Abstract
Mitochondria are multifunctional organelles central to both physiological and pathological processes. In malignant cancer cells, mitochondrial reprogramming establishes the metabolic foundation to meet cellular demands, which is particularly important in tumor cells with existing metabolic perturbations. To identify key mitochondrial pathways supporting cancer development, we developed mitochondria Knockout (mtKO), a robust and unbiased CRISPR screening platform to pinpoint critical mitochondria-associated pathways. The mtKO screen revealed that the mitochondrial antioxidant enzyme SOD2 is essential for cells harboring IDH1 mutations. Mechanistically, SOD2 activity determines the disease manifestation of IDH1-mutated cancers, through maintaining redox homeostasis and mitochondrial fitness. This study introduces a powerful functional genomic tool to identify mitochondrial-centered pathways and reveals the selective mitochondrial vulnerability in Krebs cycle-deficient cancers for future therapeutic intervention.
Keywords: CRISPR screen, mitochondria, metabolism, IDH1, SOD2
Mitochondria are multifunctional organelles essential for energy metabolism, electron transport, ROS generation and detoxification, calcium regulation, and apoptosis and their functions are extensively reprogrammed in cancer to support rapid proliferation and Warburg-like metabolism (1). While mitochondrial involvement in oncogenesis, malignant transformation, and tumor progression is evident, its precise role—particularly in cancers driven by Krebs cycle deficiencies such as those involving IDH, SDH, or FH mutations—remains unclear (2). IDH1-mutant cancers, for example, exhibit vulnerability to oxidative stress and ferroptosis due to altered mitochondrial function shaped by distinct metabolic networks (3). Mitochondria are largely governed by nuclear-encoded proteins imported posttranslation, yet how nuclear signaling reprograms mitochondrial function during malignant progression—especially under metabolic constraints—remains poorly understood (4). To address this, we developed the mtKO guide RNA library targeting key mitochondria-associated genes across major pathways, enabling pooled screens to identify mitochondrial dependencies in normal and malignant cells. We identified altered mitochondrial dependencies in IDH1-mutated cancer cells for maintaining redox and metabolic homeostasis and contributing to tumor aggressiveness.
Results
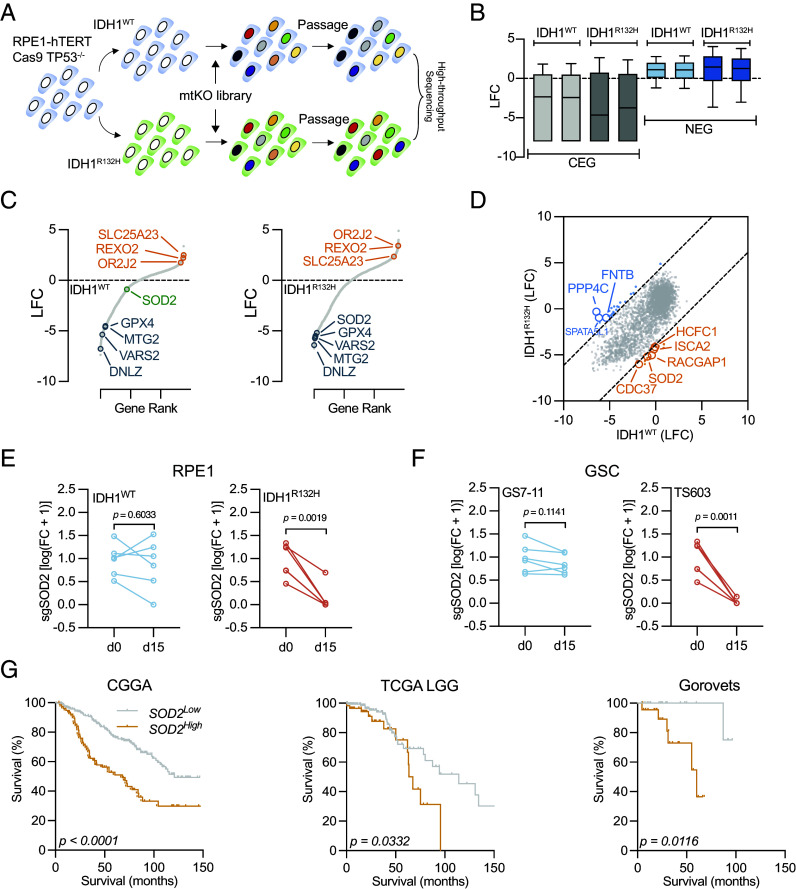
To identify essential genes among mitochondria-associated genes, we queried the MitoCarta3.0 database (5–7) and built a Cas9 guide RNA (gRNA) library targeting nuclear-encoded 1,034 mitochondrial genes to establish the mtKO library, with essential, nonessential, and nontargeting guides as controls. With this robust tool, we performed a CRISPR knockout screen in RPE1 cells harboring the pathogenic IDH1R132H mutation to explore the essentiality of mitochondrial pathways under oncogenic metabolic perturbation (Fig. 1A). High-throughput Illumina sequencing demonstrated excellent performance of the mtKO library (Fig. 1B). We identified consistent depletion of genes such as DNLZ, GPX4, and MTG2 (Fig. 1C). Conversely, enrichment was observed for SLC25A23 and REXO2. Notably, we found a differential dependency on superoxide dismutase 2 (SOD2), a key mitochondrial antioxidant enzyme. We observed that SOD2 exhibited mild depletion in IDH1WT cells, whereas a strong and significant depletion was found in IDH1R132H cells (Fig. 1 D and E). Besides, mtKO screens on patient-derived glioma stem cells (GSC) confirmed the connection between IDH1 mutation status and SOD2 dependency. The SOD2 guide RNA depletion was found significant in IDH1R132H TS603 cells, whereas it was insignificant in IDH1WT GS7-11 cells (Fig. 1F). This essential role of SOD2 is further evidenced in patient cohorts with IDH1-mutant glioma, where elevated SOD2 expression correlates with poorer outcomes and reduced overall survival (Fig. 1G).
Fig. 1.
mtKO screen to identify mitochondrial essential pathway in IDH1-mutant cells. (A) Schematic illustration shows mtKO screen in IDH1-mutant cells. (B) Bar graph shows changes in guide RNA abundancy for core essential genes (CEG) and nonessential genes (NEG). Each bar represents one biological replicate. (C) Gene-level guide RNA depletion in wild type (IDH1WT) and mutant (IDH1R132H) screens. Significantly depleted (blue) or enriched (orange) were highlighted. Nonsignificant gene was labeled in green. (D) Plot of gene depletion based on RPE1 IDH1WT and IDH1R132H screen. (E) Quantitative changes of SOD2 guide RNAs through the RPE1 cell mtKO screen. (F) Quantitative changes of SOD2 guide RNAs through the GSC mtKO screen. (G) Kaplan–Meier analysis reveals the predictive value of SOD2 expression in IDH1-mutant glioma datasets CGGA, TCGA LGG, and Gorovets (GSE35158).
To investigate the role of SOD2 in IDH1-mutated cancers, we generated SOD2 knockout (KO) derivatives of the IDH1-mutated cholangiocarcinoma cell line JJ012. Transcriptomic analysis revealed that SOD2 loss led to significant downregulation of pathways associated with cancer aggressiveness, including the replisome, electron transport chain (ETC), ribosome, and RNA metabolism (Fig. 2A). Additionally, SOD2 deficiency resulted in notable alterations in cellular proliferation and metabolic profiles, characterized by reduced gene set enrichment for oxidative phosphorylation (OXPHOS), ferroptosis pathway, E2F-mediated transcription, and mitochondria transportation pathway (MT TRANSPORT, Fig. 2B).
Fig. 2.

Validation of gene essentiality of SOD2. (A) RNA sequencing-based network analysis shows depleted genes and pathways in SOD2 KO vs. parental JJ012 cells. (B) GSEA on the significantly depleted pathways. (C) Mitochondrial ROS measurement in JJ012 parental (Par) and SOD2 KO cells using MitoSox flow cytometry. (D) Statistical analysis on MitoSox signal measured in C (**P < 0.01). (E) Statistical analysis on MitoSox signal ROS measurement in GSC (**P < 0.01). (F) Mitochondrial membrane potential measurement in JJ012 cells using JC-1 flow cytometry (**P < 0.01). (G) Dose response curve for JJ012 cells exposed to RSL3. (H) Phase contrast microscopy shows the morphology of JJ012 cells exposed to RSL3 and/or Ferr-1. (I) Cell proliferation measurement in JJ012 cells. (J) Measurement of JJ012 xenograft. (**P < 0.01). (K) Quantification of EdU positive labeled GSC with SOD2 RNA interference (**P < 0.01).
To investigate the role of SOD2 in the aggressive phenotype of IDH1-mutant cancers, we assessed mitochondrial function in SOD2-deficient (SOD2KO) cells. SOD2 loss significantly increased mitochondrial reactive oxygen species (ROS) levels (Fig. 2 C and D). Similarly, SOD2 depletion led to markedly elevated mitochondrial ROS in IDH1R132H GSC403 cells, but not in IDH1WT GS7-11 cells (Fig. 2E). Assessment of mitochondrial membrane potential (ΔΨm) using JC-1 dye revealed a significantly impaired mitochondrial function in SOD2KO cells (Fig. 2F). Furthermore, SOD2KO cells showed increased sensitivity to RSL3-induced cell death, which was rescued by the ferroptosis inhibitor ferrostatin-1 (Fer-1, Fig. 2H). Further, SOD2 loss significantly impaired the proliferation of IDH1R132H JJ012 cells in vitro and reduced the oncogenic potential of JJ012 subcutaneous xenografts (Fig. 2 I and J). Similarly, SOD2 depletion reduced proliferation in IDH1R132H GSC403 and TS603 cells, but not in IDH1WT GSC268 or GS7-11 cells (Fig. 2K). Collectively, these findings indicate that SOD2 supports the survival and growth of IDH1-mutant cancer cells by limiting mitochondrial ROS and protecting against ferroptosis, thereby promoting tumor progression.
Discussion
In this study, we developed a gRNA library, mtKO, to investigate essential mitochondrial pathways cancer cells with Krebs cycle deficiency as an alternative approach from existing platform (8). Further, we identified SOD2 as a novel metabolic “Achilles heel” in IDH1-mutated cells. SOD2 was found to be indispensable in the IDH1-mutated genetic background but not in their wild-type counterparts (Fig. 1 C and D), which predicts unfavorable disease outcomes (Fig. 1E).
Our study emphasizes the critical role of antioxidant pathways in cancers with Krebs cycle deficiencies. Evolutionarily conserved mechanisms, notably the Nrf2-guided glutathione metabolism, are vital for these malignancies, influencing disease progression and therapeutic responses (9, 10). Our findings highlight the mitochondrial antioxidant enzyme SOD2 as central to maintaining organelle function (Fig. 2 A and B), through detoxifying mitochondrial reactive oxygen species, preventing ferroptosis, and contributing to disease manifestation (Fig. 2 C–K). Targeting SOD2-guided antioxidant pathway may be valuable to impact the metabolic foundation of these malignancies with improved outcome.
Materials and Methods
Development of mtKO Library.
The list of human mitochondria-associated genes was obtained from MitoCarta 3.0 database (7). A total of 6,204 guides targeting 1,034 of these genes were obtained from the Brunello Human CRISPR Knockout Pooled Library (Addgene #73178) or designed using Guidescan (11). To establish the complete mtKO library, the following controls were included: guides targeting core essential genes (CEG) (1,409 genes, 8,454 guides), nonessential genes (NEG) (405 genes, 2,430 guides), and intergenic nontargeting regions (435 guides) (12, 13). See SI Applendix for details.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NCI and funded in whole or in part with Federal funds from the NIH, NCI, under Contract No. HHSN261201500003I. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. We appreciate the in-depth editing from Dr. Michael Aregger from the Functional Genomics Section, Molecular Targets Program, CCR, NCI.
Author contributions
C.Y. designed research; K.K., J.Z., F.L., M.Z., H.S., W.Z., and C.Y. performed research; R.C. contributed new reagents/analytic tools; K.K., J.Z., F.L., D.L.R., H.A., and C.Y. analyzed data; and K.K., J.Z., F.L., and C.Y. wrote the paper.
Competing interests
The authors declare no competing interest.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Harrington J. S., Ryter S. W., Plataki M., Price D. R., Choi A. M. K., Mitochondria in health, disease, and aging. Physiol. Rev. 103, 2349–2422 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Yang C., Oncometabolites in cancer: Current understanding and challenges. Cancer Res. 81, 2820–2823 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Liu Y., et al. , Protein kinase B (PKB/AKT) protects IDH-mutated glioma from ferroptosis via Nrf2. Clin. Cancer Res. 29, 1305–1316 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei W., et al. , Nuclear-embedded mitochondrial DNA sequences in 66, 083 human genomes. Nature 611, 105–114 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvo S. E., Clauser K. R., Mootha V. K., MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, D1251–1257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doench J. G., et al. , Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rath S., et al. , MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman M., et al. , A method for benchmarking genetic screens reveals a predominant mitochondrial bias. Mol. Syst. Biol. 17, e10013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Lang F., Yang C., NRF2 in human neoplasm: Cancer biology and potential therapeutic target. Pharmacol. Ther. 217, 107664 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Schmidlin C. J., Shakya A., Dodson M., Chapman E., Zhang D. D., The intricacies of NRF2 regulation in cancer. Semin. Cancer Biol. 76, 110–119 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez A. R., et al. , GuideScan software for improved single and paired CRISPR guide RNA design. Nat. Biotechnol. 35, 347–349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aregger M., Xing K., Gonatopoulos-Pournatzis T., Application of CHyMErA Cas9-Cas12a combinatorial genome-editing platform for genetic interaction mapping and gene fragment deletion screening. Nat. Protoc. 16, 4722–4765 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao M. S., et al. , Genome-scale exon perturbation screens uncover exons critical for cell fitness. Mol. Cell 84, 2553–2572.e2519 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.