Abstract
Congenital scoliosis (CS), a severe form of early-onset scoliosis (EOS), arises from vertebral malformations during embryogenesis, driven by complex genetic and environmental interactions. This review synthesizes recent advances in understanding CS etiology, diagnosis, and treatment. Genetically, CS is linked to mutations in TBX6, GDF3, DSTYK, and COL11A2, alongside copy number variations (CNVs) and epigenetic modifications such as allele-specific methylation in SVIL and TNS3. Maternal hypoxia, toxin exposure, and nutritional deficiencies further contribute to pathogenesis. Diagnosis incorporates advanced imaging techniques—such as X-rays, magnetic resonance imaging (MRI), and computed tomography (CT)—as well as genetic testing, with whole-exome sequencing identifying mutations in 18.6% of cases. Conservative management, including casting and bracing (e.g., alternating cast and brace treatment [ARCBT] and 3D-printed orthoses), effectively delays progression in mild-to-moderate cases. Surgical interventions—such as hemivertebra resection, hybrid techniques (HT), and growth-modulating technologies including magnetic controlled growth rods (MCGR) and the Shilla method—have demonstrated improved outcomes in patients with severe deformities. HT combines posterior osteotomy with dual growing rods, achieving significant Cobb angle correction (81.4° to 40.1°) and spinal growth (1.23 cm/year) with fewer complications. MCGR reduces repeated surgeries but shows variable impacts on quality of life. Emerging approaches, including apical control techniques and robotics, highlight the shift toward personalized care. This review underscores the need for multidisciplinary strategies to optimize outcomes, emphasizing early diagnosis, tailored treatments, and long-term monitoring to address CS complexity.
Keywords: Congenital scoliosis, Genetic mutations, Hybrid surgical techniques, Spinal dysraphism, Pediatric neurosurgery, Growth modulation
Background
Congenital scoliosis (CS) is a form of early-onset scoliosis (EOS) caused by skeletal abnormalities during gestational weeks 4 to 6, often leading to additional deformities in other systems [1]. Its etiology involves both genetic and environmental factors [2]. Genetic mutations, such as in the TBX6 gene, along with single nucleotide polymorphisms (SNPs) and copy number variations (CNVs), play a significant role [3, 4]. Environmental influences, including prenatal hypoxia, alcohol exposure, and vitamin deficiencies, are also linked to CS development [3, 5, 6]. Diagnosis relies on imaging techniques like X-rays, CT scans, and MRI, while advances in genetic methods, such as whole-exome and whole-genome sequencing, provide more detailed diagnostic insights [7].
Treatment options for CS range from conservative management—such as casting and bracing—for mild-to-moderate cases, to surgical interventions—including in situ fusion, hemivertebra resection, and the use of a vertical expandable prosthetic titanium rib (VEPTR)—for more severe deformities [8–13].
Given its progressive nature, regular follow-up is essential for monitoring and adjusting treatment plans to improve patient outcomes [14–16].
This review discusses recent advancements in the etiology, diagnosis, and treatment of CS, aiming to provide clinicians with the latest insights for personalized care.
Materials and methods
We conducted a narrative literature review in July 2025, adhering to the SANRA (Scale for the Assessment of Narrative Review Articles) guidelines [17].We searched PubMed/MEDLINE, Embase, and Cochrane Library using the terms: “congenital scoliosis” OR “hemivertebra” OR “early-onset scoliosis” OR “growth rod” OR “spinal surgery”. The search spanned January 1955 to July 2025 and was limited to English-language publications. We included clinical studies (prospective cohorts, retrospective series, and randomized trials) on CS etiology, diagnosis, or treatment. We excluded systematic reviews, meta-analyses, and nonclinical biomechanical research. After removing duplicates, two authors independently screened titles and abstracts; relevant full texts were reviewed for inclusion. Discrepancies were resolved by consensus.
Overview of etiology
CS is a type of EOS caused by abnormal spinal development during the embryonic period. It is marked by severe deformities and rapid progression, often accompanied by malformations in other organs [18]. CS is classified into Type I, Type II, and Type III [19], with incidence rates between 0.5‰ and 2‰ [20–23]. Type I defects reflect failure of vertebral formation (for example, a hemivertebra or butterfly vertebra). Type II defects result from failure of segmentation, yielding fused or block vertebrae (such as an unsegmented bar). Type III defects combine both processes (for instance, an anterolateral unsegmented bar on one side with a contralateral hemivertebra) [24]. While typically sporadic, cases among identical twins suggest a complex interaction between genetic and environmental factors. For example, one study documented CS in one twin with Chiari malformation, while the other was unaffected, highlighting this complexity [25]. Family studies further indicate a low recurrence risk, supporting the multifactorial nature of CS [26]. A deeper understanding of how genetic and environmental factors interact is crucial to unraveling the etiology of CS.
Genetic factors
The genetic landscape of CS is increasingly complex, involving a variety of mutations, SNPs, and CNVs. Early studies, such as those by Qiu et al., examined genes like MESP2, HES7, and DUSP6, but found no significant mutations, suggesting these genes may not play a major role in sporadic CS within the Chinese Han population [27]. A 16p11.2 deletion affecting the TBX6 gene has been identified as a key risk factor for CS, particularly in the Han Chinese population, with additional SNPs within TBX6 further elevating risk (rs2289292, rs3809624, rs3809627) when combined with heterozygous deletions [28, 29]. Similarly, CNVs in TBX6, NOTCH2, and other genes have been found to contribute to vertebral malformations [30].
Other genes, including LMX1A and FBN1, also play significant roles in spinal development, with mutations linked to CS [31, 32]. Recent advancements have expanded this genetic framework. For instance, mutations in the GDF3 gene were shown to influence vertebral development, with four variants displaying varying degrees of pathogenicity [33]. Likewise, DSTYK gene mutations in zebrafish models revealed abnormal notochord formation, implicating the mTORC1/TFEB pathway as a key player in scoliosis pathogenesis [34].
Epigenetics has also come to the forefront, with studies like Zhang et al. identifying allele-specific methylation in the SVIL gene, suggesting an interaction between genetic and environmental factors [35]. Similarly, differential methylation in the TNS3 gene promoter region was noted in monozygotic twins discordant for CS, underscoring the role of epigenetic regulation [36].
Recent studies have continued to broaden our understanding of the genetic basis of CS. Novel variants in the FGFR1 and PTK7 genes have been associated with vertebral malformations and scoliosis [37, 38]. Additionally, CNVs identified in Southern Chinese cohorts have provided further insight into the familial genetic patterns of CS [30]. Most recently, Rebello et al. identified COL11A2 as a candidate gene, using CRISPR/Cas9 technology to model CS-like vertebral fusions in zebrafish [39].
Different CS types have distinct etiologies. Type I (formation) anomalies are often driven by genetic disruptions in vertebral development. For example, TBX6 mutations or deletions (at 16p11.2) lead primarily to hemivertebrae or butterfly vertebrae [24]. In contrast, Type II (segmentation) anomalies are mainly linked to defects in somitogenesis signaling. Notch‐pathway genes—such as DLL3, HES7, MESP2 and related factors—when mutated cause segmentation failures like vertebral bars or block vertebrae [40].
In summary, the genetic factors underlying CS are diverse, involving mutations in TBX6, GDF3, DSTYK, and COL11A2, among others, along with CNVs and epigenetic changes. Ongoing research using advanced sequencing technologies and functional assays continues to shed light on this complex disorder, offering potential for more targeted therapeutic approaches.
Environmental factors
Maternal factors during pregnancy play a significant role in the pathogenesis of CS. Hypoxia is considered a primary factor, with studies in mouse models showing that hypoxic conditions disrupt the normal formation of embryonic vertebral cartilage, leading to spinal curvature [5]. Other maternal exposures, such as alcohol consumption, vitamin deficiency, and the use of antiepileptic drugs, also interfere with developmental pathways, affecting spinal development [5, 41–44]. Further evidence suggests that prenatal exposure to nano titanium dioxide may cause skeletal abnormalities, indicating the potential influence of environmental toxins on embryonic development [45]. Additionally, correlations between adrenal hypertrophy, hypoxia, and early scoliosis highlight the need for further research into these mechanisms [46]. In conclusion, maternal health and environmental exposures are key contributors to CS development. Ongoing research is essential for better understanding these influences and developing prevention and treatment strategies.
Treatment options for congenital scoliosis
Treatment for CS includes conservative and surgical approaches. Conservative treatments, such as casts and braces, are generally considered for milder cases, while severe deformities often require surgical intervention.
Conservative treatment
Recent studies on conservative treatment have explored its role in controlling the progression of scoliosis in early-onset cases. Parot et al. observed that chest wall abnormalities and spinal scoliosis could be managed without surgery through braces and regular monitoring, thereby avoiding surgery and highlighting the importance of follow-up care for potential complications [47]. Braces such as the Milwaukee and Boston braces, as demonstrated by Chêneau et al., have shown effectiveness in managing smaller vertebral anomalies in patients with congenital scoliosis [48].
Kawakami et al. demonstrated that ARCBT significantly reduced the progression of EOS compared to brace-only treatment (BT), providing an alternative approach for early intervention [49]. Similarly, Wang et al. confirmed that brace treatment could delay CS progression, preserving growth and postponing surgery [50]. Swain et al. introduced a flexible spinal orthosis for children, offering an innovative and comfortable treatment option [51]. Personalized 3D-printed braces, when combined with traditional physical therapy, were reported by Jin et al. to be 100% effective for adults with newly developed scoliosis [52]. Caredda et al. highlighted the effectiveness of orthotic braces, such as the Milwaukee and Boston braces, in managing CS caused by complete hemivertebra segmentation, advocating for early brace treatment over observation [53].
These findings emphasize that conservative treatments, particularly casting and bracing, can delay surgery and provide personalized care for CS patients. However, further research is necessary to tailor treatment plans based on individual patient needs and ensure long-term success.
Surgical treatment approaches
Surgery for CS is indicated when conservative treatment fails, symptoms are severe, or the curve progresses rapidly. Surgical methods include fusion, non-fusion, hybrid techniques, and growth modulation. Surgery is generally performed when rapid curve progression occurs despite nearing skeletal maturity. Preoperative evaluation and communication about risks, such as infection or nerve injury, are critical, as are postoperative rehabilitation strategies to improve spinal stability and quality of life.
Innovations like robot-assisted surgery and customized graft materials are enhancing surgical outcomes, with ongoing research focusing on more personalized approaches. Interdisciplinary cooperation remains essential for comprehensive CS management [54].
In situ fusion surgery
Historically, in situ fusion surgery has been used to treat CS, particularly in young children. However, its use has decreased due to limitations such as restricted lung development and a high rate of reoperations (25.6%, according to Goldberg) [55–57]. The"Crankshaft Phenomenon", where incomplete fusion leads to worsening scoliosis, is another concern. While effective for minor deformities in younger patients, in situ fusion is now less common, as modern techniques offer more durable results.
Hemivertebra resection short-segment fusion
Hemivertebra resection with short-segment fusion is highly effective for young CS patients (Fig. 1). Studies show that early intervention can reduce the need for long fusions and prevent curve progression [58, 59]. Innovative techniques like the use of titanium cages for anterior support have improved corrective outcomes while minimizing complications [60–62]. Research has demonstrated significant improvements in scoliosis correction without major complications, especially when the surgery is performed early [63–66].
Fig. 1.

Posterior hemivertebra resection and scoliosis correction (T8–T12) in a 3-year-old girl diagnosed with a congenital T10 hemivertebra. a Preoperative anteroposterior (AP) radiograph showing the congenital hemivertebra at T10. b Preoperative lateral radiograph demonstrating sagittal alignment. c Immediate postoperative AP radiograph revealing satisfactory coronal correction. d Immediate postoperative lateral radiograph showing maintained sagittal alignment. e AP radiograph at 2-year follow-up, demonstrating sustained coronal correction. f Lateral radiograph at 2-year follow-up, confirming preserved sagittal alignment
Growth rod technology
Growth rods, including single and dual systems, allow for spinal correction while enabling continued growth (Fig. 2). Dual growth rod (DGR) technology offers improved stability and fewer complications compared to single rods [67]. Studies have shown excellent outcomes with this approach, including curve correction and increased spinal height, particularly with frequent adjustments [68–70].
Fig. 2.
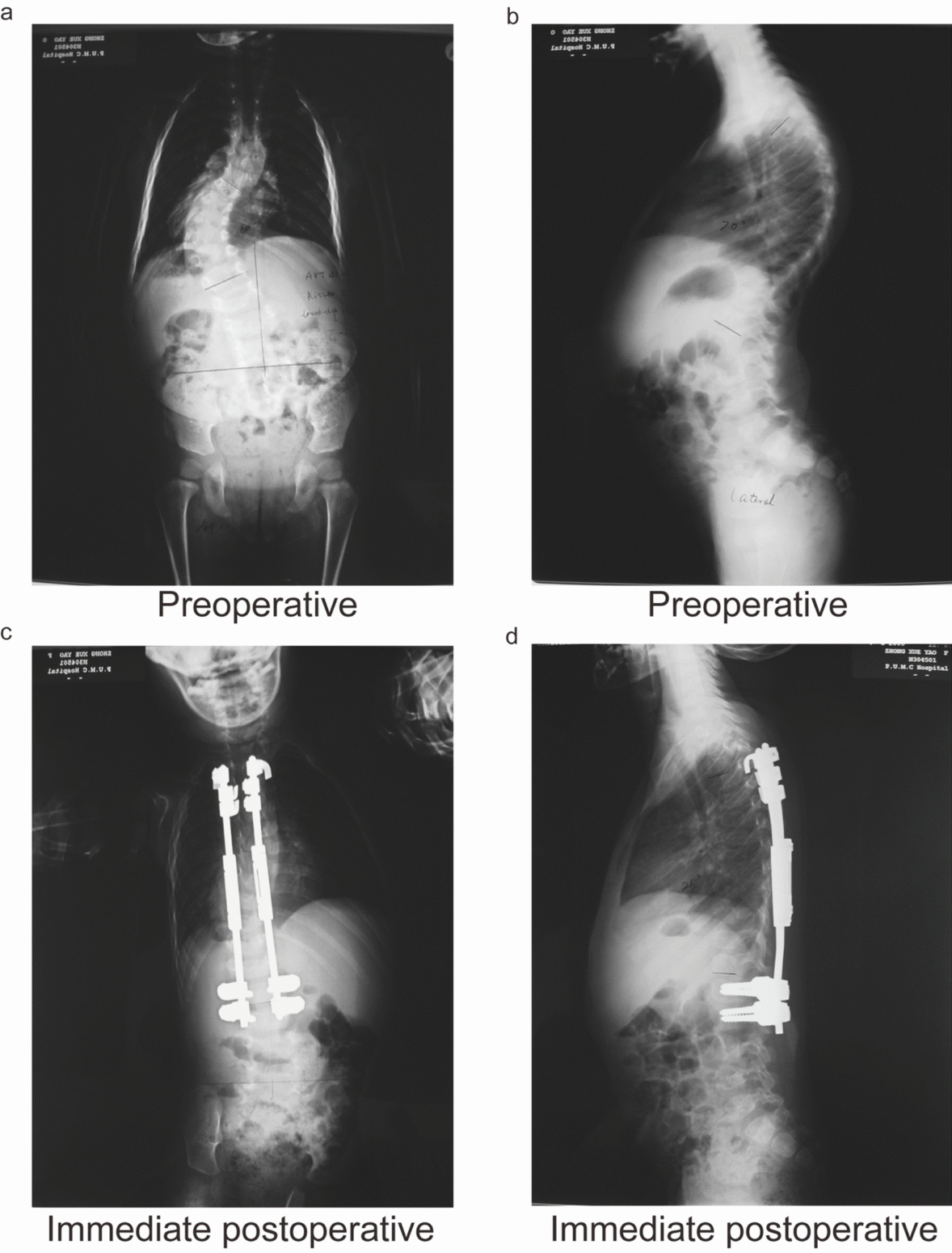
Posterior spinal correction with Isola growth-friendly instrumentation and bone grafting in a 4-year-old girl diagnosed with congenital scoliosis. a Preoperative anteroposterior (AP) radiograph showing congenital spinal curvature. b Preoperative lateral radiograph illustrating sagittal alignment. c Immediate postoperative AP radiograph demonstrating improved coronal correction. d Immediate postoperative lateral radiograph confirming maintained sagittal balance
The Shilla technique focuses on dynamic realignment of the deformity’s apex to reduce corrective loss. It has been compared to traditional growth rod systems for effectiveness [71]. The modified Luque Trolley system is another self-growing rod technique that avoids the need for repeat surgeries [72].
MCGR has transformed EOS treatment, reducing the need for repeated surgeries while maintaining correction [73]. However, some studies show no significant improvement in Health-Related Quality of Life (HRQoL) [74]. Despite this, MCGR remains a promising alternative, offering long-term correction with fewer complications [75–79]. Cost analyses also suggest that MCGR may reduce cumulative healthcare costs compared to traditional methods [80]. While there are varying opinions, MCGR represents a significant advance in EOS management.
Hybrid technique
The HT is an advanced surgical method developed to overcome limitations of traditional dual growing rods (TDGR) in treating severe, long-segment congenital early-onset scoliosis (CEOS). Key issues with TDGR, such as insufficient main curve correction, persistent apical deformities, inadequate sagittal plane control, and frequent implant failures due to asymmetric growth forces, are mitigated by HT. Combining posterior osteotomy or vertebrectomy with short fusion and dual growing rods, HT improves deformity correction, especially at the apex, while reducing mechanical complications and preserving spinal growth. Initially proposed by Wang et al., HT demonstrated significant improvements in scoliosis correction (from 81.4° to 40.1°) and spinal growth (T1–S1 length increased by 1.23 cm/year), with a low complication rate over a 53.3-month follow-up [9]. Sun et al. further confirmed its success, reporting a major curve correction from 86.4° to 37.3° and consistent growth (1.31 cm/year), with fewer complications in dual GR cases [81]. Another study by Sun et al. comparing HT to TDGR showed superior Cobb angle correction, thoracic kyphosis improvement, and fewer complications such as rod breakage in the HT group [82]. Most recently, Wang et al. affirmed HT’s superior apical control and deformity correction, though noting a slightly higher risk of dural and neurological complications compared to TDGR [83]. Overall, HT has been proven effective for severe CEOS, offering enhanced deformity correction and fewer mechanical complications while maintaining spinal growth.
Apical control techniques
The TDGR technique, combined with apical control techniques (ACTs), has been utilized to improve deformity correction in EOS by addressing apical vertebral rotation and enhancing curve control. Kamaci et al. demonstrated the efficacy of the TDGR in controlling both coronal and sagittal plane deformities while improving apical vertebral rotation [84]. Building on this, Zhao et al. explored the combination of TDGR and Apical Convex Control Pedicle Screws (ACPS), showing superior correction of apex deformity with fewer complications compared to TDGR alone [85]. Wang et al. confirmed that ACT, including hybrid techniques, could significantly enhance curve correction without hindering spinal growth, while maintaining lower complication rates [86]. Yang et al. further supported the combined approach, showing that TDGR with apical pedicle screws improved primary curve correction and spinal growth in EOS patients [87]. Wang et al. expanded on this by comparing outcomes of CEOS patients treated with TDGR and ACT, finding better deformity correction and fewer mechanical complications [88]. Li et al. confirmed these findings, demonstrating that TDGR with ACT provided better apical control and reduced complications, leading to fewer revision surgeries and avoidance of final fusion [89].
Conclusions
CS is a multifactorial disorder rooted in disrupted vertebral development during embryogenesis. Genetic studies implicate mutations in TBX6, GDF3, DSTYK, and COL11A2, alongside CNVs and epigenetic dysregulation, while environmental triggers such as maternal hypoxia and toxin exposure exacerbate risk. Diagnosis leverages advanced imaging and genetic sequencing, with MRI critical for detecting spinal cord anomalies. Conservative therapies, including innovative bracing (e.g., ARCBT, 3D-printed orthoses), effectively manage mild cases, delaying surgical needs. For severe deformities, surgical advancements like HT and MCGR offer superior correction and growth preservation. HT, combining osteotomy with growth rods, achieves 50% Cobb angle reduction and 1.23 cm/year spinal growth, outperforming traditional methods. MCGR minimizes repeat surgeries but requires further quality-of-life assessments. Apical control strategies and robotics enhance precision, reducing complications. Future research must address gene–environment interactions, and long-term outcomes of emerging therapies. A multidisciplinary approach—integrating genetics, imaging, and personalized interventions—is vital to improving prognosis for CS patients. Clinicians should prioritize early diagnosis, tailored treatment algorithms, and lifelong surveillance to mitigate progression and associated comorbidities.
Acknowledgements
We sincerely appreciate the participation of all patients and their families, whose support was invaluable to this study.
Abbreviations
- ACPS
Apical convex control pedicle screws
- ACTs
Apical control techniques
- ARCBT
Alternating cast and brace treatment
- BT
Brace-only treatment
- CEOS
Congenital early-onset scoliosis
- CS
Congenital scoliosis
- CT
Computed tomography
- CNVs
Copy number variations
- DGR
Dual growth rod
- EOS
Early-onset scoliosis
- HRQoL
Health-related quality of life
- HT
Hybrid techniques
- MCGR
Magnetic controlled growth rods
- MRI
Magnetic resonance imaging
- SNPs
Single nucleotide polymorphisms
- TDGR
Traditional dual growing rods
- VEPTR
Vertical expandable prosthetic titanium rib
Author contributions
Z.P. developed the research concept and drafted the initial version of the manuscript. H.Z. was in charge of collecting and organizing the figures. S.W. and J.Z. reviewed and approved the final manuscript for submission.
Funding
National Key Research and Development Program of China, 2023YFC2507700; National High Level Hospital Clinical Research Funding, 2022-PUMCH-D-004; CAMS Innovation Fund for Medical Sciences (CIFMS), 2021-I2M-1–051.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study received approval from the Institutional Review Board of Peking Union Medical College Hospital. Written informed consent was provided by the legal guardians of all participating patients. The research was conducted in accordance with the ethical principles outlined in the 1964 Declaration of Helsinki and its later revisions.
Consent for publication
Written consent from the patients’ legal guardians was obtained for the publication of any images included in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shengru Wang, Email: wangshengru@foxmail.com.
Jianguo Zhang, Email: jgzhang_pumch@yahoo.com.
References
- 1.Weiss HR, Moramarco M. Congenital scoliosis (mini-review). Curr Pediatr Rev. 2016;12(1):43–7. 10.2174/1573396312666151117121011. [DOI] [PubMed] [Google Scholar]
- 2.Sparrow DB, Chapman G, Smith AJ, et al. A mechanism for gene–environment interaction in the etiology of congenital scoliosis. Cell. 2012;149(2):295–306. 10.1016/j.cell.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Hensinger RN. Congenital scoliosis: etiology and associations. Spine (Phila Pa 1976). 2009;34(17):1745–50. 10.1097/BRS.0b013e3181abf69e. [DOI] [PubMed] [Google Scholar]
- 4.Wu N, Ming X, Xiao J, et al. TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med. 2015;372(4):341–50. 10.1056/NEJMoa1406829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Yu X, Shen J. Environmental aspects of congenital scoliosis. Environ Sci Pollut Res. 2015;22:5751–5. [DOI] [PubMed] [Google Scholar]
- 6.Loder RT, Hernandez MJ, Lerner AL, et al. The induction of congenital spinal deformities in mice by maternal carbon monoxide exposure. J Pediatr Orthop. 2000;20(5):662–6. 10.1097/00004694-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Batra S, Ahuja S. Congenital scoliosis: management and future directions. Acta Orthop Belg. 2008;74(2):147–60. [PubMed] [Google Scholar]
- 8.Yaszay B, Orien M, Shufflebarger HL, et al. Efficacy of hemivertebra resection for congenital scoliosis a multicenter retrospective comparison of three surgical techniques. Spine (Phila Pa 1976). 2011;36(24):2052–60. 10.1097/BRS.0b013e318233f4bb. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Zhang J, Qiu G, Wang Yp, Weng X, Guo J-g. One-stage posterior osteotomy with short segmental fusion and dual growing rod technique for severe rigid congenital scoliosis: the preliminary clinical outcomes of a hybrid technique. Spine. 2014;39: E294–E299. [DOI] [PubMed] [Google Scholar]
- 10.Berger-Groch J, Weiser L, Kunkel POS, Stuecker R, Jungesblut OD. Vertical expandable rib-based distraction device for correction of congenital scoliosis in children of 3 years of age or younger: a preliminary report. J Pediatr Orthop. 2020;40(8):e728–33. 10.1097/bpo.0000000000001597. [DOI] [PubMed] [Google Scholar]
- 11.Murphy RF, Moisan A, Kelly DM, Warner WC Jr, Jones TL, Sawyer JR. Use of vertical expandable prosthetic titanium rib (VEPTR) in the treatment of congenital scoliosis without fused ribs. J Pediatr Orthop. 2016;36(4):329–35. 10.1097/bpo.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 12.Peng Z, Du Y, Zhang H, Han B, Wang S, Zhang J. Surgical outcomes of hemivertebra resection with mono-segment fusion in children under 10 years with congenital scoliosis: a retrospective study stratified by the Crankshaft phenomenon. BMC Musculoskelet Disord. 2025;26(1):210. 10.1186/s12891-025-08375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Z, Du Y, Zhang H, Li C, Wang S, Zhang J. Two-level versus multi-level fusion in posterior hemivertebra resection for congenital early-onset scoliosis: a 10-year comparative analysis of clinical outcomes and complication rates. J Orthop Surg Res. 2025;20(1):553. 10.1186/s13018-025-05971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Kim HS, Moon ES, et al. Scoliosis imaging: what radiologists should know. Radiographics. 2010;30(7):1823–42. [DOI] [PubMed] [Google Scholar]
- 15.Sanders JO. Maturity indicators in spinal deformity. J Bone Joint Surg Am. 2007;89(Suppl 1):14–20. [DOI] [PubMed] [Google Scholar]
- 16.Burnei G, Gavriliu S, Vlad C, et al. Congenital scoliosis: an up-to-date. J Med Life. 2015;8:388–97. [PMC free article] [PubMed] [Google Scholar]
- 17.Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaskwhich D, Ali RM, Patel TC, Green DW. Congenital scoliosis. Curr Opin Pediatr. 2000;12(1):61–6. [DOI] [PubMed] [Google Scholar]
- 19.Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop Jan-Feb. 2007;27(1):106–16. 10.1097/BPO.0b013e31802b4993. [DOI] [PubMed] [Google Scholar]
- 20.Shands AR Jr, Eisberg HB. The incidence of scoliosis in the state of Delaware; a study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg Am. 1955;37(6):1243–9. [PubMed] [Google Scholar]
- 21.Wynne-Davies R. Familial (idiopathic) scoliosis. A family survey. J Bone Joint Surg Br. 1968;50(1):24–30. [PubMed] [Google Scholar]
- 22.Wynne-Davies R. Congenital vertebral anomalies: aetiology and relationship to spina bifida cystica. J Med Genet. 1975;12(3):280–8. 10.1136/jmg.12.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giampietro PF, Blank RD, Raggio CL, et al. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res. 2003;1(2):125–36. 10.3121/cmr.1.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao R, Zhao JR, Xue X, Ma D. Deciphering the etiology of congenital scoliosis: a genetic and epigenetic perspective. World J Orthop. 2025;16(6):104853. 10.5312/wjo.v16.i6.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho W, Shepard N, Arlet VM. The etiology of congenital scoliosis: genetic vs. environmental—a report of three monozygotic twin cases. Eur Spine J. 2018;27:533–7. [DOI] [PubMed] [Google Scholar]
- 26.Connor JM, Conner AN, Connor RAC, et al. Genetic aspects of early childhood scoliosis. Am J Med Genet. 1987;27(2):419–24. [DOI] [PubMed] [Google Scholar]
- 27.Qiu XS, Zhou S, Jiang H, et al. Mutation analysis of MESP2, HES7 and DUSP6 gene exons in patients with congenital scoliosis. Stud Health Technol Inform. 2012;176:52–5. [PubMed] [Google Scholar]
- 28.Chen W, Liu J, Yuan D, et al. Progress and perspective of TBX6 gene in congenital vertebral malformations. Oncotarget. 2016;7:57430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fei Q, Wu Z, Wang H, et al. The association analysis of TBX6 polymorphism with susceptibility to congenital scoliosis in a Chinese Han population. Spine. 2010;35:983–8. [DOI] [PubMed] [Google Scholar]
- 30.Lai W, Feng X, Yue M, et al. Identification of copy number variants in a southern Chinese cohort of patients with congenital scoliosis. Genes. 2021. 10.3390/genes12081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin M, Zhao S, Liu G, et al. Identification of novel FBN1 variations implicated in congenital scoliosis. J Hum Genet. 2019;65:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu N, Yuan S, Liu J, et al. Association of LMX1A genetic polymorphisms with susceptibility to congenital scoliosis in Chinese Han population. Spine. 2014;39:1785–91. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Li X, Niu Y, Wu Z, Qiu G. Functional and in silico assessment of GDF3 gene variants in a Chinese congenital scoliosis population. Med Sci Monit. 2018;24:2992–3001. 10.12659/msm.910232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Zhou Y, Zhang R, et al. Dstyk mutation leads to congenital scoliosis-like vertebral malformations in zebrafish via dysregulated mTORC1/TFEB pathway. Nat Commun. 2020;11(1):479. 10.1038/s41467-019-14169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Chen Y, Wu Y, et al. A twin-pair analysis indicates congenital scoliosis is associated with allele-specific methylation in the SVIL gene. Mol Med Rep. 2020;22(3):2093–100. 10.3892/mmr.2020.11273. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Zhang HQ, Tang M, et al. Abnormal TNS3 gene methylation in patients with congenital scoliosis. BMC Musculoskelet Disord. 2022;23(1): 797. 10.1186/s12891-022-05730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Z, Yang Y, Wang S, et al. The mutational landscape of PTK7 in congenital scoliosis and adolescent idiopathic scoliosis. Genes. 2021. 10.3390/genes12111791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Chai X, Yan Z, et al. Novel FGFR1 variants are associated with congenital scoliosis. Genes. 2021. 10.3390/genes12081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebello D, Wohler E, Erfani V, et al. COL11A2 as a candidate gene for vertebral malformations and congenital scoliosis. Hum Mol Genet. 2023;32(19):2913–28. 10.1093/hmg/ddad117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda K, Kou I, Mizumoto S, et al. Screening of known disease genes in congenital scoliosis. Mol Genet Genomic Med. 2018;6(6):966–74. 10.1002/mgg3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giampietro PF, Raggio CL, Blank RD, McCarty C, Broeckel U, Pickart MA. Clinical, genetic and environmental factors associated with congenital vertebral malformations. Mol Syndromol. 2013;4(1–2):94–105. 10.1159/000345329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Sun S, Wang D, et al. Suppression of retinoic acid receptors may contribute to embryonic skeleton hypoplasia in maternal rats with chronic vitamin A deficiency. J Nutr Biochem. 2010;21(8):710–6. 10.1016/j.jnutbio.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Huot C, Gauthier M, Lebel M, Larbrisseau A. Congenital malformations associated with maternal use of valproic acid. Can J Neurol Sci. 1987;14(3):290–3. 10.1017/s0317167100026639. [DOI] [PubMed] [Google Scholar]
- 44.de Cornulier M, de Lacour F, Avet-Loiseau H, et al. Vertebral involvement and fetal alcohol syndrome. Pediatrie. 1991;46(10):685–9 (Atteintes vertébrales et syndrome d'alcoolisme foetal). [PubMed] [Google Scholar]
- 45.Hong F, Zhou Y, Zhao X, Sheng L, Wang L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int J Nanomedicine. 2017;12:6197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ugur F, Topal K, Albayrak M, Topal M. Is obstructive sleep apnea-associated adenoid hypertrophy linked to scoliotic attitudes in children? Cureus. 2023;15: e47307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parot R, Bouhafs A, Garin C, Dubois R, Kohler R. Scoliosis and congenital diaphragmatic agenesis. Rev Chir Orthop Reparatrice Appar Mot. 2002;88(8):760–6 (Scolioses associées aux aplasies de coupole diaphragmatique). [PubMed] [Google Scholar]
- 48.Chêneau J, Grivas TB, Engels G, Fritsch HS. Wedged vertebrae normalization in congenital scoliosis due to application of external forces by brace. Scoliosis. 2007. 10.1186/1748-7161-2-s1-s29. [Google Scholar]
- 49.Kawakami N, Koumoto I, Dogaki Y, et al. Clinical impact of corrective cast treatment for early onset scoliosis: is it a worthwhile treatment option to suppress scoliosis progression before surgical intervention? J Pediatr Orthop. 2018;38(10):e556–61. 10.1097/bpo.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Feng Z, Wu Z, Qiu Y, Zhu Z, Xu L. Brace treatment can serve as a time-buying tactic for patients with congenital scoliosis. J Orthop Surg Res. 2019;14(1):194. 10.1186/s13018-019-1244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swain P, Mohanty S, Rout BK. An indigenous design for management of congenital scoliosis—a case study. Int J Health Sci Res. 2021;11(5):196–9. 10.52403/ijhsr.20210530. [Google Scholar]
- 52.Jin H, Zhang Z, Gao Y, et al. Case series: 3D printed orthopedic brace combined with traditional manipulative physiotherapy to treat new-onset scoliosis in adults. Medicine (Baltimore). 2022;101(1): e28429. 10.1097/md.0000000000028429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caredda M, Bandinelli D, Falciglia F, Giordano M, Aulisa AG. The conservative treatment of congenital scoliosis with hemivertebra: report of three cases. Front Pediatr. 2022;10: 951832. 10.3389/fped.2022.951832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winter RB, Moe JH, Lonstein JE. Posterior spinal arthrodesis for congenital scoliosis. An analysis of the cases of two hundred and ninety patients, five to nineteen years old. J Bone Joint Surg Am. 1984;66(8):1188–97. [PubMed] [Google Scholar]
- 55.Kesling KL, Lonstein JE, Denis F, et al. The crankshaft phenomenon after posterior spinal arthrodesis for congenital scoliosis: a review of 54 patients. Spine (Phila Pa 1976). 2003;28(3):267–71. 10.1097/01.Brs.0000042252.25531.A4. [DOI] [PubMed] [Google Scholar]
- 56.Murphy RF, Mooney JF 3rd. The Crankshaft phenomenon. J Am Acad Orthop Surg. 2017;25(9):e185–93. 10.5435/jaaos-d-16-00584. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg CJ, Moore DP, Fogarty EE, Dowling FE. Long-term results from in situ fusion for congenital vertebral deformity. Spine (Phila Pa 1976). 2002;27(6):619–28. 10.1097/00007632-200203150-00011. [DOI] [PubMed] [Google Scholar]
- 58.Chang D-G, Kim J-H, Ha K-Y, Lee J-s, Jang JS, Suk SI. Posterior hemivertebra resection and short segment fusion with pedicle screw fixation for congenital scoliosis in children younger than 10 years: greater than 7-year follow-up. Spine. 2015;40:E484–91. [DOI] [PubMed] [Google Scholar]
- 59.Peng Z, Du Y, Zhang H, Li C, Wang S, Zhang J. Predictive modeling and long-term outcomes in optimizing fusion strategies for congenital scoliosis: a retrospective analysis of posterior hemivertebra resection. Int Orthop. 2025. 10.1007/s00264-025-06595-0. [DOI] [PubMed] [Google Scholar]
- 60.Peng Z, Zhao Y, Zhang H, et al. Long-term results of single-stage posterior hemivertebra resection and short segment fusion using pedicle screws fixation in thoracolumbar congenital early-onset scoliosis: an 8.97-year average follow-up study. BMC Musculoskelet Disord. 2024;25(1):824. 10.1186/s12891-024-07931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao B, Su Q, Hai Y, et al. Posterior thoracolumbar hemivertebra resection and short-segment fusion in congenital scoliosis: surgical outcomes and complications with more than 5-year follow-up. BMC Surg. 2021;21:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue X, Zhao S. Revision surgery for lumbar hemivertebra in a 7-year-old child with 10-year follow-up—a case report. Medicine. 2017;96: e8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu F, Canavese F, Liang F, et al. Effects of posterior hemivertebra resection and short segment fusion on the evolution of sagittal balance in children with congenital scoliosis. J Pediatr Orthop B. 2021;31:64–71. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Zhang J, Qiu G, et al. Posterior-only hemivertebra resection with anterior structural reconstruction with titanium mesh cage and short segmental fusion for the treatment of congenital scoliokyphosis: the indications and preliminary results. Spine (Phila Pa 1976). 2017;42(22):1687–92. 10.1097/brs.0000000000002197. [DOI] [PubMed] [Google Scholar]
- 65.Zhuang Q, Zhang J, Li S, Wang S, Guo J, Qiu G. One-stage posterior-only lumbosacral hemivertebra resection with short segmental fusion: a more than 2-year follow-up. Eur Spine J. 2016;25(5):1567–74. 10.1007/s00586-015-3995-x. [DOI] [PubMed] [Google Scholar]
- 66.Peng Z, Han B, Wang S, Zhang J. The progress of research on crankshaft phenomenon. J Orthop Surg Res. 2025;20(1):188. 10.1186/s13018-025-05586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976). 2005;30(17 Suppl):S46-57. 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 68.Akbarnia BA, Breakwell L, Marks DS, et al. Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine. 2008;33:984–90. [DOI] [PubMed] [Google Scholar]
- 69.Akgül T, Dikici F, Sar C, Talu U. Domani̇ç Ü (2014) Growing rod instrumentation in the treatment of early onset scoliosis. Acta Orthopaed Belg. 2014;80(4):457–63. [PubMed] [Google Scholar]
- 70.Sun L, Sun B-s, Zhang X, et al. Dual growing rod technique for the treatment of scoliosis in children. Chin J Pediatr Surg. 2009;30:555–8. [Google Scholar]
- 71.Agarwal A, Aker L, Ahmad AA. Active apex correction (modified SHILLA technique) versus distraction-based growth rod fixation: what do the correction parameters say? Spine Surg Related Res. 2019;4:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ouellet JA. Surgical technique: modern Luqué trolley, a self-growing rod technique. Clin Orthopaed Related Res. 2011;469:1356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akbarnia BA, Pawelek JB, Cheung KM, et al. Traditional growing rods versus magnetically controlled growing rods for the surgical treatment of early-onset scoliosis: a case-matched 2-year study. Spine Deform. 2014;2(6):493–7. 10.1016/j.jspd.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 74.Bekmez Ş, Afandiyev A, Dede O, Karaismailoğlu E, Demirkiran HG, Yazici M. Is magnetically controlled growing rod the game changer in early-onset scoliosis? A preliminary report. J Pediatr Orthopaed. 2019;39:e195–200. [DOI] [PubMed] [Google Scholar]
- 75.Marquez-Lara A, Bachman D, Noble MB, et al. Maintenance of curve correction and unplanned return to the operating room with magnetically controlled growing rods: a cohort of 24 patients with follow-up between 2 and 7 years. Spine Deformity. 2023;11:715–21. [DOI] [PubMed] [Google Scholar]
- 76.Diekhöner L, Meyer CS, Eiskjær SP. The magnetic field strength and the force distance dependency of the magnetically controlled growing rods used for early onset scoliosis. Sci Rep. 2023;13:3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grabala P, Helenius IJ, Chamberlin K, Galgano MA. Less-invasive approach to early-onset scoliosis—surgical technique for magnetically controlled growing rod (MCGR) based on treatment of 2-year-old child with severe scoliosis. Children. 2023;10:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grabala P, Chamberlin K, Grabala M, Galgano MA, Helenius IJ. No benefits in using magnetically controlled growing rod as temporary internal distraction device in staged surgical procedure for management of severe and neglected scoliosis in adolescents. J Clin Med. 2023;12:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ellinger F, Tropp H, Gerdhem P, Hallgren HB, Ivars K. Magnetically controlled growing rod treatment for early-onset scoliosis: analysis of 52 consecutive cases demonstrates improvement of coronal deformity. J Spine Surg. 2023;9:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luhmann SJ, McAughey EM, Ackerman SJ, Bumpass DB, McCarthy R. Cost analysis of a growth guidance system compared with traditional and magnetically controlled growing rods for early-onset scoliosis: a US-based integrated health care delivery system perspective. ClinicoEcon Outc Res. 2018;10:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun X, Xu L, Chen ZH, et al. Hybrid growing rod technique of osteotomy with short fusion and spinal distraction: an alternative solution for long-spanned congenital scoliosis. Spine. 2019;44:707–14. [DOI] [PubMed] [Google Scholar]
- 82.Sun X, Xu L, Chen ZH, et al. Comparison of hybrid and traditional growing rod techniques in the treatment of early-onset congenital scoliosis. Zhonghua Wai Ke Za Zhi. 2019;57(5):342–7. 10.3760/cma.j.issn.0529-5815.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Wang S, Zhao Y, Yang Y, et al. Hybrid technique versus traditional dual growing rod technique to treat congenital early-onset scoliosis: a comparative study with more than 3 years of follow-up. J Neurosurg Spine. 2023;38(2):199–207. 10.3171/2022.8.Spine22618. [DOI] [PubMed] [Google Scholar]
- 84.Kamaci S, Demirkiran G, Ismayilov V, Olgun ZD, Yazici M. The effect of dual growing rod instrumentation on the apical vertebral rotation in early-onset idiopathic scoliosis. J Pediatr Orthop. 2014;34(6):607–12. 10.1097/bpo.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Y, Du Y, Yang Y, et al. Dual growing rods combined with the apical convex control pedicle screw technique versus traditional dual growing rods for the surgical treatment of early-onset scoliosis: a case-matched 2-year study. Neurosurgery. 2023;93(2):436–44. 10.1227/neu.0000000000002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang S, Zhao Y, Lin G, Du Y, Yang Y, Zhang J. Traditional dual growing rods with 2 different apical control techniques in the treatment of early-onset scoliosis. Neurospine. 2023;20(3):1061–72. 10.14245/ns.2346406.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y, Su Z, Wang S, et al. Clinical outcomes of the traditional dual growing rod technique combined with apical pedicle screws in the treatment of early-onset scoliosis: preliminary results from a single center. J Neurosurg Pediatr. 2023;31(4):358–68. 10.3171/2022.12.Peds22383. [DOI] [PubMed] [Google Scholar]
- 88.Wang S, Zhao Y, Du Y, et al. Dual growing rods and the apical control technique for treating congenital early-onset scoliosis: lessons learned. J Bone Joint Surg Am. 2024;106(4):304–14. 10.2106/jbjs.23.00201. [DOI] [PubMed] [Google Scholar]
- 89.Li C, Ye X, Yang Y, et al. Outcomes of traditional dual growing rods with apical control techniques for the treatment of early-onset scoliosis: comparison with patients treated with traditional dual growing rods only with a minimum 2-year follow-up after graduation. Neurosurgery. 2024. 10.1227/neu.0000000000003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


