Abstract
Background
Cognitive impairment is a common complication of type 2 diabetes mellitus (T2DM); however, its underlying mechanisms are unclear. This study investigated the association between serum moesin levels, cognitive impairment, and glucose fluctuations in T2DM patients.
Methods
A total of 229 T2DM patients and 150 healthy controls were enrolled, and patients with T2DM were further categorized into those with mild cognitive impairment (MCI, n = 71) and without MCI (non-MCI, n = 158). An enzyme-linked immunosorbent assay (ELISA) was used to evaluate the serum levels of moesin, high-sensitivity C-reactive protein (hs-CRP), and brain-derived neurotrophic factor (BDNF) in all participants.
Results
Serum moesin levels were significantly elevated in T2DM patients compared to those in healthy controls (P < 0.001) and further increased in the MCI group compared to those in the non-MCI group (P < 0.001). Receiver operating characteristic (ROC) analysis identified an optimal moesin cutoff of 113.49 ng/mL (AUC = 0.866) for distinguishing MCI from T2DM, with 76.1% sensitivity and 86.7% specificity. Correlation analysis demonstrated that moesin was positively correlated with triglyceride, LDL-C, IMT, hs-CRP, and glucose variability markers (MAGE, MBG, SD, and MODD) but negatively correlated with years of education, BDNF, time in range (TIR), and Montreal Cognitive Assessment (MoCA) scores (P < 0.05). Multivariate logistic regression identified BMI, years of education, diabetes duration, FBG, hs-CRP, BDNF, MAGE, SD, and moesin as independent predictors of MCI in T2DM (P < 0.05).
Conclusions
These findings suggest that elevated serum moesin levels are associated with cognitive impairment in patients with T2DM, potentially mediated by glucose fluctuations, inflammation, and vascular dysfunction.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-025-01876-5.
Keywords: Moesin, Type 2 diabetes mellitus, Cognitive impairment, Glucose fluctuations, Mild cognitive impairment
Background
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by insulin resistance, hyperglycemia, and systemic inflammation. Apart from its well-known vascular and metabolic complications, T2DM is increasingly recognized as a significant risk factor for cognitive decline and dementia, including Alzheimer’s disease (AD) and vascular cognitive impairment (VCI) [1]. Notably, cognitive deterioration in T2DM cannot be fully explained by chronic hyperglycemia alone. Recent evidence highlights the role of glycemic variability-acute glucose fluctuations, as key contributors to neurodegeneration [2]. These glucose swings exacerbate oxidative stress, endothelial dysfunction, and blood-brain barrier (BBB) breakdown, all of which may accelerate cognitive decline in diabetic individuals [3].
Moesin, a member of the ezrin-radixin-moesin (ERM) protein family, plays a pivotal role in linking the actin cytoskeleton to the plasma membrane and is essential for maintaining endothelial cell integrity and cytoskeletal dynamics [4]. Phosphorylated moesin regulates cellular shape, permeability, and immune signaling, particularly in vascular and neural tissues [5]. Emerging studies have suggested that moesin expression is upregulated under conditions involving inflammation, vascular injury, and neurodegeneration [6]. Importantly, elevated moesin levels have been observed in models of cerebrovascular disease and Alzheimer’s pathology, suggesting its potential involvement in linking vascular impairment to neuronal dysfunction [7, 8].
Recent research has also indicated the presence of moesin in circulation, with serum levels potentially reflecting endothelial activation or injury [9]. However, the clinical relevance of circulating moesin, particularly in the context of cognitive impairment and glycemic instability in T2DM, remains poorly understood. Given the overlapping pathophysiological pathways of vascular dysfunction, inflammation, and neurodegeneration in diabetes, serum moesin may serve as a promising biomarker that connects metabolic dysregulation with cognitive decline.
This study aimed to investigate the association between serum moesin level, cognitive performance, and glycemic variability in patients with T2DM. Understanding this relationship may illuminate novel pathophysiological mechanisms underlying diabetes-associated cognitive impairment and identify moesin as a potential biomarker or therapeutic target for the early detection and management of neurovascular complications in diabetic populations.
Methods
Study population
A total of 229 patients with T2DM were enrolled in this study between January 2021 and June 2024 at the Department of Endocrinology and Metabolism, Shanghai Pudong New Area Gongli Hospital. The American Diabetes Association criteria were used to diagnose T2DM [10]. Following the MoCA examination, all individuals with T2DM were allocated to two groups: those with and without cognitive decline. Supplementary Table 1 presented information on the comorbidities and primary medications of diabetic patients. The exclusion criteria were as follows: (1) T1DM or other secondary diabetes (such as after pancreatectomy); (2) severe liver and kidney dysfunction or those who have received renal replacement therapy; (3) patients undergoing anti-tumor treatment; (4) patients diagnosed with dementia or those who have taken anti-dementia drugs or have potential comorbidities that affect cognitive function (such as depression, vitamin B1, B12, or folate deficiency); (5) carotid artery stenosis ≥ 80% of the lumen area; and (6) individuals with uncontrolled thyroid dysfunction. (7) Patients had been infected with active infectious disease (COVID, influenza) within one month. Additionally, we selected 150 participants who underwent physical examinations and were confirmed not to have T2DM to serve as a healthy control group. The protocol for this study was approved (GLYYls2022-017) by the Research Ethics Committee of Shanghai Pudong New Area Gongli Hospital. The participating volunteers provided written informed consent.
The montreal cognitive assessment (MoCA)
Cognitive function was assessed using the MoCA [11], which ranges from 0 to 30. The MoCA was selected because of its higher sensitivity for detecting mild cognitive impairment (MCI) than the Mini-Mental State Examination (MMSE) and the Rey Auditory Verbal Learning Test (RAVLT) [12–15]. Participants with T2DM who scored ≥ 26 were classified as cognitively normal, whereas those scoring < 26 were considered cognitively impaired. To exclude individuals with probable dementia, only participants with MoCA scores ≥ 21 were included. This cutoff was based on previous studies indicating that scores between 21 and 26 are indicative of early cognitive decline consistent with MCI, whereas scores < 21 typically reflect moderate-to-severe impairment or dementia [11, 12, 15]. Focusing on this score range allowed for a more homogeneous sample of individuals with early-stage cognitive impairment, enhancing the specificity of our analysis.
Clinical data assessment
Upon admission, comprehensive clinical data were gathered from all patients, including age, sex, body mass index (BMI), educational background, diabetes duration, blood pressure, and smoking or alcohol consumption habits. Information on alcohol consumption and smoking status was collected during the enrolment process. BMI was calculated as weight divided by height squared (kg/m2). Fasting blood glucose (FBG) levels were assessed using the glucose oxidase technique. Glycosylated hemoglobin (HbA1c) was measured using high-performance liquid chromatography (HPLC) (BK-LCI1100 Biobase, China). The blood lipid profile, which includes triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), along with serum creatinine, was analyzed using an automatic biochemistry analyzer (Hitachi 7170, Tokyo, Japan). Carotid intima-media thickness (IMT) was evaluated using high-resolution B-mode ultrasonography (Philips HDI 4000 ultrasound, Bothell Everett Highway, USA).
Glucose fluctuations assessment
Continuous Glucose Monitoring (CGM) is used to monitor glucose fluctuations. For three days, each patient had a CGM (Dexcom, San Diego, CA, USA) sensor attached to them, and their glucose levels were recorded four times daily. The parameters of the characteristic glucose pattern for each individual were calculated, including the mean amplitude of glycemic excursions (MAGE), mean blood glucose (MBG), and standard deviation of glucose (SD) of MBG [16]. Total Indicator Runout (TIR) was measured using a dial indicator and a reference surface, and the Mean of Daily Differences (MODD) was calculated from a series of glucose readings.
ELISA detection of serum moesin and other proteins
Fasting blood samples were obtained from 229 patients with T2DM and 150 healthy control participants. After centrifuging the venous blood samples for 10 min at 3,000 rpm to extract the serum, we stored them at -80 °C. Serum moesin (CSB-EL015048HU, CUSABIO, Wuhan, China), hs-CRP (DCRP00B, R&D Systems, USA), and BDNF (DBD00, R&D Systems, USA) levels were measured using an ELISA kit, and absorbance at 450 nm was assessed by a microplate reader (BK-EL10E Biobase, China).
Statistical analysis
Categories are shown as numbers (percentages), and quantitative data are shown as the mean ± standard deviation (SD). SPSS 20.0 (SPSS Inc., Chicago, Illinois, USA) was used for all statistical analyses. For comparing groups, the t-test, Mann-Whitney U test, or χ2 test was used for quantitative and categorical data, respectively. Pearson’s test was used to analyze correlations. For mild cognitive impairment (MCI), independent risk variables were examined using univariate and multivariate logistic regression models. A receiver operating characteristic (ROC) curve was used to determine the moesin cutoff value that separates T2DM participants with MCI from those without MCI. Statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Baseline characteristics
A Student’s t-test was used to compare individuals with T2DM and healthy controls. The results revealed that several biomarkers were significantly elevated in the T2DM group, including body mass index (BMI), duration of diabetes (years), systolic blood pressure (SBP), fasting blood glucose (FBG), glycated hemoglobin (HbA1c), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), intima-media thickness (IMT), creatinine, high-sensitivity C-reactive protein (hs-CRP), brain-derived neurotrophic factor (BDNF), and moesin levels (Table 1). However, there were no significant differences in age, sex, years of education, alcohol consumption, or smoking status between the T2DM group and the healthy controls (P > 0.05).
Table 1.
Baseline characteristics of the healthy controls and subjects with T2DM
| Parameters | Healthy controls (n = 150) |
T2DM (n = 229) |
P-value |
|---|---|---|---|
| Age (years) | 61.25 ± 9.24 | 62.37 ± 9.63 | 0.261 |
| Sex (male, %) | 79 (52.7%) | 121 (52.8%) | 0.974 |
| BMI (kg/m2) | 24.27 ± 2.77 | 25.21 ± 2.83 | **0.001 |
| Education (years) | 13.41 ± 2.55 | 13.65 ± 2.79 | 0.390 |
| Diabetes duration (years) | - | 6.91 ± 2.23 | ***<0.001 |
| Smoking, n (%) | 26 (17.3%) | 53 (23.1%) | 0.173 |
| Drinking, n (%) | 35 (23.3%) | 64 (27.9%) | 0.317 |
| SBP (mmHg) | 125.77 ± 17.47 | 131.43 ± 21.46 | **0.005 |
| FBG (mmol/L) | 5.63 ± 1.04 | 7.70 ± 1.30 | ***<0.001 |
| HbA1c (%) | 5.46 ± 1.13 | 8.53 ± 1.66 | ***<0.001 |
| TG (mmol/L) | 1.91 ± 0.25 | 2.44 ± 0.31 | ***<0.001 |
| TC (mmol/L) | 4.52 ± 0.64 | 5.16 ± 0.72 | ***<0.001 |
| LDL-C (mmol/L) | 2.69 ± 0.47 | 3.36 ± 0.61 | ***<0.001 |
| HDL-C (mmol/L) | 1.35 ± 0.25 | 1.24 ± 0.19 | ***<0.001 |
| IMT (mm) | 0.84 ± 0.12 | 1.01 ± 0.20 | ***<0.001 |
| Creatinine (µmol/L) | 61.68 ± 7.38 | 65.86 ± 7.29 | ***<0.001 |
| hs-CRP (µg/mL) | 1.07 ± 0.22 | 1.27 ± 0.28 | ***<0.001 |
| BDNF (ng/mL) | 47.09 ± 6.17 | 39.75 ± 5.86 | ***<0.001 |
| Moesin (ng/mL) | 56.35 ± 8.73 | 109.45 ± 17.89 | ***<0.001 |
Continuous data are represented as Mean ± SD, and analyzed using t test or Wilcoxon-Mann-Whitney test. Categorical data are represented as frequency (percentage), and analyzed using the chi-square test. Abbreviation: BMI, body mass index; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; hs-CRP, high-sensitivity C-reactive protein; BDNF, brain-derived neurotrophic factor
*P < 0.05, **P < 0.01, ***P < 0.001
We also used the Student’s t-test to compare T2DM patients in two groups: those without MCI (n = 158) and those with MCI (n = 71). The results showed that individuals in the T2DM with MCI group had significantly elevated levels of various health indicators, including BMI, SBP, FBG, TG, TC, LDL-C, IMT, creatinine, hs-CRP, MAGE, MBG, SD, mean of daily differences (MODD), and moesin. In contrast, education (years), BDNF, TIR, and MoCA scores were significantly lower in the T2DM with MCI group (Table 2). However, age, sex, duration of diabetes, alcohol consumption, smoking, HbA1c, and HDL-C did not significantly differ between the T2DM patients in the MCI group and those in the non-MCI group (P > 0.05).
Table 2.
Baseline characteristics of T2DM with and without MCI
| Parameters | T2DM without MCI (n = 158) |
T2DM with MCI (n = 71) |
P-value |
|---|---|---|---|
| Age (years) | 61.87 ± 8.63 | 63.48 ± 11.56 | 0.296 |
| Sex (male, %) | 86 (54.4%) | 35 (49.3%) | 0.472 |
| BMI (kg/m2) | 24.93 ± 2.76 | 25.83 ± 2.90 | *0.026 |
| Education (years) | 14.29 ± 2.86 | 12.23 ± 2.03 | ***<0.001 |
| Diabetes duration (years) | 6.75 ± 2.21 | 7.27 ± 2.25 | 0.105 |
| Smoking, n (%) | 33 (20.9%) | 20 (28.2%) | 0.227 |
| Drinking, n (%) | 40 (25.3%) | 24 (33.8%) | 0.186 |
| SBP (mmHg) | 129.00 ± 20.92 | 136.85 ± 21.79 | *0.010 |
| FBG (mmol/L) | 7.53 ± 1.27 | 8.07 ± 1.28 | **0.004 |
| HbA1c (%) | 8.48 ± 1.64 | 8.63 ± 1.70 | 0.519 |
| TG (mmol/L) | 2.41 ± 0.31 | 2.50 ± 0.30 | *0.032 |
| TC (mmol/L) | 5.06 ± 0.68 | 5.37 ± 0.75 | **0.003 |
| LDL-C (mmol/L) | 3.23 ± 0.57 | 3.66 ± 0.58 | ***<0.001 |
| HDL-C (mmol/L) | 1.25 ± 0.19 | 1.22 ± 0.21 | 0.219 |
| IMT (mm) | 0.96 ± 0.16 | 1.14 ± 0.24 | ***<0.001 |
| Creatinine (µmol/L) | 65.10 ± 7.12 | 67.57 ± 7.41 | *0.017 |
| hs-CRP (µg/mL) | 1.23 ± 0.26 | 1.36 ± 0.31 | **0.002 |
| BDNF (ng/mL) | 41.69 ± 5.27 | 35.41 ± 4.67 | ***<0.001 |
| MAGE (mmol/L) | 4.00 ± 0.88 | 5.14 ± 0.96 | ***<0.001 |
| MBG (mmol/L) | 8.43 ± 1.69 | 9.78 ± 2.01 | ***<0.001 |
| SD (mmol/L) | 1.88 ± 0.64 | 2.28 ± 0.66 | ***<0.001 |
| TIR (%) | 76.17 ± 17.49 | 68.78 ± 7.26 | ***<0.001 |
| MODD (mmol/L) | 1.96 ± 0.68 | 2.29 ± 0.55 | ***<0.001 |
| Moesin (ng/mL) | 102.03 ± 12.36 | 125.94 ± 17.30 | ***<0.001 |
| MoCA | 27.86 ± 1.48 | 23.17 ± 1.48 | ***<0.001 |
Abbreviation: BMI, body mass index; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; hs-CRP, high-sensitivity C-reactive protein; BDNF, brain-derived neurotrophic factor; MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose; SD, standard deviation of glucose; TIR, time in range; MODD, mean of daily differences; MoCA, Montreal Cognitive Assessment; MCI, mild cognitive impairment
*P < 0.05, **P < 0.01, ***P < 0.001
Patients with T2DM have elevated serum moesin levels
Serum moesin levels in T2DM patients (n = 229) and healthy controls (n = 150) were assessed using ELISA. In addition, we classified all T2DM patients into those with normal conditions (non-MCI group, n = 158) and those with cognitive impairment (MCI group, n = 71). Compared to healthy controls, patients with T2DM exhibited significantly higher serum moesin levels (Fig. 1A). Furthermore, moesin levels were markedly elevated in T2DM patients with MCI compared to those without MCI (Fig. 1B). Furthermore, the examination of the Receiver Operating Characteristic (ROC) curve revealed that the ideal cutoff value for serum moesin was 113.49 ng/mL, with an Area Under the Curve (AUC) of 0.866, a sensitivity of 76.1%, and a specificity of 86.7% (Fig. 1C). Therefore, the ROC curve was used to determine the optimal cutoff value (113.49 ng/mL) for serum moesin, distinguishing T2DM patients with MCI from those without MCI.
Fig. 1.
Serum moesin levels are higher in T2DM patients. (A) Comparison of serum moesin levels between healthy controls (n = 150) and T2DM patients (n = 229). (B) Comparison of serum moesin levels between T2DM patients with normal conditions (non-MCI group, n = 158) and T2DM patients with cognitive impairment (MCI group, n = 71). (C) The ROC curve was used to obtain the optimal cutoff value of serum moesin, distinguishing T2DM patients with MCI from those without MCI. A t-test was applied. ***P < 0.001. MCI: mild cognitive impairment
Correlation between serum moesin and clinical indicators associated with cognitive impairment
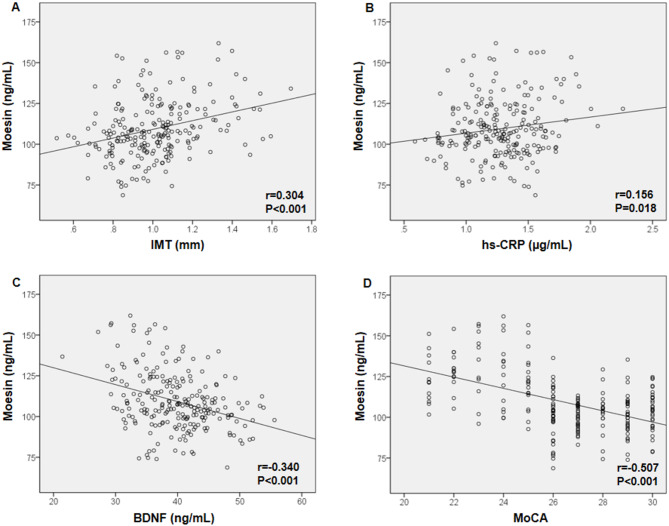
Several variables were positively correlated with blood moesin levels in a study that included 229 participants with T2DM, including TG (r = 0.197, P = 0.003), TC (r = 0.158, P = 0.016), LDL-C (r = 0.304, P < 0.001), IMT (r = 0.304, P < 0.001), hs-CRP (r = 0.156, P = 0.018), MAGE (r = 0.378, P < 0.001), MBG (r = 0.305, P < 0.001), SD (r = 0.190, P = 0.004), and MODD (r = 0.199, P = 0.002). On the other hand, moesin levels were negatively related with education (years) (r=-0.169, P = 0.010), BDNF (r=-0.340, P < 0.001), TIR (r=-0.211, P = 0.001) and MoCA (r=-0.507, P < 0.001) (Table 3). Moreover, blood moesin levels were negatively associated with BDNF and MoCA but positively correlated with IMT and hs-CRP, according to Pearson’s correlation test (Fig. 2).
Table 3.
Correlation between serum Moesin and clinical indicators
| Parameters | All T2DM subjects (n = 229) | |
|---|---|---|
| r | P | |
| Age (years) | 0.103 | 0.119 |
| BMI (kg/m2) | 0.076 | 0.252 |
| Education (years) | -0.169 | *0.010 |
| Diabetes duration (years) | 0.046 | 0.489 |
| SBP (mmHg) | 0.129 | 0.052 |
| FBG (mmol/L) | 0.114 | 0.085 |
| HbA1c (%) | 0.074 | 0.264 |
| TG (mmol/L) | 0.197 | **0.003 |
| TC (mmol/L) | 0.158 | *0.016 |
| LDL-C (mmol/L) | 0.304 | ***<0.001 |
| HDL-C (mmol/L) | -0.057 | 0.391 |
| IMT (mm) | 0.304 | ***<0.001 |
| Creatinine (µmol/L) | 0.114 | 0.086 |
| hs-CRP (µg/mL) | 0.156 | *0.018 |
| BDNF (ng/mL) | -0.340 | ***<0.001 |
| MAGE (mmol/L) | 0.378 | ***<0.001 |
| MBG (mmol/L) | 0.305 | ***<0.001 |
| SD (mmol/L) | 0.190 | **0.004 |
| TIR (%) | -0.211 | ***0.001 |
| MODD (mmol/L) | 0.199 | **0.002 |
| MoCA | -0.507 | ***<0.001 |
The correlation between serum Moesin and continuous variables was analyzed using the Pearson correlation test. Abbreviation: BMI, body mass index; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; hs-CRP, high-sensitivity C-reactive protein; BDNF, brain-derived neurotrophic factor; MoCA, Montreal Cognitive Assessment; TIR, time in range; MODD, mean of daily differences
*P < 0.05, **P < 0.01, ***P < 0.001
Fig. 2.
Correlation between serum moesin levels and clinical indicators related to cognitive impairment. A Pearson correlation test was performed on moesin with (A) IMT, (B) hs-CRP, (C) BDNF, and (D) MoCA in all T2DM patients. IMT: intima-media thickness, hs-CRP: high-sensitivity C-reactive protein, BDNF: brain-derived neurotrophic factor. MoCA: Montreal Cognitive Assessment
Correlation between serum moesin and clinical indicators associated with glucose fluctuations
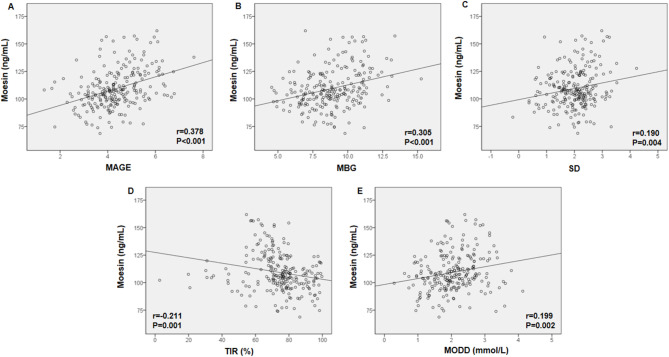
Pearson’s correlation test was used to evaluate the association between serum moesin levels and clinical markers associated with glucose fluctuations. The results indicated that serum moesin levels were positively related to MAGE, MBG, SD, and MODD and adversely associated with TIR in all T2DM patients (Fig. 3).
Fig. 3.
Correlation between serum moesin levels and clinical indicators related to glucose fluctuations. Pearson’s correlation test was performed on moesin with (A) MAGE, (B) MBG, (C) SD, (D) TIR, and (E) MODD in all T2DM patients. MAGE: mean amplitude of glycemic excursions, MBG: mean blood glucose, SD: standard deviation, TIR: time in range, MODD: mean of daily differences
Logistic regression analysis in predicting the onset of MCI in T2DM individuals
Logistic regression analysis was performed to determine the independent risk factors for MCI in patients with T2DM. The results of the univariate logistic regression study revealed that education (years) (OR = 0.620, 95%CI = 0.429–0.895; P = 0.011), duration of diabetes (OR = 1.516, 95%CI = 1.034–2.223; P = 0.033), BDNF (OR = 0.758, 95%CI = 0.637–0.903; P = 0.002), MAGE (OR = 4.384, 95%CI = 1.711–11.230; P = 0.002), SD (OR = 6.372, 95%CI = 1.435–28.302; P = 0.015), and moesin (OR = 1.068, 95%CI = 1.009–1.130; P = 0.023) (Table 4). In addition, the multivariate logistic regression analysis results demonstrated that BMI (OR = 1.299, 95%CI = 1.049–1.608; P = 0.016), education (years) (OR = 0.698, 95%CI = 0.537–0.907; P = 0.007), duration of diabetes (years) (OR = 1.401, 95%CI = 1.070–1.833; P = 0.014), FBG (OR = 1.583, 95%CI = 1.001–2.503; P = 0.049), hs-CRP (OR = 9.163, 95%CI = 1.036–81.072; P = 0.046), BDNF (OR = 0.752, 95%CI = 0.655–0.864; P < 0.001), MAGE (OR = 4.269, 95%CI = 1.977–9.220; P < 0.001), SD (OR = 3.962, 95%CI = 1.266–12.401; P = 0.018), and moesin (OR = 1.106, 95%CI = 1.053–1.161; P < 0.001) (Table 5).
Table 4.
Logistic univariate regression for T2DM with MCI
| Characteristics | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Age (years) | 1.036 | 0.958–1.120 | 0.381 |
| Sex (male, %) | 0.211 | 0.041–1.101 | 0.065 |
| BMI (kg/m2) | 1.173 | 0.897–1.533 | 0.244 |
| Education (years) | 0.620 | 0.429–0.895 | *0.011 |
| Diabetes duration (years) | 1.516 | 1.034–2.223 | *0.033 |
| Smoking, n (%) | 3.370 | 0.507–22.389 | 0.205 |
| Drinking, n (%) | 3.431 | 0.600-19.605 | 0.166 |
| SBP (mmHg) | 1.012 | 0.981–1.044 | 0.451 |
| FBG (mmol/L) | 1.352 | 0.761–2.403 | 0.304 |
| HbA1c (%) | 1.108 | 0.707–1.736 | 0.655 |
| TG (mmol/L) | 3.101 | 0.235–40.967 | 0.390 |
| TC (mmol/L) | 1.501 | 0.471–4.781 | 0.492 |
| LDL-C (mmol/L) | 3.292 | 0.779–13.913 | 0.105 |
| HDL-C (mmol/L) | 0.712 | 0.009–55.844 | 0.879 |
| IMT (mm) | 16.096 | 0.354-731.513 | 0.154 |
| Creatinine (µmol/L) | 1.025 | 0.928–1.132 | 0.631 |
| hs-CRP (µg/mL) | 4.957 | 0.243-101.069 | 0.298 |
| BDNF (ng/mL) | 0.758 | 0.637–0.903 | **0.002 |
| MAGE | 4.384 | 1.711–11.230 | **0.002 |
| MBG | 1.383 | 0.923–2.074 | 0.116 |
| SD | 6.372 | 1.435–28.302 | *0.015 |
| TIR (%) | 0.988 | 0.937–1.042 | 0.653 |
| MODD (mmol/L) | 1.990 | 0.498–7.948 | 0.330 |
| Moesin (ng/mL) | 1.068 | 1.009–1.130 | *0.023 |
Abbreviation: BMI, body mass index; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; hs-CRP, high-sensitivity C-reactive protein; BDNF, brain-derived neurotrophic factor; MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose; SD, standard deviation of glucose; TIR, time in range; MODD, mean of daily differences; MCI, mild cognitive impairment; CI: confidence interval
*P < 0.05, **P < 0.01, ***P < 0.001
Table 5.
Logistic multivariate regression for T2DM with MCI
| Characteristics | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| BMI (kg/m2) | 1.299 | 1.049–1.608 | *0.016 |
| Education (years) | 0.698 | 0.537–0.907 | **0.007 |
| Diabetes duration (years) | 1.401 | 1.070–1.833 | *0.014 |
| FBG (mmol/L) | 1.583 | 1.001–2.503 | *0.049 |
| hs-CRP (mg/L) | 9.163 | 1.036–81.072 | *0.046 |
| BDNF (ng/mL) | 0.752 | 0.655–0.864 | ***<0.001 |
| MAGE | 4.269 | 1.977–9.220 | ***<0.001 |
| SD | 3.962 | 1.266–12.401 | *0.018 |
| Moesin (ng/mL) | 1.106 | 1.053–1.161 | ***<0.001 |
Abbreviation: BMI, body mass index; FBG, fasting blood glucose; hs-CRP, high-sensitivity C-reactive protein; BDNF, brain-derived neurotrophic factor; MAGE, mean amplitude of glycemic excursions; SD, standard deviation of glucose; MCI, mild cognitive impairment
*P < 0.05, **P < 0.01, ***P < 0.001
Discussion
This study investigated the role of serum moesin in patients with T2DM, particularly in relation to MCI. Our findings demonstrated that serum moesin levels were significantly elevated in T2DM patients compared to healthy controls and were further increased in T2DM patients with MCI. Additionally, moesin exhibited significant correlations with various metabolic, inflammatory, and glycemic variability markers, suggesting its potential as a biomarker for cognitive decline in T2DM.
Our results showed that serum moesin levels were higher in T2DM patients than in healthy controls, consistent with previous studies indicating that moesin, a cytoskeletal protein, may be involved in inflammatory and metabolic dysregulation in diabetes [17]. Notably, moesin levels were higher in T2DM patients with MCI, suggesting an association with cognitive dysfunction. These findings align with research demonstrating that endothelial dysfunction and chronic low-grade inflammation contribute to neurodegeneration in patients with diabetes [18]. ROC curve analysis further supported the diagnostic potential of moesin, with an AUC of 0.866, indicating good discriminatory power for identifying MCI among T2DM patients. Although the ROC curve for moesin indicates promising predictive potential, its performance should be interpreted in the context of established biomarkers such as plasma Aβ42/Aβ40 and neurofilament light chain (NfL), which generally demonstrate higher diagnostic accuracy but require greater financial and technical resources [19, 20]. In contrast to cognitive screening tools like the MoCA, moesin may offer a more objective and scalable option, especially in resource-limited settings where access to trained evaluators is constrained [12].
Moesin, a member of the ezrin-radixin-moesin (ERM) protein family, primarily functions as a cytoskeletal linker, anchoring the actin cytoskeleton to the plasma membrane and playing key roles in cell shape, adhesion, and signaling [21]. Although moesin is typically an intracellular protein, recent studies have suggested its presence in the extracellular environment, including the bloodstream, particularly under pathological conditions such as inflammation, sepsis, or cancer [22]. The secretion mechanism of moesin is not fully understood because it lacks classical secretion signals. However, it is reported to be released via non-classical pathways, such as vesicle-mediated exocytosis, microvesicle shedding, or during cell damage or apoptosis, where membrane integrity is compromised [22, 23]. The serum concentration of moesin has been observed to correlate inversely with its expression in the cell membrane, particularly in immune and endothelial cells. During inflammatory activation or endothelial dysfunction, moesin detaches from the membrane and is redistributed within the cytoplasm or released extracellularly, leading to a reduction in membrane-associated moesin and a corresponding increase in circulating moesin levels [22, 24].
Moesin levels were positively correlated with lipid profiles (TG, TC, and LDL-C), IMT, hs-CRP, and glycemic variability indices (MAGE, MBG, SD, and MODD) and negatively correlated with BDNF, TIR, and MoCA scores. These associations suggest that moesin may be influenced by metabolic dysregulation, vascular damage, and chronic inflammation, all of which have been implicated in the cognitive decline associated with diabetes [25]. The inverse relationship between moesin and BDNF is particularly noteworthy, as BDNF is a neuroprotective factor, and its reduction is linked to neurodegeneration [26]. The strong positive correlation between moesin and glycemic variability (MAGE, SD, and MODD) is consistent with previous studies showing that glucose fluctuations exacerbate oxidative stress and endothelial dysfunction, accelerating cognitive impairment in diabetes [27]. Our findings further support the notion that maintaining stable glucose levels may mitigate cognitive decline in patients with T2DM.
Logistic regression analysis identified moesin as an independent risk factor for MCI in T2DM, along with BMI, education level, diabetes duration, FBG, hs-CRP, BDNF, and glycemic variability. This is consistent with prior research suggesting that inflammatory markers (e.g., hs-CRP) and metabolic dysregulation contribute to cognitive impairment [28]. The association between moesin levels and MCI remained significant even after adjusting for confounding factors, reinforcing the potential role of moesin in diabetic neurodegeneration.
Previous studies have highlighted the role of endothelial dysfunction and inflammation in diabetic complications, including cognitive decline [29]. Our findings extend this knowledge by introducing moesin as a novel biomarker for metabolic disturbances and cognitive impairment. While prior research has focused on traditional markers such as hs-CRP and HbA1c, our study suggests that moesin may offer additional predictive value, to assess glycemic instability-related cognitive risk. However, some discrepancies exist. Unlike previous reports [2], we did not find a significant association between HbA1c levels and MCI in our cohort. This finding highlights the potential role of acute glucose fluctuations in cognitive decline, rather than chronic hyperglycemia. Large-scale studies, such as the Atherosclerosis Risk in Communities (ARIC) study and Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial, have emphasized the impact of glucose dysregulation on cognitive function. Specifically, the ARIC study found that variability in glycemic control was associated with an increased risk of dementia, independent of the average glucose levels [30]. Likewise, the ACCORD-MIND trial reported no significant cognitive benefits from intensive glucose-lowering, suggesting that minimizing glycemic variability may be a more effective strategy for preserving cognitive function in patients with T2DM [31].
Furthermore, moesin plays a pivotal mechanistic role in linking vascular impairment to neurodegeneration through its involvement in endothelial dysfunction, BBB disruption, and inflammatory signaling. Moesin regulates cytoskeletal dynamics and cell adhesion in endothelial cells, and its activation, typically via phosphorylation, facilitates actin remodeling, resulting in increased vascular permeability and compromised BBB integrity [32]. This dysfunction permits the translocation of peripheral inflammatory mediators and immune cells into the brain parenchyma, thereby exacerbating neuroinflammation, which is a key contributor to neurodegenerative processes [33]. Additionally, moesin interacts with Toll-like receptor (TLR) signaling pathways, particularly TLR4, amplifying pro-inflammatory cascades that further damage neuronal structures [34]. Activation of the RhoA/ROCK signaling pathway has also been shown to phosphorylate moesin, leading to cytoskeletal reorganization and increased vascular permeability, which further compromises the BBB and promotes neuroinflammation [35]. Another study demonstrated that dysregulation of the RhoA/ROCK/moesin axis contributes to endothelial dysfunction and has been implicated in cerebrovascular pathology and neurodegenerative diseases, potentially linking moesin to cognitive decline [36]. Elevated moesin expression has been observed in models of cerebrovascular disease and Alzheimer’s pathology, supporting its role in bridging vascular injury and neuronal degeneration [37].
Further exploration of moesin as a biomarker holds significant therapeutic implications, particularly in the context of vascular dysfunction, neuroinflammation, and metabolic disorders. As a key regulator of cytoskeletal remodeling, cell adhesion, and endothelial barrier integrity, moesin is critically involved in the pathogenesis of diseases such as diabetes mellitus, neurodegenerative disorders, and cerebrovascular dysfunctions [6, 32, 38]. Its phosphorylation status modulates endothelial permeability and leukocyte transmigration, linking moesin to inflammatory cascades and BBB disruption [36]. Therapeutically, targeting the upstream pathways that regulate moesin activation, such as the RhoA/ROCK signaling axis, may offer new avenues for mitigating inflammation-induced tissue damage and cognitive decline [39]. Moreover, monitoring serum moesin levels could aid in early disease detection, progression monitoring, and treatment response evaluation, potentially facilitating precision medicine strategies in various clinical settings.
Limitations
However, this study had several limitations. First, the relatively small sample size limited the generalizability of the findings and necessitated cautious interpretation. Second, the cross-sectional design precludes assessment of temporal or causal relationships. Third, data on participants’ antidiabetic medications were not collected, despite the fact that certain drugs (e.g., GLP-1 receptor agonists and SGLT2 inhibitors) have established cardiovascular and neuroprotective effects that could influence cognitive outcomes. Future studies should consider the use of medication to control for these potential confounders. Additionally, longitudinal research is required to determine whether elevated moesin levels precede cognitive decline. Reverse causality may have influenced the observed associations, as cognitive impairment itself could alter lifestyle or metabolic factors-such as physical activity or glucose metabolism-that affect moesin expression [40]. Finally, the molecular mechanisms by which moesin contributes to neurodegeneration remain unclear and require further investigation.
Conclusions
In summary, our study demonstrated that serum moesin levels are elevated in T2DM patients, particularly those with MCI, and are associated with metabolic, inflammatory, and glycemic instability markers. Moesin may serve as a promising biomarker for identifying patients with T2DM at a higher risk of cognitive impairment. Future studies should explore therapeutic strategies targeting moesin-related pathways to mitigate neurodegeneration in diabetes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank everyone who participated in this study.
Author contributions
Each author played a vital role in the work presented, whether it involved the initial idea, designing the study, carrying out the research, gathering data, analyzing and interpreting the results, or contributing to all these aspects. They participated in drafting, revising, or critically evaluating the manuscript, provided their final approval for the version to be published, agreed on the journal for submission, and accepted responsibility for every part of the work.
Funding
This study was supported by the Shanghai Pudong New Area Health Commission General Project (Grant No. PW2021A-33).
Data availability
The datasets utilized and examined in this study can be obtained from the corresponding author upon reasonable requests.
Declarations
Ethics approval and consent to participate
The Ethics Committee of Shanghai Pudong New District Gongli Hospital approved this study (GLYYls2022-017). The authors adhered to all standard procedures in accordance with the 1964 Declaration of Helsinki. Informed consent has been obtained from all individuals participating in this investigation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sridhar GR, Lakshmi G, Nagamani G. Emerging links between type 2 diabetes and alzheimer’s disease. World J Diabetes. 2015;6(5):744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32(2):221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan XY, Wang XG. Mild cognitive impairment in type 2 diabetes mellitus and related risk factors: a review. Rev Neurosci. 2017;28(7):715–23. [DOI] [PubMed] [Google Scholar]
- 4.McClatchey AI. ERM proteins at a glance. J Cell Sci. 2014;127(Pt 15):3199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kása A, Csortos C, Verin AD. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers. 2015;3(1–2):e974448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adyshev DM, Dudek SM, Moldobaeva N, Kim KM, Ma SF, Kasa A, et al. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am J Physiol Lung Cell Mol Physiol. 2013;305(3):240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckmann A, Ramirez P, Gamez M, Gonzalez E, De Mange J, Bieniek KF, et al. Moesin is an effector of tau-induced actin overstabilization, cell cycle activation, and neurotoxicity in alzheimer’s disease. iScience. 2023;26(3):106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Torbey MT. Angiogenesis and Blood-Brain barrier permeability in vascular remodeling after stroke. Curr Neuropharmacol. 2020;18(12):1250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6(9):1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015; 38 Suppl: S8-S16. [DOI] [PubMed]
- 11.Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI working group of the European consortium on alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 13.Arevalo-Rodriguez I, Smailagic N, Roqué I, Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;2015(3):1–68. [DOI] [PMC free article] [PubMed]
- 14.Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to alzheimer’s disease. J Am Geriatr Soc. 2014;62(4):679–84. [DOI] [PubMed] [Google Scholar]
- 15.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal cognitive assessment in community dwelling older adults residing in the southeastern US. Int J Geriatr Psychiatry. 2009;24(2):197–201. [DOI] [PubMed] [Google Scholar]
- 16.Xia W, Luo Y, Chen YC, Chen H, Ma J, Yin X. Glucose fluctuations are linked to disrupted brain functional architecture and cognitive impairment. J Alzheimers Dis. 2020;74(2):603–13. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Chen Z, Liu L, Tang H, Zhu H, Tang S. Role of Moesin in the effect of glucagon-like peptide-1 on advanced glycation end products-induced endothelial barrier dysfunction. Cell Signal. 2022;90:110193. [DOI] [PubMed] [Google Scholar]
- 18.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, et al. High performance plasma amyloid-β biomarkers for alzheimer’s disease. Nature. 2018;554(7691):249–54. [DOI] [PubMed] [Google Scholar]
- 20.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870–81. [DOI] [PubMed] [Google Scholar]
- 21.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11(4):276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Wang J, Zhang L, Zhu J, Zeng Y, Huang JA. Moesin is a novel biomarker of endothelial injury in sepsis. J Immunol Res. 2021; 2021: 6695679. [DOI] [PMC free article] [PubMed]
- 23.Marostica G, Gelibter S, Gironi M, Nigro A, Furlan R. Extracellular vesicles in neuroinflammation. Front Cell Dev Biol. 2021;8:623039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solinet S, Mahmud K, Stewman SF, Ben El Kadhi K, Decelle B, Talje L, et al. The actin-binding ERM protein Moesin binds to and stabilizes microtubules at the cell cortex. J Cell Biol. 2013;202(2):251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umegaki H. Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin Interv Aging. 2014;9:1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-Derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15(10):18381–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schram MT, Euser SM, de Craen AJ, Witteman JC, Frölich M, Hofman A, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55(5):708–16. [DOI] [PubMed] [Google Scholar]
- 29.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to alzheimer disease. Diabetes. 2014;63(7):2262–72. [DOI] [PubMed] [Google Scholar]
- 30.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161(11):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. Effects of intensive glucose Lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Liu Q, Ma M, Chen LJ, Yu J, Xiong K, et al. Involvement of Moesin phosphorylation in ischemia/reperfusion induced inner blood-retinal barrier dysfunction. Int J Ophthalmol. 2020;13(4):545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zawawi KH, Kantarci A, Schulze-Späte U, Fujita T, Batista EL Jr, Amar S, et al. Moesin-induced signaling in response to lipopolysaccharide in macrophages. J Periodontal Res. 2010;45(5):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Fan A, Yuan Y, Chen L, Guo X, Huang X, et al. Role of Moesin in advanced glycation end Products-Induced angiogenesis of human umbilical vein endothelial cells. Sci Rep. 2016;6:22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Liu H, Chen B, Li Q, Huang X, Wang L, et al. RhoA/ROCK-dependent Moesin phosphorylation regulates AGE-induced endothelial cellular response. Cardiovasc Diabetol. 2012;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Liu H, Du J, Chen B, Li Q, Guo X, et al. Advanced glycation end products induce Moesin phosphorylation in murine brain endothelium. Brain Res. 2011;1373:1–10. [DOI] [PubMed] [Google Scholar]
- 38.McRobert EA, Gallicchio M, Jerums G, Cooper ME, Bach LA. The amino-terminal domains of the ezrin, radixin, and Moesin (ERM) proteins bind advanced glycation end products, an interaction that May play a role in the development of diabetic complications. J Biol Chem. 2003;278(28):25783–9. [DOI] [PubMed] [Google Scholar]
- 39.Mulherkar S, Tolias KF. RhoA-ROCK signaling as a therapeutic target in traumatic brain injury. Cells. 2020;9(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets utilized and examined in this study can be obtained from the corresponding author upon reasonable requests.