Abstract
Inhibitors of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, also known as statins, are lipid-lowering agents widely used in the prevention of coronary heart disease. Recent experimental and clinical data, however, indicate that the overall benefits of statin therapy may exceed its cholesterol-lowering properties. We postulate that statins may ameliorate the detrimental effects of high glucose (HG)-induced proliferation of mesangial cells (MCs), a feature of early stages of diabetic nephropathy, by preventing Rho isoprenylation. Rat MCs cultured in HG milieu were treated with and without simvastatin, an HMG-CoA reductase inhibitor. Simvastatin inhibited HG-induced MC proliferation as measured by [3H]thymidine incorporation. This inhibitory effect was reversed with geranylgeranyl pyrophosphate, an isoprenoid intermediate of the cholesterol biosynthetic pathway. At the cell-cycle level, the HG-induced proliferation of MCs was associated with a decrease in cyclin dependent kinase (CDK) inhibitor p21 protein expression accompanied by an increase in CDK4 and CDK2 kinase activities. Simvastatin reversed the down-regulation of p21 protein expression and decreased CDK4 and CDK2 kinase activities. Exposure of MCs to HG was associated with an increase in membrane-associated Ras and Rho GTPase protein expression. Cotreatment of MCs with simvastatin reversed HG-induced Ras and Rho membrane translocation. Immunofluorescence microscopy revealed that the overexpression of the dominant-negative RhoA led to a significant increase in p21 expression. Our data suggest that simvastatin represses the HG-induced Rho GTPase/p21 signaling in glomerular MCs. Thus, this study provides a molecular basis for the use of statins, independently of their cholesterol-lowering effect, in early stages of diabetic nephropathy.
The 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, or statins, are potent inhibitors of cholesterol biosynthesis that are extensively used in the treatment of hypercholesterolemia (1). Several studies have demonstrated the beneficial effects of statins in reducing cardiovascular-related morbidity and mortality in patients with and without coronary artery disease (1, 2). More recently, it has been suggested that statins may confer renoprotection in a variety of glomerular diseases, including diabetic nephropathy, through their lipid-lowering properties (3, 4). It is usually assumed that the beneficial effects of statins result from the competitive inhibition of cholesterol synthesis. However, by inhibiting the synthesis of l〈-mevalonic acid, statins may also exert additional beneficial effects by preventing the synthesis of various isoprenoids, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP; ref. 5). Both FPP and GGPP are important lipid attachments for the posttranslational modifications of a variety of small GTPase proteins, such as Ras and Rho GTPases (6). Recent studies suggest that some of the cholesterol-independent or “pleiotropic” effects of statins are mediated via small GTPases (7, 8).
By cycling between inactive GDP- and active GTP-bound states, small GTPase proteins function as critical relays in the transduction of signals emanating from membrane receptors. Among the small GTPases, the Ras and Rho family of proteins hierarchically and/or coordinately regulate various cellular processes such as apoptosis, differentiation, and cellular proliferation (9, 10). The role of GTPases in the high glucose (HG)-mediated proliferation of glomerular mesangial cells (MCs), an early feature of diabetic nephropathy, has not been previously elucidated. We hypothesize that the possible beneficial effect of statins in diabetic nephropathy is independent of its antihyperlipidemic effect, and is mediated by reversing the up-regulation of Rho GTPases.
Several in vivo studies have recently unraveled the significance of MC proliferation in the early stages of diabetic nephropathy (11, 12). It seems that mesangial hypercellularity precedes an increase in the extracellular matrix proteins and glomerular sclerosis, hallmarks of diabetic nephropathy. Moreover, in vitro studies have indicated that HG ambience stimulates MC proliferation and extracellular matrix expansion (13, 14). However, the precise molecular mechanism of this hyperplastic phenotype at the cell-cycle level has not been previously explored. Cell-cycle progression is regulated by the activity of the cyclin-dependent kinases (CDK), which consist of a kinase core and an associated cyclin subunit (15). In the early G1 phase of the cell cycle, the D-type cyclins activate CDK4 and CDK6 as cells leave the quiescent phase. CDK2 complex forms later as cells prepare to begin DNA synthesis. The activities of the CDK family are not only controlled by positive regulators, or cyclins, but are also under negative control of a number of different CDK inhibitors (16). The latter bind to specific cyclin-CDK complexes and inhibit their activities. The CDK inhibitors are classified on the basis of their sequence homology and substrate specificity. Two families of CDK inhibitors have been identified (16). The CDK inhibitor p21, a 21-kDa protein, is a member of the CIP/KIP family. The protein levels of p21 are low in most quiescent cells. However, p21 protein levels increase during cell proliferation (17). Recent studies have shown that p21 can inhibit cell proliferation by inhibiting CDKs or by a direct inhibitory effect on the proliferating cell nuclear antigen (16, 17).
The purpose of this study is to determine the role of isoprenoid intermediates and cell-cycle regulatory proteins in the HG-induced proliferation of glomerular MCs and the modulatory effects of simvastatin during cell cycle progression.
Materials and Methods
Cell culture media and supplies were purchased from Life Technologies (Grand Island, NY). Simvastatin was obtained from Merck Sharp & Dohme. Mevalonate, FPP, and GGPP were purchased from Sigma. Simvastatin prodrug was activated to its active form, as described (18). Mevalonate was chemically activated by alkaline hydrolysis (18). The antibody detection kit (chemiluminescence) and the nylon nucleic acid membrane (Hybond) were purchased from Amersham Pharmacia. RhoA mutants were kindly provided by Jacob Sznajder (Northwestern University, Chicago, IL).
Cell Culture.
Glomeruli were isolated from rat kidneys, and isolated MCs were maintained in a DMEM (DMEM/F12, Invitrogen), supplemented with 10% heat-inactivated FBS, in the presence of a humidified 5% CO2 incubator at 37°C. The cells used in these experiments were from 5th-15th passages. The MCs were rendered quiescent by incubation in the serum-free medium for 48 h, and the trypan blue exclusion test was used to monitor the viability of cells. For each experiment, cultured MCs were placed in DMEM containing low glucose (5 mM glucose), high glucose (30 mM glucose), and high glucose with simvastatin. In experiments with simvastatin, the cells were preincubated with simvastatin for 24 h before high-glucose exposure.
[3H]Thymidine Incorporation.
The quiescent rat MCs were incubated with normal glucose (NG, 5 mM), HG (30 mM), and HG and simvastatin (1 to 10 μM); DNA synthesis was assessed by [3H]thymidine incorporation. Briefly, cells were transferred into six-well plates at a density of 5 × 104 cells per well and maintained for 24 h; then, cells were incubated in serum-free media for 48 h to synchronize in G0 growth phase. [3H]thymidine (1 μCi/ml; 1 Ci = 37 GBq) was added, and the cells were incubated for an additional 24 h. Then, they were processed for determination of incorporated radioactivity after TCA precipitation.
Western Blotting.
The quiescent rat glomerular MCs were washed with PBS and microcentrifuged for 15 min at 1,500 × g at 15ο C. For preparation of whole-cell lysate, the pellet was resuspended in 300 μl of lysis buffer (50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/25 mM EDTA/5 mM EGTA/0.25% sodium deoxycholate/1 mM DTT/1 mM sodium fluoride/0.1 mg/ml PMSF/2 μg/ml leupeptin/0.234 trypsin inhibitor unit (TIU)/ml aprotinin/1 μg/ml pepstatin A). For preparation of plasmalemmal proteins, membrane fractions were first purified as described (12). Briefly, the cells were washed with PBS and resuspended in 10 mM Tris⋅HCl, pH 7.4/10 mM NaCl/1.5 mM MgCl2/0.02 mM PMSF, and homogenized in a Dounce homogenizer. The homogenate was centrifuged at 1,000 × g for 10 min. The supernatant was overlaid on 35% sucrose solution and centrifuged for 60 min at 18,000 × g. The membrane fraction at the interface was removed and resuspended in 10 mM Tris⋅HCl, pH 7.4/250 mM sucrose (buffer A). The sample then was centrifuged, and the pellets were resuspended in buffer A and stored at 4°C. Finally, the protein concentration in the total cell lysate and plasmalemmal fractions were measured by Bradford assay. Equal amounts of protein (50 μg) from different variables were subjected to SDS/PAGE analysis and then electroblotted onto nylon membranes. The blots then were individually probed with anti-p21, anti-p27 (PharMingen), anti-cyclin D1 and anti-cyclin A (Santa Cruz Biotechnology), and anti-pan-Ras and anti-Rho antibodies (Upstate Biotechnology, Lake Placid, NY) in Tris-buffered saline containing 0.5% Tween 20; autoradiograms were prepared by using an enhanced chemiluminescence system.
CDK Assay.
The CDK assay was performed as described (19). Briefly, cell protein extracts were prepared by lysis in Hepes buffer (50 mM Hepes, pH 7.0/150 mM NaCl/0.2 mM PMSF/100 μM sodium orthovanadate/10 mM β-glycerophosphate/1 mM sodium fluoride/0.33 TIU/ml aprotinin). After preclearing the protein extract (200 μg) with 30 μl of protein A agarose, immunoprecipitation was carried out with 3 μg of anti-CDK4 or anti-CDK2 (Santa Cruz Biotechnology) for 1 h at 4°C. CDK4 kinase activity in the immunoprecipitates was determined by using retinoblastoma protein as a substrate, whereas CDK2 kinase activity was determined by using histone H1 as the substrate (Santa Cruz Biotechnology). Immune complexes were resuspended in 35 μl of kinase buffer containing 1 μg of glutathione S-transferase retinoblastoma (GST-Rb) (or histone H1), 1 μM cold ATP, 0.5 mCi/ml [γ32P]ATP (Amersham Pharmacia), 50 mM Tris⋅HCl, 10 mM MgCl2, and 1 mM DTT; incubation was carried out for 30 min at 30°C, and the reaction was terminated by heating at 95°C for 5 min. Phosphorylated products were analyzed by SDS/10% PAGE, and autoradiograms were prepared.
RhoA Mutant Transfection.
MCs were seeded on cover glasses placed into six-well dishes. Cells cultured in HG (30 mM) media were transfected with 5 μg of pcDNA3-wtRhoA (wild type RhoA) or pcDNA3-N19RhoA (dominant-negative RhoA mutant) by using Lipofectamine (Life Technologies) following manufacturer's instructions. Transfected MCs were selected in the presence of the neomycin analogue G418 (500 μg/ml). For immunocytochemistry, cells were fixed with 3.7% (vol/vol) formaldehyde in PBS and permeabilized with 0.1% Triton X-100. They were then incubated with mouse monoclonal anti-Rho A (Santa Cruz Biotechnology), and anti-p21 (PharMingen) for 1 h. After washing the cells with PBS, rhodamine-conjugated goat anti-mouse IgG and fluorescein-conjugated goat anti-mouse IgG (Zymed) were individually added in different wells for 1 h. Cells then were washed with PBS and mounted onto glass slides and examined by a UV-light microscope equipped with epi-illumination.
Data Analysis.
Band intensities were analyzed densitometrically with the Eagle Eye II system (Stratagene). All values were expressed as means ± SE. Paired and unpaired student's t tests were used to determine the statistical significance.
Results
Effect of Simvastatin on HG-Induced DNA Synthesis.
To determine the dose-response effect of simvastatin on HG-induced MC proliferation, HG-exposed cells were treated with increasing concentrations of simvastatin (1, 5, and 10 μM) for 24 h (Fig. 1A). At 1 μM concentration of simvastatin, DNA synthesis, as assessed by [3H]thymidine incorporation, was reduced by 42 ± 8% compared with cells grown in HG media. Cotreatment with 5 and 10 μM of simvastatin reduced the DNA synthesis by 65 ± 5% and 77 ± 3%, respectively (P < 0.05). The [3H]thymidine incorporation under basal conditions (normal glucose) was 51.8 ± 4.5% (P < 0.05), relative to cells incubated in HG milieu.
Figure 1.
(A) Effect of simvastatin (1, 5, and 10 μM) on HG-induced MC proliferation, as assessed by [3H]thymidine incorporation (n = 10). *, P < 0.05, compared with the HG. (B) Effect of HG, HG in combination with simvastatin (SIMV), or l〈-mevalonate (MEV) on [3H]thymidine incorporation, as compared with NG (n = 18). *, P < 0.05, compared with NG.
To assess whether the effect of simvastatin on HG-induced proliferation can be ameliorated by mevalonate, the cells were exposed to NG (5 mM), HG (30 mM), HG and 1 μM simvastatin, and HG in combination with 1 μM simvastatin and 200 μM mevalonate. The cells exposed to HG-media exhibited significant increase in [3H]thymidine incorporation (169 ± 18%, P < 0.05) as compared with cells cultured in NG media. The addition of 1 μM of simvastatin decreased the observed increase in DNA synthesis with HG to almost basal levels (113 ± 20%). The inhibitory effect of simvastatin on DNA synthesis was reversed when cells were cotreated with 200 μM of mevalonate (Fig. 1B). Mevalonate alone did not have any effect on [3H]thymidine incorporation (data not shown).
Effect of HG and Simvastatin on Cell-Cycle Regulatory Proteins.
To determine the modulatory effect of simvastatin on cell-cycle regulatory proteins in HG-induced proliferating MCs, the effect of simvastatin on CDK inhibitors p21 and p27, as well as in cyclin D1 and cyclin A, was investigated. HG exposure decreased p21 protein expression significantly as compared with NG after 6, 12, and 24 h to 59 ± 26%, 17 ± 9%, and 29 ± 10%, respectively, as assessed by Western blot analyses (Fig. 2A). Cotreatment with simvastatin reversed the down-regulation of p21, resulting in a significant increase in p21 protein expression after 6, 12, and 24 h of treatment to 178 ± 46%, 77 ± 13%, and 76 ± 13% (respectively) compared with NG (Fig. 2A). In contrast, protein levels of p27, cyclin D1, and cyclin A were not significantly changed with HG exposure (Fig. 2B).
Figure 2.
(A) Immunoblot showing the time-dependent effect of HG (30 mM) and simvastatin (1 μM) on CDK inhibitor p21 after 6, 12, and 24 h of treatment. (B) Immunoblots showing the time-dependent effects of HG (30 mM) and simvastatin (1 μM) on the expression of cell-cycle regulatory proteins cyclin A and D1 and CDK inhibitor p27 after 6, 12, and 24 h of exposure. The blots in A and B are representative of three separate experiments.
Effect of HG and Simvastatin on CDK Activity.
To determine whether simvastatin can reverse the effects of HG on CDK activity, the effect of simvastatin on CDK4 and CDK2 activities was investigated. By using GST-Rb fusion protein as a substrate for CDK4 activity and histone H1 for CDK2 activity, the HG treatment increased CDK4 and CDK2 activities by 6- and 2-fold, respectively. Cotreatment with simvastatin reversed CDK4 activity reducing it by almost four-fold (Fig. 3a). However, simvastatin reversed CDK2 activity to a very limited extent (Fig. 3b).
Figure 3.
Effects of HG (30 mM) and simvastatin (1 μM) on CDK4 (a) and CDK2 (b) after 24 h of exposure. The data reflect the results of duplicate experiments.
Effect of HG and Simvastatin on Ras and Rho Membrane Translocation.
Small GTPases of the Ra and Rho family have been shown to regulate the cell-cycle progression (11, 20, 21). To investigate the role of small GTPase in HG-induced proliferation of MCs, cells were exposed to HG milieu, and total amount and membrane-associated proteins of Ras and Rho GTPases were determined. HG treatment increased the protein expression of membrane-associated Ras by ≈three-fold in MCs without any discernible change in the total amount of cellular Ras GTPase (Fig. 4A). HG stimulation also caused an ≈3-fold increase in the protein expression of membrane-associated Rho GTPase without significantly affecting the total amount of Rho (Fig. 4B). Cotreatment with simvastatin reversed HG-induced increase in the Ras and Rho membrane-associated protein expression (Figs. 4 A and B).
Figure 4.
(A) Immunoblots showing the effect of HG and simvastatin on Ras GTPase protein expression assessed in the total cell lysate (a, cytosol) and cell membrane fraction (b, membrane) after 12 and 24 h of treatment. (B) Immunoblots showing the effect of HG and simvastatin on Rho GTPase protein expression assessed in the total cell lysate (a, cytosol) and cell membrane fraction (b, membrane) after 12 and 24 h of treatment.
Relevance of Rho GTPase in HG-Induced DNA Synthesis.
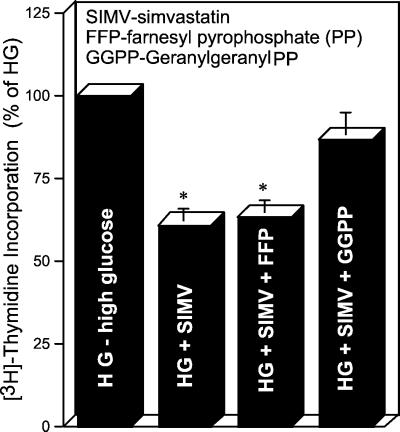
It has been shown previously that Rho geranylgeranylation is required for its membrane-associated GTPase activity (9, 10). To determine the role of Rho geranylgeranylation in HG-induced DNA synthesis, cells were treated with various isoprenoids, including FFP, GGPP, and squalene. Treatment with GGPP (5 μM) almost completely reversed (86 ± 8%) the inhibitory effect of simvastatin in MCs exposed to HG, as assessed by [3H]thymidine incorporation (Fig. 5), whereas the treatment with FFP (5 μM) did not reverse the simvastatin-induced decrease of DNA synthesis in cells exposed to HG. Also, the treatment with squalene (5 μM) failed to reverse the simvastatin-induced decrease of DNA synthesis (data not shown).
Figure 5.
Effect of SIMV (1 μM), FFP (5 μM), and GGPP (5 μM) on HG-induced DNA synthesis, as measured by [3H]thymidine incorporation (n = 10).
To establish a direct role of Rho GTPase in the HG-induced down-regulation of p21, MCs maintained in HG media were individually transfected with a wtRhoA and a dominant-negative RhoA (N19RhoA mutant); the latter acts by competitively inhibiting the interaction of endogenous RhoA with its exchange factors. The MCs transfected with the N19RhoA mutant showed a significant increase in p21 expression as compared with cells expressing wtRhoA mutant, as assessed by immunofluorescence microscopy (Fig. 6). All experiments were performed in triplicate and were repeated two times.
Figure 6.
Immunofluorescence photomicrographs showing (A) expression of CDK inhibitor p21 in MCs transfected with wild-type Rho (wtRhoA) and (B) dominant-negative Rho mutant (N19Rho) in HG milieu (30 mM). Arrows indicate the expression of p21 in the nuclei.
Discussion
This study indicates that statins inhibit HG-induced MC proliferation by preventing geranylgeranylation of Rho GTPase and by reversing Rho GTPase-induced down-regulation of CDK-inhibitor p21. The ability to inhibit MC proliferation indicates that statins may have beneficial effects beyond their cholesterol-lowering properties in early diabetic nephropathy. The findings also suggest the involvement of a previously uncharacterized Rho-dependent intracellular signaling pathway in HG-induced proliferation of MC (Fig. 7).
Figure 7.
Proposed HG-induced signaling leading to membrane translocation of Rho GTPase protein and down-regulation of CDK inhibitor p21. The schema is derived from the data included in Figs. 1, 2, 4, and 6. The results in Fig. 4 indicate that the HG milieu leads to the activation and translocation of Ras and Rho GTPases. The data in Figs. 1 and 2 suggest that HG induces proliferation of MCs via inhibition of p21. The results given in Fig. 6 indicate that MCs transfected with dominant-negative RhoA (N19RhoA mutant) show a significant increase in P21, suggesting a relationship between RhoA and p21 in HG-induced MC proliferation.
In recent years, it has become increasingly clear that the HG-signaling pathway is cell specific (22). The deleterious effects of hyperglycemia are characteristically observed in cells and tissues that do not depend on insulin for glucose entry, and, hence, are not capable of down-regulating the HG-signaling pathway, as in neuronal, retinal, and renal cells (23). Regarding the MC response to the HG ambience, a number of studies have investigated various signaling pathways in glomerular MCs that are affected in response to HG (24–27). This study indicates that a Rho/p21-dependent signaling pathway is involved in the HG-induced proliferation of MCs, which is seen in the early stages of diabetic nephropathy. The data presented in this study also is consistent with previous reports of a possible relationship between the Rho GTPase signaling pathway and cell-cycle regulatory proteins (28–31). For instance, an increased expression and activity of RhoA has been associated with reduced p27 expression in the vasculature of hypertensive rats (28). Rho GTPase also has been shown to be crucial for cyclin D1 expression in G1 phase of the cell cycle in NIH 3T3 cells (29). Similarly, Olson et al. (30) have reported that RhoA is required for the serum-dependent inhibition of p21 that occurs in Swiss 3T3 cells microinjected with H-Ras V12 (30). These results seem to indicate that the correlation between the Rho GTPase signaling pathway and different cell-cycle regulatory proteins is cell specific and possibly growth factor dependent. Data presented in this study extend these observations by demonstrating that exposure of MCs to HG environment activates the Rho GTPase signaling pathway by stimulating CDK2 and CDK4 kinase activity and by down-regulating the CDK inhibitor, p21. The concomitant activation of Ras and Rho by HG also is consistent with previous studies suggesting a possible relationship between Ras and Rho GTPase proteins, where the activation of RhoA was elucidated as a downstream effect of activated Ras (31, 32).
With respect to the biological effects of statins relative to Rho GTPase and cell-cycle proteins, Laufs et al. (7) have shown an increase in RhoA GTPase activity in platelet-derived growth factor (PDGF)-induced vascular smooth muscle cells (VSMC) that was attenuated with simvastatin treatment. Similarly, up-regulation of transforming growth factor-β in cultured heart cells by statins have been reported to be associated with the inhibition of the RhoA GTPase signaling pathway (8). Moreover, it has been shown that a dominant-negative mutant of RhoA is associated with increased levels of p27 in VSMCs stimulated with PDGF (7). Another HMG-CoA reductase inhibitor, lovastatin, has been shown to increase the levels of p21 in prostate cancer cells (33). These differences could be cell specific, but they indicate a direct correlation between the Rho GTPase pathway and CDK inhibitors p21 and p27, albeit their roles may vary in different disease processes. In our study, the finding of overexpression of a dominant-negative Rho mutant (N19RhoA) mimicking the effect of simvastatin on p21 protein expression supports the contention of activation of a Rho GTPase/p21-simvastatin-inhibitable pathway by HG. In regard to the cholesterol-independent “pleiotropic” effects of statins, they are most likely mediated through inhibition of Rho geranylgeranylation. Moreover, geranylgeranylation, and not farnesylation, has been reported to be primarily responsible for releasing the G1/S phase block (34). This observation is consistent with our findings that in the presence of simvastatin, addition of GGPP, but not FPP, restored the higher rate of DNA synthesis under the influence of HG ambience.
In support of the judicious use of statins, the initial clinical studies on the effects of statins on the progression of diabetic nephropathy were encouraging (35–37). In a study from Hong Kong (36), it was suggested that despite no significant effect on proteinuria, a decline in renal function was attenuated by lovastatin. In another crossover study with simvastatin, a significant decrease in proteinuria in type 2 diabetic patients was observed (37). Initially, the beneficial effects of statins were attributed solely to improved lipid alterations in the renal cortical tissue and decreased lipid-induced accumulation of the macrophages in the kidneys, because high levels of plasma low density lipoprotein cholesterol, triglycerides, and apolipoprotein B are seen in patients with diabetic nephropathy. However, this study provides a new rationale for the use of statins in the early stages of diabetic nephropathy, independently of their cholesterol-lowering properties. Finally, the findings of this study should give impetus for future investigations to study the effects of statins in the prevention of nephropathy in diabetic patients.
Acknowledgments
We thank Dr. J. Sznajder of Northwestern University for providing Rho mutants. This work was supported by National Institutes of Health Grants DK28492 and DK60635 and a grant from Merck & Co., Inc.
Abbreviations
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- FPP
farnesyl pyrophosphate
- GGPP
geranylgeranyl pyrophosphate
- CDK
cyclin-dependent kinase
- HG
high glucose
- MC
mesangial cell
- NG
normal glucose
References
- 1.Maron D J, Fazio S, Linton M F. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen T R. Am Heart J. 1999;138:177–182. doi: 10.1016/s0002-8703(99)70340-6. [DOI] [PubMed] [Google Scholar]
- 3.Harris K P, Purkerson M L, Yates J, Klahr S. Am J Kidney Dis. 1990;15:16–23. doi: 10.1016/s0272-6386(12)80587-7. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske B L, O'Donnell M P, Cleary M P, Keane W F. Kidney Int. 1988;33:667–672. doi: 10.1038/ki.1988.51. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein J L, Brown M S. Nature (London) 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 6.Casey P J. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 7.Laufs U, Marra D, Node K, Liao J K. J Biol Chem. 1999;274:21926–21933. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 8.Park H J, Galper J B. Proc Natl Acad Sci USA. 1999;96:11525–11530. doi: 10.1073/pnas.96.20.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Aelst L, D'Souza-Schorey C. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 10.Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, Giardina G, Di Fiore P P. EMBO J. 2000;19:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young B A, Johnson R J, Alpers C E, Eng E, Gordon K, Floege J, Couser W G, Seidel K. Kidney Int. 1995;47:935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]
- 12.Awazu M, Ishikura K, Hida M, Hoshiya M. J Am Soc Nephrol. 1999;10:738–745. doi: 10.1681/ASN.V104738. [DOI] [PubMed] [Google Scholar]
- 13.Sodhi C P, Phadke S, Batlle D, Sahai A. Am J Physiol Renal Physiol. 2001;280:F667–F674. doi: 10.1152/ajprenal.2001.280.4.F667. [DOI] [PubMed] [Google Scholar]
- 14.Wolf G, Sharma K, Chen Y, Erickson M, Ziadeh F N. Kidney Int. 1992;42:647–656. doi: 10.1038/ki.1992.330. [DOI] [PubMed] [Google Scholar]
- 15.Sherr C J. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 16.Sherr C J, Roberts J M. Genes Dev. 1996;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 17.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi M M, Collinge M, Pardi R, Bender J B. J Immunology. 2000;165:2712–2718. doi: 10.4049/jimmunol.165.5.2712. [DOI] [PubMed] [Google Scholar]
- 19.Danesh F R, Ye M, Salmi S, Lapointe M, Batlle D. Kidney Int. 1999;56:1282–1285. doi: 10.1046/j.1523-1755.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 20.Pruitt K, Der C J. Cancer Lett (Shannon, Irel) 2001;171:1–10. doi: 10.1016/s0304-3835(01)00528-6. [DOI] [PubMed] [Google Scholar]
- 21.Coleman M L, Marshall C J. Nat Cell Biol. 2001;3:E250–E251. doi: 10.1038/ncb1101-e250. [DOI] [PubMed] [Google Scholar]
- 22.Saltiel A R, Kahn A. Nature (London) 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 23.Brownlee M. Nature (London) 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 24.Booth A A, Khalifah R G, Todd P, Hudson B G. J Biol Chem. 1977;28:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava S K, Hair G A, Das B. Proc Natl Acad Sci USA. 1985;82:7222–7226. doi: 10.1073/pnas.82.21.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddi A S, Bollimeni J. Kidney Int. 2001;59:1342–1353. doi: 10.1046/j.1523-1755.2001.0590041342.x. [DOI] [PubMed] [Google Scholar]
- 27.Kang S-W, Adler S G, Nast C C, LaPage J, Gu J-L, Nadler J L, Natarajan R. Kidney Int. 2001;59:1354–1362. doi: 10.1046/j.1523-1755.2001.0590041354.x. [DOI] [PubMed] [Google Scholar]
- 28.Seasholtz T M, Zhang T, Morissette M R, Howes A L, Yang A H, Brown J H. Circ Res. 2001;89:488–495. doi: 10.1161/hh1801.096337. [DOI] [PubMed] [Google Scholar]
- 29.Welsh C F, Roovers K, Villanueva J, Liu Y, Schwartz M A, Assoian R K. Nat Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 30.Olson M F, Paterson H F, Marshall C J. Nature (London) 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 31.Nobes C D, Hall A. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 32.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee S J, Ha M J, Lee J, Nguyen P, Choi Y H, Pirnia F, Kang W K, Wang X F, Kim S J, Trepel J B. J Biol Chem. 1998;273:10618–10623. doi: 10.1074/jbc.273.17.10618. [DOI] [PubMed] [Google Scholar]
- 34.Lefer A M, Scalia R, Lefer D J. Cardiovasc Res. 2001;49:281–287. doi: 10.1016/s0008-6363(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 35.Grandaliano G, Biswas P, Choudhury G G, Abboud H E. Kidney Int. 1993;44:503–508. doi: 10.1038/ki.1993.274. [DOI] [PubMed] [Google Scholar]
- 36.Lam K S, Cheng J K, Janus E D, Pang R W. Diabetologia. 1995;38:604–609. doi: 10.1007/BF00400731. [DOI] [PubMed] [Google Scholar]
- 37.Tonolo G, Ciccaresse M, Brizzi P, Puddu L, Secchi G, Calvia P, Atzeni M M, Melis M G, Maioli M. Diabetes Care. 1997;20:1891–1895. doi: 10.2337/diacare.20.12.1891. [DOI] [PubMed] [Google Scholar]