Abstract
Background
Cancer immunotherapy includes vaccines generated through distinct approaches, each with advantages and limitations. Those made of autologous or allogeneic whole cells do not require prior identification of antigens, that is, immunize against undetermined (agnostic) tumor antigens. However, they often exhibit low adjuvanticity and modest antigenicity. Viruses have emerged as elicitors and enhancers of immune responses. Oncolytic viruses are replicating anticancer agents, most often administered intratumorally. They derepress the immunosuppressive tumor microenvironment through different mechanisms, and some promote antitumor immunity—a strategy termed oncolytic immunotherapy. Tropism-retargeted oncolytic herpes simplex viruses (here ReHVs), generated in our laboratory, specifically target a tumor-associated antigen (TAA) of choice that serves as receptor for ReHV entry into the cancer cell. ReHVs do not cause off-target infections in preclinical models, are fully replication-competent and able to contrast the antiviral innate responses they elicit, and prime T cells against tumors.
Methods
We developed an ReHV-mediated immunotherapeutic platform (Re-IP) that consists of thymidine kinase-positive cancer cells ex vivo infected with ad hoc designed HER2-tropic ReHV implanted ectopically to immunize mice against cancer without direct tumor treatment.
Results
In a therapeutic-like setting, Re-IP robustly primed anticancer T cells that infiltrated distant untreated tumors and inhibited their growth. Tumor growth inhibition required CD8+ cells. Re-IP vaccinated against both the gnostic TAA (here HER2) employed for ReHV retargeting and a broader repertoire of agnostic tumor antigens, also sensitizing tumors to checkpoint blockade. Ectopically implanted uninfected cancer cells failed to elicit an immune response, highlighting the adjuvant effect of ReHV infection. Re-IP was effective in herpes simplex virus (HSV)-preimmune mice, unlike systemic treatments with oncolytic HSVs, which are blunted by prior antiviral immunity. Re-IP safety rested in the absence of replicating virus in off-target tissues and in tumors whose growth was inhibited.
Conclusions
Ectopically administered Re-IP adjuvants cancer cells’ immunogenicity without the need for direct tumor treatment. The induced T-cell immunity inhibits the growth of distant untreated tumors and remains effective in HSV-preimmune mice. In humans, this approach might be applied to elicit anticancer T-cell responses against hard-to-reach, unresectable, or metastatic lesions and to enhance immune cell activation and expansion in adoptive therapies.
Keywords: Oncolytic virus, Immunotherapy, Vaccine
WHAT IS ALREADY KNOWN ON THIS TOPIC
Anticancer immunotherapy seeks to overcome the tumor microenvironment immunosuppression and activate the patient’s own immune system against cancer. Oncolytic viruses enhance this response by vaccinating patients against both gnostic and agnostic antigens, though their efficacy depends on the virus type, modifications, and administration route.
WHAT THIS STUDY ADDS
The ex vivo infection of suitable cancer cells with an HER2-retargeted retargeted oncolytic herpes simplex viruses (ReHV) and their ectopic implantation resulted in priming of anticancer T cells that infiltrated distant untreated tumors and inhibited their growth. These findings provide a promising proof-of-principle option for boosting a broad antitumor T response to gnostic and agnostic antigens without the need to directly treat the tumors. ReHV-mediated immunotherapeutic platform (Re-IP) demonstrates superior efficacy and potency compared with other viruses in similar platforms, is effective despite prior antiviral immunity, and is safe, with no off-target effects.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This preclinical study suggests a potential re-evaluation and re-positioning of whole cell-based vaccines, previously limited by low immunogenicity. Re-IP remains effective in herpes simplex virus (HSV)-preimmune mice, overcoming a key barrier faced by systemic oncolytic HSV therapy and other oncolytic viruses derived from common human viruses (eg, measles, human adenoviruses) or animal viruses after repeated use. This platform offers a novel strategy for immunizing at extratumor sites, targeting untreatable cancers and enhancing adoptive cell therapies.
Background
Cancer immunotherapy consists of the passive administration of immune molecules or cells, or the active elicitation of immune response through vaccination.1 2 Vaccines can be formulated with autologous or allogeneic whole cells, antigens or genetic material, each with advantages and disadvantages.3 4 A prerequisite and limitation of genetic and peptide vaccines is the need for prior identification and validation of cancer antigens. Conversely, whole cell platforms have the advantage of not requiring the prior identification of antigen, immunizing also against unidentified (agnostic) antigens, but are marred by insufficient immunogenicity, that is, insufficient adjuvanticity and/or poor antigenicity.5 6 Examples of autologous vaccines include GVAX and VIGIL, formulated with the patient’s own cancer cells genetically modified to increase T-cell response.7,9 Immune cell-based vaccines include sipuleucel10 11 and the T-cell-based lifileucel.12
Non-specific stimuli to the immune system by viral or bacterial infections can enhance immune responses to cancers—or to heterologous infectious agents—leading to clinical benefits for patients. Supporting evidence includes the historic Coley’s toxin, BCG for bladder cancer treatment,13 and viral infections that induced short-lived remissions in human cancers, repeatedly reported also in COVID-19 patients.14 15 These considerations prompted efforts to employ widely used and safe antiviral vaccines to elicit anticancer immune responses.16 17
Oncolytic viruses (OVs) are genetically modified or natural viruses introduced as anticancer agents,18,20 with some clinically approved for specific indications.21 22 The retargeted oncolytic herpes simplex viruses (ReHVs), generated in our laboratory, are cancer-specific OVs. They are tropism-retargeted to a cancer-specific tumor-associated antigen (TAA) of choice (eg, HER2, PSMA, EGFR, EGFRVIII) that serves as receptor for ReHV entry into the cell, no longer infect through the natural herpes simplex virus (HSV) receptors nectin 1 or herpesvirus entry mediator (HVEM), carry no attenuation, and are fully replication-competent.23,27 ReHVs induce durable anticancer immunity.28 29 Their specific tropism ensures that they do not cause off-tumor and off-target infections in murine models.29 30 In patients, OncoVEXGM-CSF has shown off-tumor spread.31
OVs serve as stimuli to the immune system and as immunotherapeutic agents by their ability to turn immunologically “cold” tumors into “hot” through modifications of the otherwise immunosuppressive TME. In this way, they contribute to inhibiting the growth of treated tumors and reducing the growth of distant untreated tumors, a phenomenon defined as oncolytic immunotherapy.632,36 The underlying mechanisms vary among different OVs, particularly between large DNA viruses, such as HSV, and small RNA viruses.6 33 37 The oncolytic immunotherapy property was exploited in the design of infected cell vaccine platforms that consisted of cells infected with vesiculoviruses, in particular maraba virus (MARAV), VSV-IFNβ and NDV, implanted at extratumor sites to induce systemic antitumor immunity.38,40 Recent studies have shown that vesiculovirus-based platform exerts only moderate T-cell priming efficiency.37 In contrast, herpesvirus-based OVs stand as quite proficient; clinical validation of their immunotherapeutic effects is evident from the durable and distant responses observed with OncoVEXGM-CSF treatment,21 41 its successful application as neoadjuvant,42 and the link in patients with glioblastoma between survival and antiviral immune profiles. In a totally different approach, the human cytomegalovirus—also a herpesvirus— is being investigated for its ability to favor strong T-cell responses within the OV context.43
ReHVs orchestrate several changes to the tumor immune profile, shifting it toward a Th1-antitumor phenotype. These changes include an increase in infiltrating effector CD8+ T cells, a decrease in intratumoral T regulatory cells, enhanced expression of co-immunostimulatory markers and downregulation of Ido1, activation of the interferon (IFN)-γ cascade, expression of proinflammatory cytokines, of IFN-α and IFN-β and their pathways, and of tumor necrosis factors.26 30 44 The resulting T-cell response is directed against both the selected TAA and agnostic tumor cell antigens, likely driven by increased antigenicity—viral and cellular proteins become accessible to dendritic cells (DCs) and other antigen-presenting cells following virus-induced immunogenic cell death—and enhanced adjuvanticity, as pattern recognition receptors activation and danger signals generate a proinflammatory microenvironment that supports the initiation of adaptive immune responses. These antitumor effects can be strengthened by engineering the virus to make infected cells produce immune-boosting molecules, such as interleukin-12 (IL-12),27 28 granulocyte-macrophage colony-stimulating factor (GM-CSF), or IFN; see study performed by Wang et al.45 Collectively, the intratumoral infection with ReHVs likely acts as an adjuvant and contributes to prime the anticancer T-cell response.
OncoVEXGM-CSF has shown clinical efficacy against distant tumors.21 41 46 However, it requires intratumoral administration and not all types of distant tumors respond. There is a clinical need to extend the benefits of OV-promoted T-cell responses to patients whose cancers cannot be treated in situ, to broadly enhance anticancer immunity to patients, and to boost the immune response to recipients of adoptive cell therapies. The aims of the current study were to ascertain whether ReHVs can adjuvant a therapeutic immune response also on ectopic injection of ex vivo-infected cancer cells, that is, in the absence of direct tumor treatment, while providing an adequate safety profile, and to ascertain whether the elicited response is sufficient to inhibit the growth of distant untreated tumors.
Materials and methods
Virus and cells
SK-OV-3, CT-26 (wt-CT26), CT26-HER2, and RS cells were described.26 CT26-HER2-TK cells were generated by transduction with a lentivirus encoding the HSV-1 thymidine kinase (TK), amplified with primers TK_F and TK_R as described26 and detailed in online supplemental information. TK conferred susceptibility to acyclovir (ACV) quantified by means of alamarBlue Cell Viability Reagent (Thermo Fisher Scientific).47 The HA-CT26-16pep construct was designed by joining back-to-back 16 aminoacidic sequences (25–27 amino acids in length), linked by SGSG spacers, with an initial methionine and an HA-tag at the C-terminus, as detailed in online supplemental information. The sequences included in HA-CT26-16pep were: Seq #4, 5, 10, 11, 18, 23, and 28 from48; CT26-M20, CT26-M27, and CT26-M68 from49; Smc3 (IETQQRKFKASRASILSEMKMLKEK) derived from50 CT26-ME2, CT26-ME3, CT26-ME5, CT26-ME8, and CT26-ME10 from.49 The DNA was synthesized (Genart, Thermo Fisher Scientific) and cloned into pCDNA3.1(−). R-375 was derived from R-337 by GalK recombineering51 52 as detailed in online supplemental information. CT26-HER2-TK-pep cells were generated from CT26-HER2-TK cells by transfection with pCDNA3.1(−)-HA-CT26-16pep, followed by selection with neomycin. Secretion of mIL-12p70 was determined by ELISA.47
In vivo experiments
Animal experiments were performed according to European directive 2010/63/UE and Italian laws 26/2014. The experimental protocols were reviewed and approved by the University of Bologna Animal Care and Use Committee (‘‘Comitato per il Benessere degli Animali”, CoBA) and approved by the Ministry of Health with Ethics Approval Code No. 63/2021-PR to Professor Anna Zaghini on January 28, 2021. BALB/c mice transgenic for human HER2 (BALB/c-TG) were described.47 Both male and female mice were enrolled. The experimental endpoints were described.47 The establishment and monitoring of subcutaneous tumors was described.44 The ReHV-mediated immunotherapeutic platform (Re-IP) regimen, combined with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), depletion of CD4+ and CD8+ cells are detailed in online supplemental information. Ex vivo and statistical analyses are detailed in online supplemental information.
Results
Engineering of an ad hoc designed HER2-retargeted oHSV (HER2-ReHV) for CT26 tumor targeting
In previous experiments, we reported that intratumoral (i.t.) administration of the HER2-retargeted ReHV named R-337, armed with IL-12p70 fusion protein,29 resulted in antitumor efficacy against both the primary treated tumor and distant untreated challenge tumor, due in part to immunotherapeutic effects.29 44 R-337 was improved as follows, thus generating R-375 (figure 1A): deletion of the α−47 open reding frame to increase major histocompatibility complex (MHC)-I antigen presentation in humans and improve MHC-dependent immune recognition; a ~3,000 nucleotide deletion in each copy of the latency-associated transcript (LAT) locus to provide genomic space for transgene addition; shift of the mIL-12p70 gene to the UL26-UL27 intergenic locus; insertion of an HA-tagged sequence encoding 16 known neoantigens from CT26 cancer cells (referred to as HA-CT26-16pep)48,50 under the control of the HCMV promoter in the US1-US2 intergenic locus. The expression of the HA-CT26-16pep in R-375-infected SK-OV-3 cells was confirmed by western blotting and immunofluorescence assay (figure 1B); the expression level was comparable to that induced by R-337 (figure 1C). To confer safety to the immunotherapeutic platform, we engineered the HSV TK suicide gene into CT26-HER2 cells and generated the CT26-HER2-TK cells sensitive to ACV (figure 1D).
Figure 1. Genome organization of R-375, production of HA-CT26-16pep and mIL-12 p70, and efficacy of the retargeted oncolytic herpes simplex viruses-based immunotherapy platform (Re-IP) in a prophylactic setting. (A) Schematic of R-375 genome: insertion sites for GCN4 in gB, mIL-12p70, EGFP, CT26 16 epitopes’ string with HA-tag (HA-CT26-16pep), and scFv to HER2 in the gD gene with deletion of amino acids 30 and 3829; deletions in the LAT regions (∆LAT) and α−47 ORF. (B) HA-CT26-16pep expression in SK-OV-3 cells infected with R-375, R-337, or uninfected by western blot (WB) and immunofluorescence (IFA) using an anti-HA antibody. (C) mIL-12p70 quantification in supernatants of SK-OV-3 cells at 24 or 48 hours post-infection. (D) CT26-HER2 or CT26-HER2-TK cell viability after 48 hours treatment with acyclovir, assessed by alamarBlue and expressed as the percentage of viable cells relative to the corresponding untreated cells. (E–K) Re-IP efficacy in a prophylactic regimen. (E) Treatment: mice received three i.p. (intraperitoneal) doses of Re-IP (2×106 CT26-HER2-TK cells infected ex vivo with R-375 for each injection) or vehicle on days 7, 5, and 3 days before tumor engraftment, and then subcutaneously engrafted with CT26-HER2 tumors (5×105 CT26-HER2 cells). Vehicle: n=5; Re-IP: n=7. (F, G) Tumor growth kinetics, with numbers of mice showing complete response (CR, tumor clearance or volume <80 mm3 throughout the experiment) and partial response (PR, tumor volume <50% of the vehicle group for at least two consecutive measurements). (H) Tumor volumes on day 28. (I) Kaplan-Meier survival curve. (J, K) Immune response analysis: splenocyte response against CT26-HER2 and wt-CT26 cells measured by IFN-γ secretion (J) and serum antibodies (K) measured by flow cytofluorimetry and expressed as mean fluorescence intensity (MFI). (L) Body weight values of mice in the vehicle and Re-IP groups, measured from the day of tumor engraftment. Average body weight of tumor-free mice was included as a reference (gray line). (H–K) Statistics: Mann-Whitney test (Shapiro-Wilk test for normality distribution failed) (H), log-rank Mantel-Cox test (I), Welch’s test (Shapiro-Wilk test passed, F test for variance equality failed) (J, K). IFN, interferon; LAT, latency-associated transcript.
Tumor growth inhibition and anticancer immune response by prophylactic ReHV-mediated immunotherapy platform
The first series of in vivo experiments aimed to ascertain whether immunotherapeutic protection could be achieved without direct tumor treatment in a prophylactic setting, that is, before tumor engraftment and in the absence of structured TME. CT26-HER2-TK cells were infected ex vivo with R-375 and injected intraperitoneally (i.p.) 7, 5, and 3 days prior to tumor engraftment, to mimic a prophylactic setting. Mice were then implanted subcutaneously (s.c.) with untreated CT26-HER2 cells (schedule depicted in figure 1E). Since the s.c. tumor was not treated directly with R-375, any reduction in tumor growth in this setting would be immune-mediated and result from the T-cell response elicited by the i.p.-injected ex vivo-infected tumor cells. As shown in figure 1F,G, the prophylactic treatment resulted in complete responses (CR) in five out of seven mice and partial responses (PR) in two mice, thus achieving 100% response rate in the treated group. Figure 1H shows a highly significant reduction in tumor size in Re-IP-treated mice on day 28. Kaplan-Meier survival analysis showed a 70% survival rate (figure 1I). Furthermore, the treatment elicited a robust T-cell response, as evidenced by splenocyte reactivity to both CT26-HER2 and wt-CT26 cells (figure 1J), indicating that Re-IP primed the immune system to recognize both HER2-CT26 and wt-CT26 tumor antigen repertoire. The treatment also elicited an antibody (Ab) response, primarily directed against CT26-HER2 cells and to a lesser extent against wt-CT26 cells (figure 1K). The treatment did not cause any significant decrease in average body weight (figure 1L), arguing against systemic toxicity.
Therapeutic protection by early Re-IP
Next, we ascertained whether the ReHV-trained immunotherapy platform could confer immune protection also in a therapeutic-like setting. Mice were s.c. engrafted with CT26-HER2 and wt-CT26 tumors, on opposite flanks. 1, 8, and 12 days later they received i.p. injections of Re-IP, followed by ACV, that killed both the CT26-HER2-TK cells and R-375 (schedule in figure 2A). Again, we underscore that the s.c. tumors were not directly treated with R-375 and, because they lacked the suicide TK gene, were unaffected by ACV. Re-IP inhibited the CT26-HER2 tumor growth in 100% of the treated mice (four CR and four PR, figure 2B–D). Surprisingly, the growth of wt-CT26 tumors was also inhibited in the majority of treated mice (two CR and five PR, figure 2E–G). The difference in tumor size between the treated and control groups on day 23 was highly significant for both tumors (figure 2D,G). The T-cell response, measured as splenocyte reactivity, was robust against both CT26-HER2 and wt-CT26 antigens (figure 2H), suggesting that Re-IP also primed the immune system against the wt-CT26 tumor antigen repertoire and effectively functioned as a vaccine against the untreated CT26 tumors. Importantly, the T-cell response to both HER2+-CT26 and wt-CT26 cells correlated with the observed antitumor effects. In contrast to the T response, the Ab response was strong against CT26-HER2 but low to null against wt-CT26 cells (figure 2I), indicating poor correlation with the therapeutic outcome. Because the mice were transgenic/tolerant to human HER2, Re-IP vaccination broke tolerance to HER2. The treatment did not cause any decrease in average body weight (figure 2J), again arguing against systemic toxicity. Cumulatively, these results document for the first time that tumor growth inhibition, along with robust T-cell and B-cell responses, can be achieved without direct tumor treatment when a retargeted oncolytic herpesvirus is employed for ex vivo infection of tumor cells to prime a systemic immune response.
Figure 2. Re-IP efficacy in early therapeutic setting. (A) Treatment: mice engrafted with CT26-HER2 and wt-CT26 tumors (3×105 cells) in opposite flanks received three intraperitoneally doses of Re-IP (3×106 infected cells/injection) or vehicle, plus two doses of acyclovir to kill thymidine kinase-positive Re-IP cells and R-375. Vehicle: n=5; Re-IP: n=8. (B, C) CT26-HER2 tumor growth kinetics, with CR and PR. (D) CT26-HER2 tumor volumes on day 23. (E, F) wt-CT26 tumor growth kinetics, with CR and PR. (G) wt-CT26 tumor volumes on day 23. (H, I) Immune response as splenocyte (H) and serum (I) reactivity. (J) Average body weight values of mice in the vehicle and Re-IP groups. (D, G–I) Statistics: Mann-Whitney test (D, G, I), two-tailed t-test (Shapiro-Wilk test passed, F test passed) (H). CR, complete response; IFN, interferon; MFI, mean fluorescence intensity; PR, partial response; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform.
Protection by Re-IP in combination with checkpoint inhibitor (CPI) is effective in a later therapeutic-like setting
In humans, cancer treatments are administered to patients with established tumors. To mimic a therapeutic setting, the Re-IP schedule shown in figure 2 was applied to mice implanted with tumors 5 days earlier. The cumulative 21-day time frame made it difficult to apply a longer interval between tumor implantation and start of the therapy. The regimen included three Re-IP+ACV treatments, combined with anti-CTLA-4 monoclonal antibody (MAb) (figure 3A). As shown in figure 3B–D, Re-IP led to a strong decrease in CT26-HER2 tumor growth (5/8 CR, 3/8 PR). The anti-CTLA-4 monotherapy exerted only a minor effect (0/7 CR, 4/7 PR). Remarkably, a highly significant protection was also observed towards wt-CT26 tumors (figure 3E–G), which express the tumor antigens but not the TAA. The survival curve showed that about 60% of the mice were fully protected from both tumors (figure 3H). The remaining Re-IP-treated mice succumbed to the wt-CT26 tumors, while CT26-HER2 tumors were present but far from the experimental endpoint. Similarly to the previous experiment, the strong T-cell response seen in splenocytes was directed to both CT26-HER2 cells and the wt-CT26 agnostic antigens in (figure 3I), whereas the Ab response was significant only against CT26-HER2 tumors (figure 3J). Cumulatively, Re-IP was effective in a therapeutic-like setting.
Figure 3. Re-IP efficacy in a therapeutic-like setting starting on day 5 after tumor implantation and combination with anti-CTLA-4 CPI. (A) Treatment: mice engrafted with CT26-HER2 and wt-CT26 tumors (3×105 cells) received three intraperitoneally doses of Re-IP (3×106 infected cells/injection) or vehicle, plus acyclovir and anti-CTLA-4 (α-CTLA-4) or vehicle. Vehicle: n=11; vehicle+α-CTLA-4: n=7; Re-IP+α-CTLA-4: n=8. (B–D) CT26-HER2 tumor growth kinetics, with CR and PR. (E–G) wt-CT26 tumor growth kinetics, with CR and PR. (H) Kaplan-Meier survival curve. (I, J) Immune response, as splenocyte (I) and serum (J) reactivity. (H–J) Statistics: log-rank Mantel-Cox test corrected for multiple comparisons comparing all groups (H) and the Kruskal-Wallis test with Dunn’s correction (Shapiro-Wilk test failed) comparing each group to vehicle (I, J). CPI, checkpoint inhibitor; CR, complete response; CTLA-4, cytotoxic T-lymphocyte associated protein 4; IFN, interferon; MFI, mean fluorescence intensity; PR, partial response; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform.
To ascertain whether the Re-IP immune protection was long-lasting, the CR mice were re-challenged 7 weeks later simultaneously with CT26-HER2 and wt-CT26 tumors. Online supplemental figure 1 shows that 100% of the re-challenged mice were fully protected and did not develop any tumor, whereas all the control naïve mice developed tumors (online supplemental figure 1A–D). The results indicate that Re-IP conferred durable protection, most likely through the activation of memory T cells.
Mechanistic insight: contribution of single Re-IP components
Next, we investigated the specific contribution of each Re-IP component to anticancer efficacy and immunotherapy. We first assessed the role of ReHV infection by testing whether uninfected CT26-HER-TK cells sufficed to elicit a T-cell response and inhibit tumor growth. To this end, mice received three i.p. doses of CT26-HER-TK cells without prior ex vivo infection with R-375, plus ACV. The schedule (figure 4A) mirrored that shown in figure 3, except that infection was omitted. Tumor growth kinetics (figure 4B–G) and survival curves (figure 4H) showed that i.p. treatment with uninfected CT26-HER2-TK/ACV did not significantly reduce the growth of either HER2-expressing or HER2-negative tumors, nor did it improve survival or trigger a detectable T-cell response (figure 4I). Although treated mice developed antitumor Abs (figure 4J), these did not correlate with therapeutic benefit, consistent with observations in figure 3. These findings underscore that infection with R-375 was critical and provided the essential adjuvant functions to CT26-HER2-TK cells for priming the effective T-cell response.
Figure 4. Contribution of Re-IP component. (A–J) Effects of uninfected cells platform. (A) Mice engrafted with CT26-HER2 and wt-CT26 tumors (3×105 cells) received three i.p. doses of uninfected CT26-HER2-TK cells (3×106 cells/injection), followed by acyclovir and α-CTLA4. Vehicle+α-CTLA4: n=5; uninfected CT26-HER2-TK cells+α-CTLA4: n=7. (B–C) CT26-HER2 tumor growth kinetics, with CR and PR. (D) CT26-HER2 tumor volumes on day 21. (E–F) wt-CT26 tumor growth kinetics, no CR or PR. (G) wt-CT26 tumor volumes on day 21. (H) Kaplan-Meier survival. (I, J) Immune response: splenocyte (I) and serum (J) reactivity. (L–U) Antitumoral effects of Re-IP with R-371 or HA-CT26-peptide-expressing uninfected cells. (L) Mice engrafted with CT26-HER2 tumors received three i.p. doses of either CT26-HER2-TK infected with R-371 virus (purple) or the uninfected CT26-HER2-TK cells expressing HA-CT26-peptide (orange), followed by acyclovir. (L–O) Antitumor effects of Re-IP with R-371 expressing the mIL-12p70 payload only. Vehicle: n=8; Re-IP with R-371: n=5. (L, M) Tumor growth kinetics and (N) tumor volumes on day 27. (O) Kaplan-Meier survival. (P–U) Effects of uninfected cells expressing HA-CT26-16peptide on tumor growth. Vehicle: n=5; uninfected CT26-HER2-TK-pep: n=5. (P) HA-CT26-peptide expression confirmed by αHA flow cytometry. (Q, R) Tumor growth kinetics and (S) tumor volumes on day 18. (T) Kaplan-Meier survival. (U) Immune response: splenocyte reactivity. (D, G–J, O, P, S, T) Statistics: Mann-Whitney test (D, J, O, U), two-tailed t-test (G, I, S), log-rank Mantel-Cox test (H, P, T). CR, complete response; CTLA-4, cytotoxic T-lymphocyte associated protein 4; IFN, interferon; i.p., intraperitoneally; MFI, mean fluorescence intensity; PR, partial response; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform; TK, thymidine kinase.
IL-12 is a potent immunostimulatory cytokine and does not cause systemic toxicity when expressed as a viral payload.53 Several studies from our laboratory demonstrated that in s.c. cancers treated i.t. with ReHVs, virus-encoded IL-12p70 greatly contributed to immune protection and reduced tumor growth.27,29 To investigate the enhancing role of IL-12p70 in the Re-IP setting, we engineered R-371, an ReHV that carries the same genetic modifications as R-375—hence encodes mIL-12p70—but lacks the HA-CT26-16pep and employed it in Re-IP (figure 4K). Figure 4L–N shows that the regimen partially reduced tumor growth, as seen also in the survival curve (figure 4O); this extends the evidence on the immune potentiating effect of the virus-encoded mIL-12p70 to the immunotherapeutic platform.
To pinpoint the contribution of the polypeptide that encodes multiple CT26 cell epitopes, we engineered CT26-HER2-TK cells that constitutively express HA-CT26-16pep under the CMV promoter (CT26-HER2-TK-pep). Peptide expression was confirmed by flow cytometry (figure 4P). Mice bearing HER2-positive tumors received three i.p. injections of these cells, followed by ACV (figure 4K). As shown in figure 4Q,R, CT26-HER2-TK-pep cells reduced tumor growth to a limited extent, with 1/5 mouse achieving CR and 1/5 PR. However, differences in tumor volume (Figure 4S) and survival curves (figure 4T) were not significantly different. The splenic reactivity against tumor cells was also limited (figure 4U). These results suggest a moderate contribution of the selected multiepitope string to Re-IP efficacy outside the infected cell context.
Cumulatively, the results highlight the critical contribution of the ex vivo infection with ReHVs, extend the immune-potentiating role of mIL-12p70 to Re-IP, and argue in favor of a moderate effect of the multiepitope peptide in the absence of infection, hinting that Re-IP likely mounted T response against wt-CT26 cell antigenic repertoire through immunogenic cell death and virus adjuvant functions.
Re-IP safety
The observed inhibition of wt-CT26 tumor growth (figures2 3) cannot be ascribed to direct infection with R-375 leaked from the Re-IP site, since the virus strictly requires HER2 as portal of entry and cannot infect wt-CT26 cells. To further assess the safety of the platform, we investigated whether the growth inhibition of CT26-HER2 tumors was caused by R-375 possibly escaped from the infected CT26-HER2-TK cells, by searching for replicating R-375 in the s.c. tumor specimens. Mice bearing both CT26-HER2 and wt-CT26 tumors received a single Re-IP dose (ACV treatments were purposely omitted to maximize the presence of virus that might escape from the vaccination site, if any) as well as the anti-CTLA-4 MAb (figure 5A). R-375 replication was quantified in tumor specimen by reverse transcription quantitative polymerase chain reaction (RT-qPCR) to glycoprotein C (gC), an abundant late viral protein. Neither CT26-HER2 nor wt-CT26 tumor specimens expressed gC messenger RNA (mRNA) (figure 5B), ruling out leakage of replicating virus from the vaccination site to s.c. tumors. As a further safety parameter, we checked whether HER2-positive tumor cells or infectious R-375 were present in the peritoneal liquid or adherent to peritoneal tissues on a complete Re-IP regimen in tumor-free mice (figure 5C). The results show no detectable CT26-HER2-TK cells or infectious R-375, measured as plaque-forming units (PFUs), in the peritoneal wash or in peritoneal tissues (figure 5D–F). These findings rule out viral leakage or replication beyond that in the Re-IP cells. Together with the lack of systemic toxicity as inferred by the body weight curves, the results argue in favor of the safety of the Re-IP platform.
Figure 5. Safety of Re-IP. (A, B) Absence of replicating R-375 in CT26-HER2 and wt-CT26 tumors. (A) Mice engrafted with CT26-HER2 and wt-CT26 tumors (3×105 cells) received a single i.p. dose of Re-IP (3×106 infected cells) or vehicle. Vehicle: n=2; Re-IP: n=6. (B) Replicating R-375 in tumors was assessed by RT-qPCR for glycoprotein C messenger RNA. (C–F) Absence of CT26-HER2+ cells and infectious R-375 in peritoneal specimens. (C) Tumor-free mice received three i.p. doses of Re-IP or vehicle plus acyclovir and α-CTLA-4. (D) Flow cytometry analysis of peritoneal washing, showing HER2+ cells percentages. (E) Infectious R-375 titration of peritoneal washing (PFU/mL). (F) Flow cytometry analysis of single-cell suspensions from peritoneal tissues, showing HER2+ cell percentages. CTLA-4, cytotoxic T-lymphocyte associated protein 4; gC, glycoprotein C; HSV, herpes simplex virus; i.p., intraperitoneally; PFU, plaque-forming unit; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Re-IP efficacy is immune-mediated
To provide formal proof that the Re-IP-mediated anticancer effects were immune-mediated, we investigated immune cell infiltration into the tumors and the effect of depletions of specific immune cell subpopulations. Mice bearing both CT26-HER2 and wt-CT26 tumors received the Re-IP treatment, including ACV (schedule in figure 6A). Both CT26-HER2 and the wt-CT26 tumors showed a marked increase in tumor-infiltrating lymphocytes in the Re-IP arm, with a notable increase in CD8-positive subpopulations and, to a lesser extent, CD4-positive cells (figure 6B–D). The natural killer (NK) cell infiltration was modest, as expected,29 and yet significantly increased (wt-CT26) or tended to increase (CT26-HER2) in the treated group.
Figure 6. Re-IP efficacy is immune-mediated. (A–D). Tumor infiltration by CD4, CD8, and NK cells. (A) Mice engrafted with wt-CT26 tumors (3×105 cells) received Re-IP (9×106 infected cells/injection) or vehicle, plus acyclovir. At sacrifice, tumor samples were processed into single-cell suspensions, tumor-infiltrating leukocytes were isolated by Ficoll centrifugation and analyzed by flow cytometry. Vehicle: n=3; Re-IP: n=3. (B–D) Percentages of CD4+ (B), CD8+ (C), and CD335+ (D) cells within the CD45+ population. (E–S). CD4 and CD8 depletion effects on Re-IP efficacy. (E) Treatment as in (A) with α-CD4 or α-CD8 antibodies plus αCTLA-4. Vehicle: n=8, Re-IP: n=8, CD4-depleted: n=7; CD8-depleted: n=8. (F–I) CT26-HER2 tumor growth kinetics, with CR and PR. (J) CT26-HER2 tumor volumes on day 32. (K–N) wt-CT26 tumor growth kinetics, with CR and PR. (O) wt-CT26 tumor volumes on day 26. (P, Q) Immune response as splenocyte (P) and serum (Q) reactivity. (R, S) Splenic CD4+ (R) or CD8+ (S) cell counts. (B–D, J, O–S) Statistics: two-tailed t-test (B–D), Kruskal-Wallis test with Dunn’s correction comparing each group to vehicle (J, O, P, R), Brown-Forsythe and Welch analysis of variance with Dunnett’s T3 test (Shapiro-Wilk test passed, Bartlett’s test failed) comparing each group to vehicle (Q, S). CR, complete response; CTLA-4, cytotoxic T-lymphocyte associated protein 4; IFN, interferon; MFI, mean fluorescence intensity; PR, partial response; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform.
A depletion experiment was carried out in mice treated as in previous experiments plus αCD4 or αCD8 MAbs (figure 6E). To enhance the therapeutic effect, a higher number of infected cells was administered at each i.p. injection. CD8 depletion completely abrogated the anticancer effect induced by Re-IP, while CD4 depletion had no significant impact on tumor growth inhibition (figure 6F–J and K–O for CT26-HER2 or wt-CT26 tumors, respectively). Splenocyte reactivity to CT26-HER2 and to wt-CT26 in the vaccinee arm was abrogated by CD8 cell depletion (figure 6P, compare red to violet circles). Serum Ab reactivity to CT26-HER2 was decreased by CD4 but not by CD8 cell depletion, as expected (figure 6Q). The counts reported in figure 6R,S confirm the specificity of the depletion, although depleting one subpopulation slightly affected the other non-depleted population. Altogether, the results confirm that the anticancer efficacy exerted by Re-IP was primarily mediated by effector CD8+T cells.
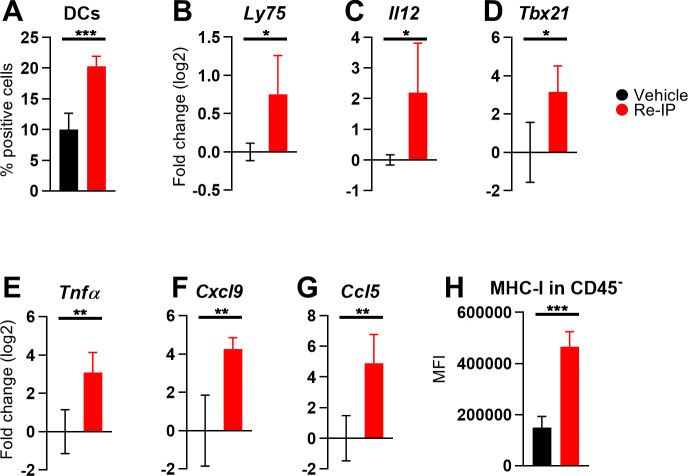
Next, we analyzed immune cells and related factors in TME by flow cytometry and RT-qPCR. We observed an increase in the CD103+ DC subset (figure 7A), together with elevated Ly75 and cell-encoded Il12 mRNA levels (figure 7B,C), suggesting activation of cross-presenting cDC1s. The accumulation of Tbx21, Tnfα (figure 7D,E), and Il12 transcripts points to a polarization of CD4+ T cells toward an antitumor Th1 phenotype. Moreover, the overexpression of Cxcl9, Ccl5 (figure 7F,G), and Tbx21 in tumor specimens from Re-IP-treated mice highlights an inflamed TME, recruitment and activation of effector immune populations, including CD8+ T cells and NK cells. Finally, MHC-I upregulation in the tumor and stromal CD45− populations suggests enhanced tumor immunogenicity (figure 7H).
Figure 7. Immune modifications in the tumor microenvironment induced by Re-IP monotherapy. Mice, tumor engraftment, and treatments are described in figure 6. For RT-qPCR, tumor homogenates were employed for RNA extraction; samples for flow cytometry were processed as detailed in figure 6. Vehicle: n=4; Re-IP: n=4. (A) Percentage of CD103+ cells within the CD45+ population. (B–G) RT-qPCR data on complementary DNA from total RNA using TaqMan probes specific for Ly75 (B), Il12 (C), Tbx21 (D), Tnfα (E), Cxcl9 (F), and Ccl5 (G). Data are normalized to the Rpl13a housekeeping gene and expressed as log2 fold change relative to the means of vehicle group. (H) MFI values of MHC-I on CD45− population by flow cytometry. (A–H) Statistics: two-tailed t-test. DC, dendritic cell; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Expansion of antigenic repertoire
The above results underscore the potent T-cell priming induced by Re-IP against CT26 tumors. To explore whether the antigenic repertoire of the vaccine can be expanded, we included a lysate of Renca cancer cells in the Re-IP treatment and tested the therapy on mice bearing CT26-HER2, wt-CT26, and Renca tumors (schedule in online supplemental figure 2A). As expected, the treatment led to partial inhibition of CT26-HER2 and wt-CT26 tumor growth (online supplemental figure 2B–G). In addition, 40% of Renca tumors exhibited delayed growth (online supplemental figure 2H–J), with a significant reduction in tumor size in the Re-IP vaccinee arm. Since most mice died from wt-CT26 tumors, a Renca-adjusted Kaplan-Meier survival curve was calculated, by considering only Renca-related deaths or estimating when the Renca tumor would have reached the endpoint volume of 1,500 mm3. This Renca-adjusted Kaplan-Meier graph showed significant survival benefit in the Re-IP vaccinee group compared with control (online supplemental figure 2K). Splenocytes displayed reactivity to Renca cells (online supplemental figure 2L), in addition to the expected reactivity against CT26-HER2 and wt-CT26 cells. The Re-IP+Renca lysate also stimulated the development of Abs against all three types of tumor cells (online supplemental figure 2M). These findings hint that the addition of a heterologous lysate during the Re-IP treatment can effectively expand the repertoire of vaccinal epitopes, potentially enhancing the immune response against multiple tumor types.
Re-IP is efficacious in HSV-preimmune (IMM) mice
A high percentage of the human population is seropositive for HSV. The extent to which prior immunity modifies the efficacy of oncolytic herpes simplex viruses (oHSVs) is a debated issue.2754,56 To preliminarily assess whether Re-IP could be effective in HSV-seropositive mice, they were immunized to HSV before tumor engraftment and standard Re-IP treatment (figure 8A). Anti-HSV-Ab and HSV-neutralizing Abs were quantified (figure 8B,C). Re-IP efficacy was tested separately for wt-CT26 and CT26-HER2 tumors due to different growth rates.
Figure 8. Re-IP is effective in HSV-preimmune mice. (A) CT26-HER2 tumors: mice were immunized to HSV with two intraperitoneally injections of purified HSV-1(F) virions (1×105 PFUs replication-competent+2×105 PFUs UV-inactivated) 15 and 8 days before tumor implantation. On day −1, anti-HSV-1 immunity was assessed in naïve and immunized mice. On day 0, mice were implanted with CT26-HER2 tumors (6×105 cells) and treated with Re-IP (9×106 infected cells/injection) and acyclovir. Vehicle naïve: n=7; Re-IP naïve: n=8; vehicle HSV-immune: n=8; Re-IP HSV-immune: n=8. (B) HSV-1 antibody titers in immune mice determined by cell-ELISA, with MAb to HSV gD (HD1) and naïve mice sera. (C) Neutralizing antibody (NAb) titers assessed by plaque reduction assay using R-8102 (HSV-1(F)-derived β-galactosidase virus) in the presence of serially diluted sera, with IC50 from infection inhibition curves. (D–G) CT26-HER2 tumor growth kinetics, with CR and PR. (H) Tumor volumes on day 34. (I, J) Kaplan-Meier survival curves in naïve (I) and HSV-immune mice (J). (K, L) Immune response to CT26-HER2 cells: splenocyte reactivity (K) and serum antibodies (L). (M) wt-CT26 tumors: mice were immunized as in (A), implanted with wt-CT26 tumors (3×105 cells) and treated with Re-IP (3×106 infected cells/injection), acyclovir, and αCTLA-4. Vehicle naïve: n=10; Re-IP naïve: n=12; vehicle HSV-immune: n=7; Re-IP HSV-immune: n=9. (N–Q) wt-CT26 tumor growth kinetics, with CR and PR. (T) Tumor volumes on day 29. (S, T) Kaplan-Meier survival curves in naïve (S) and HSV-IMM mice (T). (U, V) Immune response towards wt-CT26 cells: splenocyte (U) and serum (V) reactivity. (H–L, R–V) Statistics: Mann-Whitney test (H, K, R, U), log-rank Mantel-Cox test (I, J, S, T), or Welch’s test (L, V). CR, complete response; CTLA-4, cytotoxic T-lymphocyte associated protein 4; HSV, herpes simplex virus; IFN, interferon; MAb, monoclonal antibody; MFI, mean fluorescence intensity; PR, partial response; Re-IP, retargeted oncolytic herpes simplex viruses-mediated immunotherapeutic platform.
To enhance the immune response to the CT26-HER2 tumors, the number of infected cells for each Re-IP dose was tripled. Re-IP exerted high protection in naïve mice and provided about 50% protection in HSV-immune mice (figure 8D–G). The protective effect in both HSV-naïve and HSV-immune mice was further supported by tumor volumes on day 34 and the survival curves (figure 8H–J), confirming the partial efficacy of Re-IP in HSV-immune conditions. Splenocyte activation and Ab reactivity were similar in both arms (figure 8K,L).
wt-CT26 tumor growth (schedule in figure 8M) was highly reduced in HSV-naïve mice (figure 8N,O). In HSV-immune mice, the same schedule protected about 50% of the mice (figure 8P,Q). The tumor volumes on day 29 confirmed the efficacy in both HSV-naïve and HSV-immune mice (figure 8R), as did the Kaplan-Meier curves (figure 8S,T). Concordantly, both splenocyte reactivity and Abs responses against wt-CT26 tumors in HSV-immune mice were similar to those observed in naive mice (figure 8U,V).
Discussion
Here we set-up an ReHV-based Re-IP that consists of the ectopic implantation of cancer cells ex vivo-infected with an IL-12-armed HER2-retargeted ReHV. We show that Re-IP effectively primed robust anticancer T-cell responses against both the gnostic immunodominant HER2 TAA and a broader repertoire of tumor cell antigens expressed by the HER2-negative wt-CT26 murine cancer cells. The immune response inhibited the growth of distant untreated subcutaneous tumors, regardless of whether they expressed HER2. The protection was exerted in a therapeutic-like setting, correlated with T-cell and DC infiltration and activation within the tumors, antitumoral Th1 polarization, upregulation of pro-inflammatory markers (eg, Il12, Cxcl9, Tnfα, and Ccl5), and increased tumor antigenicity, and synergized with checkpoint blockade. Formal evidence that the tumor growth inhibition was immune-mediated rested on its abrogation following CD8+cell depletion. The ex vivo-infected cells were rendered safe by the transgenic HSV TK gene that conferred sensitivity to acyclovir. The safety profile was documented through the lack of systemic adverse effects, the absence of virus from the peritoneal cavity and off-tumor tissues, and of replicating virus from s.c. distant untreated tumors.
A striking feature of Re-IP was the strong T-cell response induced by intraperitoneal administration of the ex vivo-infected cancer cells, a feature consistent with the antitumor T response observed on i.t. or systemic administration of ReHVs that results from the subversion of the TME immunosuppressive mechanisms and leads to DCs maturation and antigen presentation to T cells.29 44 57 The T-cell response elicited by the Re-IP immunotherapeutic platform and the extents of tumor growth inhibition were comparable in magnitude to those achieved with direct tumor treatments.26 29 44 Importantly, the ectopic implantation of uninfected cancer cells and their killing by ACV or the presence of CT26 epitopes in the absence of infection failed to induce the anticancer immunization, providing compelling evidence that ReHV infection served as the adjuvant in T-cell response. This was not surprising in view of the strong immune suppression strategies put in place by cancer cells.58 This requirement for ReHV infection reflects the need for multiple immune-activating signals expressed in response to viral infection, including the release of cytokines, chemokines, and interferons further enhanced by the virus-encoded mIL-12p70, the release of damage-associated molecular patterns (DAMPS) and pathogen-associated molecular patterns (PAMPS) consequent to the virus-mediated immunogenic cell death, and the subsequent recruitment and activation of immune cell populations (eg, T and NK cells). The requirement for ReHV infection in the Re-IP cells is in agreement with earlier clinical findings (eg, early G-VAX experimentation prior to GM-CSF adjuvant), in which cancer cells alone failed to vaccinate against cancer.7 Immuno-evasion of uninfected CT26 cells was documented for current murine models by a detailed immunoprofiling study.44
The capacity of herpes-based Re-IP to prime a strong anticancer T-cell response overcomes limitations encountered with earlier platforms based on vesiculoviruses. In-depth studies demonstrated that VSV-IFNβ was scarcely efficacious in priming antitumor T response unless the virus encoded a TAA (or simply ovalbumin) and, in this way, shifted the strong dominant antiviral innate response in favor of an otherwise weak-immunosubdominant antitumor T response.37 Similarly, MARAV-based vaccines were moderately efficient in priming robust T-cell response, even when armed with IL-12.40 To circumvent this limitation, MARAV has been used as a boost following TAA-armed adenovirus priming. When administered intratumorally, vesiculoviruses were additionally armed with a TAA.37 59 Although the current study was designed to provide proof-of-principle and not optimization of the platform, three doses of Re-IP were sufficient to confer strong anticancer immunization and tumor growth inhibition. In contrast, the MARAV-based platform required six doses, further highlighting its lower T-cell priming ability.40 The presence of a TAA (here HER2) in the murine cancer cells is inherent to the Re-IP model, since the TAA serves as the virus portal of entry; even more importantly, the presence of a cellular TAA is intrinsic to the human tumors for which ReHVs have been developed. We propose that the cell-encoded TAA (HER2) may have contributed to the antitumor T-priming ability, analogously to the VSV-IFNβ-encoded TAA.
At a difference from the vesiculovirus-based vaccine platform, the experimental model developed in this study allowed us to differentiate between the T response—and tumor growth inhibition—to HER2 and that to agnostic tumor antigens. Remarkably, the antitumor effector T response was addressed also against the agnostic tumor antigen repertoire. This is in accordance with previous findings on direct tumor treatments with ReHVs that elicited antitumor responses to the gnostic TAA as well as to the agnostic tumor cell antigens,26 29 44 and supports the contention that the Re-IP platform seems to overcome the limits in adjuvanticity that marred some of the whole cell-based vaccines. The gnostic T cell response to the TAA (here HER2) raises the issue of potential-related toxicities. Recent lessons argue against this concern. In humans, the central tolerance to self-antigens, including TAAs, eliminates the high-affinity T cell receptor (TCR) T cells and leaves at best low-affinity TCR T cells. If activated during gnostic vaccination and break of tolerance, these cells are not expected to cause severe off-tumor adverse effects, including autoimmune diseases. As seen in chimeric antigen receptor T cell (CAR-T) treatments, the switch from a high-affinity CAR to a low-affinity CAR strongly reduced adverse effects.60 More importantly, the thousands of patients treated with OVs, particularly oHSVs—that induce in situ vaccination—inevitably carried cancers that do express TAAs, even though patients are not stratified with respect to TAAs. Thus, melanoma (treated with T-VEC) expresses MART-1, MAGE-A3 and gp100, glioblastoma puntiforme (treated with a number of oHSVs) expresses EGFRvIII, HER2, IL-13-R2α or combinations thereof; prostate CAs express PSMA, etc. And yet, there have not been specific descriptions of autoimmune diseases induced by OVs, especially by oHSVs, and no incidence of autoimmunity across thousands of patients as reviewed in.61
To our knowledge, immunotherapeutic platforms based on ex vivo infection with oHSVs were not attempted before. When employed for direct tumor treatments, ReHVs appear to be more efficacious than OncoVEXGM-CSF. Thus, OncoVEXGM-CSF monotherapy was moderately effective in the CT26 model against the primary treated tumor and ineffective against the untreated challenge tumor.62 OncoVEXGM-CSF is armed with GM-CSF, whereas R-375 is armed with IL-12. Since the efficacy of singly armed (IL-12 or GM-CSF) ReHVs was similar one to the other,27 we attribute the superior efficacy of ReHVs relative to OncoVEXGM-CSF to differences other than the immunomodulatory cargo. In particular, ReHVs have a modified tropism and no genetic modification other than those in the glycoproteins that mediate virus entry. They are fully replication-competent and fully competent to contrast the innate immunity as they deploy the full range of genes that HSV encodes to dampen the antiviral innate responses that it elicits. This contrasts with most oHSVs, prototyped by OncoVEXGM-CSF, which carry genetic deletions or alterations in anti-innate genes, particularly the γ134.5 gene. In essence, fully competent HSVs and ReHVs set limits to their own antiviral innate responses. Considering this sophisticated herpes balance, we propose that numerous functions put in place by fully competent HSVs or ReHVs, but not by most oHSVs nor by smaller viruses like the vesiculoviruses to dampen its own antiviral innate response may act to favor the switch from antiviral innate to anticancer immunity and additionally result in higher intratumoral viral replication. Further to this, ReHVs have shown a high safety profile in mice, as they specifically target cancer cells even when administered systemically.27 30 In contrast, OncoVEXGM-CSF causes off-tumor infection in non-cancerous tissues even in clinical settings.31 Altogether, ReHVs appear to be well suited for application as ectopic infected cell-based immunotherapeutic platforms.
In the current model, the TK gene was engineered in the Re-IP cells for safety; through it, acyclovir effectively killed both the Re-IP cells and the virus. A key question centers on the clinical feasibility of using autologous cancer cells and TK transduction to derive a human Re-IP platform. The ex vivo infection with ReHV delivers into the autologous cells the virus-encoded TK gene. The TK gene might further be transduced into the cells in a site-specific fashion by CRISPR-Cas9, or, alternatively, with the protocols employed for GM-CSF transduction in human ovary cancer cells in the VIGIL platform from Gradalis (approved by US Food and Drug Administration (FDA) in 2025; www.gradalisinc.com/product/vigil-platform)63; the same is being applied to induce immunity in patients with breast cancer,64 or for the ligand to MAGE-A4 in the TCR-T therapy by Tecelra (approved by FDA in 2024; www.tecelra.com). GM-CSF transduction is also performed in the autologous G-VAX approach.65 Expression of the TK gene encoded by the non-replicative AdV named CAN 209 did not cause any adverse effect in Phase 3 clinical investigation (Candel Therapeutics; https://candeltx.com/platforms/). CAN 209 is expected to receive investigational approval from the FDA in a short time. Alternative safety options, for example, prior irradiation of cells, may also be envisioned. Ganciclovir is routinely administered to patients, even newborns, for long periods.
OVs, and in particular oHSVs, have been in clinical use or trials for about 15 years and have shown efficacy in specific indications, particularly against high mutational burden skin carcinomas. Because of the limits highlighted above, it is generally thought that more potent viruses and regimen optimization, including combination therapies, are required to improve the efficacy and spectrum of indications that can be tackled with OVs. The current findings have the potential to open new clinical options by applying ReHVs as adjuvants for immunization at extratumor sites, in order to treat hard-to-reach, unresectable or metastatic lesions, provided that a sample from the patient’s own cancer is available. Re-IP might also be employed in combination with direct ReHVs/OVs treatments or to improve the efficacy of adoptive cell therapies. Re-IPs advantage is that it works even in HSV-pre-immune animals.
Supplementary material
Footnotes
Funding: This work was supported by PRIN-MIUR to TG and ERC-POC to GC-F.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature New Biol. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesano A, Augustin R, Barrea L, et al. Advances in the understanding and therapeutic manipulation of cancer immune responsiveness: a Society for Immunotherapy of Cancer (SITC) review. J Immunother Cancer. 2025;13:e008876. doi: 10.1136/jitc-2024-008876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MJ, Svensson-Arvelund J, Lubitz GS, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer . 2022;3:911–26. doi: 10.1038/s43018-022-00418-6. [DOI] [PubMed] [Google Scholar]
- 4.Fan T, Zhang M, Yang J, et al. Therapeutic cancer vaccines: advancements, challenges, and prospects. Signal Transduct Target Ther. 2023;8:450. doi: 10.1038/s41392-023-01674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren D, Xiong S, Ren Y, et al. Advances in therapeutic cancer vaccines: Harnessing immune adjuvants for enhanced efficacy and future perspectives. Comput Struct Biotechnol J. 2024;23:1833–43. doi: 10.1016/j.csbj.2024.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell SJ, Barber GN. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell. 2018;33:599–605. doi: 10.1016/j.ccell.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006;25:321–52. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- 8.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor−transduced allogeneic cancer cellular immunotherapy: The GVAX® vaccine for prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2006;24:419–24. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Rocconi RP, Grosen EA, Ghamande SA, et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020;21:1661–72. doi: 10.1016/S1470-2045(20)30533-7. [DOI] [PubMed] [Google Scholar]
- 10.Twardowski P, Wong JYC, Pal SK, et al. Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-T alone in patients with metastatic castrate resistant prostate cancer. Cancer Treat Res Commun. 2019;19:100116. doi: 10.1016/j.ctarc.2018.100116. [DOI] [PubMed] [Google Scholar]
- 11.Madan RA, Antonarakis ES, Drake CG, et al. Putting the Pieces Together: Completing the Mechanism of Action Jigsaw for Sipuleucel-T. JNCI. 2020;112:562–73. doi: 10.1093/jnci/djaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarnaik AA, Hamid O, Khushalani NI, et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J Clin Oncol. 2021;39:2656–66. doi: 10.1200/JCO.21.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-guerin in the Treatment of Superficial Bladder Tumors. J Urol. 1976;116:180–2. doi: 10.1016/S0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 14.Bluming A, Ziegler J. REGRESSION OF BURKITT’S LYMPHOMA IN ASSOCIATION WITH MEASLES INFECTION. Lancet. 1971;298:105–6. doi: 10.1016/S0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 15.Shin DH, Gillard A, Van Wieren A, et al. Remission of liquid tumors and SARS-CoV-2 infection: A literature review. Mol Ther Oncolytics. 2022;26:135–40. doi: 10.1016/j.omto.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melero I, Gato M, Shekarian T, et al. Repurposing infectious disease vaccines for intratumoral immunotherapy. J Immunother Cancer. 2020;8:e000443. doi: 10.1136/jitc-2019-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fusciello M, Ylösmäki E, Feola S, et al. A novel cancer vaccine for melanoma based on an approved vaccine against measles, mumps, and rubella. Mol Ther Oncolytics. 2022;25:137–45. doi: 10.1016/j.omto.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter WD, Martuza RL, Feigenbaum F, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73:6319–26. doi: 10.1128/JVI.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreansky SS, He B, Gillespie GY, et al. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci U S A. 1996;93:11313–8. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toda M, Martuza RL, Kojima H, et al. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol. 1998;160:4457–64. [PubMed] [Google Scholar]
- 21.Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7:145. doi: 10.1186/s40425-019-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todo T, Ito H, Ino Y, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med. 2022;28:1630–9. doi: 10.1038/s41591-022-01897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menotti L, Avitabile E, Gatta V, et al. HSV as A Platform for the Generation of Retargeted, Armed, and Reporter-Expressing Oncolytic Viruses. Viruses . 2018;10:352. doi: 10.3390/v10070352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menotti L, Nicoletti G, Gatta V, et al. Inhibition of human tumor growth in mice by an oncolytic herpes simplex virus designed to target solely HER-2-positive cells. Proc Natl Acad Sci U S A. 2009;106:9039–44. doi: 10.1073/pnas.0812268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanni P, Gatta V, Menotti L, et al. Preclinical therapy of disseminated HER-2⁺ ovarian and breast carcinomas with a HER-2-retargeted oncolytic herpesvirus. PLoS Pathog. 2013;9:e1003155. doi: 10.1371/journal.ppat.1003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannini A, Parenti F, Bressanin D, et al. Towards a Precision Medicine Approach and In Situ Vaccination against Prostate Cancer by PSMA-Retargeted oHSV. Viruses. 2021;13:2085. doi: 10.3390/v13102085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Lucia M, Cotugno G, Bignone V, et al. Retargeted and Multi-cytokine-Armed Herpes Virus Is a Potent Cancer Endovaccine for Local and Systemic Anti-tumor Treatment. Mol Ther Oncolytics. 2020;19:253–64. doi: 10.1016/j.omto.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leoni V, Vannini A, Gatta V, et al. A fully-virulent retargeted oncolytic HSV armed with IL-12 elicits local immunity and vaccine therapy towards distant tumors. PLoS Pathog. 2018;14:e1007209. doi: 10.1371/journal.ppat.1007209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vannini A, Leoni V, Sanapo M, et al. Immunotherapeutic Efficacy of Retargeted oHSVs Designed for Propagation in an Ad Hoc Cell Line. Cancers (Basel) 2021;13:266. doi: 10.3390/cancers13020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vannini A, Parenti F, Barboni C, et al. Efficacy of Systemically Administered Retargeted Oncolytic Herpes Simplex Viruses-Clearance and Biodistribution in Naïve and HSV-Preimmune Mice. Cancers (Basel) 2023;15:4042. doi: 10.3390/cancers15164042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andtbacka RHI, Amatruda T, Nemunaitis J, et al. Biodistribution, shedding, and transmissibility of the oncolytic virus talimogene laherparepvec in patients with melanoma. EBioMedicine. 2019;47:89–97. doi: 10.1016/j.ebiom.2019.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalhout SZ, Miller DM, Emerick KS, et al. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol. 2023;20:160–77. doi: 10.1038/s41571-022-00719-w. [DOI] [PubMed] [Google Scholar]
- 33.Coffin RS. From virotherapy to oncolytic immunotherapy: where are we now? Curr Opin Virol. 2015;13:93–100. doi: 10.1016/j.coviro.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–9. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 35.Hennessy ML, Bommareddy PK, Boland G, et al. Oncolytic Immunotherapy. Surg Oncol Clin N Am. 2019;28:419–30. doi: 10.1016/j.soc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Todo T, Rabkin SD, Sundaresan P, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10:2741–55. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 37.Webb MJ, Sangsuwannukul T, van Vloten J, et al. Expression of tumor antigens within an oncolytic virus enhances the anti-tumor T cell response. Nat Commun. 2024;15:5442. doi: 10.1038/s41467-024-49286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemay CG, Keller BA, Edge RE, et al. Oncolytic Viruses: The Best is Yet to Come. CCDT. 2018;18:109–23. doi: 10.2174/1568009617666170206111609. [DOI] [PubMed] [Google Scholar]
- 39.Schirrmacher V. New Insights into Mechanisms of Long-term Protective Anti-tumor Immunity Induced by Cancer Vaccines Modified by Virus Infection. Biomedicines. 2020;8:55. doi: 10.3390/biomedicines8030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pol JG, Atherton MJ, Bridle BW, et al. Development and applications of oncolytic Maraba virus vaccines. Oncolytic Virother. 2018;7:117–28. doi: 10.2147/OV.S154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33:2780–8. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 42.Soliman H, Hogue D, Han H, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat Med. 2023;29:450–7. doi: 10.1038/s41591-023-02210-0. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Nace R, Ariail E, et al. Oncolytic α-herpesvirus and myeloid-tropic cytomegalovirus cooperatively enhance systemic antitumor responses. Mol Ther. 2024;32:241–56. doi: 10.1016/j.ymthe.2023.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianni T, Leoni V, Sanapo M, et al. Genotype of Immunologically Hot or Cold Tumors Determines the Antitumor Immune Response and Efficacy by Fully Virulent Retargeted oHSV. Viruses. 2021;13:1747. doi: 10.3390/v13091747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Borlongan M, Kaufman HL, et al. Cytokine-armed oncolytic herpes simplex viruses: a game-changer in cancer immunotherapy? J Immunother Cancer. 2024;12:e008025. doi: 10.1136/jitc-2023-008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman HL, Shalhout SZ, Iodice G. Talimogene Laherparepvec: Moving From First-In-Class to Best-In-Class. Front Mol Biosci. 2022;9:834841. doi: 10.3389/fmolb.2022.834841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vannini A, Parenti F, Forghieri C, et al. Decrease in Heparan Sulphate Binding in Tropism-Retargeted Oncolytic Herpes Simplex Virus (ReHV) Delays Blood Clearance and Improves Systemic Anticancer Efficacy. Cancers (Basel) 2024;16:1143. doi: 10.3390/cancers16061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Alise AM, Leoni G, Cotugno G, et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat Commun. 2019;10:2688. doi: 10.1038/s41467-019-10594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature New Biol. 2015;520:692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castle JC, Loewer M, Boegel S, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics. 2014;15:190. doi: 10.1186/1471-2164-15-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vannini A, Petrovic B, Gatta V, et al. Rescue, Purification, and Characterization of a Recombinant HSV Expressing a Transgenic Protein. Methods Mol Biol. 2020;2060:153–68. doi: 10.1007/978-1-4939-9814-2_8. [DOI] [PubMed] [Google Scholar]
- 52.Menotti L, Leoni V, Gatta V, et al. oHSV Genome Editing by Means of galK Recombineering. Methods Mol Biol. 2020;2060:131–51. doi: 10.1007/978-1-4939-9814-2_7. [DOI] [PubMed] [Google Scholar]
- 53.Vannini A, Leoni V, Campadelli-Fiume G. In: Tumor microenvironment, 1290. Birbrair A, editor. Springer International Publishing; 2021. Targeted delivery of IL-12 adjuvants immunotherapy by oncolytic viruses. [DOI] [PubMed] [Google Scholar]
- 54.Delman KA, Bennett JJ, Zager JS, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther. 2000;11:2465–72. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- 55.Ling AL, Solomon IH, Landivar AM, et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature New Biol. 2023;623:157–66. doi: 10.1038/s41586-023-06623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ricca JM, Oseledchyk A, Walther T, et al. Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy. Mol Ther. 2018;26:1008–19. doi: 10.1016/j.ymthe.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vannini A, Parenti F, Forghieri C, et al. Innovative retargeted oncolytic herpesvirus against nectin4-positive cancers. Front Mol Biosci. 2023;10:1149973. doi: 10.3389/fmolb.2023.1149973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature New Biol. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 59.Jonker DJ, Hotte SJ, Abdul Razak AR, et al. Phase I study of oncolytic virus (OV) MG1 maraba/MAGE-A3 (MG1MA3), with and without transgenic MAGE-A3 adenovirus vaccine (AdMA3) in incurable advanced/metastatic MAGE-A3-expressing solid tumours: CCTG IND.214. JCO. 2017;35:e14637. doi: 10.1200/JCO.2017.35.15_suppl.e14637. [DOI] [Google Scholar]
- 60.Ghorashian S, Kramer AM, Onuoha S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408–14. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 61.Gianneschi G, Scolpino A, Oleske J. Risk of autoimmunity, cancer seeding, and adverse events in human trials of whole-tissue autologous therapeutic vaccines. Cancer Pathog Ther . 2025;3:129–34. doi: 10.1016/j.cpt.2024.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moesta AK, Cooke K, Piasecki J, et al. Local Delivery of OncoVEXmGM-CSF Generates Systemic Antitumor Immune Responses Enhanced by Cytotoxic T-Lymphocyte-Associated Protein Blockade. Clin Cancer Res. 2017;23:6190–202. doi: 10.1158/1078-0432.CCR-17-0681. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Zhang L, Zhao Y, et al. Efficacy and safety of Gemogenovatucel-T (Vigil) immunotherapy for advanced ovarian carcinoma: A systematic review and meta-analysis of randomized controlled trials. Front Oncol. 2022;12:945867. doi: 10.3389/fonc.2022.945867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson KS, Erick TK, Chen M, et al. The feasibility of using an autologous GM-CSF-secreting breast cancer vaccine to induce immunity in patients with stage II-III and metastatic breast cancers. Breast Cancer Res Treat. 2022;194:65–78. doi: 10.1007/s10549-022-06562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soiffer RJ, Kooshesh KA, Ho V. Whole tumor cell vaccines engineered to secrete GM‐CSF (GVAX) ImmunoMedicine . 2021;1:e1025. doi: 10.1002/imed.1025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.