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pleguezuelos-Beltrán P. Regenerative Cosmetics: Skin Tissue Engineering for Anti-Aging, Repair, and Hair Restoration. Cosmetics. 2024;11:121. doi: 10.3390/cosmetics11040121. [DOI] [Google Scholar]
- 111.Yuen W.Y., Di Zenzo G., Jonkman M.F., Pas H.H. New versatile monoclonal antibodies against type XVII collagen endodomain for diagnosis and subtyping COL17A1 -associated junctional epidermolysis bullosa. J. Eur. Acad. Dermatol. Venereol. 2016;30:1426–1427. doi: 10.1111/jdv.13270. [DOI] [PubMed] [Google Scholar]
- 112.Yao Z., Huang Y.t., Zhao L., Fu Z., Zhao M., Song X.m., Jia J., Wang S., Li J.p., Zhu Z.f., et al. Therapeutic effects of tyroservatide on metastasis of lung cancer and its mechanism affecting integrin focal adhesion kinase signal transduction. Drug Des. Devel. Ther. 2016;649:649. doi: 10.2147/DDDT.S86284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang L.-K., Wang M.-J.J. Image thresholding by minimizing the measures of fuzziness. Pattern Recogn. 1995;28:41–51. doi: 10.1016/0031-3203(94)E0043-K. [DOI] [Google Scholar]
- 114.Lachowski D., Matellan C., Gopal S., Cortes E., Robinson B.K., Saiani A., Miller A.F., Stevens M.M., del Río Hernández A.E. Substrate Stiffness-Driven Membrane Tension Modulates Vesicular Trafficking via Caveolin-1. ACS Nano. 2022;16:4322–4337. doi: 10.1021/acsnano.1c10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Raw images and cytometry data have been deposited at the e-cienciaDatos repository of the Madroño consortium and are publicly available as of the date of publication. The accession number is listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
This paper does not report any original code.
-
•
The software used to analyze the reported data are disclosed in the key resources table.