Abstract
Background
The sophisticated tumour microenvironment is responsible for the malignant progression and poor prognosis of hepatocellular carcinoma (HCC). Discovering new therapeutic targets is desired for the preferable treatment of HCC patients.
Methods
To characterize the HCC microenvironment, the single-cell transcriptomes of HCC tissues and corresponding noncancerous tissues were analysed. Differentially expressed genes (DEGs), enriched pathways and subgroups were analysed in B cells. Moreover, heterogeneity between malignant and normal hepatocytes was further investigated, which revealed potential biomarkers for HCC progression. The candidate biomarkers were further explored in datasets from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. Serum amyloid A2 (SAA2) was detected and further validated in HCC tissues by immunohistochemistry (IHC) and western blot analysis. The biological roles of SAA2 were further investigated in HCC cells.
Results
The number of B cells in HCC tissues was significantly lower than that in noncancerous tissues, which may result in an immunosuppressive status of the HCC microenvironment. Differentially expressed genes (DEGs) and functional enrichment analysis revealed that B cells might participate in the immunosuppression of HCC by regulating lipid metabolism. Analysis on B cell subgroups demonstrated Naïve B cells were significantly reduced in HCC tissues compared with noncancerous tissues, which indicated that Naïve B cells might be pivotal in the B cell-related immunosuppressive landscape in HCC. Further analysis of hepatocytes revealed highly expressed genes in normal hepatocytes derived from noncancerous liver tissues, which were validated in datasets from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. Interestingly, serum amyloid A2 (SAA2) was highly expressed in normal liver tissues compared with HCC tissues. The results were validated in clinical HCC samples by immunohistochemistry (IHC) and western blot assays. Moreover, investigations in HCC cells revealed that SAA2 acted as a tumour suppressor in HCC progression.
Conclusions
Taken together, the present findings elucidated the B cell-related immunosuppressive landscape in HCC and identified SAA2 as a novel suppressor in HCC, providing a better understanding of the HCC landscape and suggesting a promising therapeutic target for HCC patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06869-6.
Keywords: B cell-related immunosuppressive landscape, Hepatocellular carcinoma, Serum amyloid A2, Single-cell RNA sequencing, Suppressor, Tumour microenvironment
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide [1, 2]. Many HCC patients are diagnosed at an advanced stage due to concealed progression. Thus, radical treatment, such as hepatectomy or liver transplantation, is infeasible for advanced HCC patients [3]. Even if HCC patients undergo surgical resection, they also have high rates of recurrence and metastasis. To improve the prognosis of HCC patients, several tyrosine kinase inhibitors, immune checkpoint inhibitors, and monoclonal antibodies have been developed and have been demonstrated to strongly improve the treatment of HCC and prolong the survival of advanced HCC patients [4]. However, clinical data have revealed that HCC patients may not benefit from existing therapies and that the prognosis of HCC patients continues to be poor [5]. The efficacy of targeted therapies is limited by the considerable heterogeneity of the HCC microenvironment. Therefore, the complicated HCC microenvironment warrants further exploration, and the discovery of new therapeutic targets may pave the way for new treatments for HCC.
Single-cell RNA sequencing (scRNA-seq) has developed rapidly in recent years and has become an indispensable technology for exploring the tumour microenvironment, intercellular heterogeneity, and novel targeted genes [6]. Through single-cell capture and high-throughput sequencing, scRNA-seq performs unbiased and high-resolution transcriptome analysis of individual cells, revealing the heterogeneity of single cells in terms of biological function and gene expression [7]. Moreover, the complex interactions among cellular components in the tumour microenvironment can be accurately measured by scRNA-seq [8]. Numerous studies have utilized scRNA-seq to elucidate the heterogeneity of malignant tumour cells and immune cells in the tumour microenvironment. To date, the microenvironment discrepancy and cell heterogeneity revealed by scRNA-seq have advanced the study of new cell types, cell differentiation trajectories, biomarkers, and immune checkpoints, providing a cellular and molecular basis for therapeutic improvement [9, 10].
The tumour microenvironment of HCC is composed of malignant hepatocytes, immune cells, stromal cells, and chemokines [11]. The dynamic crosstalk among these components is involved in the malignant potential of HCC. Infiltrating immune cells regulate the tumour ecosystem through various pathways and differentially expressed genes [12]. Prior studies have focused on the behaviours of various immune cells in the HCC landscape, such as T cells, macrophages, and natural killer (NK) cells [13–15]. However, as a vital component of tumour immunity, tumour-infiltrating B cells are poorly characterized and need further investigation. Moreover, crucial events and regulators involved in the malignant process of hepatocytes are still not known.
In the present study, we anatomized the tumour microenvironment of HCC by collecting 73,707 full-length single-cell transcriptomes from 5 primary HCC patients. T cells, B cells, NK cells, and neutrophils in HCC tissues were significantly decreased compared with those in paired corresponding noncancerous tissues, suggesting an immunosuppressive status in HCC. Analysis of differentially expressed genes (DEGs) and enriched pathways in B cells revealed that B cells may participate in the immunosuppressive landscape of HCC by regulating lipid metabolism. Analysis on B cell subgroups indicated that Naïve B cells might be pivotal in the B cell-related immunosuppressive landscape in HCC. Investigation of the heterogeneity between malignant and normal hepatocytes revealed potential biomarkers for HCC progression. The candidate genes were further explored in datasets from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. Serum amyloid A2 (SAA2) was detected and further validated by immunohistochemistry (IHC) and western blot analysis. Additional experiments in HCC cells demonstrated that SAA2 was a novel suppressor in HCC.
Materials and methods
HCC tissues and noncancerous tissues collected from HCC patients
Five patients pathologically diagnosed with HCC at Shandong Provincial Hospital Affiliated to Shandong First Medical University were recruited for scRNA-seq in the present study. All patients were treated initially and had not previously received any treatments, such as chemotherapy or radiation. Paired fresh HCC tissues and corresponding noncancerous tissues were obtained immediately after surgical resection and stored at cryogenic temperature for subsequent scRNA-seq procedures. Another cohort of 37 paired HCC tissues and corresponding noncancerous liver tissues for IHC and western blot assays was also collected from Shandong Provincial Hospital Affiliated to Shandong First Medical University. Among these samples, 13 pairs of HCC tissues and corresponding noncancerous tissues were used for the IHC assay, and another 24 pairs of matched tissues were used for western blot analysis. The HCC tissues were at least 2 cm inside the tumour edges, and the corresponding noncancerous tissues were at least 2 cm from paired tumour tissues. The study protocol was approved by the Ethics Committee of Shandong Provincial Hospital, and informed consent was obtained from all patients according to the committee’s regulations. All methods were performed in accordance with the relevant guidelines and regulations of the committee.
Pulverization of tissue samples, single-cell isolation, and preparation of suspensions
Fresh HCC tissues and paired corresponding noncancerous tissues from surgical resection were immersed in a refrigerated container filled with complete medium containing 90% Dulbecco’s modified Eagle’s medium (DMEM; Cat# 11054001, Gibco) and 10% foetal bovine serum (FBS; Cat# 16140071, Gibco), and transported to the laboratory as soon as possible. After being washed three times with 1 x PBS (Cat# 10010023, Gibco), the tissues that met the experimental requirements were dissected into small pieces (diameter of 1–3 mm3) using surgical scissors on a clean UV-sterilized table. The tissues were then enzymatically digested with 1 mg/mL collagenase I (Cat# 17100-017, Gibco), 1 mg/mL collagenase II (Cat# 17101-015, Gibco), 60 U/mL Hyaluronidase (Cat# H3757, Sigma), 10 U/mL liberase (Cat# 05401127001, Roche) and 0.02 mg/mL DNase I (Cat# 11284932001, Roche) on ice for 90 min at 37 °C, with agitation. After digestion, the samples were sieved through 100 μm and 40 μm cell strainers, followed by centrifugation at 300 × g for 5 min. After the supernatant was removed, the pelleted cells were suspended in red blood cell lysis buffer (Cat# 00-4333-57, Invitrogen) to lyse red blood cells. After washing with DPBS (Cat# 21-031-CV, Corning) containing 0.5% BSA (Cat# A600332, Shenggong), the cell pellets were resuspended in DPBS containing 0.5% BSA, stained and counted, yielding a standard single-cell suspension.
Single-cell capture, library preparation, and scRNA-seq data preprocessing
scRNA-seq was performed by Genechem Technology. The Chromium instrument and the Single Cell 3’ Reagent Kit V3.1 (Chromium Next GEM Single Cell 3’ Kit v3.1, 16 rxns PN-1000268) were used to prepare individually barcoded single-cell RNA-Seq libraries following the manufacturer’s protocol (10× Genomics). Briefly, sample partitioning and molecular barcoding were performed on a Chromium Controller (10× Genomics), and cellular suspensions were loaded together with the single-cell 3’ gel beads (Dynabeads™ MyOne™ SILANE PN-2000048) on a single-cell 3’ chip (Chromium Next GEM Chip G Single Cell Kit, 48 rxns PN-1000120) to generate Gel Beads in emulsion droplets. RNA from the barcoded cells was subsequently reverse-transcribed and sequencing libraries were constructed using reagents from the Chromium Single Cell Reagent Kit V3.1 (10× Genomics). Sequencing was performed with an Illumina system (NovaSeq) according to the manufacturer’s instructions. The Cell Ranger analysis pipeline (version 6.0.2) was used to create sequencing libraries from single-cell transcriptomes without specifying a targeted panel. After sequencing, the analysis pipeline used FASTQ files, a reference genome file (GRCh38) and a transcriptome annotation file (GENCODE v32/Ensembl 98) for sequence alignment. The pipeline generated a unique molecular identifier (UMI) count matrix, which was processed using the Scanpy Python package (version 1.8) for further analysis [16]. To remove low-quality cells, several criteria, such as the number of genes and mitochondrial counts, were used for quality control (QC) before data analysis. Cells with UMIs and number of genes lower than the thresholds (UMIs ≤ 100000; 200 ≤ number of genes ≤ 8000) were filtered out. Following visual inspection of the distribution of cells by the fraction of mitochondrial genes expressed, low-quality cells in which > 20% of the counts belonged to mitochondrial genes were discarded. Library size normalization was performed with the pp.normalize_total function (sc.pp.normalize_total, data, target_sum = 1e4) in Scanpy to obtain normalized counts [16]. Specifically, the global-scaling normalization method was used to normalize the gene expression measurements for each cell.
Clustering and visualization of single cells
Highly variable genes were identified using the method described by Macosko et al. [17]. The most variable genes were selected using the pp.highly_variable_genes function in Scanpy [16]. Principal component analysis (PCA) was performed to reduce the dimensionality of the dataset with the tl.pca function (n_pcs = 50) in Scanpy [16]. Graph-based clustering was performed to cluster cells according to their gene expression profiles using the pp.neighbors function (sc.pp.neighbors, adata, n_neighbors = 10, use_rep=’X_harmony’, and n_pcs = 50) in Scanpy [16]. The cells were visualized using the 2-dimensional and 3-dimensional uniform manifold approximation and projection (UMAP) algorithm with the tl.umap function (sc.tl.umap, data, n_components = 2, min_dist = 0.5, spread = 1, copy = True) in Scanpy [16]. The FindAllMarkers function (test.use = wilcox) in Seurat was used to identify marker genes of each cluster [18]. For a given cluster, FindAllMarkers identified positive markers compared with all other cells (adjusted P value < 0.05 and |foldchange| > 2 were set as the thresholds of significant marker genes).
Functional enrichment analysis
The g: Profiler2 package in R (enricher function, pAdjustMethod = ‘BH’, minGSSize = 10, maxGSSize = 500, pvalueCutoff = 0.05, qvalueCutoff = 0.2) was used to perform a functional enrichment analysis of the marker genes [19]. The marker genes for each cluster were mapped to known functional pathways, and the hypergeometric distribution was used to check whether these biological processes were overrepresented, including pathways from Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Reactome [20–22].
TCGA and GTEx databases analysis
The bulk gene expression profiles and corresponding clinical data of 374 HCC samples and 50 paracancerous samples were downloaded from TCGA database. Data from another 110 healthy controls were obtained from the GTEx database. Statistical analysis of the obtained data was performed with R software (version 4.3.1). Collation of expression matrices, ID transformation, and differential gene analysis were performed using the tiny-array package in R. The differential expressed genes were clustered and displayed in a heatmap with the pheatmap package in R. The boxplots were drawn via a boxplot function in R.
IHC and western blot assays
IHC and western blot assays were performed as described previously [23]. The specific primary antibodies used in these assays included antibodies against SAA2 (Cat# 13192-1-AP, Proteintech), and GAPDH (Cat# 6004-1-Ig, Proteintech).
Construction of genetically modified stable cell lines
Lentivirus-mediated SAA2 interference (Sh-SAA2-1 and Sh-SAA2-2) or its mock control (Sh-NC) and lentivirus-mediated SAA2 overexpression (SAA2-OE) or its mock control (SAA2-NC) were transfected into HCC cells, according to the manufacturer’s protocol (Jinbai’ao, Shandong, China). To construct HCC cell lines with stable interference of SAA2, HepG2 cells were transfected with Sh-SAA2 and selected with puromycin (2 µg/ml; Cat# A1113803, Gibco), and HepG2 cells stably transfected with Sh-NC were used as the mock control. To obtain HCC cell lines stably overexpressing SAA2, HepG2 cells were transfected with SAA2-OE and selected with puromycin (2 µg/ml), while HepG2 cells transfected with SAA2-NC were used as the mock control. Western blot analysis was used to validate stable SAA2 interference or overexpression in HepG2 cell lines.
Cell proliferation, invasion and colony formation assays
Cell proliferation, invasion, and colony formation assays were performed as previously described [23].
Statistical analysis
Differences between two groups were analysed utilizing Student’s t test or the Mann-Whitney U test. Statistical analyses were conducted using R software (version 4.3.1). All the statistical tests were two-tailed, and a p value < 0.05 was considered statistically significant.
Results
Overview of study design
Although the HCC microenvironment has been extensively explored, the tumour ecosystem at the single-cell level remains to be further characterized. The present study investigated the HCC microenvironment at the cellular level using the study design shown in Fig. 1A. To construct a comprehensive single-cell reference for HCC, 5 newly treated HCC patients were recruited for scRNA-seq. The detailed clinical and pathological information, including sex, age, tumour size, alpha-fetoprotein (AFP) levels, Child‒Pugh stage, and Barcelona Clinic Liver Cancer (BCLC) stage, was shown in Supplementary Table S1. These patients were pathologically diagnosed with HCC, and their surgical HCC specimens and paired corresponding noncancerous tissues were collected. Sequencing libraries of pooled single cells were obtained and subjected to a sequencing run. The Chromium platform (10× Genomics) was used to capture single cells in prepared single-cell suspensions. The single cells were collected in the form of droplets, marked with specific barcodes and then subjected to a series of procedures. The captured mRNAs were reverse-transcribed into cDNA and amplified for single-cell library preparation (Fig. 1B). QC was applied with key metrics, including the number of genes, total counts, and percentage of mitochondrial counts (Supplementary Figure S1). After applying the QC criteria, a total of 73,707 single cells were retained and included in the downstream analysis, of which 36,323 single cells were from HCC tissues and the rest were from corresponding noncancerous tissues (Supplementary Table S2). These identified cells produced an average of 2,783 detected genes. The cell compositions in the HCC ecosystem were subsequently resolved, and the DEGs and corresponding signaling pathways were analysed in the cell clusters closely related to the immunosuppressive landscape of HCC, especially B cells. Transcriptome analysis of the differences between malignant and normal hepatocytes revealed promising genes involved in protecting hepatocytes from cancer. The potential biomarkers of HCC progression were subsequently validated in TCGA and GTEx databases (Fig. 1C). IHC, western blot, and cell experiments were performed to identify promising suppressors in HCC.
Fig. 1.
Overview of study design. (A) Complete study design. HCC, hepatocellular carcinoma; scRNA-seq, single-cell RNA sequencing; DEGs, differentially expressed genes; IHC, immunohistochemistry. (B) Schematic representation of scRNA-seq profiling. (C) Validating process in The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases
Identification of cell compositions by single-cell transcriptome analysis
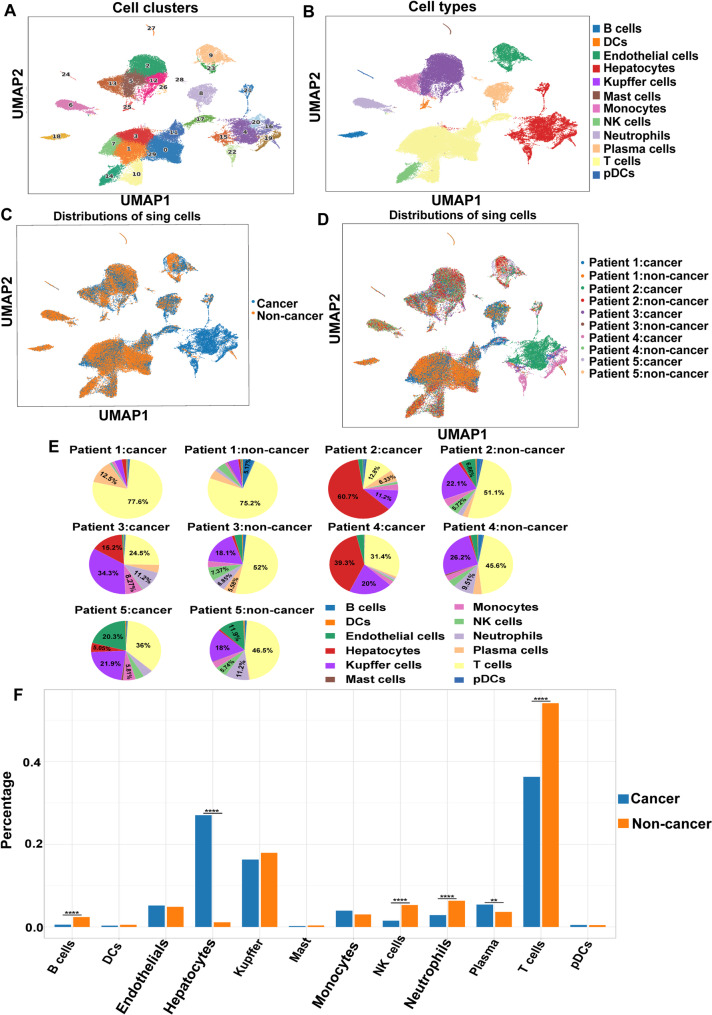
Analysis of cell compositions in the scRNA-seq data identified 30 cell clusters (Fig. 2A), and individual cell clusters were analysed according to their marker genes. The marker genes of top 10 expression levels for each cell cluster were showed in Supplementary Figure S2. The 30 cell clusters were classified into 12 major cell populations on the basis of the expression of known marker genes (Supplementary Figure S3) and enriched pathways, including 9 immune cell types and 3 nonimmune cell types (Fig. 2B). The identified immune cells included T cells, B cells, dendritic cells (DCs), mast cells, monocytes, NK cells, neutrophils, and plasmacytoid dendritic cells (pDCs), while the nonimmune cells included hepatocytes, endothelial cells, and Kupffer cells. The distributions of the identified cell lineages were compared between HCC tissues and paired corresponding noncancerous tissues (Fig. 2C). The distributions and differences of single cells among individual HCC patients were shown in Fig. 2D and E, which revealed that all of these cell types were shared among the patients, albeit at different proportions.
Fig. 2.
Cell components of HCC microenvironment identified by scRNA-seq. (A) Uniform manifold approximation and projection (UMAP) plot showing distributions of cell clusters. (B) UMAP plot exhibiting annotations and distributions of cell types. (C) UMAP plot presenting cell distribution in HCC tissues and noncancerous tissues. (D) UMAP plot presenting cell distribution among individual patients. (E) Pie charts showing proportions of cell types in HCC tissues and noncancerous tissues of different patients. (F) Histogram appearing differences of cell types distributions between HCC tissues and non-cancer tissues. ****p < 0.0001, **p < 0.01
The scRNA-seq data demonstrated that various cell types presented significantly different distributions in HCC tissues and paired corresponding noncancerous tissues (Fig. 2F). For example, there was a larger proportion of malignant hepatocytes in HCC tissues than normal hepatocytes in corresponding noncancerous tissues. Similarly, the proportion of plasma cells in HCC tissues was significantly higher than that in corresponding noncancerous tissues. In contrast, the proportions of T cells, B cells, NK cells, and neutrophils in HCC tissues were decreased compared with those in corresponding noncancerous tissues. The reduction in infiltrating immune cells, including T cells, B cells, NK cells, and neutrophils, in HCC tissues reflected the heterogeneity of the immune microenvironment and was responsible for the immunosuppression status of the HCC microenvironment.
B cell-related immunosuppressive landscape in HCC
In addition to the differences in cell composition between HCC tissues and paired noncancerous tissues, the differences in immune cells, which were closely related to the HCC immune microenvironment, were further explored (Fig. 3A). The percentage of T cells was markedly lower in HCC tissues than in noncancerous tissues (36.3% versus 54.1%, respectively), whereas the proportion of NK cells in noncancerous tissues (5.31%) was greater than that in HCC tissues (1.52%). Compared with that in paracancerous samples, the percentage of neutrophils in HCC samples was also significantly lower (2.86% versus 6.33%). Similarly, the proportion of B cells in HCC samples (0.55%) decreased more than twofold compared with that in noncancerous samples (2.38%).
Fig. 3.
Description and function enrichment of B cells. (A) Pie diagram demonstrating cell type proportions in HCC and noncancerous tissues. (B) The volcano plot showing DEGs of B cells. (C) Heatmap presenting DEGs of B cells in HCC and noncancerous tissues (adjusted p-value ≤ 0.05). (D-E) Gene Ontology (GO) analysis of the DEGs of B cells. The up-regulated genes (D) and down-regulated genes (E) were enriched in different pathways. (F) UMAP plot showing distributions of B cell subclusters. (G) Histogram showing B cell subgroups in HCC and noncancerous tissues. (H) Histogram showing differences of B cell subgroups between HCC and noncancerous tissues. *p < 0.05
B cells play vital roles in antigen presentation, immune regulation, and the tumour immune microenvironment. Because B cells were significantly decreased in HCC tissues in the present study, we conducted a detailed analysis of the DEGs and functional enrichment of B cells. Analysis of the DEGs in B cells between HCC tissues and paired noncancerous tissues revealed a total of 22,256 upregulated or downregulated genes in B cells (Supplementary Table S3). According to the adjusted p value (≤ 0.05) and foldchange thresholds (≥ 1.5) to screen significant DEGs, 31 upregulated genes and 19 downregulated genes were identified and subsequently analysed (Fig. 3B). The generated heatmap displays the top 25 upregulated genes and 19 downregulated genes with remarkable changes (Fig. 3C). Seven highly expressed genes namely, APOA2, APOC3, APOE, APOC1, APOC2, APOA1, and FABP1, play important roles in the process of lipid metabolism. Abnormal lipid metabolism leads to excessive deposition of lipid components and accelerates damage to cells, which is an important inducer of nonalcoholic fatty liver disease (NAFLD) and a crucial factor in HCC. These genes mediate lipid transport, metabolism and homeostasis, as well as reduce serum cholesterol content and stabilize the high-density lipoprotein (HDL) structure [24]. Lipid metabolism homeostasis plays a positive role in enhancing the activity of immune cells in antitumour immunity [25]. Abnormal lipid metabolism can inhibit the antitumour ability of immune cells and even redifferentiate immune cells into protumour phenotypes [26].
Differential gene products derived from B cells were further evaluated by GO analysis. The enriched pathways of the upregulated genes were mainly related to high density lipoprotein particle remodeling (GO:0034375), chylomicron and protein lipid complex assembly (GO:0034378, GO:0065005), and lipid metabolic processes (GO:0006641, GO:0034370, GO:0034384, and GO:0006638) (Fig. 3D). The major enriched pathways of the downregulated genes included nuclear transcribed mRNA catabolic process (GO:0000956), negative regulation of cell division (GO:0051782), and cotranslational protein targeting to membrane (GO:0006613) (Fig. 3E).
The investigation of DEGs and functional enrichment pathways revealed that B cells played a role in regulating lipid metabolism, which might be a crucial process involved in the B cell-related immunosuppressive landscape in HCC tissues. Compared with those in noncancerous tissues, the number of B cells in the microenvironment of HCC tissues was markedly lower, which might lead to abnormal lipid metabolism. The antitumor immunity related to B cells was also reduced, which might be responsible for HCC progression.
To investigate the subgroups of B cells which played vital roles in immune microenvironment, further analysis on subgroups of B cell clusters was conducted. A total of 1087 B cells were grouped into 18 cell subclusters according to their expression patterns (Fig. 3F). The marker genes of each B cell subcluster were analysed (Supplementary Table S4, Supplementary Figure S4). Four major B cell subgroups were identified on the basis of the expression of known marker genes (Supplementary Table S5), including pre-B cells, Naïve B cells, memory B cells, cycling B cells. The distributions of the identified cell subgroups were compared between HCC tissues and paired corresponding noncancerous tissues (Fig. 3G and H). The results showed Naïve B cells were significantly reduced in HCC tissues compared with noncancerous tissues, which indicated that Naïve B cells might be pivotal in the B cell-related immunosuppressive landscape in HCC.
Transcriptome heterogeneity of malignant and normal hepatocytes
The number of malignant hepatocytes in HCC tissues was greater than normal hepatocytes in corresponding noncancerous tissues. To investigate the difference between malignant and normal hepatocytes, the hepatocyte cluster was selected for subgroup analysis. Single cells in hepatocyte cluster were classified into 22 cell subclusters on the basis of differences in their expression patterns (Fig. 4A). The marker genes of top 10 expression levels for each hepatocyte cluster were showed in heatmap (Supplementary Figure S5). The distribution of the cell subclusters in HCC and noncancerous samples as well as among individual patients was visualized in two-dimensional images (Fig. 4B and C). Subclusters 0, 1, 2, 3, and 4 were enriched mainly in HCC tissues, whereas subclusters 5, 13, and 16 were enriched mostly in corresponding noncancerous tissues (Supplementary Figure S6). Further investigation of subcluster distribution revealed that subclusters 0, 1, 2, 3, and 4 were distributed only in HCC tissues (Fig. 4D). Among the differentially distributed subclusters, subclusters 0, 1, 3, and 4 were derived entirely from Patient 2, and subcluster 2 was derived entirely from Patient 4 (Supplementary Table S6). However, subclusters 5, 13, and 16 were derived from all five patients, with significantly greater proportions of these single cells in noncancerous tissues (34.39%, 16.83%, and 36.1%, respectively) than in HCC tissues (5.21%, 2.33%, and 0%, respectively). Therefore, the biological functions and gene expression of subclusters 5, 13, and 16 were further investigated.
Fig. 4.
Analysis of heterogeneity between malignant hepatocytes and normal hepatocytes. (A) UMAP plot appearing subclusters of hepatocytes. (B-C) UMAP plot showing hepatocytes distributions in HCC tissues and noncancerous tissues (B), as well as in individual patients (C). (D) UMAP plot showing distributions of hepatocytes subclusters in HCC tissues and noncancerous tissues
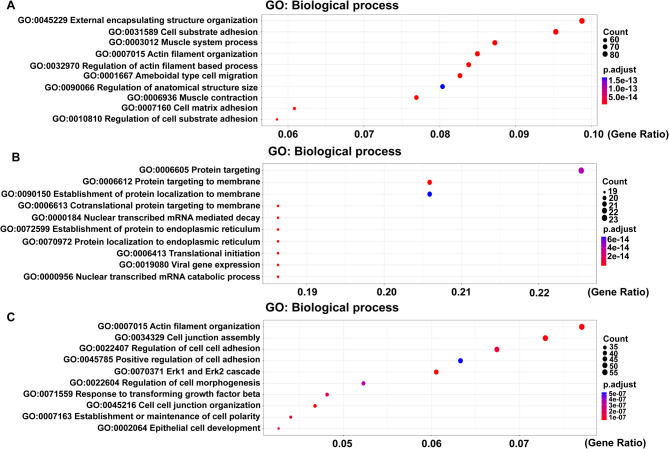
GO analysis was performed to understand the biological functions of different cell subpopulations. The main pathways enriched in subcluster 5 were cell matrix adhesion (GO:0007160), external encapsulating structure organization (GO:0045229), and ameboidal type cell migration (GO:0001667) (Fig. 5A). Subcluster 13 was mostly associated with viral gene expression (GO:0019080), nuclear transcribed mRNA catabolic process (GO:0000956), and protein targeting to membrane (GO:0006612) (Fig. 5B). The Erk1 and Erk2 cascade (GO:0070371), regulation of cell cell adhesion (GO:0022407), and response to transforming growth factor β (GO:0071559) were the main pathways enriched in subcluster 16 (Fig. 5C).
Fig. 5.
The highly expressed genes in hepatocytes subclusters. (A) GO analysis of the highly expressed genes in hepatocyte subcluster 5. (B) GO analysis of the highly expressed genes in hepatocyte subcluster 13. (C) GO analysis of the highly expressed genes in hepatocyte subcluster 16
The DEGs in hepatocyte subclusters 5, 13, and 16 were identified. The top 10 genes with the highest expression in hepatocytes subgroup 5 were ACTG2, WFDC1, CASQ2, CSPG4, PTH1R, FOXS1, CD248, SGCA, TCF21, and HSPB2. Various genes, including MT1G, MT1E, MT1H, SAA1, SAA2, HAMP, SDS, and C1QA/B/C, were identified as highly expressed genes in hepatocyte subgroup 13. For subcluster 16, the highly expressed genes included TACSTD2, KRT19, SFRP5, MMP7, CHST4, SLC5A1, PRSS22, RAB25, UCA1, and KRT80. Because hepatocytes in subclusters 5, 13, and 16 were significantly more distributed in noncancerous tissues than in HCC tissues, the significantly overexpressed genes of these three subclusters might be candidate biomarkers for protecting hepatocytes from cancer.
Validation in TCGA and GTEx databases
To validate the candidate biomarkers, bulk RNA data and corresponding clinical information from 374 HCC patients and 160 healthy controls were obtained from TCGA and GTEx databases and used as a validation dataset. The top 10 significantly overexpressed genes of subclusters 5, 13, and 16 were analysed in the validation datasets. Differential expression analysis was performed by R software. Nine genes—MT1G, MT1E, MT1H, SFRP5, PTH1R, HAMP, SAA1, SAA2, and SDS—were overexpressed in normal liver tissues from healthy controls (Fig. 6A and B). The screening and validation process suggested that these 9 genes might be potential biomarkers of HCC progression.
Fig. 6.
Validation in TCGA and GTEx databases. (A) Heatmap appearing genes expression in the HCC patients and healthy controls. (B) Boxplots presenting genes expression in the HCC patients and healthy controls. ****p < 0.0001
Many studies have described the effects of MT1G, MT1E, and MT1H on the development and prognosis of HCC. Previous studies have revealed that the expression of MT1G is downregulated in HCC, which is consistent with the present findings. The molecular mechanism involved is hypermethylation of the MT1G promoter, which leads to chromatin condensation and gene silencing [27]. As a ferroptosis-related gene, MT1G plays an important role in inhibiting the formation, proliferation and invasion of HCC, and it affects the survival of HCC patients via improving the activity and stability of p53, thus representing a potential novel biomarker [28]. Wei et al. investigated the effects of MT1G on HCC development and sorafenib resistance, and they reported that MT1G activation enhances the anticancer effects of sorafenib by modulating the epigenetics of the KLF4-HIF1-α-CA9 axis [29]. Similarly, MT1E is silenced in HCC tissues due to promoter hypermethylation, which induces the apoptosis of liver cancer cells and inhibits HCC progression [30]. Zheng et al. reported that MT1H inhibits the invasion and proliferation of hepatic cells by regulating the Wnt/β-catenin signalling pathway [31]. According to previous reports, the upregulation of MT1H also has tumour suppressive effects on various tumour types, including colon cancer, prostate cancer, and oral cancer [32–34]. In addition, epigenetic silencing of SFRP5 promotes the activation of the Wnt signalling pathway using the hepatitis B virus (HBV) X protein, leading to the development of HCC [35]. PTH1R has been reported to be an important cause of poor prognosis in patients with HCC [36]. Knockdown of HAMP in human liver cancer cell lines results in increased cell proliferation and migration [37]. SAA1 has been suggested to be a predictor of favourable prognosis in patients with HCC [38]. These findings are in accordance with the present findings, indicating that MT1G, MT1E, MT1H, SFRP5, PTH1R, HAMP, and SAA1 might be key biomarkers of HCC progression. SDS is L-serine dehydratase/L-threonine deaminase, which catalyzes the pyridoxal-phosphate-dependent dehydrative deamination of L-threonine and L-serine to ammonia and alpha-ketobutyrate and pyruvate, respectively [39]. We conducted survival analysis between the expression of 9 potential biomarkers and prognosis of HCC patients in data from TCGA database. The results showed that the expression of SDS had a correlation with the overall survival (OS) and disease-free survival (DFS) of HCC patients, thus indicated that SDS was a novel prognostic predictor for HCC patients (Supplementary Figure S7).
Verification of SAA2 expression in clinical HCC tissues
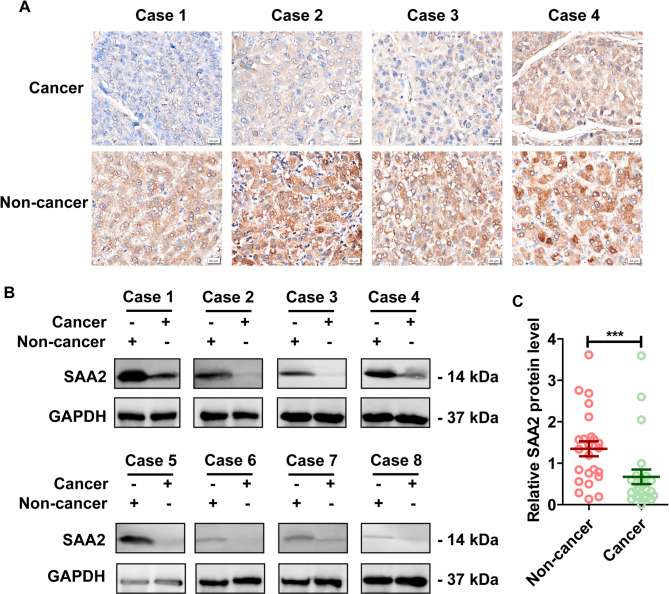
SAA2 is a member of SAA protein, which is one of the most prominent members of the acute phase response (APR) during which their serum levels rise dramatically after trauma, infection and other stimuli [40]. In the present study, we were interested in the role of SAA2 in the HCC progression. The single-cell transcriptome data and the validation using TCGA and GTEx databases suggested that SAA2 might be a novel biomarker for HCC. For further validation, clinical HCC tissues and corresponding noncancerous liver tissues from 37 HCC patients were collected, and IHC and western blot assays were performed to assess the expression level of SAA2. First, IHC was conducted on paired tissues from one cohort of 13 clinical HCC patients to detect the location and expression of SAA2. SAA2 was located primarily in the cytoplasm of HCC cells and hepatocytes, and the expression level of SAA2 in HCC tissues was significantly lower than that in corresponding noncancerous liver tissues (Fig. 7A). Second, paired tissues from another cohort of 24 HCC patients were used to measure the protein level of SAA2 by a western blot analysis (Fig. 7B). The SAA2 protein levels in the HCC tissues were significantly lower than those in the corresponding noncancerous liver tissues, which was in accordance with the IHC data (Fig. 7C). Taken together, these findings revealed significantly lower SAA2 expression levels in HCC tissues than in normal controls, suggesting that SAA2 might be involved in HCC progression.
Fig. 7.
The expression of SAA2 was reduced in clinical HCC tissues. (A) IHC staining showing the location and expression of SAA2 in HCC tissues and corresponding noncancerous liver tissues from 13 clinical HCC patients. Presented images are representative figures of investigated HCC cases. (B) Western blot showing protein level of SAA2 in HCC tissues and corresponding noncancerous liver tissues from 24 clinical HCC patients. Presented images are representative blots from 8 HCC patients. (C) Statistical analysis of SAA2 expression by western blot assay in HCC tissues and corresponding noncancerous tissues of all the investigated patients. ***p < 0.001
SAA2 acted as a tumour suppressor in HCC cells
Because SAA2 was markedly downregulated in HCC tissues compared with normal liver tissues, we investigated whether SAA2 plays a role in HCC cells. A loss-of-function model was constructed by transfecting Sh-SAA2-1 and Sh-SAA2-2 into HepG2 cells, and a gain-of-function model was generated by transfecting SAA2-OE into HepG2 cells. Successful knockdown of SAA2 in HepG2 cells (Fig. 8A) significantly increased the proliferation (Fig. 8B), invasion (Fig. 8C), and colony formation (Fig. 8D). In contrast, SAA2 overexpression significantly inhibited HepG2 cells (Fig. 8E), proliferation (Fig. 8F), invasion (Fig. 8G), and colony formation (Fig. 8H). These findings demonstrated that SAA2 functions as a tumour suppressor and inhibits the malignant behaviours of HCC cells.
Fig. 8.
SAA2 inhibited the malignant behaviors of HCC cells. (A-D) HepG2 cells were used to construct loss-of-function model, and successful knockdown of SAA2 was detected by western blot (A). The proliferation (B), invasion (C), and colony formation (D) of HepG2 cells in the loss-of-function model were further detected and analyzed. (E-H) HepG2 cells were used to construct gain-of-function model, and successful overexpression of SAA2 was detected by western blot (E). The proliferation (F), invasion (G), and colony formation (H) of HepG2 cells in the gain-of-function model were further detected and analyzed. ***p < 0.001
In summary, the compositions of different cell types were investigated in the microenvironments of HCC tissues and corresponding noncancerous tissues, revealing the heterogeneity of infiltrating immune cells as well as malignant and normal hepatocytes. The present study revealed the B cell-related immunosuppressive landscape in HCC, suggesting that B cells might participate in the immunosuppression of HCC by regulating lipid metabolism. Moreover, the present results revealed the differences between malignant hepatocytes in HCC tissues and normal hepatocytes in noncancerous tissues. Furthermore, the present study identified SAA2 as a novel suppressor in HCC, suggesting that it might become a potential biological target in future HCC therapies.
Discussion
High recurrence and mortality rates hinder the prognosis of HCC patients. Because intratumor heterogeneity is responsible for various responses to drugs, the heterogeneity of HCC tumours should be explored [41]. The interaction between immune cells and tumour cells influences the proliferation and differentiation of tumour cells, thus leading to complex tumour microenvironments [42]. To date, the tumour microenvironment in HCC remains to be further characterized, although progress in cancer surveillance and treatment strategies has been made.
To elucidate the tumour microenvironment of HCC, the present study utilized scRNA-seq to analyse cell heterogeneity and gene expression in HCC tissues and paired corresponding noncancerous tissues. In the present study, a complete single-cell transcriptome atlas was created to describe the HCC microenvironment. Analysis of the cellular and genetic differences in infiltrating immune cells, malignant hepatocytes and normal hepatocytes revealed that all these cell types were distributed differently between HCC tissues and noncancerous tissues. Compared with those in noncancerous tissues, T cells, B cells, and NK cells were markedly reduced, whereas malignant hepatocytes were significantly increased in HCC tissues. The characteristics of the immune ecosystem revealed that the infiltration of immune cells was disparate between HCC tissues and noncancerous tissues, which might be responsible for the immunosuppressive microenvironment involved in HCC progression.
B cells are vital immune cells that play crucial roles in antigen presentation and immune regulation. In the present study, decreased cells were significantly decreased in HCC tissues. To determine whether the reduction in B cells in HCC tissues contributes to the immunosuppressive status of the HCC ecosystem, we investigated the DEGs and relevant enriched functional pathways in B cells. The upregulated genes were enriched mainly in pathways related to lipid metabolism. Abnormal lipid metabolism not only is an important cause of NAFLD but also promotes the damage of hepatocytes and facilitates their progression into HCC [43]. Thus, the present data indicated that B cells might participate in the immunosuppressive landscape of HCC and lead to HCC deterioration by regulating lipid metabolism.
Single-cell analysis revealed large differences in the proportions of malignant hepatocytes in HCC tissues and normal hepatocytes in corresponding noncancerous tissues. To evaluate the differences between HCC cells and normal hepatocytes, as well as to elucidate the malignant process of hepatocytes, subgroup analysis of the hepatocyte lineage was performed. Hepatocytes in subclusters 5, 13 and 16 were derived predominantly from noncancerous tissues. DEG analysis was conducted to identify and evaluate the highly expressed genes of these cell subpopulations. The detected genes were validated with bulk RNA data from TCGA and GTEx databases. The screened and validated genes—MT1G, MT1E, MT1H, SFRP5, PTH1R, HAMP, SAA1, SAA2, and SDS—may be potential biomarkers for HCC. SAA2 is a new promising biomarker of HCC progression. IHC and western blot assays were performed to verify the expression of SAA2 in clinical tissues, which revealed that the expression level of SAA2 was significantly lower in HCC tissues than in noncancerous liver tissues. Thus, the loss of SAA2 expression might be responsible for the occurrence and progression of HCC. Further investigations in HCC cells indicated that SAA2 may inhibit the malignant behaviours of HCC cells, suggesting that SAA2 may be a target for treating HCC.
SAA2 is a member of the SAA protein family, and it is a normal component of serum and is synthesized mainly in the liver [44]. SAA2 is a well-known biomarker of inflammation and is required for HDL and cholesterol transport. Moreover, SAA2 plays roles in cancer metastasis, intestinal physiology, lung inflammation, maternal–foetal health, local tissue changes in atherosclerosis, and tissue remodelling through interactions between metalloproteinases and specific receptors [44]. SAA2 has been reported as a candidate biomarker in non-small cell lung cancer [45]. However, the role of SAA2 in HCC progression is unclear and needs to be further explored. The present study revealed that SAA2 was markedly decreased in HCC tissues. This decrease was validated with bulk RNA data from TCGA and GTEx databases, as well as IHC and western blot data from clinical tissues. Further assays in HCC cells suggested that SAA2 is a novel suppressor of HCC progression.
Conclusions
In conclusion, the present results elucidated the B cell-related immunosuppressive landscape and revealed the differences between malignant hepatocytes in HCC tissues and normal hepatocytes in noncancer liver tissues. The present study identified a novel suppressor, SAA2, which plays a protective role in HCC progression. These findings provide a molecular basis for the development of novel targets for HCC therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- AFP
Alpha-fetoprotein
- APR
Acute phase response
- BCLC
Barcelona Clinic Liver Cancer
- DCs
Dendritic cells
- DEGs
Differentially expressed genes
- DFS
Disease-free survival
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Foetal bovine serum
- GO
Gene Ontology
- GTEx
Genotype-Tissue Expression
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HDL
High-density lipoprotein
- IHC
Immunohistochemistry
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- NAFLD
Nonalcoholic fatty liver disease
- NK
Natural killer
- OS
Overall survival
- PCA
Principal component analysis
- pDCs
Plasmacytoid dendritic cells
- QC
Quality control
- SAA2
Serum amyloid A2
- scRNA-seq
Single-cell RNA sequencing
- TCGA
The Cancer Genome Atlas
- UMAP
Uniform manifold approximation and projection
- UMI
Unique molecular identifier
Author contributions
Wenhao Hou: Writing – original draft, Writing – review and editing, Data curation. Jiaqi Jiang: Writing – original draft, Writing – review and editing, Data curation. Liyuan Zhu: Writing – review and editing, Data curation, Validation. Hongwei Xu: Writing – review and editing, Resources, Supervision. Lihui Zhu: Writing – review and editing, Conceptualization, Methodology, Resources, Supervision.
Funding
This work was supported by the Natural Science Foundation of Shandong Province [no. ZR2021QH185]; and the National Natural Science Foundation of China [no. 82103075].
Data availability
All data generated and analyzed are available from the corresponding author upon reasonable request. The RNA-seq data from this study have been deposited in the Gene Expression Omnibus (GEO) and assigned the identifier GEO: GSE299340 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE299340).
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Shandong Provincial Hospital, and informed consent was obtained from all patients according to the committee’s regulations. All methods were performed in accordance with the relevant guidelines and regulations of the committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenhao Hou and Jiaqi Jiang contributed equally to this work and shared first authorship.
References
- 1.Ganesan P, Kulik LM. Hepatocellular carcinoma: new developments. Clin Liver Dis. 2023;27:85–102. [DOI] [PubMed] [Google Scholar]
- 2.Feng R, Su Q, Huang X, Basnet T, Xu X, Ye W. Cancer situation in china: what does the China cancer map indicate from the first National death survey to the latest cancer registration? Cancer Commun (Lond). 2023;43:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR, Zhong CQ, Wang K, Chai ZT, Wei XB, et al. Patterns, treatments, and prognosis of tumor recurrence after resection for hepatocellular carcinoma with microvascular invasion: a multicenter study from China. HPB (Oxford). 2022;24:1063–73. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629–52. [DOI] [PubMed] [Google Scholar]
- 5.Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72:215–29. [DOI] [PubMed] [Google Scholar]
- 6.Lei Y, Tang R, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Applications of single-cell sequencing in cancer research: progress and perspectives. J Hematol Oncol. 2021;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slovin S, Carissimo A, Panariello F, Grimaldi A, Bouche V, Gambardella G, Cacchiarelli D. Single-Cell RNA sequencing analysis: A Step-by-Step overview. Methods Mol Biol. 2021;2284:343–65. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Abbas-Aghababazadeh F, Chen YA, Fridley BL. Statistical and bioinformatics analysis of data from bulk and Single-Cell RNA sequencing experiments. Methods Mol Biol. 2021;2194:143–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404–e421416. [DOI] [PubMed] [Google Scholar]
- 10.Ho DW, Tsui YM, Chan LK, Sze KM, Zhang X, Cheu JW, Chiu YT, Lee JM, Chan AC, Cheung ET, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun. 2021;12:3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Chen DP, Fu T, Yang JC, Ma D, Zhu XZ, Wang XX, Jiao YP, Jin X, Xiao Y, et al. Single-cell morphological and topological atlas reveals the ecosystem diversity of human breast cancer. Nat Commun. 2023;14:6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–e845820. [DOI] [PubMed] [Google Scholar]
- 13.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al. Landscape of infiltrating T cells in liver Cancer revealed by Single-Cell sequencing. Cell. 2017;169:1342–e13561316. [DOI] [PubMed] [Google Scholar]
- 14.Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D. Redefining tumor-Associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. 2020;11:1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Zhao R, Qin R, Sun H, Huang Q, Liu L, Tian Z, Nashan B, Sun C, Sun R. Panoramic comparison between NK cells in healthy and cancerous liver through single-cell RNA sequencing. Cancer Biol Med. 2022;19:1334–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol 2018, 19. [DOI] [PMC free article] [PubMed]
- 17.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. Highly parallel Genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. G:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:W191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gene Ontology C. The gene ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32:D258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milacic M, Beavers D, Conley P, Gong C, Gillespie M, Griss J, Haw R, Jassal B, Matthews L, May B, et al. The reactome pathway knowledgebase 2024. Nucleic Acids Res. 2024;52:D672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Qin C, Li T, Ma X, Qiu Y, Lin Y, Ma D, Qin Z, Sun C, Shen X et al. The E3 ubiquitin ligase TRIM7 suppressed hepatocellular carcinoma progression by directly targeting Src protein. Cell Death Differ 2019. [DOI] [PMC free article] [PubMed]
- 24.Maiga SF, Kalopissis AD, Chabert M. Apolipoprotein A-II is a key regulatory factor of HDL metabolism as appears from studies with Transgenic animals and clinical outcomes. Biochimie. 2014;96:56–66. [DOI] [PubMed] [Google Scholar]
- 25.Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, Ping Y, Zhang Y. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol. 2021;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Li A, Lei Q, Zhang Y. Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment. J Hematol Oncol. 2019;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng B, Peng J, Kang F, Zhang W, Peng E, He Q. Ferroptosis-Related gene MT1G as a novel biomarker correlated with prognosis and immune infiltration in colorectal Cancer. Front Cell Dev Biol. 2022;10:881447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Wang G, Tan X, Ke K, Zhao B, Cheng N, Dang Y, Liao N, Wang F, Zheng X, et al. MT1G serves as a tumor suppressor in hepatocellular carcinoma by interacting with p53. Oncogenesis. 2019;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei T, Lin R, Fu X, Lu Y, Zhang W, Li Z, Zhang J, Wang H. Epigenetic regulation of the DNMT1/MT1G/KLF4/CA9 axis synergises the anticancer effects of Sorafenib in hepatocellular carcinoma. Pharmacol Res. 2022;180:106244. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Lu F, Chen Z. Identification of MT1E as a novel tumor suppressor in hepatocellular carcinoma. Pathol Res Pract. 2020;216:153213. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Jiang L, Hu Y, Xiao C, Xu N, Zhou J, Zhou X. Metallothionein 1H (MT1H) functions as a tumor suppressor in hepatocellular carcinoma through regulating Wnt/beta-catenin signaling pathway. BMC Cancer. 2017;17:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Xu P, Zhu R, Gao W, Yin W, Lan P, Zhu L, Jiao N. Multi-kingdom microbial signatures in excess body weight colorectal cancer based on global metagenomic analysis. Commun Biol. 2024;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han YC, Zheng ZL, Zuo ZH, Yu YP, Chen R, Tseng GC, Nelson JB, Luo JH. Metallothionein 1 h tumour suppressor activity in prostate cancer is mediated by euchromatin methyltransferase 1. J Pathol. 2013;230:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brazao-Silva MT, Rodrigues MF, Eisenberg AL, Dias FL, de Castro LM, Nunes FD, Faria PR, Cardoso SV, Loyola AM, de Sousa SC. Metallothionein gene expression is altered in oral cancer and May predict metastasis and patient outcomes. Histopathology. 2015;67:358–67. [DOI] [PubMed] [Google Scholar]
- 35.Xie Q, Chen L, Shan X, Shan X, Tang J, Zhou F, Chen Q, Quan H, Nie D, Zhang W, et al. Epigenetic Silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int J Cancer. 2014;135:635–46. [DOI] [PubMed] [Google Scholar]
- 36.Wang HJ, Wang L, Song SS, He XL, Pan HY, Hu ZM, Mou XZ. Decreased expression of PTH1R is a poor prognosis in hepatocellular carcinoma. Cancer Biomark. 2018;21:723–30. [DOI] [PubMed] [Google Scholar]
- 37.Joachim JH, Mehta KJ. Hepcidin in hepatocellular carcinoma. Br J Cancer. 2022;127:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Kong HF, Gao XD, Dong Z, Lu Y, Huang JG, Li H, Yang YP. Immune infiltration-associated serum amyloid A1 predicts favorable prognosis for hepatocellular carcinoma. World J Gastroenterol. 2020;26:5287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L, Bartlam M, Liu Y, Pang H, Rao Z. Crystal structure of the pyridoxal-5′‐phosphate‐dependent Serine dehydratase from human liver. Protein Sci. 2009;14:791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sack GH. Serum amyloid A (SAA) proteins. Vertebrate and invertebrate respiratory proteins, lipoproteins and other body fluid proteins. Subcellular Biochemistry]; 2020. pp. 421–36.
- 41.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27:15–26. [DOI] [PubMed] [Google Scholar]
- 42.Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang K, Wang X, Song C, He Z, Wang R, Xu Y, Jiang G, Wan Y, Mei J, Mao W. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics. 2023;13:1774–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sack GH Jr. Serum amyloid A (SAA) proteins. Subcell Biochem. 2020;94:421–36. [DOI] [PubMed] [Google Scholar]
- 45.Zhang FF, Han B, Xu RH, Zhu QQ, Wu QQ, Wei HM, Cui ZL, Zhang SL, Meng MJ. Identification of plasma SAA2 as a candidate biomarker for the detection and surveillance of non-small cell lung cancer. Neoplasma. 2021;68:1301–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed are available from the corresponding author upon reasonable request. The RNA-seq data from this study have been deposited in the Gene Expression Omnibus (GEO) and assigned the identifier GEO: GSE299340 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE299340).