Abstract
There are sparse data on the role of the vaginal microbiome (VMB) in pregnancy among pregnant women living with HIV (PWLWH) and its association with spontaneous preterm birth (sPTB). We conducted a scoping review to assess associations between vaginal microbiota and sPTB among PWLWH. Three studies were included, representing a total of 180 PWLWH out of 652 total pregnancies. All studies used modern DNA sequencing methods (16S rRNA amplification, metagenomics, or metatranscriptomics). PWLWH had higher VMB richness and diversity compared to HIV-uninfected pregnant women and higher sPTB rates in two of three studies. A higher proportion of sPTB among PWLWH was observed in those with Lactobacillus-deficient, anaerobe-dominant vaginal microbiota. In two of three studies, higher concentrations of vaginal inflammation markers were associated with increased VMB richness and diversity. HIV status was independently associated with sPTB. It is unclear if increased vaginal microbial diversity among PWLWH or increased vaginal inflammation contributes more to PTB, but HIV does appear to alter the VMB in pregnant individuals and may also affect PTB rates in microbiome-independent pathways. Given the limited number of studies, heterogeneity in sample size, sample collection methods, and inconsistent results it is difficult to causally link HIV, VMB, inflammatory cytokines, and sPTB.
Keywords: ART, HIV, microbiomes, pregnancy, preterm birth, vaginal microbiota
1 |. Introduction
Preterm birth (PTB), defined as live delivery before 37 completed weeks of pregnancy [1] is a major cause of neonatal and child morbidity and mortality across the globe. Globally, approximately 15 million babies (10%) are born preterm annually with 1 million annual deaths of children under 5 years of age due to PTB complications [1, 2]. The March of Dimes estimated annual cost of PTBs in the United States in 2020 was $26.2 billion [3].
Pregnant women living with HIV (PWLWH) have a risk for spontaneous preterm birth (sPTB) three to four times higher than those without HIV [4–6]. Apart from known PTB risk factors, including advanced maternal or paternal age [7, 8], substance use [8], structural racism [5, 8, 9], ethnicity [10], smoking [10], obesity [11], multiple gestation [12], history of a previous sPTB [12], PWLWH may have additional risk factors such as an unsuppressed HIV-RNA viral load (VL) [13], use of antiretroviral therapy (ART) [13], and placental mitochondrial toxicity [3, 5, 8, 10]. In some parts of Sub-Saharan Africa, maternal HIV infection complicates as many as one in four pregnancies and may increase the risk of PTB by 50% compared to women without HIV [14].
The vaginal microbiome (VMB) or collection of culturable and unculturable bacterial populations within the vagina is considered crucial for a healthy vagina. Vaginal lactobacilli, the predominant bacterial species, help maintain a delicately balanced mutualistic environment between other indigenous organisms and the human host by supporting an acidic vaginal pH and healthy metabolites [15–17]. Lactobacilli play a protective role in the vagina through different mechanisms including preventing the overgrowth of other microorganisms by competing with them for nutrients and tissue adherence, lowering the vaginal pH by the production of organic acids, mainly lactic acid, modulating the local immune system and producing antimicrobial substances such as peptides and bacteriocins [17, 18].
Although many factors may contribute to PTBs, the role of reproductive tract microbiomes in PWLWH and its association with PTB is still under research. Ravel et al. [19] categorized different types of VMB by community state types (CSTs) into five different categories based on composition and abundance of predominant bacterial types [19, 20] as follows: CST I-Lactobacillus crispatus, CSTII-L. gasseri, CST III-L. iners, CST V-L. jensenii, and CST IV- facultative and obligate anaerobes [15, 18]. In contrast to L. crispatus, which predominates in the VMBs of younger women (25–31 years), L. iners is found to predominately in the VMBs of older women (35 years) [21]. The VMB of pregnant individuals converges upon one that is Lactobacillus dominant over the course of pregnancy and studies have shown an association between early pregnancy non-Lactobacillus dominant CSTs and adverse pregnancy outcomes [22, 23]. Homeostatic disruptions to the baseline vaginal flora can lead to dysbiotic states including bacterial vaginosis (BV) that may double the risk of maternal infections like HIV, human papilloma virus (HPV), miscarriage, low birth weight, and PTB [12, 15, 21, 23].
Although several associations have been made between vaginal dysbiosis in people without HIV and adverse pregnancy outcomes, there are few studies investigating this link among women with HIV [14, 24]. People with HIV, who may have a Lactobacillus-deficient/anaerobe-dominant vaginal microbiota [25], have higher genital HIV VL [14, 25, 26], increased vaginal inflammation [25, 27, 28], and are more prone to vaginal microbial dysbiosis [25, 29]. It is largely unknown how ART impacts the VMB. Thus, it is logical to also investigate the role of vaginal dysbiosis in this population and its role in PTB pathogenesis.
The main objective of this scoping review is to assess the strength of the association between vaginal microbiota and VMB population dynamics, markers of mucosal inflammation, and PTB among PWLWH. By synthesizing and evidence-mapping existing literature, this review aims to identify gaps, inconsistencies, and research needed to guide future studies, clinical practice, and policy development.
2 |. Methods
2.1 |. Search Strategy and Information Sources
We conducted a comprehensive literature search, with the help of a medical librarian, to validate and extract pertinent findings from relevant articles. The World Health Organization’s definition of PTB “birth before 37 completed weeks of gestation” was used to formulate the review objective. A comprehensive search of research papers was conducted from PubMed, Scopus, and EMBASE with predefined inclusion and exclusion criteria using a search strategy outlined in Figure 1.
FIGURE 1 |.

From: Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
When the search terms “Vaginal microbiome,” “HIV,” and “PTB” were used on PubMed, a total of 1157 studies were found on Vaginal microbiomes in pregnancy, and 403 articles when “vaginal microbiota” were combined with “preterm birth” after running these search terms without “HIV.” However, to further slim down to relevant articles, Mesh terms like “HIV,” “Microbiota,” “Vaginal microbiome,” and “PTB” were used for PubMed, Scopus, and EMBASE as shown in Figure 2. A total of 24 studies were found from EMBASE and 27 studies from PubMed. After removing duplicates from both search engines, 13 articles based on Vaginal microbiomes and the risk of PTB in HIV-positive women were considered. PROSPERO, an international database of prospectively registered systematic reviews in health and social care, was also reviewed but no ongoing or completed systematic reviews were found on the same topic.
FIGURE 2 |.
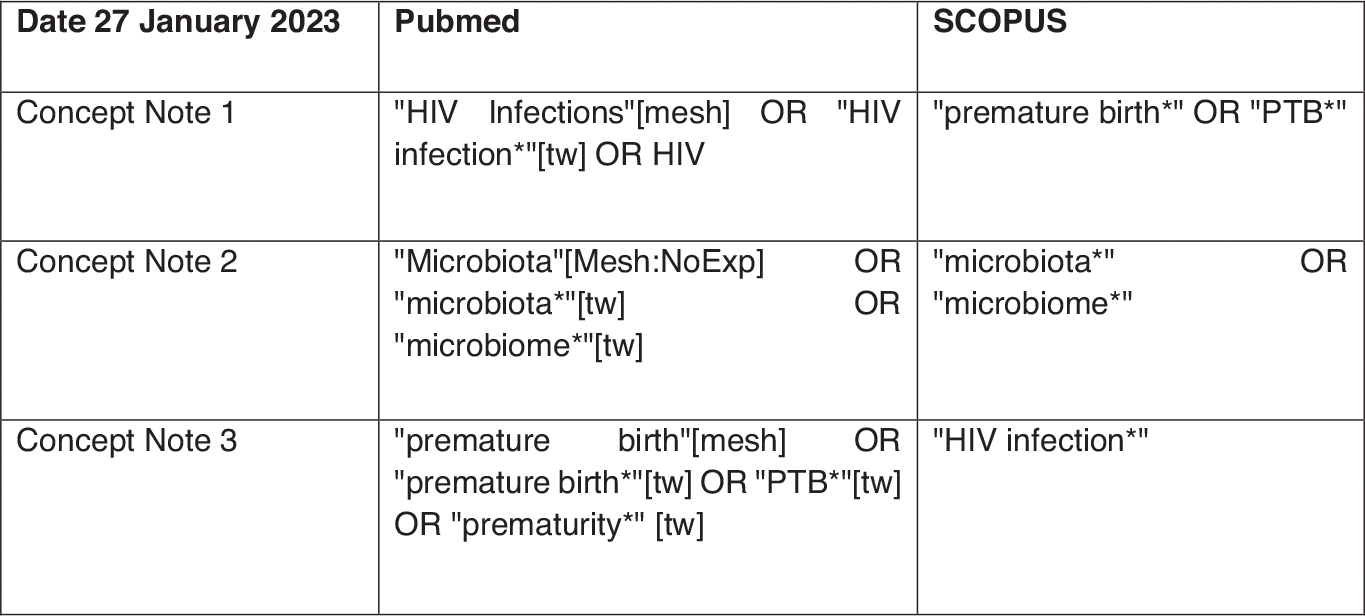
Mesh terms used to identify relevant studies.
Titles and abstracts were manually screened by the authors (F.K. & A.P.), and their references were manually checked to ensure relevant papers were not missed. The Prisma flow chart for study selection criteria is shown in Figure 1.
All studies that included pregnant women with HIV, used modern DNA sequencing technology (versus Nugent scoring) [30] to assess vaginal microbiota, and were written in the English language between January 2010 and April 2023 were included. Modern DNA technology was defined as the use of any of the following sequencing methods: shotgun metagenomics sequencing, amplicon sequencing/marker gene sequencing, and meta-transcriptomic sequencing [31]. Original prospective cohort and observational studies with quantitative data regarding maternal demography, pregnancy outcome, for example, twin deliveries and gestational age (GA) at birth were considered. All study settings were eligible for inclusion. Any study with ambiguous eligibility criteria, an inappropriate control group or no control group (HIV/non-HIV), or used only Nugent Score to diagnose BV instead of the latest DNA sequencing method was excluded. After applying the exclusion and inclusion criteria, only three studies were selected for the review with their specific characteristics and methods of swab collection outli in Table 1. These studies were conducted in hospital settings in the United Kingdom (2021) [24], Zambia (2022) [14], and Zimbabwe (2020) [29].
TABLE 1 |.
Study characteristics and methods of swab collection.
| Study design | Study setting | Study population | Specimen type | GA (weeks) at swab collection | Participant characteristics examined | ART at conception | DNA extraction | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Short et al. (2021) [24] | Prospective observational | HIV specialist & general antenatal clinics of ten London hospitals (UK) | 53 PWLWH 22 HUPW |
High vaginal swab. Soft cup for CVF in PWLWH |
For PWLWH T1: 16.0–21.9 T2:22.0–26.9 T3: 27–31.9 For HUPW vaginal sampling occurred at one second trimester time point |
Maternal age Ethnicity BMI Smoker Parity PTB risk factors Birth outcome |
n = 41/53 | 16s rRNA gene amplification at V1-V2 hypervariable regions |
| Price et al. [14] | Prospective cohort | Women & newborn hospital of the UTH in Lusaka (Zambia) | Total participants n = 461 Lost to follow up 88 HIV−ve 278 (term n = 259, sPTB n = 19) HIV +ve n = 85 (term n = 75, sPTB n = 10) |
Mid vaginal swab | Between 16 and 20 mean GA at first sample collection was 18 weeks (IQR: 17–19). Repeat specimen collection at 32 weeks for random subset of 66 PWLWH. 47 (71%) of these had cytokine analysis | Maternal age BMI GA Parity HIV seropositive Preconceptional ART |
n = 85 n = 44/75 (term) n = 6/10(sPTB) |
(WGS)sequencing |
| Gudza-Mugabe et al. [29] | Prospective cohort | Harare & Chitungwiza central hospital antenatal clinics (Zimbabwe) | Eligible women = 420 Overall = 356 passed sequencing quality control PWLWH = 42 |
2 vaginal swabs at single time | 29 weeks, IQR (25–33) | Maternal age GA Partner smoker ART regimen Gravida Parity Blood CD4 Plasma VL |
n = 40/42 TDF, FTC, EFV (36) AZT+3TC (4) |
16s rRNA gene amplification |
Abbreviations: UTH, university teaching hospitals; WGS, whole genome shotgun.
2.2 |. Data Extraction and Assessment of Risk of Bias
The assessment of study quality was conducted by two authors (F.K. and A.P.) using a tailored checklist derived from PRISMA-ScR [32] Table S1), and Covidence was employed for this evaluative process. Any duplicates were removed and data on study characteristics (setting, country, and study design), study population (age range, ethnicity, recruitment, and specific inclusion criteria) information on exposure (i.e., factors that may affect recorded VMB composition like GA at the time of swab collection, CST, and method of analysis) and outcome characteristics (estimated gestation of birth) were examined.
2.3 |. Cohort Characteristics
The main sociodemographic variables of interest from all studies included maternal age, GA at the time of swab collection, HIV status and use of anti-retroviral therapy (ART), maternal race, birth outcome, body mass index (BMI), and birth. Substantial heterogeneity was observed in the reported VMB characteristics and classification methods (Table S1). [24]
3 |. Results
The number of pregnancies per study was 75 (Short et al., UK) [24], 221 (Price et al., Zambia) [14], and 356 (Gudza-Mugabe et al., Zimbabwe) [29]. Among these pregnancies, those occurring in PWLWH were 53 (UK),85 (Zambia), and 42 (Zimbabwe) respectively. All three studies defined PTB as delivery before 37 completed weeks of pregnancy. Although Gudza-Mugabe et al. [29] did not specify if the PTBs were spontaneous or provider-initiated, Price et al. [14] excluded all provider-initiated PTBs from the analysis, and Short et al. [24] included both due to an overall small number of PTBs (n = 6). None of the studies provided information on early or late preterm. However, Price et al. mentioned that 16 had sPTB between 34 and 36 weeks and 14 had sPTB in < 34 weeks. All used modern DNA sequencing methods for the microbiome samples and calculated mean relative abundance (MRA), a component of biodiversity and measurement of the commonality or rarity of a species as compared to other species in defined locations [33]. The number of VMB subgroups in the three included studies are five, six, and seven (Table 2). All three studies measured different cytokines with at least 10 inflammatory markers in common: IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, and TNF-α.
TABLE 2 |.
Cross-study tapestry: Patterns in VMB classification.
| Short et al. [24] | Price et al. [14] | Gudza-Mugabe et al. [29] | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Group | VMB | MRA HUPW | MRA PWLWH | Group | VMB | MRA overall | Group | VMB | MRA overall |
|
| |||||||||
| VMB I | L. crispatus | 54% | 15% | mgClust1 | L. iners | 89% | CST I | L. crispastus | 30.6% |
| VMB II | L. gasseri | NA | NA | mgClust 2 | Mix of L. iners & Gardenella “others” | 54% & 26% | CST III | L. Iners | 25.8% |
| VMB III | L. iners | 32% | 36% | mgClust 3 | Gardenella type I | 70% | CST IVA | G. Vaginalis, BVAB1, and mixed diverse anaerobes | 25.85 |
| VMB IIIb | L. iners with presence of Gardnerella vaginalis | 1% | 15% | mgClust 4 | P. bivia, A. vaginae, & Gardenella | NA | CST IVB | G. Vaginalis, L. iners, A. vaginae and mixed diverse anaerobes | 15.4% |
| VMB IV | Diverse, high proportions of Atopobium, Gardnerella, Prevotella spp. and others | NA | 21% | mgClust 5 | L. iners, Gardenella type 2, & Ca. Lachnocurva Vaginae | NA | CST V | L. Jensenii dominant | 2.2% |
| VMB V | L. jensensii | 9% | NA | mgClust 6 | Gardenella type 2, L. iners, & P. bivia. | NA | |||
| mgClust 7 | L. crispastus, with few other lactobacilli | 86% | |||||||
Note: overall = clustering of all samples including HIV +ve.
Abbreviations: MRA, mean relative abundance; NA, not available; NR, not reported; VMB, vaginal microbiome.
The study by Short et al. [24] was a cross-sectional study conducted in the United Kingdom that sampled 75 women in total; 53 were PWLWH compared to 22 HIV uninfected pregnant women (HUPW). The majority of these women were Black (81% [43/53] vs. 23% [5/22]) with a median age 34 years (range 21–42) of PWLWH. Majority PWLWH (79%, 42/53) had fully suppressed VL, mean CD4 count within normal range [668 cells/mcL(range 356–1,505 cells/mcL) and 77% (41/53) were on ART at the time of conception while 23% (12/53) initiated during pregnancy. VMB samples were collected at three time points for PWLWH (16.0–21.9, 22.0–26.9, and 27–31.9 weeks of gestation), and one time point (second trimester) for HUPW. The GA was confirmed by ultrasound at 10–14 weeks. These samples were evaluated by using 16S rRNA gene sequencing and amplifying V1–V2 hypervariable regions (metataxonomics) [24]. Across all samples (n = 139, HIV = 117, uninfected = 22), 102 bacterial taxa were identified. The classification of all samples into the six vaginal microbiota types (VMB 1–6) based on relative abundance profiles was done by performing hierarchical clustering analysis (Ward linkage) on rarefied species level count data using ClustViz [34]. Cervico-vaginal fluid was collected in total of 79 matched samples collected from 49 PWLWH at three timepoints [24].
Analysis for vaginal microbiota of HUPW was dominated by Lactobacillus spp. with 54 % (12/22) having VMB I-type communities, 32% (7/22) VMB-III, 9% (2/22) VMB-V, and only one VMB IIIb sample. Conversely, the most common VMB in PWLWH was VMB-III (36%, 19/53), followed by VMB-IV (21%, 11/53), VMB-IIIb (15%, 8/53), and VMB-I (15%, 8/53) (Table 2). Higher vaginal bacterial diversity and richness and depleted amounts of Lactobacillus spp. were seen in PWLWH compared to HUPW. A positive correlation between vaginal bacterial diversity and richness and pro-inflammatory cytokines IL-1β and IL-8 were seen in PWLWH. There was one stillbirth and 6 (12%) PTBs among PWLWH compared to none among HUPW. All PTBs (n = 6) in PWLWH happened in the setting of VMB-III, VMB-IIIb, and VMB-IV with higher levels of IL-1β and TNF-α, while women with VMB-I, II, and V type delivered at term.
In contrast to HUPW, PWLWH demonstrated higher levels of Gardnerella and Prevotella spp. and lower levels of Lactobacillus species, specifically L. crispatus which is considered protective against adverse pregnancy outcomes such as PTB. These differences remained statistically significant even after correcting for multiple comparisons. The authors concluded that increased rates of unfavorable vaginal microbiota can cause vaginal inflammation in pregnancy which can lead to higher rates of PTB among PWLWH.
The second study was conducted by Price et al. [14] a prospective cohort study in Zambia, sampled 221 participants for secondary analysis, of which 192 (87%) delivered at term and 29 (13%) had sPTB. The GA was confirmed on ultrasound. Of 85 PWLWH (38% of cohort), 50/85 (59%) were on ART prior to conception, 39/85 (78%) had undetected VL, and 10 experienced sPTB (10/29, 34.4%). The first sample was collected at a median GA of 18 weeks (IQR:17–19) followed by repeat specimen collection between 24 and 36 weeks of gestation from a random subset of 66/85 PWLWH. Among these 66 PWLWH, 32 (48%) had undetected VL and had started ART before pregnancy while out of remaining 34(52%) PWLWH with detectable VL, 5(15%) had initiated ART before pregnancy. No other information is available if the ART was started for the remaining participants during pregnancy. They identified 201 bacterial species total, with L. iners found in all samples, all species of Gardenella genus in 99% of samples with mean Shannon diversity index (SDI) 0.93 (IQR: 0.47–1.50) across all 287 samples. They classified these microbiomes into seven metagenomic clusters (mgClust) using the non-redundant VIRGO catalog [35] (Table 2).
In this study, Gardnerella “other” was most common in baselined samples of HUPW (n = 77/136, 57%) followed by PWLWH with an undetectable virus (n = 16/44.36%) and least common in PWLWH with a detectable virus (n = 10/41, 24%). However, Gardenella type 2 was most common in PWLWH (n = 24/41, 59%) followed by PWLWH with undetectable virus (22/44, 50%) and least common in HUPW (n = 30/136, 22%). They analyzed associations between specific microbial MRA and sPTB among baseline samples collected at 16–20 weeks of gestation for both HUPM and PWLWH. The study found that MRA of L. iners > 26% (PR 2.5; 95% CI: 1.2, 5.2; p = 0.02), Gardnerella “other” > 0.3%, and Gardnerella type 2 > 50% (PR 2.6; 95% CI 1.1–6.4; p = 0.03) were significantly associated with sPTB respectively (p ≤ 0.03), and this association remained significant in models that were adjusted for HIV and VL [14]. The MRA of L. iners and Gardnerella “other” were similar by maternal HIV status, but women with HIV had higher MRA of Gardnerella type 2 (23%; p = 0.001). Women with term delivery had higher MRA of Gardnerella type 1 as compared to those who experienced sPTB (14% vs. 3%; p = 0.001). Gardnerella type 1 MRA also remained higher among HUPW in contrast to PWLWH (15% vs. 10%; p = 0.08). Finally, MRA of L. crispatus was alike between samples from term compared to sPTB (13% vs. 15%; p = 0.9), but was lesser among women with HIV compared to those without HIV (3% vs. 20%; p < 0.001) [14].
In a group of 192 individuals delivered at term, 10 (5%) in baseline samples belonged to metagenomics Cluster 2 while 5 out of 16 (33%) with sPTB between 34 and 36 weeks were in the metagenomics cluster 2 (PR 6.9 [95% CI: 2.6–18.3], p < 0.001). Higher levels of vaginal inflammation, measured by vaginal inflammatory scores created by confirmatory factor analysis [36], were among the sPTB cohort (median 0.90, IQR: 0.03–1.28) compared to the term birth cohort (median 0.50, IQR: 0.73–1.04). The concentrations of IL-1β, IL-10, and sCD 14 were found to be linked with increased SDI values. Conversely, IL-2, IL12p70, SLPI, and IL-6 showed a slight association with reduced SDI values. Mean SDI was higher in PWLWH with undetectable VL (1.17; p = 0.01) and highest in those with detectable VL (1.31; p < 0.001) in contrast to HUPW (0.74). It is worth noting that 9(14%) PWLWH with repeat samples at 32 weeks, had sPTB and the SDI increased between baseline and repeat sample collection. PWLWH had higher alpha diversity and anaerobe-rich vaginal microbiota in contrast to women without HIV. The study observed that women who started ART before conception had reduced vaginal inflammation compared to those who started during pregnancy (p = 0.02).
The third cross-sectional study conducted at two hospitals in Zimbabwe by Gudza-Mugabe et al. [29] recruited 420 eligible women, of which 356 fulfilled the criteria and 42/356 (12%) were PWLHW. The mean CD4 count of PWLWH was 495 cells/mm3. Majority of them (95%, 40/42) were on ART with 36/40 (90%) on the fixed-dose combination of tenofovir, lamivudine, and efavirenz and 4/40(10%) were on combivir. No details were provided about the remaining two women if they started ART during pregnancy. There were 42 PTBs reported with 33 (15.3%) in HUPW and 9 (31.0%) in PWLWH. Of these 9 PTBs among PWLWH, 7 (77.8%) were among those taking ART preconceptionally. The median gestation age for single-time vaginal sample collection was 29 weeks (IQR 25–33). However, the authors acknowledged that as GA was not confirmed via ultrasound, this might be inaccurate. A total of 356 samples were analyzed at the V4 hypervariable region of the 16sRNA gene and the VMB was clustered into five CSTs as described by Romero et al. [37] (Table 2).
The study reports that PWLWH had significantly more diverse vaginal microbiota than HUPW (mean log2 SDI of 1.66 versus 1.04, p = 0.004). Measurement of 27 cytokines in the vaginal fluid was clustered in high and low inflammatory groups by using PAM clustering of cytokines. No association was found between VMB and cytokine concentrations with an R > 0.5. Similarly, no association was observed between HIV status and the high inflammation group. PTB was independently associated with HIV (OR 3.80; 95% CI, 1.08–12.82; p = 0.004). Similarly, CST-IVA and CST-IVB, more common in PWLWH, were also independently significantly associated with HIV status (OR 6.08; 95% CI, 1.87–24.2; p = 0.005 and OR 6.31; 95% CI, 1.55–28.91; p = 0.018 respectively). The authors found a correlation between HIV status and PTB (OR, 2.73; 95%CI, 1.01–7.42), but it was independent of vaginal microbiota.
4 |. Discussion
To our knowledge, this is the first scoping review to examine the association of VMB diversity and richness in HIV-positive women and PTB deliveries. All three studies included in the review confirmed increased vaginal microbial diversity among PWLWH. Despite methodological differences among these studies in terms of categorizing the VMB, Short et al. and Price et al. found a correlation between non-Lactobacillus dominant VMBs and inflammatory markers that may be responsible for sPTB [14]. However, strong correlations are difficult to make due to the heterogeneity of methodologies among the sample population of these studies.
The study by Short et al. [24] found that a higher abundance of anaerobic Prevotella, Sneathia, and Dialister was associated with PTB while the absence of L. crispatus was not linked with PTB. However, strong conclusions cannot be drawn due underpowered sample size and small number of PTBs in PWLWH. These results are in contrast to Gudza-Mugabe et al. [29] who did not find any association between vaginal microbiota, vaginal cytokine, and PTB. This disparity may be explained partly by variations in study design, particularly the GA of sample collection and the technique used for swab collection or genital tract fluid collection, which was not explicitly reported and underpowered the sample size.
ART significantly reduces the risk of perinatal transmission of HIV though there are conflicting results in the literature regarding the link between ART exposure and premature birth [5]. To inform better clinical management strategies for PWLWH, it may be important to ascertain the relative contributions of these factors, such as timing of ART initiation, on the VMB during pregnancy. The effects may be small so individual studies may not be adequately powered to assess any versus no ART exposure on the VMB or even classify exposures by different drug classes.
The absence of increased vaginal inflammation linked to HIV infection in this cohort raises the possibility that pregnancy itself may reduce inflammation linked to vaginal microbiota enough to overcome any effects from HIV. A potential explanation is that during pregnancy, endocrine and immunological systems behave somewhat paradoxically, shifting to both higher and lower levels to adjust for a modified inflammatory response and successful reproduction [38]. Future studies should investigate whether pregnancy-specific factors help to reduce inflammation in this population. The finding of an independent association between HIV and sPTB independent of VMB type is in accord with other studies that report a positive correlation of HIV infection and increased chances of PTB [5, 6, 39].
Vaginal dysbiosis may be associated with an increased risk for PTB among PWLWH during pregnancy though HIV may increase PTB risk independently of the VMB. The distribution of these VMB among PWLWH also varies based on their status of viral suppression.
4.1 |. Limitations
Results should be interpreted with caution due to intra-study heterogeneity. Among populations of PWLWH, living in a high versus low-income setting may result in vastly different VMB composition and chemical and environmental exposures as well as ART regimens and opportunities to initiate therapy. Additionally, future studies should seek to describe study population demographics beyond self-reported race. Race is a social versus biological construct, and thus obscures the impact of additional influences such as socioeconomic status, chemical and environmental exposure, and acute and chronic life stressors [9]. Other sources of heterogeneity within the included studies include their design, GA at sampling, number of samples collected per participant, and the VMB classification. Furthermore, the GA at which vaginal samples were collected was not clearly defined in all of the studies or incorporated in the analyses, which could interfere with interpretation and reproducibility of results. Although all three studies demonstrated strengths in their methodologies, potential biases such as selection bias at collecting samples on different gestations or only once during pregnancy and variations in data collection approaches were identified. These limitations make it difficult to draw strong conclusions on the association between VMBs, HIV status, and PTB.
Future longitudinal studies should account for additional factors that impact VMB composition: timing of most recent antibiotic use, timing of most recent intercourse, vaginal douching, diet, and BMI [40]. Additional data in PWLWH will facilitate an enhanced comprehension of pathophysiology and individualized risk profiles, thereby enabling the customization of interventions tailored to distinct populations.
5 |. Conclusion
Although it is unclear if increased microbial diversity in PWLWH or increased vaginal inflammation contribute more to PTB, HIV does appear to alter the VMB in pregnant individuals but also affects PTB in microbiome-independent pathways. Given the limited number of studies, heterogeneity in sample size, sample collection methods, difference in analysis, and inconsistent results it is hard to establish a firm association between HIV, VMBs, cytokines, and adverse pregnancy outcomes in PWLWH. Based on the proportion of PTB by HIV status, these studies are underpowered to detect the outcome differences by PTB. Therefore, at this time we cannot definitively say that HIV-positive status will lead to vaginal dysbiosis thus sPTB. Future studies will also need to investigate the role and timing of ART initiation and its effect on vaginal microbiota. Reporting VMB classifications in studies in a standardized manner would help to create uniformity for future research studies. Future research with robust research methodologies, diverse participant’s characteristics, and larger sample size may ultimately be required to identify factors that may predict or modulate PTB risk in pregnant individuals with HIV.
Supplementary Material
Additional supporting information can be found online in the Supporting Information section.
Funding:
A.M.P. was funded by National Institute of Allergy and Infectious Diseases (K23AI155296).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Preterm birth, accessed May 4, 2023, https://www.who.int/news-room/fact-sheets/detail/preterm-birth. [Google Scholar]
- 2.Gudnadottir U, Debelius JW, Du J, et al. , “The Vaginal Microbiome and the Risk of Preterm Birth: A Systematic Review and Network Meta-analysis,” Scientific Reports 12 (2022): 7926, 10.1038/s41598-022-12007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A profile of prematurity of United States. March of Dimes | PeriStats, accessed May 4, 2023, https://www.marchofdimes.org/peristats/reports/united-states/prematurity-profile?reg=99. [Google Scholar]
- 4.Tukei VJ, Hoffman HJ, Greenberg L, et al. , “Adverse Pregnancy Outcomes among HIV-Positive Women in the Era of Universal Antiretroviral Therapy Remain Elevated Compared With HIV-Negative Women,” Pediatric Infectious Disease Journal 40, no. 9 (2021): 821–826, 10.1097/INF.0000000000003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones AJ, Eke UA, and Eke AC, “Prediction and Prevention of Preterm Birth in Pregnant Women Living With HIV on Antiretroviral Therapy,” Expert Review of Anti-Infective Therapy 20, no. 6 (2022): 837–848, 10.1080/14787210.2022.2046463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert AYK, Elwood C, Wagner EC, et al. , “Investigation of Factors Associated With Spontaneous Preterm Birth in Pregnant Women Living With HIV,” Aids 34, no. 5 (2020): 719, 10.1097/QAD.0000000000002464. [DOI] [PubMed] [Google Scholar]
- 7.Kaltsas A, Moustakli E, Zikopoulos A, et al. , “Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring,” Genes 14, no. 2 (2023): 486, 10.3390/genes14020486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain VG, Monangi N, Zhang G, and Muglia LJ, “Genetics, Epigenetics, and Transcriptomics of Preterm Birth,” American Journal of Reproductive Immunology (New York, NY: 1989) 88, no. 4 (2022): e13600, 10.1111/aji.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadley M, Oppong AY, Coleman J, and Powell AM, “Structural Racism and Adverse Pregnancy Outcomes Through the Lens of the Maternal Microbiome,” Obstetrics and Gynecology 142, no. 4 (2023): 911–919, 10.1097/AOG.0000000000005345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, and Lumbiganon P, “The Global Epidemiology of Preterm Birth,” Best Practice & Research Clinical Obstetrics & Gynaecology 52 (2018): 3–12, 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Dinsmoor MJ, Ugwu LG, Bailit JL, et al. , “Short-Term Neonatal Outcomes of Pregnancies Complicated by Maternal Obesity,” American Journal of Obstetrics and Gynecology MFM 5, no. 4 (2023): 100874, 10.1016/j.ajogmf.2023.100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mboya IB, Mahande MJ, Obure J, and Mwambi HG, “Predictors of Singleton Preterm Birth Using Multinomial Regression Models Accounting for Missing Data: A Birth Registry-Based Cohort Study in Northern Tanzania,” PLoS ONE 16, no. 4 (2021): e0249411, 10.1371/journal.pone.0249411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elenga N, Djossou FL, and Nacher M, “Association Between Maternal Human Immunodeficiency Virus Infection and Preterm Birth,” Medicine 100, no. 4 (2021): e22670, 10.1097/MD.0000000000022670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JT, Vwalika B, France M, et al. , “HIV-associated Vaginal Microbiome and Inflammation Predict Spontaneous Preterm Birth in Zambia,” Scientific Reports 12 (2022): 8573, 10.1038/s41598-022-12424-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Lu Y, Chen T, and Li R, “The Female Vaginal Microbiome in Health and Bacterial Vaginosis,” Frontiers in Cellular and Infection Microbiology 11 (2021): 631972, 10.3389/fcimb.2021.631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redelinghuys MJ, Geldenhuys J, Jung H, and Kock MM, “Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities,” Frontiers in Cellular and Infection Microbiology 10 (2020): 354, https://www.frontiersin.org/articles/10.3389/fcimb.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceccarani C, Foschi C, Parolin C, et al. , “Diversity of Vaginal Microbiome and Metabolome During Genital Infections,” Scientific Reports 9 (2019): 14095, 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verstraelen H, Vieira-Baptista P, De Seta F, Ventolini G, Lonnee-Hoffmann R, and Lev-Sagie A, “The Vaginal Microbiome: I. Research Development, Lexicon, Defining “Normal” and the Dynamics Throughout Women’s Lives,” Journal of Lower Genital Tract Disease 26, no. 1 (2021): 73–78, 10.1097/LGT.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravel J, Gajer P, Abdo Z, et al. , “Vaginal Microbiome of Reproductive-Age Women,” Proceedings of the National Academy of Sciences 108, no. Suppl 1 (2011): 4680–4687, 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.France MT, Ma B, Gajer P, et al. , “VALENCIA: A Nearest Centroid Classification Method for Vaginal Microbial Communities Based on Composition,” Microbiome 8, no. 1 (2020): 166, 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redelinghuys MJ, Ehlers MM, Dreyer AW, and Kock MM, “Normal Flora and Bacterial Vaginosis in Pregnancy: An Overview,” Critical Reviews in Microbiology 42, no. 3 (2016): 352–363, 10.3109/1040841X.2014.954522. [DOI] [PubMed] [Google Scholar]
- 22.Freitas AC, Chaban B, Bocking A, et al. , “The Vaginal Microbiome of Pregnant Women Is Less Rich and Diverse, With Lower Prevalence of Mollicutes, Compared to Non-Pregnant Women,” Scientific Reports 7, no. 1 (2017): 9212, 10.1038/s41598-017-07790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebedeva OP, Popov VN, Syromyatnikov MY, et al. , “Female Reproductive Tract Microbiome and Early Miscarriages,” APMIS 131, no. 2 (2023): 61–76, 10.1111/apm.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Short CES, Brown RG, Quinlan R, et al. , “Lactobacillus-Depleted Vaginal Microbiota in Pregnant Women Living with HIV-1 Infection Are Associated With Increased Local Inflammation and Preterm Birth,” Frontiers in Cellular and Infection Microbiology 10 (2021): 596917, 10.3389/fcimb.2020.596917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chávez-Torres M, Gómez-Palacio-Schjetnan M, Reyes-Terán G, et al. , “The Vaginal Microbiota of Women Living With HIV on Suppressive Antiretroviral Therapy and Its Relation to High-Risk Human Papillomavirus Infection,” BMC Microbiology 23 (2023): 21, 10.1186/s12866-023-02769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell C, Balkus JE, Fredricks D, et al. , “Interaction Between Lactobacilli, Bacterial Vaginosis-Associated Bacteria, and HIV Type 1 RNA and DNA Genital Shedding in U.S. and Kenyan Women,” Aids Research and Human Retroviruses 29, no. 1 (2013): 13–19, 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosmann C, Anahtar MN, Handley SA, et al. , “Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated With Increased HIV Acquisition in Young South African Women,” Immunity 46, no. 1 (2017): 29–37, 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgdorff H, Tsivtsivadze E, Verhelst R, et al. , “Lactobacillus-Dominated Cervicovaginal Microbiota Associated With Reduced HIV/STI Prevalence and Genital HIV Viral Load in African Women,” ISME Journal 8, no. 9 (2014): 1781–1793, 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudza-Mugabe M, Havyarimana E, Jaumdally S, et al. , “Human Immunodeficiency Virus Infection Is Associated With Preterm Delivery Independent of Vaginal Microbiota in Pregnant African Women,” Journal of Infectious Diseases 221, no. 7 (2020): 1194–1203, 10.1093/infdis/jiz584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, and Hillier SL, “Reliability of Diagnosing Bacterial Vaginosis Is Improved by a Standardized Method of Gram Stain Interpretation,” Journal of Clinical Microbiology 29, no. 2 (1991): 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight R, Vrbanac A, Taylor BC, et al. , “Best Practices for Analysing Microbiomes,” Nature Reviews Microbiology 16, no. 7 (2018): 410–422, 10.1038/s41579-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 32.Tricco AC, Lillie E, Zarin W, et al. , “PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation,” Annals of Internal Medicine 169, no. 7 (2018): 467–473, 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 33.Barlow JT, Bogatyrev SR, and Ismagilov RF, “A Quantitative Sequencing Framework for Absolute Abundance Measurements of Mucosal and Lumenal Microbial Communities,” Nature Communications 11, no. 1 (2020): 2590, 10.1038/s41467-020-16224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metsalu T and Vilo J, “ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap,” Nucleic Acids Research 43, no. Web Server issue (2015): W566–W570, 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma B, France MT, Crabtree J, et al. , “A Comprehensive Non-Redundant Gene Catalog Reveals Extensive Within-Community Intraspecies Diversity in the human Vagina,” Nature Communications 11, no. 1 (2020): 940, 10.1038/s41467-020-14677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittenhouse KJ, Mwape H, Nelson JAE, et al. , “Maternal HIV, Antiretroviral Timing, and Spontaneous Preterm Birth in an Urban Zambian Cohort: The Role of Local and Systemic Inflammation,” AIDS (London, England) 35, no. 4 (2021): 555–565, 10.1097/QAD.0000000000002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Hassan SS, Gajer P, et al. , “The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different From That of Non-Pregnant Women,” Microbiome 2 (2014): 4, 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schminkey DL and Groer M, “Imitating a Stress Response: A New Hypothesis About the Innate Immune System’s Role in Pregnancy,” Medical Hypotheses 82, no. 6 (2014): 721–729, 10.1016/j.mehy.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Brocklehurst P and French R, “The Association Between Maternal HIV Infection and Perinatal Outcome: A Systematic Review of the Literature and Meta-Analysis,” BJOG: An International Journal of Obstetrics and Gynaecology 105, no. 8 (1998): 836–848, 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Theis KR, Gomez-Lopez N, et al. , “The Vaginal Microbiota of Pregnant Women Varies With Gestational Age, Maternal Age, and Parity,” Microbiology Spectrum 11, no. 4: e03429–22, 10.1128/spectrum.03429-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Short CES, Quinlan RA, Wang X, et al. , “Vaginal Microbiota, Genital Inflammation and Extracellular Matrix Remodelling Collagenase: MMP-9 in Pregnant Women with HIV, a Potential Preterm Birth Mechanism Warranting Further Exploration,” Frontiers in Cellular and Infection Microbiology 11 (2021): 750103, 10.3389/fcimb.2021.750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price JT, Vwalika B, Hobbs M, et al. , “Highly Diverse Anaerobe-Predominant Vaginal Microbiota Among HIV-Infected Pregnant Women in Zambia,” PLoS ONE 14, no. 10 (2019): e0223128, 10.1371/journal.pone.0223128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayigga L, Kateete DP, Anderson DJ, Sekikubo M, and Nakanjako D, “Diversity of Vaginal Microbiota in Sub-Saharan Africa and Its Effects on HIV Transmission and Prevention,” American Journal of Obstetrics and Gynecology 220, no. 2 (2019): 155–166, 10.1016/j.ajog.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Wijgert J and Jespers V, “The Global Health Impact of Vaginal Dysbiosis,” Research in Microbiology 168, no. 9 (2017): 859–864, 10.1016/j.resmic.2017.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


