Abstract
Background
The optimal duration of immunotherapy (ITH) remains undefined for patients with metastatic melanoma, and debates on de-escalation strategies are ongoing. Patients in the pivotal KEYNOTE and CheckMate trials who experienced a complete response (CR) on ITH had long-term responses, even after treatment was terminated early because of toxicity or at the physician’s discretion.
Objective
Our study explores the duration of planned ITH drug holidays—intentional ITH suspension until disease progression off treatment—in patients with unresectable and metastatic melanoma treated for at least 6 months with ITH.
Patients and Methods
We enrolled 222 patients who received anti-programmed cell death protein-1-based ITH, experienced stable disease, partial response, or complete response during ITH, and had no treatment-limiting toxicities.
Results
At a median follow-up of 63 months since the first ITH cycle, median overall survival after the drug holiday start (OS3) was not reached, and the 5-year OS3 rate was 79.3%. Median progression-free survival since the start of drug holiday (PFS3) was not reached, with a 3-year PFS3 rate of 65% for all patients, and the highest rate was in the complete response group (72.3%). After the drug holidays, upon disease progression off treatment, the objective response rate to ITH reintroduction was 58.9%. Again, durable, ongoing objective responses were achieved on ITH reintroduction after drug holidays. The best radiological response achieved before drug holidays correlated with the duration of ITH drug holidays, with the longest duration of disease control without treatment for complete responders.
Conclusions
Drug holidays in patients with unresectable and metastatic melanoma after an objective response or prolonged disease stabilization during ITH result in durable control of the disease, particularly in patients with complete response.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-025-01156-2.
Key Points
| Our study explores the impact of planned immunotherapy drug holidays (intentional suspension of immunotherapy until disease progression off treatment) on survival outcomes and disease control in patients with unresectable and metastatic melanoma. |
| Median progression-free survival (PFS) after the start of drug holidays was not reached, with a 3-year PFS probability of 65%. |
| The duration of drug holidays depends on response to immunotherapy, as patients with complete response had the most favorable prognosis, with a 3-year PFS probability of 72.3%. |
| After the drug holidays, the objective response rate to reintroduction of immunotherapy upon disease progression off treatment was 58.9%. |
Introduction
To date, long-term clinical results and length of response in patients with melanoma who stop immunotherapy (ITH) in the absence of disease progression have not been reported. ITH has been attributed to providing long-term responses, with CD8+ T-memory cells playing a pivotal role in this phenomenon. ITH has a specific impact on the T-cell repertoire in the tumor niche [1]. High levels of memory CD8+ T cells predict longer progression-free survival (PFS; hazard ratio [HR] 0.64) [2], and expression of stem-like transcriptional signatures of early memory CD8+/CD4+T cells predict objective responses to ITH [3]. These cell data suggest that, despite ITH suspension, prolonged response against neoplastic cells will be maintained. Recently, some information has emerged regarding the clinical outcomes of patients who discontinued ITH after complete response (CR) or because of unacceptable adverse events (AEs) [4, 5], confirming long-term efficacy in such settings.
The optimal duration of ITH for patients with unresectable and metastatic melanoma has not been fully defined, and de-escalation strategies are under debate. In general, “drug holiday” refers to the pre-planned interruption of pharmacotherapy for a defined period and with a specific clinical aim [6]. Therefore, ITH drug holidays are defined as an agreed intentional cessation of ITH for a period in patients with clinical benefits of therapy (stable disease [SD]/partial response [PR]/CR), until potential disease progression, and are differentiated from early treatment cessation because of toxicity or progression [7, 8]. The significant advantages expected to result from drug holidays is to decrease the risk of developing AEs or to alleviate existing AEs [6]. It is expected to improve a patient’s quality of life by allowing them to recover from the cumulative treatment toxicity and increase everyday role functioning [6, 9]. At the same time, drug holidays are expected to decrease the economic burden on the healthcare system that is attributed to ITH and treatment of its AEs, especially in the elderly population [8, 10, 11]. This clinical benefit of drug holidays was first investigated for chemotherapy and later for kinase inhibitors in patients treated for colon cancer (capecitabine) [12], prostate cancer (cabazitaxel) [13], thyroid cancer (lenvatinib) [14], or renal cell cancer (sunitinib) [15]. It was proven that drug holidays in selected patient populations result in overall prolonged oncological treatment and contributes to prolonged overall survival (OS) [13]. In the case of drug holidays from tyrosine kinase inhibitors, treatment cessation started at objective remission (PR or CR) and treatment reinduction after disease progression showed significant efficacy in renal cell carcinoma [16]. In the melanoma field, it was also suggested that drug holidays increased drug sensitivity; in particular, it overcome the mitogen-activated protein kinase inhibitor-addiction phenotype while patients were receiving BRAFi/MEKi treatment [17], but data for ITH have not been widely explored.
Currently, most clinical trials for patients with advanced and unresectable tumors, including melanoma, are designed with a treatment duration of 2 years for immune checkpoint inhibitors (ICIs). The initial insights into treatment discontinuation, specifically ITH drug holidays after 24 months of therapy, come from pembrolizumab data from the KEYNOTE-001 and -006 trials, where 67 and 103 patients, respectively, discontinued treatment without evidence of progressive disease or treatment-limiting AEs [18–21]. At the same time, data for nivolumab in the CheckMate trials indicated that, in 11% of patients, treatment cessation was initiated at the patient’s request, and in 8%, it was attributed to achieving maximum clinical benefit, including CR in 16 patients [22]. Notably, in the KEYNOTE trials, durable responses after early (≥6 months) treatment termination were reported in patients who experienced a CR on pembrolizumab therapy. Patients in CheckMate 069 experienced durable responses: of patients undergoing 2 years of ITH and alive at 7.5 years, 77% (106/138) of those receiving nivolumab with ipilimumab, 70% (80/115) of those receiving nivolumab, and 45% (27/60) of those treated with ipilimumab were still treatment-free and never received subsequent systemic second-line therapy [23].
Although examples of benefits from a drug holiday include reduced toxicity and treatment costs and increased patient quality of life, there are also potential risks, including fast disease progression. Data regarding the clinical outcomes of patients who have discontinued anti-programmed cell death protein (PD)-1 ITH in real-world scenarios are sparse. Therefore, the aim of our study was to describe the survival disease control upon drug holidays in patients with melanoma receiving anti-PD-1-based ITH in routine clinical practice.
Materials and methods
Patients and the Schedule of Treatment
Patients who were diagnosed with unresectable stage III or stage IV cutaneous or mucosal melanoma (or melanoma of unknown primary) started first-line ITH treatment between August 3, 2016, and January 5, 2024, as per unified national treatment guidelines. Since September 1, 2020, the Polish Ministry of Health has allowed for drug holidays as a part of the drug reimbursement program, under which all the patients were treated [24–26]. Since that date, patients have been able to start a drug holiday. The patients were followed-up prospectively since their first computed tomography (CT) scan on the drug holiday; thus, the first patient was enrolled into the study on December 1, 2020. Data regarding treatment before December 1, 2020, were collected retrospectively. A total of 222 consecutive patients from major melanoma centers in Poland who had advanced unresectable and metastatic melanoma treated outside clinical trials with anti-PD-1-based ITH and subsequent ITH drug holidays were enrolled in this study [27–29]. ITH drug holidays were defined as pre-planned elective discontinuation of ITH with no evidence of disease progression and without AEs limiting treatment, after at least 6 months of ITH and with confirmed Response Evaluation Criteria in Solid Tumors (RECIST) response and at least two ITH doses administered after the first attainment of clinical benefit [30]. The first CT scan was performed at 3 months after ITH initiation. All patients were referred for drug holidays after confirmatory CT imaging performed at least 4 weeks after SD/PR/CR described in the 6th month of ITH treatment. Patients who stopped ITH treatment because of toxicity/AEs, pseudo-progression, or non-compliance were excluded from analysis. We used RECIST 1.1 to analyze treatment response [31]. Treatment and drug holidays were regulated by the National Reimbursement Program Protocol and united for all centers participating in the analysis [24, 32, 33]. During treatment and drug holidays, all patients underwent imaging no later than every 16 weeks.
Statistical Methods
The primary objective of this study was to describe survival outcomes and disease control in patients referred to drug holidays after experiencing clinical benefit with ITH. Multiple survival outcomes were defined:
OS1 was calculated from the first ITH start date to the date of death. PFS1 was defined as time since ITH initiation until RECIST disease progression or death.
PFS2 and OS2 were computed since the date of best overall response (BOR): PFS2 until RECIST disease progression or death, and OS2 until death.
OS3 was the time between the last pre-holiday ITH infusion and death, whereas PFS3 was the time between the last pre-holiday ITH infusion and RECIST disease progression or death. PFS3 defined the duration of the ITH drug holiday.
Among patients who experienced disease progression during the drug holiday and were eligible to re-start ITH, PFS4 and OS4 were measured since the ITH re-start: PFS4 until RECIST disease progression or death, and OS4 until death.
For all PFS and OS endpoints, death was considered an event, regardless of the cause. Patients who were alive at the last database update were censored at the date of the last follow-up. We calculated the follow-up on drug holidays using the reversed Kaplan–Meier method, from the first ITH infusion until disease progression, death from any cause, or last follow-up. Data cut-off was January 31, 2025.
We used descriptive statistics to report patients’ baseline characteristics. Overall response rate (ORR) was defined as the proportion of patients achieving PR or CR as per RECIST 1.1. We performed survival analyses using the Kaplan–Meier method [34]. We correlated the survival outcomes with clinical data. We used the log-rank test and Cox’s proportional hazards model to identify potential predictive biomarkers [35]. All analyses and visualizations were performed in the R language statistical environment, version 4.4.2, using the “tidyverse,” “survival,” and “survminer” packages (see Table 1).
Table 1.
Patient characteristics.
| Variable | Value | n (%) |
|---|---|---|
| Total number of patients | 222 | |
| Best overall response | CR | 95 (42.8) |
| PR | 62 (27.9) | |
| SD | 65 (29.3) | |
| Treatment regimen | Nivolumab | 103 (46.4) |
| Nivolumab + ipilimumab | 20 (9.0) | |
| Pembrolizumab | 99 (44.6) | |
| Line of treatment, in which ITH was used | First-line | 196 (88.3) |
| Second-line | 26 (11.7) | |
| Sex | Female | 103 (46.4) |
| Male | 119 (53.6) | |
| Brain metastases | Absent | 208 (93.7) |
| Present | 14 (6.3) | |
| BRAFV600 mutational status | Negative | 147 (66.2) |
| Positive | 75 (33.8) | |
| Histological type | Cutaneous | 173 (79.0) |
| MUP | 32 (14.6) | |
| Mucosal | 14 (6.4) | |
| Number of involved sites | 1 | 122 (55.0) |
| 2 | 62 (27.9) | |
| 3 | 23 (10.4) | |
| 4 | 11 (5.0) | |
| 5 | 1 (0.5) | |
| 6 | 1 (0.5) | |
| 7 | 2 (0.9) | |
| Clinical stage | III | 42 (18.9) |
| IV | 180 (81.1) | |
| Age, years | Mean ± standard deviation | 64.40 ± 13.99 |
| Age, categorical | > 65 | 120 (54.1) |
| ≤ 65 | 102 (45.9) | |
| Duration of treatment, months | Mean ± standard deviation | 28.19 ± 12.34 |
| Time until the BOR | Half-year | 47 (21.2) |
| Longer | 57 (25.7) | |
| SD | 55 (24.8) | |
| Unknown | 63 (28.4) | |
| Time until the BOR, months | Mean ± standard deviation | 13.09 ± 14.02 |
| Treatment regimen, simplified | Anti-PD-1 plus anti-CTLA-4 | 20 (9.0) |
| Anti-PD-1 monotherapy | 202 (91.0) | |
| Duration of treatment | > 2 years | 134 (60.4) |
| ≤ 2 years | 88 (39.6) | |
| BOR and duration of treatment | CR and > 2 years | 60 (27.0) |
| CR and ≤ 2 years | 35 (15.8) | |
| PR and > 2 years | 38 (17.1) | |
| PR and ≤ 2 years | 24 (10.8) | |
| SD and > 2 years | 36 (16.2) | |
| SD and ≤ 2 years | 29 (13.1) | |
BOR best overall response, CR complete response, CTLA-4 cytotoxic T-lymphocyte-associated protein 4, ITH immunotherapy, MUP melanoma of unknown primary, PD-1 programmed cell death protein, PR partial response, SD stable disease
Results
Patients’ Characteristics and Pre-holiday ITH
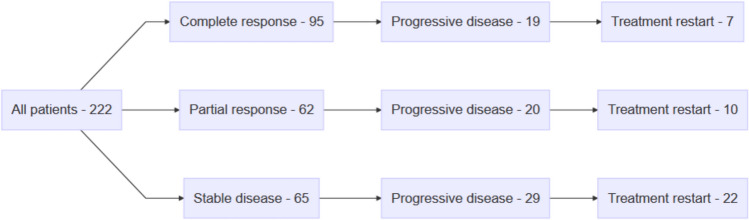
A total of 222 patients (103 female, 119 male) treated with ITH for advanced unresectable or metastatic melanoma were referred for ITH drug holidays (Fig. 1). The median age of the patients was 66 years (interquartile range 56–74), and 147 (66.2%) had BRAF-negative melanoma. Among them, 42 patients (18.9%) had unresectable stage III and 180 (81.1%) stage IV melanoma. Most patients (88.3% [n = 196]) received first-line therapy. A total of 103 (43.4%) patients received nivolumab, 20 (9%) received nivolumab + ipilimumab, and 99 (44.6%) received pembrolizumab. The BOR was CR in 95 (42.8%) patients, PR in 62 (27.9%), and SD in 65 (29.3%) (see Fig. 1 in the electronic supplementary material [ESM]). The median time on ITH, before drug holidays, was 26 months (range 6–72), and 134 (60.4%) patients were referred for drug holidays after ≥ 24 months of ITH. Table 1, Table 2, and Fig. 2 in the ESM contain complete baseline characteristics. Median follow-up after ITH initiation was 63 months (95% confidence interval [CI] 59.9–66.9).
Fig. 1.
Illustration of the flow of patients.
At 3 years since ITH initiation, PFS1 was 90.8% (95% CI 86.9–94.8), at 5 years it was 72.4% (95% CI 66.1–79.2), and the respective OS1 rates were 97.6% (95% CI 95.5–99.7) and 86.3% (95% CI 81.3–91.6) (Figs. 3, 4, 5, 6, 12, and 13 in the ESM). Median OS1 and PFS1 were not reached. In subgroup analysis, within 4 years since treatment initiation, only patients with SD as best response and treated for <2 years reached median PFS (Fig. 21 in the ESM).
Survival since BOR
At 1 year since attainment of BOR, PFS2 was 95.9% (95% CI 92.1–99.9), and the 2-year PFS2 was 92.6 (95% CI 87.5–98). At these same timepoints, the OS2 rates were 97% (95% CI 93.6–100) and 95.8% (95% CI 91.9–99.9), respectively. Median PFS2 and OS2 were not reached (Figs. 2, 7, 8, 14, and 15 in the ESM).
Survival During ITH Drug Holidays
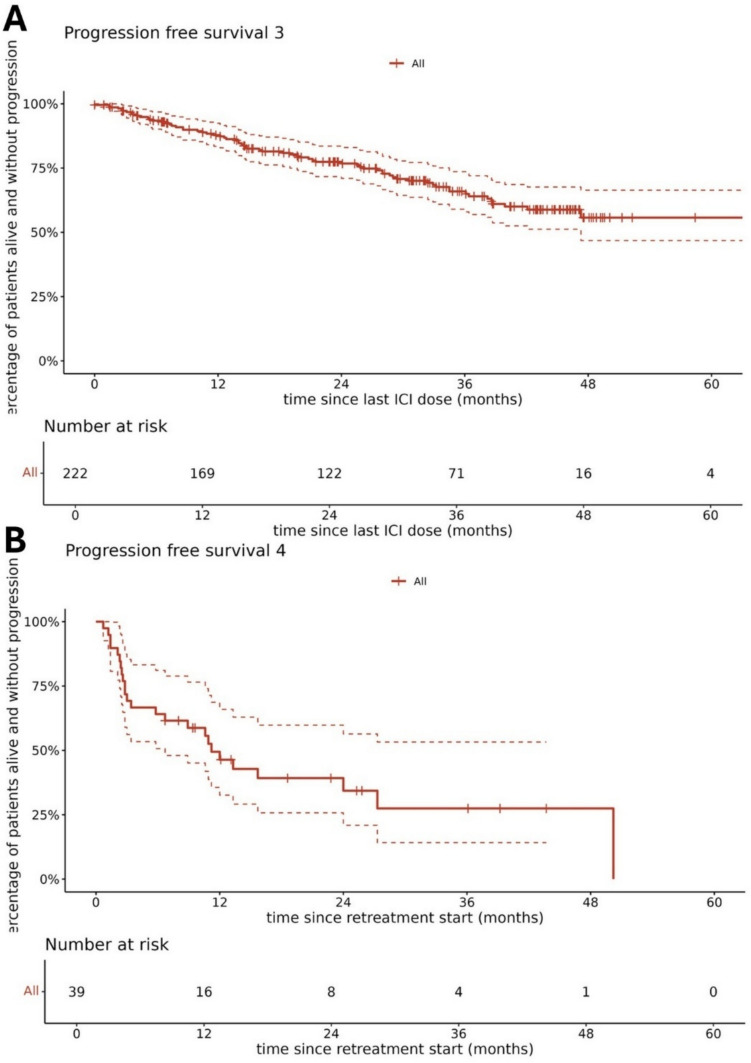
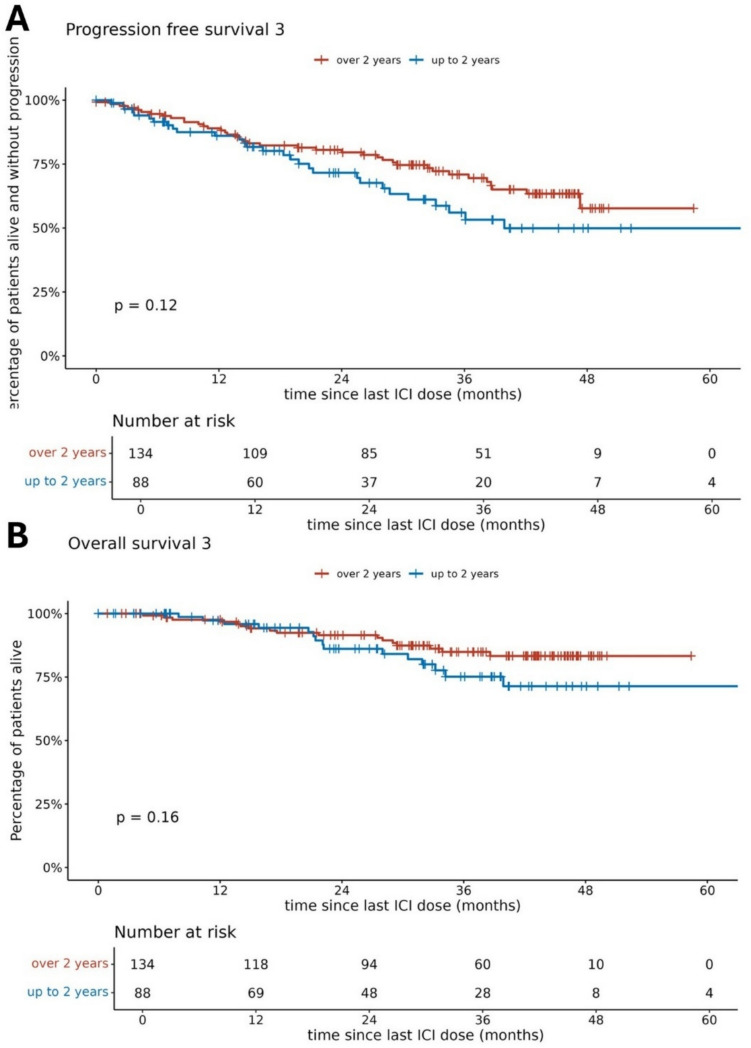
The median PFS3 was not reached (Fig. 2). The estimated 1-, 2-, and 3-year PFS3 rates were 87.3% (95% CI 82.9–92), 76.8% (95% CI 71–83), and 65% (95% CI 58–72.8), respectively. Patients treated with ITH for ≥ 24 months before drug holidays did not have significantly longer PFS3 than those treated for < 24 months in either the univariate analysis (HR 1.46 [95% CI 0.90–2.37]; p = 0.122) or the multivariate analysis (HR 1.13 [95% CI 0.68–1.88]; p = 0.637) (Fig. 3). However, in the univariate analysis, patients treated for <24 months had significantly shorter OS (HR 3.52 [95% CI 1.67–7.39]; p = 0.001). At the median follow-up of 63 months (95% CI 59.9–66.9), median OS3 was not reached, and the 5-year OS3 rate was 79.3% (95% CI 72.9–86.3). At the time of analysis, 154 (69.4%) patients were still on an ITH drug holiday.
Fig. 2.
A Progression-free survival 3 on d immunotherapy drug holidays and B progression-free survival 4 upon immunotherapy reinduction. ICI immune checkpoint inhibitor.
Fig. 3.
A Progression-free survival 3 and B overall survival dependent on immunotherapy duration before drug holidays.
PFS3 was significantly longer in patients with CR (72.3% at 3 years [95% CI 61.7–84.7]) than in patients with PR (64.3% at 3 years [95% CI 51.7–80.1]) or SD (54.9% at 3 years [95% CI 43.2–69.9]) before the drug holiday. Median PFS3 has not been reached in patients achieving a CR, PR, or SD during ITH treatment. Nevertheless, compared with patients obtaining a CR before ITH drug holidays, a significantly shorter PFS3 was observed in patients achieving an SD (HR 2.07 [95% CI 1.15–3.71]; p = 0.015), but there was no significant difference when compared with patients obtaining a PR (HR 1.64 [95% CI 0.87–3.07]; p = 0.124) (Figs. 3, 4; Table 3, Table 4, and Figs. 16, 17, and 22 in the ESM).
Fig. 4.
A Progression-free survival 3 and B overall survival on immunotherapy (ITH) drug holidays dependent on best overall response to ITH. CR complete response, ICI immune checkpoint inhibitor, PR partial response, SD stable disease
Outcomes After Re-treatment with ITH
Among 68 patients who had a PFS event during an ITH drug holiday, 39 (57.4%) were re-challenged with pembrolizumab or nivolumab (Fig. 4; Fig. 22 in the ESM). After ITH drug holidays, the objective response rate to ITH reinduction was 58.9% with a PFS4 1-year rate of 46.4% (95% CI 32.6–65.9), and the median PFS was 11.2 months (95% CI 6.7–not reached). The PFS4 was not influenced by the best response achieved during the pre-holiday ITH or by the length of pre-holiday ITH (Fig. 5). The BOR on ITH retreatment was CR in 16 (41%) patients, PR in seven (17.9%) patients, and SD in 16 (41%) patients. Of the 23 post-drug-holiday objective responses, three CRs (13%) were experienced by patients who experienced CR during their initial anti-PD-1 therapy. Durable, ongoing objective responses were achieved on post-drug-holiday therapy. At the time of analysis, median PFS4 was not experienced by patients treated for >2 years who had CR or PR. Patients who experienced CR at re-treatment were progression free at last follow-up (Fig. 21 in the ESM). PFS4 was not influenced by the length of the drug holiday duration (HR 1.00 [95% CI 0.97–1.04]; p = 0.875.
Fig. 5.
Forest plot of multivariable Cox proportional hazards model for PFS3. CI confidence interval, CR complete response, HR hazard ratio, ICI immune checkpoint inhibitor, PR partial response, SD stable disease.
The OS4 at 1 year since the restart of ITH was 77.6% (95% CI 65–92.7) and 61.7% (95% CI 46.3–82.3%) at 2 years. OS4 did not depend on either pre-holiday treatment duration or the BOR achieved then. At the time of analysis, 14 of 39 (35.9%) re-challenged patients had died from melanoma progression after ITH retreatment.
Discussion
Our study constitutes one of the most extensive real-world, observational cohorts of patients who opted for an ITH drug holiday – discontinuation of anti-PD-1 therapy—outside of a clinical trial. In most clinical trials, ITH is halted after 24 months of treatment, as per protocol. Our patients were referred to ITH drug holidays in cases of longer disease control and in the absence of immune toxicities after a median of 26 months on ITH. Clear and uniform indications for discontinuing ITH in treatment guidelines are lacking [24, 36]. Over recent years, a novel outcome measure—treatment-free survival (TFS) – has come to the attention of researchers. TFS is defined as the time from ITH discontinuation to the time of subsequent systemic therapy initiation (or death), which is in concordance with our PFS3.
As shown by long-term analysis of CheckMate trials at the 36-month mark, a significant proportion of the 1077 patients who stopped ITH treatment was still alive without the need for subsequent therapy initiation, most of whom were, per se, on drug holidays because they had finished their therapy, per protocol, not because of toxicity. Specifically, 47% of those on nivolumab plus ipilimumab, 37% on nivolumab alone, and 15% on ipilimumab demonstrated this outcome. The median TFS/PFS3 was notably longer for the nivolumab plus ipilimumab group (11.1 months) than for those receiving nivolumab (4.6 months), with a difference of 6.5 months (95% CI 5.0–8.0). Similarly, TFS/PFS3 for the nivolumab plus ipilimumab group surpassed that of the ipilimumab monotherapy group, with a difference of 8.7 months (95% CI 0.8–4.1) [37]. These data were confirmed by a recent Canadian real-world evidence report. During the 36-month follow-up, patients treated with nivolumab–ipilimumab experienced a longer duration without the need for additional ITH therapy, with a mean time of 12.4 months (95% CI 8.8–16.0) after ITH discontinuation. In comparison, patients receiving nivolumab monotherapy had a mean PFS3 of 8.9 months (95% CI 4.4–13.5), and patients treated with pembrolizumab had a mean PFS3 of 11.1 months (95% CI 8.5–13.8) [38]. All these data align with the results from the KEYNOTE-001 trial in patients who discontinued pembrolizumab upon achieving CR or PR. Of these patients, 90% were still in CR after a median follow-up of 5 years. At the long-term analysis, only 9% of patients had experienced disease progression [18]. At the same time, in KEYNOTE-006, after 2 years of pembrolizumab therapy and ≥ 3 years off treatment, 76% of patients with CR or PR had ongoing response after 5 years of follow-up [39]. Of the 103 patients who initially completed the 2-year course of pembrolizumab with at least SD, 27 experienced tumor relapse. The median PFS3 in this pembrolizumab trial was 33.3 months. Individuals with SD experienced a relapse sooner than those who achieved CR or PR [39, 40], as was also the case with our patients. In general, clinical trials and our data both prove that ITH may have a prolonged effect in disease control beyond the active treatment. Of note, the rate of CRs reported in our study (42.8%) is far superior to rates observed in Checkmate 066 and 067 and KEYNOTE-006 with anti-PD-1 monotherapy or its combination with anti-cytotoxic T-lymphocyte-associated protein (CTLA)-4, where the rate of CR ranged between 14.9 and 23.6% [22, 41, 42]. Nevertheless, this is only because our study included only patients referred to elective ITH discontinuation, and this group consisted only of individuals achieving response or prolonged stabilization. Therefore, high rates of responses were an obvious and expected finding.
It has been suggested that shorter durations of anti-PD-1 treatment might be equally as effective as extended ITH lasting ≥ 2 years [43], but our results do not confirm this hypothesis. In the NCT02673970 trial by Jansen et al. [30], the duration of anti-PD-1 therapy was shorter (median 12 months) and was also effective. Moreover, a median ITH duration of 22 months resulted in outcomes comparable to ours [44]. In the trial of 1 year of anti-PD-1 treatment, after a median follow-up of 18 months from the start of a drug holiday, 22% (n = 40) of patients experienced disease progression [30]. After a median of 12 months on anti-PD-1 treatment, the median time to disease progression was 12 months (range 2–23) [30], whereas the median PFS was not reached after a median 27 months of treatment in our cohort. After a median of 12 months on anti-PD-1 therapy, the estimated 1- and 2-year PFS rates after discontinuation (PFS3) were 90% and 71%, respectively [30], whereas they were 87.3% and 76.8%, respectively, in our cohort. After 12 months of therapy, reported relapse patterns encompassed solitary non-central nervous system (CNS) progression in six patients, CNS (brain) only progression in four patients, and multifocal non-CNS progression in three patients. Among these 13 patients, nine promptly underwent successful localized treatment for the solitary site of progression. This intervention included stereotactic radiosurgery or stereotactic body radiation therapy for three patients and surgical resection of the tumor for six patients [43]. Another report from the Huntsman Cancer Institute showed that disease progressed in 25% of patients (13 cases) who received ITH for 12 months, with a median time to progression after treatment discontinuation of 3.9 months (range 0.7–30.9). After a median follow-up of 20.5 months, 75% of patients maintained a status free from disease progression in this trial [43]. Results like ours come from the data of the report on 86 cases that included patients with and without melanoma brain metastases and who achieved CR and chose to stop ITH after a median of 22 months. In this study, disease recurred in seven patients from the group, but the median duration of survival off treatment was not reached. The median off-treatment response time was 19 months for everyone and shorter for cases with brain metastases, with a median of 17 months (range 7–41). After a median follow-up of 38 months (range 9–70), seven patients (5.6%) had died, but only one (0.8%) from melanoma [44]. In the Italian report, the median treatment time was even longer, at 33 months. In this study, 128 patients halted the anti-PD-1 treatment upon achieving CR, ten patients with SD, and 35 patients made the decision independently. After a mean follow-up period of 21 months (range 1–81), only 7.8% of those who discontinued treatment in CR experienced disease progression, whereas 20.6% faced progression after discontinuation based on the patient’s choice (including two in CR, four in PR, and one in SD) [45]. After a median 33 months of therapy, respective PFS at 12 and 24 months was 97.5% and 94.1% for individuals achieving CR before ITH discontinuation, 93.9% and 82.1% for those with PR, and 80.2% and 50.1% for those with SD [45]. A strength of our report is the high number of patients receiving ITH in the first line of treatment, whereas in the NCT02673970 trial, anti-PD-1 therapy was administered as a first-line therapy in 80 (43%) patients [30]. Based on these data, we opted for treatment discontinuation after 2 years of ITH as it was expected to be a safe choice with an acceptable risk of disease progression, with the depth of response, regardless of BRAF mutational status being a predictor of a prolonged positive outcome [18–21, 46]. We believe that the prolonged effect of ITH, also off treatment, should be attributed to CD8+ memory cells, as these cells enable long-term recognition of (melanoma) antigens after initial response. In particular, the infiltration of the tumors by memory cells – CD103+CD8+ T cells during anti-PD-1 ITH – is associated with a good prognosis [2, 47, 48].
Melanoma ITH registration trials reported that, in palliative therapy, selected clinical factors may correlate with potential long-term ITH efficacy. Data from phase II and III pivotal clinical trials confirmed that patients with high programmed death ligand-1 expression and smaller tumor burdens more frequently experienced long-term clinical benefits. Nevertheless, it was also noted that a sustained response can still occur irrespective of baseline characteristics [5]. Moreover, the Italian report found a correlation between the recurrence of melanoma during drug holidays and the primary site, with mucosal melanoma as a negative factor (HR 15.57). Moreover, a reduced incidence of relapses was reported in patients with M1b disease who experienced a CR (HR 3.84) [45]. In other real-world data, disease progression during drug holidays after 1 year of ITH in a multivariable analysis was associated with factors such as younger age, a history of brain metastasis, and elevated lactate dehydrogenase levels at the time of anti-PD-1 discontinuation [43]. We and others anticipated the rise in significance of metabolic response on 18f-FDG-positron emission tomography (PET)/CT in evaluating drug holiday candidates [46].
Few studies have also described the response after ITH reinduction. The long-term follow-up of the KEYNOTE-006 study assessed the clinical outcomes of patients who received a second course of pembrolizumab after treatment discontinuation. Among the 16 who received re-treatment with pembrolizumab, the ORR was 56%, and four patients experienced CR, five experienced PR, five had SD, and two had disease progression. The 2-year PFS with pembrolizumab re-treatment (equivalent of PFS4 in our analysis) was 62.5% [40], which is higher than the 34.3% in our population. Patients with CR or PR as the BOR to the first course of pembrolizumab in KEYNOTE-006 tended to respond better to the second course than those with SD, which is consistent with our data. Despite the constraints imposed by the limited and varied published data, we are opting for treatment discontinuation after 2 years, as it seems to be a safe choice with an acceptable risk of disease progression. Re-introducing treatment can rekindle antitumor activity in a specific group of patients following a pause in their treatment. The depth of response is currently confirmed as the most reliable predictor of a prolonged positive outcome.
van Zeij et al. [49] compared survival outcomes of patients discontinuing the treatment electively or because of treatment-limiting toxicity. They reported that the PFS and OS rates were lower in patients who discontinued the treatment because of AEs (48% and 72% at 18 months, respectively) than in patients discontinuing the treatment electively: 18-month PFS was 62% and OS 91% [49]. In our study, the PFS3 was 76.8% at 24 months after ITH discontinuation.
For patients with melanoma, the de-escalation of therapy in the form of drug holidays is safe and may be offered to those whose disease responds to first-line ITH. Patients who experienced CR before a drug holiday have a high probability of a second response at re-treatment, as suggested in lung cancer trials before including KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. It is not expected that a treatment break will completely decrease the risk of ITH toxicity because of the specific mode of action of checkpoint inhibitors. Late toxicities emerging after discontinuation of anti-PD-1 therapies have been described. Most importantly, most patients with melanoma who used drug holidays with no evidence of disease progression were still progression free at 24 months after discontinuation. Patients whose initial ITH duration was <24 months and those who did not have an objective response have a higher risk of progression. ITH re-challenge enables disease control after initial progression on drug holiday. Re-initiating ITH appears to be safe and shows potential for achieving disease control. Treatment breaks after the initial response may be considered in carefully clinically selected patients with disease control. In patients with a CR and who are treated for >6 months, the risk of relapse after treatment discontinuation was low. In the future, PET–CT and liquid biopsy (e.g., circulating tumor DNA), or tissue biopsy may become recommended techniques for assessing the effectiveness of ITH with ICIs before a drug holiday. The introduction of ITH using nivolumab, pembrolizumab, and ipilimumab has significantly enhanced the prognosis for individuals facing advanced melanoma. Nonetheless, these treatments place a substantial financial strain on both patients and healthcare systems, so the first financial analyses of treatment de-escalation have been conducted. Cost-effectiveness analysis suggests that tailoring treatment de-escalation based on treatment response in patients with advanced melanoma could result in significant cost savings for the healthcare system. The authors even suggested that such an approach is a potentially cost-effective treatment mode applicable across diverse resource settings. Nevertheless, it was pointed out that clinical trials should focus on generating additional evidence regarding the noninferiority of treatment breaks to support this strategy further [50].
Our study has several limitations. First, the number of patients treated with combined nivolumab and ipilimumab, which is currently the preferred first-line ITH, was relatively low. Another limitation was the availability of drug holidays in the drug reimbursement program: patients treated before a certain time point could not be referred to drug holidays, so these patients had a longer duration of ITH to comply with program regulations. Also, data from these patients were collected retrospectively.
In conclusion, our study supports the elective discontinuation of ITH in patients with advanced melanoma with CR, PR, or SD. At 3 years since drug holidays were introduced, the PFS3 was 65%. In patients experiencing progression, the activity of ITH was still observed, with a 58.9% ORR to the ITH re-challenge. Patients with a CR had the most favorable survival upon ITH discontinuation, suggesting that drug holidays should primarily be considered in this group of patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest
PR has served on an advisory board in a personal capacity for Blueprint Medicines; has served as an invited speaker and on an advisory board in a personal capacity and received honoraria for lectures from BMS, MSD, and Pierre Fabre; has served as an invited speaker and on an advisory board in a personal capacity for Merck and Sanofi; has served as an invited speaker in a personal capacity for Novartis; has received funding and a research grant to his institution from, and has a financial interest in, BMS; has received a research grant to his institution, has a financial interest in, and has received a research grant for ISS from Pfizer; serves as an officer for the American Society of Oncology; and is a member of the board of directors of the Polish Society of Surgical Oncology. AMC, ŁG, RD, NKK, KG, BCS, JM, MZięt, GKW, KK have delivered lectures for and received fees, honoraria, and travel accommodations from BMS, MSF, and Pierre Fabre. PT, KO, PB, and MZiel have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
Patients were treated under National Treatment Reimbursement Program B.59 as per the Announcement of the Minister of Health. All patients signed informed consent for the treatment. Data analysis was approved by the local ethical committee in Maria Sklodowska-Curie National Research Institute of Oncology (approval no. KB/430-74/20 from October 23, 2020).
Consent to Participate
All patients signed informed consent for the treatment, data processing, utilization for scientific research purposes, and publication.
Consent for Publication
All patients signed informed consent for the treatment, data processing, utilization for scientific research purposes, and publication.
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
The code created and used during the current study is available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: AMC, PT, KO, and PR. Methodology: AMC, PT, and PR. Software: AMC and PT. Data curation: AMC, KO, and PB. Investigation: all authors. Validation: AMC, PT, KO, and PB. Formal analysis: PT, AMC, and PR. Supervision: AMC and PR. Funding acquisition: PR. Visualization: PT. Project administration: AMC. Resources: AMC and PR. Writing – original draft: AMC, PT, PB, and PR. Writing – review and editing: all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Krzysztof Ostaszewski and Piotr Błoński equally contributed to the work.
References
- 1.Ribas A, Shin DS, Zaretsky J, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. 2016;4(3):194–203. 10.1158/2326-6066.CIR-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Y, Tan A, Feng J, et al. Prognostic impact of memory CD8(+) T cells on immunotherapy in human cancers: a systematic review and meta-analysis. Front Oncol. 2021;11: 698076. 10.3389/fonc.2021.698076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanmeerbeek I, Borras DM, Sprooten J, et al. Early memory differentiation and cell death resistance in T cells predicts melanoma response to sequential anti-CTLA4 and anti-PD1 immunotherapy. Genes Immun. 2021;22(2):108–19. 10.1038/s41435-021-00138-4. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Marabelle A, Herrscher H, et al. Immunotherapy discontinuation-how, and when? Data from melanoma as a paradigm. Nat Rev Clin Oncol. 2020;17(11):707–15. 10.1038/s41571-020-0399-6. [DOI] [PubMed] [Google Scholar]
- 5.Boutros C, Belkadi-Sadou D, Marchand A, et al. Cured or not? Long-term outcomes of immunotherapy responders. Focus on melanoma. Curr Oncol Rep. 2023;25(9):989–96. 10.1007/s11912-023-01429-x. [DOI] [PubMed] [Google Scholar]
- 6.Howland RH. Medication holidays. J Psychosoc Nurs Ment Health Serv. 2009;47(9):15–8. 10.3928/02793695-20090804-01. [DOI] [PubMed] [Google Scholar]
- 7.Marron TU, Ryan AE, Reddy SM, et al. Considerations for treatment duration in responders to immune check-point inhibitors. J Immunother Cancer. 2021. 10.1136/jitc-2020-001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar Das D. Clinical outcomes of early immunotherapy cessation in metastatic melanoma. Oncology Times. 2021;43(11):28,43-2943. 10.1097/01.COT.0000754700.05681.e4. [Google Scholar]
- 9.Beaulieu E, Spanjaart A, Roes A, et al. Health-related quality of life in cancer immunotherapy: a systematic perspective, using causal loop diagrams. Qual Life Res. 2022;31(8):2357–66. 10.1007/s11136-022-03110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghate SR, Li Z, Tang J, et al. Economic burden of adverse events associated with immunotherapy and targeted therapy for metastatic melanoma in the elderly. Am Health Drug Benefits. 2018;11(7):334–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Bateni SB, Nguyen P, Eskander A, et al. Changes in health care costs, survival, and time toxicity in the era of immunotherapy and targeted systemic therapy for melanoma. JAMA Dermatol. 2023;159(11):1195–204. 10.1001/jamadermatol.2023.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams RA, Fisher DJ, Graham J, et al. Capecitabine versus active monitoring in stable or responding metastatic colorectal cancer after 16 weeks of first-line therapy: results of the randomised FOCUS4-N trial. J Clin Oncol. 2021;39(33):3693–704. 10.1200/jco.21.01436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madan RA, Pal SK, Sartor O, et al. Overcoming chemotherapy resistance in prostate cancer. Clin Cancer Res. 2011;17(12):3892–902. 10.1158/1078-0432.Ccr-10-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuyama C, Enokida T, Ueda Y, et al. Planned drug holidays during treatment with lenvatinib for radioiodine-refractory differentiated thyroid cancer: a retrospective study. Front Oncol. 2023;13:1139659. 10.3389/fonc.2023.1139659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal K, Derosa L, Albiges L, et al. Drug holiday in metastatic renal-cell carcinoma patients treated with vascular endothelial growth factor receptor inhibitors. Clin Genitourinary Cancer. 2018;16(3):e663–7. 10.1016/j.clgc.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Nagyiványi K, Budai B, Küronya Z, et al. Outcome of restarted sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective trial and combined case reports from literature. Pathol Oncol Res. 2019;25(1):241–7. 10.1007/s12253-017-0345-9. [DOI] [PubMed] [Google Scholar]
- 17.Hong A, Moriceau G, Sun L, et al. Exploiting drug addiction mechanisms to select against MAPKi-resistant melanoma. Cancer Discov. 2018;8(1):74–93. 10.1158/2159-8290.Cd-17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–8. 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid O, Robert C, Daud A, et al. Long-term outcomes in patients with advanced melanoma who had initial stable disease with pembrolizumab in KEYNOTE-001 and KEYNOTE-006. Eur J Cancer. 2021;157:391–402. 10.1016/j.ejca.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36(17):1668–74. 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 21.Long GV, Schachter J, Arance A, et al. Long-term survival from pembrolizumab (pembro) completion and pembro re-treatment: Phase III KEYNOTE-006 in advanced melanoma. J Clin Oncol. 2020;38(15_suppl):10013. 10.1200/JCO.2020.38.15_suppl.10013. [Google Scholar]
- 22.Robert C, Long GV, Brady B, et al. Five-year outcomes with nivolumab in patients with wild-type BRAF advanced melanoma. J Clin Oncol. 2020;38(33):3937–46. 10.1200/JCO.20.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi FS, Chiarion-Sileni V, Lewis KD, et al. Long-term survival in advanced melanoma for patients treated with nivolumab plus ipilimumab in CheckMate 067. J Clin Oncol. 2022;40(16 suppl):9522. 10.1200/JCO.2022.40.16_suppl.9522. [Google Scholar]
- 24.Rutkowski P, Wysocki PJ, Nasierowska-Guttmejer A, et al. Cutaneous melanomas-guidelines for diagnostic and therapeutic management. Oncol Clin Pract. 2019;15(1):1–19. [Google Scholar]
- 25.Rutkowski P, Wysocki PJ, Nasierowska-Guttmejer A, et al. Cutaneous melanomas-guidelines for diagnostic and therapeutic management. Oncol Clin Pract. 2017;13(6):241–58. [Google Scholar]
- 26.Rutkowski P, Wysocki PJ, Kozak K, et al. Expert recommendations on diagnostic-therapeutic management of melanoma patients. Oncol Clin Pract. 2022;18(6):357–92. [Google Scholar]
- 27.Cybulska-Stopa B, Piejko K, Ostaszewski K, et al. Long-term clinical evidence of comparable efficacy and toxicity of nivolumab and pembrolizumab in advanced melanoma treatment. Melanoma Res. 2023;33(3):208–17. 10.1097/cmr.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 28.Kozak K, Teterycz P, Galus Ł, et al. Real-world outcomes in advanced melanoma patients treated with first-line nivolumab plus ipilimumab. J Clin Oncol. 2023;41(16_suppl): e21552. 10.1200/JCO.2023.41.16_suppl.e21552. [Google Scholar]
- 29.Czarnecka AM, Teterycz P, Mariuk-Jarema A, et al. Treatment sequencing and clinical outcomes in BRAF-positive and BRAF-negative unresectable and metastatic melanoma patients treated with new systemic therapies in routine practice. Target Oncol. 2019;14(6):729–42. 10.1007/s11523-019-00688-8. [DOI] [PubMed] [Google Scholar]
- 30.Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30(7):1154–61. 10.1093/annonc/mdz110. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Rutkowski P, Wysocki PJ, Kozak K, et al. Postępowanie diagnostyczno-terapeutyczne u chorych na czerniaki — zalecenia ekspertów. Onkol Prakt Klin Edu. 2021:ahead of print.
- 33.Rutkowski P, Wysocki PJ, Nasierowska-Guttmejer A, et al. Czerniaki skóry - wytyczne postępowania diagnostyczno-terapeutycznego. Onkol Prakt Klin Edu. 2020;6(4):225–45. [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. 10.1080/01621459.1958.10501452. [Google Scholar]
- 35.Cioci AC, Cioci AL, Mantero AMA, et al. Advanced statistics: multiple logistic regression, Cox proportional hazards, and propensity scores. Surg Infect (Larchmt). 2021;22(6):604–10. 10.1089/sur.2020.425. [DOI] [PubMed] [Google Scholar]
- 36.Michielin O, van Akkooi ACJ, Ascierto PA, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1884–901. 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 37.Regan MM, Werner L, Rao S, et al. Treatment-free survival: a novel outcome measure of the effects of immune check-point inhibition-a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2019;37(35):3350–8. 10.1200/jco.19.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta M, Stukalin I, Meyers D, et al. Treatment-free survival after nivolumab vs pembrolizumab vs nivolumab-ipilimumab for advanced melanoma. JAMA Netw Open. 2023;6(6): e2319607. 10.1001/jamanetworkopen.2023.19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–51. 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 40.Robert C, Carlino MS, McNeil C, et al. Seven-year follow-up of the phase III KEYNOTE-006 study: pembrolizumab versus ipilimumab in advanced melanoma. J Clin Oncol. 2023;41(24):3998–4003. 10.1200/JCO.22.01599. [DOI] [PubMed] [Google Scholar]
- 41.Long GV, Carlino MS, McNeil C, et al. Pembrolizumab versus ipilimumab for advanced melanoma: 10-year follow-up of the phase III KEYNOTE-006 study. Ann Oncol. 2024;35(12):1191–9. 10.1016/j.annonc.2024.08.2330. [DOI] [PubMed] [Google Scholar]
- 42.Wolchok Jedd D, Chiarion-Sileni V, Rutkowski P, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2025;392(1):11–22. 10.1056/NEJMoa2407417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pokorny R, McPherson JP, Haaland B, et al. Real-world experience with elective discontinuation of PD-1 inhibitors at 1 year in patients with metastatic melanoma. J Immunother Cancer. 2021. 10.1136/jitc-2020-001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimitriou F, Zaremba A, Allayous C, et al. Sustainable responses in metastatic melanoma patients with and without brain metastases after elective discontinuation of anti-PD1-based immunotherapy due to complete response. Eur J Cancer. 2021;149:37–48. 10.1016/j.ejca.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 45.Rubatto M, Fava P, Stanganelli I, et al. Discontinuation of anti-PD1 in advanced melanoma: an observational retrospective study from the Italian Melanoma Intergroup. Eur J Cancer. 2023;187:25–35. 10.1016/j.ejca.2023.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Jansen Y, van der Veldt AAM, Awada G, et al. Anti-PD-1: when to stop treatment. Curr Oncol Rep. 2022;24(7):905–15. 10.1007/s11912-022-01264-6. [DOI] [PubMed] [Google Scholar]
- 47.Paolini L, Tran T, Corgnac S, et al. Differential predictive value of resident memory CD8(+)T cell subpopulations in patients with non-small-cell lung cancer treated by immunotherapy. J Immunother Cancer. 2024. 10.1136/jitc-2024-009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards J, Wilmott JS, Madore J, et al. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res. 2018;24(13):3036–45. 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 49.van Zeijl MCT, van den Eertwegh AJM, Wouters M, et al. Discontinuation of anti-PD-1 monotherapy in advanced melanoma-Outcomes of daily clinical practice. Int J Cancer. 2022;150(2):317–26. 10.1002/ijc.33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartun Z, Kunz WG, Heinzerling L, et al. Cost-effectiveness of response-adapted de-escalation of immunotherapy in advanced melanoma. JAMA Dermatol. 2022;158(12):1387–93. 10.1001/jamadermatol.2022.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.