Abstract
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is a life-saving therapy for critically ill patients, but it carries an increased risk of thrombosis due to blood interacting with non-physiological surfaces. While the relationship between clinical variables and thrombosis remains unclear, our study aimed to identify which factors are most predictive of thrombosis. The Extracorporeal Life Support Organization Registry was queried to obtain a cohort of VV-ECMO patients aged 18 years and older from 2015 to 2019. Patients who were over 80-years-old, at the extremes of weight, who received less than 24 h of ECMO, multiple rounds of ECMO, or had missing data were excluded. Multivariate logistic regression modeling was used to assess predictors of thrombosis and mortality. A total of 9809 patients were included in the analysis, with a mean age of 47.1 ± 15.1 years and an average ECMO run time of 305 ± 353 h. Thrombosis occurred in 19.9% of the cohort, with circuit thrombosis (8.6%) and membrane lung failure (6.1%) being the most common. Multivariate analysis showed that ECMO runs over 14 days (OR: 2.62, P < 0.001) and pregnancy-related complications (OR: 1.79, P = 0.004) were associated with an increased risk of thrombosis. Risk factors for circuit thrombosis included incremental unit increases in the pump flow rate at 24 h (OR: 1.07 [1.00–1.14], P = 0.044) and specific cannulation sites. Increased body weight (OR: 1.02 [1.00–1.04], P = 0.026) and increased duration on ECMO (OR: 3.82 [3.12–4.71], P < 0.001) were predictive of membrane lung failure. Additionally, patients with thrombosis were at increased likelihood of in-hospital mortality (OR: 1.52, P < 0.001). This study identified multiple thrombotic risk factors in VV-ECMO, suggesting that future studies investigating the impact of pregnancy associated complications and ECMO flow rate on hemostasis would be illuminating.
Keywords: Extracorporeal circulation, Thrombosis, Cardiology
Introduction
Venovenous extracorporeal membrane oxygenation (VV ECMO) is a form of mechanical life support for patients with severe respiratory failure [1]. The use of VV ECMO has increased significantly in recent years, in part to support patients with coronavirus disease 2019 (COVID-19) experiencing severe respiratory failure [2, 3]. However, despite the potentially lifesaving benefits of ECMO [4, 5], pathologic blood clotting (thrombosis) is a commonly encountered complication, with reported incidences of over 25% in some studies [6–8]. Clot buildup can lead to decreased or ceased circuit blood flow, membrane lung failure, and pulmonary or systemic emboli, all of which can result in significant morbidity and mortality [1].
The incidence of thrombotic complications during ECMO can be influenced by a variety of patient-, circuit-, and management-related factors. For example, patients receiving frequent transfusions of blood products or who develop severe thrombocytopenia, a marker of critical illness, are at increased risk for thrombotic events while on ECMO [6, 9]. Additionally, ECMO exposes blood to non-biologic material and non-pulsatile flow; this in turn leads to platelet activation and initiation of the intrinsic pathway of coagulation and systemic inflammatory responses [10, 11]. Inappropriate maintenance of the circuit (e.g. insufficient monitoring for air bubbles, tube compression or kinks, etc.) can also contribute to thrombosis [3]. Lastly, the intensity and duration of anticoagulation therapy, as well as the type of anticoagulant used, may also impact the risk of clotting in ECMO, although these decisions underscore the precarious balance regarding the risk of bleeding imparted by anticoagulation [6]. At present, there is no evidence-based consensus for anticoagulation practices or monitoring coagulation status in ECMO patients [11, 12]. The risk of clotting with ECMO use is a multifactorial issue that requires careful attention to patient-, circuit-, and management-related factors to optimize outcomes. Additionally, our current ability to identify the subset of patients with the highest risk of thrombosis, and who could benefit the most from prophylaxis or targeted interventions, is limited.
Our hypothesis is that by utilizing data from the ELSO Registry, we will be able to identify clinically relevant variables that accurately predict thrombosis in adult patients receiving VV-ECMO for respiratory failure. Specifically, the objective of this work is to determine which clinical factors are most predictive of thrombosis in this patient population.
Methods
Study design and data source
We queried the ELSO Registry for adults (≥ 18 years old) on VV ECMO between 1/1/2015 and 12/31/2019. Data included information on patient demographics, cannulation strategies, ECMO specific factors, and clinical outcomes. Patients were excluded if their records were missing critical data, including: age, weight, sex, race, and relevant clinical data. We also excluded patients if they were ≥ 80 years old, had a weight outside of the limits defined by the ELSO Registry (< 10.0 kg or > 500.0 kg), or received multiple ECMO runs (Fig. 1).
Fig. 1.
Exclusion criteria used for defining the primary analysis population. VV-ECMO, venovenous (VV) extracorporeal membrane oxygenation. ECPR, extracorporeal cardiopulmonary resuscitation
Thrombotic events
We defined thrombotic events using the Complications Codes and the Registry Data Definitions provided by ELSO (Supplemental Table 1) [13, 14]. Thrombotic events were classified as: circuit thrombosis, membrane failure, hemolysis, CNS infarction, hemofilter clot, ischemia, or pump failure.
Statistical analysis
For continuous variables, data were reported as mean ± standard deviation (SD). For categorical variables, data were reported as frequency and the percentage of total. Since pump flow rates were correlated to body weight (Supplemental Fig. 1), normalized flow rates were calculated for unadjusted analyses by dividing the pump flow rates at 24 h by the weight of the patients. To compare differences between cohorts, P-values were calculated using Pearson’s Chi-squared tests and Welch two sample t-tests. Statistical significance was defined as P < 0.05 for simple demographic comparisons.
A multivariate logistic regression model was used to determine clinically meaningful predictors of thrombosis. Select risk factors for thrombosis were selected a priori based on known relevance for the regression model. Variable categories with a low frequency of observations (< 1%) were binned as “other”, with the exception of cannulation sites as these were primary predictors of interest. For multiple logistic regression models, statistical significance was defined as an adjusted P < 0.05. The covariates in the final model were evaluated for collinearity using the variance inflation factor. All statistics were calculated using R (R Foundation for Statistical Computing, Version 4.2).
Results
Primary analysis population characteristics
There were 16,453 ECMO patients in the ELSO Registry during the five-year study period. After excluding patients with missing data or those who met exclusion criteria, the primary analysis population was 9809 patients. This population was stratified by incidence of a thrombotic event (Fig. 1). The mean age of patients in the primary analysis population was 47.1 (± 15.1) years old and the cohort was predominantly male (61.3%). A full list of the cohort demographics is included in Table 1.
Table 1.
Demographic information for the primary analysis population of adult patients on VV-ECMO
| Characteristic | All patients | No thrombosis | Thrombosis | P-Value2 |
|---|---|---|---|---|
| n = 98091 | n = 78541 | n = 19551 | ||
|
| ||||
| Age (years) | 47.1 (15.1) | 47.3 (15.2) | 46.2 (14.9) | 0.004 |
| Weight (kg) | 89.60 (29.77) | 89.19 (29.63) | 91.25 (30.28) | 0.007 |
| Sex | ||||
| Male | 6009 (61.3%) | 4784 (60.9%) | 1225 (62.7%) | 0.163 |
| Female | 3800 (38.7%) | 3070 (39.1%) | 730 (37.3%) | 0.163 |
| Race | ||||
| White | 5827 (59.4%) | 4652 (59.2%) | 1175 (60.1%) | 0.499 |
| Black | 1260 (12.9%) | 1025 (13.1%) | 235 (12.0%) | 0.238 |
| Asian | 1198 (12.2%) | 971 (12.4%) | 227 (11.6%) | 0.384 |
| Hispanic | 744 (7.6%) | 582 (7.4%) | 162 (8.3%) | 0.207 |
| Multiple | 327 (3.3%) | 281 (3.6%) | 46 (2.4%) | 0.009 |
| Other | 453 (4.6%) | 343 (4.4%) | 110 (5.6%) | 0.021 |
| Prior cardiac arrest | 852 (8.7%) | 711 (9.1%) | 141 (7.2%) | 0.011 |
| Prior transplant | 541 (5.5%) | 433 (5.5%) | 108 (5.5%) | 1.000 |
| Time on ECMO (hours) | 305 (353) | 262 (295) | 479 (489) | < 0.001 |
| Pump flow rate (L/min) | 4.08 (0.91) | 4.06 (0.90) | 4.19 (0.94) | < 0.001 |
| Primary diagnosis | ||||
| Diseases of the respiratory system | 7217 (73.6%) | 5779 (73.6%) | 1438 (73.6%) | 1.000 |
| Diseases of the circulatory system | 490 (5.0%) | 401 (5.1%) | 89 (4.6%) | 0.344 |
| Injury and poisoning | 481 (4.9%) | 380 (4.8%) | 101 (5.2%) | 0.588 |
| Infectious and parasitic diseases | 465 (4.7%) | 369 (4.7%) | 96 (4.9%) | 0.737 |
| Pregnancy related complication | 133 (1.4%) | 93 (1.2%) | 40 (2.1%) | 0.005 |
| Endocrine, nutritional and metabolic diseases | 123 (1.3%) | 98 (1.3%) | 25 (1.3%) | 1.000 |
| Other | 900 (9.2%) | 734 (9.4%) | 166 (8.5%) | 0.260 |
| Survived ECMO | 6481 (66.1%) | 5378 (68.5%) | 1103 (56.4%) | < 0.001 |
| Year on ECMO | ||||
| 2015 | 1157 (11.8%) | 841 (10.7%) | 316 (16.2%) | < 0.001 |
| 2016 | 1597 (16.3%) | 1166 (14.9%) | 431 (22.1%) | < 0.001 |
| 2017 | 1759 (17.9%) | 1364 (17.4%) | 395 (20.2%) | 0.004 |
| 2018 | 2441 (24.9%) | 2043 (26.0%) | 398 (20.4%) | < 0.001 |
| 2019 | 2855 (29.1%) | 2440 (31.1%) | 415 (21.2%) | < 0.001 |
| Cannulation site | ||||
| Femoral vein | 6695 (68.3%) | 5389 (68.6%) | 1306 (66.8%) | 0.130 |
| Internal jugular vein | 7462 (76.1%) | 5913 (75.3%) | 1549 (79.2%) | < 0.001 |
| Subclavian vein | 124 (1.3) | 106 (1.4%) | 18 (0.9%) | 0.160 |
| Discontinuation reason | ||||
| Expected recovery | 6935 (70.7%) | 5729 (72.9%) | 1206 (61.7%) | < 0.001 |
| Died or poor prognosis | 2596 (26.5%) | 1897 (24.2%) | 699 (35.8%) | < 0.001 |
| Organ transplant | 105 (1.1%) | 98 (1.3%) | 7 (0.4%) | < 0.001 |
| ECMO complication | 83 (0.9%) | 56 (0.7%) | 27 (1.4%) | 0.006 |
| Resource limitation | 13 (0.1%) | 8 (0.1%) | 5 (0.3%) | 0.185 |
| Transition to VAD support | 10 (0.1%) | 8 (0.1%) | 2 (0.1%) | 1.000 |
| Unknown | 67 (0.7%) | 58 (0.7%) | 9 (0.5%) | 0.237 |
Mean (SD); n (%)
Welch two sample t-test; Pearson’s Chi-squared test
VV-ECMO, venovenous extracorporeal membrane oxygenation. VAD, ventricular assist device
Prevalence of thrombotic complications
Of the patients in the primary analysis population, 1955 patients had a qualifying thrombotic event (19.9%). The most common thrombotic events were circuit thrombosis (8.6%), followed by membrane lung failure (6.1%) and hemolysis (5.1%). Other, less frequent patient-specific thrombotic events include ischemia (1.0%) and blood pump failure (0.9%). The full breakdown of thrombotic events is listed in Table 2. Unadjusted data visualizing the distributions of pump flow rates at 24 h is presented in Fig. 2. Higher blood flow rates at 24 h were associated with an increased incidence of circuit exchange and mortality (P < 0.001 for all).
Table 2.
Thrombotic complications for adult patients on VV-ECMO
| Characteristic | All patients n = 98091 |
|---|---|
|
| |
| Circuit thrombosis | 843 (8.6%) |
| Membrane lung failure | 600 (6.1%) |
| Hemolysis | 501 (5.1%) |
| CNS infarction | 134 (1.4%) |
| Hemofilter clot | 107 (1.1%) |
| Ischemia | 100 (1.0%) |
| Blood pump failure | 87 (0.9%) |
n (%)
VV-ECMO, venovenous extracorporeal membrane oxygenation
Fig. 2.
Violin plot showing normalized flow rates stratified by the incidence of a thrombotic event (a), circuit thrombosis (b), membrane lung failure (c), or in-hospital mortality (d). Statistical significance is indicated by one asterisk (*) for P < 0.05
Multivariate logistic regressions to assess predictors of thrombotic events
Predictors of thrombosis (as a composite of all thrombotic events)
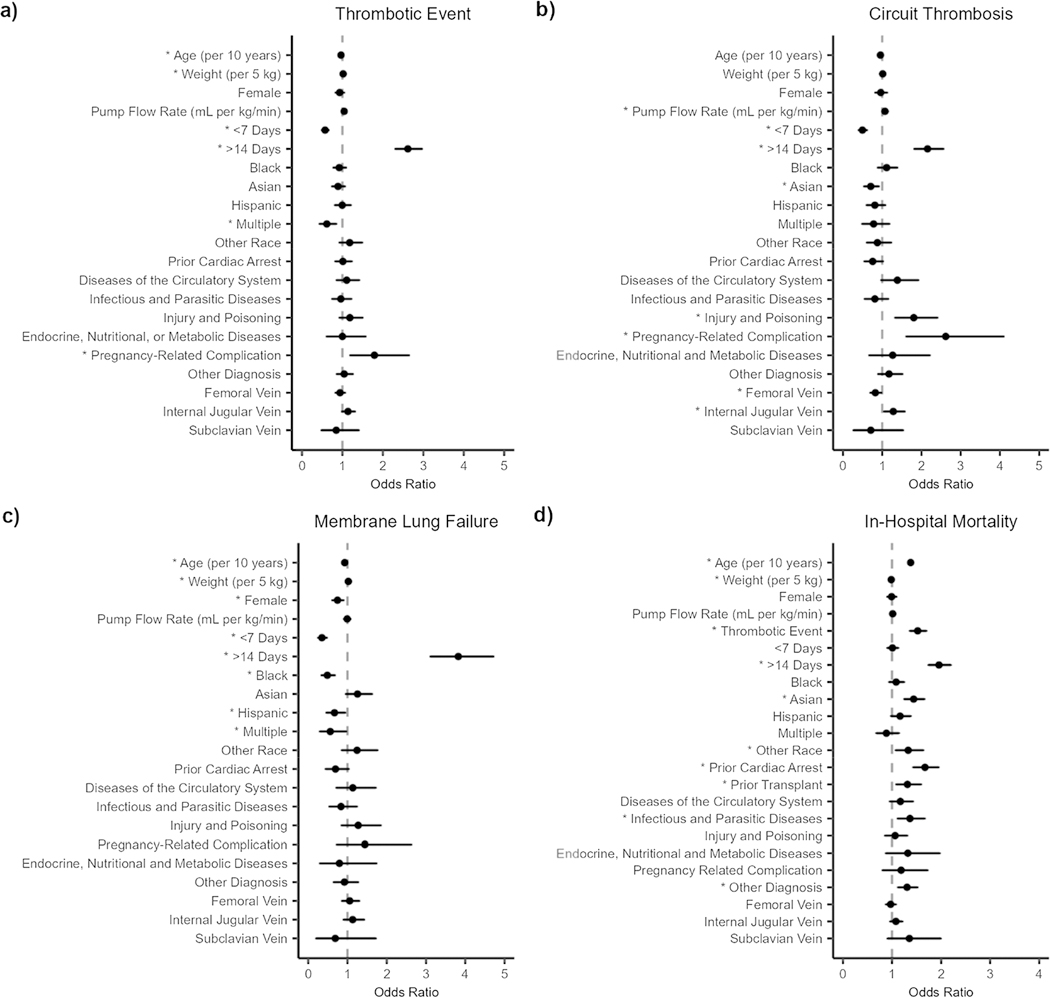
After controlling for potential risk factors for thrombotic events, risk factors included higher body weight (OR: 1.02 [1.01–1.03], P = 0.004), being on ECMO greater than 14 days (OR: 2.62 [2.31–2.96], P < 0.001), and a primary diagnosis of pregnancy-related complications (OR: 1.79 [1.19–2.64], P = 0.004). Patients that were older (up to 80 years old) (OR: 0.96 [0.93–1.00], P = 0.047) or on ECMO for less than 7 days (OR: 0.57 [0.50–0.66], P < 0.001) were at a lower risk for thrombotic events (Fig. 3a).
Fig. 3.
Forest plot showing the effect of covariates on the incidence of a thrombotic event as a composite of all thrombotic events (a), circuit thrombosis (b), membrane lung failure (c), or in-hospital mortality (d). The closed symbol represents the odds ratio and the whiskers represent the 95% confidence interval. The dashed line indicates the covariate had no effect compared to the reference patient. Statistical significance is indicated by one asterisk (*) for P < 0.05
Predictors of circuit thrombosis and membrane lung failure
Risk factors for circuit thrombosis included incremental unit increases in the pump flow rate at 24 h (OR: 1.07 [1.00–1.14], P = 0.044), being on ECMO greater than 14 days (OR: 2.15 [1.82–2.56], P < 0.001), a primary diagnosis of pregnancy-related complications (OR: 2.62 [1.62–4.09], P < 0.001), a primary diagnosis of injury or poisoning necessitating ECMO use (OR: 1.80 [1.33–2.40], P < 0.001), and cannulation of the internal jugular vein relative to cannulation of the femoral vein (OR: 1.28 [1.05–1.57], P = 0.023). Patients on ECMO for less than 7 days (OR: 0.50 [0.40–0.61], P < 0.001) or had a cannula placed in the femoral vein (OR: 0.83 [0.70–0.97], P = 0.022] were at a lower risk for of circuit thrombosis (Fig. 3b).
Risk factors for membrane lung failure included higher body weight (OR: 1.02 [1.00–1.04], P = 0.026) and being on ECMO greater than 14 days (OR: 3.82 [3.12–4.71], P < 0.001). Patients that were older (up to 80 years old) (OR: 0.93 [0.87–0.99], P = 0.017), on ECMO for less than 7 days (OR: 0.35 [0.26–0.47], P < 0.001), or female (0.75 [0.62–0.90], P = 0.002) were at a lower risk for membrane lung failure (Fig. 3c).
Predictors of in‑hospital mortality
Risk factors for in-hospital mortality included thrombosis (OR: 1.52 [1.36–1.70], P < 0.001), older age (OR: 1.38 [1.34–1.42], P < 0.001), being on ECMO greater than 14 days (OR: 1.96 [1.74–2.20], P < 0.001), having a history of cardiac arrest (OR: 1.67 [1.43–1.95], P < 0.001) or organ transplant (OR: 1.31 [1.09–1.59], P = 0.011), and a primary diagnosis of infectious and parasitic diseases necessitating ECMO (OR: 1.36 [1.12–1.67], P = 0.007). Patients with a higher body weight (OR: 0.98 [0.97–0.99], P = 0.011) were at a lower risk for in-hospital mortality (Fig. 3d).
Discussion
In this analysis of patients on VV ECMO, we corroborated other studies which found that time on ECMO and weight are predictive of thrombosis [15–17]. Likewise, our analysis identified well known thrombotic risk factors, such as pregnancy [18]. Unique to our analysis, we found that pump flow rate at 24 h was predictive of circuit thrombosis, which, to the best of our knowledge, has not been reported previously. This work adds to the growing literature showing that both patient-specific and device-specific risk factors are predictive of thrombosis in VV ECMO. Notably, we found that patients who developed a thrombotic event had a higher mortality, highlighting the critical need to effectively identify, prevent and treat thrombotic events.
Multiple variables determine ECMO blood flow rate including patient size, native cardiac output, and target arterial oxygen content [19]. After controlling for patient weight and other confounders, we determined that higher ECMO blood flow rates at 24 h were predictive of circuit thrombosis. This is initially counterintuitive as blood stasis and low flow states are well-known risk factors for thrombosis [20, 21]; however, extracorporeal devices have significant and often unpredictable effects on blood rheology and hemostatic balance. For instance, preclinical work using ex vivo ECMO models demonstrate that lower flow rates (2.5 L/min vs 4 L/min) increased hemolysis and lead to the loss of low high-molecular weight vWF multimers and ristocetin-induced platelet aggregation [22]. This observation by Ki et al. suggests that lower flow rates may tip the hemostatic balance towards bleeding. There are potential confounders for this observation that we were unable to measure due to a lack of available data in the ELSO Registry. For instance, it is possible patients on higher blood flow rates are less predisposed to be on anticoagulation or receive lower doses of anticoagulation then those receiving lower flow rates.
There are several limitations of our analysis that should be noted. While comprehensive, the ELSO Registry has the potential for incomplete data, biases that can limit the translatability of the findings, and limited clinical variables that may impact study findings. For instance, the inability to control for anticoagulation use (to assess for the absence of anticoagulation, as well as the type and dose used) and to assess for peripheral thrombosis (e.g. deep vein thrombosis, pulmonary embolism) is a major limitation. Data were additionally constrained to the period from 2015 to 2019, as ELSO database requests are limited to five-year periods. Another limitation is the ability to only assess blood flow rate at a finite time point, as mean blood flow rates for longer observation periods may be more informative.
In conclusion, this large analysis of patients on VV-ECMO showed that higher pump flow rates at 24 h correlated with an increased risk of circuit thrombosis. Thus, larger translational work assessing the impact of blood flow rate on blood rheology and hemostasis would be illuminating. This work is hypothesis generating, but requires further study to determine the true associations between ECMO blood flow rate and thrombosis.
Supplementary Material
Highlights.
Thrombosis Incidence and Types: The paper analyzed data from 9809 venovenous extracorporeal membrane oxygenation (VV-ECMO) patients and found a thrombosis incidence of 19.9%. Circuit thrombosis (8.6%) and membrane lung failure (6.1%) were the most common types of thrombotic events.
Predictive Factors for Thrombosis: Multivariate logistic regression modeling identified several predictive factors for thrombosis. Longer ECMO runs (over 14 days) and pregnancy-related complications were associated with an increased risk of thrombosis. Additionally, incremental increases in pump flow rate at 24 h and specific cannulation sites were identified as risk factors for circuit thrombosis.
Predictive Factors for Membrane Lung Failure: Higher body weight and increased duration on ECMO were found to be predictive factors for membrane lung failure. Conversely, being older (up to 80 years old), being on ECMO for less than 7 days, and being female were associated with a lower risk of membrane lung failure.
Impact on In-Hospital Mortality: Patients with thrombosis were found to have an increased likelihood of in-hospital mortality (OR: 1.52, P < 0.001). Other risk factors for mortality included older age, longer ECMO duration, history of cardiac arrest or organ transplant, and a primary diagnosis of infectious and parasitic diseases necessitating ECMO.
Funding
This work has been supported by grants from the National Institutes of Health (R01HL101972, R01HL151367).
Conflict of interest
J.J. Shatzel reports receiving consulting fees from Aronora, Inc. The Oregon Health & Science University Conflict of Interest in Research Committee has reviewed and managed this potential conflict of interest. The remaining authors declare no potential conflict of interest.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11239-023-02909-4.
Declarations
References
- 1.Makdisi G, Wang IW (2015) Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 7(7):E166–E176. 10.3978/j.issn.2072-1439.2015.07.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization ELS (2022) ECLS Registry Report International Summary 2022. https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx
- 3.Abruzzo A, Gorantla V, Thomas SE (2022) Venous thromboembolic events in the setting of extracorporeal membrane oxygenation support in adults: a systematic review. Thromb Res 212:58–71. 10.1016/j.thromres.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 4.Combes A, Hajage D, Capellier G et al. (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378(21):1965–1975. 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 5.Peek GJ, Mugford M, Tiruvoipati R et al. (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374(9698):1351–1363. 10.1016/s0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 6.Olson SR, Murphree CR, Zonies D et al. (2021) Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J 67(3):290–296. 10.1097/mat.0000000000001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunez JI, Gosling AF, O’Gara B et al. (2022) Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med 48(2):213–224. 10.1007/s00134-021-06593-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treml B, Breitkopf R, Bukumirić Z, Bachler M, Boesch J, Rajsic S (2022) ECMO predictors of mortality: a 10-year referral centre experience. J Clin Med 11(5):1224. 10.3390/jcm11051224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohs TCL, Liu P, Raghunathan V et al. (2022) Severe thrombocytopenia in adults undergoing extracorporeal membrane oxygenation is predictive of thrombosis. Platelets 33(4):570–576. 10.1080/09537104.2021.1961707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y (2015) Contact pathway of coagulation and inflammation. Thromb J 13:17. 10.1186/s12959-015-0048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sniderman J, Monagle P, Annich GM, MacLaren G (2020) Hematologic concerns in extracorporeal membrane oxygenation. Res Pract Thromb Haemost 4(4):455–468. 10.1002/rth2.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy JH, Staudinger T, Steiner ME (2022) How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med 48(8):1076–1079. 10.1007/s00134-022-06723-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization ELS (2023) Codes for extracorporeal life support (ECLS) complications. https://www.elso.org/registry/supportdocuments/eclscomplicationscode.aspx
- 14.Organization ELS (2022) Extracorporeal life support organization (ELSO) registry data definitions. https://www.elso.org/portals/0/files/pdf/elso%20registry%20data%20definitions%2005_17_22.pdf
- 15.Kirklin JK, Naftel DC, Pagani FD et al. (2015) Pump thrombosis in the thoratec heartmate II device: an update analysis of the INTERMACS registry. J Heart Lung Transplant 34(12):1515–1526. 10.1016/j.healun.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 16.Boyle AJ, Jorde UP, Sun B et al. (2014) Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 heartmate II outpatients. J Am Coll Cardiol 63(9):880–888. 10.1016/j.jacc.2013.08.1656 [DOI] [PubMed] [Google Scholar]
- 17.Chung M, Cabezas FR, Nunez JI et al. (2020) Hemocompatibility-related adverse events and survival on venoarterial extracorporeal life support: an ELSO registry analysis. JACC Heart Fail 8(11):892–902. 10.1016/j.jchf.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegers HMG, Middeldorp S (2020) Contemporary best practice in the management of pulmonary embolism during pregnancy. Ther Adv Respir Dis 14:1753466620914222. 10.1177/1753466620914222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badheka A, Stucker SE, Turek JW, Raghavan ML (2017) Efficacy of flow monitoring during ECMO. ASAIO J 63(4):496–500. 10.1097/mat.0000000000000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cushman M (2007) Epidemiology and risk factors for venous thrombosis. Semin Hematol 44(2):62–69. 10.1053/j.seminhematol.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmon CT (2009) Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev 23(5):225–229. 10.1016/j.blre.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ki KK, Passmore MR, Chan CHH et al. (2019) Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensive Care Med Exp 7(1):51. 10.1186/s40635-019-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.