Abstract
We report a new method for the enantio- and diastereoselective synthesis of α-allene quaternary centers in fully substituted cyclohexanones at the α-positions. This reaction involves asymmetric 1,2-carbonyl addition to 2-O-propargyl enones using a mixture of Grignard reagents and the PMP-H8-BINOL ligand. The resulting magnesium alkoxide chelate intermediate then activated the propargyl vinyl ether moiety, thereby triggering a cascade propargyl Claisen rearrangement in a diastereoselective manner. The synthetic applications of this method in the context of complex molecules are also demonstrated.
The Claisen rearrangement is a powerful method for the creation of carbon–carbon bonds at the α-position of carbonyl compounds. , Proceeding through [3,3]-sigmatropic rearrangement of allyl vinyl ethers, this reaction has been proven invaluable for constructing carbon quaternary centers, which is challenging in organic synthesis. A notable variant of the Claisen rearrangement involves the conversion of propargyl vinyl ethers to yield an α-allene functionality. Allenes are characterized by two perpendicular double bonds that are linked to a central sp-hybridized carbon atom. This distinct structural feature provides allenes with unusual chemical reactivity that can be harnessed in various synthetic transformations. Nonetheless, the propargyl Claisen rearrangement presents unique challenges. Specifically, the transition state of this pericyclic reaction must navigate the geometric constraints imposed by the linear arrangements of the sp-hybridized carbons in the propargyl group.
The significance of propargyl Claisen rearrangement is evident in the stereoselective construction of α-allene quaternary centers, although such examples are rare (Scheme ). For instance, Feng reported asymmetric propargyl Claisen rearrangement of O-propargyl β-ketoesters 1a using Ni(II)–ligand complex 2a, which formed product 1b in high enantioselectivity. In a related approach, Xie and Guo employed Co(II)–ligand 2b as a catalyst for this transformation. Within the context of diastereoselective propargyl Claisen rearrangement, advancements have been primarily associated with the total synthesis of natural products. In these cases, stereochemical information from conformationally restricted propargyl vinyl ether substrates is strategically deployed to direct the α-allene quaternary stereochemical outcome. For example, the propargyl Claisen rearrangement was a critical step in Ley’s total synthesis of azadirachtin, wherein complex substrate 3 was transformed to product 4 at 180 °C under microwave irradiation.
1. Synthesis of α-Allene Quaternary Centers via Propargyl Claisen Rearrangement.
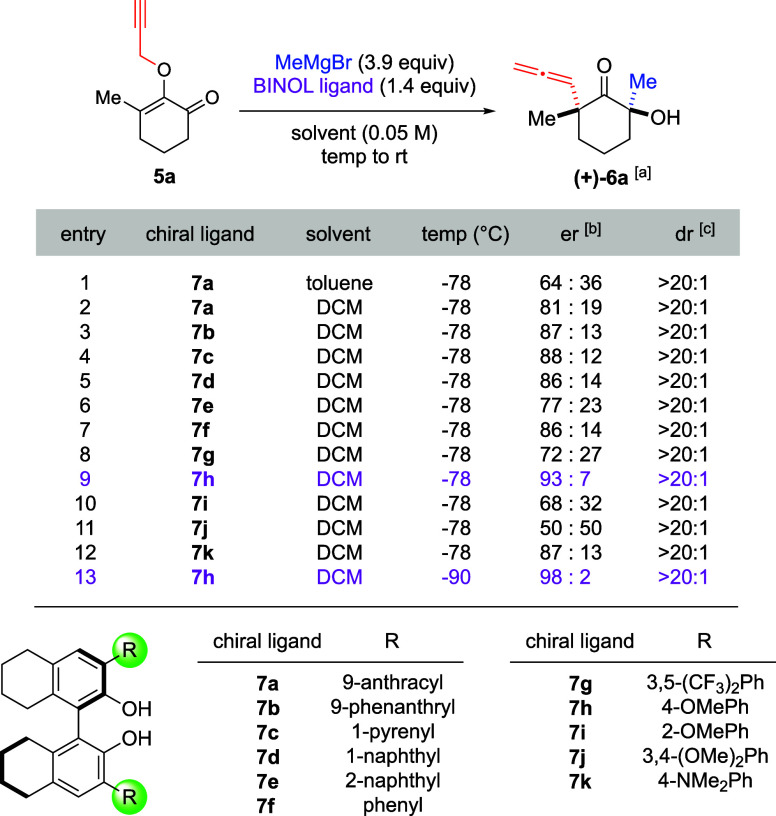
The limited examples of diastereoselective synthesis of α-allene quaternary centers via propargyl Claisen rearrangement prompted us to explore a new strategy with a focus on using simple monocyclic achiral propargyl vinyl ethers, specifically 2-O-propargyl enone 5, as starting materials. We proposed that treatment of substrate 5 with a Grignard reagent would produce a magnesium-chelate intermediate 7 as a product of the 1,2-carbonyl addition reaction. This chelation would then activate the [3,3]-sigmatropic rearrangement, thereby forming the α-allene quaternary centers while also guiding diastereoselectivity. Scheme presents our initial findings. To test our hypothesis, we introduced methylmagnesium bromide to a precooled solution of 2-O-propargyl enone 5a in three different solvents, i.e., THF, Et2O, and DCM. Once the substrate was fully consumed, the reaction mixture was warmed to room temperature, which triggered the propargyl Claisen rearrangement to furnish α-allene ketone 6a. Interestingly, DCM was found to be effective in generating the product in 82% yield as a single diastereomer. We then explored the temperature effects (0 and −78 °C) for the Grignard addition. Although the product yields were comparable, the lower temperature afforded cleaner crude reaction mixtures. Remarkably, the use of a slight excess of the Grignard reagent (1.3 equiv), which necessitated full consumption of substrate 5, did not lead to overaddition to the emerging ketone functionality in 6a.
2. Racemic Synthesis.
a Products were formed with >20:1 diastereomeric ratio based on 1H NMR analysis of the crude reaction mixtures.
b The Grignard addition was conducted at 0 °C.
c The propargyl Claisen rearrangement was performed at reflux.
d The reaction was performed with 1.1 equiv of Grignard reagent.
e The reaction yielded the 1,2-carbonyl addition product.
This simple procedure was found to be applicable across a broad scope of reactions. Our survey began with varying the R substituent in the enone moiety. When treated with methylmagnesium bromide, substrates containing long-chain octyl, branched isobutyl, and benzyl groups formed products 6b to 6d in moderate to good yields (entries 2–4). We then proceeded with aromatic substituents, including phenyl, 4-OMe-phenyl, 4-F-phenyl, and 3-benzothiophene (entries 5–8). In these cases, the resulting α-allene ketones 6e–6h were isolated in 65–75% yields. Notably, certain aromatic rings seemed to affect the propargyl Claisen rearrangement, requiring warming the reaction mixtures to reflux. Continuing with the alkyne substituents, methyl, phenyl, 2-thiophene, and bromo groups were introduced at the Ra position, which decorated the α-allene group. As indicated in entries 9–12, ketones 6i–6l were isolated with 60–83% yields. Lastly, various Grignard reagents were also examined (entries 13–20). Organomagnesium reagents bearing octyl, cyclohexyl, benzyl, and allyl groups were tolerated, furnishing α-allene ketones 6m–6p in high yields. For benzyl-containing product 6o, the propargyl Claisen rearrangement required reflux conditions. We noted differing reactivities between sp2 and sp hybridized nucleophiles. While vinylmagnesium bromide resulted in product 6q in 70% yield, the 1-propynyl counterpart formed 6r with a lower yield. An unexpected outcome was noted when comparing phenyl and 4-F-phenyl. While 4-F-phenylmagnesium bromide formed α-allene ketone 6t in 61% yield, the phenyl variant only yielded the 1,2-carbonyl addition product. In fact, the propargyl Claisen rearrangement did not occur.
The enantioselective version of our method could be achieved by regulating the facial selectivity of the Grignard addition to the 2-O-propargyl enone substrates. In this pursuit, we took inspiration from Nakajima, who reported asymmetric 1,2-carbonyl addition to ketones by combining substituted BINOL ligands with a 3-fold excess of Grignard reagents. For our studies, we opted to evaluate the H8–BINOL variant to simplify the synthesis of ligand libraries, thereby accelerating the optimization process. Results of our reaction optimization are summarized in Table . The pilot experiment involved the reaction of methylmagnesium bromide (3.9 equiv) to substrate 5a in the presence of 9-anthracyl-H8-BINOL ligand 7a (1.4 equiv) at −78 °C. Once the starting material was consumed, the mixtures were allowed to warm to room temperature to initiate the propargyl Claisen rearrangement. We conducted these reactions in two noncoordinating solvents, i.e., toluene and DCM (entries 1 and 2), and found that the use of DCM led to stronger enantioinduction (81:19 er) compared to toluene (64:36 er). Equally significant, the resulting product (+)-6a was formed as a single diastereomer. This promising outcome prompted us to screen various substituents in the H8–BINOL ligands. Other bulky groups, such as 9-phenanthryl 7b and 1-pyrenyl 7c, only marginally improved enantioselectivity (entries 3 and 4). Interestingly, similar trends were noted with smaller substituents in ligands 7d–7f (entries 5–7), with the most intriguing result arising from a simple phenyl group in ligand 7f, which afforded the α-allene ketone with 86:14 er.
1. Reaction Optimization for Enantioselective Synthesis.
Yields were not quantified due to volatility of the products.
Enantiomeric ratio was measured by chiral HPLC of the p-nitrobenzoate ester derivative of the products. See Supporting Information.
Products were formed with >20:1 diastereomeric ratio based on 1H NMR analysis of the crude reaction mixtures.
The surprising efficacy of the phenyl group prompted an evaluation of its stereoelectronic effects. As shown in entries 8 and 9, the inclusion of 3,5-(CF3)2Ph groups in ligand 7g resulted in an erosion of enantioselectivity. In contrast, 4-OMePh in ligand 7h improved er to 93:7. The positioning of the methoxy group within the phenyl ring is crucial. For example, 2-OMePh in ligand 7i (entry 10) dropped enantioselectivity to 68:32. Efforts to enhance enantioinduction by incorporating two methoxy groups at the 3,4-positions in ligand 7j proved ineffective, as the resulting α-allene ketone was formed as a racemic mixture (entry 11). We also examined the 4-NMe2Ph variant, which yielded enantioselectivity comparable to that of the parent ligand 7f (entry 12). Overall, our ligand screening identified PMP-H8-BINOL 7h as the most effective ligand. The enantioselectivity was further enhanced by lowering the reaction temperature to −90 °C. Under these conditions, the α-allene quaternary center in ketone (+)-6a was produced with 98:2 er (entry 13).
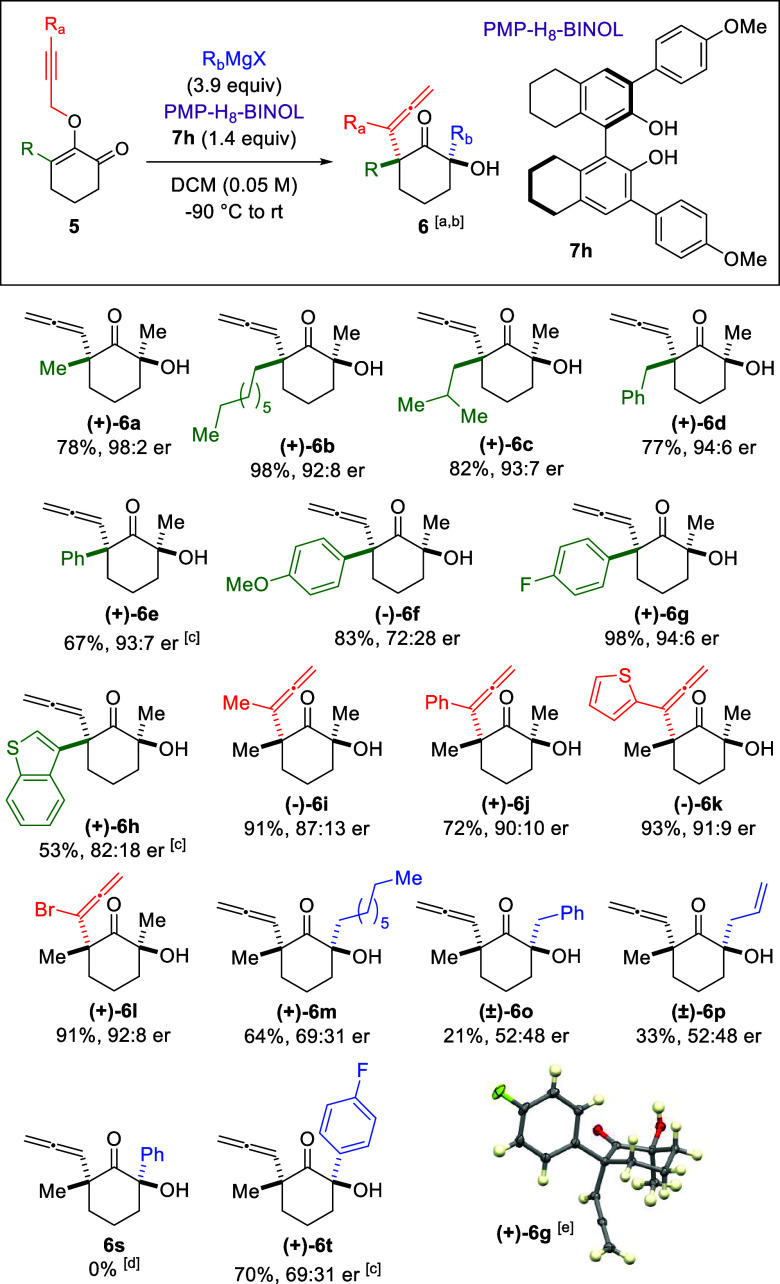
Using these optimized conditions, we surveyed the scope of reaction by first assessing effects of the R substituent on the enone moiety with methylmagnesium bromide as the nucleophile (Scheme ). Substrates featuring octyl and isobutyl groups afforded α-allene ketone (+)-6b and (+)-6c in 98% and 82% yields, respectively, with comparable 92:8 and 93:7 er. Similarly, the benzyl variant yielded product (+)-6d in 77% yield with a 94:6 er. Next, we examined aromatic and heteroaromatic substituents. Except the 4-OMe-phenyl in (−)-6f, which was isolated with 72:28 er, the phenyl (+)-6e, the 4-F-phenyl (+)-6g, and the 3-benzothiophene (+)-6h were obtained with good yields and enantioselectivity. Efforts to place substitutions at the Ra position of the resulting α-allene functionality with methyl, phenyl, 2-thiophene, and bromo were successful. As depicted in α-allene ketones (−)-6i to (+)-6l, these products were isolated in 72–93% yields with er ranging between 87:13 and 92:8. As detailed in Supporting Information, the absolute and relative stereochemistry of these enantiomerically enriched products was deduced by analogy using X-ray crystallography of (+)-6g, (+)-6j, and (+)-6l. Similar to the racemic synthesis, the propargyl Claisen rearrangement for some substrates required reflux conditions to proceed.
3. Enantioselective Synthesis.
a Enantiomeric ratio was measured by chiral HPLC of the p-nitrobenzoate ester derivative of the products. See Supporting Information.
b Products were formed with >20:1 diastereomeric ratio based on 1H NMR analysis of the crude reaction mixtures.
c The propargyl Claisen rearrangement was performed at reflux.
d The reaction only yielded the 1,2-carbonyl addition product.
e The ellipsoid contour was set at a 50% probability level.
While the asymmetric conditions proved effective with methylmagnesium bromide, efforts to employ larger nucleophiles led to a loss in enantioinduction. For instance, octylmagnesium bromide yielded product (+)-6m with 69:31 er. Surprisingly, the benzyl and allyl counterparts formed α-allene ketones (±)-6o and (±)-6p as a racemic mixture in low yields. We also assessed aromatic nucleophiles, such as phenyl and 4-F-phenyl. While phenylmagnesium bromide did not lead to the propargyl Claisen rearrangement, which is consistent with the racemic synthesis (Scheme , entry 19), the 4-F-phenyl adduct (+)-6t was isolated in 70% yield with 69:31 er.
Scheme showcases additional synthetic studies that feature our method. The utility of this magnesium-chelate activation of propargyl Claisen rearrangement within the context of complex molecules was exemplified through the functionalization of formestane-derived substrate (+)-8 with methylmagnesium bromide. As shown in the resulting product (+)-9a, which was isolated in 66% yield as a single diastereomer, the reaction of (+)-8 with 2.6 equiv of the Grignard reagent led to diastereoselective 1,2-carbonyl addition at both C3 and C17 positions, while installing the α-allene group at the C5 carbon. Subjecting the substrate with 1.1 equiv of methylmagnesium bromide remarkably led to methyl addition at the C3 carbonyl, followed by the propargyl Claisen rearrangement to furnish α-allene ketone (−)-9b in 40% yield, which was isolated as a single diastereomer. This intriguing chemoselectivity was most likely the result of the chelation effect between the magnesium metal and the two neighboring oxygen atoms at the C3 and C4 positions, which consequently differentiated the rate of the Grignard addition at the C3 carbonyl compared to that of the C17. Next, we carried out scale-up reactions of substrate 5j on a one-gram quantity using both racemic and enantioselective protocols. As expected, the racemic conditions furnished α-allene ketone 6j in 83% yield with >20:1 dr. The enantioselective version also proceeded smoothly to produce (+)-6j in 93% yield with 94:6 er and >20:1 dr. Significantly, the PMP-H8-BINOL ligand 7h was successfully recovered in pure form with a mass recovery of 91%, thus underscoring the cost-effectiveness and practicality of our method.
4. Additional Synthetic Studies.
The α-allene ketone functionality in product 6j was then subjected to further synthetic elaboration to create stereochemically complex derivatives. For example, carbonyl reduction of 6j with NaBH4 produced stereotriad 10, where the diastereochemical outcome was directed by the hydroxy stereocenter at the opposing α-position. Following this step, intramolecular cyclization catalyzed by AgOTf effectively yielded heterocycle 11. The electron-rich double bond in the resulting ring structure could then be cleaved oxidatively using catalytic RuCl3 and NaIO4 in wet reaction medium, which afforded highly functionalized product 12. Both racemic and enantioenriched substrate (±)-6j and (+)-6j were subjected to this sequence, generating their respective products in comparably high yields. We also subjected our method to heterocyclic substrate motif 13, which formed pyranone (+)-14 as a single diastereomer, albeit with 68:32 er. This enantioinduction is surprisingly lower than that of the cyclohexanone counterpart (+)-6g.
In conclusion, we have developed enantio- and diastereoselective synthesis of α-allene quaternary centers in fully substituted cyclohexanones at the α-positions. Our method featured the reaction of simple 2-O-propargyl enones with a mixture of Grignard reagents and PMP-H8-BINOL ligand, resulting in a cascade sequence of 1,2-carbonyl addition and propargyl Claisen rearrangement. The utility of this chemistry was demonstrated through its synthetic studies in complex-molecule settings.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award no. R01GM127649. Generous financial support from Louisiana State University is gratefully acknowledged. E.A.-G. is a recipient of the LSAMP BD fellowship funded by the National Science Foundation under the award no. HRD-0832999. E.A.-G. is a recipient of the NSF GRFP. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 2136519.
The data underlying this study are available in the published article and its Supporting Information.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.5c02006.
Experimental procedures, characterization data, chiral HPLC chromatograms, X-ray crystallographic data, and 1H and 13NMR spectra for all new compounds (PDF)
‡.
E.A.-G. and A.S. contributed equally.
The authors declare no competing financial interest.
References
- Claisen L.. The rearrangement of phenol-allyl-ather in C-allyl-phenole. Ber. Dtsch. Chem. Ges. 1912;45:3157–3166. doi: 10.1002/cber.19120450348. [DOI] [Google Scholar]
- a Liu Y., Liu X., Feng X.. Recent Advances in Metal-Catalysed Asymmetric Sigmatropic Rearrangements. Chem. Sci. 2022;13:12290–12308. doi: 10.1039/D2SC03806D. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kobzev M. S., Titov A. A., Varlamov A. V.. Synthesis of Heterocyclic Systems Involving [3,3]-Sigmatropic Rearrangements. Russ. Chem. Bull. 2021;70:1213–1259. doi: 10.1007/s11172-021-3208-1. [DOI] [Google Scholar]; c Bilska-Markowska M., Kaźmierczak M., Koroniak H.. Synthesis of γ,δ-Unsaturated Amino Acids by Claisen rearrangement - Last 25 Years. ARKIVOC. 2021;2021:37–72. doi: 10.24820/ark.5550190.p011.335. [DOI] [Google Scholar]; d Castro A. M. M.. Claisen Rearrangement over the Past Nine Decades. Chem. Rev. 2004;104:2939–3002. doi: 10.1021/cr020703u. [DOI] [PubMed] [Google Scholar]; e Lee H., Kim K. T., Kim M., Kim C.. Recent Advances in Catalytic [3,3]-Sigmatropic Rearrangements. Catalysts. 2022;12:227. doi: 10.3390/catal12020227. [DOI] [Google Scholar]
- a Zheng H., Wang Y., Xu C., Xu X., Lin L., Liu X., Feng X.. Stereodivergent Synthesis of Cicinal Quaternary-Quaternary Stereocenters and Bioactive Hyperolactones. Nat. Commun. 2018;9:1968. doi: 10.1038/s41467-018-04123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Uyeda C., Jacobsen E. N.. Enantioselective Claisen Rearrangements with a Hydrogen-Bond Donor Catalyst. J. Am. Chem. Soc. 2008;130:9228–9229. doi: 10.1021/ja803370x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li C. X., Ragab S. S., Liu G. D., Tang W. J.. Enantioselective Formation of Quaternary Carbon Stereocenters in Natural Product Synthesis: A Recent Update. Nat. Prod. Rep. 2020;37:276–292. doi: 10.1039/C9NP00039A. [DOI] [PubMed] [Google Scholar]; b Zeng X. P., Cao Z. Y., Wang Y. H., Zhou F., Zhou J.. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016;116:7330–7396. doi: 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]; c Liu Y. Y., Han S. J., Liu W. B., Stoltz B. M.. Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res. 2015;48:740–751. doi: 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Quasdorf K. W., Overman L. E.. Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocentres. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Peterson E. A., Overman L. E.. Contiguous Stereogenic Quaternary Carbons: A Daunting Challenge in Natural Products Synthesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11943–11948. doi: 10.1073/pnas.0402416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Black D. K., Landor S. R.. 1256. Allenes. Part X. The Claisen–Cope Rearrangement of Propargyl Vinyl Systems. J. Chem. Soc. 1965;0:6784–6788. doi: 10.1039/JR9650006784. [DOI] [Google Scholar]; b Saucy G., Marbet R.. Über eine neuartige Synthese von β-Ketoallenen durch Reaktion von tertiären Acetylencarbinolen mit Vinyläthern eine ergiebige methode zur darstellung des Pseudojonons und verwandter verbindungen. Helv. Chim. Acta. 1967;50:1158–1167. doi: 10.1002/hlca.19670500423. [DOI] [Google Scholar]; c Tejedor D., Méndez-Abt G., Cotos L., García-Tellado F.. Propargyl Claisen Rearrangement: Allene Synthesis and Beyond. Chem. Soc. Rev. 2013;42:458–471. doi: 10.1039/C2CS35311C. [DOI] [PubMed] [Google Scholar]
- a Brummond K. M., DeForrest J. E.. Synthesizing Allenes Today (1982–2006) Synthesis. 2007;2007:795–818. doi: 10.1055/s-2007-965963. [DOI] [Google Scholar]; b Yu S., Ma S.. How Easy are the Syntheses of Allenes? Chem. Commun. 2011;47:5384–5418. doi: 10.1039/C0CC05640E. [DOI] [PubMed] [Google Scholar]; c Huang X., Ma S.. Allenation of Terminal Alkynes with Aldehydes and Ketones. Acc. Chem. Res. 2019;52:1301–1312. doi: 10.1021/acs.accounts.9b00023. [DOI] [PubMed] [Google Scholar]; d Li S., Yuan K., Zhang G., Guo R.. Recent Advances in the Synthesis of Chiral Allenes via Asymmetric 1,4-Difunctionalization of 1,3-Enynes. Eur. J. Org. Chem. 2024;27:e202301316. doi: 10.1002/ejoc.202301316. [DOI] [Google Scholar]; e Singh J., Saxena B., Sharma A.. Visible Light Promoted Synthesis of Allenes. Catal. Sci. Technol. 2024;14:5143–5160. doi: 10.1039/D4CY00361F. [DOI] [Google Scholar]; f Li Y., Bao H.. Radical Transformations for Allene Synthesis. Chem. Sci. 2022;13:8491–8506. doi: 10.1039/D2SC02573F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yu S., Ma S.. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem., Int. Ed. 2012;51:3074–3112. doi: 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]; b Krause N., Winter C.. Gold-Catalyzed Nucleophilic Cyclization of Functionalized Allenes: A Powerful Access to Carbo- and Heterocycles. Chem. Rev. 2011;111:1994–2009. doi: 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]; c Deng S.-M., Zhao Y.-X., Wang C.. When Transition-Metal-Catalyzed C–H Activation Meets Allene Chemistry. Tetrahedron Chem. 2023;8:100049. doi: 10.1016/j.tchem.2023.100049. [DOI] [Google Scholar]; d Jamwal P., Vaid H., Ananda Rao G., Gurubrahamam R., Chen K.. Asymmetric Organocatalytic Reactions of Activated Racemic Allenes. Asian J. Org. Chem. 2022;11:e202200622. doi: 10.1002/ajoc.202200622. [DOI] [Google Scholar]; e Singh J., Sharma A., Sharma A.. Visible Light Mediated Functionalization of Allenes. Org. Chem. Front. 2021;8:5651–5667. doi: 10.1039/D1QO00955A. [DOI] [Google Scholar]; f Ye J., Ma S.. Palladium-Catalyzed Cyclization Reactions of Allenes in the Presence of Unsaturated Carbon–Carbon Bonds. Acc. Chem. Res. 2014;47:989–1000. doi: 10.1021/ar4002069. [DOI] [PubMed] [Google Scholar]; g Muñoz M. P.. Silver and Platinum-Catalysed Addition of O–H and N–H bonds to Allenes. Chem. 2014;43:3164–3183. doi: 10.1039/c3cs60408j. [DOI] [PubMed] [Google Scholar]
- a Liu Y., Hu H., Zheng H., Xia Y., Liu X., Lin L., Feng X.. Nickel(II)-Catalyzed Asymmetric Propargyl and Allyl Claisen Rearrangements to Allenyl- and Allyl-Substituted β-Ketoesters. Angew. Chem., Int. Ed. 2014;53:11579–11582. doi: 10.1002/anie.201404643. [DOI] [PubMed] [Google Scholar]; b Wang Y.-P., Zhang X.-P., Xie M.-S., Guo H.-M.. Cobalt(II)-Catalyzed Enantioselective Propargyl Claisen Rearrangement: Access to Allenyl-Substituted Quaternary β-Ketoesters. Org. Lett. 2023;25:7105–7109. doi: 10.1021/acs.orglett.3c02496. [DOI] [PubMed] [Google Scholar]; c Cao T., Deitch J., Linton E. C., Kozlowski M. C.. Asymmetric Synthesis of Allenyl Oxindoles and Spirooxindoles by a Catalytic Enantioselective Saucy–Marbet Claisen Rearrangement. Angew. Chem., Int. Ed. 2012;51:2448–2451. doi: 10.1002/anie.201107417. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cao T., Linton E. C., Deitch J., Berritt S., Kozlowski M. C.. Copper(II)- and Palladium(II)-Catalyzed Enantioselective Claisen Rearrangement of Allyloxy- and Propargyloxy-Indoles to Quaternary Oxindoles and Spirocyclic Lactones. J. Org. Chem. 2012;77:11034–11055. doi: 10.1021/jo302039n. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu Y., Liu X., Hu H., Guo J., Xia Y., Lin L., Feng X.. Synergistic Kinetic Resolution and Asymmetric Propargyl Claisen Rearrangement for the Synthesis of Chiral Allenes. Angew. Chem., Int. Ed. 2016;55:4054–4058. doi: 10.1002/anie.201511776. [DOI] [PubMed] [Google Scholar]; f Lai Z.-W., Liu C., Sun H., You S.-L.. Asymmetric Synthesis of 3-Allyloxindoles and 3-Allenyloxindoles by Scandium(III)-Catalyzed Claisen Rearrangement Reactions. Chin. J. Chem. 2017;35:1512–1516. doi: 10.1002/cjoc.201700486. [DOI] [Google Scholar]; g Wang L., Zhou Y., Su Z., Zhang F., Cao W., Liu X., Feng X.. [3,3]-Sigmatropic Rearrangements of Naphthyl 1-Propargyl Ethers: para-Propargylation and Catalytic Asymmetric Dearomatization. Angew. Chem., Int. Ed. 2022;61:e202211785. doi: 10.1002/anie.202211785. [DOI] [PubMed] [Google Scholar]
- a Durand-Reville T., Gobbi L. B., Gray B. L., Ley S. V., Scott J. S.. Highly Selective Entry to the Azadirachtin Skeleton via a Claisen Rearrangement/Radical Cyclization Sequence. Org. Lett. 2002;4:3847–3850. doi: 10.1021/ol0201557. [DOI] [PubMed] [Google Scholar]; b Matoba H., Watanabe T., Nagatomo M., Inoue M.. Convergent Synthesis of Taxol Skeleton via Decarbonylative Radical Coupling Reaction. Org. Lett. 2018;20:7554–7557. doi: 10.1021/acs.orglett.8b03302. [DOI] [PubMed] [Google Scholar]; c Jeker O. F., Carreira E. M.. Total Synthesis and Stereochemical Reassignment of (±)-Indoxamycin B. Angew. Chem., Int. Ed. 2012;51:3474–3477. doi: 10.1002/anie.201109175. [DOI] [PubMed] [Google Scholar]; d Ley S. V., Abad-Somovilla A., Anderson J. C., Ayats C., Bänteli R., Beckmann E., Boyer A., Brasca M. G., Brice A., Broughton H. B., Burke B. J., Cleator E., Craig D., Denholm A. A., Denton R. M., Durand-Reville T., Gobbi L. B., Göbel M., Gray B. L., Grossmann R. B., Gutteridge C. E., Hahn N., Harding S. L., Jennens D. C., Jennens L., Lovell P. J., Lovell H. J., de la Puente M. L., Kolb H. C., Koot W.-J., Maslen S. L., McCusker C. F., Mattes A., Pape A. R., Pinto A., Santafianos D., Scott J. S., Smith S. C., Somers A. Q., Spilling C. D., Stelzer F., Toogood P. L., Turner R. M., Veitch G. E., Wood A., Zumbrunn C.. The Synthesis of Azadirachtin: A Potent Insect Antifeedant. Chem. Eur. J. 2008;14:10683–10704. doi: 10.1002/chem.200801103. [DOI] [PubMed] [Google Scholar]; e Veitch G. E., Beckmann E., Burke B. J., Boyer A., Maslen S. L., Ley S. V.. Synthesis of Azadirachtin: A Long but Successful Journey. Angew. Chem., Int. Ed. 2007;46:7629–7632. doi: 10.1002/anie.200703027. [DOI] [PubMed] [Google Scholar]; f Watanabe H., Mori N., Itoh D., Kitahara T., Mori K.. Synthetic Study Towards Azadirachtin: An Efficient and Stereoselective Construction of the AB Rings with Full Functionality. Angew. Chem., Int. Ed. 2007;46:1512–1516. doi: 10.1002/anie.200604097. [DOI] [PubMed] [Google Scholar]; g Ley S. V., Gutteridge C. E., Pape A. R., Spilling C. D., Zumbrunn C.. Chemistry of Insect Antifeedants from Azadirachta indica (Part 22): Functionalisation of the Decalin Fragment of Azadirachtin via a Claisen Rearrangement Reaction. Synlett. 1999;1999:1295–1297. doi: 10.1055/s-1999-2802. [DOI] [Google Scholar]
- a Osakama K., Nakajima M.. Asymmetric Direct 1,2-Addition of Aryl Grignard Reagents to Aryl Alkyl Ketones. Org. Lett. 2016;18:236–239. doi: 10.1021/acs.orglett.5b03379. [DOI] [PubMed] [Google Scholar]; b Monasterolo C., O’Gara R., Kavanagh S. E., Byrne S. E., Bieszczad B., Murray O., Wiesinger M., Lynch R. A., Nikitin K., Gilheany D. G.. Asymmetric Addition of Grignard Reagents to Ketones: Culmination of the Ligand-Mediated Methodology Allows Modular Construction of Chiral Tertiary Alcohols. Chem. Sci. 2022;13:6262–6269. doi: 10.1039/D1SC06350B. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Collados J. F., Solà R., Harutyunyan S. R., Maciá B.. Catalytic Synthesis of Enantiopure Chiral Alcohols via Addition of Grignard Reagents to Carbonyl Compounds. ACS Catal. 2016;6:1952–1970. doi: 10.1021/acscatal.5b02832. [DOI] [Google Scholar]; d Luderer M. R., Bailey W. F., Luderer M. R., Fair J. D., Dancer R. J., Sommer M. B.. Asymmetric Addition of Achiral Organomagnesium Reagents or Organolithiums to Achiral Aldehydes or Ketones: A Review. Tetrahedron: Asymmetry. 2009;20:981–998. doi: 10.1016/j.tetasy.2009.03.015. [DOI] [Google Scholar]; e Riant O., Hannedouche J.. Asymmetric Catalysis for the Construction of Quaternary Carbon Centres: Nucleophilic Addition on Ketones and Ketimines. Org. Biomol. Chem. 2007;5:873–888. doi: 10.1039/b617746h. [DOI] [PubMed] [Google Scholar]
- a Interestingly, vinyl- or 1-propynylmagnesium bromide did not react with 2-O-propargyl enone 5a under the enantioselective conditions.; b Unsubstituted 2-O-propargyl enone 5 (R and Ra = H) proved to be an ineffective substrate for this method. When treated with methylmagnesium bromide under both racemic and asymmetric conditions, the reactions resulted in complex mixtures, affording the corresponding propargyl Claisen rearrangement product in poor yields.
- Carlsen P. H. J., Katsuki T., Martin V. S., Sharpless K. B.. A Greatly Improved Procedure for Ruthenium Tetroxide Catalyzed Oxidations of Organic compounds. J. Org. Chem. 1981;46:3936–3938. doi: 10.1021/jo00332a045. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.