ABSTRACT
Timing of pre-emptive kidney transplantation (PKT) and the role of estimated glomerular filtration rate (eGFR) change in outcome prediction remains a subject of debate. This study aimed to assess potential factors, with special attention to uraemic burden, which may be associated with 5-year outcomes. In our retrospective observational cohort study, first PKT adults registered in the CRISTAL database between 2013 and 2019 were analysed to elucidate the role of eGFR and other associating factors with death and graft loss. Recipient-, donor- and transplantation-related features were analysed by using multivariable logistic regression analysis. A conditional inference tree was applied for risk stratification. A total of 2327 first PKT [52.8 years (interquartile range 43–64), 38% female) were included. The mean percentage of PKT over time was 14%. Primary kidney disease (congenital anomalies, glomerulonephritis and other causes versus autosomal dominant polycystic kidney disease), donor age and number of DR mismatches associated with combined 5-year outcomes [odds ratio 2.64 (95% confidence interval 1.42–4.93); 1.94 (1.1–4.93); 1.76 (1.06–2.92); 1.03 (1.02–1.05); 1.67 (1.1–2.53); P < .05], whereas donor type was not associated with outcomes. By supervised decision-tree analysis, >30% risk of failure in PKT was attributed to high recipient risk, higher donor age, uraemic burden index (UBI)—a novel parameter defined by the product of eGFR change and the logarithmic time on the waiting list—and two DR mismatches. In conclusion, eGFR and donor type were not associated with death or graft failure in PKT. UBI can potentially be a novel parameter of uraemic burden and contribute to predict 5-year risk of failure. Clinical decisions based on objective risk estimations might be crucial to approach the ‘PKT in due course’ concept.
Keywords: biomarker, eGFR, kidney transplantation, outcome, pre-emptive
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Pre-emptive kidney transplantation (PKT) offers survival benefits and improved graft outcomes compared with non-pre-emptive transplantation (non-PKT) or dialysis.
‘Early’ PKT, defined by estimated glomerular filtration rate, may not always be advantageous for every individual compared with non-PKT.
This study adds:
In addition to recipient and donor-specific factors, the uraemic burden index (UBI), a new biomarker characterizing cumulative uraemic exposure, should be considered as a predictor of 5-year outcome.
Several factors are associated with an increased risk of PKT: high-risk recipients, older donors, higher UBI scores and poorer DR matching.
Potential impact:
Identifying individuals at higher risk of transplant failure or mortality is essential to prevent unnecessary post-transplantation risks.
Appropriate timing and optimal decision-making based on comprehensive risk assessments may minimize unnecessary risks and help to distribute our limited supply of donor organs, including access to scarce living donor sources.
INTRODUCTION
Recent literature supports prioritizing transplantation beyond other renal replacement therapies. This approach offers survival benefits and improved graft outcomes compared to non-pre-emptive transplantation (non-PKT) or dialysis [1–4]. However, some studies suggest that individual patient assessment is necessary to determine if pre-emptive kidney transplantation (PKT) is beneficial compared with other strategies [5, 6]. Notably, some researchers propose individual risk assessment to define a safe transplantation window [7]. Despite benefits, only ≈5–19% of kidney transplants are pre-emptive, suggesting more conservative practices than guidelines recommend. Followed by Norway (20.0%), Denmark (18.3%), Sweden (16.5%) and the UK (15.3%), France is fifth in line [8], ahead of the USA (9%) [9]. It is unclear whether these differences stem solely from varying allocation system rules or if they are influenced by factors such as donor availability, economic considerations or regional variations in clinical decision-making practices.
Kidney Disease: Improving Global Outcomes guidelines recommend PKT when estimated glomerular filtration rate (eGFR) is <10 ml/min/1.73 m2 in adults or <15 ml/min/1.73 m2 in children. The decision for PKT should consider multiple factors beyond eGFR, including overall health, chronic illnesses, donor availability and post-transplant care adherence. Nevertheless, other clinical indicators, such as uraemic symptoms, should also be considered [10–12]. The clinical decision of PKT by defining a transplantation window has been based on eGFR and individual risk–benefit analysis, without applying a specific risk percentage threshold for outcome prediction. The role of eGFR remains ambiguous for defining ‘early’ or ‘late’ PKT [7, 13–16].
Recent observational studies and medico-economic analyses suggest that PKT may not always be advantageous for every individual compared with non-PKT [17]. The limited supply of deceased donors and varying access to living donors raise questions about optimal resource allocation for best patient outcomes. It is crucial to balance this with avoiding the potential pitfalls of unsuccessful PKT. Addressing the ambiguity surrounding the ‘transplant first’ theory and identifying individuals at higher risk of transplant failure or mortality are essential to prevent unnecessary post-transplantation risks. It remains to be determined whether significant outcome differences exist among individuals with varying levels of uraemic burden.
Exploratory analysis of PKT and stratification of patients at potential risk of failure based on their eGFR at the time of transplantation have not yet been examined in the French National Transplant Registry (CRISTAL) database. Our aim was to assess potential factors, with special attention toward uraemic burden that may be associated with the risk of 5-year outcomes in patients transplanted pre-emptively.
MATERIALS AND METHODS
Study population and data collection
All adult pre-emptive kidney-only transplant patients between 1 January 2013 and 31 December 2018 were included. Data were obtained from CRISTAL, which was initiated in 1996 and is maintained by the Agence de la Biomédecine (Saint-Denis, France). CRISTAL prospectively collects demographic, clinical and laboratory data for all organ transplant recipients, donors and transplantations in France, as well as transplant outcomes. Data are recorded at waitlisting, procurement, transplantation and annually thereafter [18, 19]. All patients who received their first kidney transplant without prior dialysis and who were >18 years of age at the time of PKT were incorporated into the final cohort. Patients awaiting or who had received a previous combined solid organ transplant were excluded.
All parameters of traditional and non-traditional factors available in CRISTAL at the time of waitlisting and at the time of transplantation were recorded. The body mass index (BMI) of recipients and donors was calculated as kg/m2. eGFR was calculated using the new race-free creatinine-based equation [20]. A history of cardiovascular disease (CVD) was defined as the presence of one of following conditions: chronic heart failure, acute coronary syndrome, acute myocardial infarction, angina pectoris and arrhythmia. Primary kidney disease categories were identified as autosomal dominant polycystic kidney disease (ADPKD), congenital anomalies of the kidney and urinary tract (CAKUT), diabetes mellitus, hypertension/renal vascular disease (RVD), glomerulonephritis, other primary renal disease (PRD) and unknown or missing.
Novel parameter: uraemic burden index (UBI)
To characterize the uraemic burden on the waiting list, we implemented a more sophisticated method: the product of the delta eGFR (the difference between eGFR at PKT and eGFR at waitlisting) and the logarithm of the time on the waiting list. We transformed waitlist time logarithmically to create a normally distributed parameter (Supplementary Fig. 3). A negative value denotes a decrease in eGFR, and thus the progression of chronic kidney disease (CKD). Figure 6 illustrates that a more negative surface area suggests a higher uraemic burden. The delta eGFR, multiplied by a unit of waiting list time, could serve as a distinguishing factor between patients with the same delta eGFR but different waiting times.
Figure 6:
Decision tree for overall graft loss (death and failure) in the (a) combined, (b) deceased and (c) living donor PKT group. WL: waitlisting; Recipient: recipient score; MM DR: DR mismatch (0, 1, 2).
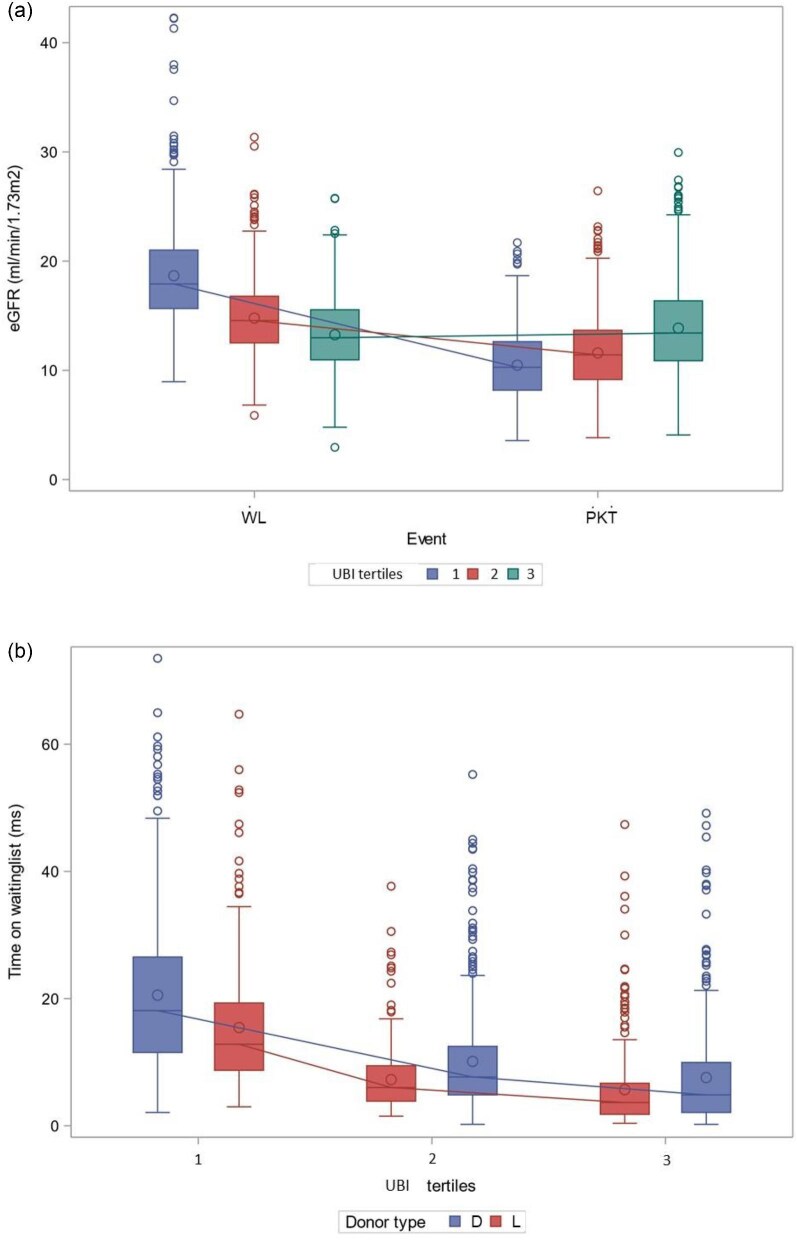
Supplementary Table 5 shows baseline characteristics classified by UBI tertiles, with the highest uraemic burden represented by the lowest tertile. Figures 4a and b display eGFR values per UBI tertile and waiting time classified by UBI tertiles and donor type, respectively.
Figure 4:
UBI, i.e. ΔeGFR * log(time on WL), where ΔeGFR = eGFR at PKT − eGFR at WL. WL: waitlisting.
Outcomes
The start of observation (t0) was the time of PKT, and the end of follow-up was when events occurred. The events of interest were death from any cause, graft loss (leading to initiation of dialysis or retransplantation) and the combination of death or graft loss, whichever came first, referred to as PKT failure (overall graft loss). Overall mortality and death-censored graft loss were also analysed. Patients lost to follow-up or who survived were censored at the time of the last visit or exactly at 5 years after PKT.
Statistical analysis
Data were expressed as mean or median with interquartile range (IQR), based on distribution. Statistically significant differences between groups were calculated using the Student's t-test for normally distributed continuous variables and the Mann–Whitney U test for non-normally distributed continuous variables or ordinal data. The chi-squared test was used for categorical variables and Fisher's exact test was applied when sample sizes were small. For multiple comparisons, Bonferroni correction was applied to adjust P-values. Assumptions about potential confounders were based on Supplementary Table 6 and biological evidence.
For outcome assessment, Kaplan–Meier estimation was used. Then we analysed potential factors associated with these outcomes using univariable and multivariable logistic regression. All analyses were conducted on the entire cohort and separately for deceased and living donor PKT subgroups. Lastly, we carried out a decision tree analysis. Two-tailed P-values <.05 were considered to be statistically significant. SAS Enterprise Guide (SAS Institute, Cary, NC, USA) and R (R Foundation for Statistical Computing, Vienna, Austria) were used for the statistical analysis.
Definition of unnecessary PKT: decision tree analysis for classification of risk categories
We employed the recursive partitioning (rpart) algorithm, a supervised classification method, to stratify PKT recipients into distinct risk groups for graft loss and mortality. Recipient and donor score, transplantation-specific factors and the UBI were included as predictors. For donor score, we performed multivariable logistic regression for overall graft loss, mortality and death-censored graft loss, subsequently including donor age, height, weight, cause of death, donor hypertension and donor diabetes. For recipient scores, we included the following in the baseline multivariable stepwise logistic model: recipient age, sex, BMI, CKD primary disease, CVD and diabetes mellitus. Only the significant parameters were included in the final score, which were calculated as the following:
Overall graft loss:
 |
 |
Overall mortality:
 |
 |
Death-censored graft loss:
 |
 |
where CVD is 0 (no) or 1 (yes).
Altogether nine decision trees were reported (Fig. 6, Supplementary Figs. 4 and 5) The algorithm recursively split the data based on these variables, creating a tree structure that optimally separated patients into risk groups. The rpart algorithm offers good interpretability, but it may be prone to overfitting. To address this, the pruning method (minbucket = 7, mincriterion = 0.9, minsplit = 7, maxdepth = 4, cp = 0.001) was set accordingly.
The age difference between recipient and donor was used in logistic regression models. The distribution is shown in Supplementary Fig. 3.
Missing data characteristics
Fully conditional specification (FCS) was implied for multiple imputations to address missing data of serum creatinine at PKT. Otherwise, variables with <15% missingness were included in the analysis. We generated 13 imputed datasets, each with a complete set of plausible values for 284 missing data points based on the observed relationships between age, sex and creatinine at the time of transplantation, which enabled inclusion of 12% of cases. Missing data are shown in Supplementary Table 9.
Propensity score matching: sensitivity analysis
A 1:1 greedy matching algorithm was employed, using the logit of the propensity score (LPS) with a 0.2 standard deviation caliper width for propensity score matching between deceased and living donor PKT to reduce confounding and selection bias in estimating the effect of donor type on outcomes. The model included recipient age at transplant, sex, baseline CKD, CVD, eGFR at transplant and DR mismatches (Supplementary Tables 7 and 8 and Supplementary Fig. 1).
Ethics
This study was conducted with the ethical approval and authorization of the Agence de la Biomedicine scientific advisory board. By law, data collection is mandatory by all organ procurement organizations and transplant centres in France. All data are anonymized and studies using the data do not require institutional review board approval. The study was conducted according to French law stating that research studies based on the CRISTAL national registry are part of transplant assessment activity and do not require institutional review board approval. Kidney allocation policies are described in detail at https://www.agence-biomedecine.fr/.
RESULTS
Descriptive analysis
During the study period, a total of 16 292 first kidney transplantations were performed, 14% of which were PKT from deceased (n = 978) or living (n = 1348) donors. Altogether, 2327 adult patients [median age 52.8 years (IQR 43–64), 38% female, P = .001] who received their first, single PKT from either a living (42%) or deceased donor were included in our retrospective observational analysis. Figure 1 represents the flow chart of patient inclusion. Figure 2 shows that the proportion of PKT increased over time. Figure 3 shows how eGFR at waitlisting and at PKT changed over time. Table 1 shows the baseline characteristics of the entire study cohort and subgroups by ‘early’ and ‘late’ transplant and by donor type. Descriptive statistics by sex are shown in Supplementary Table 1. Furthermore, features of the cohort are classified by eGFR quintiles at the time of waitlisting, PKT, transplant year and CKD baseline disease in Supplementary Tables 1–4.
Figure 1:
Flow chart of patient selection from the CRISTAL database. *Start of follow-up (T0) is at the time of PKT, the censoring date is the post-transplant fifth year or lost to follow-up. The primary event is the overall graft loss (graft loss or death, whichever comes first).
Figure 2:
(a) Number of transplantations between 2013 and 2018 (P < .05), (b) proportion of PKT per donor type and (c) if PKT versus non-PKT by year and (d) proportion of deceased and living donor PKTs within all transplantations between 2013 and 2019. (e) The number of living and deceased donor PKTs by the year of transplantation (P < .0001)). D: deceased; L: living.
Figure 3:
(a) eGFR at the time of waitlisting and at time of PKT by transplant year. (b) Least square mean of eGFR by years 15.5 at waitlisting versus 11.8 ml/min/1.73 m2 at PKT (P < .05) and 2013 versus 2016, 2017 and 2018 (P < .05) and by CKD baseline disease categories [(c) waitlisting: 2 versus 1, 5 or 7 years; P < .05; PKT: P = not significant). Baseline CKD categories: 1- ADPKD, 2- CAKUT, 3- diabetes mellitus, 4- hypertension/RVD, 5- glomerulonephritis, 6- ‘other progressive renal disease’ (e.g. tubulointerstitial diseases, medullar cystic disease, pyelonephritis, tubular dysgenesis, trauma, tumour, Balkan nephropathy, tuberculosis, goutte, nephrocalcinosis, myeloma, amyloidosis, systemic disease, toxic nephropathy, other), 7- unknown or missing.
Table 1:
Demographic data by median indication eGFR at PKT (a) and by donor type (b).
| A | ||||
|---|---|---|---|---|
| Variables | Overall | >= median eGFR | < median eGFR | |
| N = 2327a | N = 1162a | N = 1165a | P-valueb | |
| Recipient characteristics at the time of PKT | ||||
| Age (years) | 52.76 (43, 64) | 52.39 (41, 64) | 53.14 (44, 65) | .231 |
| Sex (n,%) | .001 | |||
| Male | 1451/2327 (62%) | 763/1162 (66%) | 688/1165 (59%) | |
| Female | 876/2327 (38%) | 399/1162 (34%) | 477/1165 (41%) | |
| Height (m) | 1.70 (1.64, 1.76) | 1.70 (1.64, 1.76) | 1.70 (1.63, 1.76) | .331 |
| Weight (kg) | 73.79 (63.10, 83.30) | 74.37 (64.00, 84.00) | 73.19 (62.90, 83.00) | .057 |
| BMI (kg/m2) | 25.45 (22.49, 28.09) | 25.53 (22.65, 28.09) | 25.37 (22.40, 28.09) | .386 |
| Serum Creatinine (umol/L) | 478.28 (373.86, 553.95) | 376.60 (325.00, 426.00) | 579.70 (482.00, 649.00) | <.001 |
| eGFR (ml/min/1.73 m2) | 11.90 (9.07, 14.12) | 14.94 (12.73, 16.19) | 8.87 (7.63, 10.37) | <.001 |
| CMV | .001 | |||
| IgG pos | 1192/2282 (52%) | 556/1139 (49%) | 636/1143 (56%) | |
| IgG neg | 1090/2282 (48%) | 583/1139 (51%) | 507/1143 (44%) | |
| EBV | .377 | |||
| IgG pos | 2157/2266 (95%) | 1074/1133 (95%) | 1083/1133 (96%) | |
| IgG neg | 109/2266 (4.8%) | 59/1133 (5.2%) | 50/1133 (4.4%) | |
| Blood group | .095 | |||
| A | 1162/2320 (50%) | 610/1160 (53%) | 552/1160 (48%) | |
| B | 223/2320 (9.6%) | 101/1160 (8.7%) | 122/1160 (11%) | |
| AB | 123/2320 (5.3%) | 60/1160 (5.2%) | 63/1160 (5.4%) | |
| O | 812/2320 (35%) | 389/1160 (34%) | 423/1160 (36%) | |
| Primary kidney disease | .482 | |||
| Autosomal dominant polycystic kidney disease | 557/2320 (24%) | 264/1160 (23%) | 293/1160 (25%) | |
| CAKUT | 196/2320 (8.4%) | 103/1160 (8.9%) | 93/1160 (8.0%) | |
| Diabetes mellitus | 589/2320 (25%) | 287/1160 (25%) | 302/1160 (26%) | |
| Hypertension or renal vascular disease | 130/2320 (5.6%) | 69/1160 (5.9%) | 61/1160 (5.3%) | |
| Glomerulonephritis | 177/2320 (7.6%) | 86/1160 (7.4%) | 91/1160 (7.8%) | |
| other | 366/2320 (16%) | 198/1160 (17%) | 168/1160 (14%) | |
| unknown or missing | 305/2320 (13%) | 153/1160 (13%) | 152/1160 (13%) | |
| Smoking | .332 | |||
| Never | 644/952 (68%) | 324/468 (69%) | 320/484 (66%) | |
| Ever | 308/952 (32%) | 144/468 (31%) | 164/484 (34%) | |
| Yes | 0/952 (0%) | 0/468 (0%) | 0/484 (0%) | |
| Recipient characteristics at the time of waitlisting | ||||
| Cardiovascular disease* | 217/2327 (9.3%) | 119/1162 (10%) | 98/1165 (8.4%) | .129 |
| Chronic heart failure | 26/2266 (1.1%) | 12/1137 (1.1%) | 14/1129 (1.2%) | .680 |
| Acute coronary syndrome | 97/2271 (4.3%) | 56/1141 (4.9%) | 41/1130 (3.6%) | .132 |
| Acute myocardial infarction | 57/2278 (2.5%) | 26/1145 (2.3%) | 31/1133 (2.7%) | .477 |
| Angina pectoris | 21/2283 (0.9%) | 13/1145 (1.1%) | 8/1138 (0.7%) | .279 |
| Arrhythmia | 70/2282 (3.1%) | 39/1145 (3.4%) | 31/1137 (2.7%) | .346 |
| TIA/Ischemic stroke | 112/2284 (4.9%) | 61/1145 (5.3%) | 51/1139 (4.5%) | .347 |
| Peripheral artery disease | 70/2267 (3.1%) | 38/1130 (3.4%) | 32/1137 (2.8%) | .450 |
| Dyslipidemia | 846/2174 (39%) | 410/1084 (38%) | 436/1090 (40%) | .298 |
| Diabetes mellitus (without insulin) |
281/2286 (12%) | 141/1148 (12%) | 140/1138 (12%) | .988 |
| Diabetes mellitus (with and without insulin) |
127/2286 (5.6%) | 62/1148 (5.4%) | 65/1138 (5.7%) | .745 |
| Hypertension (Primary and secondary) |
1557/2255 (69%) | 773/1132 (68%) | 784/1123 (70%) | .433 |
| Cirrhosis hepatis | 14/2261 (0.6%) | 6/1127 (0.5%) | 8/1134 (0.7%) | .600 |
| Chronic Pulmonary Disease | 8/2257 (0.4%) | 4/1134 (0.4%) | 4/1123 (0.4%) | >.999 |
| Neurologic disorder | 80/2283 (3.5%) | 43/1142 (3.8%) | 37/1141 (3.2%) | .497 |
| Uropathy | 416/2275 (18%) | 211/1139 (19%) | 205/1136 (18%) | .767 |
| Weight (kg) | 74.33 (63.00, 84.00) | 75.13 (64.00, 85.00) | 73.51 (63.00, 83.00) | .024 |
| Height (m) | 1.69 (1.63, 1.77) | 1.69 (1.63, 1.77) | 1.69 (1.63, 1.77) | .877 |
| BMI (kg/m2) | 25.45 (22.49, 28.09) | 25.53 (22.64, 28.09) | 25.38 (22.40, 28.09) | .407 |
| Serum Creatinine (umol/L) | 374.57 (307, 425) | 345.80 (291.00, 391.00) | 404.55 (332, 461) | <.001 |
| eGFR (ml/min/1.73 m2) | 15.57 (12.55, 17.89) | 17.02 (14.25, 19.23) | 14.06 (11.18, 16.28) | <.001 |
| Delta eGFR | −3.59 (−6.02, −0.71) | −2.12 (−4.47, 0.29) | −5.12 (−7.39, −2.19) | <.001 |
| UBI | −8.74 (−14.23, −0.59) | −5.37 (−9.68, 0.25) | −12.24 (−18.26, −2.85) | <.001 |
| Time on waiting list (ms) | 13.80 (4.49, 17.54) | 12.96 (4.16, 16.33) | 14.64 (4.89, 18.33) | .008 |
| Donor characteristics | ||||
| Donortype | .157 | |||
| Deceased | 1348/2326 (58%) | 656/1161 (57%) | 692/1165 (59%) | |
| Living | 978/2326 (42%) | 505/1161 (43%) | 473/1165 (41%) | |
| Donor Age (ys) | 53.89 (44, 65) | 53.69 (44, 65) | 54.09 (44.00, 65) | .545 |
| Donor Sex (n,%) | .834 | |||
| Males | 1129/2326 (49%) | 561/1161 (48%) | 568/1165 (49%) | |
| Females | 1197/2326 (51%) | 600/1161 (52%) | 597/1165 (51%) | |
| Donor Weight (kg) | 72.86 (61.50, 82.00) | 72.87 (61.00, 82.00) | 72.84 (62.00, 82.70) | .959 |
| Donor Height (m) | 1.68 (1.61, 1.75) | 1.68 (1.61, 1.75) | 1.68 (1.60, 1.75) | .573 |
| Donor BMI (kg/m2) | 26.36 (22.31, 28.04) | 26.80 (22.15, 28.06) | 25.92 (22.42, 27.97) | .242 |
| Donor Blood group | .368 | |||
| A | 1090/2326 (47%) | 565/1161 (49%) | 525/1165 (45%) | |
| B | 182/2326 (7.8%) | 90/1161 (7.8%) | 92/1165 (7.9%) | |
| AB | 90/2326 (3.9%) | 43/1161 (3.7%) | 47/1165 (4.0%) | |
| O | 964/2326 (41%) | 463/1161 (40%) | 501/1165 (43%) | |
| CMV | .562 | |||
| IgG pos | 655/1218 (54%) | 316/597 (53%) | 339/621 (55%) | |
| IgG neg | 563/1218 (46%) | 281/597 (47%) | 282/621 (45%) | |
| EBV | .976 | |||
| IgG pos | 1177/1218 (97%) | 577/597 (97%) | 600/621 (97%) | |
| IgG neg | 41/1218 (3.4%) | 20/597 (3.4%) | 21/621 (3.4%) | |
| Alcoholism | 306/1218 (25%) | 162/597 (27%) | 144/621 (23%) | .112 |
| Hypertension | 426/1204 (35%) | 201/590 (34%) | 225/614 (37%) | .350 |
| Diabetes mellitus | 104/1195 (8.7%) | 53/584 (9.1%) | 51/611 (8.3%) | .655 |
| Cause of death | .769 | |||
| Trauma | 311/1218 (26%) | 148/597 (25%) | 163/621 (26%) | |
| Vascular | 691/1218 (57%) | 337/597 (56%) | 354/621 (57%) | |
| Anoxia | 197/1218 (16%) | 103/597 (17%) | 94/621 (15%) | |
| Others | 19/1218 (1.6%) | 9/597 (1.5%) | 10/621 (1.6%) | |
| Transplantation characteristics | ||||
| Cold Ischemic Time (hs) | 10.13 (2.32, 15.92) | 9.82 (2.25, 15.37) | 10.44 (2.37, 16.17) | .067 |
| Warm Ischemic Time (Min) | 41.13 (26.00, 52.00) | 41.78 (25.50, 54.00) | 40.46 (26.00, 50.00) | .591 |
| Machine perfusion | 493/2322 (21%) | 243/1158 (21%) | 250/1164 (21%) | .771 |
| MM A | .384 | |||
| 0 | 388/2309 (17%) | 204/1153 (18%) | 184/1156 (16%) | |
| 1 | 1209/2309 (52%) | 589/1153 (51%) | 620/1156 (54%) | |
| 2 | 712/2309 (31%) | 360/1153 (31%) | 352/1156 (30%) | |
| MM B | .886 | |||
| 0 | 274/2309 (12%) | 133/1153 (12%) | 141/1156 (12%) | |
| 1 | 1030/2309 (45%) | 516/1153 (45%) | 514/1156 (44%) | |
| 2 | 1005/2309 (44%) | 504/1153 (44%) | 501/1156 (43%) | |
| MM DR | .442 | |||
| 0 | 734/2309 (32%) | 375/1153 (33%) | 359/1156 (31%) | |
| 1 | 1146/2309 (50%) | 557/1153 (48%) | 589/1156 (51%) | |
| 2 | 429/2309 (19%) | 221/1153 (19%) | 208/1156 (18%) | |
| MM DQ | .299 | |||
| 0 | 755/1432 (53%) | 390/742 (53%) | 365/690 (53%) | |
| 1 | 618/1432 (43%) | 327/742 (44%) | 291/690 (42%) | |
| 2 | 59/1432 (4.1%) | 25/742 (3.4%) | 34/690 (4.9%) | |
| cPRA (%) | 15.29 (0, 19) | 14.74 (0, 16) | 15.84 (0, 23) | .337 |
| Outcomes | ||||
| Overall mortality | 162/2327 (7.0%) | 84/1162 (7.2%) | 78/1165 (6.7%) | .613 |
| Overall graftloss | 295/2327 (13%) | 152/1162 (13%) | 143/1165 (12%) | .559 |
| Death censored graftloss | 133/2327 (5.7%) | 68/1162 (5.9%) | 65/1165 (5.6%) | .777 |
| B | ||||
| Variables | Overall (N = 2326)a | Deceased (n = 1348)a | Living (n = 978)a | P-valueb |
| Recipient characteristics at the time of PKT | ||||
| Age (years) | 52.77 (43, 64) | 55.79 (45, 68) | 48.61 (39, 59) | <.001 |
| Sex (n,%) | <.001 | |||
| Male | 1451/2326 (62%) | 793/1348 (59%) | 658/978 (67%) | |
| Female | 875/2326 (38%) | 555/1348 (41%) | 320/978 (33%) | |
| Height (m) | 1.70 (1.64, 1.76) | 1.69 (1.62, 1.75) | 1.72 (1.65, 1.78) | <.001 |
| Weight (kg) | 73.79 (63.10, 83.30) | 73.94 (63.80, 83.55) | 73.57 (63.00, 83.00) | .564 |
| BMI (kg/m2) | 25.45 (22.49, 28.09) | 25.87 (22.89, 28.57) | 24.87 (21.74, 27.47) | <.001 |
| Serum Creatinine (umol/L, at KTX) | 478.36 (373.90, 553.95) | 474.32 (367.00, 548.50) | 483.93 (383.20, 559.00) | .136 |
| eGFR (ml/min/1.73 m2) | 11.90 (9.07, 14.12) | 11.75 (8.89, 14.00) | 12.11 (9.42, 14.21) | .027 |
| CMV | <.001 | |||
| IgG pos | 1191/2281 (52%) | 739/1324 (56%) | 452/957 (47%) | |
| IgG neg | 1090/2281 (48%) | 585/1324 (44%) | 505/957 (53%) | |
| EBV | .215 | |||
| IgG pos | 2156/2265 (95%) | 1257/1314 (96%) | 899/951 (95%) | |
| IgG neg | 109/2265 (4.8%) | 57/1314 (4.3%) | 52/951 (5.5%) | |
| Blood group | <.001 | |||
| A | 1162/2320 (50%) | 745/1347 (55%) | 417/973 (43%) | |
| B | 223/2320 (9.6%) | 112/1347 (8.3%) | 111/973 (11%) | |
| AB | 123/2320 (5.3%) | 83/1347 (6.2%) | 40/973 (4.1%) | |
| O | 812/2320 (35%) | 407/1347 (30%) | 405/973 (42%) | |
| Primary kidney disease | <.001 | |||
| Autosomal dominant polycystic kidney disease | 557/2320 (24%) | 303/1347 (22%) | 254/973 (26%) | |
| CAKUT | 196/2320 (8.4%) | 98/1347 (7.3%) | 98/973 (10%) | |
| Diabetes mellitus | 589/2320 (25%) | 326/1347 (24%) | 263/973 (27%) | |
| Hypertension or renal vascular disease | 130/2320 (5.6%) | 86/1347 (6.4%) | 44/973 (4.5%) | |
| Glomerulonephritis | 177/2320 (7.6%) | 128/1347 (9.5%) | 49/973 (5.0%) | |
| other | 366/2320 (16%) | 206/1347 (15%) | 160/973 (16%) | |
| unknown or missing | 305/2320 (13%) | 200/1347 (15%) | 105/973 (11%) | |
| Smoking | .945 | |||
| Never | 644/952 (68%) | 358/530 (68%) | 286/422 (68%) | |
| Ever | 308/952 (32%) | 172/530 (32%) | 136/422 (32%) | |
| Yes | 0/952 (0%) | 0/530 (0%) | 0/422 (0%) | |
| Recipient characteristics at the time of waitlisting | ||||
| Cardiovascular disease | 217/2326 (9.3%) | 147/1348 (11%) | 70/978 (7.2%) | .002 |
| Chronic heart failure | 26/2265 (1.1%) | 17/1309 (1.3%) | 9/956 (0.9%) | .430 |
| Acute coronary syndrome | 97/2270 (4.3%) | 71/1312 (5.4%) | 26/958 (2.7%) | .002 |
| Acute myocardial infarction | 57/2277 (2.5%) | 34/1316 (2.6%) | 23/961 (2.4%) | .774 |
| Angina pectoris | 21/2282 (0.9%) | 12/1317 (0.9%) | 9/965 (0.9%) | .958 |
| Arrhythmia | 70/2281 (3.1%) | 46/1316 (3.5%) | 24/965 (2.5%) | .168 |
| TIA/Ischemic stroke | 112/2283 (4.9%) | 74/1319 (5.6%) | 38/964 (3.9%) | .068 |
| Peripheral artery disease | 70/2266 (3.1%) | 54/1304 (4.1%) | 16/962 (1.7%) | <.001 |
| Dyslipidemia | 846/2173 (39%) | 557/1253 (44%) | 289/920 (31%) | <.001 |
| Diabetes mellitus (without insulin) |
281/2285 (12%) | 191/1320 (14%) | 90/965 (9.3%) | <.001 |
| Diabetes mellitus (with and without insulin) |
127/2285 (5.6%) | 78/1320 (5.9%) | 49/965 (5.1%) | .392 |
| Hypertension (Primary and secondary) |
1557/2254 (69%) | 964/1305 (74%) | 593/949 (62%) | <.001 |
| Cirrhosis hepatis | 14/2260 (0.6%) | 8/1311 (0.6%) | 6/949 (0.6%) | .947 |
| Chronic Pulmonary Disease | 8/2256 (0.4%) | 6/1304 (0.5%) | 2/952 (0.2%) | .480 |
| Neurologic disorder | 80/2282 (3.5%) | 50/1317 (3.8%) | 30/965 (3.1%) | .378 |
| Uropathy | 416/2274 (18%) | 268/1312 (20%) | 148/962 (15%) | .002 |
| Weight (kg) | 74.33 (63.00, 84.00) | 74.33 (64.00, 84.00) | 74.33 (63.00, 84.00) | .997 |
| Height (m) | 1.69 (1.63, 1.77) | 1.68 (1.62, 1.75) | 1.71 (1.65, 1.78) | <.001 |
| BMI (kg/m2) | 25.45 (22.49, 28.09) | 25.88 (22.89, 28.57) | 24.86 (21.74, 27.48) | <.001 |
| Serum Creatinine (umol/L) | 374.54 (307.00, 425.00) | 368.72 (301.00, 415.00) | 382.19 (312.00, 434.00) | .004 |
| eGFR (ml/min/1.73 m2) | 15.57 (12.55, 17.89) | 15.43 (12.45, 17.76) | 15.75 (12.71, 18.14) | .112 |
| Delta eGFR | −3.59 (−6.02, −0.72) | −3.55 (−6.06, −0.66) | −3.66 (−6.02, −0.80) | .593 |
| UBI | −8.74 (−14.23, −0.59) | −9.13 (−15.19, −0.59) | −8.23 (−12.55, −0.60) | .115 |
| Time on waiting list (ms) | 13.81 (4.49, 17.54) | 16.29 (5.26, 20.82) | 10.38 (3.70, 12.82) | <.001 |
| Donor characteristics | ||||
| Donor Age (ys) | 53.89 (44.00, 65.00) | 55.50 (44.00, 70.00) | 51.67 (44.00, 60.00) | <.001 |
| Donor Sex (n,%) | <.001 | |||
| Males | 1129/2326 (49%) | 783/1348 (58%) | 346/978 (35%) | |
| Females | 1197/2326 (51%) | 565/1348 (42%) | 632/978 (65%) | |
| Donor Weight (kg) | 72.86 (61.50, 82.00) | 73.98 (62.50, 84.50) | 71.30 (60.00, 80.00) | <.001 |
| Donor Height (m) | 1.68 (1.61, 1.75) | 1.69 (1.62, 1.76) | 1.67 (1.60, 1.73) | <.001 |
| Donor BMI (kg/m2) | 26.36 (22.31, 28.04) | 25.79 (22.21, 28.41) | 27.15 (22.46, 27.59) | .121 |
| Donor Blood group | <.001 | |||
| A | 1090/2326 (47%) | 748/1348 (55%) | 342/978 (35%) | |
| B | 182/2326 (7.8%) | 104/1348 (7.7%) | 78/978 (8.0%) | |
| AB | 90/2326 (3.9%) | 70/1348 (5.2%) | 20/978 (2.0%) | |
| O | 964/2326 (41%) | 426/1348 (32%) | 538/978 (55%) | |
| CMV | NA | |||
| IgG pos | 655/1218 (54%) | 655/1218 (54%) | 0/0 (NA%) | |
| IgG neg | 563/1218 (46%) | 563/1218 (46%) | 0/0 (NA%) | |
| EBV | NA | |||
| IgG pos | 1177/1218 (97%) | 1177/1218 (97%) | 0/0 (NA%) | |
| IgG neg | 41/1218 (3.4%) | 41/1218 (3.4%) | 0/0 (NA%) | |
| Alcoholism | 306/1218 (25%) | 306/1218 (25%) | 0/0 (NA%) | NA |
| Hypertension | 426/1204 (35%) | 426/1204 (35%) | 0/0 (NA%) | NA |
| Diabetes mellitus | 104/1195 (8.7%) | 104/1195 (8.7%) | 0/0 (NA%) | NA |
| Cause of death | NA | |||
| Trauma | 311/1218 (26%) | 311/1218 (26%) | 0/0 (NA%) | |
| Vascular | 691/1218 (57%) | 691/1218 (57%) | 0/0 (NA%) | |
| Anoxia | 197/1218 (16%) | 197/1218 (16%) | 0/0 (NA%) | |
| Others | 19/1218 (1.6%) | 19/1218 (1.6%) | 0/0 (NA%) | |
| Cold Ischemic Time (hs) | 10.13 (2.32, 15.92) | 15.42 (11.33, 18.65) | 2.20 (1.23, 3.08) | <.001 |
| Warm Ischemic Time (Min) | 41.13 (26.00, 52.00) | 42.76 (25.00, 55.00) | 38.23 (26.00, 48.00) | .031 |
| Machine perfusion | 493/2322 (21%) | 492/1344 (37%) | 1/978 (0.1%) | <.001 |
| MM A | <.001 | |||
| 0 | 388/2309 (17%) | 168/1347 (12%) | 220/962 (23%) | |
| 1 | 1209/2309 (52%) | 684/1347 (51%) | 525/962 (55%) | |
| 2 | 712/2309 (31%) | 495/1347 (37%) | 217/962 (23%) | |
| MM B | <.001 | |||
| 0 | 274/2309 (12%) | 114/1347 (8.5%) | 160/962 (17%) | |
| 1 | 1030/2309 (45%) | 541/1347 (40%) | 489/962 (51%) | |
| 2 | 1005/2309 (44%) | 692/1347 (51%) | 313/962 (33%) | |
| MM DR | <.001 | |||
| 0 | 734/2309 (32%) | 517/1347 (38%) | 217/962 (23%) | |
| 1 | 1146/2309 (50%) | 614/1347 (46%) | 532/962 (55%) | |
| 2 | 429/2309 (19%) | 216/1347 (16%) | 213/962 (22%) | |
| MM DQ | <.001 | |||
| 0 | 755/1432 (53%) | 483/843 (57%) | 272/589 (46%) | |
| 1 | 618/1432 (43%) | 327/843 (39%) | 291/589 (49%) | |
| 2 | 59/1432 (4.1%) | 33/843 (3.9%) | 26/589 (4.4%) | |
| cPRA (%) | 15.29 (0, 19) | 16.19 (0, 20.5) | 14.04 (0, 17) | .059 |
| Outcomes | ||||
| Overall mortality | 162/2326 (7.0%) | 134/1348 (9.9%) | 28/978 (2.9%) | <.001 |
| Overall graftloss | 295/2326 (13%) | 219/1348 (16%) | 76/978 (7.8%) | <.001 |
| Death with functioning graft | 96/2326 (4.1%) | 78/1348 (5.8%) | 18/978 (1.8%) | <.001 |
| Death censored graftloss | 133/2326 (5.7%) | 85/1348 (6.3%) | 48/978 (4.9%) | .152 |
aMean (Q1, Q3); n / N (%).
bWelch Two Sample t-test; Pearson's Chi-squared test; Fisher's exact test.
Categorical variables are given as number of yes (1) / all patients in the group.
*Cardiovascular disease includes chronic heart failure ± acute coronary syndrome ± acute myocardial infarction ± angina pectoris ± arrhythmia.
N: number of cases; CMV: cytomegalovirus; EBV: Ebstein Barr virus; TIA: transient ischaemic attack; MM: mismatch; cPRA: calculated panel reactive antibody.
Novel biomarker of UBI
The lower the UBI, the higher the uraemic burden (Fig. 4). Figure 5 shows eGFR at waitlisting versus PKT by UBI tertiles. Supplementary Table 5 shows that younger patients with longer waiting times are in the lowest tertile, and there is no significant difference in donor type between tertiles.
Figure 5:
eGFR at the time of waitlisting and at the time of PKT by UBI tertiles: (a) eGFR at waitlisting versus PKT (P < .0001) [tertile 1 versus 2 and 3 (P < .001), but P = .053 tertile 2 versus 3] and the time on the waitlist classified by donor type and grouped by UBI tertiles [(b) both P < .0001)]. WL: waitlisting; ms: months.
Outcome of PKT
Survival at 5 years was 93.3% and 6.4% lost their graft. Causes of mortality were non-transplant-related malignant disease (23.7%); non-transplant-related other causes (psychiatric, bleeding or haemorrhage, cirrhotic disease; 22.4%); unknown, undetermined or missing causes (18.6%) and infection (14.1%), independent of PKT. The fifth cause was cardio- or cerebrovascular and thromboembolic origin not related to PKT (10.9%). A total of 3.2% of deaths were related to PKT and 7.1% due to COVID-19. Overall, graft loss was 12.6%. Death-censored graft loss was 5.9%, 2.21% of which were living donor transplants. The number of unfavourable outcomes increased over time (Supplementary Table 3). A total of 1.4% were lost to follow-up.
Univariable and multivariable regression analysis
The results of the univariable logisic regression analysis are shown in Table 2. Table 3 shows that primary CKD, donor age and DR mismatch were associated with overall failure of PKT at 5 years, while the overall mortality was determined mainly by recipient age, CVD and CKD primary disease and death-censored graft loss was associated with recipient and donor age and DR mismatch in the whole cohort and in deceased PKT recipients. Regarding living donor PKT, donor age and cold ischaemia time associated with the overall graft loss, recipient age and cold ischaemia time associated with mortality, and recipient age, cold ischaemia time and UBI related to death-censored graft loss. Age difference was dropped from the final model due to multicollinearity.
Table 2:
Univariable logistic regression model for overall graft loss, overall mortality and death-censored graft loss in the entire cohort and subgroups by donor type.
| All |
Deceased |
Living |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | OR | 95% CI |
P-value | OR | 95% CI |
P-value | OR | 95% CI |
P-value |
| Overall graft loss | |||||||||
| Age at PKT | 1.051 |
1.041–
1.061 |
<.0001 | 1.065 |
1.052–
1.078 |
<.0001 | 1.007 | 0.990– 1.024 |
.437 |
| Sex | 0.967 | 0.751– 1.245 |
.792 | 0.850 | 0.631– 1.144 |
.283 | 1.075 | 0.656– 1.762 |
.773 |
| BMI at PKT | 1.037 |
1.009–
1.066 |
.009 | 1.038 |
1.005–
1.071 |
.022 | 0.998 | 0.944– 1.056 |
.949 |
| CKD baseline disease 2 | 1.282 | 0.754– 2.179 |
.358 | 1.319 | 0.676– 2.574 |
.418 | 1.322 | 0.547– 3.196 |
.535 |
| CKD baseline disease 3 | 1.065 | 0.714– 1.590 |
.757 | 1.138 | 0.701– 1.847 |
.6 | 0.900 | 0.435– 1.860 |
.776 |
| CKD baseline disease 4 | 3.042 |
1.844–
5.020 |
<.0001 | 2.398 |
1.297–
4.432 |
.005 | 4.375 |
1.837–
10.422 |
.0009 |
| CKD baseline disease 5 | 3.155 |
2.008–
4.958 |
<.0001 | 3.217 |
1.907–
5.426 |
<.0001 | 1.690 | 0.589– 4.852 |
.323 |
| CKD baseline disease 6 | 1.421 | 0.928– 2.177 |
.106 | 1.296 | 0.763– 2.203 |
.338 | 1.653 | 0.802– 3.406 |
.173 |
| CKD baseline disease 7 | 1.988 |
1.307–
3.026 |
.001 | 2.232 |
1.368–
3.639 |
.001 | 0.902 | 0.343– 2.371 |
.834 |
| Cardiovascular disease (ref 0) | 2.076 |
1.464–
2.943 |
<.0001 | 2.134 |
1.436–
3.173 |
.0002 | 0.740 | 0.326– 1.677 |
.471 |
| Diabetes mellitus (ref 0) | 2.027 |
1.472–
2.792 |
<.0001 | 1.867 |
1.289–
2.704 |
.001 | 1.950 |
1.009–
3.768 |
.047 |
| Time on waiting list | 1.008 |
1.001–
1.015 |
.02 | 1.004 | 0.995– 1.012 |
.394 | 1.008 | 0.989– 1.026 |
.409 |
| Donor type (ref 0) | 0.434 | 0.330–0.572 | <.0001 | ||||||
| Donor age | 1.053 | 1.043–1.063 | <.0001 | 1.051 | 1.040–1.062 | <.0001 | 1.030 | 1.007–1.053 | .009 |
| Donor height | 0.258 | 0.098–0.681 | .006 | 0.084 | 0.023–0.300 | .001 | 0.796 | 0.115–5.526 | .817 |
| Donor weight | 0.999 | 0.992–1.007 | .881 | 0.996 | 0.987–1.005 | .356 | 1.003 | 0.988–1.018 | .72 |
| Donor BMI | 0.999 | 0.991–1.007 | .792 | 1.018 | 0.991–1.046 | .2 | 0.997 | 0.983–1.011 | .669 |
| Donor COD 2 (ref 1) | 1.740 | 1.186–2.555 | .005 | ||||||
| Donor COD 3 | 1.060 | 0.623–1.805 | .829 | ||||||
| Donor COD 4 | 0.821 | 0.183–3.689 | .7964 | ||||||
| Donor HT (ref 0) | 2.353 | 1.732–3.197 | <.0001 | ||||||
| Donor DM (ref 0) | 1.272 | 0.768–2.107 | .3498 | ||||||
| CIT | 1.036 | 1.020–1.052 | <.0001 | 0.997 | 0.972–1.022 | .798 | 1.320 | 1.119–1.557 | .001 |
| cPRA | 0.997 | 0.993–1.002 | .231 | 0.997 | 0.991–1.002 | .208 | 0.997 | 0.987–1.007 | .525 |
| MM A 1 (ref 0) | 1.013 | 0.712–1.443 | .942 | 0.869 | 0.554–1.364 | .542 | 0.985 | 0.546–1.778 | .959 |
| MM A 2 | 1.272 | 0.877–1.844 | .205 | 0.994 | 0.625–1.579 | .979 | 1.146 | 0.579–2.269 | .696 |
| MM B 1 (ref 0) | 1.158 | 0.750–1.789 | .508 | 0.855 | 0.495–1.478 | .575 | 1.535 | 0.729–3.233 | .259 |
| MM B 2 | 1.505 | 0.981–2.309 | .061 | 1.060 | 0.624–1.800 | .831 | 1.520 | 0.695–3.326 | .295 |
| MM DR 1 (ref 0) | 0.867 | 0.647–1.160 | .334 | 1.068 | 0.762–1.498 | .702 | 0.731 | 0.402–1.329 | .304 |
| MM DR 2 | 1.841 | 1.330–2.547 | .0002 | 2.513 | 1.703–3.708 | <.0001 | 1.470 | 0.777–2.782 | .236 |
| Age difference | 0.990 | 0.980–1.000 | .045 | 0.976 | 0.961–0.991 | .003 | 0.990 | 0.974–1.006 | .203 |
| UBI | 1.009 | 0.997–1.020 | .132 | 1.020 | 1.006–1.034 | .005 | 0.987 | 0.969–1.005 | .16 |
| eGFR at PKT | 1.007 | 0.976–1.039 | .659 | 1.005 | 0.968–1.042 | .806 | 1.031 | 0.972–1.094 | .312 |
| Overall mortality | |||||||||
| Age at PKT | 1.110 | 1.091 | 1.128 | <0.0001 | 1.097 | 1.077 | 1.117 | <0.0001 | 1.125 |
| Sex | 0.793 | 0.565–1.113 | .18 | 0.778 | 0.537–1.128 | .186 | 0.438 | 0.165–1.164 | .098 |
| BMI at PKT | 1.054 | 1.018–1.091 | .003 | 1.031 | 0.991–1.072 | .129 | 1.092 | 1.004–1.188 | .039 |
| CKD baseline disease 2 | 0.727 | 0.311–1.697 | .461 | 1.033 | 0.425–2.509 | .943 | <0.001 | <0.001–>999.999 | .967 |
| CKD baseline disease 3 | 0.870 | 0.499–1.518 | .624 | 0.878 | 0.466–1.654 | .686 | 0.801 | 0.241–2.658 | .717 |
| CKD baseline disease 4 | 4.444 | 2.468–8.002 | <.0001 | 3.070 | 1.523–6.188 | .002 | 9.185 | 3.013–28.003 | <.0001 |
| CKD baseline disease 5 | 3.380 | 1.915–5.965 | <.0001 | 3.099 | 1.655–5.803 | .0004 | 1.759 | 0.344–8.981 | .497 |
| CKD baseline disease 6 | 1.255 | 0.704–2.240 | .441 | 1.208 | 0.621–2.350 | .578 | 1.333 | 0.400–4.443 | .639 |
| CKD baseline disease 7 | 2.221 | 1.299–3.796 | .004 | 2.277 | 1.259–4.120 | .007 | 0.803 | 0.159–4.042 | .789 |
| Cardiovascular disease (ref 0) | 3.003 | 2.005–4.497 | <.0001 | 2.850 | 1.827–4.446 | <.0001 | 2.232 | 0.752–6.624 | .148 |
| Diabetes mellitus (ref 0) | 2.479 | 1.680–3.657 | <.0001 | 1.939 | 1.247–3.013 | .003 | 4.172 | 1.782–9.766 | .001 |
| Time on waiting list | 1.000 | 0.990–1.011 | .959 | 0.993 | 0.982–1.005 | .26 | 0.995 | 0.959–1.032 | .776 |
| Donor type (ref 0) | 0.267 | 0.176–0.405 | <.0001 | ||||||
| Donor age | 1.078 | 1.064–1.092 | <.0001 | 1.066 | 1.051–1.081 | <.0001 | 1.076 | 1.034–1.119 | .0003 |
| Donor height | 0.267 | 0.080–0.893 | .03 | 0.142 | 0.032–0.631 | .01 | 0.248 | 0.024–2.533 | .239 |
| Donor weight | 0.997 | 0.987–1.007 | .599 | 0.995 | 0.985–1.006 | .389 | 0.991 | 0.964–1.019 | .511 |
| Donor BMI | 0.998 | 0.986–1.010 | .758 | 1.010 | 0.976–1.044 | .573 | 0.995 | 0.966–1.026 | .763 |
| Donor COD 2 (ref 1) | 1.677 | 1.044–2.695 | .03 | ||||||
| Donor COD 3 | 1.057 | 0.547–2.044 | .868 | ||||||
| Donor COD 4 | <0.001 | <0.001–>999.999 | .986 | ||||||
| Donor HT (ref 0) | 2.155 | 1.480–3.137 | <.0001 | ||||||
| Donor DM (ref 0) | 1.034 | 0.537–1.991 | .919 | ||||||
| CIT | 1.050 | 1.030–1.070 | <.0001 | 0.994 | 0.964–1.026 | .722 | 1.343 | 1.043–1.729 | .02 |
| cPRA | 0.995 | 0.988–1.001 | .109 | 0.994 | 0.987–1.001 | .07 | 0.997 | 0.982–1.013 | .728 |
| MM A 1 (ref 0) | 1.574 | 0.935–2.652 | .09 | 1.100 | 0.622–1.947 | .743 | 3.206 | 0.727–14.138 | .129 |
| MM A 2 | 1.823 | 1.058–3.141 | .03 | 0.997 | 0.549–1.809 | .991 | 5.820 | 1.275–26.575 | .02 |
| MM B 1 (ref 0) | 1.505 | 0.781–2.901 | .222 | 1.110 | 0.528–2.334 | .784 | 2.327 | 0.523–10.348 | .267 |
| MM B 2 | 2.352 | 1.239–4.464 | .009 | 1.482 | 0.721–3.045 | .284 | 3.148 | 0.696–14.234 | .1236 |
| MM DR 1 (ref 0) | 0.899 | 0.609–1.328 | .593 | 1.091 | 0.716–1.662 | .687 | 1.124 | 0.354–3.570 | .843 |
| MM DR 2 | 2.018 | 1.329–3.065 | .001 | 2.414 | 1.507–3.869 | .0002 | 3.461 | 1.110–10.792 | .03 |
| Age difference | 1.010 | 0.996–1.024 | .156 | 0.985 | 0.966–1.004 | .119 | 1.050 | 1.019–1.081 | .001 |
| UBI | 1.017 | 1.001–1.033 | .03 | 1.020 | 1.003–1.037 | .02 | 1.013 | 0.976–1.051 | .495 |
| eGFR at PKT | 1.016 | 0.976–1.058 | .447 | 1.026 | 0.982–1.073 | .249 | 1.004 | 0.911–1.106 | .939 |
| DCGL | |||||||||
| Age at PKT | 1.001 | 0.989–1.013 | .861 | 1.020 | 1.004–1.036 | .01 | 0.961 | 0.941–0.981 | .0002 |
| Sex | 1.219 | 0.855–1.738 | .275 | 1.000 | 0.640–1.562 | .999 | 1.641 | 0.913–2.951 | .098 |
| BMI at PKT | 1.010 | 0.971–1.051 | .613 | 1.040 | 0.992–1.090 | .104 | 0.939 | 0.872–1.011 | .094 |
| CKD baseline disease 2 | 1.924 | 0.983–3.768 | .056 | 1.716 | 0.665–4.431 | .265 | 2.169 | 0.830–5.668 | .114 |
| CKD baseline disease 3 | 1.290 | 0.742–2.241 | .366 | 1.536 | 0.755–3.124 | .236 | 0.964 | 0.394–2.358 | .947 |
| CKD baseline disease 4 | 1.123 | 0.448–2.818 | .804 | 1.088 | 0.346–3.427 | .885 | 1.162 | 0.246–5.491 | .849 |
| CKD baseline disease 5 | 2.307 | 1.190–4.473 | .013 | 2.522 | 1.135–5.604 | .023 | 1.591 | 0.422–6.005 | .493 |
| CKD baseline disease 6 | 1.557 | 0.860–2.819 | .144 | 1.380 | 0.617–3.088 | .433 | 1.801 | 0.747–4.344 | .19 |
| CKD baseline disease 7 | 1.542 | 0.826–2.880 | .174 | 1.809 | 0.841–3.888 | .129 | 0.966 | 0.296–3.153 | .955 |
| CVD (ref 0) | 0.963 | 0.523–1.772 | .903 | 0.966 | 0.473–1.970 | .924 | 0.859 | 0.260–2.836 | .803 |
| Diabetes mellitus (ref 0) | 1.333 | 0.814–2.185 | .254 | 1.497 | 0.846–2.646 | .166 | 0.878 | 0.308–2.504 | .808 |
| Time on waiting list | 1.014 | 1.005–1.023 | .002 | 1.014 | 1.003–1.024 | .008 | 1.013 | 0.992–1.034 | .216 |
| Donor type (ref 0) | 0.767 | 0.533–1.104 | .153 | ||||||
| Donor age | 1.021 | 1.009–1.033 | .0004 | 1.023 | 1.010–1.037 | .0006 | 1.005 | 0.979–1.032 | .695 |
| Donor height | 0.330 | 0.086–1.266 | .106 | 0.089 | 0.016–0.497 | .006 | 2.492 | 0.144–43.184 | .53 |
| Donor weight | 1.002 | 0.991–1.013 | .719 | 0.998 | 0.985–1.011 | .734 | 1.008 | 0.991–1.026 | .342 |
| Donor BMI | 1.000 | 0.990–1.010 | .97 | 1.026 | 0.986–1.068 | .211 | 0.998 | 0.982–1.014 | .769 |
| Donor COD 2 (ref 1) | 1.639 | 0.909–2.956 | .1 | ||||||
| Donor COD 3 | 1.055 | 0.464–2.398 | .898 | ||||||
| Donor COD 4 | 2.322 | 0.491–10.985 | .288 | ||||||
| Donor HT (ref 0) | 2.190 | 1.385–3.466 | .0008 | ||||||
| Donor DM (ref 0) | 1.576 | 0.786–3.160 | .2 | ||||||
| CIT | 1.013 | 0.991–1.035 | .256 | 1.001 | 0.964–1.040 | .962 | 1.274 | 1.041–1.560 | .019 |
| cPRA | 1.000 | 0.994–1.006 | .963 | 1.001 | 0.994–1.009 | .742 | 0.997 | 0.985–1.009 | .604 |
| MM A 1 (ref 0) | 0.659 | 0.414–1.050 | .08 | 0.624 | 0.321–1.210 | .163 | 0.683 | 0.353–1.323 | .259 |
| MM A 2 | 0.888 | 0.546–1.445 | .632 | 0.991 | 0.515–1.910 | .979 | 0.523 | 0.217–1.260 | .149 |
| MM B 1 (ref 0) | 0.919 | 0.526–1.603 | .765 | 0.654 | 0.312–1.371 | .2609 | 1.277 | 0.545–2.992 | .573 |
| MM B 2 | 0.909 | 0.520–1.590 | .738 | 0.689 | 0.336–1.414 | .3097 | 1.023 | 0.405–2.589 | .961 |
| MM DR 1 (ref 0) | 0.846 | 0.561–1.277 | .427 | 1.024 | 0.612–1.712 | .929 | 0.625 | 0.314–1.246 | .182 |
| MM DR 2 | 1.464 | 0.919–2.331 | .109 | 2.081 | 1.170–3.703 | .013 | 0.866 | 0.391–1.918 | .722 |
| Age difference | 0.970 | 0.956–0.983 | <.0001 | 0.967 | 0.944–0.991 | .007 | 0.963 | 0.945–0.981 | <.0001 |
| UBI | 0.998 | 0.984–1.013 | .843 | 1.016 | 0.995–1.037 | .139 | 0.977 | 0.958–0.997 | .002 |
| eGFR at PKT | 0.995 | 0.951–1.041 | .842 | 0.969 | 0.915–1.027 | .295 | 1.045 | 0.972–1.122 | .232 |
Significant values in bold.
COD: cause of death; HT: hypertension; DM: diabetes mellitus; MM: mismatch.
CKD baseline disease: 1, ADPKD; 2, CAKUT; 3, diabetes mellitus; 4, hypertension/RVD; 5, glomerulonephritis; 6, ‘other primary renal disease’; 7, unknown or missing.
Table 3:
Multivariable logistic regression model for overall graft loss, overall mortality and death-censored graft loss in the entire cohort and subgroups by donor type.
| All (N = 2327) | Deceased (n = 1349) | Living (n = 978) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline disease | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Overall graft loss | |||||||||||||
| Age (years) | 1.01 | 1.00– 1.03 |
0.106 | 1.05 | 1.02–1.08 | 0.001 | 0.99 | 0.97–1.02 | 0.585 | ||||
| Sex (ref. male) | 1 | 0.94 | 0.68– 1.29 |
0.705 | 1.02 | 0.69–1.50 | 0.937 | 0.81 | 0.45–1.47 | 0.496 | |||
| BMI at PKT | 1.00 | 0.96– 1.03 |
0.828 | 1.00 | 0.96–1.05 | 0.927 | 0.98 | 0.91–1.05 | 0.565 | ||||
| CKD baseline disease (ref 1) | 2 | 2.64 |
1.42–
4.93 |
0.002 | 3.61 | 1.59–8.16 | 0.002 | 1.72 | 0.61–4.79 | 0.304 | |||
| CKD baseline disease (ref 1) | 3 | 1.19 | 0.74– 1.91 |
0.484 | 1.22 | 0.67–2.20 | 0.515 | 1.00 | 0.43–2.33 | 0.998 | |||
| CKD baseline disease (ref 1) | 4 | 1.80 | 0.84– 3.86 |
0.130 | 1.19 | 0.48–2.98 | 0.707 | 4.53 | 0.90–22.80 | 0.067 | |||
| CKD baseline disease (ref 1) | 5 | 1.94 |
1.10–
3.39 |
0.021 | 1.85 | 0.96–3.57 | 0.067 | 1.28 | 0.33–5.04 | 0.723 | |||
| CKD baseline disease (ref 1) | 6 | 1.51 | 0.92–2.46 | 0.100 | 1.27 | 0.68–2.38 | 0.447 | 1.94 | 0.87–4.36 | 0.107 | |||
| CKD baseline disease (ref 1) | 7 | 1.76 | 1.06–2.92 | 0.030 | 1.82 | 0.99–3.33 | 0.054 | 1.29 | 0.43–3.88 | 0.651 | |||
| CVD (0, no) | 1 | 1.51 | 1.00–2.29 | 0.050 | 1.49 | 0.92–2.42 | 0.106 | 1.64 | 0.66–4.04 | 0.286 | |||
| DM (0, no) | 1 | 1.02 | 0.62–1.68 | 0.927 | 0.98 | 0.56–1.70 | 0.935 | 1.22 | 0.33–4.55 | 0.764 | |||
| Donor type (0, deceased; 1 living) | 1 | 0.71 | 0.42–1.22 | 0.214 | |||||||||
| Donor age (years) | 1.03 | 1.02–1.05 | 0.001 | 1.02 | 1.00–1.04 | 0.132 | 1.03 | 1.00–1.06 | 0.046 | ||||
| Donor BMI (years) | 1.00 | 0.99–1.01 | 0.717 | 1.01 | 0.97–1.04 | 0.648 | 1.00 | 0.98–1.02 | 0.704 | ||||
| CIT (hours) | 1.00 | 0.97–1.03 | 0.932 | 0.99 | 0.96–1.03 | 0.731 | 1.39 | 1.15–1.67 | 0.001 | ||||
| cPRA (%) | 1.00 | 0.99–1.01 | 0.886 | 1.00 | 0.99–1.01 | 0.584 | 1.00 | 0.99–1.01 | 0.696 | ||||
| MM DR (n; ref 0) | 1 | 1.02 | 0.71–1.45 | 0.917 | 1.05 | 0.69–1.60 | 0.824 | 0.85 | 0.42–1.71 | 0.649 | |||
| MM DR (n; ref 0) | 2 | 1.67 | 1.10–2.53 | 0.015 | 1.73 | 1.05–2.86 | 0.033 | 1.54 | 0.69–3.42 | 0.289 | |||
| UBI | 1.00 | 0.99–1.01 | 0.733 | 1.01 | 1.00–1.03 | 0.171 | 0.98 | 0.96–1.00 | 0.080 | ||||
| All | Deceased | Living | |||||||||||
| Variables | Baseline disease | OR | CI − 95% | CI + 95% | P-value | OR | CI − 95% | CI + 95% | P-value | OR | CI − 95% | CI + 95% | P-value |
| Overall mortality | |||||||||||||
| Age (years) | 1.10 | 1.07 | 1.13 | 0.001 | 1.10 | 1.06 | 1.14 | 0.001 | 1.13 | 1.05 | 1.21 | 0.001 | |
| Sex (male; ref) | 1 | 1.34 | 0.86 | 2.10 | 0.199 | 1.23 | 0.75 | 2.02 | 0.414 | 2.85 | 0.77 | 10.59 | 0.118 |
| BMI at PKT | 0.98 | 0.93 | 1.03 | 0.370 | 0.97 | 0.92 | 1.03 | 0.329 | 0.90 | 0.78 | 1.04 | 0.139 | |
| CKD baseline disease (ref 1) | 2 | 2.89 | 1.10 | 7.59 | 0.031 | 4.85 | 1.69 | 13.93 | 0.003 | 0.00 | −493.07 | 999.9 | 0.964 |
| CKD baseline disease (ref 1) | 3 | 1.09 | 0.55 | 2.14 | 0.812 | 1.18 | 0.54 | 2.57 | 0.686 | 0.95 | 0.23 | 3.88 | 0.939 |
| CKD baseline disease (ref 1) | 4 | 3.31 | 1.30 | 8.40 | 0.012 | 2.25 | 0.77 | 6.64 | 0.140 | 11.02 | 0.82 | 148.29 | 0.070 |
| CKD baseline disease (ref 1) | 5 | 1.34 | 0.64 | 2.79 | 0.439 | 1.61 | 0.71 | 3.65 | 0.253 | 0.41 | 0.04 | 4.56 | 0.470 |
| CKD baseline disease (ref 1) | 6 | 1.17 | 0.58 | 2.35 | 0.664 | 1.17 | 0.51 | 2.65 | 0.713 | 1.14 | 0.27 | 4.78 | 0.861 |
| CKD baseline disease (ref 1) | 7 | 1.59 | 0.80 | 3.14 | 0.184 | 2.03 | 0.95 | 4.34 | 0.068 | 0.29 | 0.03 | 3.06 | 0.304 |
| CVD (0, no) | 1 | 1.87 | 1.14 | 3.06 | 0.013 | 1.96 | 1.13 | 3.38 | 0.016 | 1.72 | 0.47 | 6.27 | 0.409 |
| DM (0, no) | 1 | 0.79 | 0.42 | 1.48 | 0.460 | 0.85 | 0.43 | 1.66 | 0.626 | 0.72 | 0.08 | 6.75 | 0.770 |
| Donor type (0 deceased; 1 living) | 1 | 0.54 | 0.25 | 1.13 | 0.100 | ||||||||
| Donor age (years) | 1.00 | 0.98 | 1.03 | 0.805 | 1.00 | 0.97 | 1.03 | 0.950 | 1.02 | 0.96 | 1.08 | 0.534 | |
| Donor BMI (years) | 1.00 | 0.96 | 1.03 | 0.762 | 0.99 | 0.95 | 1.04 | 0.786 | 1.00 | 0.95 | 1.05 | 0.933 | |
| CIT (hours) | 1.00 | 0.96 | 1.04 | 0.974 | 0.99 | 0.95 | 1.03 | 0.700 | 1.63 | 1.16 | 2.30 | 0.005 | |
| cPRA (%) | 1.00 | 0.99 | 1.01 | 0.820 | 1.00 | 0.99 | 1.01 | 0.844 | 1.01 | 0.99 | 1.03 | 0.615 | |
| MM DR (n; ref 0) | 1 | 1.02 | 0.62 | 1.67 | 0.939 | 1.04 | 0.61 | 1.79 | 0.884 | 0.98 | 0.24 | 3.99 | 0.977 |
| MM DR (n; ref 0) | 2 | 1.32 | 0.76 | 2.29 | 0.329 | 1.24 | 0.66 | 2.31 | 0.509 | 1.68 | 0.40 | 7.00 | 0.476 |
| UBI | 1.00 | 0.99 | 1.02 | 0.786 | 1.00 | 0.99 | 1.02 | 0.636 | 0.99 | 0.94 | 1.04 | 0.673 | |
| All | Deceased | Living | |||||||||||
| Variables | Baseline disease | OR | CI − 95% | CI + 95% | P-value | OR | CI − 95% | CI + 95% | P-value | OR | CI − 95% | CI + 95% | P-value |
| Death-censored graft loss | |||||||||||||
| Age (years) | 0.97 | 0.95 | 0.99 | 0.002 | 0.99 | 0.95 | 1.02 | 0.492 | 0.96 | 0.93 | 0.99 | 0.004 | |
| Sex (male; ref) | 1 | 0.67 | 0.44 | 1.03 | 0.069 | 0.79 | 0.45 | 1.39 | 0.418 | 0.56 | 0.28 | 1.12 | 0.101 |
| BMI at PKT | 1.03 | 0.98 | 1.08 | 0.273 | 1.04 | 0.98 | 1.11 | 0.168 | 1.00 | 0.92 | 1.09 | 0.982 | |
| CKD baseline disease (ref 1) | 2 | 1.85 | 0.84 | 4.05 | 0.125 | 1.91 | 0.62 | 5.92 | 0.261 | 1.55 | 0.48 | 4.94 | 0.460 |
| CKD baseline disease (ref 1) | 3 | 1.11 | 0.58 | 2.11 | 0.752 | 1.15 | 0.50 | 2.64 | 0.745 | 0.81 | 0.28 | 2.33 | 0.693 |
| CKD baseline disease (ref 1) | 4 | 0.43 | 0.11 | 1.76 | 0.244 | 0.32 | 0.06 | 1.79 | 0.197 | 0.59 | 0.04 | 8.23 | 0.698 |
| CKD baseline disease (ref 1) | 5 | 2.03 | 0.93 | 4.47 | 0.077 | 1.72 | 0.67 | 4.41 | 0.260 | 1.77 | 0.33 | 9.55 | 0.509 |
| CKD baseline disease (ref 1) | 6 | 1.45 | 0.76 | 2.79 | 0.263 | 1.25 | 0.52 | 3.01 | 0.615 | 1.55 | 0.56 | 4.31 | 0.403 |
| CKD baseline disease (ref 1) | 7 | 1.41 | 0.68 | 2.91 | 0.358 | 1.17 | 0.47 | 2.90 | 0.740 | 1.66 | 0.46 | 5.92 | 0.437 |
| CVD (0, no) | 1 | 0.92 | 0.45 | 1.86 | 0.810 | 0.74 | 0.31 | 1.75 | 0.495 | 1.46 | 0.41 | 5.20 | 0.556 |
| DM (0, no) | 1 | 1.39 | 0.70 | 2.78 | 0.349 | 1.26 | 0.58 | 2.76 | 0.561 | 1.82 | 0.37 | 9.02 | 0.464 |
| Donor type (0, deceased; 1, living) | 1 | 0.95 | 0.45 | 2.02 | 0.897 | ||||||||
| Donor age (years) | 1.04 | 1.02 | 1.06 | 0.001 | 1.03 | 1.00 | 1.06 | 0.0543 | 1.0178 | 0.985 45 | 1.05 | 0.285 | |
| Donor BMI (years) | 1.00 | 0.99 | 1.01 | 0.810 | 1.03 | 0.98 | 1.08 | 0.290 | 1.00 | 0.97 | 1.02 | 0.725 | |
| CIT (hours) | 1.01 | 0.96 | 1.05 | 0.752 | 1.00 | 0.96 | 1.05 | 0.986 | 1.30 | 1.03 | 1.63 | 0.027 | |
| cPRA (%) | 1.00 | 0.99 | 1.01 | 0.828 | 1.00 | 0.99 | 1.01 | 0.733 | 1.00 | 0.98 | 1.01 | 0.641 | |
| MM DR (n; ref 0) | 1 | 0.91 | 0.56 | 1.49 | 0.718 | 0.97 | 0.52 | 1.80 | 0.919 | 0.84 | 0.37 | 1.90 | 0.680 |
| MM DR (n; ref 0) | 2 | 1.81 | 1.02 | 3.21 | 0.043 | 2.06 | 1.01 | 4.18 | 0.047 | 1.46 | 0.52 | 4.06 | 0.469 |
| UBI | 1.00 | 0.98 | 1.02 | 0.985 | 1.02 | 0.99 | 1.04 | 0.176 | 0.98 | 0.96 | 1.00 | 0.048 | |
Significant values in bold.
DM: diabetes mellitus; CIT: cold ischaemic time; cPRA: calculated panel reactive antibody; MM: mismatch.
CKD baseline disease: 1, ADPKD; 2, CAKUT; 3, diabetes mellitus; 4, hypertension/RVD; 5, glomerulonephritis; 6, ‘other primary renal disease’; 7, unknown or missing.
Decision tree model
Fig. 6 and Supplementary Figs. 4 and 5 illustrate various risk scenarios identifying the risk of failed PKT at >30% based on recipient score, donor age, UBI and DR mismatch consecutively. For instance, recipient score >−1.03 and donor age >84.5 years had a 46.7% risk of failure within 5 years.
Sensitivity analysis
Our sensitivity analysis, shown in Supplementary Tables 7–8 and Supplementary Fig. 1, demonstrates that LPS-adjusted deceased and living donor PKT patient, donor and transplantation characteristics may differ. Donor type was not associated with death-censored graft loss and was excluded from the list of potential predictors for all outcomes in the multivariable model. Neither eGFR nor UBI was associated with the outcomes.
DISCUSSION
Our retrospective study suggests that PKT has become more frequent, accompanied by slight changes in practice patterns over time. The effect of donor type alone does not necessarily predict better outcomes in PKT. Although eGFR is not directly associated with transplant failure or patient mortality 5 years after PKT, more focused risk assessment strategies should be implemented. These strategies should incorporate recipient scores, donor age, number of DR mismatches and the UBI.
The number of PKT increased over time during the study period, reflecting efforts to avoid dialysis initiation. While living donor PKT numbers remained stable, deceased donor PKT became more frequent, suggesting PKT is increasingly considered the optimal treatment choice [13, 21–23]. In Europe, PKT accounts for 13–19% of all kidney transplant procedures, evenly split between living and deceased donors. Our study showed living PKT constituted >50% of all PKT, as these numbers have improved slowly over the years [8, 24, 25]. The overall proportion of PKT in our study was 14%, consistent with previously published data.
Our study confirms that eGFR at transplantation is not associated with hard outcomes in PKT. While eGFR alone is insufficient for indicating pre-emptive waitlisting or PKT, no reliable methods exist to predict PKT versus non-PKT prognosis [26]. eGFR trajectories could theoretically aid decision-making but are not yet part of routine practice. eGFR alone before transplantation poorly predicts post-transplant survival, but may indicate relative mortality risk at decision time [27–30]. The Kidney Failure Risk Index is not specifically used for PKT evaluation. Similar scores could help determine transplantation urgency, but should be used alongside other clinical factors in decision-making. UBI could be an easy-to-calculate measure of uraemic burden for further classifying CKD burden and it has the potential for use in individual clinical decision-making before starting renal replacement therapy.
The rpart-based risk classification model offers a valuable tool for identifying high-risk PKT. It incorporates recipient score, donor age, DR mismatches and UBI to estimate total graft loss risk, mortality and graft failure without death for both deceased and living donor candidates. This model could improve patient management and resource allocation in transplant programs on an individual basis. The novel UBI parameter shows promise as an easily calculable approach to estimate uraemic burden on the waiting list, potentially replacing eGFR for indicating renal replacement therapy needs. However, external validation in diverse cohorts is necessary before clinical application to ensure generalizability and validate the UBI parameter.
Kidney transplantation, including PKT, offers a clear survival advantage over dialysis. Recent data indicate that the 5-year graft survival rate after transplantation is ≈70–80%. In contrast, the 5-year survival rate for patients on dialysis is 42–52% [31]. For patients starting dialysis at <50 years of age, this survival is ≈80%, while for patients >80 years of age, it drops to ≈33%. Even if graft failure occurs (estimated at 15–20% at 5 years), PKT still have a survival advantage over dialysis. Considering these factors, an acceptable risk of PKT failure could be up to 25–30% at 5 years. It is crucial to note that the risk acceptability should be assessed individually, considering patient preferences and discussions with the transplant team during clinical decision-making. Therefore, in clinical practice, risk estimation should be performed individually at the time of the organ offer, when all potential risk factors are known (recipient risk, donor age, UBI score and DR mismatch), allowing for timely decisions to proceed with transplantation even with a ≥25–30% risk of combined graft loss. This timely clinical decision-making enables us to tailor strategies and predict the risk of unsuccessful PKT at 5-years post-transplant.
Strengths and limitations
The CRISTAL cohort is a large, meticulously documented national dataset, providing a robust resource for our research. The combination of this cohort with sophisticated statistical methods enhances the validity and applicability of our findings. The scale and quality of CRISTAL enable us to identify subtle trends often overlooked in smaller studies, offering a detailed understanding of risk factors and outcomes in PKT. There are limitations though, including the retrospective observational design focused only on PKT patients, not all waitlisted patients, which limits conclusions about the net benefits of PKT over other renal replacement therapies. Second, results should be interpreted cautiously due to the retrospective nature of the analysis, which primarily reveals practice patterns and generates hypotheses for future benchmarking studies. Third, the study cannot assess the relative risk of overall death in different patient populations. Fourth, analysis of a single national database with unique epidemiological characteristics may limit result generalizability, necessitating external validation. Fifth, the estimation of GFR using creatinine and cystatin (or the European formula) may modulate the present results. Finally, despite using UBI, lead time bias may not have been fully eliminated. UBI requires validation using eGFR slopes from the CKD diagnosis to clinical decision-making.
CONCLUSION
Our study demonstrated a gradual increase in PKT rates over time in France. We found that creatinine-based eGFR calculations alone are insufficient for accurate risk prediction in PKT. However, we introduced the UBI, a promising new biomarker that characterizes cumulative uraemic exposure and effectively distinguishes specific recipient subgroups in PKT. Utilizing decision tree analysis, we identified several factors associated with increased PKT failure risk: high-risk recipients, older donors, higher UBI scores and poorer DR matching. These findings suggest that a multifactorial approach incorporating UBI could enhance decision-making regarding PKT timing. This approach may refine risk assessment for PKT candidates at the time of organ offer or when considering elective living donor PKT.
Optimizing donor resource utilization is crucial to maximize quality-adjusted life years for recipients while minimizing unnecessary risks associated with early transplantation. While PKT remains the optimal approach, its success hinges on appropriate timing and decision-making based on comprehensive risk assessments.
Supplementary Material
ACKNOWLEDGEMENTS
O.C., a visiting scholar, was supported by the European Renal Association Long Term Fellowship Grant collaboration of the Young Nephrologist Platform and Developing Education Science and Care for Renal Transplantation in European States Working Group. The authors thank all their colleagues of the Agence de la Biomedecine and the INSERM Cardiovascular and Epidemiology Working Group who provided intellectual product or their expertise for improving the content.
Contributor Information
Orsolya Cseprekal, Department of Surgery, Transplantation and Gastroenterology, Semmelweis University, Budapest, Hungary; INSERM Unit 1018, Team 5, CESP, Hôpital Paul Brousse, Paris-Sud University and Versailles Saint-Quentin-en-Yvelines University (Paris-Ile-de-France-Ouest University, UVSQ), Villejuif, France; Agence de la Biomedicine, La Plaine Saint-Denis, Île-de-France, Paris, France.
Emilie Savoye, Agence de la Biomedicine, La Plaine Saint-Denis, Île-de-France, Paris, France.
Nasser Al Hawajri, Agence de la Biomedicine, La Plaine Saint-Denis, Île-de-France, Paris, France.
Camille Legeai, Agence de la Biomedicine, La Plaine Saint-Denis, Île-de-France, Paris, France.
Benedicte Stengel, INSERM Unit 1018, Team 5, CESP, Hôpital Paul Brousse, Paris-Sud University and Versailles Saint-Quentin-en-Yvelines University (Paris-Ile-de-France-Ouest University, UVSQ), Villejuif, France.
Ziad Massy, INSERM Unit 1018, Team 5, CESP, Hôpital Paul Brousse, Paris-Sud University and Versailles Saint-Quentin-en-Yvelines University (Paris-Ile-de-France-Ouest University, UVSQ), Villejuif, France; Association pour l'Utilisation du Rein Artificiel dans la région parisienne (AURA), Paris, France; Ambroise Paré University Hospital, APHP, Department of Nephrology Boulogne-Billancourt/Paris, Paris, France.
Christian Jacquelinet, INSERM Unit 1018, Team 5, CESP, Hôpital Paul Brousse, Paris-Sud University and Versailles Saint-Quentin-en-Yvelines University (Paris-Ile-de-France-Ouest University, UVSQ), Villejuif, France; Agence de la Biomedicine, La Plaine Saint-Denis, Île-de-France, Paris, France.
FUNDING
None.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to confidential reasons of the Agence de la Biomedecine. The data analysed in this study are subject to the following licenses: in accordance with French law, research studies based on the CRISTAL national registry are part of transplant assessment and do not require additional institutional review board approval. The database has been reported to the French National Commission on Computing and Liberty. The data presented in this study are available upon request from the corresponding author.
CONFLICT OF INTEREST STATEMENT
O.C. is a member of the CKJ Editorial Board.
REFERENCES
- 1. Chadban SJ, Ahn C, Axelrod DA et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020;104(4 Suppl 1):S11–103. 10.1097/TP.0000000000003136 [DOI] [PubMed] [Google Scholar]
- 2. Wolfe RA, Ashby VB, Milford EL et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725–30. 10.1056/NEJM199912023412303 [DOI] [PubMed] [Google Scholar]
- 3. Friedewald JJ, Reese PP. The Kidney-First Initiative: what is the current status of preemptive transplantation? Adv Chronic Kidney Dis 2012;19:252–6. 10.1053/j.ackd.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azegami T, Kounoue N, Sofue T et al. Efficacy of pre-emptive kidney transplantation for adults with end-stage kidney disease: a systematic review and meta-analysis. Ren Fail 2023;45:2169618. 10.1080/0886022X.2023.2169618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rana Magar R, Knight SR, Maggiore U et al. What are the benefits of preemptive versus non-preemptive kidney transplantation? A systematic review and meta-analysis. Transplant Rev (Orlando) 2023;37:100798. 10.1016/j.trre.2023.100798 [DOI] [PubMed] [Google Scholar]
- 6. Chiodo Ortiz C, Choubey AP, Shrivastava S et al. Preemptive renal transplant: too early is not always better-a national cohort study. Int Urol Nephrol 2022;54:2025–35. 10.1007/s11255-021-03086-0 [DOI] [PubMed] [Google Scholar]
- 7. Kiberd BA, Tennankore KK, Vinson AJ, Comparing the net benefits of adult deceased donor kidney transplantation for a patient on the preemptive waiting list vs a patient receiving dialysis. JAMA Netw Open 2022;5:e2223325. 10.1001/jamanetworkopen.2022.23325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kramer A, Boenink R, Mercado Vergara CG et al. Time trends in preemptive kidney transplantation in Europe: an era registry study. Nephrol Dial Transplant 2024;39:2100–12. 10.1093/ndt/gfae105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. King KA-O, Husain SA-O, Jin Z et al. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol 2019;14:1500–11. 10.2215/CJN.03140319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abramowicz D, Hazzan M, Maggiore U et al. Does pre-emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position statement by the Descartes Working Group and ERBP. Nephrol Dial Transplant 2016;31:691–7. 10.1093/ndt/gfv378 [DOI] [PubMed] [Google Scholar]
- 11. Segev DL, Muzaale AD, Caffo BS et al. Perioperative mortality and long-term survival following live kidney donation. JAMA 2010;303:959–66. 10.1001/jama.2010.237 [DOI] [PubMed] [Google Scholar]
- 12. Prezelin-Reydit M, Combe C, Harambat J et al. Prolonged dialysis duration is associated with graft failure and mortality after kidney transplantation: results from the French transplant database. Nephrol Dial Transplant 2019;34:538–45. 10.1093/ndt/gfy039 [DOI] [PubMed] [Google Scholar]
- 13. Akkina SK, Connaire JJ, Snyder JJ et al. Earlier is not necessarily better in preemptive kidney transplantation. Am J Transplant 2008;8:2071–6. 10.1111/j.1600-6143.2008.02381.x [DOI] [PubMed] [Google Scholar]
- 14. Ku E, McCulloch CE, Johansen KL. Starting renal replacement therapy: is it about time? Am J Nephrol 2019;50:144–51. 10.1159/000501510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grams ME, Massie A, Coresh J et al. Trends in the timing of pre-emptive kidney transplantation. J Am Soc Nephrol 2011;22:1615–20. 10.1681/ASN.2011010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irish GL, Chadban S, McDonald S et al. Quantifying lead time bias when estimating patient survival in preemptive living kidney donor transplantation. Am J Transplant 2019;19:3367–76. 10.1111/ajt.15472 [DOI] [PubMed] [Google Scholar]
- 17. Cseprekal O, Jacquelinet C, Massy Z. Push toward preemptive kidney transplantation – for sure? Clin Kidney J 2024;17:sfae335. 10.1093/ckj/sfae335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cseprekal O, Jacquelinet C, Massy Z. Push toward pre-emptive kidney transplantation – for sure? Clin Kidney J 2024;17:sfae335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strang WN, Tuppin P, Atinault A et al. The French organ transplant data system. Stud Health Technol Inform 2005;116:77–82. [PubMed] [Google Scholar]
- 20. Inker LA-O, Eneanya NA-O, Coresh J et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasiske BL, Snyder JJ, Matas AJ et al. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol 2002;13:1358–64. 10.1097/01.ASN.0000013295.11876.C9 [DOI] [PubMed] [Google Scholar]
- 22. Prezelin-Reydit M, Madden I, Macher MA et al. Preemptive kidney transplantation is associated with transplantation outcomes in children: results from the French kidney replacement therapy registry. Transplantation 2022;106:401–11. 10.1097/TP.0000000000003757. [DOI] [PubMed] [Google Scholar]
- 23. Haller MC, Kammer M, Oberbauer R. Dialysis vintage and outcomes in renal transplantation. Nephrol Dial Transplant 2019;34:555–60. 10.1093/ndt/gfy099 [DOI] [PubMed] [Google Scholar]
- 24. Boenink R, Astley ME, Huijben JA et al. The ERA Registry Annual Report 2019: summary and age comparisons. Clin Kidney J 2022;15:452–72. 10.1093/ckj/sfab273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boenink R, Kramer A, Masoud S et al. International comparison and time trends of first kidney transplant recipient characteristics across Europe: an ERA Registry study. Nephrol Dial Transplant 2024;39:648–58. 10.1093/ndt/gfad189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grams ME, Massie AB, Coresh J et al. Trends in the timing of pre-emptive kidney transplantation. J Am Soc Nephrol 2011;22:1615–20. 10.1681/ASN.2011010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jørgensen IF, Muse VP, Aguayo-Orozco A et al. Stratification of kidney transplant recipients into five subgroups based on temporal disease trajectories. Transplant Direct 2024;10:e1576. 10.1097/TXD.0000000000001576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Burgh AC, Sedaghat S, Ikram MA et al. Trajectories of kidney function and risk of mortality. Int J Epidemiol 2023;52:1959–67. 10.1093/ije/dyad111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boucquemont J, Loubère L, Metzger M et al. Identifying subgroups of renal function trajectories. Nephrol Dial Transplant 2017;32:ii185–93. [DOI] [PubMed] [Google Scholar]
- 30. Faye M, Legrand K, Le Gall L et al. Five-year symptom trajectories in nondialysis-dependent CKD patients. Clin J Am Soc Nephrol 2022;17:1588–97. 10.2215/CJN.06140522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferreira EA-O, Moreira TR, da Silva RG et al. Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: a 20-year cohort. BMC Nephrol 2020;21:502. 10.1186/s12882-020-02135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available due to confidential reasons of the Agence de la Biomedecine. The data analysed in this study are subject to the following licenses: in accordance with French law, research studies based on the CRISTAL national registry are part of transplant assessment and do not require additional institutional review board approval. The database has been reported to the French National Commission on Computing and Liberty. The data presented in this study are available upon request from the corresponding author.