Abstract
In retinal rods, light-induced isomerization of 11-cis-retinal to all-trans-retinal within rhodopsin triggers an enzyme cascade that lowers the concentration of cGMP. Consequently, cyclic nucleotide-gated (CNG) ion channels close, generating the first electrical response to light. After isomerization, all-trans-retinal dissociates from rhodopsin. We now show that all-trans-retinal directly and markedly inhibits cloned rod CNG channels in excised patches. 11-cis-retinal and all-trans-retinol also inhibited the channels, but at somewhat higher concentrations. Single-channel analysis suggests that all-trans-retinal reduces average open probability of rod CNG channels by inactivating channels for seconds at a time. At physiological cGMP levels, all-trans-retinal inhibited in the nanomolar range. Our results suggest that all-trans-retinal may be a potent regulator of the channel in rods during the response to bright light, when there is a large surge in the concentration of all-trans-retinal.
Cyclic nucleotide-gated (CNG) ion channels are directly opened by the binding of cyclic nucleotides (reviewed in refs. 1–9). They play a critical role in vision (reviewed in refs. 10 and 11) and olfaction (reviewed in refs. 12–14), and have been identified in a large variety of other tissues, including heart, brain, and kidney.
Retinoids, which are derivatives of vitamin A, represent a class of lipophilic substances with diverse and important physiological roles. They are involved not only in visual transduction (reviewed in refs. 15 and 16), but also in regulating gene transcription in development (reviewed in refs. 17–19), and in immune responses (reviewed in ref. 20). Furthermore, retinoids have been found to modulate several ion channels, including Ca2+ channels (21) and K+ channels (22) in lymphocytes, neuronal Na+ channels (23), and gap junction channels in retinal horizontal cells (24, 25). In photoreceptors, retinoids have been found to stimulate the ABCR transporter, apparently by acting as substrates (26), and to modulate Ca2+ channels with a possible role in the regulation of neurotransmitter release (27).
In rod cells, the visual process begins when 11-cis-retinal, covalently bound to opsin within the visual pigment rhodopsin, absorbs a photon and isomerizes to all-trans-retinal. This isomerization causes rhodopsin to activate a G-protein-mediated cascade that decreases [cGMP], closing CNG channels in the rod outer segment plasma membrane (reviewed in refs. 10 and 11). After isomerization, all-trans-retinal dissociates from opsin and associates with cellular retinoid binding proteins and with membranes, including the rod outer segment plasma membrane. Within the rod outer segment, all-trans-retinal is eventually converted to all-trans-retinol, which must be transported by interphotoreceptor retinoid binding protein from the rod to the retinal pigment epithelial cells (15, 28), where it can be converted back into 11-cis-retinal. The newly formed 11-cis-retinal is then shuttled to the rod where it inserts into opsin, reforming photoactivatable rhodopsin.
Exposure of rods to bright light causes a prolonged shutdown of the “dark current” (i.e., closure of CNG channels) and a desensitization of the photoresponse, known as bleaching adaptation. Behaviorally, this produces a lingering perception of light (an “after-image”) long after the bright light is terminated. Some have proposed that this desensitization results from activation of the G protein, transducin, by the free opsin protein (reviewed in ref. 29). Others suggest that a complex formed by a noncovalent interaction between liberated all-trans-retinal and opsin may activate transducin and participate in desensitization (30, 31).
Here we present evidence that there may be an additional pathway in the bleaching response that involves inhibition of rod CNG channels by all-trans-retinal. At physiological cGMP concentrations, this inhibition occurred in the nanomolar range. All-trans-retinal appeared to be a closed-state inhibitor that decreased the average open probability of cloned rod CNG channels by inactivating the channels for many seconds at a time. Other retinoids (11-cis-retinal and all-trans-retinol) also inhibited the channels, but with somewhat higher IC50 values. Preliminary reports of this work have appeared elsewhere.†,‡
Experimental Procedures
Expression of Channels in Xenopus Oocytes.
The plasmids containing cDNA for the bovine rod α (CNGA1), the rat olfactory α (CNGA2) and the bovine rod β (CNGB1) CNG channel clones were kindly provided by William N. Zagotta, Randall R. Reed, and Robert S. Molday, respectively. See Richards and Gordon (5) for other terminology for these channels. The vectors contained the untranslated sequence of the Xenopus β-globin gene to promote high protein expression in oocytes (32). cRNA was made by in vitro transcription using either Promega's RiboMAX Large Scale RNA Production system or Ambion's mMessage mMachine (Austin, TX).
Portions of ovaries were surgically removed from anesthetized Xenopus laevis frogs, and individual oocytes were isolated by treatment with 1 mg/ml collagenase type 1A (Worthington) in a low-calcium solution (82.5 mM NaCl/2.5 mM KCl/5 mM Hepes/1 mM MgCl2, pH 7.6). The cRNA was injected into Xenopus oocytes by using a Drummond “NANOJECT” injector (Broomall, PA). The injected oocytes were incubated at 14–16°C for 1–12 days before patch clamp experiments. For single-channel studies, the oocytes were stored at 4°C once a desirable level of expression was reached (usually 1 day after injection). The oocyte storage solution contained 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 5 mM Hepes, 1 mM MgCl2, 2.5 mM pyruvic acid, 100 units/ml penicillin, and 100 μg/ml streptomycin, at pH 7.6. The vitelline membrane was removed by mechanical dissection after treatment with a hypertonic solution (200 mM N-methyl-d-glucamine/2 mM KCl/10 mM EGTA/10 mM Hepes/1 mM MgCl2, pH 7.4).
Electrophysiological Solutions and Their Application.
A plastic Petri dish was the cell chamber for patch clamp experiments, and water soluble solutions were applied by using a 36-solution patch perfusion system, the RSC-100 rapid solution changer (Molecular Kinetics, Pullman, WA). Both sides of the patches were bathed in a low-divalent sodium solution consisting of 130 mM NaCl, 0.2 mM EDTA, and 2 mM Hepes buffer (pH 7.2). The solution bathing the intracellular surface of the patch contained various concentrations of cGMP (Sigma). Niflumic acid (500 μM; Sigma) was added to the extracellular (i.e., pipette) solution to block Ca2+-activated Cl− channels that are endogenous to the oocytes.
Retinoid stocks were made in 100% ethanol. All stocks were kept in brown glass vials covered with aluminum foil, and stored at −80°C or −20°C. The purity of each retinoid solution was checked by HPLC after the experiments. Because of the lipophilicity of the retinoids, they were not applied to the patches by perfusion through plastic tubing; instead, they were applied by direct addition of stock to the bathing solution in the Petri dish, followed immediately by very vigorous mixing with a transfer pipette. We found that 0.1% ethanol (the greatest concentration applied in the retinoid solutions) had no effect on cGMP-activated current or on the seal resistance. Petri dishes and agar bridges were replaced after each experiment with retinoids (i.e., between patches). All-trans-retinal and -retinol stock solutions were made up and applied to the patches in room light; the 11-cis-retinal stock was made up and applied under infrared illumination. 11-cis-retinal was generously provided by Rosalie Crouch (by means of the National Eye Institute of the National Institutes of Health, Bethesda). The other two retinoids were obtained from Sigma.
Electrophysiological Recordings and Analysis.
Standard patch clamp methods were used. Data were collected from inside-out patches in the steady state after any spontaneous increases in apparent cGMP affinity for the rod channel. These changes have been attributed to dephosphorylation by endogenous patch-affiliated phosphatases (33, 34) and were monitored by sampling the current periodically at a low concentration of cGMP (below the K1/2) while incubating the patch in saturating cGMP and waiting for the monitored current to stabilize. The time required for this stabilization ranged from approximately 10 to 40 min. The olfactory channel did not demonstrate a spontaneous change in apparent cGMP affinity.
For retinoid dose-response curves, the bath usually contained a saturating concentration of cGMP (2 mM for rod and 100 μM for olfactory channels), and each retinoid was added in small increments. The current was monitored for several minutes after each addition of retinoid to ensure that the effect of the retinoid had stabilized (i.e., that the inhibition had reached steady-state). This time may have been required for the retinoids to insert into the membrane.
Pipette openings were typically 0.5 to 20 μm in diameter, with resistances of 0.6–15 MΩ. All recordings were obtained at room temperature (20–25°C). Macroscopic currents from multichannel patches were recorded as voltage was changed from −100 to +100 mV in 50 mV steps, from a holding potential of 0 mV. The leak currents obtained in the absence of cGMP were subtracted from each record. Macroscopic currents were measured in the steady state after voltage-dependent gating (35) and before significant ion depletion effects (36). For single-channel studies, records were obtained for several seconds, with membrane voltage held continuously at +80 mV. For these recordings, the pipettes were coated with dental wax before polishing to reduce electrical noise caused by pipette capacitance.
Patch currents were recorded by using an Axopatch 1B or 200 patch clamp amplifier (Axon Instruments, Foster City, CA) with analog-to-digital converters to a Macintosh Quadra or G4 computer running Pulse software (Instrutech, Port Washington, NY). Before digitization, the data were low-pass filtered by an 8-pole bessel filter (Frequency Devices, Haverhill, MA). The filter cutoff frequency (−3-dB point) was 2 kHz for multichannel patches and 5 kHz for single-channel patches. Sampling rates were at least five times the filter cutoff frequency to prevent aliasing. Data analysis was performed by using the igorpro software package (WaveMetrics, Lake Oswego, OR). Analysis of single-channel records was performed by using standard routines included in the pulse and igor software packages.
Results
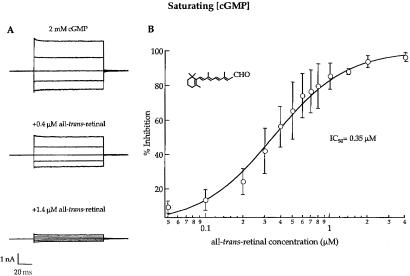
Inhibition of rod CNG channels by the retinoids was studied in inside-out patches excised from Xenopus oocytes expressing cloned homomultimeric CNGA1 channels. Fig. 1A shows families of macroscopic cGMP-activated currents recorded from a multichannel patch at several voltages. Addition of all-trans-retinal to the solution bathing the intracellular surface of the patch reduced the current in a dose-dependent manner at all voltages. Currents were recorded with saturating (2 mM) cGMP, and were monitored for several minutes after each addition of retinoid to ensure that the inhibition had reached steady-state. This length of time may have been required for the retinoids to insert into the membrane. Similar inhibition was seen with heteromeric (CNGA1/CNGB1) rod channels (data not shown). We were only able to achieve approximately 60% recovery of the current on washout (data not shown), presumably because it is difficult to remove the retinoids from the membrane. Fig. 1B presents an average dose-response relation for inhibition by all-trans-retinal for 5 patches, with all currents recorded at +100 mV in the presence of 2 mM cGMP. The IC50 for inhibition of the rod channel by all-trans-retinal at saturating cGMP was 0.35 μM.
Figure 1.
In the presence of a saturating (2 mM) concentration of cGMP, rod channels are inhibited by all-trans-retinal. Data were measured from multichannel, inside-out patches of homomultimeric (CNGA1 only) rod channels. The families of cGMP-activated currents were recorded in response to voltage jumps ranging from −100 to +100 mV in steps of 50 mV, from a holding potential of 0 mV. Currents measured in the absence of cGMP were subtracted from all traces. (A) Current families demonstrating inhibition at saturating cGMP: control, 0.4 μM all-trans-retinal (40% inhibition), and 1.4 μM all-trans-retinal (87% inhibition). (B) Dose-response relation for inhibition by all-trans-retinal in saturating cGMP. Steady-state, cGMP-activated currents were measured at +100 mV from several patches at increasing concentrations of all-trans-retinal added to the solution bathing the “inside” surface of the patch. Averaged data were fit with the Hill equation, IN/INMAX = [all-trans-retinal]n/(IC50n + [all-trans-retinal]n), where IN is percent inhibition, INMAX is maximal inhibition, IC50 is the concentration of all-trans-retinal required to achieve half maximal inhibition, and n is the Hill coefficient. Data points are averaged values from 5 patches, and plotted with SD (error bars). INMAX = 100%; IC50 = 0.35 μM; and n = 1.5.
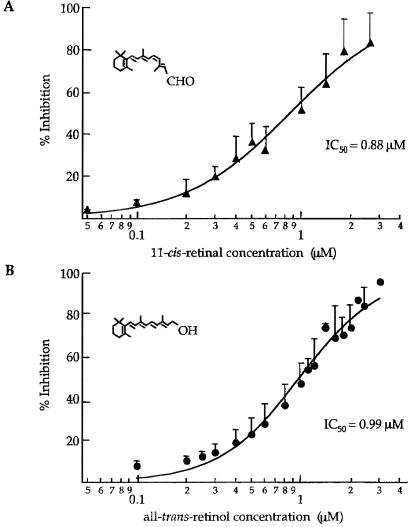
The rod CNG channel was also inhibited by 11-cis-retinal and by all-trans-retinol, although these retinoids demonstrated higher IC50's than that for all-trans-retinal. Fig. 2 shows dose-response relations for these two retinoids, measured at +100 mV. The IC50's were 0.88 μM for 11-cis-retinal and 0.99 μM for all-trans-retinol. All-trans-retinal was used for the remainder of the experiments described here because of its somewhat higher apparent affinity and its putative role in the bleaching response in rods (see Discussion).
Figure 2.
Rod channels are inhibited by other retinoids less potently than by all-trans-retinal. (A) Dose–response relation for inhibition by 11-cis-retinal in saturating cGMP. Points are mean values from 2 to 4 patches along with SD (error bars). INMAX = 100%; IC50 = 0.88 μM; and n = 1.36. (B) Dose-response relation for inhibition by all-trans-retinol in saturating cGMP. Points are mean values from 2 to 4 patches along with SD (error bars). INMAX = 100%; IC50 = 0.99 μM; and n = 1.77.
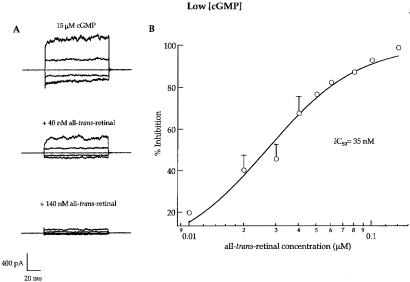
The rod channel was much more sensitive to all-trans-retinal at low cGMP concentrations that are much closer to the levels (a few micromolar) expected in vivo (reviewed in ref. 29). Fig. 3 presents data like those in Fig. 1, except that the concentration of cGMP was only 15 μM, giving currents that were only about 8% of those obtained with saturating (2 mM) cGMP. The IC50 for inhibition by all-trans-retinal at this lower cGMP concentration was 35 nM, or only a tenth of that at saturating cGMP. Thus, all-trans-retinal appears to be a more effective inhibitor at low cGMP concentrations, either because it inhibits closed channels more effectively than open channels, or because it prefers unliganded channels.
Figure 3.
Inhibition of rod channels by all-trans-retinal is more pronounced at low concentrations of cGMP. Recordings shown in A were made as those shown in Fig. 1, except that the bath concentration of cGMP was far below saturating, eliciting only ≈8% of the maximal current evoked by a saturating (2 mM) cGMP concentration. (A) Current families demonstrating inhibition at low (15 μM) cGMP: control, 40 nM all-trans-retinal (62% inhibition), and 140 nM all-trans-retinal (99% inhibition). (B) Dose–response relation for inhibition by all-trans-retinal in low cGMP. Measurements were made in a manner similar to those described in Fig. 1. Data were fit with the Hill equation as in Fig. 1. Data points with error bars (SD) are averaged values from 2 patches; other points are from a single patch. Experiments with intermediate subsaturating cGMP concentrations yielded intermediate IC50s for inhibition by all-trans-retinal. INMAX = 100%; IC50 = 35 nM; and n = 1.5.
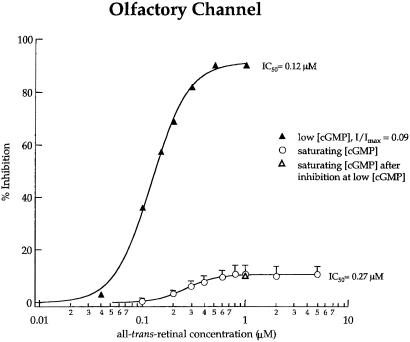
The notion that all-trans-retinal is a closed-state inhibitor is supported by results with the olfactory CNG channel. At saturating cGMP, when both rod and olfactory channels should be fully liganded, the olfactory channel has a greater open probability because of a lower free energy for opening (37). This phenomenon has been used to explain the fact that the olfactory channel is only partially inhibited by diacylglycerol (38); thus, the fully liganded olfactory channel is thought to open some of the time in the presence of such inhibitors, whereas the rod channel cannot. In other words, a more favorable energy of the opening transition makes closed-state inhibitors less effective. Fig. 4 demonstrates that at saturating (100 μM) cGMP the olfactory channel is also only slightly (10.7%) inhibited by all-trans-retinal. However, at low cGMP, when most of the channels would be closed, all-trans-retinal gives almost full (91.3%) inhibition.
Figure 4.
Inhibition of the olfactory channel suggests that all-trans-retinal is a closed-state inhibitor. Steady-state currents were measured from patches of homomultimeric (CNGA2 only) olfactory channels at +100 mV. Data obtained at saturating cGMP (open circles) are the average of 2–4 patches with SD (error bars) and were fit with the Hill equation with the following parameters: INMAX = 10.7%; IC50 = 0.27 μM; and n = 2.90. Data obtained at low cGMP (filled triangles) are from a single patch and were fit with the Hill equation with INMAX = 91.3%; IC50 = 0.12 μM; and n = 2.47. These data are consistent with those from other experiments of this type with different subsaturating cGMP concentrations. The open triangle represents the recovery of much of the cGMP-activated current following the experiment at low cGMP through the addition of a saturating amount of cGMP. This finding demonstrates a reversibility of the inhibition without removal of the retinoid.
These olfactory channel data also provide evidence that all-trans-retinal actually affects channel gating, rather than just acting as a pore blocker that prefers closed channels. If all-trans-retinal were a closed-pore blocker, it would have at least some affinity for the open pore as well, so that the weak (10.7%) inhibition at saturating [cGMP] could be increased to 100% by raising the concentration of all-trans-retinal until all channels were blocked. If, on the other hand, all-trans-retinal inhibits channel opening, then the low fractional inhibition at saturating cGMP would reflect the favorable free energy of opening for the olfactory channel, allowing opening even in the presence of retinal. Thus, raising the concentration of all-trans-retinal would not give an additional reduction in current because the fractional inhibition would be limited by the gating equilibrium. Our results suggest that all-trans-retinal inhibits channel opening, rather than simply blocking the pore, because inhibition of the olfactory channel remains constant at 10.7% as the concentration of all-trans-retinal is raised to many times the IC50.
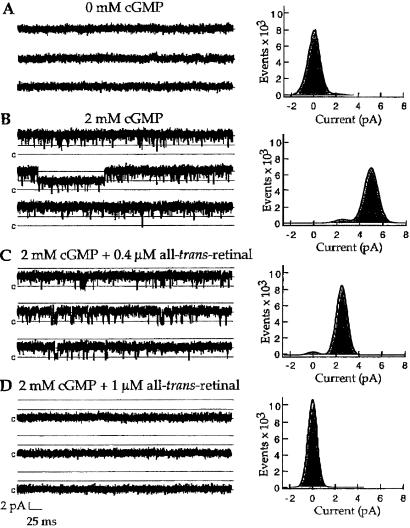
Single-channel recordings from the rod CNG channel suggest that all-trans-retinal induces a long-lived closed state (or states), effectively shutting down the channel for several seconds at a time. This inhibition is much more striking than that seen with most other inhibitors, such as tetracaine (39) and diacylglycerol (38). Fig. 5 shows that application of 0.4 μM all-trans-retinal to a patch containing two active channels in saturating [cGMP] reduced the open probability to such an extent that only one channel seemed to be active throughout many seconds of recording. This remaining channel activity demonstrated the same unitary current (i) and open probability (PO) as that measured before addition of all-trans-retinal. Raising the all-trans-retinal concentration to 1 μM completely shut down all channel activity for the duration of these recordings. Similar results were seen with six other patches containing 2–8 channels each. Two lines of evidence suggest that the extremely low channel open probability did not reflect damage to the channel protein or to the lipid bilayer by all-trans-retinal. First, in longer recordings, reopenings of the rod channel were occasionally seen. Second, after shutting down all of the olfactory channels in a multichannel patch in the presence of low cGMP, application of saturating cGMP reopened enough channels to give the same low maximal inhibition (10.7%) seen with inhibition of other patches at saturating cGMP (Fig. 4).
Figure 5.
Single-channel analysis reveals a dramatic decrease in open probability by all-trans-retinal. Raw current traces in A–D were recorded from a single inside-out patch containing 2 homomultimeric rod channels at a holding potential of +80 mV. Sampling rate was 25 kHz after filtering at 5 kHz. The line labeled c represents the zero-current level when both channels were closed. The upper two lines represent the current when one or both channels were open as determined from the fits to the histograms. Patches were bathed in the low divalent sodium solution (see Experimental Procedures) without cGMP (A), with saturating cGMP (B), and with two different all-trans-retinal concentrations at saturating cGMP as designated (C and D). Each amplitude histogram on the right was constructed from four 2.2-s traces of continuous recording. The application of 0.4 μM all-trans-retinal markedly decreases channel open probability, so that there are no simultaneous openings of two channels for the duration of this recording. There is no channel activity during the recording obtained in 1 μM all-trans-retinal. Histograms in A and D were fit with a Gaussian distribution. The histogram in B was fit by a sum of two Gaussian functions constrained so that the opening of the channels is described by a binomial distribution with the number of open channels n = 2; open probability PO = 0.97; single-channel current i = 2.49 pA; and standard deviation σ = 0.60 pA. The histogram in C was fit by similar distributions, with n = 1; PO = 0.96; i = 2.50 pA; and σ = 0.48 pA.
Discussion
Our results suggest that in addition to their effects on visual transduction via rhodopsin, retinoids can also modulate rod CNG channels, inducing long-lived closed or inactivated states lasting seconds. We have found that all-trans-retinal is a somewhat more effective inhibitor than are 11-cis-retinal and all-trans-retinol. Experiments with all-trans-retinal suggest that it is a closed-state inhibitor; (i) inhibition was less with saturating cGMP than with low cGMP; and (ii) at saturating cGMP, inhibition of rod channels was greater than that of olfactory channels, which have a more favorable free energy of opening. All-trans-retinal does not appear to destroy the channel protein or the lipid bilayer, as evidenced by the reopening of channels in the continued presence of all-trans-retinal, either spontaneously or following an increase in cGMP concentration after inhibiting the channels in low cGMP.
The molecular mechanism of retinoid inhibition of rod CNG channels remains unclear. The retinoids may interact directly with the channel protein, for instance by insertion among its α-helical transmembrane segments. Clearly, there is precedent for a protein-binding mechanism, because retinoids interact directly with visual pigments and with other proteins, including retinoid binding proteins (28, 40, 41). In particular, retinoid molecules may insert into hydrophobic crevices within the channel protein, interfering with the movements of transmembrane segments, such as S6, thought to occur in gating (42–45). Another possibility is that the retinoids indirectly interact with the channels by binding to an intermediary protein, which in turn modulates the channels. This type of mechanism is well established for the role of calcium-binding proteins in Ca2+ regulation of enzymes and ion channels, and recently has been proposed for inhibition of CNG channels by a protein kinase inhibitor (46).
Alternatively, retinoids may control the channels via interactions with the surrounding lipid bilayer, or by a combination of protein and bilayer interactions. There is increasing evidence that some lipids and other hydrophobic or amphipathic molecules can regulate the function of ion channels and other membrane proteins by altering the mechanical properties of the surrounding lipid bilayer, including its curvature at the protein/lipid interface (47, 48). There is also evidence for control of membrane protein function by lipid microdomains, such as “lipid rafts” (ref. 49; reviewed in refs. 50–52). Recent evidence suggests that lipid rafts may even be involved in the control of protein function in rod outer segments (53). Finally, retinoids have been shown to form specific domains or aggregates within bilayers (54), raising the question of whether the prolonged closures of rod CNG channels by retinoids involves their diffusion into retinoid-rich regions in the membrane.
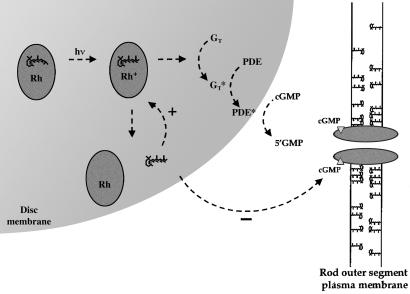
Based on our results, we propose a model (Fig. 6) in which all-trans-retinal released from rhodopsin after a bright light inhibits the CNG channels in the rod outer segment plasma membrane. All-trans-retinal most likely reaches the plasma membrane by diffusion through the cytosol, either freely or while bound to cellular retinoid-binding proteins (15). Although we do not know the concentrations of retinoids in the bilayer in these experiments, our aqueous retinoid IC50's are all below the expected critical micellar concentrations of the retinoids (55). Furthermore, because all-trans-retinal works in the nanomolar range when cGMP is somewhat higher than the physiological (dark) range, estimated at 6 μM (56), much lower all-trans-retinal concentrations should be effective when the free cGMP concentration falls in the light. Thus, it is quite conceivable that the response to bright light in rods partly involves inhibition of the CNG channels by all-trans-retinal, and perhaps by other retinoids, like all-trans-retinol, whose concentration is expected to rise near the channels as it is converted from all-trans-retinal. Potential regulation by all-trans-retinal is especially appealing, because a full bleach of the rhodopsin in a rod would be expected to liberate 3 mM all-trans-retinal in the outer segment if it is considered as a single compartment (15). Even with most of the retinoid bound to cellular retinoid binding proteins, it is reasonable to expect some to be free in the cytosol or in the plasma membrane surrounding the channels, particularly following the surge in the all-trans-retinal concentration after a strong bleach. Thus, we suggest that inhibition of the rod CNG channels by all-trans-retinal may represent a previously undescribed pathway in the rod cell's response to bright light.
Figure 6.
Model for the role of retinoid inhibition of rod CNG channels. The visual transduction pathway in photoreceptors, including two modes of positive feedback involving the all-trans-retinal liberated from rhodopsin following photoisomerization. Evidence suggests that all-trans-retinal can reenter the binding pocket in the opsin protein and stimulate transduction through a noncovalent interaction (30). We suggest that the inhibitory effect of all-trans-retinal on the cGMP-gated channel may also contribute to channel closure under certain circumstances, perhaps during the response to bright light.
Acknowledgments
We thank C. Cornwall, M. F. Crary, R. Crouch, C. Makino, T. Wensel, and N. Zimmerman for helpful discussions; and S. Gordon, K. Magleby and W. N. Zagotta for comments on an earlier version of the manuscript. We are also grateful to R. Mahajan, R. Neisa and E. Seed for technical assistance, and to J. Zimmerman for software development. This work was supported by National Eye Institute, National Institutes of Health Grant EY07774.
Abbreviation
- CNG
cyclic nucleotide-gated
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Nguitragool, W., Miri, A., McCabe, S. L., Dean, D. M. & Zimmerman, A. L. (2002) Biophys. J. 82, 277A (abstr.).
Zimmerman, A. L. & Dean, D. M. (2001) Invest. Ophthal. Vis. Sci. 42, S369 (abstr.).
References
- 1.Broillet M C, Firestein S. Ann NY Acad Sci. 1999;868:730–740. doi: 10.1111/j.1749-6632.1999.tb11352.x. [DOI] [PubMed] [Google Scholar]
- 2.Finn J T, Grunwald M E, Yau K-W. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- 3.Kaupp U B. Curr Opin Neurobiol. 1995;5:434–442. doi: 10.1016/0959-4388(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 4.Molday R S, Hsu Y-T. Behav Brain Sci. 1995;18:441–451. [Google Scholar]
- 5.Richards M J, Gordon S E. Biochemistry. 2000;39:14003–14011. doi: 10.1021/bi001639i. [DOI] [PubMed] [Google Scholar]
- 6.Wei J-Y, Roy D S, Leconte L, Barnstable C J. Progr Neurobiol. 1998;56:37–64. doi: 10.1016/s0301-0082(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 7.Yau K-W, Chen T Y. In: Handbook of Receptors and Channels: Ligand and Voltage-gated Ion Channels. North R A, editor. Boca Raton, FL: CRC; 1995. pp. 307–335. [Google Scholar]
- 8.Zagotta W N, Siegelbaum S A. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman A L. Curr Opin Neurobiol. 1995;5:296–303. doi: 10.1016/0959-4388(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 10.Pugh E N, Jr, Lamb T D. In: Handbook of Biological Physics. Stavenga D G, de Grip W J, Pugh E N Jr, editors. Vol. 3. Amsterdam: Elsevier; 2000. pp. 183–255. [Google Scholar]
- 11.Roof D J, Makino C L. In: The Principals and Practice of Ophthalmology. Alberts D M, Jakobiec F A, editors. Vol. 3. Philadelphia: Saunders; 2000. pp. 1624–1673. [Google Scholar]
- 12.Dionne V E, Dubin A E. J Exp Biol. 1994;194:1–21. doi: 10.1242/jeb.194.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Menini A. Biophys Chem. 1995;55:185–196. doi: 10.1016/0301-4622(94)00153-b. [DOI] [PubMed] [Google Scholar]
- 14.Zufall F, Firestein S, Sheperd G M. Annu Rev Biophys Biomol Struct. 1994;23:577–607. doi: 10.1146/annurev.bb.23.060194.003045. [DOI] [PubMed] [Google Scholar]
- 15.Saari J C. In: Retinoids: The Biochemical and Molecular Basis of Vitamin A and Retinod Action. Nau H, Blaner W S, editors. Vol. 139. New York: Springer; 1999. pp. 563–588. [Google Scholar]
- 16.Crouch R K, Chader G J, Wiggert B, Peppreberg D R. Photochem Photobiol. 1996;64:613–621. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 17.Mangelsdorf D, Umesono K, Evans R M. In: The Retinoids: Biology, Chemistry and Medicine. 2nd Ed. Sporn M B, Roberts A B, Goodman D S, editors. New York: Raven; 1994. pp. 319–350. [Google Scholar]
- 18.Levine E M, Fuhrmann S, Reh T A. Cell Mol Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyatt G A, Dowling J E. Invest Ophthalmol Vis Sci. 1997;38:1471–1475. [PubMed] [Google Scholar]
- 20.Ross C A, Hammering U G. In: The Retinoids: Biology, Chemistry and Medicine. 2nd Ed. Sporn M B, Roberts A B, Goodman D S, editors. New York: Raven; 1994. pp. 521–544. [Google Scholar]
- 21.Bosma M, Sidell N. J Cell Physiol. 1988;135:317–323. doi: 10.1002/jcp.1041350220. [DOI] [PubMed] [Google Scholar]
- 22.Sidell N, Schlichter L. Biochem Biophys Res Commun. 1986;138:560–567. doi: 10.1016/s0006-291x(86)80533-2. [DOI] [PubMed] [Google Scholar]
- 23.Song J-H, Narahashi T. J Pharmacol Exp Ther. 1995;275:1402–1411. [PubMed] [Google Scholar]
- 24.Zhang D-Q, McMahon D G. Proc Natl Acad Sci USA. 2000;97:14754–14759. doi: 10.1073/pnas.010325897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D-Q, McMahon D G. Mol Vis. 2001;7:247–252. [PubMed] [Google Scholar]
- 26.Sun H, Molday R S, Nathans J. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 27.Vellani V, Reynolds A M, McNaughton P A. J Physiol. 2000;529:333–344. doi: 10.1111/j.1469-7793.2000.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noy N. J Biochem. 2000;348:481–495. [PMC free article] [PubMed] [Google Scholar]
- 29.Fain G, Matthews H, Cornwall M C, Koutalos Y. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Palczewski K, Saari J. Curr Opin Neurobiol. 1997;7:500–504. doi: 10.1016/s0959-4388(97)80029-3. [DOI] [PubMed] [Google Scholar]
- 31.Surya A, Knox B. Exp Eye Res. 1998;66:599–603. doi: 10.1006/exer.1997.0453. [DOI] [PubMed] [Google Scholar]
- 32.Liman E R, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S E, Brautigan D L, Zimmerman A L. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- 34.Molokanova E, Trivedi B, Savchenko A, Kramer R H. J Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpen J W, Zimmerman A L, Stryer L, Baylor D A. Proc Natl Acad Sci, USA. 1988;85:1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerman A L, Karpen J W, Baylor D A. Biophys J. 1988;54:351–355. doi: 10.1016/S0006-3495(88)82966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon S E, Zagotta W N. Neuron. 1995;14:857–864. doi: 10.1016/0896-6273(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 38.Crary J I, Dean D M, Nguitragool W, Kurshan P T, Zimmerman A L. J Gen Physiol. 2000;116:755–768. doi: 10.1085/jgp.116.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fodor A A, Gordon S E, Zagotta W N. J Gen Physiol. 1997;109:3–14. doi: 10.1085/jgp.109.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kefalov V, Crouch R, Cornwall M. Neuron. 2001;29:749–755. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Bozic D, Malashkevich V, Kammerer R, Schulthess T, Engel J. EMBO J. 1998;17:5265–5272. doi: 10.1093/emboj/17.18.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson J P, Zagotta W N. Nature (London) 2001;412:917–921. doi: 10.1038/35091089. [DOI] [PubMed] [Google Scholar]
- 43.Flynn G E, Zagotta W N. Neuron. 2001;30:689–698. doi: 10.1016/s0896-6273(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 44.Perozo E, Cortes D M, Cuello L G. Science. 1999;285:59–61. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- 45.Unwin N. Nature (London) 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 46.Molokanova E, Savchenko A, Kramer R H. J Gen Physiol. 1999;113:45–56. doi: 10.1085/jgp.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundbaek J A, Andersen O S. J Gen Physiol. 1994;104:645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantor R. Biophys J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martens J, Navarro-Polanco R, Coppock E, Nishiyama A, Parshley L, Grobaski T, Tamkun M. J Biol Chem. 2000;275:7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- 50.Brown D A, London E. Annu Rev Cell Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson K, Dietrich C. Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- 52.Edidin M. Curr Opin Struct Biol. 1997;7:528–532. doi: 10.1016/s0959-440x(97)80117-0. [DOI] [PubMed] [Google Scholar]
- 53.Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada Y, Yoshiyama W, Liu H, Hayashi F. J Biol Chem. 2001;276:20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- 54.Boeck H D, Zidovetzki R. Biochim Biophys Acta. 1988;946:244–252. doi: 10.1016/0005-2736(88)90399-9. [DOI] [PubMed] [Google Scholar]
- 55.Noy N. In: Retinoids: The Biochemical and Molecular Basis of Vitamin A and Retinoid Action. Nau H, Blaner W S, editors. Vol. 139. New York: Springer; 1999. pp. 3–29. [Google Scholar]
- 56.Nakatani K, Yau K-W. J Physiol. 1988;395:69–71. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]