Abstract
Background
Haematophagous Diptera can transmit a wide range of diseases to both humans and animals. Some species of the Trypanosoma genus rely on these vectors for transmission, either cyclically or mechanically. Trypanosoma evansi, the causative agent of Surra, is the only African-origin trypanosome species detected in Spain to date, which is mechanically transmitted.
Methods
To assess the occurrence and distribution of potential mechanical vectors at the national level, a systematic review was conducted on the Hippoboscidae, Muscidae and Tabanidae families. The review followed the methodology established by the Food and Agriculture Organization of the United Nations (FAO) and adhered to PRISMA guidelines. Data were compiled from 43 peer-reviewed scientific publications and four citizen science digital databases.
Results
The review identified three genera belonging to the Hippoboscidae, two of the Muscidae and ten of the Tabanidae families. Genus-level distribution maps were generated for each group.
Conclusions
This atlas serves as a valuable tool for the prevention and control of vector-borne animal trypanosomosis in Spain. Nonetheless, further studies on the distribution, ecology and behaviour of haematophagous dipterans are essential to better understand their role in disease transmission and their potential impact on future outbreaks.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-025-06922-9.
Keywords: Diptera, Surra, Maps, Muscidae, Hippoboscidae, Tabanidae, Citizen science databases, Atlas
Background
Haematophagous arthropods play a critical role in both human and animal health [69]. Their blood-feeding behaviour can cause direct harm, including irritation, immune reactions and localised trauma. Indirectly, they act as vectors for a wide range of infectious agents, facilitating both mechanical and biological (i.e. cyclical) transmission [87]. Among these pathogens, protozoans of the genus Trypanosoma (Gruby, 1843; Kinetoplastida: Trypanosomatidae) are of particular concern owing to their impact on veterinary and human medicine.
Animal trypanosomosis, transmitted by vectors and manifesting as either Nagana and Surra, poses a significant barrier to livestock production across many African regions. Nagana is caused by Trypanosoma brucei (Plimmer & Bradford, 1899), Trypanosoma vivax (Ziemann, 1905) and Trypanosoma congolense (Broden, 1904), while Trypanosoma evansi (Chauvrat, 1896) causes Surra [17]. Unlike other trypanosomes, T. evansi does not need a biological vector and is instead transmitted mechanically by haematophagous insects [18, 72]. However, the parasite’s limited survival time within the insect’s mouthparts constrains transmission efficiency.
Haematophagous dipterans are the most relevant vectors of T. evansi, owing to their high mobility and frequent feeding patterns [18, 67, 87]. Several studies highlighted the role of different insect taxa in the transmission of Surra to mammals, including biting flies of the tribe Stomoxyini (Diptera: Muscidae) (Stomoxys spp. and Haematobia spp.), tabanids (Diptera: Tabanidae) (Tabanus spp., Atylotus spp. and Chrysops spp., among others) and louse flies (Diptera: Hippoboscidae) (Hippobosca spp., Lipoptena spp. and Melophagus spp.) [20].
Surra is mainly found in North Africa, Northeast Africa, Latin America, the Middle East and Asia. However, outbreaks have also been reported in Europe, including France (Aveyron Department) and Spain. In the latter country, cases were recorded in the province of Alicante (mainland Spain), linked to the movement of infected animals from the Canary Islands [15, 82]. The first confirmed case of Surra in Spain occurred in 1997 on the Island of Gran Canaria [46], which led to subsequent studies to assess the parasite’s prevalence in dromedaries and other livestock, as well as the potential role of rodents and haematophagous insects in disease maintenance [60, 73–75].
Although no new seropositive cases of Surra have been reported in the Canary Islands since 2022 [57, 83], ongoing surveillance and research into disease dynamics remain essential to prevent future outbreaks. This is particularly relevant, as Spain may act as an entry point for the disease into mainland Europe. In this context, the current study aims to compile a comprehensive atlas of the distribution of potential T. evansi vectors across Spain, within the framework of the COMBAT project (COntrolling and progressively Minimizing the Burden of Animal Trypanosomosis) [8]. This work complements the recently published national atlas of Surra in the country [57].
Methods
Search protocol and selection criteria
A systematic review was carried out in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [59], and following the methodology established by the Food and Agriculture Organization of the United Nations (FAO) for continental and national atlases of Glossina spp. and African trypanosomes [1, 11–14, 21, 42, 65, 71, 80]. In addition, scientific repositories and search engines, including PubMed®, Scopus, Google Scholar and ResearchGate were used, without date restrictions. The collection and analysis period of the data from these platforms extended from February to December 2024.
The review focused on insect taxa identified as potential mechanical vectors of T. evansi, with particular attention on the families Muscidae, Hippoboscidae and Tabanidae [15, 17–19, 56, 75, 82]. Genera within each family were selected on the basis of prior literature, including Stomoxys and Haematobia for Muscidae [17, 18], and mammophilic genera for Hippoboscidae (Hippobosca, Lipoptena, and Melophagus) [84]. For the Tabanidae, the classification followed the nomenclature proposed by Portillo (2002) for horsefly in Spain, encompassing Atylotus, Chrysops, Dasyrhamphis, Haematopota, Hybomitra, Nemorius, Pangonius, Philipomyia, Silvius, and Tabanus.
Keyword searches were conducted in Spanish, French and English, using terms such as ‘Diptera’, ‘Spain’, ‘Canary Islands’, ‘Balearic Islands’, ‘mainland Spain’, ‘Haematophagous’, ‘Muscidae’, ‘Hippoboscidae’, ‘Tabanidae’, ‘Stomoxyini’, ‘Stomoxys’, ‘Haematobia’, ‘Lipoptena’, ‘Hippobosca’, ‘Melophagus’, ‘Tabanus’, ‘Haematopota’, ‘Dasyrhamphis”, ‘Atylotus”, ‘Pangonius’, ‘Silvius’, ‘Chrysops’, ‘Hybomitra’, ‘Nemorius’, ‘Philipomyia’, ‘Trypanosoma evansi’ and ‘Surra’. Approximately less than 10% of the studies identified were excluded owing to access restrictions, often related to publication age or paywalls. However, some were retained indirectly through citations in other included sources.
Citizen science platforms
Data from citizen science platforms were obtained from four sources: iNaturalist (Nugent, 2018), GBIF.org, Biodiversidad Virtual (https://www.biodiversidadvirtual.org) and the Biodiversity Data Bank of the Canary Islands (BDBC) (https://www.biodiversidadcanarias.es/biota). Searches were conducted using insect genera or species keywords, and results were filtered by country (last accessed: 11 March 2025). These platforms primarily provide presence-only records, often accompanied by precise geographical coordinates, occurrence dates and photographic evidence.
Only records validated by experts were retained. When expert validation was unavailable, all submitted images were manually reviewed. Duplicate entries across platforms were cross-checked and excluded.
Digital repository and database
The literature retrieved through the systematic review was compiled in a digital repository, and the following information were extracted regarding the occurrence of target insect taxa:
Sources: Full reference details of each document, including authors, title, date and journal.
Site geographical data (Geo_Data_Site): Sampling locations reported in the documents, georeferenced using Google Earth or Spanish National Geographic System when not explicitly stated. Location accuracy varied, from specific coordinates to approximate central points of municipalities. For broad designations (e.g., nature reserves), the centroid of the defined area was used.
Site entomological data (Entomo_Data_Site): Descriptions of insect findings at each site, including collection date, taxonomic identification (family, genus, species, subspecies), number of individuals, trap types and survey duration.
Trap geographical data (Geo_Data_Trap): Exact coordinates of sampling sites when such detail was available in the source publications.
Trap entomological data (Entomo_Data_Trap): Entomological data associated with specific trap sites, mirroring the structure of Entomo_Data_Site but with finer spatial resolution.
Mapping
Genus-level occurrence maps for each insect family were generated using QGIS Geographic Information System (version 3.34.11, QGIS Association). The data presented on these maps were separated into two categories: literature-based records and citizen science records. To harmonise spatial reporting, particularly for entries derived from broader administrative units, the number of genera recorded per province was also indicated on the maps.
Results
This study analysed 105 species across 15 genera and three families, on the basis of a total of 2709 data records published between 1951 and 2024 throughout Spain. From the literature, 43 sources were consulted (40 scientific publications, two books and one doctoral thesis) (see Additional file 1: text S1 for more information), yielding 1088 presence records. From citizen science platforms, 1621 presence records were included. Although GBIF.org aggregates records from iNaturalist and Biodiversidad Virtual, 65% originated from Biodiversidad Virtual, 20% from iNaturalist and 15% from GBIF. Biodiversity Data Bank of the Canary Islands (BDBC) records were limited to the Canary Islands and lacked specific coordinates.
A total of 323 locations were georeferenced from the literature: 23 at the trap level or exact capture site, and 300 at broader spatial resolutions. In contrast, 90% of citizen science records were georeferenced with precise coordinates.
Some literature sources included quantitative trapping data. The total number of captured or observed insects during the study period was 20,076 individuals. Of the 1088 literature-based records, 18,455 insects were quantified: 86.5% belonged to the Muscidae family, 13.3% to the Tabanidae family and 0.2% to the Hippoboscidae. Each citizen science record was assumed to represent a single observed individual, totalling 1621 insects, of which 64.8% were Tabanidae, 19.2% Muscidae and 16% Hippoboscidae.
Table 1 summarises the families and genera included in the study, indicating the number of species per genus, number of provinces in which each genus was recorded, host species and trapping methods cited in the literature. Species-level details can be found in Additional file 2: Table S1.
Table 1.
Summary of the blood-feeding dipteran families analysed in Spain (from 1951 to 2024). N: number; ND: not described
| Family | Genus | Species (N) | Provinces (N) | Cited hosts | Cited capture methods |
|---|---|---|---|---|---|
| Hippoboscidae | Hippobosca Linnaeus, 1758 | 2 | 44 | Equids, bovids, canids, felids, human | Trap (Sticky), sweep |
| Lipoptena Nitzsch, 1818 | 3 | 16 | Cervids, human | Trap (Suction) | |
| Melophagus Latreille, 1802 | 1 | 6 | Ovis aries, cervids | ND | |
| Muscidae | Stomoxys Geoffroy, 1762 | 1 | 44 | Equids, ruminants, camelids, human | Trap (Sticky, suction, BG-sentinel, Nzi), sweep |
| Haematobia Lepeletier & Serville, 1828 | 2 | 14 | Bovids, human | Trap (Sticky, suction, CDC-Miniature light trap) | |
| Tabanidae | Atylotus Osten-Sacken, 1876 | 9 | 20 | Equids, bovids | Trap (Canopy, Malaise), sweep |
| Chrysops Meigen, 1803 | 7 | 40 | Equids, bovids, cervids | Trap (Canopy, Malaise), sweep | |
| Dasyrhamphis Enderlein, 1922 | 3 | 25 | Equids | Sweep | |
| Haematopota Meigen, 1803 | 12 | 38 | Equids, bovids | Trap (Canopy, Malaise), sweep | |
| Hybomitra Enderlein, 1922 | 12 | 23 | Equids, bovids | Trap (Malaise, H-trap), sweep | |
| Nemorius Rondani, 1856 | 1 | 3 | Equids, bovids | ND | |
| Pangonius Latreille, 1802 | 14 | 30 | Equids, human | Sweep | |
| Philipomyia Olsufjev, 1964 | 2 | 21 | Equids, bovids | Trap (Canopy, Malaise), sweep | |
| Silvius Meigen, 1820 | 3 | 8 | Equids, bovids | Trap (Malaise) | |
| Tabanus Linnaeus, 1758 | 33 | 44 | Equids, bovids, human | Trap (Canopy, Malaise, Sticky, H-trap), sweep |
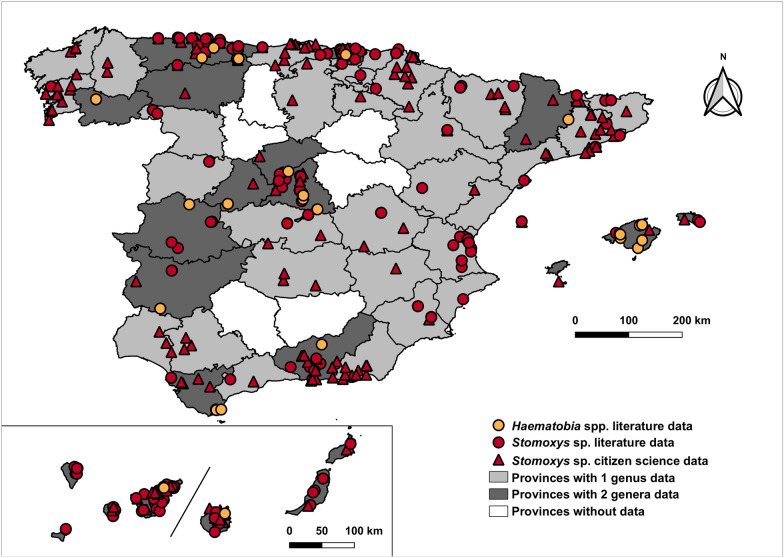
Hippoboscidae family
Three mammophilic genera were reviewed within the Hippoboscidae family (Fig. 1). The Hippobosca genus showed the highest number of observations, primarily in northern, north-eastern, central and central-eastern and southern mainland Spain, with additional sightings in both Canary Islands provinces, and in the Balearic Islands.
Fig. 1.
Geographic distribution of Hippoboscidae family in Spain (1951–2024). The map includes mainland Spain (centre), the Balearic Islands (right) and the Canary Islands (bottom left)
The Lipoptena genus was mostly observed in the northern, north-eastern and south-western mainland Spain, with no records from the Canary or the Balearic islands.
Finally, the Melophagus genus was represented by a few isolated in northern, central and central-western mainland Spain, as well as the western Canary Islands.
Muscidae family
Two genera were reviewed within the Muscidae family (Fig. 2). Sightings of the Stomoxys genus were distributed across most of Spain, especially in the northern, southern and central regions of mainland regions. In addition, all islands of the Balearic and Canary archipelagos reported sightings. Observations of the genus Haematobia were concentrated in the northern, central and southern mainland, as well as the Balearic Islands, with a smaller number of reports from the Canary Islands.
Fig. 2.
Geographic distribution of Muscidae family in Spain (1951–2024). The map shows mainland Spain (centre), the Balearic Islands (right) and the Canary Islands (bottom left)
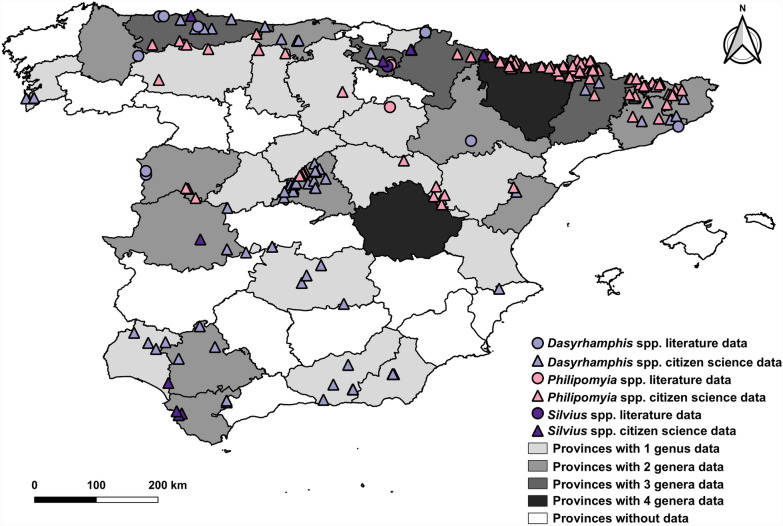
Tabanidae family
A total of ten genera within the Tabanidae family were studied. Figure 3 shows the occurrence for four of the genera: Dasyrhamphis, Philipomyia, Silvius and Nemorius. The Dasyrhamphis genus was found mainly in central, southern and north-western mainland Spain, whereas the Philipomyia genus was predominantly observed in north-eastern mainland Spain. The Silvius genus was limited to a few sightings in northern and southern mainland Spain. Finally, no specific coordinates were available for Nemorius genus; however, its presence was reported in central and northern mainland Spain.
Fig. 3.
Geographic distribution of the genera Dasyrhamphis, Philipomyia, Silvius and Nemorius in Spain (1951–2024). The map includes mainland Spain (centre) and the Balearic Islands (right)
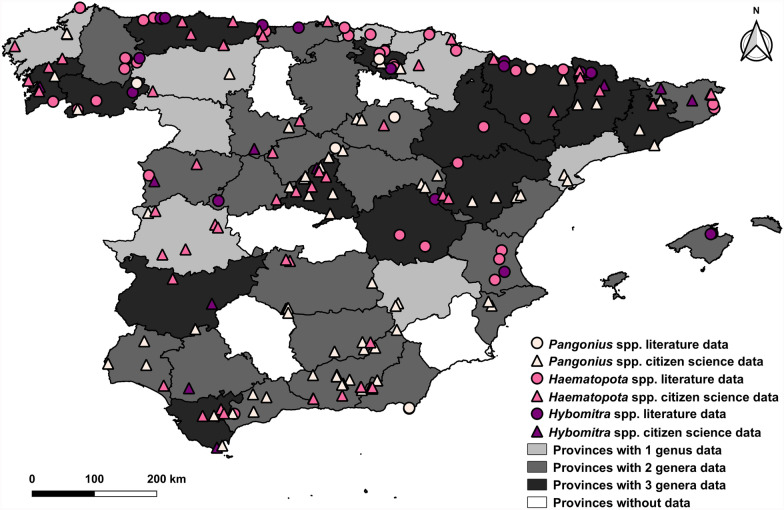
The sightings of the Pangonius, Haematopota and Hybomitra genera are shown in Fig. 4. For Pangonius, sightings are distributed in the central, southern and north-eastern regions of mainland Spain. For Haematopota and Hybomitra, sightings were recorded both in mainland Spain (especially in the north and centre) and in the Balearic Islands.
Fig. 4.
Geographic distribution of the genera Pangonius, Haematopota and Hybomitra in Spain (1951–2024). The map includes mainland Spain (centre) and the Balearic Islands (right)
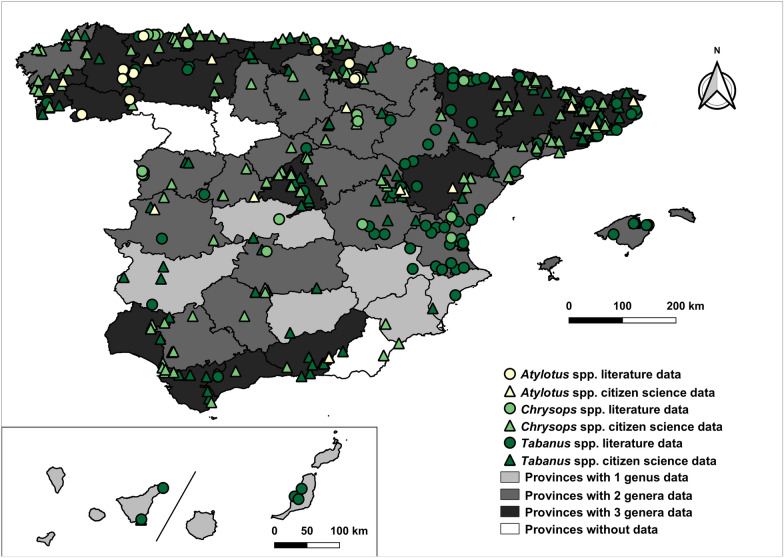
Finally, Fig. 5 shows records for the Atylotus, Chrysops and Tabanus genera. The genus Atylotus was mostly observed in northern mainland Spain, though unspecified records exist for the Balearic Islands. The genus Chrysops was mostly restricted to central and northern mainland Spain. The genus Tabanus was reported throughout Spain, including the Canary and Balearic Islands, with notable concentrations in the north-east and central mainland.
Fig. 5.
Geographic distribution of the genera Atylotus, Chrysops and Tabanus in Spain (1951–2024). The map includes mainland Spain (centre), the Balearic Islands (right) and the Canary Islands (bottom left)
Discussion
This study, developed within the framework of the COMBAT project [8], represents the first comprehensive compilation of information on the potential vectors of Surra in Spain. Previous national atlases on trypanosome control and its vectors in African countries typically included both a tsetse component and an animal infection component [1, 21, 42, 65, 71]; however, this study focused exclusively on the entomological component, specifically on the three different dipteran families that can potentially transmit Surra in Spain. The Surra atlas for Spain has already been published [57], and to date, no vectors belonging to the Glossina genus have been reported in the country.
A notable gap in the literature relating vector presence to Surra in Spain became evident during the compilation of information sources. The present data reveal a high diversity of horsefly genera in the country, particularly in mainland Spain. Among these, the Tabanus genus stands out for its species richness and high abundance, and is the only genus found across mainland Spain, the Balearic Islands and the Canary Islands. Likewise, the Hippobosca genus (especially Hippobosca equina Linnaeus, 1758), Melophagus genus (Melophagus ovinus (Linnaeus, 1758)) and Stomoxys genus (Stomoxys calcitrans (Linnaeus, 1758)) are generally distributed throughout Spain. Among these, S. calcitrans is particularly abundant and found in diverse biotopes and environmental conditions. However, while the literature describes the distribution of M. ovinus as widespread, there is a notable lack of georeferenced sightings. It is also worth noting that only one record exists for the genus Haematobia (Haematobia titilans (Bezzi, 1907)) in the Canary Islands, with no subsequent observations to date.
Given the wide distribution of all the genera recorded in this study, potential re-emergent Surra outbreaks in Spain would likely encounter an abundance of competent mechanical vectors, as seen in past episodes in the Canary Islands and Alicante [82, 83]. In such scenarios, Surra could not only spread across Spain, but also pose a threat to continental Europe, as demonstrated by the 2008 outbreak in Aveyron (France), linked to the transport of infected dromedaries from the Canary Islands [15].
In addition to their potential role Surra transmission, bites from certain haematophagous flies pose an occupational hazard and a considerable nuisance in rural areas of Europe [47, 54]. The study of these brachycerans flies in Spain has been far less extensive than that of nematoceran vectors, such as mosquitoes, sandflies and biting midges [9, 29] owing to their limited public health impact in urban settings and the lack of standardised trapping methods [77]. Another key factor is that these brachycerans rely on less refined capture methods than nematocerans do [48, 78, 85]. This methodology gap warrants further exploration to enhance our understanding of these vectors [2, 58]. For example, it would be necessary to standardise and optimise trapping methods for each of the families, especially in the case of louse flies, which generally require direct host contact for capture. In this context, carbon dioxide traps have recently proven effective for monitoring Lipoptena species in Spain [44]. For tabanids, comparative studies are underway to evaluate existing trap types and explore modifications for enhanced performance [48], also serving as mechanical control strategies. In North America, horseflies are often sampled using large open-style traps equipped with shiny black spherical targets, such as Malaise, Canopy, Box, Greenhead, Manitoba and Epps traps [7]. In Spain, tabanids were successfully collected using custom-made Canopy traps, sweep nets and Malaise traps [43].
Notably, the genera included in this atlas do not reflect the full diversity of haematophagous flies in Spain. Furthermore, the absence of data from certain regions does not necessarily imply species absence. For example, within other blood-feeding Hippoboscidae within the Ornithomyinae subfamily, primarily parasitised birds, are not included here owing to their limited relevance for Surra transmission.
This review highlights that some genera could act as potential vectors not only for Surra, but for a broad range of other diseases. For instance, the Stomoxys genus, and other muscids have been implicated in the transmission of salmonellosis, shigellosis, bacillary dysentery and even aspergillosis [5]. Stomoxys species are considered potential vectors of anthrax, a role they share with horseflies such as Chrysops spp. and Tabanus spp., both of which have been associated with tularemia transmission [6, 86]. Other studies highlight the role of the Stomoxys in the mechanical transmission of viruses (e.g., equine infectious anaemia virus, African swine fever virus, West Nile virus or Rift Valley virus), protozoa (Besnoitia besnoiti; Besnoit and Robin, 1912) and helminths (Habronema microstoma; Schneider, 1866) [5], facilitated by their frequent and persistent feeding behaviour [22]. Similarly, Hippoboscidae (Hippobosca, Melophagus and Lipoptena), have been linked to viruses (e.g., border disease virus or bluetongue virus) and bacteria (Rickettsia spp., Borrelia spp., Bartonella spp. or Corynebacterium pseudotuberculosis) [23].
The distribution maps presented in this study are subject to several limitations. They are based on local studies and citizen science data, with heterogeneous sampling efforts and methodologies. This reliance introduces potential biases and may not reflect current distributions or ecological dynamics. In addition, while citizen science data are valuable, they are not always consistent in terms of quality and geographic coverage. These issues underscore the urgent need for standardised, large-scale field surveys to produce more robust and updated distribution data. New surveys at the meso- and microscales could provide crucial insights into species ecology and interactions with potential hosts. Citizen science platforms offer significant potential for this purpose, especially those with strong expert participation and photographic validation, as demonstrated by apps already implemented in Spain [45, 62]. Advances in artificial intelligence for insects recognition also offer exciting prospects for integrating automated data processing, whether by scientists or citizens [26, 50, 70]. Ultimately, cooperation between researchers, citizens, public health authorities and stakeholders could foster a more holistic and integrated vector surveillance and control programs in Spain and beyond.
Conclusions
This work represents a valuable contribution to the understanding of the epidemiology of T. evansi in Spain, as it is the first to consolidate and integrate information on the presence of its potential dipteran vectors across the country. Documenting the occurrence of these vector enables health authorities to implement more effective control and prevention strategies, as well as to respond promptly in the event of new outbreaks. Despite the findings presented, additional studies are needed to further characterise the distribution of these vectors throughout the territory. In addition, the integration of citizen science platforms as a complementary tool for scientific research, significantly enhancing our understanding and surveillance of vector populations in Spain.
Supplementary Information
Acknowledgements
The authors would like to thank the members of the participating institutions who are contributing to the ongoing implementation of this work. Particularly the Food and Agriculture Organization of the United Nations (FAO), which contributed to the paper and participates in the COMBAT project in the framework of the Programme Against African Trypanosomosis (PAAT).
Abbreviations
- COMBAT
COntrolling and progressively minimizing the burden of animal trypanosomosis
- FAO
Food and Agriculture Organization of the United Nations
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
Author contributions
Conceptualisation: M.T.T.J., J.A.C. and G.C.; Data curation: A.M.H., M.T.T.J.; Formal analysis: A.M.H.; Funding acquisition: M.T.T.J., J.A.C. and G.C.; Investigation: A.M.H., M.T.T.J., D.B.B., P.M.A.E., C.B., M.A.G., I.R.A.; Methodology: M.P., G.C.; Project Administration: M.T.T.J.; Resources: M.T.T.J., J.A.C.; Supervision: M.T.T.J., G.C.; Validation: M.T.T.J., J.A.C., G.C.; Visualisation: A.M.H., M.T.T.J., G.C.; Writing (original draft): A.M.H., M.T.T.J., D.B.B.; Writing (review and editing): A.M.H., M.T.T.J., D.B.B., P.M.A.E., C.B., M.A.G., I.R.A., G.C., J.A.C.
Funding
This research was financially supported by the European Union’s Horizon 2020 research and innovation program under the grant agreement number [101000467] (COntrolling and progressively Minimizing the Burden of Animal Trypanosomosis [COMBAT]). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. Adrián Melián Henríquez was funded by a predoctoral formation program of Research personnel of the Canary Islands Government: ‘Agencia Canaria de Investigación, Innovación y Sociedad de la Información de la Consejería de Universidades, Ciencia e Innovación y Cultura and by the European Social Fund Plus (ESF +) Programa Operativo Integrado de Canarias 2021–2027, Eje 3 Priority Theme 74 (85%)’ (TESIS2022010062). The Article processing charge (APC) has been funded by the University of Las Palmas de Gran Canaria thanks to the direct financial support received from the Agencia Canaria de Investigación, Innovación y Sociedad de la Información, Gobierno de Canarias (SD-2302).
Data availability
Data supporting the main conclusions of this study are included in the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed SK, Rahman AH, Hassan MA, Salih SE, Paone M, Cecchi G. An atlas of tsetse and bovine trypanosomosis in Sudan. Parasit Vectors. 2016;9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarathunga DC, Grundy J, Parry H, Dorin A. Methods of insect image capture and classification: a systematic literature review. Smart Agric Technol. 2021;1:100023. [Google Scholar]
- 3.Amisigo CM, Amegatcher G, Sunter JD, Gwira T. Adipose and skin distribution of African trypanosomes in natural animal infections. Parasit Vectors. 2024;17:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aregawi WG, Agga GE, Abdi RD, Büscher P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasit Vectors. 2019;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldacchino F, Muenworn V, Desquesnes M, Desoli F, Charoenviriyaphap T, Duvallet G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite. 2013;20:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldacchino F, Desquesnes M, Mihok S, Foil LD, Duvallet G, Jittapalapong S. Tabanids: neglected subjects of research, but important vectors of disease agents. Infect Genet Evol. 2014;28:596–615. [DOI] [PubMed] [Google Scholar]
- 7.Baldacchino F, Desquesnes M, Duvallet G, Lysyk T, Mihok S. Veterinary importance and integrated management of Brachycera flies in dairy farms. In: Garros C, Bouyer J, Takken W, Smallegange RC, editors. Pests and vector-borne diseases in the livestock industry. Wageningen: Wageningen Academic Publishers; 2018. p. 55–90. [Google Scholar]
- 8.Boulangé A, Lejon V, Berthier D, Thévenon S, Gimonneau G, Desquesnes M, et al. The COMBAT project: controlling and progressively minimizing the burden of vector-borne animal trypanosomosis in Africa. Open Res Eur. 2022;2:67. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=The+COMBAT+project%3A+controlling+and+progressively+minimizing+the+burden+of+vector-borne+animal+trypanosomosis+in+Africa&btnG=. [DOI] [PMC free article] [PubMed]
- 9.Bravo-Barriga D, Ruiz-Arrondo I, Peña R, Lucientes J, Delacour-Estrella S. Phlebotomine sand flies (Diptera, Psychodidae) from Spain: an updated checklist and extended distributions. ZooKeys. 2022;1106:81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–409. [DOI] [PubMed] [Google Scholar]
- 11.Cecchi G, Paone M, Feldmann U, Vreysen MJ, Diall O, Mattioli RC. Assembling a geospatial database of tsetse-transmitted animal trypanosomosis for Africa. Parasit Vectors. 2014;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cecchi G, Paone M, Herrero R, Vreysen MJB, Mattioli RC. Developing a continental atlas of the distribution and trypanosomal infection of tsetse flies (Glossina species). Parasit Vectors. 2015;8:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cecchi G, Paone M, de Gier J, Zhao W. The continental atlas of the distribution of tsetse flies in Africa. PAAT Technical and Scientific Series, No. 12. Rome: FAO. 2024. 10.4060/cd2022en. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=The+continental+atlas+of+the+distribution+of+tsetse+flies+in+Africa&btnG=
- 14.de Gier J, Cecchi G, Paone M, Dede P, Zhao W. The continental atlas of tsetse and African animal trypanosomosis in Nigeria. Acta Trop. 2020;204:105328. [DOI] [PubMed] [Google Scholar]
- 15.Desquesnes M, Bossard G, Patrel D, Herder S, Patout O, Lepetitcolin E, et al. First outbreak of Trypanosoma evansi in camels in metropolitan France. Vet Rec. 2008;162:750–2. [DOI] [PubMed] [Google Scholar]
- 16.Desquesnes M, Biteau-Coroller F, Bouyer J, Dia ML, Foil L. Development of a mathematical model for mechanical transmission of trypanosomes and other pathogens of cattle transmitted by tabanids. Int J Parasitol. 2009;39:333–46. [DOI] [PubMed] [Google Scholar]
- 17.Desquesnes M, Holzmuller P, Lai DH, Dargantes A, Lun ZR, Jittaplapong S. Trypanosoma evansi and Surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Res Int. 2013;2013:194176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desquesnes M, Dargantes A, Lai DH, Lun ZR, Holzmuller P, Jittapalapong S. Trypanosoma evansi and Surra: a review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res Int. 2013;2013:321237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desquesnes M, Gonzatti M, Sazmand A, Thévenon S, Bossard G, Boulangé A, et al. A review on the diagnosis of animal trypanosomoses. Parasit Vectors. 2022;15:64. [DOI] [PMC free article] [PubMed]
- 20.Desquesnes M, Sazmand A, Gonzatti M, Boulangé A, Bossard G, Thévenon S, et al. Diagnosis of animal trypanosomoses: proper use of current tools and future prospects. Parasit Vectors. 2022;15:235. [DOI] [PMC free article] [PubMed]
- 21.Diarra B, Diarra M, Diall O, Bass B, Sanogo Y, Coulibaly E, et al. A national atlas of tsetse and African animal trypanosomosis in Mali. Parasit Vectors. 2019;12:466. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=A+national+atlas+of+tsetse+and+African+animal+trypanosomosis+in+Mali&btnG=. [DOI] [PMC free article] [PubMed]
- 22.Doyle MS, Swope BN, Hogsette JA, Burkhalter KL, Savage HM, Nasci RS. Vector competence of the stable fly (Diptera: Muscidae) for West Nile Virus. J Med Entomol. 2011;48:656–68. [DOI] [PubMed] [Google Scholar]
- 23.Espinoza MA, Em D, Shahi-Barogh B, Berer D, Duscher G, Vloedt L, et al. Molecular pathogen screening of louse flies (Diptera: Hippoboscidae) from domestic and wild ruminants in Austria. Parasit Vectors. 2023;16:179. [DOI] [PMC free article] [PubMed]
- 24.Fetene E, Leta S, Regassa F, Büscher P. Global distribution, host range and prevalence of Trypanosoma vivax: a systematic review and meta-analysis. Parasit Vectors. 2021;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraga J, Fernández-Calienes A, Montalvo AM, Maes I, Deborggraeve S, Büscher P, et al. Phylogenetic analysis of the Trypanosoma genus based on the heat-shock protein 70 gene. Infect Genet Evol. 2016;43:165–72. [DOI] [PubMed]
- 26.Gao Y, Xue X, Qin G, Li K, Liu J, Zhang Y, et al. Application of machine learning in automatic image identification of insects—a review. Ecol Inform. 2024;80:102539.
- 27.Garcia HA, Blanco PA, Rodrigues AC, Rodrigues CMF, Takata CSA, Campaner M, et al. Pan-American Trypanosoma (Megatrypanum) trinaperronein. sp. in the white-tailed deer Odocoileus virginianus Zimmermann and its deer ked Lipoptena mazamae Rondani, 187: morphological, developmental and phylogeographical characterisation. Parasit Vectors. 2020;13:308. [DOI] [PMC free article] [PubMed]
- 28.García K, Martínez-López B, Cecchi G, Scoglio C, Matovu E, Muhanguzi D. Prevalence of African animal trypanosomiasis among livestock and domestic animals in Uganda: a systematic review and meta-regression analysis from 1980 to 2022. Sci Rep. 2023;13:20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrido M, Veiga J, Garrigós M, Morales-Yuste M, Recuero-Gil J. Aedes albopictus in a recently invaded area in Spain: effects of trap type, locality, and season on mosquito captures. Sci Rep. 2024;14:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaston KJ. The magnitude of global insect species richness. Conserv Biol. 1991;5:283–96. [Google Scholar]
- 31.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.x35vrx. Accessed 6 Feb 2025.
- 32.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.5ng5eh. Accessed 6 Feb 2025.
- 33.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.ajvenn. Accessed 6 Feb 2025.
- 34.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.2ad2ty. Accessed 6 Feb 2025.
- 35.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.bx8bcq. Accessed 6 Feb 2025.
- 36.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.vkxgj4. Accessed 6 Feb 2025.
- 37.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.686jp6. Accessed 6 Feb 2025.
- 38.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.u7fvem. Accessed 6 Feb 2025.
- 39.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.bcusew. Accessed 6 Feb 2025.
- 40.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.27h59s. Accessed 6 Feb 2025.
- 41.GBIF.org. GBIF Home Page. 2025. 10.15468/dl.bnjuke. Accessed 6 Feb 2025.
- 42.Gebre T, Kapitano B, Beyene D, Alemu D, Beshir A, Worku Z, et al. The national atlas of tsetse flies and African animal trypanosomosis in Ethiopia. Parasit Vectors. 2022;15:491. [DOI] [PMC free article] [PubMed]
- 43.González MA, Stokes JE, Bravo-Barriga D. Diversity and abundance of tabanids in Northern Spain. Parasitol Res. 2022;121:87–96. [DOI] [PubMed] [Google Scholar]
- 44.González MA, Ruiz-Arrondo I, Magallanes S, Oboňa J, Ruiz-López M, Figuerola J. Molecular and morphological analysis revealed a new Lipoptena species (Diptera: Hippoboscidae) in southern Spain harbouring Coxiella burnetii and bacterial endosymbionts. Vet Parasitol. 2024;332:110300. [DOI] [PubMed] [Google Scholar]
- 45.González MA, López-de-Felipe M, Magallanes S, Alarcón-Elbal PM, Barceló C, Martínez-Barciela Y, et al. Distribution, identification and ecology of Phortica genus (Diptera: Drosophilidae) in Spain. Int J Vet Sci. 2025;13:1–11. [DOI] [PMC free article] [PubMed]
- 46.Gutiérrez C, Montoya-Alonso JA, Padrón M, Corbera JA, Juste MC. Descripción de un caso de Tripanosomosis en el Dromedario por T. evansi En Canarias. Med Vet. 1998;15:356–7. [Google Scholar]
- 47.Härkönen S, Laine M, Vornanen M, Reunala T. Deer ked (Lipoptena cervi) dermatitis in humans—An increasing nuisance in Finland. Alces. 2009;45:73–9. [Google Scholar]
- 48.Hennekeler V, Jones R, Skerratt LF, Fitzpatrick LA, Reid S, Bellis G. A comparison of trapping methods for Tabanidae (Diptera) in North Queensland, Australia. Med Vet Entomol. 2008;22:26–31. [DOI] [PubMed] [Google Scholar]
- 49.Hwang WS, Weirauch C. Evolutionary history of assassin bugs (Insecta: Hemiptera: Reduviidae): insights from divergence dating and ancestral state reconstruction. PLoS ONE. 2012;7:e45523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kariyanna B, Sowjanya M. Unravelling the use of artificial intelligence in management of insect pests. Smart Agric Technol. 2024;8:100517. [Google Scholar]
- 51.Kaufer A, Ellis J, Stark D, Barratt J. The evolution of trypanosomatid taxonomy. Parasit Vectors. 2017;10:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kennedy PG. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013;12:186–94. [DOI] [PubMed] [Google Scholar]
- 53.Krčmar S, Radolić V, Lajoš P, Lukačević I. Efficiency of colored modified box traps for sampling of tabanids. Parasite. 2014;21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laukkanen A, Ruoppi P, Mäkinen-Kiljunen S. Deer ked-induced occupational allergic rhinoconjunctivitis. Ann Allergy, Asthma Immunol. 2005;94:604–8. [DOI] [PubMed] [Google Scholar]
- 55.Lukeš J, Speijer D, Zíková A, Alfonzo JD, Hashimi H, Field MC. Trypanosomes as a magnifying glass for cell and molecular biology. Trends Parasitol. 2023;39:902–12. [DOI] [PubMed] [Google Scholar]
- 56.Magri A, Galuppi R, Fioravanti M. Autochthonous Trypanosoma spp. in European mammals: a brief journey amongst the neglected trypanosomes. Pathogens. 2021;10:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melián-Henríquez A, Tejedor-Junco MT, González-Martín M, Doreste MM, Martel SM, Paone M, et al. An atlas of Surra in Spain: a tool to support epidemiological investigations and disease control. Animals. 2024;14:243. [DOI] [PMC free article] [PubMed]
- 58.Mihok S, Carlson DA. New materials for improving catches of horseflies (Diptera: Tabanidae) in Nzi traps. Med Vet Entomol. 2021;35:580–94. [DOI] [PubMed] [Google Scholar]
- 59.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molina JM, Ruiz A, Juste MC, Corbera JA, Amador R, Gutiérrez C. Seroprevalence of Trypanosoma evansi in dromedaries (Camelus dromedarius) from the Canary Islands (Spain) using an antibody Ab-ELISA. Prev Vet Med. 1999;47:53–9. [DOI] [PubMed] [Google Scholar]
- 61.Moloney NM, Barylyuk K, Tromer E, Crook OM, Breckels LM, Lilley KS, et al. Mapping diversity in African trypanosomes using high resolution spatial proteomics. Nat Commun. 2023;14:4401. [DOI] [PMC free article] [PubMed]
- 62.Alert M, Escobar A, Južnič-Zonta Ž. Mosquito alert dataset. Version 1.15. CREAF—centre de recerca ecològica i aplicacions forestals. Occurr Dataset. 2024. 10.1547/t5a1os. [Google Scholar]
- 63.Mullen GR, Durden LA, editors. Medical and veterinary entomology. 3rd ed. London: Academic Press; 2019. [Google Scholar]
- 64.Nartshuk EP, Matyukhin AV, Shokhrin VP. Parasitic louse flies (Diptera, Hippoboscidae) and their associations with bird hosts in the south of the Russian far east. Entomol Rev. 2022;102:367–76. [Google Scholar]
- 65.Ngari NN, Gamba DO, Olet PA, Zhao W, Paone M, Cecchi G. Developing a national atlas to support the progressive control of tsetse-transmitted animal trypanosomosis in Kenya. Parasit Vectors. 2020;13:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogolla KO, Chemuliti JK, Wamwiri FN, Auma JE, Kurgat RK, Wanjala KB, et al. Spatial-temporal variations in parasitological prevalence and host-related risk factors of camel trypanosomiasis and its vectors in North Eastern Kenya: a repeated cross-sectional study. J Parasitol Res. 2023;2023:7218073. [DOI] [PMC free article] [PubMed]
- 67.Okello I, Mafie E, Eastwood G, Nzalawahe J, Mboera LEG. African animal trypanosomiasis: a systematic review on prevalence, risk factors and drug resistance in Sub-Saharan Africa. J Med Entomol. 2022;59:1099–143. [DOI] [PubMed] [Google Scholar]
- 68.Onyilagha C, Uzonna JE. Host immune responses and immune evasion strategies in African trypanosomiasis. Front Immunol. 2019;10:2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otranto D, Wall R. Veterinary parasitology. 5th ed. Chichester: Willey-Blackwell; 2024. [Google Scholar]
- 70.Pataki BA, Garriga J, Eritja R, Palmer JRB, Bartumeus F, Csabai I. Deep learning identification for citizen science surveillance of tiger mosquitoes. Sci Rep. 2021;11:4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Percoma L, Rayaissé JB, Gimonneau G, Bengaly Z, Pooda SH, Pagabeleguem S, et al. An atlas to support the progressive control of tsetse-transmitted animal trypanosomosis in Burkina Faso. Parasit Vectors. 2022;15:72. [DOI] [PMC free article] [PubMed]
- 72.Ramírez-Iglesias JR, Eleizalde MC, Gómez-Piñeres E, Mendoza M. Trypanosoma evansi: a clinical, parasitological and immunological evaluation of trypanosomosis using a chronic rabbit model. Open Vet J. 2012;2:78–82. [PMC free article] [PubMed] [Google Scholar]
- 73.Rodríguez N, Tejedor-Junco MT, González-Martín M, Santana A, Gutiérrez C. Cross-sectional study on prevalence of Trypanosoma evansi infection in domestic ruminants in an endemic area of the Canary Islands (Spain). Prev Vet Med. 2012;105:144–8. [DOI] [PubMed] [Google Scholar]
- 74.Rodríguez N, Tejedor-Junco MT, González-Martín M, Doreste F, Gutierrez C. Trypanosoma evansi assessment in equines: a study in one decade in an endemic area of the Canary Islands, Spain. J Equine Vet Sci. 2013;33:406–9. [Google Scholar]
- 75.Rodríguez NF, Tejedor-Junco MT, González-Martín M, Gutierrez C. Stomoxys calcitrans as possible vector of Trypanosoma evansi among camels in an affected area of the Canary Islands, Spain. Rev Soc Bras Med Trop. 2014;47:510–2. [DOI] [PubMed] [Google Scholar]
- 76.Rojo M, Hernández M, Campos F, Santamaría T, Dias S, Casanueva P. The Iberian Peninsula is an Area of Infection by Haemoproteus payevskyi and Haemoproteus nucleocondensus for the white-throated dipper Cinclus cinclus. Ardeola. 2015;62:373–82. [Google Scholar]
- 77.Ruiz-Arrondo I, Alarcón-Elbal PM, Figueras L, Delacour-Estrella S, Muñoz A, Kotter H, et al. Expansión de los simúlidos (Diptera: Simuliidae) en España: un nuevo reto para la salud pública y la sanidad animal. Boletín SEA. 2014;54:193–200.
- 78.Sasaki H. Capturing tabanids by traps: development history, visual and olfactory attractants and future of tabanid trap. Med Entomol Zool. 2016;67:205–18. [Google Scholar]
- 79. Sazmand A, Desquesnes M, Otranto D. Trypanosoma evansi. Trends in Parasitology. 2022. [DOI] [PubMed]
- 80.Shereni W, Neves L, Argilés R, Nyakupinda L, Cecchi G. An atlas of tsetse and animal African trypanosomiasis in Zimbabwe. Parasit Vectors. 2021;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stork NE. How many species of insects and other terrestrial arthropods are there on Earth? Ann Rev Entomol. 2018;63:31–45. [DOI] [PubMed] [Google Scholar]
- 82.Tamarit A, Gutierrez C, Arroyo R, Jimenez V, Zagalá G, Bosch I, et al. Trypanosoma evansi infection in mainland Spain. Vet Parasitol. 2010;167:74–6. [DOI] [PubMed]
- 83.Tejedor-Junco MT, Melián A, Puerto PP, Ramos MD, González-Martín M, Doreste MM, et al. Surveillance and control of Trypanosoma evansi in the Canary Islands: a descriptive analysis. Acta Trop. 2023;246:106990. [DOI] [PubMed]
- 84.Tolrá MC, Andersen H. Catálogo de los Díptera de España, Portugal y Andorra (Insecta). Monografías SEA, vol 8. 2002.
- 85.Tunnakundacha S, Desquesnes M, Masmeatathip R. Comparison of Vavoua, Malaise and Nzi traps with and without attractants for trapping of Stomoxys spp. (Diptera: Muscidae) and tabanids (Diptera: Tabanidae) on cattle farms. J Agric Nat Resour. 2017;51:319–23. [Google Scholar]
- 86.Turell MJ, Knudson GB. Mechanical transmission of Bacillus anthracis by stable flies (Stomoxys calcitrans) and mosquitoes (Aedes aegypti and Aedes taeniorhynchus). Infect Immun. 1987;55:1859–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams RE. Veterinary entomology: livestock and companion animals. Hoboken: Taylor and Francis; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the main conclusions of this study are included in the manuscript.