Abstract
Multiple classes of factors contribute to angiogenesis. In past years, the primary focus has been to understand the functions of individual classes of angiogenic factors. However, few studies have focused on the combinatorial roles of multiple classes of factors in angiogenesis. In this report, we have investigated the in vivo angiogenic processes regulated by two major classes of angiogenic factors, the angiopoietins and vascular endothelial growth factor (VEGF). Here we show that angiopoietin-1, a factor previously considered to be proangiogenic, can offset VEGF-induced angiogenesis in vivo. We also provide direct in vivo evidence for the synergistic effect of angiopoietin-2 and VEGF on the induction of angiogenesis. Furthermore, we show that these two classes of factors control the ratio of arterial and venous blood vessel types during angiogenesis. We believe that our study is a step toward understanding how multiple classes of factors harmonize angiogenesis and blood vessel types.
Angiogenesis is one of the most vital biological processes required for the formation and physiological functions of virtually all organs in both normal and disease states. In some human diseases such as in heart ischemia, this process can be used to restore the vital function of the affected organs (1). In other diseases such as in cancer, aberrant angiogenesis can be therapeutically blocked to prevent disease progression (2). In past years, many factors that play important roles in angiogenesis have been discovered and their critical and individual involvement in angiogenesis has been extensively investigated. However, very little is known how these multiple classes of factors coordinately regulate angiogenesis.
Among the many known classes of angiogenic factors, vascular endothelial growth factor (VEGF) and the angiopoietins represent two (3). Recently, their essential roles in angiogenesis have been confirmed by both loss-of-function and gain-of-function studies (4–8). Furthermore, it has been shown that VEGF and the angiopoietins exhibit both distinct and overlapping expression patterns during angiogenesis (9, 10). Based on this indirect evidence, it has been suggested that VEGF and the angiopoietins collaborate to regulate various aspects of angiogenesis. To support this hypothesis, it has been shown that cooverexpression of VEGF-A and angiopoietin-1 (Ang1) in postnatal mouse skin has a synergistic effect on the induction of angiogenesis (7). Furthermore, it has been shown that Ang1 can rescue the vessel leakiness caused by VEGF-A without interfering with induction of angiogenesis (7, 8). On the basis of this study, it has been suggested that VEGF-A and Ang1 collaborate to induce angiogenesis and make nonleaky mature vessels.
Another angiopoietin, angiopoietin-2 (Ang2), was previously shown to block the function of Ang1 in vitro (5). Furthermore, it was shown that VEGF-A and Ang2 were coexpressed at sites where new vessel formation is just beginning (9, 10). Based on evidence obtained from these biochemical and expression studies, it has been proposed that Ang2 makes mature vessels unstable by blocking the effects of Ang1 (5, 11). This Ang2-mediated vessel destabilization is thought to make the vessels hypersensitive to other classes of angiogenic factors such as VEGF-A (5, 11). This model is quite attractive and if proven correct could have a direct impact on devising new strategies for therapeutic angiogenesis.
Clearly, there is a paucity of more comprehensive data that support these models of collaborative functions among the VEGF-A, Ang1, and Ang2. Therefore, we performed this study to obtain more direct evidence by testing these models in vivo in a systematic manner.
Experimental Procedures
Transgenic Constructs.
Murine VEGF164, Ang1, and Ang2 were specifically expressed in the cardiac muscle by ligating cDNA encoding each into the SalI site of the αMHC/Hgh vector (kindly provided by J. Robbins Children's Hospital, Cincinnati) (12). This vector contains the 5′-promoter region and the 3′- intron of the α-myosin heavy chain gene followed by the human growth hormone polyadenylation sequence. Each plasmid construct, αMHC∷VEGF, αMHC∷Ang1, and αMHC∷Ang2, was digested with NotI to remove vector sequences. Procedures for transgene fragment purification and generation of the transgenic mice by pronuclear injections were performed as described (13, 14).
Mice.
αMHC∷VEGF, αMHC∷Ang1, and αMHC∷Ang2 single-transgenic lines.
These transgenic mice were generated by injecting each transgene fragment, and maintained by crossing to both CD1 and C57BL.
αMHC∷VEGF;αMHC∷Ang1 double-transgenic line.
Two independent methods were used to generate this double-transgenic line. Each single-transgenic line, αMHC∷VEGF and αMHC∷Ang1, was crossed to generate the double-transgenic line. In the other method, two transgenes were coinjected to generate the double-transgenic line. All results presented here were confirmed by using the double-transgenic lines generated by both methods.
αMHC∷VEGF;αMHC∷Ang2 double-transgenic line.
Two independent methods were used to generate this double-transgenic line. Each single-transgenic line, αMHC∷VEGF and αMHC∷Ang2, was crossed to generate the double-transgenic line. In the other method, two transgenes were coinjected to generate the double-transgenic line. All results presented here were confirmed by using the transgenic lines generated by both methods.
αMHC∷VEGF;αMHC∷Ang1;αMHC∷Ang2 triple-transgenic line.
This triple-transgenic line was generated by either crossing the αMHC∷VEGF;αMHC∷Ang1 double-transgenic line to the αMHC∷Ang2 single-transgenic line or crossing the αMHC∷VEGF;αMHC∷Ang2 double-transgenic line to the αMHC∷Ang1 single-transgenic line. All results presented here were confirmed by using the triple-transgenic lines generated by both methods.
ephrinB2taulacZ/+ and ephB4taulacZ/+.
These lacZ-knock-in mice were previously described and kindly provided by D. Anderson (California Institute of Technology, Pasadena) (15, 16). In this study, the ephrinB2taulacZ/+ and ephB4taulacZ/+ lines were used as lacZ-based indicator lines for arterial and venous endothelial cells, respectively (15, 16).
All analyses presented here were carried out with mice that were 1–2 months old. Multiple transgenic lines that express transgenes were generated and they all exhibited the same phenotypes presented in this study.
Genotyping.
Genotypes of the mice were routinely determined by PCR. Three 3′ specific reverse primers were used (VEGF [257] GGCTGTGCTGTAGGAAGCTC, Ang1 [260] GTCGTGTTCTGGAAGAATGAAAGT, and Ang2 [354] GACATGTAGGGGCTGGAGGAAGAT) with a 5′ upstream forward primer in the exon 3 region [259] TGGTCCACATTCTTCAGGATTC of the αMHC/Hgh vector.
Reverse Transcription (RT)-PCR.
Heart-specific expression of the transgenes was confirmed by RT-PCR. Heart, brain, kidney, liver, and spleen tissues were harvested from each transgenic line, rinsed in PBS, and stored in RNAlater (Ambion, Austin, TX) at 4°C until use. RNA from mouse tissues was prepared by using Trizol (Life Technologies, Rockville, MD) according to the manufacturer's directions. RT-PCR was performed with mouse tissue RNA by using Titan One Tube RT-PCR reagents (Roche). The primers for the angiopoietins and VEGF were as described above. The mRNA transcript-specific amplification products are approximately 300 bp smaller than the products generated by contaminating transgenes present in the genomic DNA.
Immunolabeling and LacZ Staining.
Mice were anesthetized and perfused with 0.1 M Pipes (pH 7.3) followed by 2% paraformaldehyde (PFA)/0.1 M Pipes by means of left ventricular puncture. Hearts were excised and fixed in 0.2% PFA/0.1 M Pipes overnight at 4°C. Fixed hearts were processed for cryosectioning at 7 μm. Cryosections were cleared in PBS, pH 7.3, permeabilized in 0.3% Triton X-100 (Roche) in PBS, and labeled with antibodies to CD31/PECAM-1 (PharMingen), and β-galactosidase (5′-3′, Boulder, CO). Primary antibodies were immunolocalized with Cy3- or Cy5-conjugated donkey anti-rat, and FITC-conjugated donkey anti-rabbit secondary antibodies (Jackson Immunochemicals, West Grove, PA), respectively. LacZ histochemical staining of the FLK1∷lacZ was performed as described (http://cbi.swmed.edu/ryburn/sato). Images were generated on a Zeiss Axioplan 2 microscope equipped with a Zeiss Axiocam digital camera.
Results
Transgenic mouse lines ectopically expressing either one or a combination of angiogenic factors specifically in cardiac muscle were generated (Fig. 1). The cardiac expression system was chosen because of the potential for direct applicability to therapies for human cardiac diseases where the therapeutic angiogenesis is clearly needed (1).
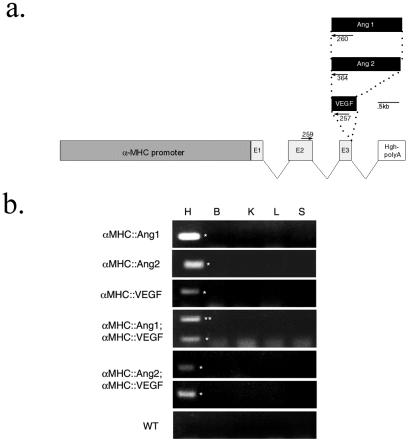
Figure 1.
Transgenic constructs and the cardiac-specific expression of the transgenes. (a) Schematic diagram of the transgenic constructs. cDNA encoding Ang1, Ang2, and VEGF was inserted downstream of the αMHC promoter/exons. (b) RT-PCR expression analysis of the transgenes in adult organs. The transgenes are expressed specifically in the heart. Integrity of each RNA sample was confirmed by amplifying for Tie2, which is expressed in all tissues (data not shown). WT, wild type; H, heart; B, brain; K, kidney; S, spleen.
First, adult hearts from the transgenic mouse line expressing VEGF-A, αMHC∷VEGF, were examined. The endothelial cells were specifically identified by immunolabeling with anti-CD31/PECAM-1 antibody. A significant increase in capillary density in the heart was observed, confirming the previous notion that VEGF-A is a potent angiogenic factor (Fig. 2 a and b). Moreover, some fluid accumulation in heart tissue was noticed during the dissection, suggesting that the vessels generated by VEGF-A might be leaky as shown (7, 8).
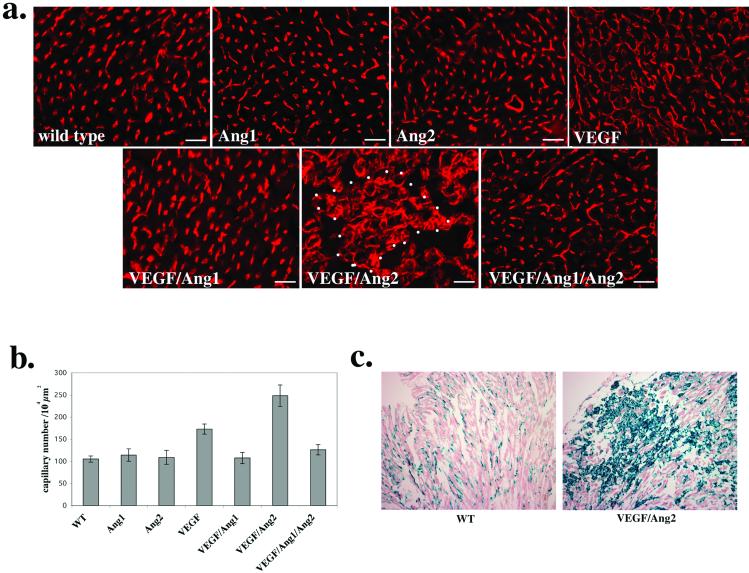
Figure 2.
Combinatorial regulation of angiogenesis by VEGF and angiopoietins. (a) Anti-CD31/PECAM-1-antibody staining of the adult heart from each transgenic line and wild-type control. The genotype of each line is indicated at the bottom left (Ang1, αMHC∷Ang1; Ang2, αMHC∷Ang2; VEGF, αMHC∷VEGF; VEGF/Ang1, αMHC∷VEGF;αMHC∷Ang1 double-transgenic line; VEGF/Ang2, αMHC∷VEGF;αMHC∷Ang2 double-transgenic line; and VEGF/Ang1/Ang2, αMHC∷VEGF;αMHC∷Ang1;αMHC∷Ang2 triple-transgenic line). An example of the aberrant clusters of capillaries in the VEGF/Ang2 double-transgenic heart is outlined by the dotted line. (Scale bar: 25 μm.) (b) Quantitation of capillary density. Capillary lumens in each transgenic line and wild-type control hearts were counted (per 104-μm2 field) from 6–13 randomly selected fields. (c) Flk1∷lacZ staining of the wild-type control and VEGF/Ang2 transgenic lines.
In contrast, transgenic Ang1 expression in the heart did not cause increased angiogenesis (Fig. 2 a and b). CD31/PECAM-1-positive capillary density in the αMHC∷Ang1 mouse heart was at almost the same level as in the wild-type mouse heart. This lack of angiogenesis in the heart is contradictory to previous data with transgenic mice overexpressing Ang1 in skin (6). This difference might be explained in part by the fact that cardiac muscle, like most other tissues, expresses a high level of endogenous Ang1 throughout life (4, 17). In contrast, skin may be a very specialized microenvironment in this context because there is almost no detectable level of endogenous Ang1 expression in skin (6).
Subsequently, the heart of the transgenic line expressing both VEGF-A and Ang1, αMHC∷VEGF;αMHC∷Ang1, was examined. In this transgenic line, capillary angiogenesis in the heart induced by VEGF-A was significantly inhibited by Ang1 (Fig. 2 a and b). This inhibition is again in contrast to the previous study in skin, where Ang1 enhanced VEGF-induced angiogenesis in a synergistic manner (7). Based on these studies, it is possible that Ang1 could work as both a positive and negative regulator of VEGF-induced angiogenesis depending on the specific organ microenvironment. In the αMHC∷VEGF;αMHC∷Ang1 double-transgenic line, no fluid accumulation was noticeable in the heart, suggesting that Ang1 might be preventing the VEGF-induced vascular leakiness as would be predicted based on previous studies (7, 8).
Next, we tested for whether the other angiopoietin, Ang2, could collaborate with VEGF-A to facilitate angiogenesis. We and others have proposed this hypothesis based on the coincidental expression patterns of VEGF-A and Ang2 during the initial phase of angiogenesis (5, 9, 11). For this purpose, the double-transgenic line αMHC∷VEGF;αMHC∷Ang2, in which both VEGF-A and Ang2 are coexpressed in the heart, was examined. In this transgenic line, cardiac capillary density was increased over the enhanced capillary density observed in the αMHC∷VEGF individuals by nearly 50% (Fig. 2 a and b). Furthermore, numerous aberrant clusters of capillaries were present in the αMHC∷VEGF;αMHC∷Ang2 transgenic heart (Fig. 2a). This phenotype was not observed in the αMHC∷Ang2 transgenic heart, where only Ang2 is ectopically expressed (Fig. 2a).
The endothelial origin of these aberrant capillary clusters was confirmed by expression of another endothelial marker, kdr/flk1, in addition to the CD31/PECAM-1 (Fig. 1c). The double-transgenic line was crossed to the flk1∷lacZ knock-in line, in which the lacZ reporter is inserted into the first exon of the kdr/flk1 gene (18, 19). In the triple-transgenic line αMHC∷VEGF;αMHC∷Ang2;flk1∷lacZ these aberrant vessel clusters stained positive for LacZ, confirming the endothelial origin of these capillary clusters (Fig. 2c).
The histology of the αMHC∷VEGF;αMHC∷Ang2 double-transgenic heart is consistent with the presence of edema (Fig. 3a). This histological cardiac phenotype may reflect the fact that capillaries in this double-transgenic mouse line remain severely leaky. Furthermore, severe fibrosis was obvious in the cardiac tissue surrounding capillaries in this double-transgenic line (Fig. 3a). This observation was confirmed by staining the tissues with Masson's trichrome (Fig. 3a).
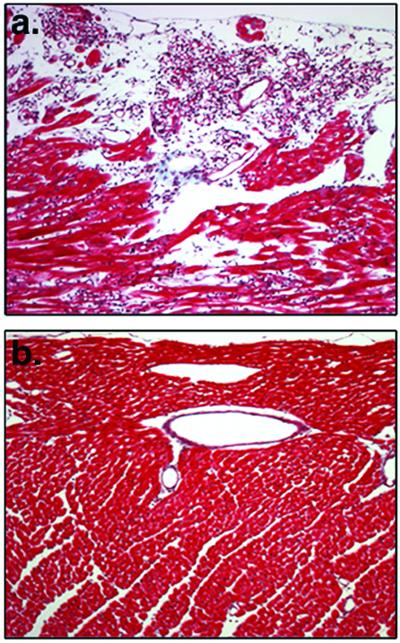
Figure 3.
Severe edema and fibrosis caused by VEGF/Ang2 is corrected by Ang1. (a) The αMHC∷VEGF;αMHC∷Ang2 double-transgenic heart stained with Masson's trichrome. This histology is consistent with the widely distributed severe edema. Furthermore, many clustered extracellular matrix (ECM) fibers were detected by the trichrome method, indicating fibrosis. (b) The αMHC∷VEGF;αMHC∷Ang1;αMHC∷Ang2 triple-transgenic heart stained with trichrome. The histology is consistent with the significant improvement of severe edema. Furthermore, the decreased incidence of ECM fibers identified by the trichrome staining indicates improvement of this pathological condition.
We subsequently tested whether Ang1 could block the aberrant and enhanced angiogenesis induced by VEGF-A and Ang2. For this purpose, the triple-transgenic line, αMHC∷VEGF;αMHC∷Ang2;αMHC∷Ang1, in which all three factors are ectopically expressed in the heart, was generated and examined. Hearts from this triple-transgenic line demonstrated significantly decreased capillary density as compared with the αMHC∷VEGF and αMHC∷VEGF;αMHC∷Ang2 lines (Fig. 2 a and b). This result indicates that Ang1 can block both VEGF-induced and VEGF/Ang2-induced angiogenesis. In this triple-transgenic line, the formation of aberrant capillary clusters present in the αMHC∷VEGF;αMHC∷Ang2 heart was significantly reduced (Fig. 2 a and c). Furthermore, the histology of the triple-transgenic heart is consistent with significant improvement of the edema and fibrosis observed in the double-transgenic heart (Fig. 3b).
Conventionally, three major parameters have been examined to assess the efficacy of the angiogenic activities of individual factors: vessel density, supporting cell (e.g., smooth muscle) recruitment, and vessel permeability. Historically, when assessing by all three criteria, vessels have been studied as one uniform class of organ structure. However, it has recently been revealed that all blood vessels, including capillaries, can be molecularly classified into two types: veins and arteries (15, 16, 20, 21). The problem of determining whether angiogenic factors have a preferential effect on assembling one type of vessel over the other (i.e., veins vs. arteries) has not been addressed.
In this study, VEGF-A is shown to induce angiogenesis in the heart. Therefore, we have studied whether VEGF-A preferentially elicits assembly of arterial- or venous-type capillaries. For this purpose, we have used two convenient knock-in mice reporters. The ephrinB2taulacZ/+ line is a mouse reporter in which the lacZ reporter is knocked-in to the ephrinB2 locus (16, 21). In this line, lacZ is preferentially expressed in the arterial-blood vessels (16, 21). The other line is an ephB4taulacZ/+ line, in which the lacZ reporter is knocked-in to the ephB4 locus and lacZ is preferentially expressed in the venous blood vessels (15, 21).
These reporters were crossed to the αMHC∷VEGF transgenic line and the double-transgenic lines, αMHC∷VEGF; ephrinB2taulacZ/+ and αMHC∷VEGF;ephB4taulacZ/+, were studied for lacZ expression (Fig. 4). Anti-β-galactosidase (LacZ) immunolabeling of the αMHC∷VEGF;ephrinB2taulac/Z+ heart showed nearly 95% of the capillaries are of an arterial type. Anti-LacZ immunolabeling of the wild-type control showed only 50% of the capillaries are of an arterial type (Fig. 4a). In the αMHC∷VEGF;ephB4taulacZ/+ heart, less than 10% of the capillaries were LacZ positive, suggesting that the increase in arterial-type capillaries is accompanied by a decrease in venous-type capillaries in the αMHC∷VEGF heart (Fig. 4b).
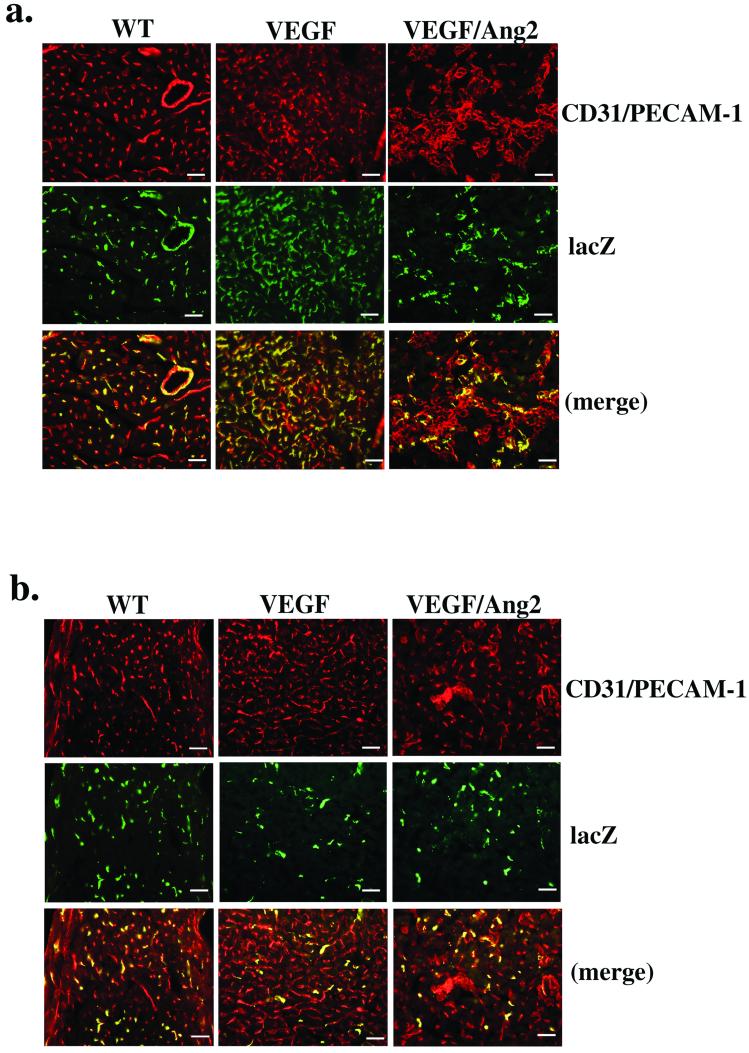
Figure 4.
Regulation of the dominance of vascular types by VEGF and VEGF/Ang2. (a) CD31/PECAM-1 (Top) and LacZ (Middle) immunolabeling of each transgenic line, αMHC∷VEGF (VEGF), αMHC∷VEGF;αMHC∷Ang2 (VEGF/Ang2), and wild type control (WT) crossed to the arterial-endothelial reporter line, ephrinB2taulacZ/+. The merged images are shown at Bottom. (b) CD31/PECAM-1- (Top) and LacZ (Middle) immunolabeling of each transgenic line, αMHC∷VEGF (VEGF), αMHC∷VEGF;αMHC∷Ang2 (VEGF/Ang2), and wild type (WT) crossed to the venous endothelial reporter line, ephB4taulacZ/+. The merged images are shown at Bottom. The percentages of either ephrinB2+ or ephB4+ capillaries were calculated by comparing number of CD31/PECAM-1-positive and the LacZ-immunopositive capillary lumens in the same section. Percentages of ephrinB2+ (i.e., arterial-type) capillaries: WT 50 ± 3.1%; and VEGF 96 ± 6 4%; VEGF/Ang2, 55 ± 17. Percentages of ephB4+ (i.e., venous-type) capillaries: WT 36 ± 6.4%; VEGF 10 ± 2.0%; and VEGF/Ang2, 12 ± 4%. (Scale bars: 25 μm.)
In the αMHC∷VEGF;αMHC∷Ang2 heart, capillary formation is further enhanced as compared with the αMHC∷VEGF single-transgenic line. Moreover, formation of aberrantly clustered capillary beds was observed in this double-transgenic-line heart. This double-transgenic line was crossed to the same lacZ reporter lines to determine the identity (i.e., arterial vs. venous) of these aberrant capillaries (Fig. 4). In the αMHC∷VEGF;αMHC∷Ang2;ephrinB2taulacZ/+ heart, lacZ immunopositive vessels constituted nearly 50% of the capillaries (Fig. 4a). This result clearly suggests that the marked increase in arterial-type capillaries induced by VEGF-A is significantly suppressed by the presence of Ang2. In the αMHC∷VEGF;αMHC∷Ang2;ephB4taulacZ/+ heart, LacZ staining remained as low as that which was observed in the αMHC∷VEGF;ephB4taulacZ/+ heart, indicating that a significant percentage of the aberrantly clustered capillaries in the double-transgenic line may express neither ephrinB2 nor ephB4 (Fig. 4b).
Discussion
In this report, we describe two findings. We demonstrate that two classes of angiogenic factors, VEGF and angiopoietins, can collaborate in the regulation of angiogensis in vivo. In this regard, we have clearly shown that Ang2 can collaborate with VEGF-A to induce capillary angiogenesis in vivo in a synergistic manner. We also describe the surprising finding that Ang1 can act as a negative regulator of VEGF-induced angiogenesis in a specific organ microenvironment: the heart. We expect that this study is a step toward understanding how multiple classes of factors contribute to the formation of new blood vessels in a combinatorial manner. Furthermore, it raises an important possibility that the combinatorial regulatory effects of multiple classes of angiogenic factors differ from one organ microenvironment to another.
Another previously unreported finding is that the expression of VEGF-A induces a predominance of ephrinB2+- (i.e., arterial) type vascular beds in the heart. This could be accomplished by preferentially assembling ephrinB2+ endothelial cells. Alternatively, this could also be accomplished by switching on ephrinB2 expression in endothelial cells, which normally express ephB4. Furthermore, this asymmetric distribution of vascular type induced by VEGF-A can be modulated by Ang2.
It has been recently reported that the symmetric distribution of ephrinB2+ and ephB4+ capillaries is essential for normal vascular development (15, 16). Therefore, our results suggest an interesting possibility when considered in the context of this previous report: the “asymmetric” distribution of ephrinB2+ and ephB4+ capillaries in the αMHC∷VEGF and αMHC∷VEGF;αMHC∷Ang2 transgenic hearts may effect aberrant vascular morphogenesis such as that clearly found in the αMHC∷VEGF;αMHC∷Ang2 heart. Subtle morphological defects may also exist in the capillaries found in the αMHC∷VEGF transgenic heart as a result of this asymmetric distribution of the vascular types. Furthermore, the presence of a significant number of ephrinB2−/ephB4− capillaries in the αMHC∷VEGF;αMHC∷Ang2 heart may also contribute to the formation of the aberrant clusters of capillaries found in this double-transgenic heart.
Previously, many angiogenic factors have been discovered and their functions have been described. However, the problem of determining whether these factors contribute to the preferential assembly and/or specification of arterial and/or venous types of capillaries has not been addressed. Based on the results presented here, this previously unreported regulatory mechanism should be investigated with regard to other angiogenic factors. Such studies would surely contribute to our further understanding of the complexity and intricacy of the regulatory mechanisms underlying angiogenesis. These studies could lead to development of future therapeutic strategies that permit the manipulation of the arterial/venous system in a specific manner to treat human diseases.
Acknowledgments
We thank the Sato lab members for discussions. We also thank the Molecular Histology Core and the Transgenic Core with help on some of the sectioning and the microinjections, respectively. Technical assistance by Jenifer Wilder and Joyce Pike is also acknowledged. This work was supported by National Institutes of Health Grant R01HL63880 (to T.N.S.) and a National Institutes of Health training grant (to R.P.V.).
Abbreviations
- VEGF
vascular endothelial growth factor
- Ang1 and Ang2
angiopoietin-1 and angiopoietin-2
- αMHC
α-myosin heavy chain
- RT-PCR
reverse transcription–PCR
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Present address: Medical University of South Carolina, Charleston, SC 29425.
References
- 1.Isner J M. Nature (London) 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Yancopoulos G D, Davis S, Gale N W, Rudge J S, Wiegand S J, Holash J. Nature (London) 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 4.Suri C, Jones P F, Patan S, Bartunkova S, Maisonpierre P C, Davis S, Sato T N, Yancopoulos G D. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 5.Maisonpierre P C, Suri C, Jones P F, Bartunkova S, Wiegand S J, Radziejewski C, Compton D, McClain J, Aldrich T H, Papadapoulos N, et al. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 6.Suri C, McClain J, Thurston G, McDonald D M, Zhou H, Oldmixon E H, Sato T N, Yancopoulos G D. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 7.Thurston G, Suri C, Smith K, McClain J, Sato T N, Yancopoulos G D, McDonald D M. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 8.Thurston G, Rudge J S, Ioffe E, Zhou H, Ross L, Croll S D, Glazer N, Holash J, McDonald D M, Yancopoulos G D. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 9.Holash J, Maisonpierre P C, Compton D, Boland P, Alexander C R, Zagzag D, Yancopoulos G D, Wiegand S J. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 10.Stratmann A, Risau W, Plate K H. Am J Pathol. 1998;153:1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holash J, Wiegand S J, Yancopoulos G D. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 12.Subramaniam A, Jones W K, Gulick J, Wert S, Neumann J, Robbins J. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 13.Schlaeger T M, Qin Y, Fujiwara Y, Magram J, Sato T N. Development (Cambridge, UK) 1995;121:1089–1098. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- 14.Schlaeger T M, Bartunkova S, Lawitts J A, Teichmann G, Risau W, Deutch U, Sato T N. Proc Natl Acad Sci USA. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerety S S, Wang H U, Chen Z F, Anderson D J. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang H U, Chen Z F, Anderson D J. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Aldrich T H, Jones P F, Acheson A, Bruno J, Radjiewski C, Compton D, Maisonpierre P C, Yancopoulos G D. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 18.Shalaby F, Rossant J, Yamaguchi T P, Breitman M, Schuh A C. Nature (London) 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 19.Shalaby F, Ho J, Stanford W L, Fischer K-D, Schuh A C, Schwartz L, Bernstein A, Rossant J. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 20.Gale N W, Baluk P, Pan L, Kwan M, Holash J, DeChiara T M, McDonald D M, Yancopoulos G D. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 21.Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone M A, Jr, Anderson D J. Dev Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]