ABSTRACT
Introduction/Aims
Pain is a recognized symptom of muscular dystrophy (MD), but little is known about prescription pain medications in this population. We describe pain experiences and pain medications prescribed for individuals with selected MDs using population‐based surveillance data collected by the Muscular Dystrophy Surveillance, Tracking, and Research Network.
Methods
Pain and prescription data were abstracted from medical records for 1282 individuals with Duchenne and Becker (DBMD) MD during 2000–2015 and congenital (CMD), distal (DD), Emery‐Dreifuss (EDMD), facioscapulohumeral (FSHD), limb‐girdle (LGMD), and myotonic (DM) MDs during 2008–2016. Percentages of individuals prescribed pain medications for ≥ 6 weeks during follow‐up were estimated. Logistic regression was used to examine associations with selected demographic and clinical characteristics.
Results
Moderate pain was observed among 34% of all people with available pain scores and varied by MD type (13%–53%). Pain medications were prescribed for 31.1%–40.2% of people 20 years and older, but less frequently (< 15%) among people less than 20 years old. Among people prescribed pain medications, the first medication was typically a non‐opioid (57%), but both non‐opioid and opioid medication classes were prescribed during follow‐up (34%). Pain medications were typically prescribed for longer than 1 year (> 85%). Impaired mobility had the strongest association with prescription pain medication.
Discussion
The prescription of pain medication is common for people with symptomatic MD. Most people were prescribed only non‐opioids. These data highlight pain management as a frequent component of MD care. Understanding modifiable factors associated with MD‐related pain and effective interventions may help improve care.
Keywords: facioscapulohumeral muscular dystrophy, limb‐girdle muscular dystrophy, muscular dystrophy, myotonic dystrophy, prescription pain medication
1. Introduction
Pain represents a shared and commonly reported symptom among people with symptomatic muscular dystrophy (MD) [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20]. Pain management may involve over‐the‐counter (OTC) treatments (e.g., non‐steroidal anti‐inflammatory drugs, aspirin, and acetaminophen), prescription pain medications (e.g., opioids, non‐opioids, antidepressants, and antiepileptic medications), or non‐pharmacologic treatments such as physical therapy or acupuncture. Systematic study of the management of pain in MD has been limited to selected MD types and relied on self‐reported treatment modalities [5, 6, 7, 8, 10, 11, 15, 21]. OTC medications have been reported as the most common methods of pain management by those with facioscapulohumeral (FSHD) and myotonic (DM) muscular dystrophies, with estimates of use ranging from 26% to 78% [7, 8, 10, 11, 15, 17, 18, 21]. Smaller proportions (6%–33%) of individuals have reported use of prescription medications to manage pain, with use patterns varying by pain severity and MD type [5, 6, 8, 11, 12, 15, 21]. Opioids have been used among those with severe pain.
To better understand chronic pain and its medical management across MDs, we used medical record surveillance data from the United States Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). We expand on previous research by including seven MD types in a large study population with longitudinal follow‐up. This work also explores factors associated with prescribing pain medications in these selected MDs.
2. Methods
Surveillance methodologies of MD STARnet have been previously described [22, 23, 24]. For this study, eligibility for MD STARnet included a newly confirmed diagnosis of congenital (CMD), distal (DD), DM, Emery‐Dreifuss (EDMD), FSHD, or limb‐girdle (LGMD) MDs during 1/1/2008–12/31/2016. Additional criteria for these MDs included residency in Colorado (CO), Iowa (IA), western New York (21 counties, [wNY]), North Carolina (33 counties in the Piedmont region of [NC]), South Carolina (SC), or Utah (UT)/Nevada (NV), and a health encounter with a healthcare provider of any type during the same period. For Duchenne and Becker (DBMD) MD, eligibility included birth and diagnosis during 1/1/2000–12/31/2015 and an eligible residency and health encounter during the same time period.
Diagnostic and clinical data were abstracted for health encounters during the respective surveillance periods from available medical records by trained medical record abstractors. A clinical review committee (CRC) comprised of clinicians experienced in treating patients with MD reviewed clinical signs and symptoms, diagnostic test results, and family history of MD to assign a case status using defined case definitions—definite: direct support by confirmatory testing (DNA analysis, muscle biopsy), probable: clinical features and inheritance consistent, but no diagnostic DNA (or laboratory) support; possible: diagnosis in clinical record without clear diagnostic laboratory support; asymptomatic: direct support of the diagnosis by DNA analysis without symptoms of MD; or manifesting carrier (DBMD only: female with direct support of the diagnosis by DNA analysis and symptoms of weakness or cardiomyopathy). The CRC also assigned MD subtypes, where appropriate, based on clinical presentation and available diagnostic testing (Table S1). Public health authority and Institutional Review Board approval were utilized for medical record abstraction for CO, IA, NY, NC, and SC. For UT/NV, Institutional Review Board approval was obtained at the University of Utah.
2.1. Pain Location and Score
Where available, pain locations and scores (0–10) were collected annually from clinical encounters. A description of the clinical history leading up to the diagnosis, which included pain experiences, was also extracted from medical records. Presence of any pain was classified as at least one pain score greater than zero at any encounter during follow‐up. We identified the maximum pain score out of all available pain scores for each person with moderate to severe pain defined as a maximum pain score at or above five [25]. Pain locations were classified as head; neck, back, and chest; upper extremities (arm, shoulder, wrist, hand, scapula); abdomen; lower extremities (hip, groin, buttocks, leg, foot); diffuse; and unknown/unspecified (mention of pain but location not stated). Month, day, and year were available for pain scores and associated locations identified from clinical encounters; dates were unavailable for pain locations determined from the clinical history descriptions.
2.2. Pain Medications
Abstractors were instructed to only abstract medications prescribed for longer than 6 weeks based on details available in the medical record and create one entry per calendar year for each recorded medication. The medication name and month and year of the health encounter from which the medication was abstracted were collected. Discontinuation of a medication was inferred from the absence of an entry for the same medication in a subsequent calendar year. Conversely, continuation or resumption of a medication was inferred from entries in consecutive calendar years or resumed entries after discontinuation, respectively, during the interceding calendar year(s). Because only the year and month were abstracted for each medication, the day was imputed to the 15th day of the month.
Each medication was reviewed by two MD STARnet clinicians (KDM, JFH). Those determined to be used for pain management in MD were assigned generic names and medication classes. Dates of medication prescriptions were compared against dates of hospitalizations, procedures (e.g., surgical treatment for scoliosis), or fractures (DBMD only) to ensure medications prescribed for less than 6 weeks for an acute problem were not abstracted. We excluded a medication record if the medication first appeared in the medical record within 6 months of a hospitalization and continuation of the medication beyond the initial 6 months could not be determined. Following review, 42 medication records were excluded from analyses. Retained medications were classified as belonging to an opioid or non‐opioid medication class. Among individuals with documentation of any pain medication, we determined the medication classes at the first recording (non‐opioid, opioid, or both [same date]) and during follow‐up. Among people with at least 5 years of follow‐up, we also calculated the percentages of people with two or more consecutive annual medication records, which approximates at least one continuous year, to compare to the clinical practice guidelines for prescribing opioids [26, 27, 28].
2.3. Demographic and Disease Characteristics
Selected demographic characteristics collected by MD STARnet were included in our study: race/ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic or Latino/Latina, other, unknown), MD STARnet site, ages at first and last health encounters, and sex (female, male). We also included selected clinical characteristics abstracted as part of the diagnostic history or health encounters following diagnosis or at any time during the follow‐up period: presence of a family history of MD (no, yes), pulmonary function (forced vital capacity [FVC] < 80% predicted [no, yes], noninvasive/invasive ventilation [no, yes]), mobility status (independent walking without assistance, walking with an assistive device, and ceased ambulation [full‐time wheelchair or documentation of ceased ambulation]), noninvasive ventilation (bilevel positive airway pressure or continuous positive airway pressure) and invasive ventilation (ventilator tracheostomy and ventilator sip). Pulmonary function and mobility status served as proxies for disease severity.
2.4. Statistical Analysis
Descriptive statistics were calculated for categorical variables (frequencies, proportions) and continuous variables (means, standard deviations) for all symptomatic MD types. For demographics (MD STARnet site, sex, and race/ethnicity), cells with counts less than 6 are suppressed. Exploratory multivariable logistic regression analysis was used to estimate adjusted odds ratios (aORs) and corresponding 95% confidence intervals for associations between demographics and clinical characteristics and prescription pain medication. A supplemental analysis describing pain experiences among people with asymptomatic MD is also presented. All analyses were performed using SAS v. 9.4 (Cary, NC, USA) [29].
3. Results
Among all eligible MD cases identified by MD STARnet (n = 1351), we excluded people classified as asymptomatic (DBMD = 19, EDMD = 1, FSHD = 2, LGMD = 4, DM = 15) due to the absence of neuromuscular symptoms that might increase pain and manifesting carriers (DBMD = 2) due to small numbers. We also excluded people with residence in NV due to limited ascertainment and follow‐up (n = 26). The final sample was comprised of 1282 people with one of the eligible MD types (Table 1).
TABLE 1.
Demographic and clinical characteristics for all MD types combined (n = 1282) in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
| Characteristics | N (%) |
|---|---|
| MD type | |
| Congenital (CMD) | 44 (3.4) |
| Distal myopathies (DD) | 22 (1.7) |
| Duchenne or Becker (DBMD) | 402 (31.4) |
| Emery‐Dreifuss (EDMD) | 21 (1.6) |
| Facioscapulohumeral (FSHD) | 170 (13.3) |
| Limb‐girdle (LGMD) | 156 (12.2) |
| Myotonic (DM) | 467 (36.4) |
| Site | |
| CO | 237 (18.5) |
| IA | 182 (14.2) |
| NC | 208 (16.2) |
| NY | 248 (19.3) |
| SC | 182 (14.2) |
| UT | 225 (17.6) |
| Race/ethnicity | |
| Non‐Hispanic White | 927 (72.3) |
| Non‐Hispanic Black | 61 (4.8) |
| Hispanic or Latino/Latina | 131 (10.2) |
| Other a | 37 (2.9) |
| Unknown | 126 (9.8) |
| Sex – adult‐onset | |
| Female | 416 (47.3) |
| Male (non‐DBMD) | 463 (52.7) |
| Family history | |
| No | 411 (32.1) |
| Yes | 871 (67.9) |
| FVC < 80% predicted | |
| No | 186 (14.5) |
| Yes | 295 (23.0) |
| Unknown b | 801 (62.5) |
| Noninvasive/invasive ventilation | |
| No | 1135 (88.5) |
| Yes | 147 (11.5) |
| Mobility status | |
| Independent ambulation without assistive device | 808 (63.0) |
| Independent ambulation with assistive device | 321 (25.0) |
| Ceased Ambulation | 147 (11.5) |
| Missing | 6 (0.5) |
| Age at first health encounter (years) | |
| 0–9 | 539 (42.0) |
| 10–19 | 124 (9.7) |
| 20–39 | 267 (20.8) |
| 40–59 | 234 (18.3) |
| ≥ 60 | 112 (8.7) |
| Age at last health encounter (years) | |
| 0–9 | 306 (23.9) |
| 10–19 | 316 (24.7) |
| 20–39 | 253 (19.7) |
| 40–59 | 250 (19.5) |
| ≥ 60 | 153 (11.9) |
Note: Due to rounding, percentages may not total to 100%.
Abbreviations: FVC, forced vital capacity; MD, muscular dystrophy.
Other includes native Hawaiian or Pacific islander, multiple races/ethnicities, or all other races/ethnicities.
FVC result coded as unknown if no testing was performed or percent predicted was not reported.
3.1. Sociodemographic and Clinical Characteristics
The sample was evenly distributed across MD STARnet sites, predominantly non‐Hispanic White, equally split by male and female for non‐DBMD MDs, and most had a known family history of MD (Table 1). A small percentage had an FVC < 80% predicted, used noninvasive or invasive ventilation, or ceased ambulation. About one‐half were less than 20 years of age at first health encounter, mostly due to the high proportion of people with DBMD. Descriptions of these characteristics by MD type are available in Table S2.
3.2. Pain Scores and Location
Pain scores were available in the medical record for 29% of people. Among people with at least one pain score, 54% reported any pain (pain score > 0) and 34% reported moderate pain (pain score ≥ 5). The percent reporting moderate pain varied by MD type and ranged from 13% (CMD) to 53% (LGMD). Of people with described pain locations, pain in the neck, chest, and back, lower extremities, and unknown/unspecified location were most frequent (Figure 1). For individual MD types, pain locations reported by about 50% or more of people included pain in the neck, chest, and back among people with DD and FSHD, and lower extremity pain among those with CMD, DBMD, DD, and LGMD (Table S3).
FIGURE 1.
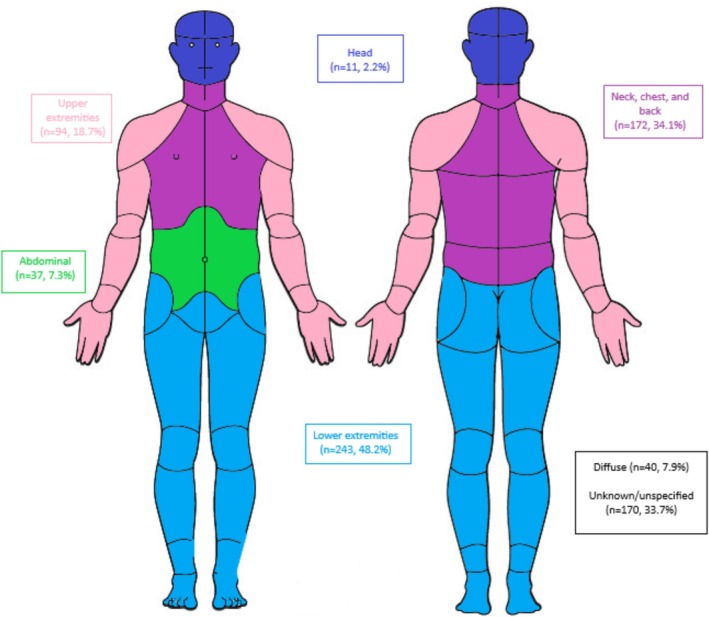
Pain locations among people diagnosed with muscular dystrophy in the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet), 2008–2016. Percentages describe the number of people identifying the pain location out of the number of people with at least one pain score (n = 1282).
3.3. Pain Medications Prescribed
In total, 36 individual medications were included (Table 2). For all MD types combined, most people did not have a prescription pain medication recorded during the surveillance period (Table 2). Among those who did have a prescription medication recorded, opiate agonists and anticonvulsants were the most frequently prescribed medication classes. The most frequent individual medications were tramadol, acetaminophen–hydrocodone, gabapentin, cyclobenzaprine, and meloxicam. Among people with pain scores, 56.3% of people with moderate to severe pain had a prescription pain medication compared to 22.1% of people with milder pain. The distributions of medication classes by MD type are available in Table S4.
TABLE 2.
Prescription pain medication frequencies for all MD types combined (n = 1282) in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016 a .
| Medication classes and individual medications | N | Total sample % | Pain medication % b |
|---|---|---|---|
| Any pain medication | 279 | 21.8 | 100.0 |
| Opiate agonists | 142 | 11.1 | 50.9 |
| Tramadol | 59 | 4.6 | 21.1 |
| Acetaminophen – hydrocodone | 49 | 3.8 | 17.6 |
| Hydrocodone | 26 | 2.0 | 9.3 |
| Oxycodone | 26 | 2.0 | 9.3 |
| Acetaminophen – oxycodone | 15 | 1.2 | 5.4 |
| Morphine | 9 | 0.7 | 3.2 |
| Fentanyl | 8 | 0.6 | 2.9 |
| Acetaminophen – codeine | 6 | 0.5 | 2.2 |
| Methadone | 5 | 0.4 | 1.8 |
| Hydromorphone | 4 | 0.3 | 1.4 |
| Oxymorphone | 4 | 0.3 | 1.4 |
| Tapentadol | 4 | 0.3 | 1.4 |
| Acetaminophen – tramadol | 3 | 0.2 | 1.1 |
| Codeine | 1 | 0.1 | 0.4 |
| Acetaminophen – propoxyphene | 1 | 0.1 | 0.4 |
| Non‐opioids | |||
| Anticonvulsants | 125 | 9.8 | 44.4 |
| Gabapentin | 111 | 8.6 | 39.4 |
| Pregabalin | 25 | 2.0 | 9.0 |
| Gabadone | 0 | 0.0 | 0.0 |
| Skeletal muscle relaxant | 91 | 7.1 | 32.6 |
| Centrally acting | |||
| Cyclobenzaprine | 38 | 3.0 | 13.6 |
| Tizanidine | 20 | 1.6 | 7.2 |
| Carisoprodol | 5 | 0.4 | 1.8 |
| Metaxalone | 3 | 0.2 | 1.1 |
| Methocarbamol | 3 | 0.2 | 1.1 |
| GABA‐derived | |||
| Baclofen | 29 | 2.3 | 10.4 |
| Miscellaneous skeletal muscle relaxants | |||
| Orphenadrine | 2 | 0.2 | 0.7 |
| NSAIDs | |||
| Oxicam | |||
| Meloxicam | 39 | 3.0 | 13.6 |
| Carboxyl‐acetic acid | |||
| Diclofenac | 23 | 1.8 | 8.2 |
| Indomethacin | 4 | 0.3 | 1.4 |
| Etodolac | 3 | 0.2 | 1.1 |
| Cox‐2 inhibitors | |||
| Celecoxib | 15 | 1.2 | 5.4 |
| Other | |||
| Nabumetone | 6 | 0.5 | 2.2 |
| Ketorolac | 3 | 0.2 | 1.1 |
| Carboxyl‐propionic | 2 | ||
| Oxaprozin | 2 | 0.2 | 0.7 |
| Carboxyl‐salicylate/sedative combinations | 1 | ||
| Butalbital – Aspirin – Caffeine | 1 | 0.1 | 0.4 |
| Antidepressants | 33 | 2.6 | 11.8 |
| Amitriptyline | 26 | 2.0 | 9.3 |
| Nortriptyline | 8 | 0.6 | 2.9 |
| Fibromyalgia agents | |||
| Milnacipran | 1 | 0.1 | 0.4 |
Abbreviation: MD STARnet, Muscular Dystrophy Surveillance, Tracking and Research Network.
Formatted in order of frequency.
All proportions among individuals with documentation of any pain medication during follow‐up.
3.4. Patterns of Pain Medication Prescriptions
To explore combinations of pain medication prescriptions, we considered prescription pain medication classes (no medication, non‐opioid only, opioid only, both) first recorded and at any time during follow‐up (Figure 2). Non‐opioid‐only medications were the most common first recorded medication. During follow‐up, the percentages of people having prescriptions for the combination of non‐opioid and opioid medications increased but non‐opioid‐only medications continued to be most common. Similar patterns were observed across the different MD types (Table S5).
FIGURE 2.
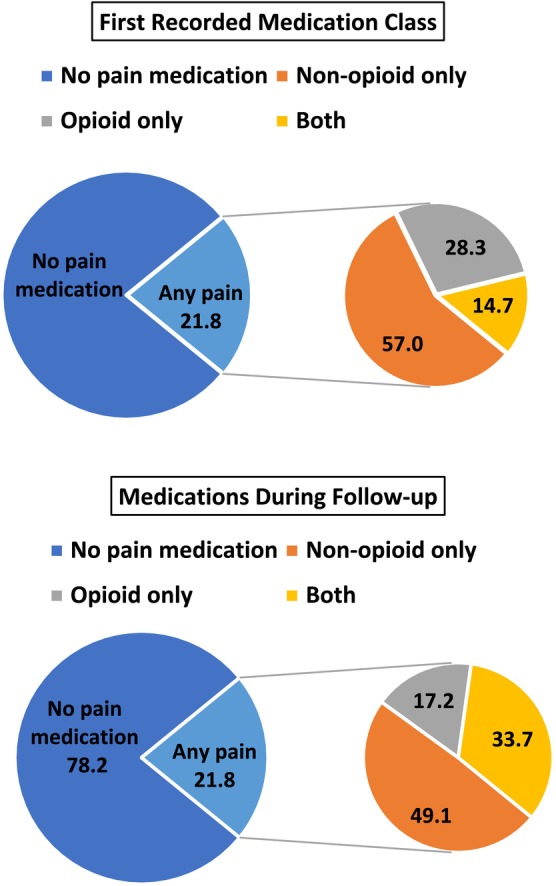
Combinations of medication classes at first record and during follow‐up for all muscular dystrophies (MD) combined in the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet), 2008–2016 (n = 1282).
To explore the chronicity of prescriptions of pain medications, we identified people with at least 5 years of follow‐up and determined percentages of people with long‐term (1 or more years) prescription pain medication (Figure 3). Among people with a prescription, nearly 90% were prescribed any pain medication or an opioid medication for 1 year or longer. Long‐term prescription pain medication tended to be similar across MD types (Table S6).
FIGURE 3.
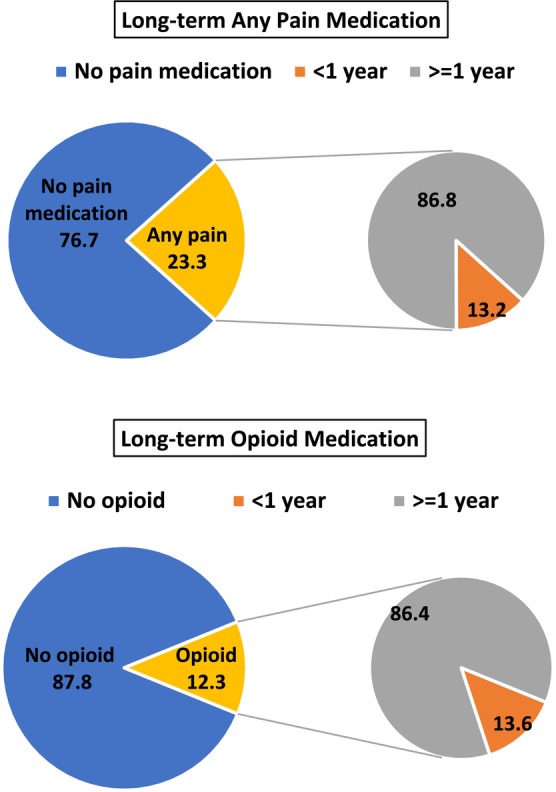
Long‐term pain medication during 5‐year follow‐up for any pain medication and opioid medication for all muscular dystrophies (MD) combined in the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet), 2008–2016 (n = 1282).
3.5. Characteristics Associated With Prescription Pain Medication
To explore associations with prescription pain medications through multivariable modeling, we included mobility status, race/ethnicity (recoded as non‐Hispanic White versus other), family history of MD, sex, and age at last health encounter (continuous); MD STARnet site was included as a random effect. In this analysis, we included people > 19 years old due to the limited use of prescription pain medications in the younger age groups (Figure 4). We excluded from analysis MD types with fewer than 5 people over the age of 19 years having prescription pain medication. Thus, the MD types analyzed included DD, FSHD, DM, and LGMD. The MD types were analyzed separately due to differences in the distributions of the characteristics examined (Table S2). Individual results for DD MD are not presented due to small numbers producing unstable models.
FIGURE 4.
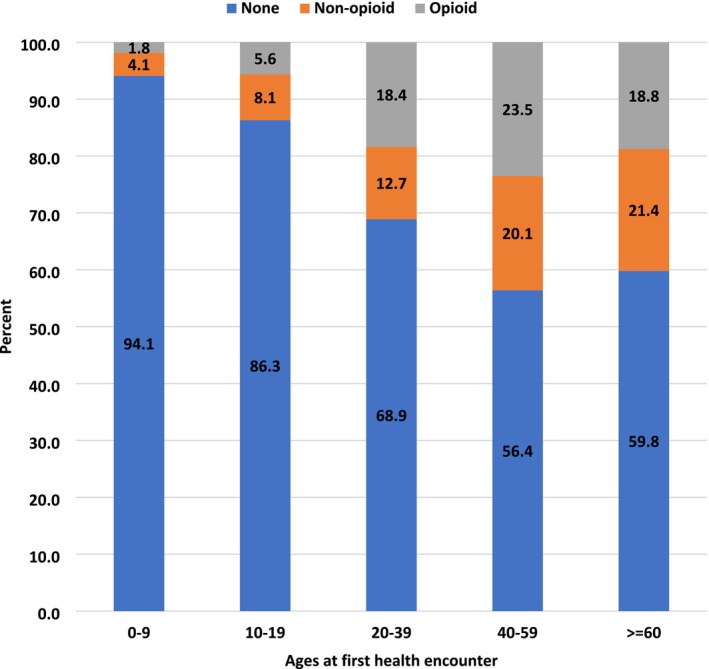
Documented prescription pain medication by age at first health encounter for all muscular dystrophies (MD) combined in the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet), 2008–2016 (n = 1282). The sum of non‐opioid and opioid totals equals the percent with any prescription medication.
Among people with LGMD, those of other race/ethnicity had higher adjusted odds (≥ 1.5) of any pain medication prescription compared to non‐Hispanic Whites (Table 3). Higher adjusted odds were found with a positive family history compared to no family history for those with FSHD and LGMD. Males had higher odds of pain medication prescription compared to females among people with FSHD, but lower odds of pain medication prescription compared to females among people with LGMD. Higher odds of prescription pain medications were found for ambulatory people using an assistive device compared to people who walked independently for FSHD, DM, and LGMD. Similar patterns of associations were found for opioid medications, except the association between sex and FSHD, which fell below our threshold (Table S7). Across all MDs, the 95% CIs were wide and most included the null value.
TABLE 3.
Logistic regression for any pain medication prescription among people 20 years or older at first health encounter in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
| FSHD (n = 129) | DM (n = 355) | LGMD (n = 98) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Any Pain Med | aOR (95% CI) | Any Pain Med | aOR (95% CI) | Any Pain Med | aOR (95% CI) | ||||
| No | Yes | No | Yes | No | Yes | ||||
| Race/ethnicity | |||||||||
| Non‐hispanic white | 67 | 39 | Reference | 162 | 93 | Reference | 41 | 35 | Reference |
| Other a | 14 | 9 | 1.1 (0.4, 3.0) | 72 | 28 | 0.7 (0.4, 1.2) | 9 | 13 | 2.5 (0.7, 8.7) |
| Family history | |||||||||
| No | 26 | 8 | Reference | 29 | 13 | Reference | 26 | 17 | Reference |
| Yes | 55 | 40 | 2.8 (1.1, 7.3) | 205 | 108 | 1.2 (0.6, 2.5) | 24 | 31 | 2.4 (0.9, 6.9) |
| Sex | |||||||||
| Female | 36 | 19 | Reference | 131 | 70 | Reference | 13 | 21 | Reference |
| Male | 45 | 29 | 1.7 (0.8, 3.8) | 103 | 51 | 0.9 (0.6, 1.4) | 37 | 27 | 0.6 (0.2, 1.8) |
| Mobility status b | |||||||||
| Independent | 68 | 30 | Reference | 180 | 73 | Reference | 38 | 15 | Reference |
| Assistive device | 10 | 16 | 3.6 (1.3, 10.0) | 48 | 42 | 1.9 (1.1, 3.3) | 11 | 28 | 3.6 (1.2, 10.5) |
| Ceased ambulation | 3 | 2 | nc | 2 | 6 | nc | 1 | 5 | nc |
Note: Models also included MD STARnet site entered as random intercept and age at final health encounter as a continuous variable.
Abbreviation: nc, not calculated.
Other includes non‐Hispanic Black, Hispanic, all other races, and unknown races/ethnicities.
May not total to the sum due to missing.
3.6. Pain Experiences Among People With Asymptomatic MD
Among people classified with asymptomatic MD (n = 41), pain descriptions were available for two, and a non‐opioid prescription pain medication was documented for one (Table S8).
4. Discussion
Our population‐based study confirms that pain is common among people with symptomatic MD. Most people in this sample did not have pain medication prescribed. We found that prescription pain medications were rarely recorded for children, and among people 20 years and older, over one in three people had pain medications prescribed. Opioids were the most common medication class; however, most people were initially prescribed non‐opioid medications. The long‐term prescription of pain medications suggests pain is a chronic condition in MD.
Chronic pain has been reported as highly prevalent among people with MDs [4]. The definition of chronic pain has varied, with most studies describing pain experiences within the preceding 3 months. Pooled prevalence rates for chronic pain from a recent review and meta‐analysis were similar (60%–70%) across MD types, although individual studies varied greatly in reported chronic pain rates [4]. This pooled prevalence is higher than we found (54% with any pain) but our value is within the range reported in individual studies. Our findings that over 50% of people with LGMD have moderate pain and 37% have a prescription pain medication are consistent with published reports on pain experiences within this patient population [4, 6, 19, 30]. Furthermore, our observation that medication prescriptions were stable or additive over time indicates that we captured chronic pain that persisted for years. However, mild chronic pain, which never required prescription medication, might have been incompletely captured in our study [6].
Relatively few studies include descriptions of prescription pain medications in the MD population and most have been limited to people with DM or FSHD [7, 8, 10, 11, 12, 18, 21]. The most frequent medication classes found in our study were similar to available reports. Opioids were prescribed for 11% of our sample, but very little is reported about opioid use in people with MD. Pain management in this population can present a difficult clinical challenge, as patients have chronic pain but are at increased risk for opioid side effects of respiratory depression and worsened constipation [31].
Few studies describe the clinical characteristics associated with pain experiences in people with MD [1, 6, 7, 8, 10, 21]. We found pain medication prescriptions were more common among people who were ambulatory but required assistance [7]. This pattern was also seen in those with a subtype of LGMD but is not reported uniformly across MD types [16]. Most studies do not report sex as a variable related to pain [4]. We found that while prescriptions for pain medications differed between men and women for each MD type, there was not a consistent pattern. The positive association between pain medication and a family history of MD is consistent with the predictions of familial pain models whereby people exposed to pain within the family are more likely to report pain [32, 33]. It is also possible that people who observe a family member affected by chronic MD‐related pain are more proactive in seeking management of their own pain.
4.1. Limitations
The use of population‐based surveillance has limitations. Although geographically diverse, the MD STARnet sites may not be representative of clinical care provided across the US. Our study relied on access to medical records to collect data, which varied by MD STARnet site and the degree to which medical care was centralized. Our study focused on prescription pain medications. It is likely that much of MD‐related pain is managed with OTC medications and non‐pharmacologic treatments [6, 8, 10, 11, 13, 15, 21, 34]. Indeed, in the US, use of selected complementary health approaches to manage pain in the general population rose steadily from 2002 to 2020 [35]. Small sample sizes also limited interpretation of some analyses, in particular the imprecision of our estimates from the logistic regression analyses. Thus, the factors found to be associated with medication prescriptions could be due to chance and should be interpreted cautiously.
Pain scores were unavailable for most people, so we were unable to fully describe the pain experiences of the people in our sample. We describe medications recorded in the medical record, but information about dose, frequency, and alternative indicators for selected medications (e.g., gabapentin for sleep; TCAs for depression) were not collected. Lastly, data collection for our study ended before the declaration of the opioid crisis as a public health emergency in 2017 and publication of the Centers for Disease Control and Prevention guidelines on prescribing opioids for chronic pain [26]. The data shown here serves as an important baseline and future data collection may be needed to evaluate changes in the use, efficacy, and safety of prescription pain medications in this population.
5. Conclusions
Our study highlights that the use of prescription medications for pain management is a component of MD clinical care and underscores the importance of establishing individual‐focused guidance for managing pain among people with MD [27, 28]. Given the significant impact of pain on quality of life, continued research into pain experiences in adult and pediatric patient populations, and how patients use prescription medication to manage pain, is warranted for people with all MD types [20, 36, 37, 38, 39].
Author Contributions
Jonathan Suhl: conceptualization, methodology, investigation, formal analysis, writing – original draft. Kristin M. Conway: conceptualization, investigation, methodology, funding acquisition, project administration, writing – original draft. Shiny Thomas: validation, formal analysis, writing – review and editing. Sonja A. Rasmussen: writing – review and editing. James F. Howard: writing – review and editing. Nicholas E. Johnson: writing – review and editing. Paul A. Romitti: methodology, investigation, project administration, funding acquisition, writing – review and editing. Katherine D. Mathews: conceptualization, investigation, funding acquisition, writing – original draft, methodology. MD STARnet: data curation.
Ethics Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest
K.D.M. receives research funding from the Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant (NIH U54 NS053672), and the Centers for Disease Control (U01 DD001248). She serves as an advisory board member for MDA and the FSH Society; is a board member for the Friedreich Ataxia Research Alliance (FARA); receives or has recently received clinical trial funding from PTC Therapeutics, Sarepta Therapeutics, Pfizer, Reata, Italfarmaco, Fibrogen, Italfarmaco, CSL Behring, AMO and Reata. S.A.R. serves on scientific advisory committees for pregnancy registries for Harmony Biosciences, Axsome Therapeutics, Biohaven Pharmaceuticals (recently acquired by Pfizer), and Myovant Sciences. N.E.J. has received grant funding from NINDS (R01NS104010, U01NS124974), NCATS (R21TR003184), CDC (U01DD001242), and the FDA (7R01FD006071). He has received grant funding from the Myotonic Dystrophy Foundation, C3 Foundation, and the Muscular Dystrophy Association. He receives royalties from the CCMDHI and the CMTHI. He receives research funds from Takeda, Sanofi Genzyme, Dyne, Vertex Pharmaceuticals, Fulcrum Therapeutics, AskBio, ML Bio, Pfizer, and Sarepta. He has provided consultation for Arthex, Takeda, Dyne, Avidity, Regenta, and Vertex Pharmaceuticals. The other authors have no conflicts of interest.
Supporting information
Table S1. Muscular dystrophy types, subtypes, and genes for case classification by the MD STARnet clinical review committee.
Table S2. Demographic and Clinical Characteristics by MD Types in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S3. Numeric pain rating score and locations for total sample and by MD type in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S4. Frequencies of prescription pain medication documented in the medical record by MD type in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S5. Medication patterns (non‐opioids only, opioids only, or non‐opioids plus opioids) in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S6. Consecutive prescription pain medications among people with at least 5 years between first and last clinical encounter in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S7. Logistic regression for opioid medication prescriptions among people 20 years or older at first health encounter in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet) (n = 582).
Table S8. Numeric pain rating score and locations for people with asymptomatic MD by MD type in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Acknowledgments
This publication was supported by the Cooperative Agreement numbers (data collection: DD001126, DD001119, DD001123, DD001116, DD001117, DD001108, DD001120, DD001054; data analysis and manuscript preparation: DD001248, DD001252, DD001255, DD001242) funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Diseases Control and Prevention. Partial support for all datasets within the Utah Population Database was provided by the University of Utah Huntsman Cancer Institute and the Huntsman Cancer Institute Cancer Center Support grant, P30 CA2014 from the National Cancer Institute. We also thank the University of Utah Health Sciences Center and Intermountain Health Care. We would like to acknowledge Joyce T Alece for her feedback on the manuscript. Preliminary analyses were presented as a poster, “Prescription Pain Medication for Individuals with Selected Muscular Dystrophies in the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet)”, at the Muscular Dystrophy Association Clinical & Scientific Conference, March 19–20, 2023 and as a poster, Pain Medication for Individuals with Facioscapulohumeral Muscular Dystrophy in the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet), at the FSHD International Research Congress, June 15–16, 2023.
Suhl J., Conway K. M., Thomas S., et al., “Pain Experiences and Prescription Pain Medications Among People With Selected Muscular Dystrophies in the Muscular Dystrophy Surveillance, Tracking, and Research Network,” Muscle & Nerve 72, no. 3 (2025): 475–484, 10.1002/mus.28460.
Funding: This work was supported by Centers for Disease Control and Prevention.
Data Availability Statement
MDSTARnet data is not available.
References
- 1. Della Marca G., Frusciante R., Vollono C., et al., “Pain and the Alpha‐Sleep Anomaly: A Mechanism of Sleep Disruption in Facioscapulohumeral Muscular Dystrophy,” Pain Medicine 14, no. 4 (2013): 487–497, 10.1111/pme.12054. [DOI] [PubMed] [Google Scholar]
- 2. George A., Schneider‐Gold C., Zier S., Reiners K., and Sommer C., “Musculoskeletal Pain in Patients With Myotonic Dystrophy Type 2,” Archives of Neurology 61, no. 12 (2004): 1938–1942, 10.1001/archneur.61.12.1938. [DOI] [PubMed] [Google Scholar]
- 3. Guy‐Coichard C., Nguyen D. T., Delorme T., and Boureau F., “Pain in Hereditary Neuromuscular Disorders and Myasthenia Gravis: A National Survey of Frequency, Characteristics, and Impact,” Journal of Pain and Symptom Management 35, no. 1 (2008): 40–50, 10.1016/j.jpainsymman.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 4. Huang M., Magni N., and Rice D., “The Prevalence, Characteristics and Impact of Chronic Pain in People With Muscular Dystrophies: A Systematic Review and Meta‐Analysis,” Journal of Pain 22 (2021): 1343–1359, 10.1016/j.jpain.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 5. Jacques M. F., Stockley R. C., Bostock E. I., Smith J., DeGoede C. G., and Morse C. I., “Frequency of Reported Pain in Adult Males With Muscular Dystrophy,” PLoS One 14, no. 2 (2019): e0212437, 10.1371/journal.pone.0212437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen M. P., Abresch R. T., Carter G. T., and McDonald C. M., “Chronic Pain in Persons With Neuromuscular Disease,” Archives of Physical Medicine and Rehabilitation 86, no. 6 (2005): 1155–1163, 10.1016/j.apmr.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 7. Jensen M. P., Hoffman A. J., Stoelb B. L., Abresch R. T., Carter G. T., and McDonald C. M., “Chronic Pain in Persons With Myotonic Dystrophy and Facioscapulohumeral Dystrophy,” Archives of Physical Medicine and Rehabilitation 89, no. 2 (2008): 320–328, 10.1016/j.apmr.2007.08.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moris G., Wood L., FernaNdez‐Torron R., et al., “Chronic Pain has a Strong Impact on Quality of Life in Facioscapulohumeral Muscular Dystrophy,” Muscle & Nerve 57, no. 3 (2018): 380–387, 10.1002/mus.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Padua L., Aprile I., Frusciante R., et al., “Quality of Life and Pain in Patients With Facioscapulohumeral Muscular Dystrophy,” Muscle & Nerve 40, no. 2 (2009): 200–205, 10.1002/mus.21308. [DOI] [PubMed] [Google Scholar]
- 10. Peric M., Peric S., Rapajic N., et al., “Multidimensional Aspects of Pain in Myotonic Dystrophies,” Acta Myologica 34, no. 2–3 (2015): 126–132. [PMC free article] [PubMed] [Google Scholar]
- 11. Suokas K. I., Haanpaa M., Kautiainen H., Udd B., and Hietaharju A. J., “Pain in Patients With Myotonic Dystrophy Type 2: A Postal Survey in Finland,” Muscle & Nerve 45, no. 1 (2012): 70–74, 10.1002/mus.22249. [DOI] [PubMed] [Google Scholar]
- 12. Tieleman A. A., Jenks K. M., Kalkman J. S., Borm G., and van Engelen B. G., “High Disease Impact of Myotonic Dystrophy Type 2 on Physical and Mental Functioning,” Journal of Neurology 258, no. 10 (2011): 1820–1826, 10.1007/s00415-011-6027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tiffreau V., Viet G., and Thevenon A., “Pain and Neuromuscular Disease: The Results of a Survey,” American Journal of Physical Medicine & Rehabilitation 85, no. 9 (2006): 756–766, 10.1097/01.phm.0000228518.26673.23. [DOI] [PubMed] [Google Scholar]
- 14. van der Kooi E. L., Kalkman J. S., Lindeman E., et al., “Effects of Training and Albuterol on Pain and Fatigue in Facioscapulohumeral Muscular Dystrophy,” Journal of Neurology 254, no. 7 (2007): 931–940, 10.1007/s00415-006-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Vliet J., Tieleman A. A., Verrips A., et al., “Qualitative and Quantitative Aspects of Pain in Patients With Myotonic Dystrophy Type 2,” Journal of Pain 19, no. 8 (2018): 920–930, 10.1016/j.jpain.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 16. Richardson M., Mayhew A., Muni‐Lofra R., Murphy L. B., and Straub V., “Prevalence of Pain Within Limb Girdle Muscular Dystrophy R9 and Implications for Other Degenerative Diseases,” Journal of Clinical Medicine 10, no. 23 (2021): 5517, 10.3390/jcm10235517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kools J., Deenen J. C., Blokhuis A. M., Verbeek A. L., Voermans N. C., and van Engelen B. G., “The Dutch Registry for Facioscapulohumeral Muscular Dystrophy: Cohort Profile and Longitudinal Patient Reported Outcomes,” Neuromuscular Disorders 33, no. 12 (2023): 964–971, 10.1016/j.nmd.2023.10.020. [DOI] [PubMed] [Google Scholar]
- 18. McNiff M. M., Hawkins S., Haase B., et al., “Facioscapulohumeral Muscular Dystrophy European Patient Survey: Assessing Patient Reported Disease Burden and Preferences in Clinical Trial Participation,” Journal of Neuromuscular Diseases 11, no. 2 (2024): 459–472, 10.3233/JND-230171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vrist L. T. H., Knudsen L. F., and Handberg C., “‘It Becomes the New Everyday Life’ ‐ Experiences of Chronic Pain in Everyday Life of People With Limb‐Girdle Muscular Dystrophy,” Disability and Rehabilitation 45, no. 23 (2023): 3875–3882, 10.1080/09638288.2022.2142679. [DOI] [PubMed] [Google Scholar]
- 20. Savas D. and Simsek T. T., “Functional Level and Its Relationship to Upper Extremity Function, Pain, and Muscle Stiffness in Children With Duchenne Muscular Dystrophy,” Irish Journal of Medical Science 192, no. 4 (2023): 1867–1873, 10.1007/s11845-022-03162-z. [DOI] [PubMed] [Google Scholar]
- 21. Solbakken G., Loseth S., Froholdt A., et al., “Pain in Adult Myotonic Dystrophy Type 1: Relation to Function and Gender,” BMC Neurology 21, no. 1 (2021): 101, 10.1186/s12883-021-02124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller L. A., Romitti P. A., Cunniff C., et al., “The Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): Surveillance Methodology,” Birth Defects Research. Part A, Clinical and Molecular Teratology 76, no. 11 (2006): 793–797, 10.1002/bdra.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Do T. N., Street N., Donnelly J., et al., “Muscular Dystrophy Surveillance, Tracking, and Research Network Pilot: Population‐Based Surveillance of Major Muscular Dystrophies at Four U.S. Sites, 2007–2011,” Birth Defects Research 110 (2018): 1404–1411, 10.1002/bdr2.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathews K. D., Cunniff C., Kantamneni J. R., et al., “Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): Case Definition in Surveillance for Childhood‐Onset Duchenne/Becker Muscular Dystrophy,” Journal of Child Neurology 25, no. 9 (2010): 1098–1102, 10.1177/0883073810371001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schweizer L., Sieber R., Nickel C. H., and Minotti B., “Ability of Pain Scoring Scales to Differentiate Between Patients Desiring Analgesia and Those Who Do Not in the Emergency Department,” American Journal of Emergency Medicine 57 (2022): 107–113, 10.1016/j.ajem.2022.04.046. [DOI] [PubMed] [Google Scholar]
- 26. Dowell D., Haegerich T. M., and Chou R., “CDC Guideline for Prescribing Opioids for Chronic Pain ‐ United States, 2016 MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report,” Recommendations and Reports 65, no. 1 (2016): 1–49, 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 27. Dowell D., Ragan K. R., Jones C. M., Baldwin G. T., and Chou R., “CDC Clinical Practice Guideline for Prescribing Opioids for Pain ‐ United States, 2022,” MMWR ‐ Recommendations and Reports 71, no. 3 (2022): 1–95, 10.15585/mmwr.rr7103a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dowell D., Ragan K. R., Jones C. M., Baldwin G. T., and Chou R., “Prescribing Opioids for Pain ‐ The New CDC Clinical Practice Guideline,” New England Journal of Medicine 387, no. 22 (2022): 2011–2013, 10.1056/NEJMp2211040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. SAS Institute Inc , “SAS 9.4. Version 9.4,” 2013.
- 30. Jensen S. M., Friborg O., Mellgren S. I., Muller K. I., Bergvik S., and Arntzen K. A., “Health‐Related Quality of Life in FKRP‐Related Limb‐Girdle Muscular Dystrophy R9,” Journal of Neuromuscular Diseases 11, no. 1 (2024): 59–74, 10.3233/JND-221629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim C. S., Park J. M., Park D., Kim D. H., and Park J. S., “Opioid Use May Be Associated With Postoperative Complications in Myotonic Dystrophy Type 1 With High‐Grade Muscular Impairment,” Scientific Reports 11, no. 1 (2021): 8, 10.1038/s41598-020-76217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson A. C., Holley A. L., Stone A., Fales J. L., and Palermo T. M., “Pain, Physical, and Psychosocial Functioning in Adolescents at Risk for Developing Chronic Pain: A Longitudinal Case‐Control Stusdy,” Journal of Pain 21, no. 3–4 (2020): 418–429, 10.1016/j.jpain.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dario A. B., Kamper S. J., O'Keeffe M., et al., “Family History of Pain and Risk of Musculoskeletal Pain in Children and Adolescents: A Systematic Review and Meta‐Analysis,” Pain 160, no. 11 (2019): 2430–2439, 10.1097/j.pain.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 34. Stokholm R. N., Handberg C., and Knudsen L. F., “Prevalence of Chronic Pain in a National Cohort of Patients With Limb‐Girdle Muscular Dystrophy: A Cross‐Sectional Study,” Disability and Rehabilitation 44, no. 25 (2022): 7802–7810, 10.1080/09638288.2021.1998669. [DOI] [PubMed] [Google Scholar]
- 35. Nahin R. L., Rhee A., and Stussman B., “Use of Complementary Health Approaches Overall and for Pain Management by US Adults,” JAMA 331, no. 7 (2024): 613–615, 10.1001/jama.2023.26775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varni J. W. and Uzark K., “Pain and Health‐Related Quality of Life in Duchenne Muscular Dystrophy: A Multiple Mediator Analysis,” European Journal of Paediatric Neurology 46 (2023): 61–66, 10.1016/j.ejpn.2023.07.003. [DOI] [PubMed] [Google Scholar]
- 37. Nichita D. and Pernet K., “Quality of Life in Duchenne Muscular Dystrophy: It's About More Than Pain Management,” Developmental Medicine and Child Neurology 65, no. 5 (2023): 595, 10.1111/dmcn.15567. [DOI] [PubMed] [Google Scholar]
- 38. Huang M., Chen T., Wang Y., et al., “Chronic Pain, Psychological Distress, and Quality of Life in Males With Duchenne Muscular Dystrophy,” Developmental Medicine and Child Neurology 65, no. 5 (2023): 640–654, 10.1111/dmcn.15404. [DOI] [PubMed] [Google Scholar]
- 39. Kim A., Park M., and Shin H. I., “Pain Characteristics Among Individuals With Duchenne Muscular Dystrophy According to Their Clinical Stage,” BMC Musculoskeletal Disorders 23, no. 1 (2022): 536, 10.1186/s12891-022-05504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Muscular dystrophy types, subtypes, and genes for case classification by the MD STARnet clinical review committee.
Table S2. Demographic and Clinical Characteristics by MD Types in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S3. Numeric pain rating score and locations for total sample and by MD type in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S4. Frequencies of prescription pain medication documented in the medical record by MD type in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S5. Medication patterns (non‐opioids only, opioids only, or non‐opioids plus opioids) in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S6. Consecutive prescription pain medications among people with at least 5 years between first and last clinical encounter in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Table S7. Logistic regression for opioid medication prescriptions among people 20 years or older at first health encounter in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet) (n = 582).
Table S8. Numeric pain rating score and locations for people with asymptomatic MD by MD type in the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet), 2008–2016.
Data Availability Statement
MDSTARnet data is not available.


