Abstract
Perineuronal nets (PNNs) are specialized, dense extracellular matrix structures that enmesh the cell bodies and dendrites of specific neurons, most notably inhibitory interneurons. Increasing evidence indicates that PNNs serve not merely as passive scaffolds but play an active and essential role in modulating synaptic plasticity and circuit physiology. They critically influence the timing of sensory system critical periods, as well as processes underlying learning, memory, and higher cognitive functions. Furthermore, dysregulation of PNN density and architecture have been associated with conditions like autism, neurodevelopmental disorders, schizophrenia and Alzheimer’s disease. Since they are extensively involved in brain function, we discuss the multitude of regulatory factors that govern the formation, maturation, and remodeling of PNNs. In particular, we focus on both molecular and cellular brain-intrinsic mechanisms, highlighting the potential contributions of microglia and astrocyte derived factors. Additionally, we consider the influence of long-range signaling cues, including the metabolic status and peripheral hormones. Analysing this complex network of interactors, we try to highlight the role of PNNs beyond neural plasticity and brain function, in a broader whole-body physiological perspective.
Keywords: Perineuronal nets, Extracellular matrix, Microglia, Astrocytes, Glycosaminoglycan, Plasticity, Metabolism
Introduction
Neural plasticity, the ability of the brain to adapt and reorganize, is essential for learning, memory, and recovery from injury. Perineuronal nets (PNNs), dense extracellular matrix (ECM) structures enwrapping the soma and dendrites of a large number of neurons in the brain, represent crucial regulators of such processes.
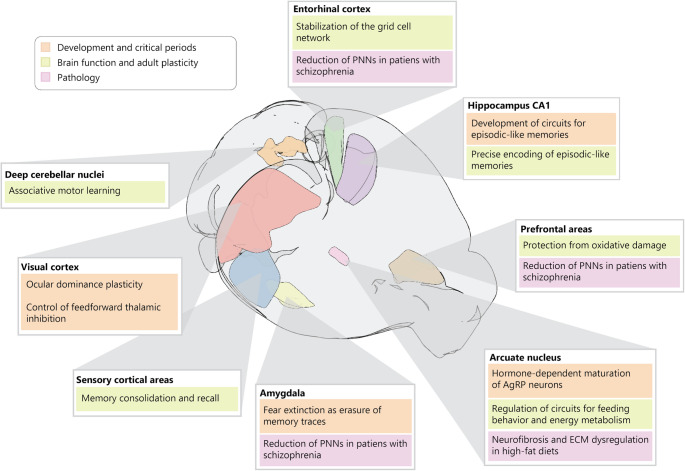
Interest in PNNs has steadily increased over the past two decades, as their involvement in key brain functions has been demonstrated across multiple brain regions (Fig. 1). By antagonizing plasticity, PNNs promote the maturation of neural circuits during early postnatal life, gating the temporal windows of maximal development called critical periods. This role was first established in ocular dominance plasticity within the visual cortex [1], and later extended to various functions, including fear extinction in the amygdala [2], AgRP neurons maturation in the arcuate nucleus of the hypothalamus [3, 4], precise encoding of episodic-like memory in the hippocampus CA1 [5, 6].
Fig. 1.
Functional roles of PNNs across multiple brain regions. Regional specificity of PNN-mediated control of neural plasticity is suggestive of the action of complex regulatory networks both in postnatal development and in adulthood. The cartoon of the mouse brain was created with Brainrender [43] and adapted for illustration purposes
During adulthood, PNNs stabilize acquired neural representations and memories in multiple regions. PNN depletion affects spatial maps in the grid cell network [7], fear memory acquisition, consolidation and extinction [8–10], cerebellar plasticity and related learning [11].
From a molecular point of view, PNNs are composed of a lattice-like scaffold of hyaluronic acid chains and chondroitin-sulfate proteoglycans (CSPGs), connected by link proteins and tenascin-R [12, 13]. While sharing some molecular constituents with the unorganized ECM of the brain, PNN-bound CSPGs display specific sulfation motives and can be isolated through biochemical methods [14]. In histological preparations, many PNNs are labeled by the lectin Wisteria floribunda agglutinin [15], which is one of the most common reagents used as PNN marker. Despite the common structural organization, PNNs can display variable proteoglycan composition, such that some PNNs can be detected by specific antibodies but not by WFA [16–18].
Due to their tight involvement in plasticity processes, PNNs are associated with various pathological conditions. In the context of brain injury, CSPG expression is upregulated in the glial scar and, in the dorsal root ganglia, acts as an inhibitor of axon growth [19–21]. Disassembly of PNNs by the bacterial enzyme chondroitinase ABC (ChABC) can promote recovery after spinal cord injury [22, 23]. In other systems, the inhibition of axon growth has been shown to limit plasticity and has been attributed to the action of the chemorepulsive molecule semaphorin 3A (SEMA3A), which can bind the PNN scaffold [24–26].
While the limitation of neuroplasticity is sometimes viewed as a constraint, it also serves essential functions. Controlled plasticity ensures stability in neural circuits, and prevents maladaptive changes. Alterations in PNNs have indeed been reported in multiple models of neurodevelopmental disorders [27–31], in which impaired plasticity severely affects cognitive, motor and behavioral function.
In addition to their plasticity-limiting role, PNNs and their molecular constituents can display a neuroprotective function [32]. The reduction of PNNs around parvalbumin-expressing (PV) interneurons has been related to an increase in oxidative stress in animal models and in post-mortem samples of patients with schizophrenia [33–35]. A reduction of WFA signal has also been reported in neurodegenerative pathologies such as Alzheimer’s disease [36]. However this notion has been challenged [37] and the reduction in the staining imputed to a change in PNN composition or sulfation pattern altering WFA reactivity [27].
Despite the general role in controlling plasticity in multiple brain areas, PNN distribution in the adult brain is not uniform. In the mouse, the highest levels are found in the midbrain, the hindbrain and the cortex, particularly in primary sensory regions [38]. PNNs are typically associated with PV inhibitory neurons, although they can also encircle different cell types, for instance pyramidal neurons in the hippocampus CA2 [39].
The regional heterogeneity in PNN distribution and composition, as well as in the time course required for their formation suggest that these structures are tightly regulated and controlled across the entire lifespan. The mechanisms underlying PNN formation and maturation are crucial for circuit-specific control of plasticity.
While the functions of PNNs in physiological and pathological contexts have been extensively reviewed [12, 40–42], here we explore the complex network of regulatory elements that govern the formation, maturation, and remodeling of PNNs across development and into adulthood. We place particular emphasis on both molecular and cellular factors within the CNS and extrinsic signals originating from peripheral tissues. Besides purely neuronal signals, we will also consider regulation by glial cells, specifically astrocytes and microglia. These cell types are now intensely studied for their capability to act locally while still sensing and integrating systemic factors. Although much attention has traditionally been given to brain-intrinsic mechanisms, emerging evidence suggests that systemic cues, such as metabolic signals, hormones, and inflammatory mediators, can also influence PNN dynamics and architecture. By examining how central and peripheral signals converge to regulate PNN formation, we aim to shed light on the multifaceted role of these ECM structures beyond neural plasticity and brain function, in broader physiological processes such as energy homeostasis.
Molecular and Physiological Factors Modulate PNN Aggregation during Development
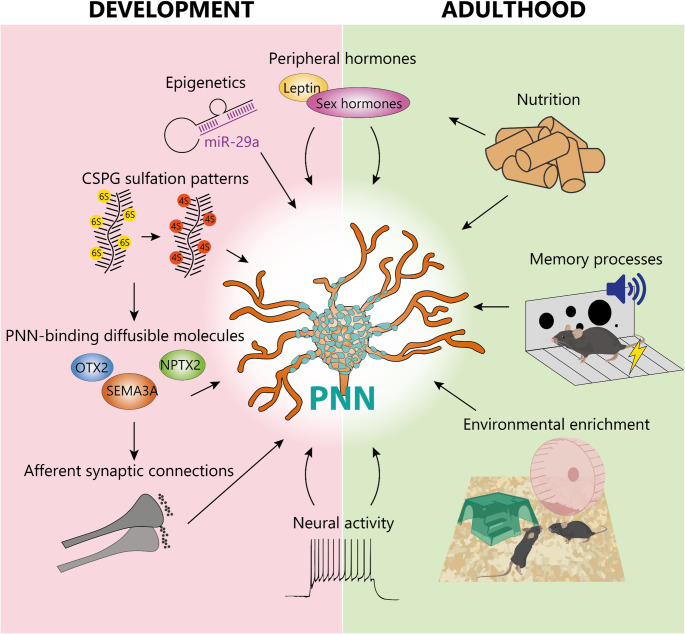
Regulating neural plasticity during early postnatal life is essential to guide neural circuits along a healthy developmental path, ultimately enhancing their functional efficiency in adulthood. For these reasons, the formation of PNNs is not a completely spontaneous event but instead an actively regulated process that must be initiated and timely controlled (Fig. 2).
Fig. 2.
Regulation of PNNs during development and adulthood. Molecular and physiological factors promoting circuit maturation during postnatal development are involved in the accumulation of PNNs. During adulthood, events requiring plasticity, like memory formation and consolidation, affect PNN maintenance and function. Notably, PNNs integrate both brain-intrinsic and systemic cues, such as nutrition and hormonal landscape
PNNs are highly complex molecular structures, composed of a variety of proteins and CSPGs. Therefore, the biosynthetic processes of each of these constituents offer multiple regulatory hubs that enable tight control over PNN formation.
The building blocks of PNNs are produced by different cell types, including neurons, astrocytes and oligodendrocytes [40]. Although many CSPGs are already present during embryonic development and serve as extracellular cues to orchestrate neurogenesis, cell migration, and axon guidance, the expression of aggrecan, brevican, and link proteins increase postnatally during PNN formation [44, 45]. These components are essential for the physiological formation of PNNs. Indeed, their removal has been shown to result in attenuated PNNs and persistent plasticity. This phenotype is observed following the knock-out of hyaluronan and proteoglycan link protein 1 (Hapln1) [45], the combined removal of multiple ECM proteins like tenascins and CSPGs [46], and the genetic deletion of aggrecan in adults [47]. In contrast, removing aggrecan from birth (germline knock-out) surprisingly had no effect on plasticity or PV neuron physiology, suggesting that the developing brain activates compensatory mechanisms to offset its loss [48]. In several systems, the expression PNN molecules is linked to neural activity during early postnatal life, which promotes circuit maturation. For instance, sensory deprivation delays critical period closure and PNN formation in the visual [1] and somatosensory [49] cortex, with concurrent reduction of the expression of aggrecan.
In addition to the production of molecular components, studies have shown the importance of sugar moiety modifications, particularly the sulfation patterns of glycosaminoglycans, as a regulatory ‘code’ that modulates PNN function. As mentioned before, CSPGs are crucial elements of PNN scaffold and are composed of a core protein to which a number of chondroitin sulfate glycosaminoglycan (CS-GAG) chains are attached. During the synthesis of CS-GAG chains, sulfate groups are added by sulfotransferase enzymes [50] at carbon 4 and/or 6 of N-acetylgalactosamine. The pattern of sulfation results in charged domains that affect the binding properties of CSPGs and influence their permissiveness towards plasticity. Particularly, chondroitin-4-sulphate (C4S) has been shown to be inhibitory towards axonal growth and plasticity while chondroitin-6-sulphate (C6S) is more permissive [51]. Consistently, CSPG sulfation changes across development and lifespan: C6S is much more abundant than C4S during embryogenesis and decreases during postnatal development, while C4S increases. Their ratio stabilizes up until the onset of critical periods, after which C4S becomes predominant in the adult brain [14, 52, 53].
Due to their proximity to the cell membrane and to the biochemical properties of CSPGs, PNNs can capture and concentrate diffusible molecules acting as long-range signaling cues. Long-range signaling is crucial for coordinating the maturation of functionally related networks. Epidermal growth factor (EGF) has been shown to suppress development of GABAergic neurons in the rodent cortex. Administration of EGF to cultured neurons and EGF overexpression in transgenic mouse models reduces aggregation of PNNs around PV neurons, increasing the enzymatic activity of matrix metalloproteinases (MMPs) and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) [54]. Signaling molecules can also integrate in the PNN scaffold and regulate developmental plasticity. One such molecule, SEMA3A, acts as an axonal chemorepellant that accumulates in PNNs during late postnatal development. This accumulation restricts the formation of new synapses on PNN-enwrapped neurons [24], thus contributing to the end of the critical period [26]. Another critical protein is orthodenticle homeobox protein 2 (OTX2), which is essential for PNN development and maintenance. The understanding of OTX2 origin has evolved; it was first identified as being transported from the retina to the primary visual cortex in an activity-dependent manner [55]. However, subsequent work revealed that OTX2 is also synthesized and secreted globally by the choroid plexus, a source whose disruption affects remote circuits like the primary visual cortex [56]. While OTX2 and SEMA3A are thought to bind 4,6 di-sulfated GAGs in the PNNs [57, 58], neuronal pentraxin 2 (NPTX2) binds to both 4,6 sulfated GAGs and HA and promotes maturation of PNNs in cell cultures [59].
The action of PNNs in critical period timing is also regulated by epigenetic mechanisms. For instance, miR-29a is a microRNA upregulated with age across different species and tissues [60] and its upregulation during PNN formation can lead to the early appearance of PNNs. Conversely, inhibiting miR-29a in adult mice promoted plasticity, reduced PNNs, and increased the expression of ECM remodeling enzymes such as MMP2, MMP9, MMP13 [61].
In some brain regions, PNNs are also associated with specific connectivity patterns. In the cortex, the highest levels of PNN expression is found in the thalamic recipient layer 4 of primary sensory cortices and in PV-rich areas connected into intracortical networks [38]. However, while it has been shown that in the primary visual cortex PNN degradation can induce an activity-dependent increase in thalamic afferents onto PV neurons [62], it is not known whether those synapses might be important for instructing or facilitating PNN development. In general, further studies will be needed to elucidate the influence of connectivity in shaping PNN function.
These regulatory elements do not act independently, but actually cooperate for proper critical period timing and circuit maturation. The changing sulfation pattern of PNNs alters significantly the interactions with signaling molecules. Mice overexpressing chondroitin-6-sulfotransferase, the enzyme responsible for the juvenile C6S sulfation, show an abnormal development of PNNs in the visual cortex, revealed by reduced reactivity to WFA, and lower accumulation of OTX2 both before and after the critical period closure. This ultimately results in impaired formation of thalamocortical synapses onto PNN + neurons and retention of ocular dominance plasticity in adulthood [63].
Together, these findings underscore that PNN formation is a tightly orchestrated process, and that these structures integrate multiple intrinsic molecular cues, activity-dependent mechanisms, and biochemical signals to ensure proper maturation of neural circuits.
Experience-dependent Regulation of Perineuronal Nets in Adulthood
While neural plasticity is most prominent during development, it remains essential in adulthood for processes such as learning, memory, and adaptive responses to experience. Therefore, multiple factors continue to regulate PNNs in the adult brain to balance stability with the need for ongoing plasticity, ensuring neural circuits remain adaptable while preserving functional integrity (Fig. 2).
As previously mentioned, the process of memory formation, consolidation and maintenance require intact PNNs, as they are impaired by PNN depletion obtained with local injection of PNN-degrading enzyme ChABC [2, 8, 11]. Consequently, experience can shape the PNN to support stable memory encoding. Nevertheless, the molecular mechanisms coupling learning to PNN expression have not been completely characterized. Memory acquisition and consolidation typically induce an increase in the expression of PNNs and of its molecular components. Auditory fear memory formation, for instance, is accompanied by an upregulation of the mRNA expression of PNN proteoglycan components like aggrecan, brevican and neurocan in the auditory cortex associated with increased numbers of aggregated PNNs [9]. PNNs around PV neurons increase 4 and 96 h after contextual fear conditioning in the hippocampal CA1 and in the anterior cingulate cortex, enhancing consolidation and recall of both recent and remote fear memory [64].
During learning, specific signaling pathways can act on plasticity to interfere with PNN expression. Neuropeptide Y (NPY) signaling has been associated with learning and memory [65]. Mice lacking NPY receptor in excitatory forebrain neurons display slower spatial learning and increased intensity of PNN in the hippocampus CA1. Deletion of PNNs with ChABC rescues the learning deficit [66].
As in development, another factor potentially capable of reshaping PNNs, promoting plasticity phenomena and paving the way for circuit rearrangements is neural activity. Indeed, in the cortex, chemogenetic inhibition of either excitatory or PV-expressing inhibitory neurons triggers a decrease in PNN density, highlighting the dependence of these structures on patterns of activity of the local microcircuit [67].
While the dependence of PNNs on neural activity has been consistently shown in multiple studies, the mechanism underlied by this regulation is not completely understood. A possible way by which PNNs can respond to neural activity is the controlled expression of activity-dependent genes that modulate the ECM. NPTX2, for instance, is a member of the neuronal pentraxin family of calcium-dependent lectins and is regulated as an immediate-early gene [68]. By binding both PNNs and AMPA receptors, it facilitates receptor clustering and excitatory neurotransmission onto PV cells [69]. In the mature brain, PNNs are engaged in a positive feedback loop mediated by PNN-dependent constant internalization of OTX2 into PV neurons, which in turn dampens adult plasticity and promotes PNN maintenance [57].
Environmental factors and experience can reactivate plasticity in the adult brain. For example, rearing mice in an enriched environment (EE)—a paradigm that enhances sensory, cognitive, and social stimulation—can promote reactivation of juvenile plasticity and, consistently, a reduction of PNNs in the visual cortex [70]. Similarly, in the cerebellum, EE is associated with a reduction of SEMA3A bound to the PNN scaffold, consistent with an increased plasticity [25]. Conversely, environmental enrichment during early postnatal life accelerates cortical development and PNN accumulation by overexpression of insulin-like growth factor 1 (IGF1) [71]. Changes of the same sign can be found in the striatum, where EE causes an increase of PNN expression [72].
Altogether, these findings demonstrate that PNNs in the adult brain are dynamic structures, shaped by learning, neural activity, and environmental experience. This regulation maintains a balance between stability and plasticity, supporting both memory consolidation and adaptive circuit remodeling. Elucidating the molecular and activity-dependent mechanisms underlying PNN dynamics will be key to understanding how experience influences long-term cognitive function and to developing strategies for modulating plasticity in neurological disorders.
The Perineuronal Nets are Dynamic Players at the Interface between Metabolism and Brain Plasticity
As described in the previous sections, PNNs were initially characterized in cortical areas and were associated with sensory processing and memory [12]. Recent evidence highlights the presence and critical importance of PNNs in brain regions governing metabolic homeostasis, including the hypothalamus. Furthermore, their regulation appears far more complex than previously thought, extending beyond local neuronal activity to involve long-range signals related to sex, hormones, diet, and overall metabolic status.
PNN-like structures have been identified within the arcuate nucleus of the hypothalamus (ARC) [3], a critical hub for integrating peripheral metabolic signals to control energy balance and glucose homeostasis [73]. These ARC PNNs are unique in several ways. They form a dense, continuous ring or ‘collar’ at the junction between the ARC and the median eminence (ME), a circumventricular organ providing neurons direct access to circulating hormones and nutrients since the blood brain barrier (BBB) composed of fenestrated microvessel loops is particularly permeable [74]. This dense clustering contrasts sharply with the sparser distribution of PNNs seen in cortical regions. Notably, ARC PNNs seem to preferentially enmesh metabolically relevant neuronal populations. The majority are GABAergic, leptin-receptor positive (LepRb+), and include most Agouti-related peptide (AgRP) neurons—key orexigenic regulators—while only a smaller fraction of anorexigenic pro-opiomelanocortin (POMC) neurons are PNN-enmeshed. Postnatal appearance of these PNN, becoming fully formed around postnatal day 28 (P28), coincides precisely with the closure of the critical period for the maturation of AgRP neuron projections and the establishment of their response to leptin [3]. Thus, the timing of ARC PNN formation is tightly linked to postnatal neurodevelopment and, likely, to hypothalamic plasticity, in line with PNN maturation in other brain regions [1, 75]. Compelling evidence indicates that this developmental process is dependent on long-range hormonal signals, specifically the adipocyte-derived hormone leptin. In leptin-deficient ob/ob mice, ARC PNN formation during the postnatal period is markedly impaired. This deficit can be rescued by systemic leptin administration, however only if given during the critical period [3]. This establishes leptin signalling as a key factor not only for AgRP neuron development [76] but also for the assembly of the surrounding PNNs. This piece of data strongly implicates PNNs in the mechanisms that close the critical developmental window for metabolic circuit wiring and suggests the PNN as potential stabilizer of hypothalamic feeding circuits.
Beyond development, hypothalamic PNNs exhibit ongoing remodelling and regulation in adulthood, influenced by sex, gonadal hormones, and metabolic status. Studies in adult mice revealed that gonadal hormones are essential for maintaining the structural integrity of PNNs specifically within the ARC. a portion of PNN-enmeshed ARC neurons express estrogen receptor α (ERα) and a majority express the androgen receptor. Indeed, surgical gonadectomy significantly reduced both WFA intensity and the number of PNN-enwrapped cells in the ARC, an effect observed in both sexes. Intriguingly, the terete hypothalamic nucleus (TE) shows significant sex dimorphism, with females displaying higher PNN WFA fluorescence intensity than males. Furthermore, PNNs in the TE are sensitive to diet in a sex-specific manner; chronic high-fat diet (HFD, 60% Kcal from fat) feeding significantly increased PNN intensity in females but had no effect in males. Finally, PNNs observed in other hypothalamic nuclei, such as the paraventricular hypothalamic nucleus(PVH), lateral hypothalamic area (LHA), ventromedial hypothalamic nucleus (VMH), and dorsomedial hypothalamic nucleus (DMH), did not show significant regulation by sex or HFD consumption [77].
The ME is another key site of PNN plasticity directly linked to the nutritional state. PNNs in the ME undergo rapid remodeling in response to acute changes in the feeding state. Kohnke et al. demonstrated that refeeding after an overnight fast triggers a rapid increase in the proliferation and differentiation of oligodendrocyte progenitor cells (OPCs) into mature oligodendrocytes specifically within the ME. Intriguingly, this nutritionally-driven oligodendrocyte differentiation is directly linked to PNN remodeling. Fasting reduces, while refeeding rapidly increases, the density of ME PNNs. This reshaping is dependent on OPC differentiation, as the deletion of the transcription factor Myelin regulatory factor (Myrf) in OPC blunts the PNN response to refeeding. Furthermore, the PNN-degrading enzyme ADAMTS4 shows increased expression in ME oligodendrocytes during fasting, potentially contributing to PNN breakdown. Importantly, this rapid PNN remodeling in the ME is functionally relevant for energy balance. Enzymatic digestion of ARC/ME PNNs using ChABC in lean mice led to increased food intake and weight gain, demonstrating that intact ME PNNs are required for normal energy homeostasis maintenance [78]. This results highlights the ME as a site of unique glial and ECM plasticity directly responsive to acute nutritional challenges, influencing central metabolic control and mouse feeding responses.
The remodelling of PNNs in key hypothalamic nuclei for metabolic homeostasis maintenance may be crucial for the development and progression of pathological states. Recent evidence indicates that established metabolic diseases (obesity and type 2 diabetes) are associated with a distinct, pathological PNN alteration in the ARC, termed neurofibrosis. In both high fat high sugar (HFHS, 45% kcal from fat) diet-induced obese (DIO) and genetically diabetic (db/db) mice, the ARC shows significant remodeling of the PNN, including increased WFA staining, elevated aggrecan deposition, and altered CS-GAG sulfation (notably a higher 4 S/6S ratio). These changes occur specifically around AgRP neurons and progress with the metabolic disease. The authors hypothesized that neurofibrosis is the result of reduced PNN turnover in obesity. In DIO mice, PNN degradation is slower than in lean controls and is associated with lower expression of ECM proteases. Functionally, ARC neurofibrosis impairs insulin access to neurons by forming a physical and electrostatic barrier. Hypothalamic insulin signaling, tested through phosphorylation of the insulin receptor and AKT, is reduced in DIO mice but restored by enzymatic PNN removal with ChABC, which mechanistically should disrupt the insulin-GAG binding and sequestration. Remarkably, stereotaxic injections of ChABC in DIO or db/db mouse models led to metabolic improvements. To increase translatability, CS-GAG synthesis inhibitor fluorosamine intranasal administration to DIO mice decreased body weight, adiposity, restored glucose tolerance and insulin sensitivity. Together, these findings suggest ARC neurofibrosis is a causal factor in neuronal insulin resistance and dysfunction in obesity, and a novel potential druggable target [79].
In contrast to this discovery, a different study demonstrated that the abundance of PNNs enmeshing ARC neurons is decreased in DIO mice compared to lean control diet fed animals. This loss was also associated with increases of ARC microgliosis and astrogliosis suggesting a link between ARC PNN loss and glial activation. ChABC-driven depletion of ARC PNNs exacerbates the metabolic impact of a HFHS diet in rats. Specifically, PNN digestion led to increased hyperphagia, greater weight gain, and elevated fat accumulation, primarily driven by enhanced food intake [80]. On the same page, compared to healthy Wistar controls, Zucker diabetic fatty (ZDF) rats, a model of type 2 diabetes, exhibit markedly reduced PNN abundance in the ARC and altered sulfation pattern of their CSPG. A single intracerebroventricular injection of fibroblast growth factor 1 (FGF1) induces sustained remission of diabetes in ZDF rats and increases ARC PNN density, normalizing toward healthy levels. Remarkably, PNN digestion through ChABC administration completely prevents diabetes remission, indicating that the integrity of ARC PNNs is essential for the sustained glucoregulatory effect of FGF1 [4].
These contrasting findings suggest that ARC PNNs may play a role in energy balance, and that their dysregulation could contribute to the development of obesity and insulin resistance. However, further investigation is needed to determine whether they are causative, and how they might be effectively targeted.
The influence of diet, particularly HFD, on PNN plasticity extends beyond the hypothalamus. Studies in the prefrontal cortex (PFC) reveal complex, region-specific, and sex-dependent effects of HFD on PNNs, which also vary based on inherent susceptibility to obesity. In obesity-prone (OP) and standard Sprague-Dawley (SD) male rats, chronic HFD reduced PNN intensity in the prelimbic (PL) and ventromedial orbitofrontal (vmOFC) subregions. In contrast, HFD increased PNN intensity in the infralimbic (IL) PFC of female OP/SD rats. Obesity-resistant (OR) rats showed opposite responses, with males exhibiting increased PNN intensity in the vmOFC and females showing decreased intensity in the IL-PFC after HFD [81]. Given that PFC PNNs typically surround PV + interneurons and regulate E/I balance, these diet- and sex-specific changes likely impact PFC function related to cognitive control, decision-making, and motivation, potentially contributing to difficulties in voluntary food consumption. HFD has been associated with excessive accumulation of PV-positive neurons and upregulation of PNN in the mouse CA3 region of the hippocampus. Treatment with indoleamine 2,3-dioxygenase (IDO) inhibitor 1-methyltryptophan (1-MT) improved performance in the tails suspension test and forced swimming test, and effectively reverses ECM changes, highlighting a PNN-related mechanism by which 1-MT exerts its antidepressant effect [82]. HFD consumption decreases the number of WFA + cells in the CA1 hippocampal region of WT mice; and a mouse model knock-out for pleiotrophin, a cytokine implicated in HFD-induced weight gain, brain alterations and cognitive impairments, displays a higher number of WFA + cells, which are not affected by HFD [83].
The studies reviewed here powerfully illustrate that PNN formation, maintenance, and remodeling are not solely governed by local neuronal activity or developmental programs within the brain. Instead, PNNs in metabolic circuits exist at a nexus, integrating both local and long-range signals. Peripheral metabolic/sexual hormones regulate PNN formation, and intriguingly the systemic metabolic status influences PNN assembly and turnover in the hypothalamus. This complex interplay underscores the role of PNNs as dynamic integrators, situated at key brain–body interfaces like the ARC-ME site, translating peripheral metabolic information into structural changes that regulate neuronal function and likely circuit stability, impacting whole-body energy homeostasis.
Astrocytes Regulate ECM in Health and Disease
Glial cells, historically viewed as passive scaffolding, are now understood to play dynamic and regulatory roles in neural signaling and plasticity. Astrocytes, the most abundant glial cell type in the brain, are crucially involved in the maintenance of potassium homeostasis and in the uptake of glutamate at synapses [84]. Moreover, growing evidence increasingly recognized them as active participants of the development, refinement and maintenance of neural circuits through different processes including a direct influence on PNN expression [85, 86] (Fig. 3).
Fig. 3.
Regulation of PNNs by glial cells. Microglia and astrocytes influence PNN development and stability both in physiological and in pathological conditions. Microglia is mostly associated with clearance of ECM molecules while astrocytes with their production. Both cell types are involved in regulation of PNNs by matrix metalloproteases and in synaptic homeostasis
Astrocytes are a substantial source of ECM components throughout development and adulthood. However, the fraction of these molecules eventually incorporated in the developing PNNs is still debated. PNN-like structures have been reported in cultures of neurons without astrocytes [87], suggesting that neuron-secreted molecules might be sufficient for the assembly of PNNs. Consistently, co-cultures experiments of wild-type astrocytes and neurons lacking expression of tenascin-R, tenascin-C, neurocan and brevican showed lower expression of aggregated PNNs [88]. In vivo knock-out of these molecules, however, had resulted in limited impairment of PNN assembly, possibly due to compensatory mechanisms [89, 90].
During the visual cortex critical period, astrocytes increase their expression of the gap junction channel subunit connexin 30 (Cx30). Through an atypical signaling pathway, Cx30 downregulates the RhoA-matrix metalloproteinase 9 (MMP9) pathway. This cascade leads to reduced levels of MMP9, an enzyme that degrades ECM components, consequently promoting the maturation and condensation of PNNs around inhibitory neurons and contributing to the closure of critical period for ocular dominance [86]. In vivo transplantation of mature astrocytes in the developing cortex results in anticipated gating of developmental plasticity [86]. An astrocyte-driven control of PNNs can be found also in subcortical regions which are typically less studied for PNN biology. In the medial nucleus of the trapezoid body (MNTB), PNNs ensheat principal neurons and the large calyx of Held terminals [91]. Disruption of fibroblast growth factor 9 signaling between neurons and astrocytes in MNTB results in higher brevican (BCAN) accumulation inside PNNs and concurrent increased size of calyx of Held terminals, indicating a role of this communication in synapse refinement [92].
In the adult brain, astrocytes are essential for synaptic homeostasis as they intimately interact with both the pre- and post- synaptic terminals, in tight association with the ECM and microglia creating structures increasingly termed ‘tripartite’ or even ‘tetrapartite’ synapses ([93], Fig. 3). The association is particularly strong in the meshes of the PNN lattice: here most afferent terminals making contacts with the postsynaptic membrane are accompanied by astrocytic processes expressing Kir4.1, glutamate and GABA transporters [94]. The degradation of PNNs impairs astrocytic transmitter and potassium uptake with consequent spillage of glutamate in the extrasynaptic space, indicating that PNNs are necessary for physiological regulation of synaptic physiology by astroglia [94].
The interplay between ECM and astrocytes is also evident in pathological conditions. In disease and neuroinflammatory conditions astrocytes transition in a so-called reactive state undergoing morphological and molecular changes [95, 96], such as the increased expression of glial fibrillary acid protein (GFAP), an intermediate filament protein, which is widely used as an astrocytic marker [97]. Reactive astrocytes also alter ECM homeostasis by increasing the expression of ECM components and matrix metalloproteases, increasing the levels of interstitial ECM while reducing aggregated PNNs [98]. In pathologies involving brain injury, reactive astrocytes take part in the formation of the glial scar [99], accumulating various CSPGs that counteract axonal regrowth [100, 101]. On the other hand, upon injury astrocytes also increase MMP2, MMP3 [102, 103]. Upon disruption of the blood brain barrier and albumin extravasation, TGFβ signaling in reactive astrocytes promote PNN degradation [104].
It is worth noting that it is still hard to disentangle the action of astrocytes on aggregated PNNs from their regulation of the diffuse ECM. Astrocytes exert a broad action on several ECM components and the regulation of PNNs can be considered part of a much broader picture. Recent evidence showed that during synaptogenesis astrocytes secrete neurocan (NCAN) that is cleaved in two fragments. The one containing the C-terminal domain is soluble and does not take part of PNN formation. This fragment strongly regulates the formation of somatostatin-positive inhibitory synapses, which is impaired in mice lacking either the entire Ncan gene or just the C-terminal sequence [105].
Collectively these studies indicate that astrocytes might be in a unique position to regulate PNN at multiple scales: from the microenvironment of the synaptic cleft, up to the larger context of the glial scar.
Microglia: Another Crucial Player Sculpting the ECM
Microglia, the resident immune cells of the central nervous system (CNS), are now known to perform functions far beyond classical immunity [106]. While their roles in pruning synapses during development and stripping them in disease are well-documented [106–108], a growing body of evidence shows they are also integral components of the synaptic microenvironment.
Microglia, the resident immune cells and macrophages of the CNS, are increasingly recognized for functions extending far beyond classical immunity [106]. While their roles in synaptic pruning during development and synaptic stripping in disease are well-studied aspects of their interaction with neurons [106–108], a growing body of evidence shows they are also integral components of the synaptic microenvironment. They are embedded within the complex meshwork of the ECM, which, as mentioned before, together with pre- and postsynaptic terminals and astrocyte processes, forms the tetrapartite synapse [109, 110]. Emerging evidence strongly suggests that microglia actively regulate and remodel both the diffuse perisynaptic ECM and the condensed PNN, adding another layer to their role as sculptors of the neural environment in both health and disease. Microglia possess a variety of instruments enabling them to dynamically interact with and modify the ECM, including PNNs. The two primary mechanisms implicated are direct phagocytosis of matrix components and the secretion of ECM-degrading enzymes.
Direct physical interaction and engulfment of ECM components by microglia is increasingly documented across various contexts. Following peripheral nerve injury (spared nerve injury, SNI model), microglia in the spinal cord dorsal horn become activated and contain WFA immunoreactivity within their lysosomes [111]. Similarly, in mouse models of Amyotrophic Lateral Sclerosis (ALS), activated microglia and astrocytes in the ventral horn show increased engulfment of the core PNN protein aggrecan, coinciding with the timing of PNN breakdown around vulnerable motor neurons [112]. In Alzheimer’s disease (AD) models (5xFAD mice) and human AD brains, microglia associated with amyloid-beta (Aβ) plaques (PAMs) contain intracellular WFA + material and inclusions of PNN components [113]. Finally, loss of PNN coincided with early microglial activation and with a reduction in synaptic plasticity in the hippocampus in a ME7 murine prion disease [114].
While disease states clearly show engulfment, microglia seem to interact with PNNs under baseline conditions. Recent work highlights a fundamental role for microglia cells in clearing perisynaptic ECM (including CSPGs like aggrecan and brevican) via phagocytosis in the healthy adult hippocampus, a process regulated by the neuron-derived cytokine IL-33, which is modulated by experience. Loss of IL-33 or its microglial receptor IL1RL1 impaired ECM engulfment and led to ECM accumulation around synapses associated with impairment in memory consolidation in mice [115]. This suggests microglia perform homeostatic surveillance and a certain extent of ECM clearance, including potentially PNN components, even during health conditions.
Importantly, experimental manipulations are able to activate microglia phagocytic capacity. Repeated anesthetic ketamine exposure, which induces PNN loss, leads to microglia closely interacting with PV + neurons and containing WFA + fragments. Live ex vivo imaging of primary somatosensory cortex coronal slices confirms that microglia cells actively survey WFA-labeled structures and accumulate WFA + material internally over time following ketamine treatment [116]. Although correlative, activation of microglia proliferation by LPS or valproic acid treatment reduces the density of PNNs in both mouse hippocampal CA1 and the visual cortex [117].
Remarkably, postnatal development microglia phagocytic activity of synapses and PNNs in the mediobasal hypothalamus have been implicated in systemic glucose tolerance. In mice, transiently depleting microglia before weaning (postnatal day [P]6–16) but not afterward (P21–31) induces glucose intolerance in adulthood due to impaired insulin responsiveness, which is linked to PNN overabundance and reduced autonomic synaptic connectivity between hypothalamic glucoregulatory neurons and the pancreatic beta cell compartment. The authors conclude that neonatal hypothalamic microglia help program the ability of the CNS to properly potentiate glucose-responsive pancreatic insulin secretion in adulthood by determining the autonomic connectivity of hypothalamic neurons, including those involved in glucose sensing, with the pancreatic beta cell compartment [118].
The signals triggering PNN engulfment are still being elucidated. While classical “eat-me” markers like phosphatidylserine (PS) mediate microglial pruning of synapses, and complement factors (C1q, C3) are involved [119], their specific role in PNN phagocytosis is less clear. The CX3CL1-CX3CR1 neuron-microglia communication axis, important for pruning, does not appear essential for SNI-induced PNN degradation in the spinal cord [111] nor for PNN changes in ALS models [112]. However, the IL-33/IL1RL1 pathway clearly drives ECM engulfment in the hippocampus [115]. It is plausible that damaged or altered PNNs expose signals that may mark them for microglial clearance. A possibility could be related to the sulfation pattern of CSPGs which is sensitive to changes in the microenvironment (e.g. metabolic signals [4, 79]), regulates distinct properties of the matrix [120–122] and may be read as a code for degradation by microglia cells.
Further evidence of the link between ECM remodelling and microglia is that microglia secrete various proteases capable of degrading ECM components, offering an alternative or complementary mechanism to direct phagocytosis. For instance, MMP9 expression is upregulated in microglia (and astrocytes) in the ventral horn of ALS mouse model, coinciding with PNN breakdown. Interestingly, MMP9 inhibition or genetic ablation is protective in ALS models [112]. ADAMTS family members, such as ADAMTS4, are also expressed by microglia and can cleave aggrecan and brevican [123, 124]. However, there is no direct evidence of ADAMTS involvement in microglia-driven reshaping of PNNs and ECM.
We could speculate that microglia dependent enzymatic degradation and phagocytosis work in concert. Proteases released by microglia could ‘prime’ the PNN by cleaving core proteins or GAG chains, creating fragments or altering the structure to make it more amenable to subsequent engulfment.
A striking feature emerging from the above-mentioned studies is that microglia could interact with PNNs and ECM both in health and disease state, suggesting that PNN degradation could be related to homeostatic function as well as to pathological conditions. Thus, the interaction between microglia and PNNs may be tightly regulated with significant functional consequences. Colony stimulating factor 1 receptor (CSF1R) signaling is critical, as both genetic haploinsufficiency as observed in the Csf1r+/- model of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP), and pharmacological inhibition impact microglial homeostasis and consequently PNN integrity. Loss of one Csf1r allele leads to microglial dyshomeostasis (reduced purinergic receptor P2Y G-protein coupled 12, P2RY12) and PNN loss, which can be rescued by eliminating the microglia altogether or, paradoxically, by further low-grade CSF1R inhibition through PLX5622. This work suggests that mitigating microglia dyshomeostasis through pharmacological modulation is beneficial to improve the phenotype of ALSP mice [125].
The switch in microglial function from homeostatic maintenance to active PNN degradation may be triggered upon encountering pathological stimuli or inflammatory cues. The precise triggers for this switch likely vary depending on the specific disease context and brain region. It could involve inflammatory signals (e.g. TNFalpha), neuronal stress signals (e.g. CX3CL1, or other chemokines), or direct interaction with pathological proteins like Aβ. Understanding the signals that govern the switch between homeostatic maintenance and pathological degradation of PNNs and ECM in general by microglia holds significant therapeutic potential for a wide range of neurological and psychiatric disorders. Modulating microglial activity, perhaps via CSF1R signaling or through P2RY12, to modulate PNN loss or promote appropriate remodeling could represent a novel strategy to preserve neuronal function, promote synaptic plasticity and combat disease progression.
Future Directions
In this review, we highlight that PNNs—complex extracellular structures involved in regulating multiple plasticity processes during development and adulthood—require tight regulation to ensure proper circuit maturation, physiological brain function, and responses to pathological states. Increasing evidence shows that PNNs are modulated by signaling molecules, neuronal activity, interactions with astrocytes and microglia, and—more recently—by hormones and metabolic factors. We can speculate that PNNs act as an integratory hub shaping neural plasticity in relation to both brain-intrinsic and peripheral cues. However, further research is needed to elucidate how these regulatory mechanisms cooperate and to identify the central nodes in this network of interactors.
We discussed how astrocytes are involved in the production of ECM molecules and how, together with microglia, control PNNs especially in a pathological context. However, the cooperative dynamics among these cellular players in orchestrating PNN regulation remain poorly understood.
Overall, PNN deposition is not solely determined by the cells enwrapped by PNNs themselves, but depends on multiple cell types and, in some cases, on signals originating from distant brain regions or even peripheral tissues. For example, in the brain, many—but not all—PV interneurons are surrounded by PNNs [38], suggesting that PNN expression is driven by mechanisms not shared across the entire cell type. Nevertheless, the molecular signals or cellular machinery that enable specific neurons to be ensheathed by PNNs remain largely unknown.
Another major challenge in studying PNN regulation stems from the complex biology of CSPGs. For instance, the sulfation pattern of CSPG sugar chains—a critical factor influencing PNN properties during development— is studied as a molecular code modulating plasticity, while other sugar modifications and their impact on PNN physiology and plasticity have not been characterized. Recent methodological advances offer promising solutions. For example, spatial omics approaches, including spatial transcriptomics [126] and proteomics [127, 128], can help characterize the molecular fingerprint of PNN-ensheated cells in different brain regions as well as the biochemical pathways responsible for their aggregation. Additionally, expansion microscopy [129, 130] could enable high-resolution imaging of ECM structures and glycan modifications within brain tissue, offering unprecedented insights into PNN architecture and molecular composition. We anticipate that these tools will significantly deepen our understanding of the molecular regulation and functional diversity of PNNs.
The complexity of PNN regulation is further compounded by regional differences in PNN composition and dynamics across brain areas. It is conceivable that region-specific connectivity patterns might cause rearrangements of PNN both in development or in adulthood, perhaps driving activity of the local circuits towards a more or less plastic configuration.
In addition to this, while attention has been focused on condensed PNNs, the regulation of the diffuse ECM remains poorly understood, as its role in neural plasticity control.
Technical advancements are already promising, particularly in the field of optical methods for the visualization of PNNs in vivo, either with fluorescent labeling of WFA or through transgenic models [131, 132], which could provide valuable means to test longitudinally the effects of specific modulatory elements on PNN biology. Recent work also showed the possibility of studying the PNN interactome by performing precipitation with WFA and mass spectroscopy analysis [86]. Nevertheless, the development of new tools for the functional and molecular characterization of PNNs will be needed to further clarify their tight control of neural plasticity in health and disease.
Acknowledgements
This work was supported by the following grants: European Union - Next Generation EU Missione 4 Componente 2 Inv. 1.5 THE CUP I53C22000780001, PNRR MUR M4 C1 Inv. 3.4 Iniziative Educative Transnazionali - TNE 00034 - Avviso n.167/2023 for dissemination of PhD program at Scuola Normale Superiore, PRIN2022 20228RMXBE, and “Sviluppo di un approccio di terapia genica per la cura del Deficit del Trasportatore della Creatina” Fondo di Beneficenza Intesa San Paolo to TP; European Union - Next Generation EU Italian Ministry of University and Research PRIN-PNRR2022 CARE P2022CXN7X CUP I53D23006920001 to PT.
Author Contributions
All authors contributed to the conceptualization of the review. VT and PT wrote the initial draft. VT prepared the figures. TP and PT provided supervision and funding. All authors contributed to the text, reviewed the final version, and approved the manuscript for submission.
Funding
Open access funding provided by Scuola Normale Superiore within the CRUI-CARE Agreement. This work was supported by the following grants: European Union - Next Generation EU Missione 4 Componente 2 Inv. 1.5 THE CUP I53C22000780001, PNRR MUR M4 C1 Inv. 3.4 Iniziative Educative Transnazionali - TNE 00034 - Avviso n.167/2023 for dissemination of PhD program at Scuola Normale Superiore, PRIN2022 20228RMXBE, and “Sviluppo di un approccio di terapia genica per la cura del Deficit del Trasportatore della Creatina” Fondo di Beneficenza Intesa San Paolo to TP; European Union - Next Generation EU Italian Ministry of University and Research PRIN-PNRR2022 CARE P2022CXN7X CUP I53D23006920001 to PT.
Data Availability
No datasets were generated or analysed during the current study.
Materials Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pizzorusso T, Medini P, Berardi N et al (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251 [DOI] [PubMed] [Google Scholar]
- 2.Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261 [DOI] [PubMed] [Google Scholar]
- 3.Mirzadeh Z, Alonge KM, Cabrales E et al (2019) Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat Metab 1:212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonge KM, Mirzadeh Z, Scarlett JM et al (2020) Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats. Nat Metab 2:1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsaran AI, Ventura S, Gallucci J et al (2025) A sensitive period for the development of episodic-like memory in mice. Curr Biol 35:2032–2048e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsaran AI, Wang Y, Golbabaei A et al (2023) A shift in the mechanisms controlling hippocampal engram formation during brain maturation. Science 380:543–551 [DOI] [PubMed] [Google Scholar]
- 7.Christensen AC, Lensjø KK, Lepperød ME et al (2021) Perineuronal nets stabilize the grid cell network. Nat Commun 12:253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson EH, Lensjø KK, Wigestrand MB et al (2018) Removal of perineuronal nets disrupts recall of a remote fear memory. Proc Natl Acad Sci U S A 115:607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee SB, Gutzeit VA, Baman J et al (2017) Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron 95:169–179e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poli A, Viglione A, Mazziotti R et al (2023) Selective disruption of perineuronal nets in mice lacking Crtl1 is sufficient to make fear memories susceptible to erasure. Mol Neurobiol 60:4105–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carulli D, Broersen R, de Winter F et al (2020) Cerebellar plasticity and associative memories are controlled by perineuronal nets. Proc Natl Acad Sci U S A 117:6855–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fawcett JW, Oohashi T, Pizzorusso T (2019) The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 20:451–465 [DOI] [PubMed] [Google Scholar]
- 13.Oohashi T, Edamatsu M, Bekku Y, Carulli D (2015) The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol 274:134–144 [DOI] [PubMed] [Google Scholar]
- 14.Deepa SS, Carulli D, Galtrey C et al (2006) Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281:17789–17800 [DOI] [PubMed] [Google Scholar]
- 15.Härtig W, Meinicke A, Michalski D et al (2022) Update on perineuronal net staining with agglutinin (WFA). Front Integr Neurosci 16:851988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews RT, Kelly GM, Zerillo CA et al (2002) Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci 22:7536–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travascio F (2016) Composition and function of the extracellular matrix in the human body. BoD – Books on Demand
- 18.Galtrey CM, Kwok JCF, Carulli D et al (2008) Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci 27:1373–1390 [DOI] [PubMed] [Google Scholar]
- 19.Kwok JCF, Dick G, Wang D, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71:1073–1089 [DOI] [PubMed] [Google Scholar]
- 20.Smith-Thomas LC, Stevens J, Fok-Seang J et al (1995) Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci 108(Pt 3):1307–1315 [DOI] [PubMed] [Google Scholar]
- 21.Asher RA, Morgenstern DA, Fidler PS et al (2000) Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci 20:2427–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradbury EJ, Moon LDF, Popat RJ et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y-Y, Xue R-R, Yao M et al (2024) A systematic review and meta-analysis of chondroitinase ABC promotes functional recovery in rat models of spinal cord injury. Nutr Neurosci 27:917–933 [DOI] [PubMed] [Google Scholar]
- 24.Vo T, Carulli D, Ehlert EME et al (2013) The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci 56:186–200 [DOI] [PubMed] [Google Scholar]
- 25.Carulli D, Foscarin S, Faralli A et al (2013) Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci 57:10–22 [DOI] [PubMed] [Google Scholar]
- 26.Boggio EM, Ehlert EM, Lupori L et al (2019) Inhibition of Semaphorin3A promotes ocular dominance plasticity in the adult rat visual cortex. Mol Neurobiol 56:5987–5997 [DOI] [PubMed] [Google Scholar]
- 27.Scarlett JM, Hu SJ, Alonge KM (2022) The loss of perineuronal nets in alzheimer’s disease: missing or hiding in plain sight?? Front Integr Neurosci 16:896400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carstens KE, Lustberg DJ, Shaughnessy EK et al (2021) Perineuronal net degradation rescues CA2 plasticity in a mouse model of Rett syndrome. J Clin Invest 131. 10.1172/JCI137221 [DOI] [PMC free article] [PubMed]
- 29.Liu P, Zhao Y, Xiong W et al (2023) Degradation of perineuronal nets in the cerebellar interpositus nucleus ameliorated social deficits in Shank3-deficient mice. Neuroscience 511:29–38 [DOI] [PubMed] [Google Scholar]
- 30.Wen TH, Afroz S, Reinhard SM et al (2018) Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing Fmr1 knock-out mice. Cereb Cortex 28:3951–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizzo R, Gurgone A, Castroflorio E et al (2016) Lack of Cdkl5 disrupts the organization of excitatory and inhibitory synapses and parvalbumin interneurons in the primary visual cortex. Front Cell Neurosci 10:261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suttkus A, Morawski M, Arendt T (2016) Protective properties of neural extracellular matrix. Mol Neurobiol 53:73–82 [DOI] [PubMed] [Google Scholar]
- 33.Cabungcal J-H, Steullet P, Morishita H et al (2013) Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A 110:9130–9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauney SA, Athanas KM, Pantazopoulos H et al (2013) Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry 74:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantazopoulos H, Woo TU, Lim MP et al (2010) Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67. 10.1001/archgenpsychiatry.2009.196 [DOI] [PMC free article] [PubMed]
- 36.Baig S, Wilcock GK, Love S (2005) Loss of perineuronal net N-acetylgalactosamine in alzheimer’s disease. Acta Neuropathol 110:393–401 [DOI] [PubMed] [Google Scholar]
- 37.Morawski M, Brückner G, Jäger C et al (2012) Involvement of perineuronal and perisynaptic extracellular matrix in alzheimer’s disease neuropathology. Brain Pathol 22:547–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupori L, Totaro V, Cornuti S et al (2023) A comprehensive atlas of perineuronal net distribution and colocalization with parvalbumin in the adult mouse brain. Cell Rep 42:112788 [DOI] [PubMed] [Google Scholar]
- 39.Lensjø KK, Christensen AC, Tennøe S et al (2017) Differential expression and cell-type specificity of perineuronal nets in hippocampus, medial entorhinal cortex, and visual cortex examined in the rat and mouse. eNeuro 4. 10.1523/ENEURO.0379-16.2017 [DOI] [PMC free article] [PubMed]
- 40.Testa D, Prochiantz A, Di Nardo A (2019) Perineuronal nets in brain physiology and disease. Semin Cell Dev Biol 89:125–135 [DOI] [PubMed] [Google Scholar]
- 41.Carulli D, Verhaagen J (2021) An extracellular perspective on CNS maturation: perineuronal nets and the control of plasticity. Int J Mol Sci 22. 10.3390/ijms22052434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorg BA, Berretta S, Blacktop JM et al (2016) Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci 36:11459–11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claudi F, Tyson AL, Petrucco L et al (2021) Visualizing anatomically registered data with brainrender. 10.7554/eLife.65751 [DOI] [PMC free article] [PubMed]
- 44.Gao R, Wang M, Lin J et al (2018) Spatiotemporal expression patterns of chondroitin sulfate proteoglycan mRNAs in the developing rat brain. NeuroReport 29:517–523 [DOI] [PubMed] [Google Scholar]
- 45.Carulli D, Pizzorusso T, Kwok JCF et al (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133:2331–2347 [DOI] [PubMed] [Google Scholar]
- 46.Gottschling C, Wegrzyn D, Denecke B, Faissner A (2019) Elimination of the four extracellular matrix molecules tenascin-C, tenascin-R, brevican and neurocan alters the ratio of excitatory and inhibitory synapses. Sci Rep 9:13939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowlands D, Lensjø KK, Dinh T et al (2018) Aggrecan directs extracellular matrix-mediated neuronal plasticity. J Neurosci 38:10102–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grødem S, Thompson EH, Røe MB et al (2025) Differential impacts of germline and adult Aggrecan knockout in PV + neurons on perineuronal nets and PV + neuronal function. Mol Psychiatry. 10.1038/s41380-025-02894-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McRae PA, Rocco MM, Kelly G et al (2007) Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci 27:5405–5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitagawa H, Ujikawa M, Tsutsumi K et al (1997) Characterization of serum beta-glucuronyltransferase involved in chondroitin sulfate biosynthesis. Glycobiology 7:905–911 [DOI] [PubMed] [Google Scholar]
- 51.Foscarin S, Raha-Chowdhury R, Fawcett JW, Kwok JCF (2017) Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging 9:1607–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fawcett JW, Kwok JCF (2022) Proteoglycan sulphation in the function of the mature central nervous system. Front Integr Neurosci 16:895493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitkänen ADBW-H (2014) Brain extracellular matrix in health and disease. Elsevier [DOI] [PubMed]
- 54.Iwakura Y, Kobayashi Y, Namba H et al (2024) Epidermal growth factor suppresses the development of GABAergic neurons via the modulation of perineuronal net formation in the neocortex of developing rodent brains. Neurochem Res 49:1347–1358 [DOI] [PubMed] [Google Scholar]
- 55.Sugiyama S, Di Nardo AA, Aizawa S et al (2008) Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134:508–520 [DOI] [PubMed] [Google Scholar]
- 56.Spatazza J, Lee HHC, Di Nardo AA et al (2013) Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep 3:1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beurdeley M, Spatazza J, Lee HHC et al (2012) Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 32:9429–9437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dick G, Tan CL, Alves JN et al (2013) Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J Biol Chem 288:27384–27395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van’t Spijker HM, Rowlands D, Rossier J et al (2019) Neuronal pentraxin 2 binds PNNs and enhances PNN formation. Neural Plast 2019:6804575 [DOI] [PMC free article] [PubMed]
- 60.Swahari V, Nakamura A, Hollville E et al (2024) miR-29 is an important driver of aging-related phenotypes. Commun Biol 7:1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Napoli D, Lupori L, Mazziotti R et al (2020) MiR-29 coordinates age-dependent plasticity brakes in the adult visual cortex. EMBO Rep 21:e50431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faini G, Aguirre A, Landi S et al (2018) Perineuronal nets control visual input via thalamic recruitment of cortical PV interneurons. Elife 7. 10.7554/eLife.41520 [DOI] [PMC free article] [PubMed]
- 63.Miyata S, Komatsu Y, Yoshimura Y et al (2012) Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci 15:414–422, S1–2 [DOI] [PubMed] [Google Scholar]
- 64.Shi W, Wei X, Wang X et al (2019) Perineuronal nets protect long-term memory by limiting activity-dependent inhibition from parvalbumin interneurons. Proc Natl Acad Sci U S A 116:27063–27073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gøtzsche CR, Woldbye DPD (2016) The role of NPY in learning and memory. Neuropeptides 55:79–89 [DOI] [PubMed] [Google Scholar]
- 66.Bertocchi I, Mele P, Ferrero G et al (2021) NPY-Y1 receptor signaling controls spatial learning and perineuronal net expression. Neuropharmacology 184:108425 [DOI] [PubMed] [Google Scholar]
- 67.Devienne G, Picaud S, Cohen I et al (2021) Regulation of perineuronal nets in the adult cortex by the activity of the cortical network. J Neurosci 41:5779–5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu D, Hopf C, Reddy R et al (2003) Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39:513–528 [DOI] [PubMed] [Google Scholar]
- 69.Chang MC, Park JM, Pelkey KA et al (2010) Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci 13:1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sale A, Maya Vetencourt JF, Medini P et al (2007) Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci 10:679–681 [DOI] [PubMed] [Google Scholar]
- 71.Ciucci F, Putignano E, Baroncelli L et al (2007) Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS ONE 2:e475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor AM, Burton TJ, Mansuri H et al (2019) Environmental enrichment from birth impacts parvalbumin expressing cells and Wisteria Floribunda agglutinin labelled peri-neuronal nets within the developing murine striatum. Front Neuroanat 13:453832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joly-Amado A, Cansell C, Denis RGP et al (2014) The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract Res Clin Endocrinol Metab 28:725–737 [DOI] [PubMed] [Google Scholar]
- 74.Jiang H, Gallet S, Klemm P et al (2020) MCH neurons regulate permeability of the median eminence barrier. Neuron 107:306–319e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker KD, Gray AR, Richardson R (2017) The development of perineuronal nets around parvalbumin gabaergic neurons in the medial prefrontal cortex and basolateral amygdala of rats. Behav Neurosci 131:289–303 [DOI] [PubMed] [Google Scholar]
- 76.Kamitakahara A, Bouyer K, Wang C-H, Simerly R (2018) A critical period for the trophic actions of leptin on AgRP neurons in the arcuate nucleus of the hypothalamus. J Comp Neurol 526:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang N, Yan Z, Liu H et al (2021) Hypothalamic perineuronal nets are regulated by sex and dietary interventions. Front Physiol 12:714104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kohnke S, Buller S, Nuzzaci D et al (2021) Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep 36:109362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beddows CA, Shi F, Horton AL et al (2024) Pathogenic hypothalamic extracellular matrix promotes metabolic disease. Nature 633:914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alonge KM, Dorfman MD, Scarlett JM et al (2022) 152-OR: evidence linking loss of hypothalamic perineuronal nets to obesity pathogenesis. Diabetes 71. 10.2337/db22-152-or
- 81.Dingess PM, Zhang Z, Sorg BA et al (2020) Sex and region-specific effects of high fat diet on PNNs in obesity susceptible rats. Physiol Behav 222:112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu J, Zhang S, Wu H et al (2024) 1-Methyltryptophan treatment ameliorates high-fat diet-induced depression in mice through reversing changes in perineuronal nets. Transl Psychiatry 14:228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cañeque-Rufo H, Fontán-Baselga T, Galán-Llario M et al (2025) Pleiotrophin deletion prevents high-fat diet-induced cognitive impairment, glial responses, and alterations of the perineuronal nets in the hippocampus. Neurobiol Dis 205:106776 [DOI] [PubMed] [Google Scholar]
- 84.Allen NJ, Eroglu C (2017) Cell biology of astrocyte-synapse interactions. Neuron 96:697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vivi E, Di Benedetto B (2024) Brain stars take the lead during critical periods of early postnatal brain development: relevance of astrocytes in health and mental disorders. Mol Psychiatry 29:2821–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ribot J, Breton R, Calvo C-F et al (2021) Astrocytes close the mouse critical period for visual plasticity. Science 373:77–81 [DOI] [PubMed] [Google Scholar]
- 87.Miyata S, Nishimura Y, Hayashi N, Oohira A (2005) Construction of perineuronal net-like structure by cortical neurons in culture. Neuroscience 136:95–104 [DOI] [PubMed] [Google Scholar]
- 88.Geissler M, Gottschling C, Aguado A et al (2013) Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci 33:7742–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song I, Dityatev A (2018) Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull 136:101–108 [DOI] [PubMed] [Google Scholar]
- 90.Rauch U, Zhou X-H, Roos G (2005) Extracellular matrix alterations in brains lacking four of its components. Biochem Biophys Res Commun 328:608–617 [DOI] [PubMed] [Google Scholar]
- 91.Blosa M, Sonntag M, Brückner G et al (2013) Unique features of extracellular matrix in the mouse medial nucleus of trapezoid body–implications for physiological functions. Neuroscience 228:215–234 [DOI] [PubMed] [Google Scholar]
- 92.Brandebura AN, Kolson DR, Amick EM et al (2022) Transcriptional profiling reveals roles of intercellular Fgf9 signaling in astrocyte maturation and synaptic refinement during brainstem development. J Biol Chem 298:102176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dityatev A, Schachner M, Sonderegger P (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11:735–746 [DOI] [PubMed] [Google Scholar]
- 94.Tewari BP, Woo AM, Prim CE et al (2024) Astrocytes require perineuronal nets to maintain synaptic homeostasis in mice. Nat Neurosci 27:1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patani R, Hardingham GE, Liddelow SA (2023) Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Reviews Neurol 19:395–409 [DOI] [PubMed] [Google Scholar]
- 96.Escartin C, Galea E, Lakatos A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel DC, Tewari BP, Chaunsali L, Sontheimer H (2019) Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci 20:282–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tewari BP, Chaunsali L, Prim CE, Sontheimer H (2022) A glial perspective on the extracellular matrix and perineuronal net remodeling in the central nervous system. Front Cell Neurosci 16:1022754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rhodes KE, Moon LDF, Fawcett JW (2003) Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience 120:41–56 [DOI] [PubMed] [Google Scholar]
- 100.Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156 [DOI] [PubMed] [Google Scholar]
- 101.Lau LW, Cua R, Keough MB et al (2013) Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 14:722–729 [DOI] [PubMed] [Google Scholar]
- 102.Muir EM, Adcock KH, Morgenstern DA et al (2002) Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res 100:103–117 [DOI] [PubMed] [Google Scholar]
- 103.Falo MC, Fillmore HL, Reeves TM, Phillips LL (2006) Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res 84:768–781 [DOI] [PubMed] [Google Scholar]
- 104.Kim SY, Senatorov VV Jr, Morrissey CS et al (2017) TGFβ signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults. Sci Rep 7:7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Irala D, Wang S, Sakers K et al (2024) Astrocyte-secreted neurocan controls inhibitory synapse formation and function. Neuron 112:1657–1675e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B (2015) Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol 36:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mordelt A, de Witte LD (2023) Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: hype or hope? Curr Opin Neurobiol 79:102674 [DOI] [PubMed] [Google Scholar]
- 108.Sakai J (2020) Core concept: how synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc Natl Acad Sci U S A 117:16096–16099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carmignoto G (2000) Reciprocal communication systems between astrocytes and neurones. Prog Neurobiol 62:561–581 [DOI] [PubMed] [Google Scholar]
- 110.Semyanov A, Verkhratsky A (2021) Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci 44:781–792 [DOI] [PubMed] [Google Scholar]
- 111.Tansley S, Gu N, Guzmán AU et al (2022) Microglia-mediated degradation of perineuronal nets promotes pain. Science 377:80–86 [DOI] [PubMed] [Google Scholar]
- 112.Cheung SW, Willis EF, Simmons DG et al (2024) Phagocytosis of aggrecan-positive perineuronal nets surrounding motor neurons by reactive microglia expressing MMP-9 in TDP-43 ALS model mice. Neurobiol Dis 200:106614 [DOI] [PubMed] [Google Scholar]
- 113.Crapser JD, Spangenberg EE, Barahona RA et al (2020) Microglia facilitate loss of perineuronal nets in the alzheimer’s disease brain. EBioMedicine 58:102919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franklin SL, Love S, Greene JRT, Betmouni S (2008) Loss of perineuronal net in ME7 prion disease. J Neuropathol Exp Neurol 67:189–199 [DOI] [PubMed] [Google Scholar]
- 115.Nguyen PT, Dorman LC, Pan S et al (2020) Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182:388–403e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Venturino A, Schulz R, De Jesús-Cortés H et al (2021) Microglia enable mature perineuronal nets disassembly upon anesthetic ketamine exposure or 60-Hz light entrainment in the healthy brain. Cell Rep 36:109313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu L, Li T, Chang J et al (2024) Microglia inversely regulate the level of perineuronal nets with the treatment of lipopolysaccharide and valproic acid. Neurosci Lett 842:137992 [DOI] [PubMed] [Google Scholar]
- 118.Valdearcos M, McGrath ER, Brown Mayfield SM et al (2025) Microglia mediate the early-life programming of adult glucose control. Cell Rep 44:115409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cornell J, Salinas S, Huang H-Y, Zhou M (2022) Microglia regulation of synaptic plasticity and learning and memory. Neural Regen Res 17:705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hrabetová S, Masri D, Tao L et al (2009) Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol 587:4029–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Properzi F, Carulli D, Asher RA et al (2005) Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci 21:378–390 [DOI] [PubMed] [Google Scholar]
- 122.Mizumoto S, Yamada S, Sugahara K (2015) Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr Opin Struct Biol 34:35–42 [DOI] [PubMed] [Google Scholar]
- 123.Gottschall PE, Howell MD (2015) ADAMTS expression and function in central nervous system injury and disorders. Matrix Biol 44–46:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Demircan K, Topcu V, Takigawa T et al (2014) ADAMTS4 and ADAMTS5 knockout mice are protected from versican but not aggrecan or brevican proteolysis during spinal cord injury. Biomed Res Int 2014:693746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arreola MA, Soni N, Crapser JD et al (2021) Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R mouse model of ALSP, which can be rescued via CSF1R inhibitors. Sci Adv 7. 10.1126/sciadv.abg1601 [DOI] [PMC free article] [PubMed]
- 126.Rao A, Barkley D, França GS, Yanai I (2021) Exploring tissue architecture using Spatial transcriptomics. Nature 596:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu B, He R, Pang K et al (2025) High-resolution spatially resolved proteomics of complex tissues based on microfluidics and transfer learning. Cell 188:734–748e22 [DOI] [PubMed] [Google Scholar]
- 128.Unterauer EM, Shetab Boushehri S, Jevdokimenko K et al (2024) Spatial proteomics in neurons at single-protein resolution. Cell 187:1785–1800e16 [DOI] [PubMed] [Google Scholar]
- 129.Chen F, Tillberg PW, Boyden ES (2015) Optical imaging. Expansion microscopy. Science 347:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wassie AT, Zhao Y, Boyden ES (2019) Expansion microscopy: principles and uses in biological research. Nat Methods 16:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benbenishty A, Peled-Hajaj S, Krishnaswamy VR et al (2023) Longitudinal imaging of perineuronal nets. Neurophotonics 10:015008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lemieux SP, Lev-Ram V, Tsien RY, Ellisman MH (2023) Perineuronal nets and the neuronal extracellular matrix can be imaged by genetically encoded labeling of HAPLN1 and bioRxiv
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.
Not applicable.
Not applicable.