Abstract
Type 2 diabetes mellitus results from an inadequate adaptation of the functional pancreatic β cell mass in the face of insulin resistance. Changes in the concentration of glucose play an essential role in the regulation of β cell turnover. In human islets, elevated glucose concentrations impair β cell proliferation and induce β cell apoptosis via up-regulation of the Fas receptor. Recently, it has been shown that the caspase-8 inhibitor FLIP may divert Fas-mediated death signals into those for cell proliferation in lymphatic cells. We observed expression of FLIP in human pancreatic β cells of nondiabetic individuals, which was decreased in tissue sections of type 2 diabetic patients. In vitro exposure of islets from nondiabetic organ donors to high glucose levels decreased FLIP expression and increased the percentage of apoptotic terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL)-positive β cells; FLIP was no longer detectable in such TUNEL-positive β cells. Up-regulation of FLIP, by incubation with transforming growth factor β or by transfection with an expression vector coding for FLIP, protected β cells from glucose-induced apoptosis, restored β cell proliferation, and improved β cell function. The beneficial effects of FLIP overexpression were blocked by an antagonistic anti-Fas antibody, indicating their dependence on Fas receptor activation. The present data provide evidence for expression of FLIP in the human β cell and suggest a novel approach to prevent and treat diabetes by switching Fas signaling from apoptosis to proliferation.
Long-term adaptation of pancreatic islets to increased demand of insulin occurs mainly by increasing β cell mass (1–3). The failure of the β cells to compensate in the face of insulin resistance leads to type 2 diabetes (2–9). In animal models of diabetes, the failure of β cell mass expansion is due to an imbalance between β cell proliferation and/or neogenesis on the one hand and β cell apoptosis on the other (10–13). In vitro studies with islets isolated from genetically predisposed animals have shown that elevated glucose concentrations are directly involved in the mechanisms responsible for the defective adaptation of β cell turnover. Indeed, elevated glucose concentrations induce β cell apoptosis in cultured islets from the diabetes-prone Psammomys obesus (10). By contrast, in rat islets, an increase in glucose concentrations to 11 mM promotes β cell survival (10, 14–16). When glucose concentrations are further increased, glucose proves to be pro- or antiapoptotic, depending on culture conditions. Investigations of β cell proliferation revealed induction of proliferation by glucose both in rat (16, 17) and P. obesus islets (10). However, unlike the long-lasting effect in rat islets, only a transient and reduced proliferative response was observed in P. obesus islets. The sensitivity of human islets to elevated glucose concentrations is similar to that of P. obesus islets, with increased glucose levels decreasing β cell proliferation and inducing β cell apoptosis (18, 19).
Recently, we showed that in human islets, the mechanism underlying glucose-induced β cell apoptosis and impaired proliferation involves the up-regulation of Fas receptors, which interact with the constitutively expressed Fas-ligand (FasL) on neighboring β cells (18). Fas-to-FasL interaction leads then to cleavage of procaspase-8 to caspase-8. Activated caspase-8, the most upstream caspase in the Fas apoptotic pathway, promotes caspase-3 activation and DNA fragmentation. However, despite abundant Fas surface expression induced by glucose, only a subpopulation of β cells undergoes apoptosis. Therefore, in apoptosis-resistant Fas-positive β cells, protective factors may interfere with activation of Fas-induced caspases. A progressive decrease of the putative protection factors may allow glucose to induce apoptosis in such β cells. Recently, an inhibitor of Fas-induced apoptosis was identified, termed cellular FLICE (caspase-8)-inhibitory protein (FLIP) (20). FLIP structurally resembles caspase-8, except that it lacks proteolytic activity. FLIP is highly expressed in tumor cells, T lymphocytes, and healthy, but not injured, myocytes, suggesting a critical role of FLIP as an endogenous modulator of apoptosis (21). Moreover, Fas signals do not always result in apoptosis but can also trigger a pathway that leads to proliferation (22). Thereby, FLIP may be pivotal in turning signals for cell death into those for cell survival/proliferation (23). These two conflicting functions of Fas are reminiscent of the dual and opposing effects of glucose on β cell turnover.
Therefore, we investigated the expression of FLIP in human pancreatic β cells of nondiabetic and diabetic patients, its regulation by glucose, and the ability of FLIP to switch Fas activation from a death signal into a proliferation signal, and so potentially to expand β cell mass.
Materials and Methods
Islet and β Cell Isolation and Culture.
Islets were isolated from pancreases of 10 organ donors at the Department of Surgery, University of Geneva Medical Center, and of two organ donors at the Division of Endocrinology and Diabetes, University Hospital of Zurich, as described (24–26). The islet purity was >95%, as judged by dithizone staining (if this degree of purity was not primarily achieved by routine isolation, islets were handpicked). The donors, aged 35–55 years, were heart-beating cadaver organ donors, and none had a previous history of diabetes or metabolic disorders. For long-term in vitro studies, the islets were cultured on extracellular matrix-coated plates derived from bovine corneal endothelial cells (27, 28) (Novamed, Jerusalem). Islets were cultured in CMRL 1066 medium containing 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 10% FCS (GIBCO), hereafter referred to as culture medium. Two days after plating, when most islets were attached and began to flatten, the medium was changed to culture medium containing 5.5 or 33.3 mM glucose. In some experiments, islets were additionally cultured with 20 ng/ml of human transforming growth factor β (TGF-β) (R & D Systems), 100 ng/ml of staurosporine (Sigma), or 500 ng/ml of antagonistic Fas antibody (ZB4; MBL, Nogoya, Japan) or transfected as described below. Human pancreatic islets were purified into single β cells (>95% purity) by targeted expression of green fluorescent protein, as described previously (29).
Liposome-Mediated Transfection of Human Islets.
Lipofectamine 2000–DNA complexes were prepared according to the manufacturer's instructions (GIBCO) using FLAG-tagged-FLIP-long- [generously provided by J. Tschopp, University of Lausanne, Switzerland (20)] or green fluorescent protein (GFP)-plasmid-DNA (pIRES 2-EGFP; CLONTECH). The solution was added to the islets at a final concentration of 3 μg of DNA/ml. After 6-h incubation, 2 ml of culture medium was added, and after 24 h, the medium was aspirated and replaced by fresh culture medium.
β Cell Replication and Apoptosis.
For β cell proliferation studies, a monoclonal antibody against the human Ki-67 antigen was used (Zymed), the free 3-OH strand breaks resulting from DNA degradation were detected by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) technique, and the activated caspase 3 was revealed with a rabbit anticleaved-caspase-3 antibody (D 175, Cell Signaling, Beverly, MA), as described in detail (10, 18, 30, 31). In parallel to the TUNEL reaction, we used the DNA-binding dye propidium iodide (Sigma) to assess the effects of glucose on necrosis.
Detection of FLIP- and Fas-Expressing β Cells.
Pancreases from routine necropsies were deparaffinized and rehydrated and endogenous peroxidase blocked by submersion in 0.3% H2O2. Sections were then incubated in methanol. Islet cultures were fixed in 4% paraformaldehyde followed by permeabilization with 0.5% Triton X-100. Both tissue sections and cultured islets were double-labeled for FLIP or Fas receptor and insulin by 1-h exposure to 10% BSA, followed by incubation with rabbit anti-FLIP antibody [prepared in our laboratory (32)] or with mouse anti-Fas antibody (Transduction Laboratories, Lexington, KY). Ductal cells were labeled with anti-cytokeratin 19 [Dako (30);]. Detection was performed by using the streptavidin–biotin–peroxidase complex (Zymed), donkey anti-rabbit or anti-mouse Cy3-conjugated antibodies (The Jackson Laboratory). Subsequently, the specimens were stained for insulin by incubation with guinea pig anti-insulin antibody (Dako), followed by detection with a fluorescein-conjugated rabbit anti-guinea pig antibody (Dako). Human cardiac tissue sections were used as positive control for FLIP (33, 34).
For mRNA in situ hybridization, we used FLIP full-length cDNA that was cloned into pCR-Script SK(+) plasmid (Stratagene) and linearized. Using RNA polymerase and RNA Digoxigenin labeling mix (Roche, Gipf-Oberfrick, Switzerland), we prepared sense and antisense digoxigenin-labeled RNA probes. Tissue sections were treated with 20 μg/ml of Proteinase K (Roche) and prehybridized for 2 h at 55°C in hybridization buffer containing 50% formamide, 5 × sodium chloride–sodium citrate, 50 μg/ml of salmon sperm (Sigma), 1 × Denhart's solution, and 250 μg/ml of RNA Type IV from calf liver (Sigma). The hybridization was performed overnight at 52°C in 100 μl of hybridization buffer containing 30 ng of digoxigenin-labeled RNA probe. Sections were then blocked with 5% milk powder at room temperature and incubated 1 h at 37°C with anti-digoxigenin-rhodamine Fab fragment (20 μg/ml; Roche), which had been blocked overnight (4°C) with 500 μg of human pancreatic tissue, followed by insulin immunostaining.
Intensity and saturation of FLIP staining were analyzed by using ANALYSIS 3.1 software (Soft Imaging System, Muenster, Germany).
Western Blot Analysis.
Equivalent amounts of protein from each treatment group were run on 15% SDS polyacrylamide gels. Proteins were electrically transferred to nitrocellulose filters and incubated with rabbit anti-FLIP, mouse anti-Fas, or goat anti-actin (C-11; Santa Cruz Biotechnology) antibodies followed by incubation with horseradish–peroxidase-linked anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology) or anti-goat IgG (Sigma).
RNA Extraction, Reverse Transcription–PCR (RT-PCR), and Sequencing of RT-PCR Product.
Total RNA was extracted by using Rneasy mini kit (Qiagen, Basel), and RT-PCR was performed by using the Superscript II Rnase H − Reverse transcriptase KIT and oligo-dT (24) (Life Technologies, GIBCO) according to the instructions from the manufacturers. The primers were 5′-GAGCAAGCCCCTAGGAATCT-3′-/5′-GCCCTGAGTGAGTCTGATCC-3′ (FLIP long). The conditions of the PCR amplification were: denaturation, 30 sec at 94°C; annealing, 30 sec at 60°C; and elongation, 30 sec at 72°C, followed for real-time PCR by quantification, 5 sec at 80°C, 45 cycles. The saturation of the PCR amplification occurred between 22 and 28 cycles. The size of the PCR amplification products was 250 bp. The purified PCR products were sequenced to confirm amplification of the correct gene. For quantitative analysis, we used the Light Cycler quantitative PCR system (Roche, Basel) and performed quantitative PCR with a commercial kit (Light Cycler–DNA Master SYBR Green I; Roche). The amount of FLIP mRNA was standardized against phosphoglycerate kinase, glyceraldehyde-3-phosphate dehydrogenase and hypoxanthine phosphoribosyltransferase with similar results. The primers were 5′-GAGTCAATCTGCCACAGAAG-3′-/5′-ATCATCTCATTGACTTTGTC-3′ (PGK), 5′-AACAGCGACACCCACTCCTC-3′-/5′-GGAGGGGAGATTCAGTGTGGT-3′ (GAPDH), and 5′-TCCATTCCTATGACTGTAG-3′-/5′-CATGATTCAAATCCCTGAAG-3′ (HPRT).
Insulin Release and Content.
To determine acute insulin release in response to glucose stimulation, islets were washed in RPMI 1640 medium containing 3.3 mM glucose and preincubated for 1 h in the same medium. The medium was then discarded and replaced with fresh medium containing 3.3 mM glucose for 1 h for basal secretion, followed by an additional 1-h incubation in medium containing 16.7 mM glucose. Thereafter, islets were extracted with 0.18 M HCl in 70% ethanol, and the acid–ethanol extracts were collected for determination of insulin content. Insulin was determined by a human insulin RIA kit (CIS biointernational, Gif-Sur-Yvette, France).
Culture Evaluation and Statistical Analysis.
Cultures were evaluated in a randomized manner by a single investigator (K.M.), who was blinded to the treatment conditions. After transfection with a vector coding for FLIP, only FLIP-expressing β cells were analyzed. Care was taken to score islets of similar size. Some larger islets did not completely spread and were several cells thick. Such larger islets were excluded because a monolayer is a prerequisite for single cell evaluation. The mean surface of the evaluated islet monolayers was 0.030 ± 0.013 mm2 and 0.028 ± 0.010 mm2 in islets cultured at 5.5 and 33.3 mM glucose, respectively (not significant). Thus, the exclusion of larger islets occurred to similar extent in each dish independently of treatment. saisam software (Microvision Instruments, Evry, France) was used to measure the areas. Data were analyzed by Student's t test or by analysis of variance with a Bonferroni correction for multiple group comparisons.
Results
FLIP Is Expressed by Human Pancreatic β Cells and Is Down-Regulated in a Diabetic Milieu.
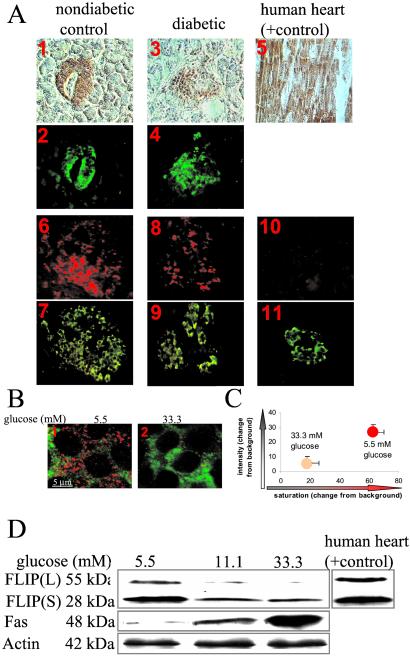
Immunodetection of human pancreatic sections from nondiabetic patients revealed the presence of FLIP localized in the β cells (Fig. 1A 1, 2 and Table 1). No specific staining was observed in the exocrine tissue. Moreover, expression of FLIP mRNA transcripts was observed by in situ hybridization in β cells (Fig. 1A 6, 7). For controls, we used a digoxigenin-labeled sense probe and found no signal (Fig. 1A 10, 11). Next, we studied expression of FLIP in sections of pancreases from six poorly controlled type 2 diabetic patients. In all pancreases, FLIP protein expression was decreased as compared with the nondiabetic controls (Table 1; for representative images, see Fig. 1A 3, 4). However, in diabetics and controls, the number of FLIP mRNA transcript-positive cells appeared comparable (Fig. 1A 8, 9). The hypothesis that glucose was responsible for the in vivo posttranscriptional regulation of FLIP was investigated in vitro in primary culture of human islets. Exposure of islets to elevated glucose concentrations for 4 days dramatically decreased expression of FLIP (Fig. 1 B and C). FLIP is expressed as a long (FLIPL) and a short (FLIPS) isoform. FLIPL is a more potent inhibitor of cell death than FLIPS (20, 34, 35). Using Western blot analysis, we found both isoforms expressed in human islets (Fig. 1D). However, treatment of islets with elevated glucose concentrations strongly down-regulated FLIPL, whereas FLIPS was only marginally affected. The ability of human β cells to express FLIP was verified by RT-PCR of RNA isolated from purified human β cells (not shown). Quantitative RT-PCR measurement revealed that FLIP RNA expression of whole islets and purified β cells was not significantly affected by changes in the ambient glucose concentration (not shown).
Figure 1.
Expression and regulation of FLIP in human islets. (A) Double immunostaining for FLIP in brown (1, 3, 5) and insulin in green (2, 4) in tissue sections of pancreases from a nondiabetic patient (1, 2) and from a patient with type 2 diabetes (3, 4) and in a tissue section of human heart (positive control, 5). In situ hybridization for FLIP mRNA in red (6, 8, 10) double immunostained for insulin in green (7, 9, 11) in tissue sections of pancreases from a nondiabetic patient with antisense probe (6) and with sense probe (negative control; 10), and from a patient with type 2 diabetes using anti-sense probe (8) (×250-fold). (B) Double immunostaining (confocal microscope) for FLIP in red and insulin in green in human islets cultured on extracellular matrix-coated dishes and exposed for 4 days to media containing 5.5 mM glucose (1) or 33.3 mM glucose (2). (C) Intensity and degree of saturation of FLIP immunostaining relative to background in β cells identified by double staining for insulin. Results are shown as mean ± SE of human islets from 3 donors exposed for 4 days to 5.5 or 33.3 mM glucose. (D) Immunoblotting of FLIPL, FLIPS, Fas, and actin. Human islets cultured in suspension at 5.5, 11.1, or 33.3 mM glucose were analyzed after 42-h incubation. Cardiac tissue was used as a positive control for FLIP. The antibodies were blotted on the same membrane after stripping. One representative of five experiments from five donors is shown.
Table 1.
Expression of FLIP in human islets of type 2 diabetic patients
| Patient no. | Age, yr | BMI, kg/m2 | FPG, mM | FLIP (saturation) | |
|---|---|---|---|---|---|
| Diabetic | 1 | 90 | 28.6 | 8.9 | 2 |
| 2 | 87 | 30.8 | 14.3 | 1 | |
| 3 | 74 | 34.1 | 21.9 | 7 | |
| 4 | 71 | 29.1 | 13.4 | 2 | |
| 5 | 69 | 29.4 | 10 | 2 | |
| 6 | 65 | 27.6 | 10.3 | 5 | |
| Nondiabetic | 7 | 50 | 19.6 | 5.5 | 27 |
| 8 | 54 | 16.7 | 5.0 | 37 | |
| 9 | 81 | 29.4 | 5.5 | 18 | |
| 10 | 77 | 29.1 | 5.0 | 12 | |
| 11 | 78 | 20.3 | 4.4 | 43 | |
| 12 | 58 | 24.5 | 4.9 | 33 |
Saturation (change from background) of FLIP immunostaining in β cells of patients with type 2 diabetes and of nondiabetic control patients. BMI, body mass index; FPG, fasting plasma glucose.
Glucose-Induced β Cell Apoptosis and Impaired Proliferation Correlate with Decreased FLIP Expression and Induction of Fas.
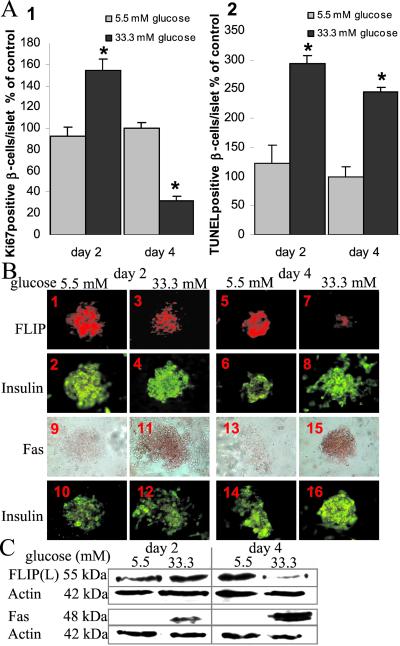
Short-time (2-day) exposure of cultured human islets to 33.3 mM glucose induced a 1.7-fold increase of the number of proliferating (Ki-67-positive) β cells, whereas prolonged (4-day) exposure resulted in a 3-fold inhibition of proliferation, relative to islets at 5.5 mM glucose (Fig. 2A1). By contrast, the time course of the effect of 33.3 mM glucose on DNA fragmentation revealed a significant increase in β cell apoptosis persisting throughout the study (2.0- and 2.3-fold increase after 2 and 4 days of treatment, respectively, compared with 5.5 mM glucose) (Fig. 2A2). Those changes in β cell turnover were accompanied by a progressive decrease of FLIP expression. Indeed, exposure of human islets to 33.3 mM glucose for 2 days had no influence on FLIP expression as compared with control at 5.5 mM (Fig. 2 B1–4 and C), but after a 4-day exposure, FLIP was almost undetectable (Fig. 2B5–8 and C). The Fas receptor was already induced by glucose after 2 days (Fig. 2 B9–12 and C) and remained highly expressed after 4 days (Fig. 2 B13–16 and C).
Figure 2.
Time course of the effect of elevated glucose concentrations on human β cell proliferation and apoptosis in correlation with the expression of FLIP and Fas. (A) Relative number of Ki-67-positive (1) and TUNEL-positive (2) β cells per islet after 2- and 4-day culture in 5.5 or 33.3 mM glucose normalized to control incubations at 5.5 mM glucose alone for 4 days (100%; in absolute values: 1.63 Ki-67-positive β cells per islet and 0.30 TUNEL-positive β cells per islet). The mean number of islets scored from each donor was 29 for each treatment condition. Islets were isolated from six organ donors. Results are shown as mean ± SE. *, P < 0.01 relative to islets at 5.5 mM glucose. (B) Double-immunostaining for FLIP in red (1, 3, 5, 7) or Fas in brown (9, 11, 13, 15) and insulin in green (2, 4, 6, 8, 10, 12, 14, 16) in human islets cultured on extracellular matrix-coated dishes and exposed for 2 (1–4, 9–12) and 4 days (5–8, 13–16) to media containing 5.5 mM (1, 2, 5, 6, 9, 10, 13, 14) or 33.3 mM glucose (3, 4, 7, 8, 11, 12, 15, 16) (×250). (C) Immunoblotting of FLIPL, Fas and actin in human islets cultured on extracellular matrix-coated dishes and exposed for 2 and 4 days to media containing 5.5 mM or 33.3 mM glucose.
FLIP Protects Human β Cells from Glucose-Induced Apoptosis and Restores β Cell Proliferation.
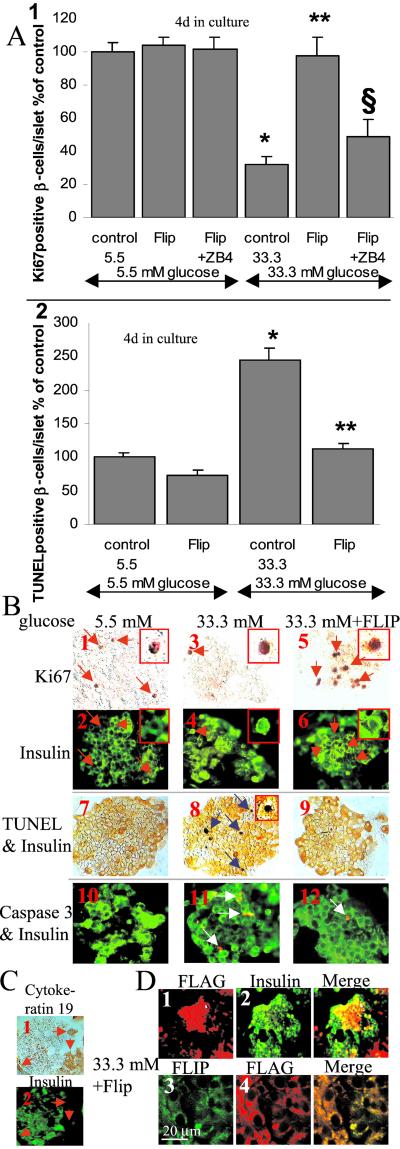
We next studied the functional role of FLIP in human β cells; specifically, we investigated the ability of FLIP to change the fate of β cells during glucose-induced Fas activation from death to proliferation. Human islets were transfected with an expression vector coding for FLIPL, exposed to elevated glucose concentrations for 4 days, and analyzed for β cell proliferation and apoptosis. Exposure of human islets to 33.3 mM glucose decreased the number of proliferating β cells by 68% relative to islets at 5.5 mM glucose (Fig. 3 A1 and 3B1–4). Transfection of human islets cultivated at 5.5 mM glucose had no influence on β cell proliferation. However, in islets induced to express Fas by exposure to 33.3 mM glucose, FLIP overexpression induced a 3.1-fold increase of β cell proliferation as compared with control at same glucose concentration (Fig. 3 A1 and B5, 6). To demonstrate that Fas receptor activation is required for FLIP-mediated proliferation, we used the antagonistic anti-Fas antibody ZB4. ZB4 inhibited the beneficial effect of FLIP at 33.3 mM glucose and had no influence on β cell proliferation in islets cultured in 5.5 mM glucose (Fig. 3A1). Finally, to show that Fas signaling by itself can lead to proliferation, Fas receptor was blocked in islets exposed for 2 days to high glucose, a time point when β cell proliferation is increased (Fig. 2A1): addition of ZB4 to cultured human islets exposed for 2 days to 33.3 mM glucose decreased the number of proliferating β cells by 45% as compared with control at 33.3 mM glucose alone, but ZB4 had no influence on β cell proliferation in islets cultured in 5.5 mM glucose (not shown).
Figure 3.
FLIP protects human β cells from glucose-induced apoptosis and restores β cell proliferation. (A) Human islets were cultured on extracellular matrix-coated dishes for 4 days in 5.5 and 33.3 mM glucose (control) after transfection with an expression vector coding for FLIP (FLIP) with or without addition of the antagonistic Fas antibody ZB4 (FLIP + ZB4). Results are means ± SE of the relative number of Ki-67-positive (1) and TUNEL-positive β cells (2) per islet normalized to control incubations at 5.5 mM glucose alone (100%; in absolute values: 1.63 Ki-67-positive β cells per islet and 0.30 TUNEL-positive β cells per islet). The mean number of islets scored from each donor was 32 for each treatment condition. Islets were isolated from six organ donors. *, P < 0.001 relative to islets at 5.5 mM glucose; **, P < 0.01 relative to islets at 33.3 mM glucose; §, P < 0.01 relative to FLIP transfected islets at 33.3 mM glucose. (B) Islets were exposed for 4 days to media containing 5.5 mM glucose (1, 2, 7, 10) or 33.3 mM glucose (3, 4, 8, 11) or 33.3 mM glucose and after transfection with FLAG-tagged FLIP (5, 6, 9, 12). Detection of β cell proliferation with anti-Ki-67 (orange; 1, 3, 5) and with anti-insulin antibody (green; 2, 4, 6). Double-immunostaining (7–9) for insulin (orange) and DNA fragmentation by the TUNEL assay (black). Double-immunostaining (10–12) for cleaved caspase-3 (red) and insulin (green). The red arrows mark cells stained positive for Ki-67 and for insulin; the blue arrows mark β cells nuclei stained positive for the TUNEL reaction; the white arrows mark β cells stained positive for cleaved caspase-3 (×250, with higher magnifications of Ki-67+- (1–6) or TUNEL+- (8) β cells in the inserts. (C) Double-immunostaining for cytokeratin 19 (1) and insulin (2) in human islets cultured for 4 days in 5.5 mM glucose. The red arrows mark ductal cells (×250). (D) Islets were transfected with FLAG-tagged FLIP and exposed for 4 days to media containing 33.3 mM glucose. Double-immunostaining for FLAG (1) and insulin (2) or FLIP (3) and FLAG (4). In 3 and 4, images are of a selected field in which essentially all cells were seen to have been transfected (FLAG positive). (×200) (1, 2); confocal microscopy (3, 4).
Subsequently, we tested the ability of FLIP to protect from glucose-induced β cell apoptosis. Exposure of cultured human islets to elevated glucose concentrations induced DNA fragmentation (TUNEL-positive) and cleavage of procaspase-3 (Fig. 3B7, 8, 10, 11). To assess the effect of glucose on necrosis, we used the DNA-binding dye propidium iodide. Exposure of islet cultures for 4 days to increasing glucose concentrations (from 5.5 to 33.3 mM) did not lead to propidium iodide uptake into the cultured cells, excluding necrosis (not shown). Lipofectamine transfection of FLIP did not significantly change baseline apoptosis at 5.5 mM glucose (Fig. 3A2). However, transfection of FLIP prevented the β cells from 33.3 mM glucose-induced apoptosis (Fig. 3 A2 and B9, 12).
Triple immunostaining of islets cultured in medium containing 33.3 mM glucose demonstrated that in all TUNEL-positive β cells, FLIP was no longer detectable (not shown). To demonstrate that glucose-induced decrease in FLIP expression is not due to unspecific proteolytic activity as a general feature of an apoptotic process, apoptosis was triggered by the kinase inhibitor staurosporine. Staurosporine-induced apoptosis has been shown to be independent of the Fas pathway and its modulation by FLIP (20). Exposure of human islets to staurosporine did not decrease FLIP expression even in TUNEL-positive β cells (not shown).
The contamination by ductal cells after 4 days in culture was of 5–15%, as determined by double immunostaining for insulin and cytokeratin 19, a marker of ductal cells (30). However, almost all ductal cells were found in the periphery of the islets and did not colocalize with β cells, as shown in Fig. 3C. Therefore, the identity of the evaluated β cells is unequivocal.
Lipofectamine 2000 transfection efficiency of human islets cultured on extracellular matrix-coated plates was evaluated with a green fluorescent protein control plasmid. Analysis of the islets showed a transfection efficiency of about 40% and no changes in the rate of apoptosis or proliferation as compared with untransfected islets (not shown). Transfection efficiency of human β cells with FLAG-tagged FLIP-plasmid-DNA was 45–50% (Fig. 3D1, 2), leading to FLIP expression in all FLAG-positive cells even when exposed to 33.3 mM glucose (Fig. 3D3, 4). Transfection of islets cultured in 33 mM glucose with a vector coding for FLIP resulted in a similar expression level of FLIP as compared with that of low-glucose-treated islets (not shown).
TGF-β Protects Human Islets from Glucose-Induced Changes in β Cell Turnover.
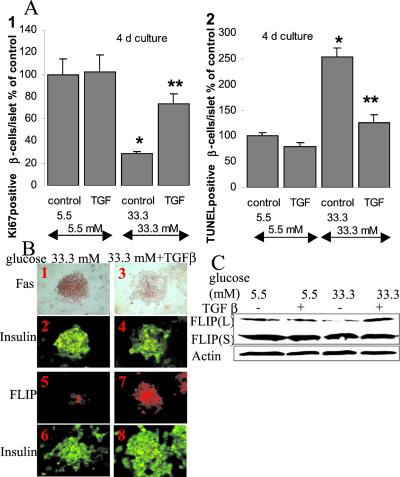
It has been shown that TGF-β induces FLIP expression and inhibits Fas expression in glial cells (36, 37). Therefore, we tested the effect of TGF-β in human islets on the expression of FLIP and Fas as well as on β cell turnover. In human islets cultured at low glucose medium (5.5 mM), addition of TGF-β had no significant influence on β cell proliferation and apoptosis (Fig. 4A). However, in islets exposed to 33.3 mM glucose for 4 days, addition of TGF-β induced a 1.8-fold increase in β cell proliferation and a 1.9-fold decrease in β cell apoptosis. In parallel, TGF-β induced FLIP and decreased Fas expression in β cells exposed for 4 days to high glucose (Fig. 4 B and C). TGF-β-induced FLIP expression was observed only at protein but not at RNA levels, as assessed by quantitative RT-PCR measurement (not shown).
Figure 4.
TGF-β protects human islets from the deleterious effects of high glucose on β cell turnover. (A) Human islets were cultured on extracellular matrix-coated dishes for 4 days in 5.5 and 33.3 mM glucose alone (control) or with TGF-β (TGF). Results are mean ± SE of the relative number of Ki-67-positive (1) and TUNEL-positive (2) β cells per islet normalized to control incubations at 5.5 mM glucose alone (100%; in absolute values: 1.04 Ki-67-positive β cells per islet and 0.31 TUNEL-positive β cells per islet). The mean number of islets scored from each donor was 23 for each treatment condition. Islets were isolated from three organ donors. *, P < 0.001 relative to islets at 5.5 mM glucose; **, P < 0.01 relative to islets at 33.3 mM glucose. (B) Double-immunostaining for Fas (brown; 1, 3) or FLIP (red; 5, 7) and insulin (green; 2, 4, 6, 8) in human islets cultured on extracellular matrix-coated dishes and exposed for 4 days to media containing 33.3 mM glucose alone (1, 2, 5, 6) or in the presence of TGF-β (3, 4, 7, 8). B originates from the same experiment as in Fig. 2B and has therefore the same controls. (×250). (C) Immunoblotting of FLIPL, FLIPS and actin. Human islets cultured in suspension at 5.5, or 33.3 mM glucose without or with 20 ng/ml of TGF-β were analyzed after 42-h incubation. The antibodies were blotted on the same membrane after stripping. One representative of three experiments from three donors is shown.
FLIP and TGF-β Improve Impaired β Cell Function Due to “Glucotoxicity.”
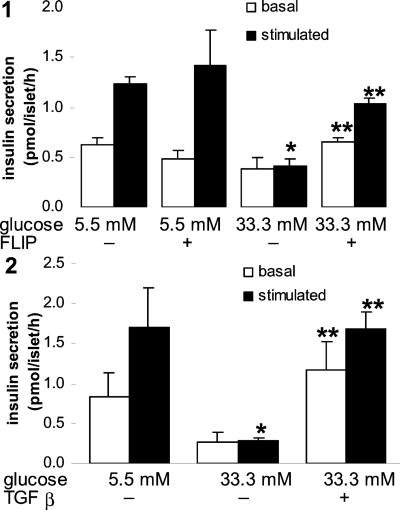
Chronic exposure of human islets to 33.3 mM glucose for 6 days completely abolished acute glucose-stimulated insulin release (Fig. 5). Transfection with FLIP or coincubation with TGF-β partly restored such glucose stimulation. Insulin content of islets cultured at high glucose tended to be lower compared with control (5.5 mM glucose) and increased after transfection with FLIP or addition of TGF-β; however, these changes were not statistically significant (data not shown).
Figure 5.
FLIP and TGF-β preserve glucose-stimulated insulin secretion in human islets exposed to high glucose for 6 days. Islets were cultured on extracellular matrix-coated dishes for 6 days in 5.5 and 33.3 mM glucose (control) or after transfection with an expression vector coding for FLIP (FLIP) (1) or with addition of 20 ng/ml TGF-β (2). Basal and stimulated insulin secretion denotes the amount secreted during a 1-h incubation at 3.3 and 16.7 mM glucose, respectively, after the 6-day culture period. Each bar represents the mean of three experiments ± SE from three separate donors. In each experiment, the data were collected from three plates per treatment. *, P < 0.01 relative to islets at 5.5 mM glucose; **, P < 0.01 relative to islets at 33.3 mM glucose.
Discussion
Elevated glucose concentrations have a dual effect on β cell turnover. Depending on duration of the exposure to glucose and on the genetic background of the islets, glucose may induce or impair β cell proliferation and has pro- or antiapoptotic effects (2, 10, 14–16, 18, 19). We now propose that this dual effect of glucose may be explained by the pivotal role of FLIP on Fas signaling. Increased glucose concentration induces Fas expression, which can interact with the constitutively expressed FasL on neighboring β cells (18). As long as a critical level of FLIP is expressed, Fas signaling would lead to β cell proliferation. When FLIP expression is decreased, Fas activation would promote apoptosis leading to a loss of β cells. The results of the present study support this hypothesis. FLIP is basely expressed in human pancreatic β cells. Short-term exposure of cultured human islets to elevated glucose concentrations promoted β cell proliferation, whereas longer exposure times resulted in a marked inhibition of the proliferative capacity of β cells in parallel with decreased FLIP expression. Up-regulation of FLIP, by incubation with transforming growth factor β or by transfection with an expression vector coding for FLIP, restored β cell proliferation and protected from glucose induced β cell apoptosis. However, addition of an antagonistic anti-Fas antibody blocked glucose-induced proliferation in FLIP-transfected β cells, indicating that Fas receptor activation is required for FLIP mediated proliferation.
The proliferative capacity of cultured human adult β cells is controversial. Brelje et al. observed 1.0–2.3 5′-bromo-2′-deoxyuridine-positive (BrdUrd+) β cells per islet (38), which is compatible with our estimate of proliferation. Using human islets attached to the matrix produced by a bladder cell line, Hayek et al. reported a much higher rate of 32% BrdUrd-positive β cells (39). However, these findings were not confirmed by Lefebvre et al., who believe that the extremely high proliferation rate observed by Hayek et al. was due to undetected contamination with ductal cells (40). In the present study, we used human preparations with very low contamination of ductal cells, and we always performed double staining for insulin to verify the identity of the β cells.
This study shows that glucose-induced decrease in FLIP expression is due neither to changes at RNA level nor to unspecific proteolytic activity as a general feature of an apoptotic process. Thus, the decrease in FLIP protein could result from increased protein turnover and degradation or from reduced protein synthesis. In macrophages, FLIP expression levels decrease on challenge with FasL (36). Possibly, Fas activation, which recruits FLIP together with caspase-8, leads to a secondary degradation of the FLIP–caspase-8 complex. This would lead to decreased FLIP expression, provided that FLIP synthesis is limited. Similar to the glucose-induced decrease in FLIP expression, TGF-β-induced expression of FLIP was observed only at protein but not at RNA levels. Because TGF-β-inhibited Fas expression, it is tempting to postulate that this protective effect of TGF-β prevented the degradation of FLIP.
Alterations in β cell mass as a reflection of a shift from proliferation to apoptosis play a crucial role in the pathophysiology of Type 1 and 2 diabetes and may further limit the outcome of islet transplantation. Fas signaling represents a final common pathway responsible for β cell apoptosis and impaired β cell proliferation in response to elevated glucose concentrations and to cytokines (18, 41–43). Clearly, in Type 1 diabetes, islet cell destruction results not solely from activation of the Fas pathway but also from other actions of cytokines and from cytotoxic T cells. Nevertheless, our results open a therapeutic approach to switch Fas engagement from a death signal to induction of proliferation, thereby preventing β cell death and possibly restoring sufficient β cell mass to overcome diabetes.
Acknowledgments
We thank G. Siegfried-Kellenberger and C. S. Manzano for technical assistance, and N. Wey and M. Hoechli for support at the microscope. This work was supported by Swiss National Science Foundation Grant 3200-067049.01 (M.Y.D.), 32-061873.00 (J.O.), and 3200-061776.00 (P.A.H.), and by the Juvenile Diabetes Research Foundation Grant 4-1999-844 (P.A.H.). M.Y.D is supported by the Max Cloetta Foundation.
Abbreviations
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end
- TGF-β
transforming growth factor β
- RT-PCR
reverse transcription–PCR
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bernard C, Berthault M F, Saulnier C, Ktorza A. FASEB J. 1999;13:1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Bonner-Weir S. J Mol Endocrinol. 2000;24:297–302. doi: 10.1677/jme.0.0240297. [DOI] [PubMed] [Google Scholar]
- 3.Polonsky K S, Sturis J, Bell G I. N Engl J Med. 1998;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 4.Cavaghan M K, Ehrmann D A, Polonsky K S. J Clin Invest. 2000;106:329–333. doi: 10.1172/JCI10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn B B. Cell. 1998;92:593–596. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 6.Cerasi E. Diabetologia. 1995;38:992–997. doi: 10.1007/BF00400591. [DOI] [PubMed] [Google Scholar]
- 7.Gerich J E. J Clin Endocrinol Metab. 2000;85:2113–2115. doi: 10.1210/jcem.85.6.6646. [DOI] [PubMed] [Google Scholar]
- 8.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz P U. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 9.Taylor S I, Accili D, Imai Y. Diabetes. 1994;43:735–740. doi: 10.2337/diab.43.6.735. [DOI] [PubMed] [Google Scholar]
- 10.Donath M Y, Gross D J, Cerasi E, Kaiser N. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 11.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky K S. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Noma Y, Mizuno A, Sano T, Shima K. Diabetes. 1996;45:941–946. doi: 10.2337/diab.45.7.941. [DOI] [PubMed] [Google Scholar]
- 13.Miralles F, Portha B. Diabetes. 2001;50 Suppl. 1,:S84–S88. doi: 10.2337/diabetes.50.2007.s84. [DOI] [PubMed] [Google Scholar]
- 14.Efanova I B, Zaitsev S V, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren P O. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 15.Hoorens A, Van d C, Kloppel G, Pipeleers D. J Clin Invest. 1996;98:1568–1574. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maedler K, Spinas G A, Dyntar D, Moritz W, Kaiser N, Donath M Y. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Bonner-Weir S, Deery D, Leahy J L, Weir G C. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Maedler K, Spinas G A, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath M Y. Diabetes. 2001;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- 19.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, et al. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 20.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, et al. Nature (London) 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 21.Tschopp J, Irmler M, Thome M. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal B B, Singh S, LaPushin R, Totpal K. FEBS Lett. 1995;364:5–8. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka T, Budd R C, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, et al. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 24.Oberholzer J, Triponez F, Mage R, Andereggen E, Buhler L, Cretin N, Fournier B, Goumaz C, Lou J, Philippe J, et al. Transplantation. 2000;69:1115–1123. doi: 10.1097/00007890-200003270-00016. [DOI] [PubMed] [Google Scholar]
- 25.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Diabetes. 1997;46:1120–1123. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- 26.Ricordi C, Lacy P E, Finke E H, Olack B J, Scharp D W. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser N, Corcos A P, Sarel I, Cerasi E. Endocrinology. 1991;129:2067–2076. doi: 10.1210/endo-129-4-2067. [DOI] [PubMed] [Google Scholar]
- 28.Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross D J, Cerasi E, Melloul D. Diabetes. 1999;48:1230–1236. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- 29.Meyer K, Irminger J C, Moss L G, de Vargas L M, Oberholzer J, Bosco D, Morel P, Halban P A. Diabetes. 1998;47:1974–1977. doi: 10.2337/diabetes.47.12.1974. [DOI] [PubMed] [Google Scholar]
- 30.Bouwens L, Lu W G, De K R. Diabetologia. 1997;40:398–404. doi: 10.1007/s001250050693. [DOI] [PubMed] [Google Scholar]
- 31.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann F, Buechner S A, Wernli M, Strebel S, Erb P. J Invest Dermatol. 2001;117:59–66. doi: 10.1046/j.0022-202x.2001.01380.x. [DOI] [PubMed] [Google Scholar]
- 33.Imanishi T, Murry C E, Reinecke H, Hano T, Nishio I, Liles W C, Hofsta L, Kim K, O'Brien K D, Schwartz S M, et al. Cardiovasc Res. 2000;48:101–110. doi: 10.1016/s0008-6363(00)00154-1. [DOI] [PubMed] [Google Scholar]
- 34.Imanishi T, McBride J, Ho Q, O'Brien K D, Schwartz S M, Han D K. Am J Pathol. 2000;156:125–137. doi: 10.1016/S0002-9440(10)64712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita H, Yoshikawa H, Shiiki K, Hamada Y, Nakajima Y, Tasaka K. Int J Cancer. 2000;88:986–991. doi: 10.1002/1097-0215(20001215)88:6<986::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Schlapbach R, Spanaus K S, Malipiero U, Lens S, Tasinato A, Tschopp J, Fontana A. Eur J Immunol. 2000;30:3680–3688. doi: 10.1002/1521-4141(200012)30:12<3680::AID-IMMU3680>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Lee S J, Zhou T, Choi C, Wang Z, Benveniste E N. J Immunol. 2000;164:1277–1285. doi: 10.4049/jimmunol.164.3.1277. [DOI] [PubMed] [Google Scholar]
- 38.Brelje T C, Scharp D W, Lacy P E, Ogren L, Talamantes F, Robertson M, Friesen H G, Sorenson R L. Endocrinology. 1993;132:879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 39.Hayek A, Beattie G M, Cirulli V, Lopez A D, Ricordi C, Rubin J S. Diabetes. 1995;44:1458–1460. doi: 10.2337/diab.44.12.1458. [DOI] [PubMed] [Google Scholar]
- 40.Lefebvre V H, Otonkoski T, Ustinov J, Huotari M A, Pipeleers D G, Bouwens L. Diabetes. 1998;47:134–137. doi: 10.2337/diab.47.1.134. [DOI] [PubMed] [Google Scholar]
- 41.Loweth A C, Williams G T, James R F, Scarpello J H, Morgan N G. Diabetes. 1998;47:727–732. doi: 10.2337/diabetes.47.5.727. [DOI] [PubMed] [Google Scholar]
- 42.Stassi G, Todaro M, Richiusa P, Giordano M, Mattina A, Sbriglia M S, Lo M A, Buscemi G, Galluzzo A, Giordano C. Transplant Proc. 1995;27:3271–3275. [PubMed] [Google Scholar]
- 43.Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa F, Inada C, Nonaka K. Diabetologia. 1996;39:1306–1312. doi: 10.1007/s001250050574. [DOI] [PubMed] [Google Scholar]