Abstract
Background
According to evidence, medicinal plants such as thyme, rosemary, and fenugreek were beneficial for human health. Recently, these plants showed a great impact in animal health, particularly in poultry.
Objectives
To map the body of literature on the impact of medicinal plants on poultry health, including growth performance, gut microbiota, and mortality.
Eligibility criteria
Articles published in the English language from January 2019 to February 2023 randomized controlled trials, quasi-experimental studies, conducted on hens, chickens, or chicks that aimed to assess the effect of medicinal plants with or without prebiotics, on health-related outcomes including growth performance, mortality rate, and gut microbiota composition.
Sources of evidence
From December 2022 to February 2023, a systematic search on PubMed, Science Direct, and Google Scholar was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews guidelines.
Charting methods
Data charting was performed using a standardized form on Excel 365 that included study identification features, population and sample size, study groups, intervention description, follow-up period, and main outcomes.
Results
After the second screening, 38 articles were included. Results showed that thyme, rosemary, and peppermint were widely tested, and they were effective in promoting body weight gain, feed conversion ratio, live body weight, and microbiota, and in reducing mortality rate and intestinal multiple resistant bacteria. Cinnamon, lemon, garlic, and fenugreek were less commonly experimented. However, some studies that they were effective in improving growth performance and improving gut microbiota in healthy chickens.
Conclusions
Various studies confirmed that 5 to 6 g/kg of thyme powder was effective in improving growth performance and gut microbiota in healthy chickens. Further experiments are needed to compare the impact of thyme to antibiotics in chickens infected with multiple drug-resistant bacteria.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-025-04760-6.
Keywords: Poultry, Microbiota, Medicinal plant, Herb, One health
Introduction
The poultry industry is under increasing pressure to improve efficiency to feed a growing population with a limited resource base in a short period. Thus, producers often use antibiotic growth promoters to meet these conditions [1]. Although antibiotic growth promoters used in poultry nutrition were prohibited worldwide, it remains used in some countries illegally. Antibiotics used in poultry feeds have resulted in the selection of numerous antibiotic-resistant bacterial species that potentially endanger human health, such as Staphylococcus, Pseudomonas, Escherichia, and Salmonella [2, 3]. Residues of antibiotics detected in food such as penicillin, tetracycline, macrolide, aminoglycoside, and amphenicol harm human health including teeth development problems in young children, food poisoning, muscle tremors, tachycardia, and aplastic anaemia in humans [2].
Antimicrobial resistance (AMR) is an urgent threat to global health that is accelerated by the misuse of antibiotics in humans and animals [4]. The Centres for Disease Control and Prevention (CDC) reported that AMR killed at least 1.27 million people worldwide and was associated with nearly 5 million deaths in 2019 [5].
Initially, the World Health Organisation launched several programmes and strategies to tackle AMR. All of them focused on AMR in human medicine. These plans did not prove their worth in any significant way, and the statistics relating to AMR were constantly expanding. To counter this, the World Health Organisation has set up a consortium with the Food and Agriculture Organisation and the Office International of Epizooties; this tripartite collaboration, which incorporates the One Health concept, represents a new paradigm for dealing with the issue of AMR. In 2019, aware of the role of the environment in the fight against antibiotic resistance, a fourth partner has been added: the United Nations Environment Programme.
Forces were joined to advance international cooperation for a One Health response on AMR. The four organizations developed a joint strategic framework for collaboration on AMR and in 2022, they signed an agreement to strengthen their cooperation and fully integrate environmental considerations into One Health efforts.
The One Health approach suggested that human, animal, and environmental health are all interconnected. Any changes in these interactions can lead to the emergence and spread of novel human and animal diseases [6]. To protect human health at a human-animal-environment interface, international organizations are working on monitoring animal infections and diseases that can be harmful to human health, such as AMR [7].
By integrating human, animal, and environmental health perspectives, the One Health approach provides a comprehensive framework for addressing AMR, ensuring more effective and sustainable interventions to combat this global health threat. When it comes to poultry production, the economic aspect is very highly taken into consideration, both on an individual level (for farmers) and on a national level (in relation to the economic impact of the poultry sector). The solutions currently available to alleviate the various infectious problems affecting poultry production, and which could eventually have a major economic impact, are focused on the use of antibiotics, something which, given the epidemiological data and existing surveillance, has had a number of consequences: These include, but are not limited to: the presence of antibiotic residues in chicken meat (toxicological risk), the development of antibiotic resistance in bacteria from the chicken's normal microbiota, and the possible transmission of these bacteria to humans via the food chain. From another angle, antibiotic residues are released into the environment via effluents generated in animal production zones. This concerns not only antibiotic residues, but also antibiotic-resistant bacteria in effluents. If we approach the issue from a One Health perspective, we can clearly see that the use of antibiotics in poultry farming is a real challenge, and that substituting them with an alternative will enable us to mitigate all the consequences in connection with the health of the animal, foodstuffs, the food chain, human health and the health of the environment.
Therefore, there is increasing demand for safe poultry products free from antibiotic residues. This fact can be achieved by developing alternatives of antibiotic growth promoters [8]. The literature suggested several antibiotics alternatives such as phytogenic feed additives, plants’ essential oils, probiotics, prebiotics, organic acids, amino acids, and enzymes [2]. However, few essential oils, including thymol, had useful antibacterial properties [2]. A recent review reported that garlic, pepper, rosemary, black cumin, oregano, fennel, peppermint, chamomile, and oxtongue have the potential to provide healthy microbiota in poultry and to increase the growth rate [9]. These plants produced several immunological and physiological changes in poultry including synthesis of antimicrobial molecules [9].
The use of plants in poultry production is a growing topic that needs special emphasis from veterinarians, and policymakers as they may be harmless growth promoter alternatives. Therefore, it is essential to highlight the effects of different plants existing in the current literature on poultry health, production performance, and AMR. This scoping review aimed to map the body of literature on the impact of medicinal plants on poultry health-related outcomes.
Material and methods
Research design
Addressing a potentially large and diverse body of literature pertaining to a broad topic is an indication for scoping review, which is appropriate with the current research question [10, 11]. Accordingly, authors established a scoping review and did not include indicators, such as Hedges’ g, used in systematic reviews and meta-analysis. This scoping review was conducted following guidelines of PRISMA Extension for Scoping Reviews [12]. This study did not require ethical approval by an institutional review board, and it was not registered in any review registry.
Information sources and search strategy
From December 2022 to February 2023, we systematically searched PubMed, Science Direct, and Google Scholar for relevant articles published in English. Since other databases, such as Embase, were not accessible in universities of Lower-middle income countries including Tunisia, search was limited to three databases. Authors used the following combinations of terms in PubMed:
("poultry"[MeSH Terms] OR"broiler"[Title/Abstract] OR"chicken"[Title/Abstract]) AND ("garlic"[MeSH Terms] OR"garlic"[MeSH Terms] OR"plants, medicinal"[MeSH Terms] OR"drug evaluation, preclinical"[MeSH Terms] OR"phytotherapy"[MeSH Terms] OR"plants, medicinal"[MeSH Terms] OR"cinnamomum zeylanicum"[MeSH Terms] OR"cinnamomum zeylanicum"[MeSH Terms] OR"curcuma"[MeSH Terms] OR"curcuma"[MeSH Terms] OR"thymus plant"[MeSH Terms] OR"rosmarinus"[MeSH Terms] OR"rosmarinus"[MeSH Terms]) AND ("drug resistance, multiple"[MeSH Terms] OR"drug resistance, microbial"[MeSH Terms] OR"drug intake"[Title/Abstract] OR"antibiotic intake"[Title/Abstract]); ("poultry"[MeSH Terms] OR"broiler"[Title/Abstract] OR"chicken"[Title/Abstract]) AND ("garlic"[MeSH Terms] OR"garlic"[MeSH Terms] OR"plants, medicinal"[MeSH Terms] OR"drug evaluation, preclinical"[MeSH Terms] OR"phytotherapy"[MeSH Terms] OR"plants, medicinal"[MeSH Terms] OR"cinnamomum zeylanicum"[MeSH Terms] OR"cinnamomum zeylanicum"[MeSH Terms] OR"curcuma"[MeSH Terms] OR"curcuma"[MeSH Terms] OR"thymus plant"[MeSH Terms] OR"rosmarinus"[MeSH Terms] OR"rosmarinus"[MeSH Terms]) AND ("body weight"[MeSH Terms] OR"body weight changes"[MeSH Terms] OR"eating"[MeSH Terms] OR"feed intake"[Title/Abstract] OR"microbiota"[Title/Abstract] OR"microbiota"[MeSH Terms]); ("poultry"[MeSH Terms] OR"broiler"[Title/Abstract] OR"chicken"[Title/Abstract]) AND ("garlic"[MeSH Terms] OR"garlic"[MeSH Terms] OR"plants, medicinal"[MeSH Terms] OR"drug evaluation, preclinical"[MeSH Terms] OR"phytotherapy"[MeSH Terms] OR"plants, medicinal"[MeSH Terms] OR"cinnamomum zeylanicum"[MeSH Terms] OR"cinnamomum zeylanicum"[MeSH Terms] OR"curcuma"[MeSH Terms] OR"curcuma"[MeSH Terms] OR"thymus plant"[MeSH Terms] OR"rosmarinus"[MeSH Terms] OR"rosmarinus"[MeSH Terms]) AND ("mortality"[MeSH Terms] OR"health status"[MeSH Terms] OR"health performance"[Title/Abstract] OR"growth performance"[Title/Abstract]).
In Science Direct and Google Scholar we used these combinations:"poultry"and"herbals"and"antimicrobial resistance"and"health status";"poultry"and"medicinal plants"and"health status"and"antimicrobial resistance";"poultry"and"medicinal plant"or"herbals"and"antibiotic use".
Inclusion criteria and study selection process
Language, incomplete data, population, as well as ranking and indexing of the journal in which the paper was published were significant factors in including studies for review.
English is the universal language of science. Most of reliable journals publish full text in English. Therefore, we included articles published in English language from January 2019 to February 2023 using automation tools (filters), randomized controlled trials, and quasi-experimental studies, conducted on hens, chickens, or chicks that aimed to assess the effect of medicinal plants with or without prebiotics, on at least one of the following outcomes: growth performance (Feed intake (FI), feed conversion ratio (FCR), body weight (BW), body weight gain (BWG)), mortality rate, intestinal microbiota composition. Studies published in reliable peer-reviewed journals (ranked Q1 or Q2 in Scopus and/or Web of Science, indexed in Scopus, Medline, or Web of Science) were included.
Articles published in a language other than English; cross-sectional studies, cohort studies, case–control studies, commentaries, editorials, letters, qualitative studies, narrative reviews, conference papers, and thesis papers were excluded. Studies conducted on quails, ducks, or poultries other than hens, studies that used probiotics alone or with medicinal plants, and studies that used only in vivo analysis of the medicinal plants’ impact were also excluded.
Two authors (MD, MMM) conducted the database search and the first screening which was based on titles and abstracts. Eligible studies were enrolled in full-text screening with referral to (WM) and (SA). The entire texts of the eligible studies were reviewed by three authors (MD, MMM, AMK), who assessed the studies'applicability. During screening process, reviewers used discussions to settle any disagreements. If not settled, a third reviewer (head of the project Prof. WM) resolved any discrepancies.
Data analysis
One author (MD) did the data extraction using a standardized form on Excel 365 approved by the team to collect the relevant data from each article. The form included study identification features (First author’s name, publication year, country of origin), population and sample size, study groups (treatment and control groups), interventions’ description, follow-up period, and main outcomes about growth performance, intestinal microbiota. All studies were critically assessed for quality by (MD) based on the paper’s methodology analysis and journal quality, with referral to (WM) and (SA).
Results
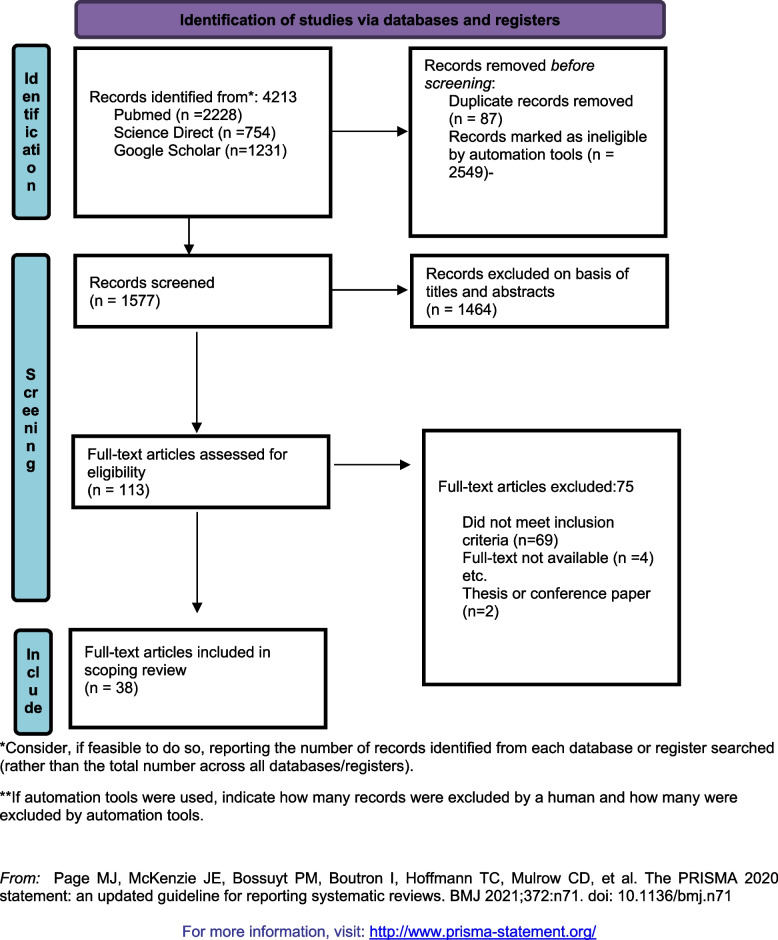
Figure 1 shows the process of study identification via databases. The database search using the predefined combinations revealed 4213 of which 2228 in PubMed, 1231 in Google Scholar, and 754 in Science Direct. Using the databases’ filters of publication year from 2019 to 2023 (present), 2549 were automatically removed. After duplicate removal, 1577 articles were screened. After the first screening, 1464 records were excluded based on title and abstract. After full-text reviewing of the remaining 113 records, 75 articles were excluded of which 69 articles did not meet the inclusion criteria, 4 full-text papers were not available, one record was a conference paper, and one record was a thesis paper. At last, 38 articles were included in this scoping review and processed for data charting (Fig. 1).
Fig. 1.
Studies’ selection process. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).**If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Table 1 presents the included studies in an ascendant chronological order. Their characteristics including the following items: authors, country, sample size, study groups, interventions’ description, follow-up period, and main outcomes.
Table 1.
Studies included in scoping review: Characteristics and main outcomes (n = 38)
| First author, year, country) | Sample size | Study groups | Follow-up periods | Interventions | Main Outcomes |
|---|---|---|---|---|---|
|
Abdelwareth et al. 2019 Egypt [13] |
384 one-day-old chicks | Two groups of 192 each; with 6 replicate pens with 8 birds | 0–21 days, 21 to 35 days |
Peppermint (Mentha piperita L.) leaf powder: Experiment 1: 4 dietary treatments that included peppermint leaves at concentrations of 0, 5, 10, or 15 g/kg. The peppermint leaves contained 1.48% essential oil of which 35.1% was menthol. Each treatment had 6 replicate pens with 8 birds Experiment 2: dietary treatments that included menthol at concentrations of 0, 26, 52, or 78 mg/kg. Each treatment had 6 replicate pens with 8 birds |
Body weight and BWG increased with the increase in dietary peppermint leaves (linear, P < 0.01) and menthol concentrations (linear, quadratic, P < 0.01) during the trial periods. Feed intake linearly increased (P < 0.01) with increasing peppermint leaves or menthol levels and, in turn, caused linear improvements (P < 0.01) in feed conversion values. Lower mortality rate was recorded in the supplementation groups and, therefore, a higher net economic return was observed. European production efficiency factor was significantly increased by peppermint leaves and menthol supplementations |
|
Akram et al. 2019 Turkey [14] |
150 day-old broiler chicks (Cobb) | Five different treatments with 3 replicates of 10 chicks each | 1 to 21 days, 22 to 35 days | Different doses of aloe vera (AV) with commercial starter (ME = 2900 kcal/kg; CP = 21%) and finisher diet (ME = 3060 kcal/kg; CP = 18%): 100 mg/kg enramycin and 60 mg/kg salinomycin (positive control), 50 ml/L AV of pH 3 (AV1), 50 ml/L AV of pH 7 (AV2), 50 ml/L AV of pH 12 (AV3) and fresh clean water (CON) | ANT and AV1 treatments caused a significant (p < 0.05) increase in feed intake at 21 and 35 days of age and feed conversion ratio at 21 days of age. The highest weight gain was obtained by AV1 at 35 days of age (p < 0.05) |
|
Ismail et al. 2019 Egypt [15] |
140 day-old unsexed Cobb 500 broiler chicks | 7 equal groups, each with 4 replicates | 1, 21, 42 days | T1: control group (basal diet), T2: 0.5 g/kg thyme essential oils, T3: 1.0 g/kg thyme essential oils, T4: 1.5 g/kg thyme essential oils, T5: 5.0 g/kg thyme, T6: 10.0 g/kg thyme, T7: 15.0 g/kg thyme | T1 and T5 were significantly better in their live body weight and BWG in comparison with other groups in the first three weeks of study and whole experimental period. T5 had better feed intake during the starter and whole experimental periods as compared to other groups. T3 had significantly better feed conversion ratio in comparison with other groups during the starter and whole experimental periods |
|
Nameghi et al. 2019 Iran [16] |
500 ten‐day‐old male broiler chicks (Ross × Ross 308; 40 ± 1.5 g) | five treatments with four replicates each | 10, 21,42 days | Control group: no supplements; 4 treatments of essential oils blend (EOB) of thyme, peppermint, and eucalyptus: EOB- 50; EOB- 100; EOB- 150; EOB- 200 | BWG and FCR during finisher and the whole period were linearly improved (p < 0.05) by EOB‐150 compared to CON and EOB‐50. There were no differences (p > 0.05) in FI and mortality rate among treatments in each phase and the entire experimental period. The inclusion of EOB‐100, EOB‐150 and EOB‐200 increased production index in a linear fashion (p < 0.001) compared to CON and EOB‐50. The ileal Escherichia coli count was lower and Lactobacillus count was higher in EOB‐200 and EOB‐150, respectively, compared to CON at 21 and 42 days of age (p < 0.05) |
|
Nouri 2019 Iran [17] |
600 mixed-sexed, one-day-old Ross 308 broiler chicks | 8 experimental groups randomly distributed with 5 replicates and 15 chickens in each pen | 0–14 days, 15–28 days, 29–42 days | Mint (MEO), thyme (TEO), and cinnamon (CEO) essential oils: Dietary treatments included two factors; form (free and chitosan nano-encapsulated (CNE)) and type (no essential oil (distilled water), MEO, TEO, and CEO) of EO to give eight experimental diets. EOs were added in amounts of 0.025%, 0.04% and 0.055% to the starter, grower, and finisher diets, respectively | Both EOs and the CNE form had significant (P < 0.05) benefits on improving BWG, and feed conversion ratio in periods of 29–42 and 1–42 days. The highest effect was (P < 0.05) obtained in birds receiving TEO, which was intensified by nano encapsulation. The thyme and cinnamon EOs and CNE significantly improved intestinal Lactobacillus spp. and E. coli populations (at 42 days) in broilers. Nano-encapsulated EOs, especially TEO and CEO had (P < 0.05) the largest effect on increasing microbial populations |
|
Shirani et al. 2019 Iran [18] |
576 one-day old Ross 308 male broiler chicks |
6 dietary treatments with 8 replicate pens per treatment and 12 birds per pen |
1 to 10 days, 11 to 24 days, 25 to 42 days | Pulicaria gnaphalodes powder (PGP): The dietary treatments consisted of a basal diet as control (CON, with no additive), CON + 0.1% PGP, CON + 0.2% PGP, CON + 0.3% PGP, CON + 0.1% probiotic mixture (PRO), and CON + 0.05% bacitracin methylene disalicylate (AGP) | Higher BWG and lower feed conversion ratio were obtained in birds fed AGP and 0.3% PGP compared with those fed CON and 0.1% PGP during grower, finisher, and the entire study (P < 0.05). On day 42, birds on PRO, 0.2 and 0.3% PGP treatments had lower counts of Escherichia coli and higher Lactobacillus spp. in ileum and caecal contents compared to the CON and 0.1% PGP |
|
Tayeb et al. 2019 Iraq [19] |
288 unsexed 7-day old broiler chicks |
8 treatment groups with 3 replicates of 12 chicks based on completely randomized design |
week 1 to week 6 |
Different levels of medical plants (Thymus vulgaris, Adiantum capillus-veneris L, Rosemarinus officinalis and their combination) added to basal diets at 7 th days old: T1 Control (no supplemented), T2 Thyme powder 5 g/kg, T3 Thyme powder 10 g/kg, T4 Adiantum powder 3 g/kg, T5 Adiantum powder 5 g/kg, T6 Rosemary powder 5 g/kg, T7 Rosemary powder 10 g/kg and T8 Mixture (Thyme, Adiantum and Rosemary) with 7.5 g/kg + 4 g/kg and 7.5 g/kg, respectively |
In the 6 th week of ages, except for T3 group, medical plants significantly improved live body weight compared to control group |
|
Vase-Khavari et al. 2019 Iran [20] |
150 one-day old Ross 308 broiler chicks |
5 treatment groups with 3 replicates per treatment in a total of 10 birds per replicate | 1 st to 6 th week of age | Three tropical medicinal plants (R. coriaria, H. persicum, and M. piperita) and superzist probiotic: (1) Control diets; (2) control diets + 0.03% w/v superzist; (3) control diets + 0.5% w/v R. coriaria; (4) control diets + 0.5% w/v H. persicum; and (5) control diets + 0.5% w/v M. piperita |
There were no differences observed in the feed intake, weight gain, feed efficiency, and economic index values for the control as well as supplemented diets of broiler chickens at 14 th, 29 th, 35 th days of age. Between 36 th and 42nd days of age, a gradual reduction (P < 0.05) in the feed efficiency, as well as economic index values, was reported across the treatments versus control diet. Supplementing broiler diets with additives (superzist and medicinal plants) slightly decreased (P < 0.05) the population of coliform bacteria in the cecum. Birds fed superzist and medicinal plants increased (P < 0.05) the total counts of lactobacilli, M. piperita being the most effective additive with log 8.95 CFU/g of Lactobacilli |
|
Witkowska et al. 2019 Poland [21] |
360 one-day old chicks | 3 groups with 120 chicks each one | 1, 42 days | Peppermint and thyme essential oil mist: control group (C – misted with pure water), two experimental groups (PO – misted with peppermint essential oil, and TO – misted with thyme essential oil) | During the entire experiment, mean BW and WG were higher in broiler chickens exposed to PO mist than in control birds and chickens exposed to TO mist. Birds exposed to TO mist were characterised by the highest FCR (P > 0.05), particularly at the last stage of the experiment |
|
Abdulbasit et al. 2020 Malaysia [22] |
210 one-day-old broiler chicks | 7 treatments: each treatment group has 3 replicates (n = 10) with a total number of 30 chicks | 1–21 days, and 21–42 days | Different dose supplementation of Piper betle leaf meal (PBLM) and Persicaria odorata leaf meal (POLM): T1 control (basal diet (BD) with no supplementation), T2 (BD + 2 g/kg PBLM); T3 (BD + 4 g/kg PBLM), T4 (BD + 8 g/kg PBLM), T5 (BD + 2 g/kg POLM), T6 (BD + 4 g/kg POLM), T7 (BD + 8 g/kg POLM) | Except for T4, graded dose inclusion of PBLM and POLM increased (P < 0.05) the body weight gain (BWG), positively modulated the gut architecture and enhanced nutrient digestibility in both stater and finisher growth phases of broiler chickens. (T3) and (T7) had significantly higher (P < 0.05) BWG with superior (P < 0.05) feed efficiency in the overall growth period, longer (P < 0.05) villi for duodenum as well as for jejunum, improved (P < 0.05) digestibility of ether extract (EE), and dry matter (DM) compared to the control group. T4 had least (P < 0.05) crude protein (CP) digestibility |
|
Abdulbasit et al. 2020 Malaysia [23] |
120 one-day-old male broiler chicks | Allocated randomly into 4 treatment groups with 5 replicates of 6 birds each | 1–21 days, 22–42 days | Different Doses of Persicaria odorata Leaf Meal (POLM)-basel diet (BD): BD + 2 g/kg POLM (Po2), BD + 4 g/kg POLM (Po4), and BD + 8 g/kg POLM (Po8) | On day 42, compared to the control, BWG was significantly increased (p < 0.05) in POLM-supplemented groups (Po2, Po4, and Po8). Feed conversion rate was decreased (p < 0.05) in POLM-supplemented diets compared to the control group. Feed intake was not affected (p > 0.05) by dietary supplementation of POLM. There was no significant difference between groups in term of mortality rate from day 1 to 42 |
|
Aldik et al. 2020 Iraq [24] |
180 unsexed broiler chickens |
4 treatments randomly distributed, each consists of 3 replicates where each replicates contains 15 chicks |
1, 42 days, 5 weeks |
Two diets were fed, initiator diet from (1 to 21 days) and final diet from 22 to 42 days. The Thymus vulgaris powder leaves were added to the diet (manual mixing) from the age of one day as follows: First treatment (control) without adding Thymus vulgaris powder leaves to the diet, second treatment: Adding Thymus vulgaris powder leaves with amount of (2 g/kg feed), third treatment: Adding Thymus vulgaris powder leaves with amount of (4 g/kg feed), and fourth treatment: Adding Thymus vulgaris powder leaves with amount of (6 g/kg feed) |
In group 4, a significant decrease in the total numbers for the aerobic bacteria and coliform bacteria in the duodenum and cecum was showed in comparison with other treatments. The treatments of Thymus vulgaris leaves showed a decrease of the total numbers of the aerobic bacteria and coliform bacteria with an increase in the numbers of Lactobacillus in comparison with the control group |
|
Ahmadian et al. 2020 Iran [25] |
280 male broiler chicks of Ross 308 genotype |
Four replicates of seven treatment groups of 10 birds/group |
1–14 days, 15–28 days, 29–42 days | Treatment 1: Basal diet (control); T1: Basal diet + thyme powder at 1% from days 29–42; T2: Basal diet + thyme powder at 2% from days 29–42; T3: Basal diet + thyme powder at 3% from days 29–42; S1: Basal diet + sumac powder at 1% from days 29–42; S2: Basal diet + sumac powder at 2% from days 29–42; S3: Basal diet + sumac powder at 3% from days 29–42 | Feed intake was reduced for chickens fed the sumac supplements, and, at the two higher doses, defeathered body weight was also reduced. Abdominal fat was reduced by 41% in chickens fed thyme and 62% in those fed sumac. There was little effect of the supplements on carcass composition. Blood glucose was reduced in the supplemented chickens |
|
Obajuluwa et al. 2020 Nigeria [26] |
250 unsexed day-old broiler chicks of Abore acre strains | 5 groups of 45 chickens |
3–4 weeks, 5–8 weeks |
T1 = Feed without additives T2 = Feed with 5 mg/Kg Larvacide T3 = Feed with 60 mg/Kg of Yohimbe bark meal T4 = Feed with 120 mg/Kg of Yohimbe bark meal T5 = Feed with 180 mg/kg of Yohimbe bark meal |
At the finisher phase, supplementation of Yohimbe resulted to a progressive reduction in the feed intake (p < 0.05) without consequential reduction in the body weights. The chickens fed Basal diet + 180 mg Yohimbe had the best Feed Conversion Ratio (FCR) |
|
Shu et al. 2020 China [27] |
300 one-day-old Arbor Acres (AA) broilers |
4 groups in 3 replicates of 25 birds per replicate |
1, 14, 28, 42 days | Bamboo leaf flavone (BLF): A regular diet with BLF supplement at 0 (control), 200 (Low group, L), 400 (Medial group, M), and 800 mg/(kg d) (High group, H) | BFL caused the changes of the gut microbial community structure, resulting in greater proportions of bacterial taxa belonging to Lactobacillus, Clostridiales, Ruminococcus, and Lachnospiraceae |
|
Yadav et al. 2020 Brazil [28] |
360 12-day-old male chicks |
6 groups: 10 birds per cage replicate and 6 replicate cages per treatment |
1, 12, 20 days of age | 3 levels of curcumin; 0, 100, and 200 mg/kg) and 2 doses of Eimeria either challenged (C) or nonchallenged (NC): nonchallenged control, NC + 100 mg/kg curcumin, NC + 200 mg/kg curcumin, challenged control, C + 100 mg/kg curcumin, and C + 200 mg/kg curcumin | The growth performance and permeability were higher (P < 0.001) in the NC and C groups, respectively. No interaction was observed between curcumin dose and cocci challenge on both parameters |
|
Yalçin et al. 2020 Turkey [29] |
108 Hyline Brown laying hens aged 36 weeks | 3 dietary groups with 6 replicates each, 6 hens per replicate | 16 weeks | Dried and ground thyme leaves (Thymus vulgaris L.) added in the isocaloric and isonitrogenousdiets at the levels of 0 (control), 1 and 2% | There were no significant differences between groups in term of feed intake, hen-day egg production, egg weight, and feed conversion ratio |
|
Amouei et al. 2021 United Kingdom [30] |
400 one-day-old Ross 308 male broiler chickens | 5 treatment groups, composed by 2 birds, with 4 replicates per each treatment |
1–21 days, 22–42 days |
Thyme essential oil (TEO) or Prebiotic (MOS) in different doses: (1) basic diet (no additive; CTR); (2) basic diet including 0.025% (0.25 g/kg) TechnoMOS® (MOS025); (3) basic diet including 0.075% (0.75 g/kg) TechnoMOS® (MOS075); (4) basic diet including 0.125% (1.25 g/kg) TechnoMOS® (MOS125); (5) basic diet including 0.075% (0.75 g/kg) thyme extract (TEO075) | No effects of prebiotic or thyme on carcass characteristics. No significant effects of treatments on weight gain on a week-by-week basis. CTR birds had greater feed intake and gained less weight during the grower phase and overall compared with MOS birds |
|
Cheng et al. 2021 China [31] |
210 one day-old broiler chicks ((Ross 308) |
7 treatment groups with 5 replicate cages (6 birds per cage) | 1, 7, 14, 21 days | Lotus leaf extract (LLE) in different doses: (1) a basal diet (blank control); (2) the basal diet supplemented with 50 mg/kg chlortetracycline (antibiotics control); (3) the basal diet supplemented with 1.0 g/kg LLE (LLE1); (4) the basal diet supplemented with 2.5 g/kg LLE (LLE2.5); (5) the basal diet supplemented with 5.0 g/kg LLE (LLE5); (6) the basal diet supplemented with 7.5 g/kg LLE (LLE7.5); (7) the basal diet supplemented with 10.0 g/kg LLE (LLE10) | In LLE5 group, the average daily weight gain was higher than that of the antibiotics and blank control groups (P < 0.05) from day 7 to 21. In LLE5 group, the abundance of Clostridiaceae and Bacteroidales S24 - 7 was increased, whereas that of Peptostreptococcaceae was reduced (P = 0.05) |
|
Youssef et al. 2021 Egypt [32] |
400 one-day-old male broiler chickens (Cobb 500) | 4 treatments, each with 5 replicates (20 chickens per replicate) | 1 st to 6 th week of age (1 to 42 days) | Control group: no phytogenic feed additives; Essential oils group: 25.0 mg/kg of an essential oil blend from star anise, rosemary, thyme, and oregano; Saponins group: 46.0 mg/kg of a Quillaja saponin blend; Essential oils plus Saponins group: a combination of both phytogenic preparations | There was no change in performance parameters among the treatments during the starter period. At the grower period and the overall experiment, the BWG of birds was significantly high in all supplemented groups compared to the control, and the best value was registered in the saponins group. Feed conversion ratio was better in saponins group from 21 to 42 days and overall periods |
|
Ahmadzadeh et al. 2022 Iran [33] |
388 Ross- 308 strain broilers | 8 treatments, 4 replicates, and 12 chickens in 5 × 2 factorial arrangements | 1 to 10 days, 11 to 24 days, 25 to 42 days | (1) Control; (2) thyme extract (2 kg/ton); (3) organic acid (GLOBACID) (3 kg/ton); (4) probiotics (protoxin containing Lactobacillus acidophilus and Lactobacillus bifidobacterium each gram of protoxin contained 0.1 × 10 CFU) (50 g/ton) and (5) prebiotics (Mannan-oligosaccharide) (2 kg/ton) with normal and reduced protein levels (10% lower than usual) | The use of feed additives along with both levels of crude protein had significant effects on performance, intestinal morphology, and faecal nutrient levels (P < 0.05). A 10% decrease in crude protein level of diet caused to decrease in daily weight gain and an increase in feed conversion ratio in the starting period (P < 0.05). Decreased dietary crude protein levels in growing and finishing period had insignificant effects on chicken’s performance (P > 0.05) |
|
Alqahtani et al. 2022 Saudi Arabia [34] |
540-day-old broiler chicks | 9 groups: From day 1 to 21, each treatment comprised ten replicates, with six birds. From 21 to 34: 270 birds and each group contain only five replicates) |
1–21 days, 21–34 days |
Rumex nervosus leaves (RNL) or Cinnamomum verum bark (CVB) in different doses: Groups 1–3: basal diet supplemented with 1, 3, or 5 g RNL/kg feed, respectively. Groups 4–6: basal diet supplemented with 2, 4, or 6 g CVB/kg feed. Group 7: basal diet with antibiotic growth promoter (AGP: Colimox'a combination of the antibiotics amoxicillin and colistin at 200 and 150 mg/kg, respectively’; Group 8: not vaccinated received basal diet (negative control); Group 9: vaccinated received basal diet (positive control); |
Broilers given 1 g RNL were numerically heavier at 34 days and gained more to a degree comparable to the AGP group (p = 0.053). CVB at 2 g resulted in the best-feed conversion up to 21 d (p = 0.04). Dietary treatments had no impact on feed intake |
|
Bahadori et al. 2022 Iran [35] |
250-day-old Ross broiler chicks (male sex) |
randomly distributed into 5 dietary treatments with 5 replicates of 10 birds |
1–10 days, 11–24 days, 25–42 days |
Control group: corn-soybean meal-based diet; control diet supplemented with 0.5 g/kg of Bacitracin as antibiotic (A), 3 g/kg of sesame meal bioactive peptides (SMBP group); 0.5 g/kg of a mixture of savory and thyme essential oils (STEO group), and combination of 3 g/kg of SMBP and 0.5 g/kg of STEO (SMBP + STEO group) |
During days 11 to 24, BWG of the broilers was influenced by experimental diets (p < 0.05) During all periods, the control group had the lowest BWG and feed intake than the other groups. In comparison with the control group, feed conversion ratio improved in broilers fed with SMBP + STEO diet. The viable counts of Lactobacilli and E. coli were significantly influenced by the experimental diets (P < 0.05) The enumeration of cecal Lactobacilli enhanced in birds which fed with SMBP + STEO diet compared with other treatments. The caecal population of E. coli was greater in broilers fed with control diet compared with other treatments |
|
Behboodi et al. 2022 Iran [36] |
300 one day-old broiler male chicks | 4 treatments with 5 replications per groups (15 birds per pen) |
1–21 days, 21–42 days |
Mixture IMX: peppermint, coneflower (Echinacea purpurea), thyme, propolis, and prebiotic. Treatments were administered through drinking water: control (A) (no IMX), 0.25 mL/L (B), 0.5 mL/L (C), and 1 mL/L (D) | Higher BWG and feed intake in groups B, C, and D (P < 0.05) with no significant difference in FCR |
|
Cong et al. 2022 Vietnam [37] |
288 Ho × Luong Phuong coloured broiler chickens aged 6 weeks old | 3 groups of 96 birds (6 replicates of 16 birds each) | one day a week from 1 st to 10 th week of age (70 days) | Control group: diet supplemented with 2% soybean oil (SBO); SIO group: a diet supplemented with 2% sacha inchi (Plukenetia volubilis L.) oil (SIO); SIM group: a diet supplemented with 2% Sacha inchi oil and 1% medicinal plant powder | The final live body weight was similar among dietary treatments (p = 0.52). Experimental diets did not result in any changes in average daily gain, average daily feed intake, and feed conversion rate of coloured broiler chickens (P ≥ 0.28) |
|
Elbaz et al. 2022 Egypt [38] |
480 broiler chicks (Ross 308) | 4 groups (120 chicks/group) randomly divided at one-day-old, | 1, 14, 28, and 35 days | Garlic and lemon essential oils: The control group received the basal diet (CON), while the other three groups received the basal diet supplemented with 200 mg/kg garlic essential oil (GEO), 200 mg/kg lemon essential oil (LEO), and their mixture (GLO) 200 mg/kg diet, respectively for 35 days | Supplementing the diet with essential oils increased caecal Lactobacillus and reduced E. coli count at 35 d of age compared to control broilers (P < 0.05). There were no significant differences in total lactic acid bacteria count. There was a significant increase in average body weight and a significant decrease in feed conversion ratio in the GEO, LEO, and GLO groups compared to the CON group. In addition, there was an increase (P < 0.05) in the AFI in the GLO and LEO groups than those in the GEO and CON groups during the overall period. The mortality rate decreased, and the European Production Efficiency Factor (EPEF) increased (P < 0.05) |
|
Farouk et al. 2022 Egypt [39] |
80 commercial Cobb broiler |
4 equal groups (n = 20 chicks/group): control non-infected (CN) group, Control infected (CI) group Rosemary infected (RI) group, Fenugreek infected (FI) group |
1 day and 6 weeks |
Rosemary leaves and fenugreek seeds. Chicks were inoculated with 0.5 ml of E. coli O78 bacterial inoculum, at 7 days old of age as follows: 0.25 ml intranasal and 0.25 ml via eye drop route. (1) CN group: fed on balanced commercial ration free from any feed additives; (2) CI group: fed on balanced commercial ration free from any feed additive and experimentally infected with E. coli at 1 week of age; (3) RI group: fed on balanced commercial ration supplied with rosemary at the level of 0.5% (5 g rosemary leaves powder/kg ration) from 1 day to 6 weeks old and experimentally infected with E. coli at 1 week of age. (4) FI group: fed on balanced commercial ration supplied with fenugreek at the level of 0.5% (5 g fenugreek seed powder/kg ration) from 1 day to 6 weeks old and experimentally infected with E. coli at 1 week of age |
The RI and FI groups revealed a significant elevation in their body weight and BWG compared with the CI group, higher activities were evident in both RI and FI groups. The highest mortality rate was recorded in CI group (25%) followed by RI (10%), and the lowest one was recorded in FI group (5%) |
|
Hamed et al. 2022 Egypt [40] |
215 one day-old Cobb broiler chicks | 14 groups (15 chicks in each group) | 1, 35, 42, and 49 days of age | GP1: Inoculated with Salmonella Enteritidis multidrug resistance strain (MDR) Strain 1; GP2: Inoculated with Salmonella Enteritidis sensitive to sulpha-trimethoprim strain (SXT) Strain 2; GP3: Strain 1 + sulpha-trimethoprim antibiotic; GP4: Strain 2 + sulpha-trimethoprim antibiotic; GP5: Strain 1 + sulpha-trimethoprim antibiotic + microemulsion; GP6: Strain 2 + sulpha-trimethoprim antibiotic + microemulsion; GP7: Only thymol oil; GP8: Strain 1 + thymol oil; GP9: Strain 2 + thymol oil; GP10: Only microemulsion; GP11: Strain 1 + microemulsion; GP12: Strain 2 + microemulsion; GP13: Negative control; GP14: Only antibiotic | Thymol oil (0.1%) and microemulsion (0.01%) decreased the count of Salmonella Enteritidis in caecal content and faecal dropping and the mortality rates after five days of treatment. Infected groups treated with Cotrimazine® + thyme oil microemulsion had a slight significant economic impact (P < 0.05) compared to Cotrimazine® alone |
|
Kairalla et al. 2022 Libya [41] |
240 1-day-old broiler chicks (Cobb500) | 4 dietary treatments. Each treatment comprised 5 replicates with 12 chicks each | 1, 21, 42 days | Graded levels of garlic (Allium sativum L.) powder: 0.0%, 0.1%, 0.2%, and 0.3% garlic | The findings showed that birds fed a diet supplemented by 0.3% garlic powder was significantly (p < 0.05) better in terms of body weight, BWG, and feed conversion ratio compared to those birds fed 0%, 0.1%, or 0.2% garlic powder |
|
Kanwal et al. 2022 Pakistan [42] |
200 four-day-old broiler chicks |
4 groups and with triplicates (T1, T2 T3 and T4). Each treated group received three (15-chicks) sessions (9 treatments) |
one day a week from 1 st to 5 th week of age (35 days) | T1: Untreated control group, T2: 1.5%Neem + 5%Moringa; T3: Treatment-T3 2.5% Neem + 5% Moringa; T4 3.5% Neem + 5% Moringa | Feed conversion ratio measured is significantly (p < 0.05) different in treated groups and in the control group. Highest FCR was recorded in control group as compared to supplementary groups. Low FCR recorded in treated groups show significant (p < 0.05) results |
|
Noruzi et al. 2022 Iran [43] |
480, day-old Ross- 308 broiler chicks | 4 dietary treatments with six replicates with 20 birds each | 1 day, 11 to 21 days, 22 to 42 days |
Four iso-caloric and iso-nitrogenous diets including two levels (0 and 250 mg/kg) of TEO and two levels (0 and 0.3 mg/kg) of SY in a 2 × 2 factorial arrangement; 1) Thyme (Thymus vulgaris L.) essential oil (TEO) group; 2) Selenium yeast (SY) group; 3) TEO x SY group; 4) Control group; All groups received basal diet from 1 to 10 days of age |
Adding SY significantly decreased feed intake in finishing period (22–42 d) (p < 0.05). There is no significant difference in BWG, feed conversion rate, and body weight between groups at growing and finishing period |
|
Yang et al. 2022 China [44] |
240 3-day old chicks |
4 treatment groups (6 replicates per group, 10 broilers each replicate) |
1 to 28 days, 29 to 56 days | Fenugreek seed extracts (FSE) (50% fenugreek polysaccharide, 15% saponin, 10% fenugreek flavones, 2% alkaloids). Groups:1) basal diet (CON group), 2) basal diet supplemented with 30 mg/kg Zinc bacitracin (ZB group), 3) basal diet supplemented with 50 (D-FSE group), 4) 100 (H-FSE group) mg/kg FSE | dietary FSE supplementation improved average daily weight gain (ADG) and ratio of feed to weight gain (F: G) (P < 0.01). FSE significantly reduced Campylobacter and Lachnoclostridium abundance (P ≤ 0.05) |
|
Gumus et al. 2023 Turkey [45] |
400 three-day-old male Ross | five treatments with four replicates of 20 birds each | 1, 3, 10, 17, 24, 31 and 42 days |
Thyme and rosemary essential oils. The feeding period was divided in starter diets, fed from 3 to 21 d of age, and finisher diets, fed from 22 to 42 days of age. 1/control group (standard commercial feed), 2/standard commercial feed + 150 mg kg- 1 thyme essential oil (TEO- 150 group), 3/300 mg kg- 1 thyme essential oil (TEO- 300 group), 4/100 mg kg- 1 rosemary essential oil (REO- 100 group) and5/200 mg kg- 1 rosemary essential oil (REO- 200 group) |
In term of performance (Body weight, BWG, and feed intake) there were no significant differences between groups. Enterobacteriaceae counts had significantly decreased on day 2 of storage in groups TEO- 150, TEO- 300 and REO- 200 (P < 0.01), |
|
Jahja et al. 2023 Indonesia [46] |
750 one-day-old (DOC) Cobb broiler chickens | 3 groups each consisting of 250 birds, with 5 replicates of each treatment containing 50 birds per replicate | 1, 7, 14, 28 days | 1) FOA group was supplemented with basal diet plus 10 mg of Origanum vulgare and 15 mg of Andrographis paniculata extract per kg of feed; 2) ZB group was supplemented with basal diet plus 6.3 mg/kg feed of Zinc bacitracin as positive control; 3) Control group was given basal diet without antimicrobial growth promoters addition as negative control | On day 28, FOA group and ZB group showed significantly higher body weight than the control group (P < 0.05). The FCR of ZB group was better than FOA group. However, FOA group displayed better microbiota profile than ZB group and negative control, with more Lactobacillus spp. and Bacillus spp., and less Escherichia coli and Salmonella spp. isolated from intestines |
|
Nameghi et al. 2023 Iran [47] |
500 fourteen-d-old male Ross 308 chicks | 5 dietary treatments randomly distributed using 4 floor pens per treatment and 25 chicks per pen (2.0 m × 1.5 m) | 14–24 days, 25–42 days | Blend of thyme and rosemary powders: Five experimental diets include of a negative control diet (NC; without supplementation of poultry by-product meal (PBPM), a positive control diet (PC; with supplementation of 7% PBPM), and three levels of additive supplementation of thyme and rosemary powders in the basal diets: 0.750% rosemary powder (PCR); 0.375% thyme powder + 0.375% rosemary powder (PCRT), and 0.750% thyme powder (PCT) | The supplementation of PBPM in broilers diets decreased average daily gain in the PC group compared to the NC group, at the periods of d 14 to 21, and 14 to 42. The FCR was increased by 0.06 units in the broilers fed 7% PBPM (PC group) compared to broilers fed basal diet (NC group) at period of d 14 to 21. The ileal E. coli count was lower in PCRT (P = 0.001) compared to PC at 21 and 42 d of age. Broilers fed thyme and rosemary powders combination diet (PCRT group) had a higher (p = 0.001) number of ileal Lactobacillus than the PC birds at d 21 and 42 |
|
Oladukun et al. 2023 Canada [48] |
288 one-day old chicks | 6 groups of 48 birds each, 8 replicate birds per treatment group |
0–14 days, 15–28 days, 29–42 days |
Six groups with 48 chicks in each: Negative control (NC), NC + infeed antibiotics, NC + infeed water essential oil, in ovo saline, in ovo essential oil, in ovo essential oil + in-water essential oil |
Alpha and beta diversity differed significantly between ileal and ceca samples except in control group. In-feed antibiotic treatment significantly increased the proportion of specific bacteria in the family Lachnospiraceae while reducing the proportion of bacteria in the genus Christensenellaceae in the caeca, compared to other treatments |
|
Safiyen et al. 2023 Nigeria [49] |
192 one day-old broiler chicks (Arbor acre) | 4 groups (six replicates per group and eight birds per replicate) | 1, 9, 18, 26 days | Citrus–coconut electrolyte blend (CCEB) composed of 52.27% of orange juice, 4.05% of lime juice, 40.45% of coconut water, 3.01% of honey and 0.22% of Himalayan salt. CCEB was added via drinking water provided daily for each group of birds at 0, 5, 10 and 15 ml per litre of water, respectively for 26 days | All productive performance parameters measured were not significantly (p < 0.05) different across treatment means |
|
Toson et al. 2023 Egypt [50] |
320 one-day-old unsexed Ross 308 broiler chicks |
4 groups (control, L, M, H) | 1, 21, 35 days |
(1) chicks received a basal diet with no addition (control); (2) chicks received a basal plus 1 g of Licorice extract/kg diet; (3) chicks received a basal diet plus 2 g of Licorice extract/kg diet; (4) chicks were received a basal diet plus 3 g of Licorice extract/kg diet. 2-phase diet: the starter phase was from day 1 to day 21 of age, while the finisher phase was from day 22 to day 35 of age |
Live BW was improved (P < 0.05) in experimental groups that received Licorice extract at 21 and 35 days of age than the other groups. BWG during starter, finisher, and the entire periods of birds received 3 g Licorice extract was higher (P < 0.05) than in the control group |
According to the literature, several plants were investigated, in a single format or combinations. The most tested plant was thyme. Seventeen studies assessed the effect of thyme of which thirteen studies [15, 17, 19, 21, 24, 25, 29, 30, 33, 40, 43, 45, 47] used thyme alone as powder extract or essential oil. Four studies tested a mixture of thyme with other plants including peppermint, and eucalyptus [16], star anise, rosemary, and oregano [32], savory [35], peppermint, coneflower, and propolis [36]. Table 2 shows that eleven studies assessed the impact of thyme on growth performance parameters.
Table 2.
Number of studies by outcomes and plants (plants ‘blends were not included in this table)
| Outcomes Plants |
Growth performance | Mortality | Gut microbiota |
|---|---|---|---|
| Thyme | 11 studies [15, 17, 19, 21, 25, 29, 30, 33, 43, 45, 47] | 2 studies [19, 40] | 4 studies [17, 24, 40, 47] |
| Rosemary | 4 studies [19, 39, 45, 47] | 2 studies [19, 39] | [45, 47] |
| Peppermint |
3 studies |
1 study [13] | [17] |
| Cinnamon | 2 studies [17, 34] | _ | 1 study [17] |
| Garlic | 2 studies [38, 41] | 1 study [38] | [38] |
| Lemon | 2 studies [38, 49] | 1 study [38] | [38] |
| Fenugreek | 2 studies [39, 44] | 1 study [39] | 1 study [44] |
Two studies showed that FI and FCR significantly improved in thyme groups in comparison with the control group [15, 33]. However, Ahmadian and colleagues found that thyme powder at 2% reduced FI [25]. Witkowska and colleagues found that FCR was improved with thyme essential oil mist [21]. Six studies showed that thyme increased BWG [15, 17, 19, 30, 33]. Hamed and colleagues showed that thyme essential oil at 0.1% reduced mortality rate in Salmonella Enteritidis [40]. Tayeb and colleagues [19] also found that 10 g/kg of thyme reduced mortality. Regarding microbiota changes, Aldik and colleagues showed that 6 g of thyme in 1 kg of feed reduced aerobic bacteria and increased Lactobacillus in comparison with the control group [24]. Nouri et al. also found that thyme essential oil improved Lactobacillus and Escherichia coli populations at 42 days [17].
Rosemary was investigated in five studies; in four studies [19, 39, 45, 47] in a single format and in one study combined with star anise, thyme, and oregano [32]. Tayeb et al. found that 10 g/kg of rosemary showed the best live body weight of chickens and 5 g/kg significantly reduced the mortality rate [19]. Farouk and colleagues noted that rosemary increased BWG and reduced mortality in comparison with the control group [39]. However, two Turkish studies revealed that rosemary and thyme had no significant effect on growth performance parameters [29, 45].
In their study, Nameghi and colleagues [47] assessed the impact of rosemary and thyme powders alone and combined on growth performance and microbiota. They demonstrated that the rosemary and thyme blend revealed the best average daily weight gain and the lowest FCR in comparison with rosemary, thyme, and control groups. Furthermore, broilers fed a combined diet of thyme and rosemary powders significantly showed a greater number of ileal Lactobacillus and a lower count of ileal E. coli than the control group at days 21 and 42.
Three studies [13, 17, 21] assessed the peppermint effect on growth performance parameters in a single format and 2 studies experimented it in combination with other plants [16, 36]. Abdelwareth et al. revealed that BWG and FI increased with.
the increase in dietary peppermint leaves and menthol concentrations. Besides, a lower mortality rate was recorded in peppermint groups [13]. Nouri also confirmed that mint essential oil significantly improved BWG at 42 days [17]. In their study, Witkowska and colleagues showed that live body weight and BWG were higher in broiler chickens exposed to peppermint oil mist than in control chickens exposed to thyme oil mist [21].
Adding to rosemary, thyme, and peppermint, other medicinal plans were rarely studied including cinnamon, lemon, garlic, fenugreek, oregano, sesame, lotus, aloe vera, bamboo, moringa, curcumin, and others specific to the local context of researchers.
Cinnamon was tested in two interventions. In their study, Alqhatani and colleagues [34] revealed that Cinnamomum verum bark at 2 g showed the best FCR up to 21 days. However, it had no impact on FI. Nouri also confirmed that cinnamon essential oil significantly improved BWG, FCR, and intestinal Lactobacillus population at 42 days [17].
In regards to garlic, Kairalla and colleagues showed that a 0.3% garlic powder-supplemented diet significantly resulted in better live body weight, BWG, and FCR compared to 0%, 0.1%, or 0.2% garlic powder [41]. Elbaz and colleagues compared the effects of garlic essential oil, lemon essential oil, and their mixture with the control group. They found that lemon essential oil and lemon-garlic essential oils mixture significantly showed the highest average body weight and average FI, and the lowest FCR and mortality rate at 35 days in comparison with garlic essential oil and control [38]. In another study, 4.05% of lemon juice was blended with 52.27% of orange juice, 40.45% of coconut water, 3.01% of honey, and 0.22% of Himalayan salt. Different doses of this blend had no significant effect on growth performance parameters [49].
Fenugreek was studied in two trials. Farouk and colleagues showed that broilers fed on a balanced commercial ration supplied with fenugreek at the level of 0.5% (5 g fenugreek seed powder/kg ration) from 1 day to 6 weeks old and experimentally.
infected with E. coli at 1 week of age showed the lowest mortality rate in comparison with rosemary and control groups infected with E. coli and the negative control group. Furthermore, the fenugreek-infected group showed significantly the best live body weight and BWG and lower FCR and FI in comparison with control and rosemary-infected groups [39]. These results are concordant with the findings of Yang et al. which revealed that dietary fenugreek seed extracts supplementation improved average daily weight gain (ADG) and the ratio of feed to weight gain, and significantly reduced Campylobacter, and Lachnoclostridium abundance [44].
Results related to the effects of oregano and Andrographis paniculata, sesame, lotus, aloe vera, bamboo, moringa, curcumin, licorice, and other country-specific plants such as Pulicaria gnaphalodes were presented in Table 1 [20, 22, 23, 26–28, 37, 42, 48, 50].
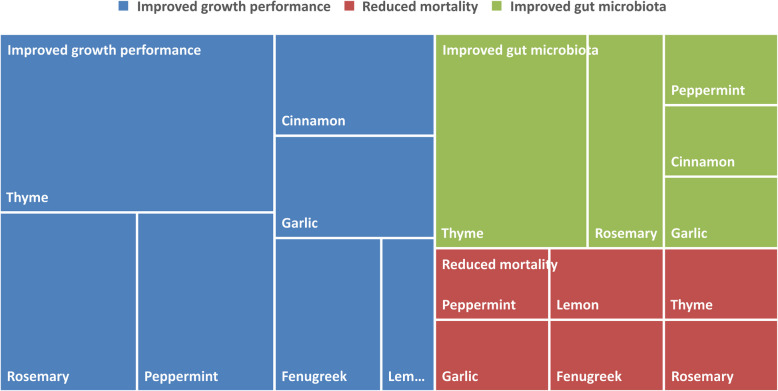
Figure 2 summarizes the number of studies that confirmed a positive effect of various medicinal plants on growth performance, mortality, and gut microbiota. It visually compares the effect of various plants on different outcomes. Larger parts showed a greater number of studies that confirmed improved outcomes. Several studies found that thyme was effective in improving growth performance, especially BW and FCR [15, 17, 19, 21, 33], and improving the quality of gut through reducing aerobic bacteria and E.coli and increasing Lactobacillus [17, 24, 40, 47]. These findings were noticed in experiments used 5 to 6 g/kg of Thyme powder [19, 24, 51]. Thyme essential oil at 0.075% in basic diet did not provide a significant difference in growth performance [30]. However, water misted with Thyme essential oil and Thyme essential oil with nanoencapsulation produced greater growth performance [17, 21]. Thyme essential oil at 0.1% with microemulsion was effective in reducing mortality and count of Salmonella Enteritidis in caecal content [40]. Different dose of Rosemary powder (5, 7.5, 10 g/kg) were also effective in improving growth performance [19, 39, 47]. Rosemary powder at 7.5 g/kg was greater in increasing Lactobacillus and reducing E. coli in intestines [47]. Rosemary powder at 5 g/kg reduced mortality in infected chicks with E. coli (10%). However, 5 g/kg of Fenugreek powder was more effective than 5 g/kg of Rosemary powder in reducing mortality in the same population (5%) [39]. Studies showed that greater dose of peppermint essential oil or leaves gave greater results in term of growth performance and reduced E. coli in intestines [13, 17, 21]
Fig. 2.
Comparison of the positive effect of various medicinal plants based on the number of studies confirming improved growth performance, reduced mortality, and improved gut microbiota
From an economic perspective, greater dose of peppermint leaves (15 g/kg) and mixture of garlic and lemon essential oils (200 mg/kg) increased the European Production Efficiency Factor [13, 38].
Discussion
This scoping review aimed to map the different growth promoter alternatives investigated in clinical trials and to compare their effectiveness on growth performance parameters, mortality, and microbiota composition in chicken production. Current findings revealed that medicinal plants, especially thyme, rosemary, and peppermint, were effective in promoting growth performance, enhancing microbiota composition, and reducing mortality rates in healthy broilers. These results are in line with a previous study that demonstrated that thyme and other medicinal plants were effective in improving growth performance and intestinal bacteria population [8].
The present scoping review revealed that thyme powder or essential oil was effective in improving body weight and Lactobacillus growth and reducing Salmonella, E. coli, and mortality rates in broiler chickens [17, 19, 21, 24, 40]. Rosemary was also effective in increasing body weight and reducing mortality [19, 39]. Indeed, the combination of thyme and rosemary produced better results than either plant alone [47]. They suggested that a blend of thyme and rosemary powders supplemented at 0.375% each into broiler diets during day 14 to 42 has the potential to be employed as a natural antioxidant in diets prepared with chicken by-product meal.
In another study, thyme essential oil supplied to poultry feed increased BWG and Lactobacillus better than mint and cinnamon essential oils [17]. The author mentioned that thyme essential oil increased Lactobacillus, FCR, and BWG in broiler chickens, especially when used as chitosan nano-encapsulation form which made it an efficient, appropriate, and affordable growth promoter alternative. Another research recorded the highest FCR when chicks drink pure water with thyme essential oil mist in comparison with adding it to basic diet (food) [21]. These discrepancies in studies’ results could be mainly explained by differences in the form, dosage of plants and experiments conditions [52]. Indeed, recent research confirms that microencapsulated essential oils improved growth performance in chickens infected with multiple resistant bacteria [53–55].
The combination of lemon and garlic essential oils resulted in the highest average body weight and enhanced intestinal microbiota in broilers than in either plant alone [38]. In broilers infected with E. coli at 1 week of age, 5 g fenugreek seed powder/kg ration free from any additives was sufficient to give the lowest mortality rate, FCR, and FI, and the best live body weight and BWG in comparison with 5 g rosemary leaves/kg ration. These findings suggested that fenugreek is likely to be more effective in infected broilers than other medicinal plants. This hypothesis needs to be tested in future research.
Other studies found that additional plants, such as aloe vera, pulicaria gnaphalodes powder, Lotus, and Origanum vulgare, had similar effect as antibiotics on growth performance and were more effective than antibiotics on gut microbiota promotion [14, 18, 31, 35, 46]. Relevant studies showed that diets supplemented with 50 ml/l Aloe vera of pH 3, 5 g/kg Lotus leaf extract, and combination of 3 g/kg of sesame bioactive peptides and 0.5 g/kg of savory and thyme essential oils produced better BWG in broilers than 100 mg/kg Enramycin and 60 mg/kg Salinomycin, 50 mg/kg Chlortetracycline, and 0.5 g/kg Bacitracin, respectively [14, 31, 35]. Nonetheless, 10 mg of Origanum vulgare and 15 mg of Andrographis paniculata extract per kg of feed, and 0.3% of Pulicaria gnaphalodes powder produced similar BWG as 6.3 mg/kg feed of Zinc bacitracin, and 0.05% Bacitracin methylene disalicylate, respectively [18, 46]. Furthermore, 10 mg of Origanum vulgare and 15 mg of Andrographis paniculata extract increased Lactobacillus and reduced E. coli and Salmonella in intestines better than Zinc bacitracin [46]. Pulicaria gnaphalodes powder at 0.3% produced higher Lactobacillus than 0.05% Bacitracin methylene disalicylate. These plants were rarely investigated in the literature. This could be related to their unavailability in different countries.
According to these data, a variety of plants including thyme, rosemary, mint, and cinnamon, reduced multiple drug resistant bacteria such as Salmonella and E.coli in gut microbiota of chickens. According to Farouk et al. 2022 [39], Fenugreek was more effective than rosemary in reducing mortality in chickens infected with E. coli. Hence, it can hold potential for future research. Considering the antibacterial properties of these plants in human and animals, future trial must consider investigating their effects on infected chickens with multi-drug resistant bacteria in comparison with antibiotics. This would be beneficial on both scientific and economic levels as the number of antibiotic substances is becoming limited on the market.
The incorporation of medicinal plants as feed additives in poultry and livestock is being investigated as a natural substitute for antibiotics. By using herbs with antimicrobial properties, farmers can help control multi-resistant bacteria while promoting gut health and enhancing overall animal performance.
Medicinal plants have demonstrated effectiveness in reducing intestinal multi-resistant bacteria in poultry and other livestock through various mechanisms [56]. These plants contain bioactive compounds such as polyphenols, alkaloids, tannins, flavonoids, and essential oils [57], which possess antimicrobial properties that inhibit the growth of resistant bacteria. For instance, oregano, thyme, garlic, and cinnamon contain active compounds like carvacrol, thymol, allicin, and cinnamaldehyde [58]. which can compromise bacterial cell membranes and disrupt essential metabolic functions.
Additionally, herbs contribute to a healthier gut microbiota by fostering the growth of beneficial bacteria (e.g., Lactobacillus, Bifidobacterium) while suppressing harmful or resistant strains. A well-balanced microbiome reduces the likelihood of multi-resistant bacteria colonization by competing for nutrients and space [59]. Furthermore, certain plant compounds can impede horizontal gene transfer, the mechanism by which bacteria exchange resistance genes [60]. By limiting this process, herbs play a role in slowing the spread of antimicrobial resistance within the gut environment.
Finally, it is well known that bacteria are economic organisms by nature, and that their investment in a process of multi-resistance to antibiotics is an energy-consuming phenomenon which will only hump them when they need to. From this point of view, we can explain that the use of antibiotics exerts a selection pressure on the bacteria, which will develop resistance to protect themselves against the antibiotic. Reducing the use of antibiotics or replacing them with other alternatives, such as medicinal plants, will create a certain level of reassurance in the bacteria, which, feeling under no threat, will not go on to multiply its resistance.
Antimicrobial resistance (AMR) is a growing concern in both human and veterinary medicine, and its impact on poultry health is profound. AMR occurs when microorganisms, such as bacteria, fungi, and parasites, evolve mechanisms that reduce or eliminate the effectiveness of drugs designed to target them. Understanding the underlying mechanisms of AMR is essential for developing strategies to combat its spread in poultry production.
One of the primary mechanisms through which AMR develops is through mutations in the genetic material of microorganisms. Mutations in the genes of bacteria can alter the structure of drug target sites, such as enzymes or cell wall components, making the bacteria less susceptible to the effects of antimicrobial agents. These mutations are often spontaneous but can be exacerbated by the selective pressure of antibiotic use. In Poultry, In Salmonella and Escherichia coli, mutations can result in resistance to common antibiotics like tetracycline, aminoglycosides, or fluoroquinolones [61]. For instance, resistance in Salmonella to quinolones has been associated with mutations in the gyrA gene, which encodes for the enzyme DNA gyrase, the target of quinolone antibiotics [62]. Some plant compounds, such as alkaloids (e.g., berberine from Berberis vulgaris) and flavonoids (e.g., quercetin from onions), have been shown to inhibit bacterial growth or enhance the efficacy of conventional antibiotics [63]. These plant-derived agents could act synergistically with existing antibiotics, potentially reducing the emergence of resistance mutations.
Horizontal Gene Transfer (HGT) is a critical mechanism by which bacteria share resistance genes, allowing resistance to spread rapidly within and between bacterial species. This process can occur through conjugation (direct transfer of plasmids), transformation (uptake of naked DNA), or transduction (bacteriophage-mediated transfer). In Poultry, Campylobacter jejuni is a common reservoir for antibiotic resistance genes, particularly those coding for resistance to macrolides and fluoroquinolones [64]. Resistance can be spread rapidly in poultry production environments through HGT, exacerbating the AMR crisis. Certain plant compounds may disrupt the mechanisms of HGT. Flavonoids (e.g., luteolin, apigenin) have been demonstrated to interfere with bacterial conjugation, reducing the transfer of resistance genes [65]. By inhibiting HGT, plant-based compounds could help slow the spread of antimicrobial resistance within poultry flocks.
Many resistant bacteria can employ efflux pumps to actively expel antimicrobial agents from their cells, rendering them ineffective. These pumps can contribute to multi-drug resistance by making the bacteria less susceptible to a wide range of antibiotics. This mechanism is prevalent in various bacterial species found in poultry, including Campylobacter and Salmonella: efflux pumps can be responsible for resistance to commonly used antibiotics like tetracycline, ampicillin, and chloramphenicol [66, 67]. Efflux pumps such as the AcrAB-TolC system in E. coli can pump out antibiotics before they can reach effective concentrations inside the bacterial cell. Certain plant compounds can inhibit bacterial efflux pumps, increasing the effectiveness of antibiotics [68]. Carvacrol (from oregano) and thymol (from thyme) have been shown to inhibit the AcrAB-TolC efflux pump in E. coli [69]. These plant compounds might serve as potential adjuncts in combating resistance by reducing the capacity of bacteria to expel antimicrobial agents.
In addition, some bacteria produce enzymes that can chemically modify and deactivate antimicrobial agents, rendering them ineffective. These enzymes include β-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferases. They can break down or alter antibiotics, preventing them from binding to their targets. In Poultry, Escherichia coli can produce extended-spectrum β-lactamases (ESBLs) that break down β-lactam antibiotics, such as penicillins and cephalosporins. This resistance mechanism has been widely reported in poultry-associated E. coli strains. Some plant compounds have been shown to inhibit the activity of antibiotic-degrading enzymes [70]. Flavonoids and saponins have demonstrated the ability to reduce the production of β-lactamases in bacterial strains, making antibiotics more effective [71, 72]. This could be a useful strategy for restoring the efficacy of β-lactam antibiotics in poultry production. For example, β-lactamase enzymes, which break down β-lactam antibiotics like penicillin, are widespread in poultry-associated pathogens. Other enzymes, such as aminoglycoside-modifying enzymes, also contribute to resistance by modifying antibiotics and preventing their action.
Since animal and human health are interconnected according to the One Health approach, there is an urgent need for the investigation of these plants’ effects on AMR in chicken. This could be beneficial in developing new strategies in reducing the exacerbation of AMR in human.
However, ethical considerations related to the use of medicinal plants in poultry feed trials should be considered. Although the papers included in this scoping review have considered ethical aspects, we would like to highlight several key ethical considerations in poultry feed trials involving medicinal plants.
Animal welfare is paramount—it is crucial to ensure that the inclusion of medicinal plants in poultry feed does not negatively impact the bird's health, behaviour, or overall well-being. Researchers must carefully monitor potential toxicity, palatability, and physiological effects to prevent undue stress or harm.
Additionally, the sustainable sourcing of plant materials must be considered, as ethical concerns extend beyond animal welfare to environmental impact. Overharvesting or unsustainable cultivation of medicinal plants can lead to biodiversity loss and ecosystem disruption. Therefore, researchers should prioritize the use of sustainably sourced, organically cultivated, or waste-derived plant materials to minimize environmental harm and promote responsible resource management.
By addressing these ethical considerations, poultry feed trials can contribute to scientific progress while upholding high standards of animal welfare, research integrity, and environmental sustainability.
Limitations
This literature review presented several limitations. First, there is a risk for potential bias in study selection related to limited number of databases and English language publications.
However, this scoping review reported recent interventions regarding growth promoter alternatives in chicken production. The included studies have heterogeneous methodologies. Three trials tested a blend of different medicinal plants. Consequently, it is difficult to neutralize the effect of each herb. In addition, eleven studies investigated different local medicinal plants such as bamboo, lotus, moringa, neem, oregano, sacha inchi, yohimbe bark meal, and tropical medicinal plants. The lack of information on these plants can lead to an underestimation of their impact as growth promoter alternatives in chicken production.
A variety of plants including rosemary, thyme, peppermint, cinnamon, fenugreek, lemon, and garlic, as a powder or essential oil, proved effectiveness in improving performance parameters and microbiota composition in broiler chickens. These plants are available in many countries worldwide. Peppermint powder and leaves provided higher European production efficiency factor, improved growth performance and reduced E.coli in intestines. Thus, it could be a greater alternative in both economic and production levels for veterinarians and farmers. Using medicinal plants in commercial poultry production becomes highly recommended especially in treating infections [73]. Veterinarians play a crucial role in awakening farmers regarding safe, healthy, and sustainable poultry production.
After mapping evidence regarding various plants and their effects on growth performance, mortality, and gut microbiota, authors recommended a future systematic review that systematically search the impact of thyme on these outcomes, considering differences in dosage, format (essential oil, powder, mist, etc.), experiments, and plant preparation to define which aspect of thyme is most effective in improving growth performance and gut microbiota. For sustainable poultry production, further clinical trials should assess the effect of 5 to 6 g/kg of thyme, 5 to 10 g/kg of rosemary, and 5 to 10 g/kg thyme-rosemary blend on other relevant outcomes such as economic return and antimicrobial resistance in healthy and infected chickens with multiple resistant bacteria.
Conclusions
This scoping review showed that thyme, rosemary, and peppermint were widely tested, and they were effective in promoting BWG, FI, FCR, live body weight, and microbiota, and in reducing mortality rate and intestinal multi-resistant bacteria. Cinnamon, lemon, garlic, and fenugreek were less tested. However, they were effective in improving BWG and FCR and reducing intestinal bacteria. Studies revealed that lemon-garlic and rosemary-thyme combinations showed the best results in terms of growth performance. Rosemary and thyme blend also showed a greater number of ileal Lactobacillus, a lower count of ileal E. coli, and a reduced mortality rate. Fenugreek was more effective than rosemary in reducing mortality in infected broilers. From one health perspective, exploring the effect of 5 to 6 g/kg of thyme, 5 to 10 g/kg of rosemary, and 5 to 10 g/kg thyme-rosemary blend on AMR in chicken infected with multi-drug resistant bacteria need to be studied by further experiments.
Supplementary Information
Acknowledgements
None.
Authors' contributions
M.D, M.M, S.A and A.M made the research of papers, analyse the results and wrote the manuscript. WM conceived the idea, monitored the work and revised the manuscript. All authors reviewed the manuscript.
Funding
This work was funded by the International Center for Antimicrobial Resistance Solutions (ICARS)- Project ID: 200005; ENVIRE Project—13 th JPIAMR transnational co-funded call for research projects within the ERA-NET JPIAMR-ACTION: “One Health interventions to prevent or reduce the development and transmission of antimicrobial resistance (AMR)”.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magnusson U, Moodley A, Osbjer K (2021) Antimicrobial resistance at the livestock–human interface: implications for Veterinary Services. OIE Rev Sci Tech 40:511–521. 10.20506/rst.40.2.3241. [DOI] [PubMed]
- 2.Mehdi Y, Létourneau-Montminy MP, Lou GM, et al. Use of antibiotics in broiler production: Global impacts and alternatives. Anim Nutr. 2018;4:170–8. 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agyare C, Boamah VE, Zumbi CN, Osei FB (2018) Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. In: Kumar Y (ed) Antimicrobial Resistance. IntechOpen.
- 4.World Health Organization (2020) Antibiotic resistance. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance#:~:text=Bacteria%2C not humans or animals,hospital stays%2C and increased mortality. Accessed 28 Jul 2023.
- 5.Centers for Disease Control and Prevention (CDC) (2022) Antimicrobial Resistance. In: About Antimicrob. Resist. https://www.cdc.gov/drugresistance/about.html. Accessed 28 Jul 2023.
- 6.World Health Organization (2022) One health. https://www.who.int/news-room/fact-sheets/detail/one-health. Accessed 23 May 2023.
- 7.United Nations (2023) Sustainable Development Goals. https://www.un.org/sustainabledevelopment/. Accessed 23 May 2023.
- 8.Seidavi A, Tavakoli M, Slozhenkina M, et al. The use of some plant-derived products as effective alternatives to antibiotic growth promoters in organic poultry production: a review. Environ Sci Pollut Res. 2021;28:47856–68. 10.1007/S11356-021-15460-7. [DOI] [PubMed] [Google Scholar]
- 9.Seidavi A, Tavakoli M, Asroosh F, et al. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ Sci Pollut Res. 2022;29:5006–31. 10.1007/S11356-021-17401-W. [DOI] [PubMed] [Google Scholar]
- 10.Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:1–7. 10.1186/S12874-018-0611-X/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak S, Thomas A. Steps for Conducting a Scoping Review. J Grad Med Educ. 2022;14:565. 10.4300/JGME-D-22-00621.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–73. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Wareth AAA, Kehraus S, Südekum KH. Peppermint and its respective active component in diets of broiler chickens: growth performance, viability, economics, meat physicochemical properties, and carcass characteristics. Poult Sci. 2019;98:3850–9. 10.3382/ps/pez099. [DOI] [PubMed] [Google Scholar]
- 14.Akram MZ, Salman M, Jalal H, et al. Growth Promoters in Broiler Production. Turkish J Vet Res. 2019;3:21–6. [Google Scholar]
- 15.Ismail FSA, El-Gogary MR, El-Morsy N. Impact of Dietary Supplementation of Different Levels of. Egypt Poult Sci J. 2019;39:365–79. [Google Scholar]
- 16.Hesabi Nameghi A, Edalatian O, Bakhshalinejad R. Effects of a blend of thyme, peppermint and eucalyptus essential oils on growth performance, serum lipid and hepatic enzyme indices, immune response and ileal morphology and microflora in broilers. J Anim Physiol Anim Nutr (Berl). 2019;103:1388–98. 10.1111/jpn.13122. [DOI] [PubMed] [Google Scholar]
- 17.Nouri A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br Poult Sci. 2019;60:530–8. 10.1080/00071668.2019.1622078. [DOI] [PubMed] [Google Scholar]
- 18.Shirani V, Jazi V, Toghyani M, et al. Pulicaria gnaphalodes powder in broiler diets: consequences for performance, gut health, antioxidant enzyme activity, and fatty acid profile. Poult Sci. 2019;98:2577–87. 10.3382/ps/pez010. [DOI] [PubMed] [Google Scholar]
- 19.Tayeb IT, Artoshi NHR, Sögüt B (2020) Performance of broiler chicken fed different levels thyme, adiantum, rosemary and their combination. Iraqi J Agric Sci 50:1522–1532. 10.36103/IJAS.V50I6.840.
- 20.Vase-Khavari K, Mortezavi SH, Rasouli B, et al. The effect of three tropical medicinal plants and superzist probiotic on growth performance, carcass characteristics, blood constitutes, immune response, and gut microflora of broiler. Trop Anim Health Prod. 2019;51:33–42. 10.1007/s11250-018-1656-x. [DOI] [PubMed] [Google Scholar]
- 21.Witkowska D, Sowinska DJ, Murawska DD, et al. Effect of peppermint and thyme essential oil mist on performance and physiological parameters in broiler chickens. S Afr J Anim Sci. 2019;49:29–39. 10.4314/sajas.v49i1.4. [Google Scholar]
- 22.Abdul Basit M, Arifah AK, Loh TC, et al. Effects of graded dose dietary supplementation of Piper betle leaf meal and Persicaria odorata leaf meal on growth performance, apparent ileal digestibility, and gut morphology in broilers. Saudi J Biol Sci. 2020;27:1503–13. 10.1016/j.sjbs.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul Basit M, Kadir AA, Loh TC, et al. Effects of inclusion of different doses of persicaria odorata leaf meal (Polm) in broiler chicken feed on biochemical and haematological blood indicators and liver histomorphological changes. Animals. 2020;10:1–18. 10.3390/ani10071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldik HBM, Suhail AI, Ali NAL. Effect of adding different levels of thymus vulgaris leaves powder to the diet, on blood physiological traits and microbial content of the gut of broiler chickens. Plant Arch. 2020;20:858–62. [Google Scholar]
- 25.Ahmadian A, Seidavi A, Phillips CJC. Growth, carcass composition, haematology and immunity of broilers supplemented with sumac berries (Rhus coriaria l.) and thyme (Thymus vulgaris). Animals. 2020;10:1–14. 10.3390/ani10030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obajuluwa OV, Sanwo KA, Egbeyale LT, Fafiolu AO. Performance, blood profile and gut morphometry of broiler chickens fed diets supplemented with Yohimbe (Pausynistalia yohimbe) and Larvacide. Vet Anim Sci. 2020;10: 100127. 10.1016/j.vas.2020.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu G, Kong F, Xu D, et al. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci Rep. 2020;10:1–10. 10.1038/s41598-020-69010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav S, Teng PY, Souza dos Santos T, et al. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult Sci. 2020;99:5936–45. 10.1016/j.psj.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yalçin S, Eser H, Onbaşilar İ, Yalçin S (2020) Effects of dried thyme (Thymus vulgaris l.) leaves on performance, some egg quality traits and immunity in laying hens. Ankara Univ Vet Fak Derg 67:303–311. 10.33988/auvfd.677150.
- 30.Amouei H, Ferronato G, Qotbi AAA, et al. Effect of essential oil of thyme (Thymus vulgaris l.) or increasing levels of a commercial prebiotic (technomos® ) on growth performance and carcass characteristics of male broilers. Animals. 2021;11:1–12. 10.3390/ani11113330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L, Zhang W, Jin Q, et al. The effects of dietary supplementation with lotus leaf extract on the immune response and intestinal microbiota composition of broiler chickens. Poult Sci. 2021;100: 100925. 10.1016/j.psj.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youssef IMI, Männer K, Zentek J. Effect of essential oils or saponins alone or in combination on productive performance, intestinal morphology and digestive enzymes’ activity of broiler chickens. J Anim Physiol Anim Nutr (Berl). 2021;105:99–107. 10.1111/JPN.13431. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadzadeh A, Nobakht A, Mehmannavaz Y. Supplementary Prebiotics, Probiotics, and Thyme (Thymus vulgaris) Essential Oil for Broilers: Performance, Intestinal Morphology, and Fecal Nutrient Composition. Probiotics Antimicrob Proteins. 2022. 10.1007/s12602-022-09927-3. [DOI] [PubMed] [Google Scholar]
- 34.Alqhtani AH, Qaid MM, Al-Garadi MA, et al. Efficacy of Rumex nervosus leaves or Cinnamomum verum bark as natural growth promoters on the growth performance, immune responsiveness, and serum biochemical profile of broiler chickens. Ital J Anim Sci. 2022;21:792–801. 10.1080/1828051X.2022.2065941. [Google Scholar]
- 35.Bahadori MM, Rezaeipour V, Abdullahpour R, Irani M (2022) Effects of sesame meal bioactive peptides, individually or in combination with a mixture of essential oils, on growth performance, carcass, jejunal morphology, and microbial composition of broiler chickens. Trop Anim Health Prod 54:. 10.1007/s11250-022-03232-5. [DOI] [PubMed]
- 36.Behboodi HR, Hosseini D, Salarieh A, et al (2022) Impact of drinking water supplementation of a blend of peppermint, coneflower (Echinacea purpurea), thyme, propolis, and prebiotic on performance, serum constituents, and immunocompetence of broiler chickens. Trop Anim Health Prod 54:. 10.1007/S11250-022-03274-9. [DOI] [PubMed]
- 37.Cong ON, Viet DN, Kim DP, Hornick JL. Effects of dietary sacha inchi (Plukenetia volubilis L.) oil and medicinal plant powder supplementation on growth performance, carcass traits, and breast meat quality of colored broiler chickens raised in Vietnam. Trop Anim Health Prod. 2022;54:1–9. 10.1007/s11250-021-02994-8. [DOI] [PubMed] [Google Scholar]
- 38.Elbaz AM, Ashmawy ES, Salama AA, et al. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet Res. 2022;18:1–12. 10.1186/s12917-022-03530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farouk SM, Abdel-Rahman HG, Abdallah OA, EL-Behidy NG,. Comparative immunomodulatory efficacy of rosemary and fenugreek against Escherichia coli infection via suppression of inflammation and oxidative stress in broilers. Environ Sci Pollut Res. 2022;29:40053–67. 10.1007/s11356-021-18358-6. [DOI] [PubMed] [Google Scholar]
- 40.Hamed EA, Abdelaty MF, Sorour HK, et al (2022) A Pilot Study on the Effect of Thyme Microemulsion Compared with Antibiotic as Treatment of Salmonella Enteritidis in Broiler. Vet Med Int 2022:. 10.1155/2022/3647523. [DOI] [PMC free article] [PubMed]
- 41.Kairalla MA, Alshelmani MI, Aburas AA. Effect of diet supplemented with graded levels of garlic (Allium sativum L.) powder on growth performance, carcass characteristics, blood hematology, and biochemistry of broilers. Open Vet J. 2022;12:595–601. 10.5455/OVJ.2022.v12.i5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanwal A, Asghar R, Babar M, et al. Growth performance of poultry in relation to Moringa oliefera and Azadirachta indica leaves powder. J King Saud Univ - Sci. 2022;34: 102234. 10.1016/j.jksus.2022.102234. [Google Scholar]
- 43.Noruzi S, Torki M, Mohammadi H. Effects of supplementing diet with Thyme (Thymuas vulgaris L.) essential oil and/or selenium yeast on production performance and blood variables of broiler chickens. Vet Med Sci. 2022;8:1137–45. 10.1002/VMS3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Chen L, Zheng K, et al. Effects of fenugreek seed extracts on growth performance and intestinal health of broilers. Poult Sci. 2022;101: 101939. 10.1016/j.psj.2022.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumus R, Gelen SU. Effects of dietary thyme and rosemary essential oils on performance parameters with lipid oxidation, water activity, pH, colour and microbial quality of breast and drumstick meats in broiler chickens. Arch Anim Breed. 2023;66:17–29. 10.5194/aab-66-17-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jahja EJ, Yuliana R, Simanjuntak WT, et al. Potency of Origanum vulgare and Andrographis paniculata extracts on growth performance in poultry. Vet Anim Sci. 2023;19: 100274. 10.1016/j.vas.2022.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nameghi AH, Edalatian O, Bakhshalinejad R (2023) A blend of thyme and rosemary powders with poultry by-product meal can be used as a natural antioxidant in broilers. Acta Sci - Anim Sci 45:. 10.4025/actascianimsci.v45i1.57126.
- 48.Oladokun S, Clark KF, Adewole DI (2022) Microbiota and Transcriptomic Effects of an Essential Oil Blend and Its Delivery Route Compared to an Antibiotic Growth Promoter in Broiler Chickens. Microorganisms 10:. 10.3390/MICROORGANISMS10050861. [DOI] [PMC free article] [PubMed]
- 49.Safiyu KK, Akinsola KL, Ibedu AJ, et al (2023) Effect of citrus-coconut electrolyte blend on growth performance, haemato-biochemical status, organs development and intestinal morphology of broiler chickens. Trop Anim Health Prod 55:. 10.1007/s11250-023-03463-0. [DOI] [PubMed]
- 50.Toson E, Abd El Latif M, Mohamed A, et al. Efficacy of licorice extract on the growth performance, carcass characteristics, blood indices and antioxidants capacity in broilers. Animal. 2023;17: 100696. 10.1016/j.animal.2022.100696. [DOI] [PubMed] [Google Scholar]
- 51.Ismaila A, Blais L, Dang-Tan T, et al (2019) Direct and indirect costs associated with moderate and severe asthma in Quebec, Canada. Can J Respir Crit Care, Sleep Med 0:1–9. 10.1080/24745332.2018.1544839.
- 52.Salahshour A, Vakili R, Poultry AN-BJ of, 2023 undefined Effect of different conditioning temperatures and times on the pellet quality, performance, intestinal morphology, ileal microbial population, and apparent. SciELO Bras Salahshour, R Vakili, AH NameghiBrazilian J Poult Sci 2023•SciELO Bras.
- 53.Moharreri M, Vakili R, … EO-J of the S, 2022 undefined Effects of microencapsulated essential oils on growth performance and biomarkers of inflammation in broiler chickens challenged with salmonella enteritidis. ElsevierM Moharreri, R Vakili, E Oskoueian, G RajabzadehJournal Saudi Soc Agric Sci 2022•Elsevier.
- 54.Moharreri M, Vakili R, … EO-A of R, 2022 undefined (2022) Evaluation of microencapsulated essential oils in broilers challenged with salmonella enteritidis: a focus on the body’s antioxidant status, gut microbiology, and. pmc.ncbi.nlm.nih.govM Moharreri, R Vakili, E Oskoueian, R GhArchives Razi Institute, 2022•pmc.ncbi.nlm.nih.gov. 10.22092/ARI.2021.354334.1634. [DOI] [PMC free article] [PubMed]
- 55.Moharreri M, Vakili R, … EO-IJ of, 2021 undefined (2021) Phytobiotic role of essential oil-loaded microcapsules in improving the health parameters in Clostridium perfringens-infected broiler chickens. Taylor Fr Moharreri, R Vakili, E Oskoueian, G RajabzadehItalian J Anim Sci 2021•Taylor Fr 20:2075–2085. 10.1080/1828051X.2021.1993093.
- 56.Abiala M, Olayiwola J, Babatunde O, et al (2016) Evaluation of therapeutic potentials of plant extracts against poultry bacteria threatening public health. SpringerM Abiala, J Olayiwola, O Babatunde, O Aiyelaagbe, S AkinyemiBMC Complement Altern Med 2016•Springer 16:. 10.1186/s12906-016-1399-z. [DOI] [PMC free article] [PubMed]
- 57.Egbu C, Mulaudzi A, Motsei L, et al (2024) Moringa oleifera products as nutraceuticals for sustainable poultry production. SpringerCF Egbu, A Mulaudzi, LE Motsei, C MnisiAgriculture Food Secur 2024•Springer 13:. 10.1186/s40066-024-00508-x.
- 58.Yuan W, Microbiology HY-A and E, 2019 undefined (2019) Effects of Sublethal Thymol, Carvacrol, and trans-Cinnamaldehyde Adaptation on Virulence Properties of Escherichia coli O157:H7. journals.asm.orgW Yuan, HG YukApplied Environ Microbiol 2019•journals.asm.org 85:. 10.1128/AEM.00271-19. [DOI] [PMC free article] [PubMed]
- 59.Aruwa C, Pillay C, Nyaga M, et al (2021) Poultry gut health–microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. SpringerCE Aruwa, C Pillay, MM Nyaga, S SabiuJournal Anim Sci Biotechnol 2021•Springer 12:. 10.1186/s40104-021-00640-9. [DOI] [PMC free article] [PubMed]
- 60.Arip M, Selvaraja M, Mogana R, et al (2022) Review on Plant-Based Management in Combating Antimicrobial Resistance - Mechanistic Perspective. Front Pharmacol 13:. 10.3389/FPHAR.2022.879495/PDF. [DOI] [PMC free article] [PubMed]
- 61.Zhou K, Sun L, Zhang X, et al (2023) Salmonella antimicrobials inherited and the non-inherited resistance: mechanisms and alternative therapeutic strategies. Front Microbiol 14:. 10.3389/FMICB.2023.1176317/PDF. [DOI] [PMC free article] [PubMed]
- 62.Eaves DJ, Randall L, Gray DT, et al (2004) Prevalence of Mutations within the Quinolone Resistance-Determining Region of gyrA, gyrB, parC, and parE and Association with Antibiotic Resistance in Quinolone. journals.asm.orgDJ Eaves, L Randall, DT Gray, A Buckley, MJ Woodward, AP White, LJV PiddockAntimicrobial agents Chemother 2004•journals.asm.org 48:4012–4015. 10.1128/AAC.48.10.4012-4015.2004. [DOI] [PMC free article] [PubMed]
- 63.Tarabishi A, Mashhoud J, Food ZT-D, 2024 undefined (2024) Quercetin and rutin as a dual approach to antibacterial and anti-biofilm activity via iron chelation mechanism. SpringerAA Tarabishi, J Mashhoud, ZS TahanDiscover Food, 2024•Springer 4:189. 10.1007/s44187-024-00265-7.
- 64.Efimochkina NR, Stetsenko VV, Sheveleva SA. Formation of the Resistance of Campylobacter jejuni to Macrolide Antibiotics. Bull Exp Biol Med. 2020;169:351–6. 10.1007/S10517-020-04885-8. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Shen W, Li Y, et al (2025) Multi-omics Analysis of Klebsiella pneumoniae Revealed Opposing Effects of Rutin and Luteolin on Strain Growth. Curr Microbiol 82:. 10.1007/S00284-024-03982-5. [DOI] [PubMed]
- 66.Mumbo M, Nyaboga E, Kinyua J, et al (2023) Antimicrobial resistance profiles of salmonella spp. and escherichia coli isolated from fresh nile tilapia (oreochromis niloticus) fish marketed for human consumption. SpringerMT Mumbo, EN Nyaboga, JK Kinyua, EK Muge, SGK Mathenge, H Rotich, G MuriiraBMC Microbiol 2023•Springer 23:. 10.1186/s12866-023-03049-8. [DOI] [PMC free article] [PubMed]
- 67.Quinn T, Bolla J, … JP-J of antimicrobial, 2007 undefined Antibiotic-resistant Campylobacter: could efflux pump inhibitors control infection? Acad Quinn, JM Bolla, JM Pagès, S FanningJournal Antimicrob Chemother 2007•academic.oup.com. [DOI] [PubMed]
- 68.Abass S, Parveen R, Irfan M, et al (2024) Mechanism of antibacterial phytoconstituents: An updated review. SpringerS Abass, R Parveen, M Irfan, Z Malik, SA Husain, S AhmadArchives Microbiol 2024•Springer 206:. 10.1007/s00203-024-04035-y. [DOI] [PubMed]
- 69.Bohnert J, … SS-T open microbiology, 2013 undefined (2013) Pimozide inhibits the AcrAB-TolC efflux pump in Escherichia coli. pmc.ncbi.nlm.nih.govJA Bohnert, S Schuster, WV KernThe open Microbiol journal, 2013•pmc.ncbi.nlm.nih.gov 83. [DOI] [PMC free article] [PubMed]
- 70.Blaak H, A M van Hoek AH, Hamidjaja RA, et al (2015) Distribution, Numbers, and Diversity of ESBL-Producing E. coli in the Poultry Farm Environment. journals.plos.orgH Blaak, AHAM van Hoek, RA Hamidjaja, RQJ van der Plaats, L Kerkhof-de HeerPloS one, 2015•journals.plos.org 10:. 10.1371/journal.pone.0135402. [DOI] [PMC free article] [PubMed]
- 71.Lateef Adebayo O, James A, Kasim SB, Jagri OP (2014) Leaf extracts of Vernonia amygdalina Del. From northern Ghana contain bioactive agents that inhibit the growth of some beta-lactamase producing bacteria in. Acad Adebayo, J Abugri, SB Kasim, PJ OnilimorBritish J Pharm Res 2014•academia.edu 4:192–202.
- 72.Intharuksa A, Kuljarusnont S, Molecules YS-, 2024 undefined Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and. mdpi.comA Intharuksa, S Kuljarusnont, Y Sasaki, D TungmunnithumMolecules, 2024•mdpi.com. [DOI] [PMC free article] [PubMed]
- 73.Jamil M, Aleem MT, Shaukat A, et al. Medicinal Plants as an Alternative to Control Poultry Parasitic Diseases. Life. 2022;12:449. 10.3390/LIFE12030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.