Abstract
The use of probiotics to reduce the use of chemical fertilizers in agriculture has proven to be a promising area of research, especially as the agricultural sector searches for sustainable alternatives to synthetic agents. However, there are few studies integrating probiotics into agricultural practices, especially in lettuce cultivation, to minimize the use of chemical fertilizers. Most previous research has focused on the effects of plant growth-promoting rhizobacteria (PGPR) and microalgae on yield, with little consideration of the combined effects of different microorganisms under the same soil and growing conditions. In this study, the effects of microbial biostimulants on the efficiency of fertilizer use in lettuce (Lactuca sativa L.) cultivation were investigated, reducing the use of chemical fertilizers by 25%. The trial was conducted as a randomized block trial in a high tunnel greenhouse and comprised eight treatments: a negative control (T0, no chemical fertilizers or microorganisms), a positive control (T1, 500 kg ha−1 NPK fertilizer) and treatments with single biostimulants — microalgae (Chlorella vulgaris, T2), plant probiotic microorganisms (PPMs, Lactobacillus spp, Rhodopseudomonas palustris, Saccharomyces cerevisiae, T3), and PGPRs (Bacillus subtilis, Bacillus megaterium, Bacillus amyloliquefaciens, T4). Further treatments combined reduced fertilizer (375 kg ha−1 NPK) with microalgae (T5), PPMs (T6) and PGPRs (T7). The results showed that T6 (PPMs + reduced fertilizer) achieved the highest plant weight (364 g) and leaf length (43.8 cm), with increases of 152% and 128%, respectively, compared to the control (T0). Chlorophyll content improved significantly in T5 (27.5%) and T7 (29.4%) compared to T0. Biomass production was 78% higher in T6 than in T1, while T5 and T7 showed a moderate increase in biomass of 42% and 47%, respectively. Cluster analysis identified T6 as the most effective treatment, outperforming T1 in growth parameters. These results demonstrate the potential of microbial biostimulants, especially in combination with reduced chemical fertilizers, to increase plant growth, biomass production and fertilizer efficiency. The study underlines their role in promoting sustainable agriculture and provides a basis for future research under different soil and environmental conditions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-06986-w.
Keywords: Chemical Fertilizer, Sustainable Agriculture, Lettuce, Microbial Inoculation, Biostimulants
Introduction
The global human population is rapidly growing, and with this population increase, the rising demand for food is also driving a greater need for synthetic fertilizers [1]. The application of chemical fertilizers (CF) is not only a crucial determinant of high agricultural production but also a widely adopted management strategy for improving soil fertility globally [2]. In recent years, the growth of global crop yields has largely depended on substantial investments in synthetic fertilizers [3]. Farmers have started to apply large quantities of fertilizer in order to achieve higher crop yields and thus increase yields [4]. However, it is known that only a fraction of the applied fertilizers is absorbed by the plants [5]. The utilization of applied fertilizers in total crop yield has been reported to be approximately 50% or less for nitrogen, less than 25% for phosphorus, and around 40% for potassium mineral fertilizers [6]. More than 55% of the increase in crop production in developing countries is attributed to the use of nitrogen-containing fertilizers [7]. As a result, farmers aiming to increase yield by enhancing nitrogen fertilization have started applying nitrogen at rates higher than the recommended levels [1]. Nevertheless, excessive and unregulated nitrogen fertilization, not aligned with crop requirements, can cause potential harm to the soil, including excessive nutrient accumulation, soil acidification, a decline in organic matter, and a sharp reduction in soil biodiversity [8, 9]. Moreover, the potential for nitrogen loss through leaching and runoff is increased [10]. Consequently, nitrogen fertilizer losses from agricultural fields contribute to water pollution, allowing these contaminants to mix with rivers and groundwater. This contamination negatively impacts ecosystems and biodiversity, thereby posing risks to both the environment and human health [11]. In addition, the excessive application of these fertilizers has a negative impact on food safety and quality, as it leads to an accumulation of nitrates and potentially toxic metals in the plants and at the same time deteriorates the physico-chemical properties of the soil, which in turn leads to reduced fertility and limited agricultural usability. These negative effects can be mitigated by minimizing their use and using bio-organic fertilizers as sustainable alternatives. Biofertilizers are products containing live microorganisms or natural compounds derived from organisms such as bacteria and fungi that enhance the chemical and biological properties of the soil, promote plant growth, and restore soil fertility [12]. Among biofertilizers, formulations based on photosynthetic organisms, including prokaryotic cyanobacteria and microalgae, have gained significant attention due to their excellent ability to enhance bioavailability, soil fertility, and crop yield [13, 14]. Moreover, their biomass contains proteins, carbohydrates, and vitamins that are suitable for producing green manure [15]. Compared to synthetic fertilizers, biofertilizers, particularly microorganisms derived from biofertilizers, offer several advantages, such as improving the efficient utilization of nitrogen, phosphorus, potassium, and other elements in the soil, enhancing crop stress tolerance, improving the quality and yield of agricultural products, and significantly inhibiting the toxicity of plant pathogens in the soil [16]. Previous research has largely focused on long-term chemical or organic fertilization, with little attention paid to the combined use of bio-organic fertilizers and reduced conventional fertilizer applications, especially in lettuce cultivation [17]. Consequently, uncertainties remain regarding the effects on yield, quality and diversity of soil bacterial and fungal communities in systems that integrate organic and inorganic fertilization [17]. In this study, lettuce (Lactuca sativa L.), a widely grown and globally popular vegetable prized for its leaf structure, was selected as a model crop [18]. Although the excessive use of fertilizers in lettuce cultivation has short-term benefits for plant growth, it also poses significant environmental risks. In this study, the effects of biofertilization with plant probiotic microorganisms (PPBs), plant growth-promoting bacteria (PGPR), and microalgae in combination with a 25% reduction in conventional fertilizer use on lettuce cultivation were investigated. The aim was to determine how reducing the use of conventional fertilizers in combination with the application of beneficial microorganisms affects crop yields and to explore the potential of these biofertilizers to improve fertilizer efficiency in order to contribute to sustainable agriculture through greenhouse experiments.
Our study aims to compare the effects of different microbial biostimulants (microalgae, plant probiotic microorganisms (PPMs) and plant growth-promoting rhizobacteria (PGPRs)) on the reduction of chemical fertilizer use under the same soil, plant and cropping conditions. This approach provides a practical way to determine which biostimulant is more effective and to minimize reliance on chemical fertilizers. Comparative studies of this type are very limited in the literature and fill a significant knowledge gap.
Materials and Methods
Experimental material and applications
This study was conducted in the high tunnel greenhouse of the Gebze Technical University Campus between December 2022 and March 2023. The coordinates of the experimental area were recorded as (40° 48′ 27.1764"N, 29° 21′ 50.8536"E). In this study, the curly lettuce variety Festival (Lactuca sativa L. var. Crispa) was used in this study. The plant material was purchased from the agricultural supplier Tarım Tedarik’s website (http://www.tarimtedarik.com).
Lettuce seedlings used in the experiment, which had 3–4 primary leaves, were planted with a spacing of 50 cm between rows and 30 cm between plants, with at least 10 plants per row. A drip irrigation system was employed. The experiment was conducted in a randomized block design with five replications. The experimental treatments included a negative control group (T0), which received no chemical fertilizers or microorganisms and served as a basis for comparison. As a positive control (T1), the traditional practice of local farmers to apply NPK fertilizer (Quatro 18.18.18) at a rate of 500 kg ha−1 together with liquid sulfur (2 L ha−1) was included to evaluate the effectiveness of microbial biostimulants in reducing dependence on chemical fertilizers. In addition, treatments including only Microalgae (T2), Plant Probiotic Microorganisms (T3), and Plant Growth-Promoting Rhizobacteria (T4) were applied as biostimulants. The primary objective of this study was to reduce the chemical fertilizer used by local farmers by 25% (375 kg ha−1 NPK 18.18.18 and 1.5 L ha−1 liquid sulfur) and to evaluate the effects of chemical fertilizers in combination with biostimulants. These combinations were applied as Microalgae + chemical fertilizer (T5), Plant Probiotic Microorganisms + chemical fertilizer (T6), and Plant Growth-Promoting Rhizobacteria + chemical fertilizer (T7), resulting in a total of eight treatments. Three different biostimulant formulations were used: PGPBs (Bacillus subtilis, Bacillus megaterium, Bacillus amyloliquefaciens; 1 × 108 cfu/ml), PPMs (Lactobacillus lactis, Lactobacillus cremoris, Lactobacillus acidophilus, Lactobacillus plantarum, Rhodopseudomonas palustris, Saccharomyces cerevisiae; 7 × 106 cfu/ml), and Microalgae (Chlorella vulgaris; 1 × 106 cfu/ml), which were obtained from Biogen Tarım Kimya A.Ş. under the commercial name Algazone for microalgae, Agrosem Dış Ticaret Kimya Tar. San. Ltd. Şti. under the commercial name Losepa for probiotic bacteria, and ARK Çevre ve Biyoteknolojik Ürünler Üretim Gıda Danışmanlık Müh. Paz. İth. İhr. San. Ve Tic. Ltd.Sti. under the commercial name Ark Biofer for PGPRs.
Microorganism applications
The microorganisms were inoculated into the roots 24 h before transplanting. After planting, they were applied to the root zone every two weeks via drip irrigation and then to the leaves every two weeks. In the plots where chemical fertilizers were applied, the fertilizers were incorporated into the surface soil one week prior to planting. The PPB treatments were applied on the first day after sowing at 5 L ha−1 by drip irrigation. After two weeks, they were applied to the root zone at 20 L ha−1 via drip irrigation. Foliar fertilization was applied to the leaves at 10 L ha−1 every two weeks after the 28th day of drip irrigation.
The PGPR treatment was applied on the first day after planting at 30 L ha−1 via drip irrigation. The foliar treatment was applied to the leaves at 30L ha−1 every two weeks after the 28th day of drip irrigation. The microalgae treatment was applied on the first day after planting with 15 L ha−1 drip irrigation. The foliar treatment was applied to the leaves at 30 L ha−1 every two weeks after the 28th day of drip irrigation. All microbial inoculations were applied a total of four times. Applications were made according to the company's recommendations. The lettuce plants were harvested on the 80th day after planting. The time of harvest was determined based on the physiological maturity of the lettuce variety used and its suitability for commercial harvest. Each group consisted of rows of 10 plants, with five replicates for each treatment. Thus, a total of 50 plants were evaluated in each treatment group. The data presented in the tables and analyzes represent the mean values calculated from these 50 plants.
The height of the lettuce plants was measured with a meter stick from the root collar to the shoot tip, with the results given in centimeters (± 0.5 cm accuracy). The plant diameter was measured with a digital caliper at the widest point of the leaf canopy. For both measurements, the average values of 50 plants in each group were calculated and analyzed.
Soil analysis
Soil samples (0–30 cm, 30 subsamples) were taken to determine certain properties of the soil. After sampling, exchangeable cations were determined using the ammonium acetate method [19] and cation exchange capacities (CEC) using the sodium acetate—ammonium acetate method [20]. Total nitrogen was determined using the Kjeldahl method [21], and plant-available phosphorus using the sodium bicarbonate method [22]. Electrical conductivity (EC) was measured in saturation extracts [23]. Soil pH and calcium carbonate content were determined using the method of [24]. Soil organic matter was determined using the Smith-Weldon method [25]. The Fe, Mn, Zn, and Cu content in the soils was determined using the DTPA method [26], and the available B was analyzed using the azomethine H method [27].
Statistical analysis
The statistical analyses of the experimental data were performed using SPSS software (Version 22). One-way analysis of variance (ANOVA) was applied to determine the significant differences between treatments, and Duncan's multiple range test (p < 0.05) was used for post hoc comparisons. In addition, Principal Component Analysis (PCA) was conducted to identify patterns and reduce dimensionality in the dataset, aiding in the interpretation of the relationships between the variables and treatments.
Results
The physical and chemical properties of the greenhouse soil used for this study can be found in SI 1. According to the analysis, the soil structure was classified as loamy and the content of organic matter, plant-available nitrogen, phosphorus, potassium, and trace elements was considered sufficient. The results of the study showed statistically significant differences (p < 0.05) between treatments in terms of physiological yield components of lettuce plants (Table 1). The highest plant weight was observed in treatment T6 (364.00 ± 47.58 g plant−1), followed by treatment T3 (273.20 ± 83.66 g plant−1). These increases compared to the control groups T0 and T1 correspond to 229.71% and 152.43% for treatment T6 and 147.46% and 89.46% for treatment T3, respectively. No statistically significant differences were found between treatments T2, T4, T5, and T7 compared to the control groups. For plant height, the highest statistically significant value was found in treatment T7 (32.40 ± 4.04 cm plant−1), which corresponds to an increase of 52.83% and 42.11% compared to treatments T0 and T1, respectively. No statistically significant differences were found in plant height between treatments T2, T3, T4, T5, and T6. For plant diameter, a statistically significant difference was found in treatment T7 (32.00 ± 4.30 cm), resulting in an increase of 113.33% and 100% compared to the control groups T0 and T1. No statistically significant differences were found in the other treatments (p < 0.05). Significant differences in root length were found in treatments T2 and T7 compared to the other treatments (p < 0.05). The highest root lengths were observed in treatments T2 (12.00 ± 0.71 cm) and T7 (11.60 ± 1.14 cm), with an increase of 185.71% and 140.00% in treatment T2 and 176.19% and 132.00% in treatment T7 compared to T0 and T1, respectively. Although chlorophyll content showed statistically significant differences between treatments, the highest chlorophyll contents were recorded in treatments T5 (204.20 ± 14.82 SPAD plant−1) and T7 (199.60 ± 8.11 SPAD plant−1). These values corresponded to an increase of 98.64% and 73.64% for T5 and 94.16% and 69.73% for T7 compared to the control groups T0 and T1. Statistically significant differences (p < 0.05) were observed in leaf length between treatments, with the largest leaf sizes recorded in treatments T6 (43.80 ± 4.71 cm) and T3 (42.80 ± 3.70 cm). Compared to T0 and T1, the increase in leaf size was 67.18% and 61.03% at T6 and 63.36% and 57.35% at T3, respectively.
Table 1.
The effect of treatments on plant growth parameters of lettuce
| Treatments | Plant Weight (g) | Plant Height (cm) | Plant Diameter (cm) | Root Height (cm) | Chlorophyll (SPAD) | Leaf (cm) |
|---|---|---|---|---|---|---|
| T0 | 110.40 ± 13.32c | 21.20 ± 1.64e | 15.00 ± 0.71d | 4.20 ± 1.10d | 102.80 ± 12.66c | 26.20 ± 2.39c |
| T1 | 144.20 ± 11.48c | 22.80 ± 1.30d,e | 16.00 ± 1.41d | 5.00 ± 1.00d | 117.60 ± 24.36c | 27.20 ± 1.48c |
| T2 | 145.40 ± 12.19c | 27.60 ± 1.14b,c | 28.40 ± 4.72a,b | 12.00 ± 0.71a | 166.00 ± 28.92b | 40.20 ± 2.86a,b |
| T3 | 273.20 ± 83.66b | 29.20 ± 2.28b | 23.40 ± 3.65c | 8.20 ± 0.71c | 160.80 ± 18.85b | 42.80 ± 3.70a |
| T4 | 148.00 ± 12.19c | 24.80 ± 1.92c,d | 26.40 ± 4.04b,c | 8.80 ± 1.30c | 178.00 ± 4.00a,b | 34.20 ± 3.35b |
| T5 | 154.40 ± 11.42c | 26.60 ± 1.14b,c | 28.00 ± 1.58a,b | 10.60 ± 1.95a,b | 204.20 ± 14.82a | 38.60 ± 5.41a,b |
| T6 | 364.00 ± 47.58a | 27.40 ± 1.14b,c | 29.60 ± 3.05a,b | 9.20 ± 0.45b,c | 179.60 ± 16.55a,b | 43.80 ± 4.71a |
| T7 | 148.00 ± 12.19c | 32.40 ± 4.04a | 32.00 ± 4.30a | 11.60 ± 1.14a | 199.60 ± 8.11a | 37.80 ± 4.09a,b |
Duncan a,b,c (p < 0.05)
Given the significant differences in yield parameters observed between treatments, a cluster analysis was performed to identify the treatments with similar characteristics. Based on the cluster analysis, the treatments were categorized into three different clusters. Treatments T0 and T1 (C2) were summarized with the following average values: Plant height: 22.00 cm, plant diameter: 15.50 cm, number of leaves: 26.70, chlorophyll content (SPAD): 110.20, root length: 4.60 cm and plant weight: 127.30 g. Treatments T3 and T6 (C0) showed similarities, with average values of plant height: 28.22 cm, plant diameter: 26.89 cm, number of leaves: 43.67, chlorophyll content (SPAD): 169.00, root length: 8.67 cm and plant weight: 333.89 g. Treatments T2, T4, T5, and T7 (C1) were also grouped together, with average values of plant height: 27.90 cm, plant diameter: 28.43 cm, number of leaves: 37.81, chlorophyll content (SPAD): 186.67, root length: 10.67 cm and plant weight: 150.48 g (Fig. 1).
Fig. 1.
Cluster analysis of physiological parameters in lettuce depend on treatments: mean scaled values of plant height, diameter, leaf quantity, chlorophyll content, root height, and plant weight across different clusters
Discussion
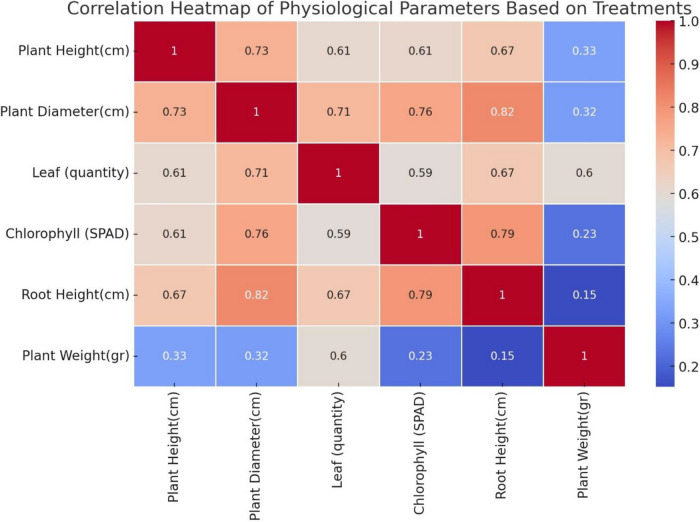
Researchers'interest in plant growth-promoting microorganisms has recently increased due to environmental concerns and the need for sustainable food production [28]. Plant growth-promoting bacteria (PGPRs) and probiotics have gained popularity as valuable tools to improve crop yields through environmentally friendly agricultural practices [29, 30]. The main objective of this study was to evaluate the effects of biostimulants containing three different groups of microorganisms on improving the efficiency of chemical fertilizers in lettuce cultivation and reducing the application rates of chemical fertilizers. Given the statistically significant differences in various physiological parameters of lettuce plants between the treatments, a cluster analysis (C) was performed better to understand the similarities between the treatments and their effectiveness. The analysis revealed that treatments T0 and T1 (C2) showed insufficient physiological growth compared to the average values of the treatments with microbial biostimulants. In addition, treatment T1, representing the group with the full dose of chemical fertilizer, showed lower results than some combinations of biostimulants and reduced chemical fertilizer, which was unexpected. This result suggests that biostimulants containing microorganisms could optimize the use of chemical fertilizers so that similar or even higher yields could be obtained with a lower use of chemical inputs. For example, a study by Pereira et al. has shown that biostimulants increase yield and fertilizer efficiency [31]. Another group identified in the analysis was treatments T3 and T6, grouped together as C0. This group had the highest average plant weight (333.89 g) and the highest number of leaves (43.67). In addition, plant height and diameter were relatively high (28.22 cm and 26.89 cm, respectively), while chlorophyll content (SPAD: 169.00) was also increased. Root length (8.67 cm) was highest in this group compared to the other clusters. These results indicate that the treatments in the C0 group promoted plant growth and biomass accumulation the most. In addition, the high leaf number and chlorophyll content indicate that these treatments improved the plant's photosynthetic capacity, resulting in overall healthier plant development. The correlation analysis also revealed a strong positive relationship between leaf number and chlorophyll content (R = 0.793**). Therefore, the C0 treatments were the most effective in terms of plant growth, leaf number, chlorophyll content, and biomass accumulation (R = 0.819**) (Fig. 2).
Fig. 2.
Correlation heatmap of physiological parameters of lettuce based on Treatments
This group showed the best development of both the aerial parts and the plant's root system. The results of this study are consistent with previous findings on the effects of PGPBs on the physiological parameters of lettuce. The study conducted by Basheer et al. on the effects of biofertilizers on the growth of sunflowers also supports the claim that beneficial microorganisms can improve nutrient availability and promote plant growth. The study highlights how biofertilizers containing effective living microorganisms can significantly improve plant growth and yield by increasing the supply of primary nutrients [32]. This is consistent with the idea that probiotic microorganisms contribute to the development of beneficial microbial communities in the rhizosphere. For example, in a hydroponic study under nutrient-poor conditions, the probiotic Streptomyces was reported to positively affect the growth and development of lettuce [33]. Other studies have shown a significant impact during the vegetative stage [34, 35]. Microorganisms can also influence the biosynthesis of other molecules, such as auxins, which positively affect the production of photosynthetic pigments [36]. In addition, some growth-related compounds, such as cytokinin [37], are influenced by phosphate-solubilizing bacteria, which benefits chlorophyll production [38]. Reis et al. [39] reported that inoculation of soybeans with Paenibacillus alvei and Lysinibacillus fusiformis increased both biomass and chlorophyll synthesis. In the present study, the high chlorophyll content observed in the presence of PGPBs can be attributed to their ability to act as phosphate solubilizers and phytohormone producers, thus increasing the concentration of photosynthetic pigments in lettuce [40]. In addition, plant probiotic actinomycetes are known bacteria that promote plant growth. They stimulate plant growth both directly and indirectly via various mechanisms and thus contribute to the overall development of the plant [41]. The direct mechanism includes the production of phytohormones such as indole-3-acetic acid, siderophores, and the solubilization of phosphate [42, 43]. In addition to producing siderophores and solubilizing phosphate, these bacteria are known to produce enzyme cocktails, including amylase, chitinase, cellulase, invertase, lipase, keratinase, peroxidase, pectinase, protease, phytase, and xylanase, which convert complex nutrients into simple mineral forms. This ability to convert nutrients makes them ideal candidates for natural fertilizers [44]. In contrast to chemical fertilizers, the use of probiotic bacteria as green manure in the field therefore improves the nutritional quality of fruit and vegetables. Therefore, reducing the use of chemical fertilizers in favor of products based on probiotic bacteria is a good alternative to minimize the excessive use of chemical fertilizers and improve food quality.
The cluster analysis also showed that treatments T2, T4, T5, and T7 (C1) had similar characteristics. The C1 cluster had an average plant height of 27.90 cm, plant diameter of 28.43 cm, and chlorophyll content of 186.67 SPAD, although plant weight (150.48 g) and number of leaves (37.81) were lower compared to the C0 group. However, root length (10.67 cm) was longer in the C1 group than in the other two groups, indicating a greater potential for water and nutrient uptake from the soil. Although the C1 treatments performed well in terms of growth, they did not significantly increase biomass compared to the C0 treatments. This may indicate that the C1 treatments primarily promoted root development and had limited effects on yield, as reflected in plant biomass. Although the C1 treatments performed best on root growth, they did not have the same effect on the aerial parts of the plant (R = 0.15, p > 0.05) (Fig. 2). A study on lettuce has shown that the interactions between rhizobacteria can influence root length under nutrient-poor conditions [45], as these microbes act as an extension of the plant’s root system. Chen et al. [46] reported that phosphate-solubilizing microorganisms increase carotenoid production when they colonize plant roots. Root growth can also be influenced by bacterial metabolic activities and the interaction between the availability of nutrients and the microbes [47–49]. Therefore, the nutrients available to the seedlings and the high bacterial population in the inoculated substrate can either limit or promote plant growth and biomass accumulation [50]. These results indicate that the colonization of plant roots by microorganisms promotes root growth and has positive effects on processes such as photosynthesis. Rostaminia et al. [51] a 10–20% increase in lettuce yield was observed when using different Pseudomonas bacterial species. The increase in lettuce weight and yield was attributed to enzymatic activity associated with bacterial hydrolysis of ACC (1-aminocyclopropane-1-carboxylic acid), which lowered ethylene levels in the root environment of the plant, resulting in higher root and shoot weight. Studies have found yield increases of 58.7% in PGPR-inoculated plants compared to non-inoculated plants [52], and a 14–25% increase in lettuce yield due to bacterial inoculation and fertilizer management [53]. The increased leaf area, number of leaves and chlorophyll content combined with improved nutrient availability and uptake have been shown to improve photosynthesis and lead to a greater accumulation of biomass and dry matter in lettuce.
In studies on microalgae, foliar application of A. platensis on sweet pepper plants grown on sandy soils increased yields in the first and second harvests compared to NPK fertilization. By the third harvest, yields of A. platensis and NPK-treated sweet pepper plants were almost identical, but by the fourth harvest, NPK-treated plants exceeded yields. This initial increase was attributed to the high free amino acid content of A. platensis and the presence of growth-promoting substances that are taken up by the leaves and act faster than nutrient uptake from the soil [54]. Consequently, algal biomass can be applied directly to plants via the leaves or roots to increase growth and overall yield. In addition, extracellular exudates of Chlorella sorokiniana increased the total dry biomass of wheat plants by 22% above ground 51% below ground, and by 30% in plant height compared to control groups. The study highlighted the biostimulatory potential of extracellular metabolites from microalgae [55]. In basil plants grown in hydroponic systems, it was reported that the use of a complete mineral fertilizer was unnecessary, as the combination of 80% mineral fertilizer plus mycorrhiza resulted in higher yields than 100% mineral fertilizer alone [56]. This study sheds light on the multifunctional role of the applied microorganisms in influencing macro- and micronutrient dynamics in lettuce plants, even under reduced mineral fertilizer conditions. The application of these microbes in combination with reduced mineral fertilization can optimize nutrient distribution to plants, allowing them to take up more nutrients [57, 58]. Some studies have reported that excessive fertilization can be detrimental to the quality of lettuce seedlings and the environment [59–63]. In addition, a study on wheat showed that the use of different amounts of nitrogen fertilizer increases cadmium uptake from the soil [64, 65]. From a human health perspective, the use of beneficial microorganisms can increase the efficiency of mineral fertilizers while reducing their application rate, resulting in healthier food production. Microorganisms such as bacteria, protists, and fungi are known to degrade heavy metal compounds and incorporate them into their metabolism with the help of specific enzymes [66]. Microbial activity can either remove heavy metals or convert them into non-toxic compounds, and soil microbes can influence the mobility and bioavailability of metals through biosorption and bioaccumulation [67–69]. These mechanisms also contribute to the immobilization of heavy metals in soil [70–72]. In addition, reducing the use of chemical fertilizers limits the potential for accumulation of toxic metals from these fertilizers in agricultural soils, thereby reducing the potential biological accumulation of these toxic metals in plants. Microbial inoculation with beneficial microorganisms is a complementary management tool to increase vegetable production. However, the combination with chemical fertilization has not yet been sufficiently investigated [17, 73]. Biostimulants are also gaining importance as potential soil conditioners to improve plant health and productivity. The response of plant growth by beneficial microorganisms to poorly soluble fertilizers can enable plants to take up less soluble nutrients more effectively. In addition, microbial populations and processes affect the efficiency of nutrient cycling by contributing to soil fertility and structure through various mechanisms, including improving mineralization and nutrient availability. They influence cation exchange capacity, nitrogen, sulfur, and phosphorus reserves, soil acidity and toxicity, and water retention capacity. Each of these factors also positively influences important productivity constraints related to soil [74, 75]. Therefore, in this study, which investigated the effects of various beneficial microorganisms on yield parameters of lettuce, a commercially important vegetable for human consumption, it was found that the application of beneficial microorganisms resulted in statistically significant yield increases with a 25% reduction in chemical fertilization compared to treatments without chemical fertilization restriction. The cluster analysis conducted among the different microbial biostimulants used in this study revealed that the PPM treatment (T6) distinctly outperformed other treatments and achieved the highest yield under conditions of reduced chemical fertilizer application. The T6 treatment resulted in a plant weight of 364 g and a leaf length of 43.8 cm, surpassing the full-dose chemical fertilizer treatment (T1) and the control group (T0), as well as combinations with other biostimulants (T5 and T7). These findings clearly demonstrate that PPMs exhibit superior performance in both growth and biomass production under reduced chemical fertilizer conditions. The remarkable effects of PPMs are likely associated with various microbial mechanisms. Probiotic microorganisms such as Lactobacillus spp., Rhodopseudomonas palustris, and Saccharomyces cerevisiae are known to enhance the development of beneficial microbial communities in the rhizosphere, optimizing soil microbial balance. These microorganisms play a key role in improving plant development by solubilizing nutrients and making them more accessible to plants. The cluster analysis results indicate that the PPM treatment is particularly superior to other applications in terms of plant growth parameters such as chlorophyll content and biomass production. This distinction confirms that PPMs occupy a unique position by activating biological mechanisms and supporting plant growth under reduced chemical fertilizer usage. An important study by Jin et al. investigated the effects of bio-organic fertilizer with probiotic microorganisms on the growth of baby Chinese cabbage. The study showed that such fertilizers can effectively reduce the need for chemical fertilizers while improving crop yield and soil microbial diversity [76]. These findings are consistent with those of Upadhayay et al. who emphasized that plant-based probiotics can mitigate the adverse effects of agrochemicals and promote sustainable agricultural practices. Their study highlights the dual role of probiotics as bio-elicitors that promote plant growth and improve soil health, providing a viable way to reduce the use of chemical fertilizer [77]. Furthermore, beneficial microorganisms such as Bacillus spp. have been shown to enhance nutrient availability in the soil. For example, Bai et al. demonstrated that the application of Bacillus subtilis improved the rhizosphere bacterial community and contributed to better nutrient uptake by plants, thereby reducing the necessity for chemical fertilizers [78]. This is particularly significant in the context of improving soil health and fertility through biological methods, as noted by He, who investigated the effects of microbial inoculants combined with chemical fertilizers on plant growth and soil nutrient dynamics [79]. A relevant study by Alori et al. deals with the role of microbial phosphorus solubilization and emphasizes that various microorganisms, including those in the rhizosphere, can improve the availability of phosphorus to plants. This microbial activity is crucial for improving plant growth and reducing the need for chemical fertilizers [80] The study shows that phosphorus-solubilizing microorganisms (PSM) play an important role in the nutrient cycle, which is essential for sustainable agricultural practices. Given these results, it was concluded that the application of probiotics, PGPR and microalgae in lettuce cultivation, especially under greenhouse conditions, improves the efficiency of chemical fertilizers and provides better nutrition, resulting in higher yields. Reducing the use of chemical fertilizers is not only economically beneficial for lettuce growers but also important for providing consumers with healthier food. Therefore, the results of this study should make an important contribution to future research on the integrated use of biostimulants in conventional agriculture. The results of this study strongly suggest that PPBs are a reliable and effective biostimulant for sustainable agricultural practices, offering significant advantages over other biostimulants. However, further studies are needed to optimize PPPs and chemical fertilizers under different soil conditions when fewer chemical fertilizers are used.
Conclusion
This study has shown that reducing the use of chemical fertilizers by 25% and combining it with the application of microbial biostimulants significantly improves plant growth and yield in lettuce cultivation. Among the treatments, combining plant probiotic microorganisms (PPMs) with reduced chemical fertilizers (T6) resulted in the highest improvements in plant weight, leaf length, and total yield compared to the control groups and other microbial applications. The results confirm that microbial inoculants such as PGPR, PPBs, and microalgae can optimize plant growth and nutrient uptake, even with reduced fertilizer use. Biostimulants not only improved growth metrics but also increased photosynthetic capacity, as evidenced in particular by the higher chlorophyll content in treatments T5 and T7. In addition, the cluster analysis showed that the microbial inoculation treatments achieved similar or better results than applying the full dose of chemical fertilizers. This result suggests that microbial inoculation could be an effective alternative to reduce the use of chemicals in sustainable agriculture.
Future studies should focus on balancing microbial inoculations with further reduced chemical fertilizers under different soil types and environmental conditions, to achieve better economic and environmental results in lettuce production.
Supplementary Information
Acknowledgements
The author would like to thank two anonymous referees who madevaluable comments and suggestions concerning our manuscript and the editor of BMC Plant Biology.
Authors’ contribution
N.K.S., A.K., and Ö.E.A. contributed equally to this work. N.K.S. developed the conceptual framework and design of the study. A.K. performed data collection and analysis, wrote the manuscript, and critically revised it. Ö.E.A. performed datacollection and analysis. All authors contributed to the interpretation and discussion of the findings, actively participated in all stages of the study, and approved the final version.
Funding
Not applicable.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Some Physical and Chemical Properties of the Trial Soil Supplementary Info 1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nurgul Kitir Sen, Email: nksen@gtu.edu.tr.
Ayhan Kocaman, Email: ayhan.kocaman.ak@gmail.com, Email: ayhankocaman@karabuk.edu.tr.
References
- 1.Özkutlu F, Turan M, Kebapci T, Ete Aydemir Ö, Kocaman A. Optimization of Urea Fertilization with Various Doses and Applications on the Yield and Quality of the Hazelnut. Appl Fruit Sci. 2024;66(3):963–71. [Google Scholar]
- 2.Yang F, Tian J, Fang H, Gao Y, Xu M, Lou Y, Zhou B, Kuzyakov Y. Functional soil organic matter fractions, microbial community, and enzyme activities in a mollisol under 35 years manure and mineral fertilization. J Soil Sci Plant Nutr. 2019;19:430–9. [Google Scholar]
- 3.Geng Y, Cao G, Wang L, Wang S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE. 2019;14(7):e0219512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruzdeva L, Matveeva E, Kovalenko T. Changes in soil nematode communities under the impact of fertilizers. Eurasian Soil Sci. 2007;40:681–93. [Google Scholar]
- 5.Sileshi GW, Jama B, Vanlauwe B, Negassa W, Harawa R, Kiwia A, Kimani D. Nutrient use efficiency and crop yield response to the combined application of cattle manure and inorganic fertilizer in sub-Saharan Africa. Nutr Cycl Agroecosyst. 2019;113:181–99. [Google Scholar]
- 6.Yildirim E, Turan M, Dursun A, Ekinci M, Kul R, Karagoz FP, Donmez MF, Kitir N. Integrated use of nitrogen fertilization and microbial inoculation: change in the growth and chemical composition of white cabbage. Commun Soil Sci Plant Anal. 2016;47(19):2245–60. [Google Scholar]
- 7.Li S, Wang Z, Hu T, Gao Y, Stewart B. Nitrogen in dryland soils of China and its management. Adv Agron. 2009;101:123–81. [Google Scholar]
- 8.Lv F, Song J, Giltrap D, Feng Y, Yang X, Zhang S. Crop yield and N2O emission affected by long-term organic manure substitution fertilizer under winter wheat-summer maize cropping system. Sci Total Environ. 2020;732:139321. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Jiang Y, Zhao F, He X, Liu H, Yu K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci Rep. 2020;10(1):9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Lv W, Bloszies S, Shi Q, Pan X, Zeng Y. Effects of fertilizer management practices on yield-scaled ammonia emissions from croplands in China: a meta-analysis. Field Crop Res. 2016;192:118–25. [Google Scholar]
- 11.Ullah H, Santiago-Arenas R, Ferdous Z, Attia A, Datta A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv Agron. 2019;156:109–57. [Google Scholar]
- 12.Pirttilä AM. Mohammad Parast Tabas H, Baruah N, Koskimäki JJ: Biofertilizers and biocontrol agents for agriculture: How to identify and develop new potent microbial strains and traits. Microorganisms. 2021;9(4):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chittora D, Meena M, Barupal T, Swapnil P, Sharma K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochemistry and biophysics reports. 2020;22:100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez AL, Weyers SL, Goemann HM, Peyton BM, Gardner RD. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021;54:102200. [Google Scholar]
- 15.Sarma S, Sharma S, Rudakiya D, Upadhyay J, Rathod V, Patel A, Narra M: Valorization of microalgae biomass into bioproducts promoting circular bioeconomy: a holistic approach of bioremediation and biorefinery. 3 Biotech 2021, 11:1–29. [DOI] [PMC free article] [PubMed]
- 16.Guo S, Wang P, Wang X, Zou M, Liu C, Hao J. Microalgae as biofertilizer in modern agriculture. Microalgae biotechnology for food, health and high value products. 2020. p. 397–411. 10.1007/978-981-15-0169-2_12.
- 17.Jin N, Jin L, Wang S, Li J, Liu F, Liu Z, Luo S, Wu Y, Lyu J, Yu J. Reduced chemical fertilizer combined with bio-organic fertilizer affects the soil microbial community and yield and quality of lettuce. Front Microbiol. 2022;13:863325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi M, Gu J, Wu H, Rauf A, Emran TB, Khan Z, Mitra S, Aljohani AS, Alhumaydhi FA, Al-Awthan YS. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce—A comprehensive review. Antioxidants. 2022;11(6):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas ED, Sanders JE, Buckner CD, Papayannopoulou T, Borgna-Pignatti C, De Stefano P, Clift R, Sullivan K, Storb R. Marrow transplantation for thalassaemia. The Lancet. 1982;320(8292):227–9. [DOI] [PubMed] [Google Scholar]
- 20.Sumner ME, Miller WP: Cation exchange capacity and exchange coefficients. Methods of soil analysis: Part 3 Chemical methods 1996, 5:1201–1229.
- 21.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies Synapse. 1996;23(1):28–38. [DOI] [PubMed] [Google Scholar]
- 22.Olsen SR: Estimation of available phosphorus in soils by extraction with sodium bicarbonate: US Department of Agriculture; 1954.
- 23.Rhoades J: Salinity: Electrical conductivity and total dissolved solids. Methods of soil analysis: Part 3 Chemical methods 1996, 5:417–435.
- 24.Morrice LM, McLean MW, Williamson FB, Long WF. β-Agarases I and II from Pseudomonas atlantica Purifications and some properties. Eur J Biochem. 1983;135(3):553–8. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DW, Sommers LE: Total carbon, organic carbon, and organic matter. Methods of soil analysis: Part 2 chemical and microbiological properties 1982, 9:539–579.
- 26.Lindsay WL, Norvell W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J. 1978;42(3):421–8. [Google Scholar]
- 27.Wolf B. Improvements in the azomethine-H method for the determination of boron. Commun Soil Sci Plant Anal. 1974;5(1):39–44. [Google Scholar]
- 28.Toan N-S, Nguyen TDP, Thu TTN, Lim DT, Dong PD, Gia NT, Khoo KS, Chew KW, Show PL. Soil mineralization as effects of plant growth promoting bacteria isolated from microalgae in wastewater and rice straw application in a long-term paddy rice in Central Viet Nam. Environ Technol Innov. 2021;24:101982. [Google Scholar]
- 29.Omar AF, Abdelmageed AH, Al-Turki A, Abdelhameid NM, Sayyed R, Rehan M. Exploring the plant growth-promotion of four Streptomyces strains from rhizosphere soil to enhance cucumber growth and yield. Plants. 2022;11(23):3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasudee K, Tokuyama S, Lumyong S, Pathom-Aree W. Actinobacteria associated with arbuscular mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and Thai jasmine rice (Oryza sativa). Front Microbiol. 2018;9:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira S, Abreu D, Moreira H, Vega A, Castro P. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon. 2020;6(10). 10.1016/j.heliyon.2020.e05106. [DOI] [PMC free article] [PubMed]
- 32.Basheer HG, Idris AE, Ahmed BEAM: Impact of Bio-Fertilizer on Growth and Yield of Two Sunflower (Helianthus annuus L.) Hybrids at Shambat, Sudan. Scholars Journal of Agriculture and Veterinary Sciences 2016, 3:332–336.
- 33.Kitwetch B, Rangseekaew P, Chromkaew Y, Pathom-Aree W, Srinuanpan S: Employing a plant probiotic actinomycete for growth promotion of lettuce (Lactuca sativa L. var. longifolia) cultivated in a hydroponic system under nutrient limitation. Plants 2023, 12(22):3793. [DOI] [PMC free article] [PubMed]
- 34.Bonaldi M, Chen X, Kunova A, Pizzatti C, Saracchi M, Cortesi P. Colonization of lettuce rhizosphere and roots by tagged Streptomyces. Front Microbiol. 2015;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Pizzatti C, Bonaldi M, Saracchi M, Erlacher A, Kunova A, Berg G, Cortesi P. Biological control of lettuce drop and host plant colonization by rhizospheric and endophytic streptomycetes. Front Microbiol. 2016;7:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos MS, Nogueira MA, Hungria M. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express. 2019;9(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudoyarova G, Arkhipova T, Korshunova T, Bakaeva M, Loginov O, Dodd IC. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front Plant Sci. 2019;10:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Zhao Q, Wang Z, Wang L, Li X, Fan Z, Zhang Y, Li J, Gao X, Shi J. Effects of nitrogen fertilizer on photosynthetic characteristics, biomass, and yield of wheat under different shading conditions. Agronomy. 2021;11(10):1989. [Google Scholar]
- 39.Reis MNO, Vitorino LC, Lourenço LL, Bessa LA. Microbial Inoculation Improves Growth, Nutritional and Physiological Aspects of Glycine max (L.) Merr. Microorganisms. 2022;10(7):1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schindler DW, Hecky RE, Findlay D, Stainton M, Parker B, Paterson M, Beaty K, Lyng M, Kasian S. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci. 2008;105(32):11254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangseekaew P, Barros-Rodríguez A, Pathom-Aree W, Manzanera M. Plant beneficial deep-sea actinobacterium, Dermacoccus abyssi MT1. 1T promote growth of tomato (Solanum lycopersicum) under salinity stress. Biology. 2022;11(2):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sathya A, Vijayabharathi R, Gopalakrishnan S: Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech 2017, 7:1–10. [DOI] [PMC free article] [PubMed]
- 43.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012(1):963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jog R, Nareshkumar G, Rajkumar S. Enhancing soil health and plant growth promotion by actinomycetes. Plant growth promoting actinobacteria: a new avenue for enhancing the productivity and soil fertility of grain legumes. 2016. p. 33–45. 10.1007/978-981-10-0707-1_3.
- 45.Oldroyd GE, Leyser O: A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368(6486):eaba0196. [DOI] [PubMed]
- 46.Chen S, Zhao H, Zou C, Li Y, Chen Y, Wang Z, Jiang Y, Liu A, Zhao P, Wang M. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front Microbiol. 2017;8:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ekin Z, Oguz F, Erman M, Oeguen E. The effect of Bacillus sp. OSU-142 inoculation at various levels of nitrogen fertilization on growth, tuber distribution and yield of potato (Solanum tuberosum L.). Afr J Biotechnol. 2009;8(18):4418–24.
- 48.Welbaum GE, Sturz AV, Dong Z, Nowak J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit Rev Plant Sci. 2004;23(2):175–93. [Google Scholar]
- 49.Marschner P, Gerendás J, Sattelmacher B. Effect of N concentration and N source on root colonization by Pseudomonas fluorescens 2–79RLI. Plant Soil. 1999;215:135–41. [Google Scholar]
- 50.Oliveira Ad. Urquiaga S, Döbereiner J, Baldani J: The effect of inoculating endophytic N 2-fixing bacteria on micropropagated sugarcane plants. Plant Soil. 2002;242:205–15. [Google Scholar]
- 51.Rostaminia M, Habibi D, Shahbzi S, Sani B, Pazoki A: Effect of three commercial bio-fertilizers prepared with Pseudomonas on yield and morphophysiological traits of lettuce (Lactuca sativa L.). 2021.
- 52.Abdel-Ilah T, Anas R, Noura B, Mohamed A, Abderrahim B, Khalid O, Abdelilah M. Beneficial effects of plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and compost on lettuce (Lactuca sativa) growth under field conditions. Gesunde Pflanzen. 2022;1:219–35. [Google Scholar]
- 53.Vetrano F, Miceli C, Angileri V, Frangipane B, Moncada A, Miceli A. Effect of bacterial inoculum and fertigation management on nursery and field production of lettuce plants. Agronomy. 2020;10(10):1477. [Google Scholar]
- 54.Aly M, Esawy MA. Evaluation of Spirulina platensis as bio stimulator for organic farming systems. Journal of Genetic Engineering and Biotechnology. 2008;6(2):1–7. [Google Scholar]
- 55.Kholssi R, Marks EA, Miñón J, Montero O, Debdoubi A, Rad C. Biofertilizing effect of Chlorella sorokiniana suspensions on wheat growth. J Plant Growth Regul. 2019;38:644–9. [Google Scholar]
- 56.Daşgan HY, Ceylan E, Dere S: Effect of mycorrhiza on reducing mineral fertilizers in hydroponic growing basil. 2022.
- 57.Aini N, Yamika WSD, Ulum B: Effect of nutrient concentration, PGPR and AMF on plant growth, yield, and nutrient uptake of hydroponic lettuce. 2019.
- 58.Ayuso-Calles M, García-Estévez I, Jiménez-Gómez A, Flores-Félix JD, Escribano-Bailón MT, Rivas R. Rhizobium laguerreae improves productivity and phenolic compound content of lettuce (Lactuca sativa L.) under saline stress conditions. Foods. 2020;9(9):1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo VM. Biological amendment, fertilizer rate, and irrigation frequency for organic bell pepper transplant production. HortScience. 2006;41(6):1402–7. [Google Scholar]
- 60.Masson J, Tremblay N, Gosselin A. Nitrogen fertilization and HPS supplementary lighting influence vegetable transplant production I Transplant growth. J Am Soc Hortic Sci. 1991;116(4):594–8. [Google Scholar]
- 61.Liptay A, Nicholls S: Nitrogen supply during greenhouse transplant production affects subsequent tomato root growth in the field. 1993.
- 62.Reddy M, Rodríguez-Kabana R, Kenney D, Kokalis-Burelle N, Martinez-Ochoa N, Kloepper J. Application for Rhizobacteria in Transplant Production and Yield Enhancement. In: XXVI International Horticultural Congress: Issues and Advances in Transplant Production and Stand Establishment Research 631. 2002. p. 219–29. 10.17660/ActaHortic.2004.631.28.
- 63.Soundy P, Cantliffe DJ, Hochmuth GJ, Stoffella PJ. Management of nitrogen and irrigation in lettuce transplant production affects transplant root and shoot development and subsequent crop yields. HortScience. 2005;40(3):607–10. [Google Scholar]
- 64.Özkutlu F. Effects of Applying Different N Sources on Cd Accumulation, Mineral Micronutrients, and Grain Yield of Durum Wheat. J Soil Sci Plant Nutr. 2024;24(3):4261–8. 10.1007/s42729-024-01831-9. [Google Scholar]
- 65.Ozkutlu F, Aydemir ÖE. Effect of Na2SO4 Application on the Growth, Yield and Cd uptake of Wheat. Akademik Ziraat Dergisi. 2024;13(1):169–74. [Google Scholar]
- 66.Abdu N, Abdullahi AA, Abdulkadir A. Heavy metals and soil microbes. Environ Chem Lett. 2017;15(1):65–84. [Google Scholar]
- 67.Zhuang X, Chen J, Shim H, Bai Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int. 2007;33(3):406–13. [DOI] [PubMed] [Google Scholar]
- 68.Pajuelo E, Rodríguez-Llorente ID, Lafuente A, Caviedes MÁ. Legume–rhizobium symbioses as a tool for bioremediation of heavy metal polluted soils. Biomanagement of metal-contaminated soils. 2011. p. 95–123. 10.1007/978-94-007-1914-9_4.
- 69.Han H, Cai H, Wang X, Hu X, Chen Z, Yao L. Heavy metal-immobilizing bacteria increase the biomass and reduce the Cd and Pb uptake by pakchoi (Brassica chinensis L) in heavy metal-contaminated soil. Ecotoxicol Environ Saf. 2020;195:110375. [DOI] [PubMed] [Google Scholar]
- 70.Mendez MO, Maier RM. Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ Health Perspect. 2008;116(3):278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merdy P, Gharbi LT, Lucas Y. Pb, Cu and Cr interactions with soil: sorption experiments and modelling. Colloids Surf, A. 2009;347(1–3):192–9. [Google Scholar]
- 72.Gadd GM, Bahri-Esfahani J, Li Q, Rhee YJ, Wei Z, Fomina M, Liang X. Oxalate production by fungi: significance in geomycology, biodeterioration and bioremediation. Fungal Biol Rev. 2014;28(2–3):36–55. [Google Scholar]
- 73.Angulo J, Martínez-Salgado MM, Ortega-Blu R, Fincheira P. Combined effects of chemical fertilization and microbial inoculant on nutrient use efficiency and soil quality indicators. Scientia Agropecuaria. 2020;11(3):375–80. [Google Scholar]
- 74.Singh JS, Pandey VC, Singh DP. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agr Ecosyst Environ. 2011;140(3–4):339–53. [Google Scholar]
- 75.Kocaman A, Turan M, Tüfenkçi Ş, Katırcıoğlu H, Güneş A, Kıtır N, Giray G, Gürkan B, Ersoy N, Yıldırım E. Development of plant-friendly vermicompost using novel biotechnological methods. J Mater Cycles Waste Manage. 2023;25(5):2925–36. [Google Scholar]
- 76.Jin L, Jin N, Wang S, Li J, Meng X, Xie Y, Wu Y, Luo S, Lyu J, Yu J: Fertilizer reduction with bio-organic fertilizer to regulate the root soil microbial community structure to improve Baby Chinese cabbage yield. bioRxiv 2022:2022.2002. 2028.482435.
- 77.Upadhayay VK, Chitara MK, Mishra D, Jha MN, Jaiswal A, Kumari G, Ghosh S, Patel VK, Naitam MG, Singh AK. Synergistic impact of nanomaterials and plant probiotics in agriculture: A tale of two-way strategy for long-term sustainability. Front Microbiol. 2023;14:1133968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai N, Zhang H, He Y, Zhang J, Zheng X, Zhang H, Zhang Y, Lv W, Li S. Effects of Bacillus subtilis A-5 and its fermented γ-polyglutamic acid on the rhizosphere bacterial community of Chinese cabbage. Front Microbiol. 2022;13:954489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He S, Zhang Y, Yang X, Li Q, Li C, Yao T. Effects of Microbial Inoculants Combined with Chemical Fertilizer on Growth and Soil Nutrient Dynamics of Timothy (Phleum pratense L.). Agronomy. 2024;14(5):1016. [Google Scholar]
- 80.Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017;8:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information. Some Physical and Chemical Properties of the Trial Soil Supplementary Info 1.