Abstract
Objective
To analyze the effect of mask usage on dry eye symptoms and meibomian gland function in patients with obstructive sleep apnea syndrome (OSAS).
Methods
OSAS patients who visited our hospital from January 2022 to December 2023 were selected as research subjects. All patients were treated with continuous positive airway pressure (CPAP) therapy. The polysomnography (PSG) results before and after treatment were analyzed, including the apnea-hypopnea index (AHI) and peripheral oxygen saturation (SpO2). Dry eye conditions and ocular surface analyzer results of patients before and after treatment were evaluated. Pearson correlation was used to assess the relationship between dry eye conditions and ocular surface analyzer results in OSAS patients, and the correlations between PSG results and dry eye conditions as well as ocular surface analyzer results were also analyzed.
Results
AHI levels in 31 OSAS patients were significantly decreased after treatment, while SpO2 levels significantly increased, with a statistically significant difference (P<0.05). After treatment, the tear breakup time (BUT), palpebral (PL) scores, and Ocular Surface Disease Index (OSDI) scores of patients were significantly higher than those before treatment (P<0.05). Additionally, after treatment, the tear meniscus height of OSAS patients was significantly lower than that before treatment, and the lipid layer thickness and eye redness index were significantly higher than those before treatment, with a statistically significant difference (P<0.05). The results of Pearson correlation analysis showed that BUT was positively correlated with lipid layer thickness and eye redness improvement, while Schirmer’s test (Sit) was positively correlated with tear meniscus height. PL scores were negatively correlated with tear meniscus height but positively correlated with the eye redness index (P<0.05). AHI from PSG results was negatively correlated with BUT, PL scores, and OSDI scores (P<0.05), while SpO2 was positively correlated with BUT, PL scores, and OSDI scores but negatively correlated with Sit (P<0.05). In the PSG results, AHI was negatively correlated with lipid layer thickness and eye redness index, and positively correlated with meibomian gland opening score (P < 0.05).
Conclusion
CPAP mask usage in OSAS patients may negatively impact dry eye symptoms and meibomian gland function, leading to decreased tear film stability and worsening symptoms of eye dryness.
Keywords: OSAS, CPAP, Mask, Dry eye symptoms, Meibomian gland function
Introduction
Obstructive sleep apnea syndrome (OSAS) is a disease caused by repeated collapse of the upper airway during sleep. It is often accompanied by symptoms such as snoring, sleep interruption, and hypoxemia, which can lead to a series of pathophysiological changes. These changes significantly increase the risk of various systemic diseases such as hypertension, stroke, and cardiovascular events [1, 2].
Continuous positive airway pressure (CPAP) is one of the main methods for treating OSAS. This method can effectively reduce the apnea hypopnea index (AHI) and respiratory disturbance index (RDI), and improve the patient’s sleep quality and daytime sleepiness symptoms [3, 4]. Dry eye is a multifactorial disease of the ocular surface. Its main characteristics are tear film instability and ocular surface damage, which can lead to symptoms such as eye discomfort and decreased vision. The mechanism of occurrence is relatively complex, primarily including insufficient tear secretion, rapid tear evaporation, and meibomian gland dysfunction [5]. Among them, meibomian gland dysfunction is a major cause of dry eye, as the lipid layer secreted by the meibomian glands reduces tear evaporation and maintains tear film stability. Studies have shown [6] that the prevalence of dry eye disease is significantly higher in patients with OSAS compared to the general population. This association may be attributed to OSAS-related intermittent hypoxia, which induces abnormal neural regulation. Specifically, it suppresses basal tear secretion via the trigeminal-parasympathetic pathway, while sleep fragmentation reduces blink frequency, thereby prolonging the tear film renewal cycle.
However, during CPAP therapy, two physical factors, including the mechanical pressure exerted by the mask on the face (particularly the meibomian gland area) and the retrograde flow of pressurized air through the nasolacrimal duct, may act synergistically on ocular tissues [7, 8]. Existing studies have shown that CPAP therapy masks (such as nasal masks/oral-nasal masks) have a significantly different physical mechanism of action compared to protective masks (such as N95 masks) due to the requirements of continuous positive airway pressure and edge sealing. CPAP masks can cause deformation of the meibomian glands under pressure-sensitive thresholds (> 10 cmH₂O) [9], and when air leakage exceeds 24 L/min, tear film evaporation rates increase to 1.8 times the baseline level [10, 11]. Although some mechanistic studies have been conducted, the differential effects of various mask designs remain to be further explored. Therefore, this study aims to investigate changes in dry eye symptoms and meibomian gland function before and after mask usage in OSAS patients through a controlled study, providing clinical treatment references.
Study subjects and methods
Study subjects
Patients with OSAS who visited our hospital from January 2022 to December 2023 were selected as study subjects. Inclusion criteria: ① Meet the diagnostic criteria for OSAS [12]; ② Aged 18 years or older; ③ Willing to participate in this study and sign the informed consent. Exclusion criteria: ① Combination with other eye diseases, such as glaucoma, retinopathy, etc.; ② Recent use of medications that affect dry eye symptoms or meibomian gland function; ③ Contraindications to CPAP treatment such as facial trauma and severe nasal diseases. ④Exclude long-term CPAP users (usage time > 3 months) to avoid confounding effects of previous ocular surface adaptation. A total of 31 patients with OSAS were included. The patient inclusion process is shown in Fig. 1.
Fig. 1.
Flow chart of patient inclusion
Methods
(1) CPAP therapy: All patients included in the study were OSAS patients receiving CPAP therapy for the first time and had not used CPAP devices prior to treatment. All patients underwent a one-week CPAP adaptation therapy (pressure 8–12 cmH₂O) prior to PSG monitoring to ensure mask compliance. The types of CPAP masks used in the study included nasal masks (ResMed AirFit N20, 74.2%) and oronasal mask masks (ResMed AirFit F20, 25.8%), both made of silicone. Leakage data were recorded in real-time using the device’s built-in sensor (ResMed S10 AutoSet).
(2) Data collection: Demographic data such as age, gender, body mass index (BMI), systolic blood pressure, diastolic blood pressure, blood sugar, and severity of OSAS were collected from all patients. Patients were divided into three groups based on the apnea–hypopnea index (AHI): mild (5–14.9 times/h, mean AHI ± SD, 10.35 ± 2.94), moderate (15–29.9 times/h, mean AHI ± SD, 22.48 ± 4.57), and severe (≥ 30 times/h, mean AHI ± SD, 40.83 ± 9.57).
(3) Polysomnography (PSG) results: Before treatment and the day after treatment, polysomnography (Beijing Yihe Jiaye, Poly Pro YH-2000 A) was used to monitor AHI (normal range: 0–5 times/h) and peripheral oxygen saturation (SpO2) (normal range: 95-98%). AHI was calculated by the number of apnea and hypopnea events per hour. Post-treatment PSG monitoring was conducted one week after CPAP therapy to assess the long-term effects of wearing the mask.
(4) Dry eye condition: The breakup time (BUT), Schirmer I test (Sit), palpebral (PL), and Ocular Surface Disease Index (OSDI) were observed before and after treatment.
①BUT: A 0.125% sodium fluorescein solution was applied to the conjunctival sac, and the patient was asked to blink several times to evenly distribute the sodium fluorescein on the corneal and conjunctival surfaces. Then, the patient was asked to keep eyes open while a slit-lamp microscope with cobalt blue light was used to observe the corneal surface, and the time from the last blink to the appearance of the first dry spot was recorded. Normal tear film break-up time is over 10 s; less than 10 s indicates tear film instability, with abnormal values below 8 s indicating significant instability, and values below 4 s suggesting severe dry eye.
②Sit: There are two ways of testing, including the active and passive tear secretion test. The active detection method was to place a filter paper strip on the lower meibomian gland margin for 5 min, and the wetted length was measured. Normal secretion is 10–15 mm; less than 10 mm indicates low secretion, and less than 5 mm suggests dry eye. In passive secretion testing, the strip was placed in the conjunctival sac for about 5 min with closed eyes. Tear secretion below 10 mm indicates low secretion, and repeated measures below 5 mm may diagnose dry eye.
③PL: Fluorescein staining was used to divide the cornea into four quadrants, with each quadrant scored from 0 to 3. A score of 0 indicates no staining; 1 indicates 1–30 punctate stains; 2 indicates more than 30 punctate stains but no coalescence; and 3 indicates coalescence, filaments, or ulcers.
④OSDI: The OSDI questionnaire consists of 12 items, divided into three subscales: ocular symptoms, vision-related functions, and environmental triggers. Patients score each item on a scale of 0 (never) to 4 (always), with the total OSDI score calculated as: OSDI = (total score of answered questions × 100) / (total number of questions answered × 4). Based on the total score, OSDI scores are classified as normal (0–12), mild dry eye (13–22), moderate dry eye (23–32), or severe dry eye (33 or above).
(5) Ocular surface analyzer results.
The tear meniscus height, lipid layer thickness, eye redness index and meibomian gland opening score were evaluated by ocular surface comprehensive analyzer (Keratograph 5 M).
① Tear meniscus height: An ocular surface comprehensive analyzer (e.g., Keratograph 5 M) was used to take the image of the concave arc formed at the junction of the tear and the meibomian gland margin, and indirectly evaluated the tear secretion by measuring the tear retention height. A normal tear meniscus height is ≥ 0.2 mm, with lower values indicating reduced tear secretion.
② Lipid layer thickness: Assessed using an ocular surface interferometer (e.g., Lipi -View). A normal lipid layer thickness is greater than 100 nm. Thicknesses between 61 and 100 nm suggest a 50% likelihood of meibomian gland dysfunction (MGD), while values of ≤ 60 nm indicate a 90% likelihood of MGD.
③ Eye Redness Index: The eye redness index was measured by a non-invasive ocular surface comprehensive analyzer (e.g., Keratograph 5 M), which evaluates the redness of the eyes by analyzing the degree of conjunctival congestion. While specific scoring criteria were not found in the referenced data, the index typically classifies redness severity by analyzing the degree of congestion.
④ Meibomian gland opening score: Meibomian gland opening score was assessed by observing the condition of the meibomian gland openings. The method involved having the patient look upward, and then examining and grading the opening condition of the 8 meibomian glands located in the central third of the lower meibomian gland. The scoring system is as follows: 0 points for normal gland openings (rounded, with no blockages); 1 point for openings with lipid capping; and 2 points for blocked or narrowed openings with protruding lipid plugs.
Statistical methods
Analysis was performed using SPSS 22.0 software. All measurement data were first subjected to the Shapiro–Wilk normality test (P > 0.05). Data that met the criteria for normal distribution were expressed as mean ± standard deviation (SD), and intergroup comparisons were performed using the independent samples t-test; data that did not meet the criteria for normal distribution were analyzed using the Mann-Whitney U test. Correlation analysis was performed using Pearson linear correlation. When performing stratified analysis of air leakage volume and ocular surface indicators, patients were divided into a high air leakage group (n = 11) and a low air leakage group (n = 20) based on a cutoff of 24 L/min. Covariance analysis (ANCOVA) was used to control for confounding factors such as BMI and AHI. P < 0.05 was considered statistically significant.
Results
Analysis of general data of OSAS patients
A total of 31 OSAS patients were included in the study, with 21 males and 10 females. The average BMI of the patients was (26.71 ± 4.33) kg/m², which exceeds the normal range and falls within the overweight category. Blood pressure analysis showed that the average systolic pressure was (131.23 ± 12.50) mmHg, slightly above the normal range, while the average diastolic pressure was (75.50 ± 10.23) mmHg. The severity of the patients’ conditions was as follows: 8 cases (25.81%) had mild OSAS, 11 cases (35.48%) had moderate OSAS, and 12 cases (38.71%) had severe OSAS. Additional general information is provided in Table 1.
Table 1.
General information of OSAS patients
| General Information | OSAS Patients (n = 31) | |
|---|---|---|
| Age (years) | 50.23 ± 10.74 | |
| Gender | Male | 21(67.74) |
| Female | 10(32.26) | |
| BMI (kg/m2) | 26.71 ± 4.33 | |
| Systolic Blood Pressure (mmHg) | 131.23 ± 12.50 | |
| Diastolic Blood Pressure (mmHg) | 75.50 ± 10.23 | |
| Fasting Blood Glucose (mmol/L) | 6.24 ± 2.03 | |
| OSAS Severity | Mild | 8(25.81) |
| Moderate | 11(35.48) | |
| Severe | 12(38.71) | |
| Mask type | Nasal mask | 23(74.20) |
| Oronasal mask | 8(25.80) | |
| Leakage rate (L/min) | Nasal mask | 10.80 ± 2.71 |
| Oronasal mask | 14.72 ± 4.11 |
Analysis of PSG results before and after treatment in OSAS patients
To further analyze the PSG results of OSAS patients before and after CPAP treatment, this study conducted PSG testing on 31 OSAS patients before treatment and the day after treatment to assess AHI and SpO2 levels. In the 31 OSAS patients, the mean SpO₂ level during wakefulness was 89.30 ± 3.75%. Before CPAP treatment, the average AHI was 22.80 ± 4.55 events/h, and the SpO2 level was 85.90 ± 4.07%. After CPAP treatment, the AHI significantly decreased to 11.07 ± 3.62 events/h, although it remained within the abnormal range; meanwhile, the SpO2 level markedly increased to 90.72 ± 4.15%. An independent samples t-test showed that the differences between pre- and post-treatment values were statistically significant (P < 0.05).
Analysis of changes in dry eye conditions in OSAS patients before and after treatment
To further analyze changes in dry eye conditions among OSAS patients after CPAP treatment, 31 patients were assessed both before treatment and a day after treatment. The evaluations included BUT, Schirmer I test (Sit), PL score, and OSDI score. The results showed no statistically significant difference in Sit between pre- and post-treatment (P > 0.05). After treatment, patients showed a significant increase in BUT (P < 0.05), while both PL scores and OSDI scores were significantly higher than before treatment, indicating improved tear film stability but worsened dry eye symptoms (P < 0.05, Table 2).
Table 2.
Analysis of changes in dry eye condition in OSAS patients before and after treatment
| Time | Cases | BUT(s) | Sit(mm/5min) | PL Score | OSDI Score |
|---|---|---|---|---|---|
| Before Treatment | 31 | 10.46 ± 2.15 | 12.83 ± 2.25 | 2.32 ± 0.88 | 18.01 ± 3.85 |
| After Treatment | 31 | 12.99 ± 3.32 | 12.02 ± 3.09 | 4.02 ± 1.56 | 24.58 ± 8.22 |
| t | 3.561 | 1.180 | 5.285 | 4.030 | |
| P | 0.001 | 0.243 | <0.001 | <0.001 |
Ocular surface analyzer examination results of OSAS patients before and after treatment
To further analyze the changes in the ocular surface analyzer examination results of OSAS patients before and after CPAP treatment, 31 OSAS patients were examined with the ocular surface analyzer before treatment and the day after treatment. The examination items included tear meniscus height, lipid layer thickness, eye redness index, and meibomian gland opening score. The results showed that after treatment, the tear meniscus height of OSAS patients was significantly lower than before treatment, and the lipid layer thickness and eye redness index were significantly higher than before treatment. An independent samples t-test on the two sets of data revealed statistically significant differences (P < 0.05). However, the comparison of meibomian gland opening scores before and after treatment showed no statistically significant difference (P > 0.05, Table 3).
Table 3.
Results of ocular surface analyzer examination of OSAS patients before and after treatment
| Time | Cases | Tear Meniscus Height (mm) | Lipid Layer Thickness (nm) | Eye Redness Index | Meibomian Gland Opening Score |
|---|---|---|---|---|---|
| Before Treatment | 31 | 0.26 ± 0.06 | 64.33 ± 10.72 | 0.65 ± 0.12 | 0.65 ± 0.10 |
| After Treatment | 31 | 0.22 ± 0.02 | 70.20 ± 11.55 | 0.78 ± 0.14 | 0.62 ± 0.11 |
| t | 3.521 | 2.191 | 3.925 | 1.124 | |
| P | 0.001 | 0.032 | <0.001 | 0.266 |
Correlation analysis between dry eye symptoms and meibomian gland function after CPAP treatment
To further analyze the correlation between dry eye symptoms and meibomian gland function in OSAS patients before and after CPAP treatment, Pearson correlation analysis was performed. The results showed that BUT was positively correlated with lipid layer thickness and improvement of eye redness (r = 0.357, 0.286, P < 0.05). Sit was positively correlated with tear meniscus height (r = 0.294, P < 0.05), PL score was negatively correlated with tear meniscus height (r=-0.336, P < 0.05), and positively correlated with eye redness index (r = 0.251, P < 0.05), see Table 4. The correlation coefficient graph is shown in Fig. 2.
Table 4.
Correlation analysis of dry eye symptoms and meibomian gland function after CPAP treatment
| Indicator | BUT | Sit | PL score | OSDI score | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Tear Meniscus Height | -1.780 | 0.162 | 0.294 | 0.020 | -0.336 | 0.008 | -0.183 | 0.154 |
| Lipid Layer Thickness | 0.357 | 0.004 | 0.039 | 0.763 | 0.101 | 0.434 | 0.172 | 0.183 |
| Eye Redness Index | 0.286 | 0.025 | 0.106 | 0.414 | 0.251 | 0.049 | 0.197 | 0.125 |
| Meibomian Gland Opening Score | -2.229 | 0.073 | -0.229 | 0.073 | -0.191 | 0.137 | -0.202 | 0.058 |
Fig. 2.
Correlation coefficient matrix of dry eye symptoms and meibomian gland function after CPAP treatment
Correlation between PSG results and changes in dry eye conditions
To further analyze the correlation between PSG results and changes in dry eye conditions in OSAS patients after CPAP treatment, Pearson correlation analysis was conducted. The results indicated that in the PSG results, the AHI was negatively correlated with BUT, PL score, and OSDI score (r=-0.444, -0.350, -0.378; P < 0.05). Additionally, SpO2 was positively correlated with BUT, PL score, and OSDI score (r = 0.308, 0.435, 0.288; P < 0.05) and negatively correlated with Sit (r=-0.300; P < 0.05) (Table 5; Figs. 3 and 4).
Table 5.
Correlation between PSG results and changes in dry eye conditions
| Indicator | AHI | SpO2 | ||
|---|---|---|---|---|
| r | P | r | P | |
| BUT | -0.444 | < 0.001 | 0.308 | 0.015 |
| Sit | 0.155 | 0.230 | -0.300 | 0.018 |
| PL Score | -0.350 | 0.005 | 0.435 | < 0.001 |
| OSDI Score | -0.378 | 0.002 | 0.288 | 0.023 |
Fig. 3.
Correlation between AHI and changes in dry eye condition in PSG results. A: Correlation between AHI and BUT; B: Correlation between AHI and Sit; C: Correlation between AHI and PL score; D: Correlation between AHI and OSDI score
Fig. 4.
Correlation between SpO2 and changes in dry eye condition in PSG results. A: Correlation between SpO2 and BUT; B: Correlation between SpO2 and Sit; C: Correlation between SpO2 and PL score; D: Correlation between SpO2 and OSDI score
Correlation between PSG results and ocular surface analyzer examination results
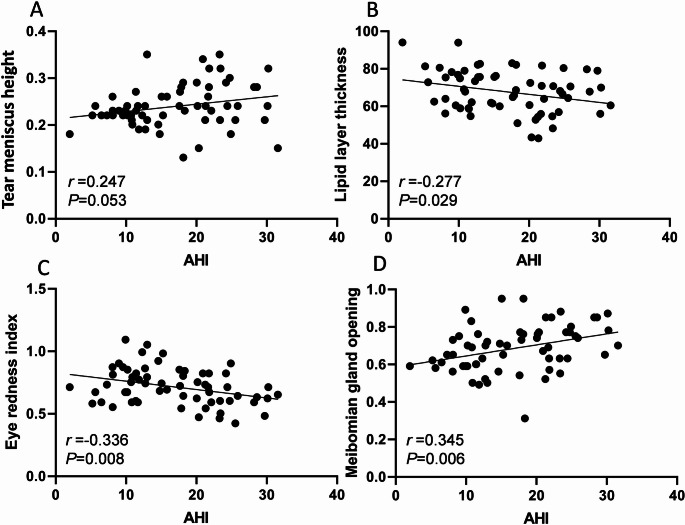
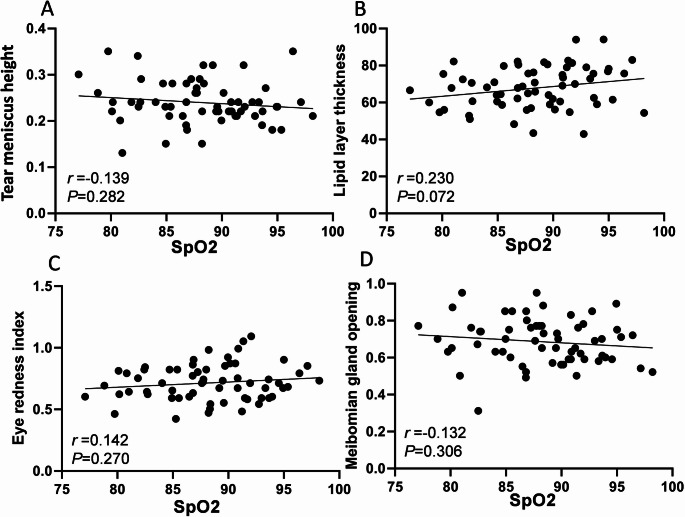
The correlation between PSG results and ocular surface analyzer examination results before and after CPAP treatment in OSAS patients was further analyzed. The results of Pearson correlation analysis showed that AHI in PSG results was negatively correlated with lipid layer thickness and eye redness index (r=-0.277, -0.336, P<0.05), and positively correlated with meibomian gland opening score (r = 0.345, P<0.05). SpO2 had no correlation with tear meniscus height, lipid layer thickness, eye redness index, and meibomian gland opening score (P>0.05, Table 6; Figs. 5 and 6).
Table 6.
Correlation between PSG results and ocular surface analyzer examination results
| Indicator | AHI | SpO2 | ||
|---|---|---|---|---|
| r | P | r | P | |
| Tear Meniscus Height | 0.247 | 0.053 | -0.139 | 0.282 |
| Lipid Layer Thickness | -0.277 | 0.029 | 0.230 | 0.072 |
| Eye Redness Index | -0.336 | 0.008 | 0.142 | 0.270 |
| Meibomian Gland Opening Score | 0.345 | 0.006 | -0.132 | 0.306 |
Fig. 5.
Correlation between AHI in PSG results and ocular surface analyzer examination results. A: Correlation between AHI and tear meniscus height; B: Correlation between AHI and lipid layer thickness; C: Correlation between AHI and eye redness index; D: Correlation between AHI and meibomian gland opening score
Fig. 6.
Correlation between SpO2 in PSG results and ocular surface analyzer examination results. A: Correlation between SpO2 and tear meniscus height; B: Correlation between SpO2 and lipid layer thickness; C: Correlation between SpO2 and eye redness index; D: Correlation between SpO2 and meibomian gland opening score
Discussion
OSAS is a common sleep disorder. Studies have shown [13] that OSAS patients suffer from repeated breathing pauses and hypopnea at night, which leads to decreased blood oxygen saturation, thereby affecting tear secretion and tear film stability, and increasing the risk of dry eye syndrome.
In addition, OSAS patients often have changes in sleep structure, such as a decrease in rapid eye movement (REM) sleep and an increase in non-rapid eye movement (NREM) sleep. These changes can also affect tear secretion and tear film stability [14]. Studies have also found that CPAP treatment may indirectly alleviate dry eye symptoms by improving sleep quality and blood oxygen saturation [15]. In addition, the mask changes the direction of the gas, causing the airflow to be directed toward the eye above the mask. This increased airflow will also accelerate the evaporation of the tear film, causing irritation of the eye surface [16].
The results of this study showed that the AHI level of 31 OSAS patients after treatment was significantly lower than that before treatment, and the SpO2 levels were significantly higher than that before treatment, indicating that OSAS patients have significant clinical efficacy after CPAP treatment. The CPAP mask delivers continuous positive pressure airflow into the airway, so that the airway maintains a positive pressure state throughout the respiratory cycle, thereby treating OSAS. Furthermore, daytime hypoxemia in this study population was primarily caused by OSAS itself and was associated with persistent hypoxia resulting from nocturnal apnea events. Although BMI did not meet obesity criteria, the severity of OSAS (38.71% of patients were severe) was significantly correlated with the severity of hypoxemia, suggesting that the hypoxic characteristics of this population are directly related to the progression of OSAS rather than metabolic factors. Further analysis of the results of this study showed that the BUT time of patients after treatment was significantly higher than that before treatment. The extension of BUT time indicates that CPAP treatment can relieve dry eye syndrome, which is related to CPAP treatment improving the sleep quality of OSAS patients, alleviating hypoxia symptoms, and thus reducing systemic inflammatory response. In addition, CPAP treatment may also produce a certain positive pressure effect on the eye, promote the secretion and distribution of tears, and thus improve dry eye symptoms [17]. However, the patient’s PL score and OSDI score were worse than before treatment, indicating that CPAP treatment may cause new damage to the patient’s eyes. The possible reason is that during CPAP treatment, due to the effect of pressure, gas may overflow from the edge of the mask, and the backflow of gas continuously impacts and stimulates the ocular surface tissue. This continuous stimulation may induce ocular surface diseases such as dry eye (DE) and conjunctivitis [9, 16, 18]. In addition, the use of masks may affect blink frequency, thereby affecting the secretion of meibomian glands [19]. Frequent blinking helps the renewal of tear film and the distribution of lipid layer, while the use of CPAP masks may reduce blink frequency, thereby affecting the normal secretion and function of meibomian glands [11, 20, 21]. Once the meibomian glands are obstructed, it leads to reduced secretion of lipids on the surface of the tear film, resulting in tear film instability and rapid evaporation of moisture, ultimately causing dry eyes and potentially leading to damage or rupture of the ocular surface [22]. Therefore, the use of a CPAP mask may exacerbate meibomian gland dysfunction and further worsen dry eye symptoms. Clinically, for patients experiencing dry eye symptoms and meibomian gland dysfunction, the mask should provide adequate coverage to minimize air flow stimulation to the eyes; the part of the mask in contact with the face should be made of soft materials to reduce pressure on the meibomian glands. Additionally, the mask should feature adjustable functionalities to accommodate different facial profiles for improved comfort.
The results of this study showed that the tear meniscus height of OSAS patients after treatment was significantly lower than before treatment, while the lipid layer thickness and eye redness index were significantly higher than before treatment. The meibomian gland opening score did not change before and after treatment, indicating that CPAP treatment can improve the lipid layer thickness and eye redness index of OSAS patients, but has no effect on the meibomian gland opening score. According to existing studies [23], there are significant abnormalities in the function and morphology of meibomian glands in OSAS patients, including a high incidence of meibomian gland atrophy and morphological abnormalities, and these changes are related to the severity of OSAS. In addition, as the degree of hypoxia in OSAS patients increases, the loss of meibomian glands becomes more obvious. During the use of CPAP masks, a certain amount of airflow may be generated, which may directly or indirectly affect the eye environment and cause dry eyes. Dry eyes are an important cause of meibomian gland dysfunction, because a dry environment accelerates tear evaporation, affects the stability of the tear film, and thus has a negative impact on meibomian gland function [24, 25]. Unlike protective masks (such as N95 masks), CPAP therapy masks require high airtightness to ensure positive pressure ventilation. Their continuous use at night (average 6–8 h) and dynamic leakage characteristics (leakage volume in this study: nasal mask 10.80 ± 2.71 L/min; oronasal mask 14.72 ± 4.11 L/min) result in more sustained mechanical stimulation of the ocular surface. This unique mode of action explains why CPAP masks are more likely to cause BUT shortening and increased OSDI scores, a conclusion supported by specialized CPAP studies [11, 26]. In addition, while the mask provides positive pressure airflow, it may also exert a certain amount of pressure on the eyes. This pressure may directly affect the discharge of meibomian gland secretions, leading to meibomian gland dysfunction [27]. Physicians should choose an appropriate type and size of mask based on the patient’s specific conditions, such as the severity of the disease, meibomian gland function, and dry eye symptoms. They should also pay attention to the patient’s feedback to timely adjust mask parameters for optimal treatment effectiveness.
The results of Pearson correlation analysis showed that BUT was positively correlated with lipid layer thickness and improvement of eye redness, Sit was positively correlated with tear meniscus height, PL score was negatively correlated with tear meniscus height, and positively correlated with eye redness index. Meibomian gland dysfunction is one of the common causes of dry eye, and the morphological structure and function of meibomian glands in OSAS patients also changed significantly. Studies have shown that [28–30] the morphological structure of meibomian glands and the stability of tear film in OSAS patients have changed, which may be due to the decreased sleep quality and increased tear evaporation caused by OSAS. OSAS not only directly affects the stability of tear film and increases the risk of dry eye, but also further aggravates dry eye symptoms by affecting the function of meibomian glands. Therefore, OSAS patients need to pay attention to the improvement of tear film stability and meibomian gland function during treatment to reduce the occurrence and development of dry eye symptoms. Additionally, the results of this study indicate that in PSG results, AHI negatively correlated with BUT, PL scores, and OSDI scores, while SpO2 positively correlated with BUT, PL scores, and OSDI scores, but negatively correlated with the Sit score. In PSG results, AHI negatively correlated with lipid layer thickness and eye redness index, suggesting that CPAP treatment impacts dry eye symptoms and meibomian gland function, which can further influence clinical treatment outcomes. During the use of CPAP masks, leaks or gas backflow through the nasolacrimal duct can irritate the eyes and meibomian glands, increasing the risk of dry eye disease [31]. In addition, the sealing and pressure distribution of the mask may also affect the blood circulation and tear secretion of the eyes, thereby aggravating the symptoms of dry eye. Additionally, the seal quality and pressure distribution of the mask may also affect ocular blood circulation and tear secretion, further exacerbating dry eye symptoms. Therefore, in the treatment of OSAS, the choice of mask material is crucial for alleviating dry eye symptoms and improving meibomian gland function. Ideal materials should possess good breathability, comfort, and durability, while avoiding irritation to the eyes and surrounding skin. Commonly used mask materials on the market include silicone and foam. Researchers can conduct comparative analyses to select materials that minimally affect the eyes, thus reducing the occurrence of dry eye symptoms. CPAP therapy may indirectly improve BUT by alleviating hypoxia, but the design of this study aimed to elucidate the negative effects of mask-related physical factors (such as edge pressure and airflow backflow) on the ocular surface. The correlation between prolonged BUT and increased SpO₂ after treatment (r = 0.308, P < 0.05) suggests a protective effect from improved hypoxia, but the exacerbation of dry eye symptoms caused by the mask (increased PL scores and OSDI) indicates that mask-related factors play a dominant role. Although the impact of CPAP therapy on ocular surface parameters was observed, the dose–response relationship between air leakage rate and ocular symptoms was not quantitatively analyzed. Previous studies have shown that when the leakage rate exceeds 24 L/min, the incidence of ocular irritation symptoms increases significantly [11, 21]. Moreover, different mask types may have varying effects, with oronasal masks more likely than nasal masks to cause airflow disturbance in the palpebral fissure area [26]. In addition, the treatment pressure setting (8–12 cmH2O on average in this study) may have a nonlinear association with tear film evaporation rate [27, 29], warranting stratified analysis in studies with larger samples. Future research should systematically document these parameters to clarify their modulatory roles.
In summary, when OSAS patients use CPAP masks for treatment, it may have a negative impact on dry eye symptoms and meibomian gland function, leading to decreased tear film stability and aggravated symptoms such as dry eyes. This study has a relatively small sample size, and the results may have certain biases. The study period is short, and the long-term effects of mask usage on dry eye symptoms and meibomian gland function remain unclear. Insufficient comparisons among different types of masks may overlook more effective treatment options. Future research could expand the sample size and lengthen the study period to conduct a more comprehensive comparison of different types of masks, aiming to provide more effective treatment strategies for OSAS patients. Moreover, exploring other adjunctive therapies, such as pharmacological or physical treatments, could further enhance treatment efficacy.
Author contribution
Shu-Yi Wei: Conceptualization, Data curation, Formal analysis, Resources, Writing– original draft. Zhen Wu: Conceptualization, Data curation, Investigation, Supervision, Writing– original draft. Yao Xu: Funding acquisition, Project administration, Software, Visualization, Writing– review and editing. Dan Li: Funding acquisition, Methodology, Project administration, Validation, Writing– review and editing. Yimin Xia: Data curation, Formal analysis, Resources, Validation, Writing– review and editing.
Funding
This study was funded by The Program of the Suzhou Industrial Park Cultivation Project of China (No. JL201803), The Program of the Suzhou Kowloon Hospital Shanghai Jiao Tong University School of Medicine (No. SZJL202116 and No. SZJL202106), The Research Programs of Science and Technology Commission Foundation of Suzhou (SKY2023112) and Suzhou Clinical Key Disease Project (LCZX202236).
Data availability
The data that support the findings of this study are available from the corresponding author, Yao Xu and Dan Li, upon reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants after a detailed explanation of the study’s purpose, methods, potential risks, and benefits.
Conflict of interest
The authors report there are no conflicts of interest in this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yao Xu, Email: 604062040@qq.com.
Dan Li, Email: zoe19900@163.com.
Yimin Xia, Email: yimin_xia@163.com.
References
- 1.Lv R, Liu X, Zhang Y, Dong N, Wang X, He Y et al (2023) Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct Target Ther 8(1):218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N et al (2021) Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American heart association. Circulation 144(3):e56–e67 [DOI] [PubMed] [Google Scholar]
- 3.Teiga P, Chatelain S, Heinzer R, Lambercy K (2020) [Obstructive sleep apnea syndrome: CPAP or mandibular advancement Device?]. Rev Med Suisse 16(709):1865–1869 [PubMed] [Google Scholar]
- 4.Nokes B, Cooper J, Cao M (2022) Obstructive sleep apnea: personalizing CPAP alternative therapies to individual physiology. Expert Rev Respir Med 16(8):917–929 [DOI] [PubMed] [Google Scholar]
- 5.Hakim FE, Farooq AV (2022) Dry eye disease: an update in 2022. JAMA 327(5):478–479 [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Li S, Li M, Zeng S, Chen B, Zhang L (2022) Evaluation of the ocular surface and meibomian gland in obstructive sleep apnea hypopnea syndrome. Front Med (Lausanne) 9:832954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulutas HG, Balikci Tufekci A, Gunes A (2022) Evaluation of corneal, ocular surface, and meibomian gland changes in obstructive sleep apnea syndrome. J Fr Ophtalmol 45(2):191–200 [DOI] [PubMed] [Google Scholar]
- 8.Acar M, Firat H, Acar U, Ardic S (2013) Ocular surface assessment in patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath 17(2):583–588 [DOI] [PubMed] [Google Scholar]
- 9.Itokawa T, Okajima Y, Iwashita H, Koji K, Suzuki T, Hori Y (2023) Association between mask-associated dry eye (MADE) and corneal sensations. Sci Rep 13(1):1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzam SH, Nama A, Badarni H, Asael H, Dahoud WA, Mimouni M et al (2022) Assessment of dry eye disease in N95 versus surgical face mask wearers during COVID-19. Indian J Ophthalmol 70(3):995–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry SA, Mann DL, Beare R, McIntyre R, Beatty C, Thomson LDJ et al (2023) Oronasal vs nasal masks: the impact of mask type on CPAP requirement, pharyngeal critical closing pressure (P(crit)), and upper airway Cross-Sectional areas in patients with OSA. Chest 164(3):747–756 [DOI] [PubMed] [Google Scholar]
- 12.Carter A, Davenport-Jones L (2024) Obstructive sleep apnoea/hypopnoea syndrome: a feasibility study to review NICE guidelines. Br Dent J [DOI] [PubMed]
- 13.Pu Q, Wu Z, Li AL, Guo XX, Hu JJ, Li XY (2022) Association between poor sleep quality and an increased risk of dry eye disease in patients with obstructive sleep apnea syndrome. Front Med (Lausanne) 9:870391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q, Chen Z, Xie C, Liu L, Wei R (2021) The association between dry eye disease with depression, anxiety and sleep disturbance during COVID-19. Front Psychiatry 12:802302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaca EE, Akcam HT, Uzun F, Ozdek S, Ulukavak Ciftci T (2016) Evaluation of ocular surface health in patients with obstructive sleep apnea syndrome. Turk J Ophthalmol 46(3):104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavigok E, Ozcan AA, Ulas B (2023) Obsructive sleep apnea syndrome: is it a risk factor for ocular surface disease and ocular comorbidities? Int Ophthalmol 43(7):2329–2334 [DOI] [PubMed] [Google Scholar]
- 17.Schabus M, Griessenberger H, Gnjezda MT, Heib DPJ, Wislowska M, Hoedlmoser K (2017) Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia. Brain 140(4):1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannaccare G, Pellegrini M, Borselli M, Senni C, Bruno A, Scorcia V (2022) Diurnal changes of noninvasive parameters of ocular surface in healthy subjects before and after continuous face mask wearing during the COVID-19 pandemic. Sci Rep 12(1):12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esen Baris M, Guven Yilmaz S, Palamar M (2022) Impact of prolonged face mask wearing on tear break-up time and dry eye symptoms in health care professionals. Int Ophthalmol 42(7):2141–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali Momin SN, Siddiqui R (2022) Mask-associated dry-eye in COVID-19 pandemic: A case report and review of the literature. J Pak Med Assoc 72(5):981–982 [DOI] [PubMed] [Google Scholar]
- 21.Larsen PS, Heeboll J, Meyer KE (2023) Measured air flow leakage in facemask usage. Int J Environ Res Public Health.;20(3) [DOI] [PMC free article] [PubMed]
- 22.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK et al (2017) TFOS DEWS II definition and classification report. Ocul Surf 15(3):276–283 [DOI] [PubMed] [Google Scholar]
- 23.Muhafiz E, Olcen M, Erten R, Bozkurt E (2020) Evaluation of meibomian glands in obstructive sleep Apnea-Hypopnea syndrome. Cornea 39(6):685–690 [DOI] [PubMed] [Google Scholar]
- 24.Boccardo L (2022) Self-reported symptoms of mask-associated dry eye: A survey study of 3,605 people. Cont Lens Anterior Eye 45(2):101408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard JD, Nichols KK (2023) Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther 12(3):1397–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh NP, Walker RJ, Cowan F, Davidson AC, Roberts DN (2014) Retrograde air escape via the nasolacrimal system: a previously unrecognized complication of continuous positive airway pressure in the management of obstructive sleep apnea. Ann Otol Rhinol Laryngol 123(5):321–324 [DOI] [PubMed] [Google Scholar]
- 27.Al-Dolat W, Abu-Ismail L, Khamees A, Alqudah N, Abukawan MM, Alrawashdeh HM et al (2022) Is wearing a face mask associated with symptomatic dry eye disease among medical students during the COVID-19 era? An online survey. BMC Ophthalmol 22(1):159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Chen J, Qin G, Qi Y, Chen Y, Li M et al (2023) Tear film lipid layer changes following combined effect of heated eye mask with intense pulsed light therapy for evaporative dry eye: A randomized control study. Photobiomodul Photomed Laser Surg 41(8):435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Wu Q, Zhai Z, Yang Y, Wei A, Xu J et al (2020) Comparison of the meibomian gland openings by optical coherence tomography in obstructive meibomian gland dysfunction and normal patients. J Clin Med.;9(10) [DOI] [PMC free article] [PubMed]
- 30.Moshirfar M, West WB Jr., Marx DP (2020) Face Mask-Associated ocular irritation and dryness. Ophthalmol Ther 9(3):397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey SK, Sharma V (2021) Mask-associated dry eye disease and dry eye due to prolonged screen time: are we heading towards a new dry eye epidemic during the COVID-19 era? Indian J Ophthalmol 69(2):448–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Yao Xu and Dan Li, upon reasonable request.