Abstract
We found that the second intron of Stat5a was one of the common integration sites of the endogenous ecotropic murine leukemia virus, i.e., SL/Kh virus integration-1 (Svi1), in early pre-B lymphomas in SL/Kh mice. The high expression of STAT5A induced by Svi1 integration and activation accelerated the transcription of its target genes such as c-Myc. Transfection of the constitutively active Stat5a mutant cDNA, but not of the wild-type cDNA, to the bone marrow cells induced colony formation of pre-B cells in a methylcellulose medium and escaped from dependence on IL-7. Such growth depended on a genetic factor in the SL/Kh strain. Consitutively high expression of Stat5a either by retrovirus integration or transfection of active mutant cDNA can be lymphomagenic to early pre-B cells in collaboration with a certain genetic background factor of mice.
Keywords: provirus‖hot spot‖lymphomagenesis
The SL/Kh strain of mice develop spontaneous pre-B lymphomas at >90% incidence by 6 mo of age (1, 2). There are two morphological distinct types: the major lymphomas, which show systemic lymphatic tissue involvement, and the minor type, which shows restricted growth in bone marrow (BM) (1). Both are pre-B lymphomas expressing BP-1 and B220 (2, 3). Several lines of evidence indicate that the endogenous murine leukemia virus (MuLV) plays an etiologic role in SL/Kh lymphomas; in particular, somatically acquired proviruses frequently are observed in lymphoma DNAs (3, 4).
To elucidate the mechanism of lymphomagenesis, we cloned the virus-host junctional segments from lymphoma DNAs by an inverse PCR technique and performed sequence analysis. In three of 60 SL/Kh lymphomas, clonal ecotropic provirus integration was found within a 400-bp stretch in the second intron of the Stat5a gene, a member of the STAT family. In this article, we examined the effect of the provirus integration in this hot spot, named SL/Kh virus integration-1 (Svi1). The effect of the constitutive high expression of Stat5a on early B cells was further verified by colonial growth of early pre-B cells in soft agar medium and loss of IL-7 dependence, when SL/Kh BM cells were transfected with an active Stat5a mutant cDNA. It was shown that the immortalization of early pre-B cells by Stat5a depended on an unidentified host factor in SL/Kh mice. There are several reports on the relevance of Stat5a in hemopoietic malignancies (5–12). Stat5b is a human myeloid cell oncogene, but neither Stat family member has been shown to have a direct pathogenetic role in lymphoid disease. Herein, we report that Stat5a is one of the hot spots of MuLV integration and subsequent constitutive up-regulation of Stat5a perturbs signaling in early B lineage leading to lymphoma development in a certain genetic background.
Materials and Methods
Mice.
SL/Kh is an inbred strain of mice with a high incidence of spontaneous pre-B lymphomas. Previously, we reported their origin (13) and virus expression (1), the pathology of lymphomas (1, 2), and host genetic factors in lymphomagenesis (3, 4, 14). They were maintained by brother–sister mating in our laboratory.
Southern Hybridization.
High Mr DNAs were extracted from lymphoma and normal kidney tissues, and 2.5- to 5-μg aliquots of DNAs were digested with a restriction enzyme of NcoI, SacI, PvuII, or SacII (New England Biolabs) for 16 h and transferred to a N+ Hybond nylon membrane (Amersham Pharmacia) after electrophoresis. MuLV env and gag probes were kindly provided by H. Ikeda (National Institute of Animal Health, Tsukuba, Japan). The Stat5a probe A was located in the segment including its first and second exons. The probe was obtained by PCR from the genomic DNA of SL/Kh mice and labeled with Megaprime DNA-labeling system (Amersham Pharmacia).
Inverse PCR.
Genomic DNA (100 ng) from each lymphoma was first digested with SacII for 2 h and self-ligated with T4 ligase (Takara Shuzo, Kyoto) at 14°C overnight. Amplification of the virus-host junctions was performed in a volume of 50 μl containing 2.5 mM deoxynucleotide triphosphate. Each primer had 10 pmol/μl and 0.25 unit of Taq DNA polymerase (expand long template PCR system, Roche Diagnostics).
PCR amplification was carried out in three steps under the following conditions in a thermal cycler (Perkin–Elmer/Cetus): The first step was 10 cycles (30 s at 94°C, 40 s at 62°C, and 4 min at 68°C) preceded by an initial denaturation step (1 min at 95°C); the second step was 20 cycles (30 s at 94°C, 40 s at 62°C, and 4 min plus extended 20 s by one cycle at 68°C); and the final step was elongation (10 min at 72°C). In some cases, nested PCR procedures were added to the above.
The primers for inverse PCR were located within the AKV-MLV (AKV murine leukemia virus) genomes. Their sequences were: 5B4, GAGGGCTTGGACCTCTCGTCTCCTAAAAAACCACG and 5F1, GTCTCTCCCAAACTCTCCCCCTCTCCAACC in the first set, and 5F2, CCT CCTCTGACGGAGATGGCGACAGAGAAGAGG and 5B1, GAGGGCTTGGAC CTCTCGTCTCCTAAAAGAACCACG in the second step of cycles for the nested PCR. The PCR products were resolved by electrophoresis in 1% agarose gel and stained with ethidium bromide; they were then subcloned into the pCR 2.1-TOPO vector (Invitrogen), and sequence analysis reaction was performed with Thermo-sequenase (Amersham Pharmacia).
Northern Blot Analysis, 3′ Rapid Amplification of cDNA Ends (RACE) and Reverse Transcription–PCR (RT-PCR) Analysis.
Total RNA was extracted from lymphoma tissues by ISOGEN (Nippon Gene, Toyama, Japan). Samples containing equal amounts of RNA (20 μg) were size-fractionated by electrophoresis on a 1.0% formaldehyde-agarose gel, transferred to a N+ Hybond nylon membrane, and hybridized with a probe B (containing exons 3 and 4). Ethidium bromide staining of rRNA bands was used to ensure equal RNA loading, and β-actin RNA was used as an internal control. The probes were labeled with the kit (Amersham Pharmacia) mentioned above.
A 3′ RACE assay was performed with a 3′ full RACE core set (Takara) with the primers located within a long terminal repeat of retroviral genome. The products from the 3′ RACE assay were checked by the additional poly(A) sequence of the terminus specific to mRNA.
RT-PCR was performed with a one-step RT kit (Invitrogen). The primers for RT-PCR of β-actin were according to Wei et al. (15).
The probes for Northern hybridization were amplified with RT-PCR primers: c-Myc, TCTGTACCTCGTCCGATTC and TGCCTCTTCTCCACAGACAC; Bcl-xL, TGGATCCTGGAAGAGAATCG and AGATCACTGAACGCTCTCCG; and Pim-1, TTCTGGACTGGTTCGAGAGG and TAGCGAATCCACTCTGGAGG. The primers for examining the phenotype markers were as follows: Igα, ATCACATGGTGGTTCAGCC and TCTCCAATGTGGAGGTTGC; CD19, TGTCTCTTCTGAGAAGCTGGC and AACCAGAAGTGGACCTGTGG; HSA(CD24), AACATCTAGAGAGTCGCGCC and CTGGTGGTAGCGTTACTTGG; and IL-7R α chain, CTCTCAGAATGATGGCTCTGG and CTGTGCAGGAAGATCATTGG.
Abs.
FITC, phycoerythrin, or biotin-conjugated rat mAbs against mouse B220 (RA3–6B2), BP-1, and anti-phosophorylated tyrosine (PY20) Ab were purchased from PharMingen. Anti-IL-7R was obtained from Santa Cruz Biotechnology. Anti-phosphorylated STAT5A was purchased from New England BioLabs and anti-STAT5A was purchased from Zymed and Santa Cruz Biotechnology. Streptavidin-peroxidase conjugate was obtained from Dako.
Western Blotting.
Lymphoma cells were washed once with a PBS and lysed in an immunoprecipitation buffer [50 mmol/liter Tris (pH 7.4) containing 150 mmol/liter NaCl, 5 mmol/liter EDTA, 1% Triton X-100] on ice for 15 min. Tissue lysates were clarified by microfuge centrifugation. The proteins were separated by 10% SDS/PAGE using the radioimmunoprecipitation assay buffer and a Laemli buffer system and transferred electrophoretically onto poly(vinylidene difluoride) membranes (Bio-Rad). The membrane was incubated with a primary Ab in PBS for 1 h at room temperature and then incubated with a secondary Ab with biotin-conjugated goat anti-rabbit IgG (1/10,000 dilution; Dako) for 1 h at room temperature. After this step, the membrane was incubated with peroxidase-conjugated streptavidin (Sigma) for 1 h at room temperature. The detected bands were visualized by enhanced chemiluminescence (ECL, Amersham Pharmacia).
Electrophoretic Mobility Shift Assay.
A 32P-labeled β-casein probe (5′-TAG ATT TCT AGG AAT TCG-3′, 5′-CGA ATT CCT AGA AAT CTA-3′) was preincubated with 5 μg of cellular and nuclear extracts in a final volume of 20 μl containing 10 mM Hepes (pH 7.8), 50 mM KCl, 1 mM EDTA, 5 mM MgCl, 10% (vol/vol) glycerol, 5 mM DTT, and 1 μg of poly (dI-dC) (Amersham Pharmacia) on ice for 30 min, and Abs were subsequently added. Complexes were resolved by electrophoresis in 4.5% polyacrylamide gel.
Flow Cytometry.
The single-cell suspension was prepared from the right-femur BM plug and adjusted to 106 cells/ml. Total BM cell yield was in the range of 0.8 × 107-1.2 × 107 cells/femur, and no significant difference was noted between SL/Kh mice. BM cells were doubly stained with FITC-labeled anti-BP-1 (clone 6C3) and phycoerythrin-labeled anti-B220 (clone RA3–6B2; PharMingen) and analyzed with a FACScan (Becton Dickinson).
The Time Course of STAT5A Phosphorylation After Stimulation by Anti-IL-7R.
A 10-μl aliquot of 200 μg/ml anti-IL-7R was added to the cultured Svi1 lymphoma cells, and the cell lysates were blotted to the poly(vinylidene difluoride) membrane (Bio-Rad). The density of the band visualized by enhanced chemiluminescence (ECL, Amersham Pharmacia) on the immunoblot membranes was measured by a LumiVision Imager (Taitec, Tokyo)
Construction of an Expression Vector and Transfection and Colony Growth Assay.
A constitutive active form of murine Stat5a cDNA (1*6-Stat5a) was described by Onishi et al. (16) and Ariyoshi et al. (17). The puro-pMX vectors for murine wild-type (wt) Stat5a and mutant 1*6-Stat5a was constructed according to Kitamura and coworkers (16–18). These cDNAs were kindly given to us by Y. Kitamura (Institute of Medical Science, University of Tokyo). The Stat5a cDNA was subcloned to pcDNA 3.1 expression vector (Invitrogen) that was introduced by Matsumura et al. (19). BM cells used for transfection assay were harvested from 3-wk-old mice. They were washed once with PBS, and a 400-μl cell suspension (7 × 106 cells/ml) was transfected with 2–8 μg of an expression vector of murine wt Stat5a or mutant 1*6-Stat5a in 220 μl of PBS with 10 mg/ml DEAE-dextran (Sigma). After washing, they were suspended in 10 ml of a semisolid medium.
Either Methocult (Stemcell Technology, Vancouver, Canada) containing IL-7 or a StemPro methylcellulose medium (GIBCO) without IL-7 was used as a basic semisolid medium in the colony assay. Recombinant murine IL-3, IL-7, and stem cell factor (PeproTech, Rocky Hill, NJ) were added to the StemPro methylcellulose medium at 100 ng/ml depending on the assay. In relevant cultures, the number of cells in each colony increased rapidly. To detect early growth, before 48 h, we defined the colony as a cluster of >5 cells, but after 48 h, only those of >20 cells.
Transfected BM cells were suspended in 100 μl of an SFO2 medium (modified mixture of RPMI medium 1640, DMEM, and Ham's F-12 medium, supplemented with insulin and transferrin; Sankyo) and were cultured in 96-well plates for 48–480 h at 37°C in the presence or absence of IL-7. The viability of the cultured cells was estimated by a trypan blue dye exclusion test.
Results
Integration to the Second Intron of Stat5a.
Our previous study (3) revealed that SL/Kh lymphoma DNA contains seven copies of the endogenous ecotropic MuLV provirus and one or more additional copies of the somatically acquired provirus. To characterize the provirus integration sites and nearby host genes, host-virus junctions were amplified by inverse PCR from 60 independent primary lymphoma DNAs.
Sequencing of the flanking host segments of the host-virus junctions from three of 60 cases of SL/Kh lymphomas revealed that they were from a 400-bp segment within the second intron of the Stat5a gene, immediately upstream of the translation region (20). We named this segment SL/Kh virus integration-1 (Svi1) as illustrated in Fig. 1 A and B. The proviruses integrated in Svi1 had general structure shared by most endogenous ecotropic MuLVs without a viral oncogene (data not shown) and were integrated in the direction of transcription.
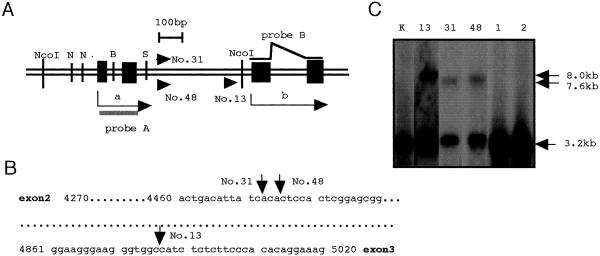
Figure 1.
The genome structure of Svi1 and provirus integration. (A) The virus integration site Svi1 within the second intron of Stat5a in SL/Kh lymphomas nos. 13, 31, and 48. All proviruses were in the direction of transcription (arrowheads). N, B, and S: restriction sites of NarI, BssHII, and SacII, respectively. A closed bar represents the Stat5a probe A for Southern hybridization. The probe B for Northern hybridization was synthesized by RT-PCR with primers set in exons 3 and 4; arrow a indicates the transcription of normal Stat5a mRNA, and arrow b indicates the normal translation of the STAT5A protein and the altered transcription of Stat5a mRNA in Svi1 lymphomas. (B) Genomic sequence of the second intron of Stat5a and the sites of retroviral integration are shown individually by arrows. (C) Southern blot of NcoI-digested DNAs with probe A including the first and second exon of Stat5a gene. The 3.2-kb bands represent the germ-line Stat5a gene and clonal rearranged bands of 7.6 kb to ≈8.0 kb in Svi1 lymphomas nos. 13, 31, and 48 but not in non-Svi1 lymphomas nos. 1 and 2. Note that the MuLV provirus has internal NcoI sites. Lane K represents germ-line DNA from normal SL/Kh kidney.
Southern hybridization of NcoI-digested DNAs with a probe containing exons 1 and 2 of Stat5a (probe A) revealed clonal rearrangements of the Stat5a gene in three lymphomas nos. 13, 31, and 48 but not in lymphomas nos. 1 and 2 (Fig. 1C). We named the lymphomas nos. 13, 31, and 48 with clonally rearranged Stat5a gene as Svi1 lymphomas. The lymphomas nos. 1 and 2 had multiple provirus integrations but not in Svi1.
High Expression of Stat5a Gene and Its Target Genes in Svi1 Lymphomas.
To evaluate the effect of retroviral provirus integration into Svi1, we examined the transcription of Stat5a mRNA. By the cDNA library screening with probe B, a 2.4-kb product of Stat5a cDNA lacking the first two untranslated exons was recovered from Svi1 lymphomas. In the sequence analysis of the recovered cDNA, no mutation was detected in the exons from the third to the last encoding STAT5A protein. Subsequently it was examined whether or not the retroviral long terminal repeat mRNA was fused with Stat5a mRNA as a leading sequence for the initiation of transcription. A 3′ RACE assay using the primer in the 3′-long terminal repeat of the Svi1-integrated provirus detected the expression of the retroviral long terminal repeat mRNA; however, none of them fused with the Stat5a mRNA according to the sequence analysis. Northern blot demonstrated a high expression of Stat5a mRNA in the Svi1 lymphomas with the probe B located at exons 3–4 (Fig. 2A) and the transcription in the Svi1 lymphomas was >10 times as high as in other lymphomas without clonal Svi1 integration by densitometry of the blots. The integrated proviral genome functioned as an enhancer of the transcription of Stat5a mRNA, which initiated exactly from the first base of exon 3.
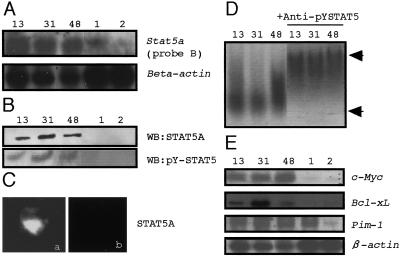
Figure 2.
The expression and transcription activity of Stat5a in Svi1 lymphomas. (A) Northern hybridization of lymphoma RNA hybridized with the Stat5a probe B and the β-actin probe. Lanes 13, 31, and 48, Svi1 lymphomas; lanes 1 and 2, non-Svi1 lymphomas. (B) Western blot with anti-STAT5A and anti-Tyr-694 phosphorylated-STAT5A (pY-STAT5A) of the nuclear fractions of the lymphomas. Lanes 13, 31, and 48: Svi1 lymphomas; lanes 1 and 2: non-Svi1 lymphomas. (C) Nuclear localization of pY-STAT5A. Positive signal was seen only with Svi1 lymphoma (a) but not in non-Svi1 control (b). (D) A gel-shift assay demonstrating the DNA-binding activity of the STAT5A with the β-casein promoter, which contained a GAS element. Lanes 13, 31, and 48: the probe plus the nuclear extraction of nos. 13, 31, and 48 lymphomas. The lower arrow indicates the shift by nuclear extracts from Svi1 lymphomas, and the upper arrow indicates the supershift by addition of the anti-pY-STAT5A Ab to the nuclear extracts. (E) Northern blot of the target genes of STAT5A. β-Actin was a positive control.
Western blotting of lymphoma extracts demonstrated a high expression of normal-sized STAT5A (98 kDa) and tyrosine phosphorylation exclusively in Svi1 lymphomas (Fig. 2B) but no variant form of STAT5A. In immunohistochemistry, the SAT5A signal was intense in the nucleus of Svi1 lymphoma cell (Fig. 2C). STAT5A binds DNA at a interferon-γ-activated sequence (GAS) element and thus induces enhanced transcription of the GAS-bearing target genes. A gel-shift assay using the β-casein promoter containing a GAS element demonstrated the DNA-binding activity in each Svi1 lymphoma (Fig. 2D). Moreover, target genes of STAT5A, such as Bcl-xL (21), Pim-1 (22), and c-Myc (23) were expressed in Svi1 lymphomas (Fig. 2E). Especially, the expression of c-Myc and Bcl-xL in Svi1 lymphomas was 12 and five times as high as that in controls. Thus, the role of STAT5A in Svi1 lymphomagenesis may be to induce the high expression of the oncogenes or antiapoptotic genes.
The Phenotype of Svi1 Lymphomas and IL-7R Expression.
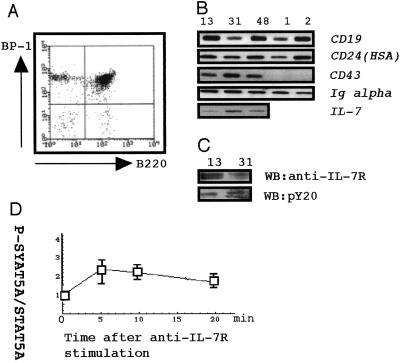
Phenotype analysis revealed that Svi1 lymphomas expressed B220, BP-1 (Fig. 3A), CD19, CD24, CD43, Igα (Fig. 3B), and IL-7R (Fig. 3C). These observations implied that Svi1 lymphomas had phenotypes similar to early pre-B cells of CD43+IL-7R+. In contrast, non-Svi1 controls were of CD43−IL-7R− late pre-B cells. In lymphoma tissues, we could detect IL-7 (Fig. 3B). By stimulating cultured Svi1 lymphoma cells with an anti-IL-7R Ab 24 h after withdrawal of IL-7 from the medium, the ratio of phosphorylated STAT5A to whole STAT5A transiently increased (Fig. 3C), suggesting the STAT5A was phosphorylated in a time-dependent way by the stimulation of IL-7R. This finding implied the employment of an IL-7R-signaling pathway in Svi1 lymphoma cells.
Figure 3.
Phenotypes of Svi1 lymphomas. (A) Flow cytometry of lymphoma cells doubly stained with phycoerythrin-labeled anti-B220 and FITC-labeled anti-BP-1. (B) RT-PCR of CD19, CD24 (HSA), CD43, Igα, and IL-7. Lanes 13, 31, and 48, Svi1 lymphoma; lanes 1 and 2, non-Svi1 lymphoma. (C) Western blot with anti-IL-7R and with pY20 by using the same membrane for the blot with anti-IL-7R after stripping. (D) The time course of STAT5A phosphorylation of a cultured Svi1 lymphoma after stimulation of IL-7R by anti-IL-7R. The sequentially harvested cells were stained with either anti-pY-STAT5A or anti-STAT5A, and the intensity of staining was measured by a Lumi Vision imager. The ratios of tyrosine phosphorylated STAT5A to total STAT5A were standardized by setting the values at time 0 as 1.0.
Colony Growth of BM Cells in a Semisolid Medium by Transfecting STAT5A cDNA.
Enhanced expression of Stat5a by provirus integration seemed the basic mechanism of Svi1 lymphoma induction. To examine the effect of the high expression of the Stat5a gene in BM cells, we transfected them with expression vectors bearing either wt or constitutively active mutant 1*6-Stat5a cDNA and cultured them in a semisolid Methocult medium containing IL-7 optimalized for the culture of pre-B cells. The transfection was done similarly to BM cells of NFS, C57BL/6, C3H, AKR, and SL/Kh mice at 3 wk of age. The SL/Kh BM cells transfected with mutant Stat5a cDNA (4 μg for 2 × l07 BM cells) started to grow after 48 h and reached a plateau at 96 h, forming colonies in the semisolid medium (Fig. 4A). In Fig. 4, the number of colonies represents an average of six dishes each containing 2.8 × l06 transfected BM cells. The number of colonies increased linearly to the dose of cDNA within a range of 2 to ≈8 μg per 2 × 107 BM cells but the dose did not affect the kinetics itself.
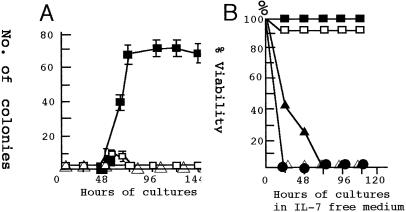
Figure 4.
Colony formation of BM cells by transfection of the expression vector carrying Stat5a cDNA. (A) Kinetics of the colony formation of BM cells transfected with Stat5a cDNA. The “numbers of colony” represent colonies formed by 2.8 × l06 transfected BM cells. ■, Colonies of BM cells transfected with 4 μg of the 1*6-Stat5a expression vector; □, colonies of SL/Kh BM cells transfected with 4 μg of the wt Stat5a expression vector. The dose of cDNA was between 2 μg and 8 μg for 2 × l07 BM cells. ▵, Colonies from SL/Kh BM cells transfected with 4 μg of the empty vector. (B) The survival of the colony cells in the IL-7-free medium. The colony cells harvested 96 h after transfection of 1*6-Stat5a were incubated in a medium with (■) or without (□) IL-7. Similar cells harvested 48 h after transfections were incubated in the medium with (▴) or without (▵) IL-7. These cells died quickly of apoptosis. The viability of the BM cells transfected with wt Stat5a was as low as 10% when harvested 48 or 72 h after transfection and no cells survived after transfer to IL-7-free medium (●).
In contrast, the transfection of wt Stat5a to SL/Kh BM cells induced weak and transient colony growth after 48 h as seen in Fig. 4A, but the most of the cells died quickly by apoptosis before forming larger colonies. The BM cells from NFS, C57BL/6, C3H, and AKR strain mice started to grow 48 h after transfection with either wt or mutant Stat5a cDNA, but, thereafter, they rapidly died of apoptosis (Table 1). Therefore, colony growth of BM cells by mutant Stat5a may require a host factor in SL/Kh.
Table 1.
Requirement for BM cells to form colonies in semisolid medium
| Transfected with
|
Medium supplemented with
|
No. of colonies* | |||
|---|---|---|---|---|---|
| Activated Stat5a | WT Stat5a | IL-7 | IL-3 | SCF | |
| + | − | + | − | − | 40 ± 5 |
| + | − | − | − | − | 0 |
| + | − | − | − | + | 0 |
| + | − | − | + | − | 0 |
| − | − | + | − | − | 2 ± 1 |
| − | + | + | − | − | 8 ± 2 |
| − | + | − | − | − | 0 |
| − | + | − | + | − | 0 |
| − | + | − | − | + | 0 |
The basic medium used in this experiment was a methylcellulose medium (GIBCO) supplemented as indicated in the table.
Colonies were counted at 96 hr in culture.
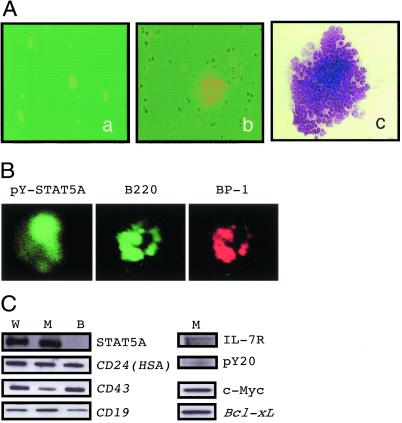
Fig. 5A shows representative colonies at 24 h (a) and 72 h (b) of culture after the transfection of 1*6-Stat5a cDNA. The colony was a packed uniform collection of lymphoblasts (c), but no granulocyte or macrophage colony was observed under these culture conditions by 144 h. They were positively stained anti-pY-STAT5A, anti-BP-1, and anti-B220 (Fig. 5B). Phenotype markers were further examined for colony cells induced by transfection of wt (W), active mutant Stat5a cDNA (M), or normal BM cells (B) by RT-PCR. Both W and M colony cells were positive for STAT5A in Western blot and RT-PCR assay detected expression of HSA (CD24), CD19, CD43, and IL-7R (Fig. 5C). Consequently, the colony cells induced by the transfection of Stat5a cDNA, either the wt or constitutively active mutant form, shared the same phenotype of early pre-B cells, although the colony growth of the cells transfected with the former was just transient.
Figure 5.
Phenotype of the colony cells. (A) Representative colony at 48 h (a) and a colony at 96 h (b and c) in culture after the transfection of 1*6-Stat5a cDNA. (Aa and Ab) Phase-contrast microscopy = ×200. (Ac) Giemsa stain = ×200. (B) The colonies formed by SL/Kh BM cells transfected 1*6-Stat5a were immunostained with anti-pYSTAT5A, anti-B220, or anti-BP1. (C) Expression of STAT5A shown by Western blot and RT-PCR assays of CD19, CD24 (HSA), CD43, c-Myc, Bcl-xL, and IL-7R and with pY20 using the same membrane for the blot with anti-IL-7R after stripping in the colony cells; lane W, BM cells transfected with wt Stat5a; lane M, BM cells transfected with mutant 1*6-Stat5a, and lane B, fresh BM cells with an empty vector. The cells for lanes W and M were harvested at 48 h after transfection.
For the colony growth of BM cells transfected with mutant Stat5a, the presence of IL-7, but not of IL-3 or stem cell factor, was absolutely required. Such a requirement of IL-7 was limited only in the early stage of colony growth because the colony cells harvested after 96 h in culture no longer required IL-7 to survive in the IL-7-free medium (Fig. 4B). In contrast, the colony cells harvested 48 h after transfection failed to maintain viability in the IL-7-free medium and the addition of IL-7 slightly delayed cell death. The colony cells of wt Stat5a cDNA transfectant showed <20% viability when harvested at 48 h and did not survive irrespective of the presence of IL-7.
These observations indicate that the transfectant of the mutant Stat5a require IL-7 for initial growth and possibly survival, but beyond certain critical points, they convert to immortalized cells by losing IL-7 dependence.
Discussion
We found that a retrovirus integration into the immediate upstream of the protein-coding region of Stat5a induces enhanced expression of the gene, contributing to lymphoma development. This segment Svi1, a 400-bp stretch in the second intron of Stat5a, is one of the hot spots in which three of 60 SL/Kh lymphomas had a clonally integrated proviral MuLV genome. The phenotype study on Svil lymphomas indicated that they belonged to early pre-B cells, unlike the controls of non-Svi1 lymphomas in SL/Kh showing more mature pre-B phenotypes without IL-7R expression.
STAT5A is a member of the Signal Transducer and Activator of Transcription family in cytokine signaling (19, 23). STAT5A regulates proliferation, differentiation, and apoptosis in hematopoietic cells (18). Binding of appropriate ligands to the cytokine receptors induces the phosphorylation of STAT5A. For instance, the signals mediated by the IL-2 receptor in T cells (23), or that by IL-7 receptor in early pre-B cells (24) use STAT5A. Consistent detection of phosphorylated IL-7R in Svi1 lymphomas suggests that its activation either by IL-7 in microenvironments or else is a likely explanation for STAT5A activation. The active STAT5A forms a dimer that translocates into the nucleus and exerts transcriptional activity by binding to the GAS element in the promoter of target genes such as c-Myc, Pim-1, and Bcl-xL. The most direct and plausible mechanism of transformation of Svi1 lymphomas with excess expression of STAT5A is, therefore, by increasing the expression of these target genes related to tumorigenesis. Among them, c-Myc was selectively expressed: Hence, it is one of the strongest candidate genes that is the downstream target of STAT5A and is responsible for Svi1 lymphomagenesis. The relevance of c-Myc with cell cycle progression mediated by STAT family has been documented (25–27).
Consistent with the above hypothesis is the fact that transfection of constitutively active Stat5a mutant cDNA induced colony formation and immortalization of SL/Kh BM cells in a semisolid medium in the presence of IL-7. The cells composing colonies shared the phenotype of early pre-B cells with Svi1 lymphomas. Transient growth of early pre-B cells that form small colonies was observed after transfection of either wt Stat5a or mutant 1*6-Stat5a cDNA in the presence of IL-7; however, they disappeared rapidly by apoptosis within 60–72 h. In this experiment, IL-7 was required in the early growth phase, but not always for the maintaining colony survival. The colony cells survived even after transfer to the IL-7-free medium. Therefore, STAT5A, both wt and 1*6-Stat5a mutant, initiate the growth of early pre-B cells in the presence of IL-7, but only the mutant Stat5a can induce immortalization, i.e., loss of dependence on IL-7. This model is, however, of partial significance because the Stat5a driven by virus integration is a wt. The true reason virus-driven wt Stat5a induces lymphomas when its transfection to BM cells is ineffective has remained obscure. One explanation is that the expression of wt transgene is transient, so that it may be not effective even the dose is increased. On the other hand, the virus-driven expression of wt Stat5a is constititutive and high enough to transform the pre-B target cells by activation through an intracellular signaling network somehow unique to SL/Kh mice.
It is noteworthy that such immortalization of pre-B cells by the active mutant of Stat5a was observed only with BM cells of the SL/Kh strain but not with others. Schwaller et al. (5) infected BM cells with retrovirus bearing constitutively active Stat5a mutant and induced myelo-proliferation but not any lymphoid tumors in the C57BL/6 and BALB/c background. Therefore, a genetic factor unique to the SL/Kh strain seems to determine the susceptibility of BM pre-B cells to the mutant Stat5a function. We have reported that the SL/Kh mice show a transient expansion of BM pre-B cells with a peak at ≈4–6 wk of age (14). This expansion is controlled by a quantitative trait locus Bomb1 on the middle segment of chromosome 3 (14). Elucidation of such host gene and the human homolog would provide deeper insight of the cell-signaling network in hemopoietic cells that determines the target of transformation.
Acknowledgments
We thank Drs. T. Honjo and K. Ikuta for critical reading of the manuscript and Dr. M. Osaka for helpful discussions. The puro-pMX vectors carrying Stat5a cDNA were kindly provided by Dr. Y. Kitamura. Our experiments were assisted by Miss M. Takeuchi. This work was supported by a Grant-in-Aid for Cancer Research from the Ministry of Education, Culture, Science, and Technology, Japan, and a grant from the Ministry of Health, Welfare and Labor, Japan. We are grateful for generous financial support from the Eiko Norihara Memorial Fund.
Abbreviations
- BM
bone marrow
- MuLV
murine leukemia virus
- GAS
interferon-γ-activated sequence
- wt
wild type
- RT-PCR
reverse transcription–PCR
- RACE
rapid amplification of cDNA ends
References
- 1.Hiai H, Kaneshima K, Nakamura H, Oguro B Y, Moriwaki K, Nishizuka Y. Jpn J Cancer Res. 1982;73:704–707. [PubMed] [Google Scholar]
- 2.Shimada M O, Yamada Y, Nakakuki Y, Okamoto K, Fukumoto M, Honjo T, Hiai H. Leuk Res. 1993;17:573–578. doi: 10.1016/0145-2126(93)90087-2. [DOI] [PubMed] [Google Scholar]
- 3.Hiai H, Yamada Y, Abujiang P, Lu L-M, Kamoto T, Tsuruyama T. In: Animal Models of Cancer Predisposition Syndromes. Hiai H, Hino H, editors. Basel: Karger; 1998. pp. 64–77. [Google Scholar]
- 4.Yamada Y, Shimada M O, Toyokuni S, Okamoto K, Fukumoto M, Hiai H. Cancer Res. 1994;54:403–407. [PubMed] [Google Scholar]
- 5.Schwaller J, Parganas E, Wang D, Cain D, Aster J C, Williams I R, Lee C K, Gerthner R, Kitamura T, Frantsve J, et al. Mol Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 6.Migone T S, Lin J X, Cereseto A, Mulloy J C, O'Shea J J, Franchini G, Leonard W J. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 7.Sun S C, Maggirwar S B, Harhaj E W, Uhlik M. Oncogene. 1999;18:1401–1409. doi: 10.1038/sj.onc.1202430. [DOI] [PubMed] [Google Scholar]
- 8.Takemoto S, Mulloy J C, Cereseto A, Migone T S, Patel B K, Matsuoka M, Yamaguchi K, Takatsuki K, Kamihira S, White J D, et al. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Lee J M, Zong Y, Borowitz M, Ng M H, Ambinder R F, Hayward S D. J Virol. 2001;75:2929–2937. doi: 10.1128/JVI.75.6.2929-2937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin T S, Mahajan S, Frank D A. Oncogene. 2000;19:2496–2504. doi: 10.1038/sj.onc.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura N, Fujii M, Tsukahara T, Arai M, Ohashi T, Wakao H, Kannagi M, Yamamoto N. Oncogene. 1999;18:2667–2675. doi: 10.1038/sj.onc.1202608. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Lee J M, Wang Y, Huang D P, Ambinder R F, Hayward S D. Proc Natl Acad Sci USA. 1999;96:9339–9344. doi: 10.1073/pnas.96.16.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abujiang P, Yamada Y, Haller O, Kobayashi K, Kamoto T, Lu L M, Ogawa M, Ishimoto A, Katoh H, Kanehira K, et al. Lab Anim Sci. 1996;46:410–417. [PubMed] [Google Scholar]
- 14.Lu L M, Shimada S, Higashi S, Zeng Z Z, Hiai H. Cancer Res. 1999;59:2593–2595. [PubMed] [Google Scholar]
- 15.Wei C, Zeff R, Goldschneider I. J Immunol. 2000;164:1961–1970. doi: 10.4049/jimmunol.164.4.1961. [DOI] [PubMed] [Google Scholar]
- 16.Onishi M, Nosaka T, Misawa K, Mui A L, Gorman D, McMahon M, Miyajima A, Kitamura T. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariyoshi K, Nosaka T, Yamada K, Onishi M, Oka Y, Miyajima A, Kitamura T. J Biol Chem. 2000;275:24407–24413. doi: 10.1074/jbc.M909771199. [DOI] [PubMed] [Google Scholar]
- 18.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui A L, Kitamura T. EMBO J. 1999;17:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestelland R G, Kanakura Y. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Robinson G W, Wagner K U, Garrett L, Wynshaw-Boris A, Hennighausen L. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 21.Dumon S, Santos S C, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, Mollat P, Gisselbrecht S, Gouilleux F. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- 22.Yip-Schneider M T, Horie M, Broxmeyer H E. Blood. 1995;85:3494–3502. [PubMed] [Google Scholar]
- 23.Matikainen S, Sareneva T, Ronnin T, Lehtonen A, Koskinen P J, Julkunen I. Blood. 1999;93:1980–1991. [PubMed] [Google Scholar]
- 24.Steven D L, Ray M K, Sherree L F, Deborah E I, Steven F Z, Roger M P, Andrew G F. J Immunol. 1999;162:677–683. [Google Scholar]
- 25.Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano T, Ishihara K, Hibi M. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 27.Bowman T, Broome M A, Sinibaldi D, Wharton W, Pledger W J, Sedivy J M, Irby R, Yeatman T, Courtneidge S A, Jove R. Proc Natl Acad Sci USA. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]