Abstract
In recent decades, advanced therapeutic modalities such as therapeutic cells, viral vectors, and extracellular vesicles (exosomes), have emerged as effective therapies for intractable diseases. These therapeutic modalities produced through bioprocesses must be purified from contaminants. Effective separation methods are essential for optimizing therapeutic modalities. This review highlights innovative temperature-modulated separation methods enabled by the thermoresponsive polymer poly(N-isopropylacrylamide) (PNIPAAm). The design of PNIPAAm-modified interfaces plays a pivotal role in ensuring precise and efficient separation. We summarize the recent advancements in the application of temperature-modulated separation methods for cells, viral vectors, and exosomes, with a focus on the design of PNIPAAm interfaces.
Graphical abstract
Keywords: Thermoresponsive polymer, Thermoresponsive interface, Temperature-responsive chromatography, Polymer brush, Regenerative medicine, Biomaterials
Introduction
Biopharmaceuticals such as antibody drugs, cellular drugs, viral vectors, and exosomes have emerged as new therapeutic modalities in addition to conventional small-molecule drugs. Since these modalities are produced through bioprocesses, they must be purified to remove impurities. However, current purification processes for these modalities are expensive and may compromise drug activity. Therefore, there is a need for an efficient method to separate and purify these drugs at a lower cost while preserving their activity.
To address this issue, we developed a separation and analysis method using the thermoresponsive polymer poly(N-isopropylacrylamide) (PNIPAAm). PNIPAAm exhibits temperature-dependent transitions between hydrophilic and hydrophobic states owing to hydration and dehydration. This reversible behavior allows PNIPAAm to extend or shrink depending on temperature changes (Fig. 1) [1–7]. The thermoresponsive properties of PNIPAAm have been utilized in various biomedical applications. These include temperature-controlled drug and gene delivery systems [8–15], biosensors and bioimaging systems [16–23], nanoactuators [24–30], bioseparation tools [31–41], cell separation materials [42–48], and cell culture substrates for tissue engineering [49–67].
Fig. 1.
Illustration of A temperature-responsive poly(N-isopropylacrylamide) (PNIPAAm) and B PNIPAAm brush-modified interfaces
The thermoresponsive properties of PNIPAAm find application in temperature-responsive chromatography [68–73]. The chromatography system uses a PNIPAAm-modified stationary phase, where temperature changes regulate the interaction between the stationary phase and analytes. Hydration and dehydration of PNIPAAm-modified stationary phase with temperature results in shifts between hydrophilic and hydrophobic states. Hydrophobic analytes like steroids can be separated by temperature-responsive chromatography through hydrophobic interactions [68, 74]. Additionally, incorporating hydrophobic monomers into PNIPAAm enhances the hydrophobic interactions with the analyte. The enhanced hydrophobic interaction enables the separation of hydrophilic analytes such as insulin [75], amino acid phenylthiohydantoins [76], the benzoic acid family [77, 78] and benzodiazepines and barbiturates [79].
The introduction of ionic groups to PNIPAAm has enabled the development of temperature-responsive ion-exchange chromatography for separating ionic analytes. Specifically, temperature-responsive anion-exchange chromatography involves the integration of cationic monomers into PNIPAAm. This chromatographic technique can separate acidic biomolecules such as adenosine nucleotides and oligonucleotides through temperature-modulated electrostatic interaction [41, 80, 81]. Similarly, temperature-modulated cation-exchange chromatography incorporates anionic monomers into PNIPAAm. This enables the separation of basic biomolecules such as catecholamines and angiotensins [82, 83]. Temperature-responsive mixed-mode chromatography is another technique, which utilizes a combination of PNIPAAm-modified beads and ionic polymer-modified beads [84, 85]. This chromatography technique can be modulated via electrostatic interactions by changing the bead composition of the column. Temperature-responsive chromatography has been applied in therapeutic drug monitoring, particularly in hospitals, since it functions without organic solvents in the mobile phase [86–89].
Therefore, various temperature-responsive chromatography methods have been developed, emphasizing the need for designing modified thermoresponsive polymers for separating new medical modalities. This review summarizes the recent advances in separation technologies for therapeutic cells, viral vectors, and exosomes, along with the progress in the design of separation materials.
Temperature-modulated cell separation with thermoresponsive ionic polymer brushes
Regenerative medicine is an effective approach for treating various intractable diseases [90–101]. These therapies are performed using cell suspensions or cellular tissue transplantation. Cells with therapeutic effects are typically mixed with contaminants, necessitating effective cell separation methods for regenerative applications. Various cell separation methods have been developed [102–115].
Centrifugation, which separates cells based on differences in specific gravity, is the simplest method and allows for large-scale separation of the cells. However, its low cell selectivity results in limited separation accuracy. Microfluidic devices offer an alternative approach, separating cells according to their size [109]. Here, fluid distribution is achieved by creating two types of microchannels, allowing the continuous separation of cells with different sizes.
More precise cell separation techniques include the use of antibodies labeled with fluorescent markers or conjugated to magnetic particles [102, 103]. Fluorescence-activated cell sorting (FACS) uses a fluorescence labelled antibody having affinity for target cells. Cells are fluorescently stained using these extracellular markers and then separated based on their fluorescence color or intensity. However, this separation method modifies the cell surface with fluorescent antibodies, resulting in the loss of cellular characteristics. Magnetic-activated cell sorting (MACS) has also been used as a precise cell separation method. MACS uses antibody-conjugated magnetic beads [103]. Magnetic beads are conjugated to the cell surface through interactions between the antibody and cell surface. The magnetic bead-modified cells are separated from the unmodified cells using magnetic force. However, the magnetic bead modification of cell surfaces also results in the loss of cell characteristics. Thus, FACS and MACS technologies involve cell modifications, which can alter their inherent properties and potentially diminish their therapeutic efficacy. Consequently, there is a growing demand for cell separation methods that preserve the integrity of the cell surface.
A promising alternative involves PNIPAAm-modified surfaces as a novel cell separation tool. The PNIPAAm-modified interface can control cell adhesion and detachment by temperature-dependent hydration and dehydration [49, 116]. Additionally, different cell types exhibit varying adhesion behaviors on PNIPAAm surfaces [117], making them efficient cell separation materials.
A PNIPAAm homopolymer brush-modified glass substrate has been used as a temperature-modulated cell-separation tool that does not require cell modification [42]. For example, vascular endothelial cells and myoblasts were separated based on their different cell desorption rates from the PNIPAAm brushes upon decreasing temperature.
Additionally, a thermoresponsive cationic random copolymer brush, poly(NIPAAm-co-N,N-dimethylaminopropylacrylamide (DMAPAAm)-co-tert-butylacrylamide)(tBAAm), has been used to separate mesenchymal stem cells (MSCs). MSCs have strong anionic properties, which can easily interact with the cationic polymer [44]. The selective adhesion of MSCs to the copolymer brush allowed for their targeted recovery, simply by lowering the temperature and inducing hydration and extension of the copolymer brush.
A thermoresponsive cationic copolymer brush has been prepared by random copolymerization of cationic and thermoresponsive monomers. This ensured a uniform incorporation of cationic groups into the thermoresponsive polymers. In temperature-responsive chromatography for protein separation and purification, packing materials prepared by block copolymerization (with bottom ionic segments and upper thermoresponsive segments) exhibited more effective protein adsorption and desorption than randomly copolymerized packing materials [34, 35, 118, 119]. This was due to the effective utilization of PNIPAAm shrinkage and extension. At high temperatures, the upper PNIPAAm segment shrinks, leading to effective protein adsorption onto the bottom ionic polymer brush. In contrast, at low temperatures, the upper PNIPAAm segment extends, preventing protein interactions with the ionic polymer. These results indicate that block-copolymer brushes are effective polymer structures for temperature-modulated separation of biomolecules.
Therefore, thermoresponsive cationic block copolymer brushes were investigated as separation materials for umbilical cord-derived mesenchymal stem cells (UCMSCs) (Fig. 2A) [120]. A poly(N,N-dimethylaminopropylacrylamide) (PDMAPAAm)-b-PNIPAAm brush was prepared on a glass substrate using two-step activator regeneration via electron-transfer atom-transfer radical polymerization (ARGET-ATRP) [121, 122]. These copolymer brushes have a cationic basal segment and a thermoresponsive upper segment. At 37 °C, the upper PNIPAAm segment shrinks, expressing the cationic property on the outermost copolymer brush. This enabled the adhesion of anionic UCMSCs to the copolymer brushes. Upon reducing the temperature to 20 °C, the adhered UCMSCs selectively detach due to the extension of upper PNIPAAm and weakened interaction between UCMSCs and the bottom cationic polymer segment. In contrast, fibroblasts and macrophages remain adhered to the copolymer brush even when the PNIPAAm is extended. Using the properties of the block copolymer brush, UCMSCs can be successfully separated from contaminant cells by temperature modulation [120].
Fig. 2.
Temperature-modulated cell separation using thermoresponsive ionic polymer brushes. A UCMSC separation using a thermoresponsive cationic block copolymer brush-grafted glass substrate B BMMSC separation from differentiated adipocytes using a mixed polymer brush composed of PNIPAAm- and PDMAPAAm-modified glass substrates
Thermoresponsive anionic copolymer brushes have also been developed as vascular-cell separation materials [123]. This is because the anionic group effectively interacts with the vascular cells [124]. A poly(acrylic acid) (PAAc)-b-PNIPAAm brush was prepared on a glass substrate through a two-step ARGET-ATRP of the tert-butyl acrylate (tBA) and PNIPAAm, followed by deprotection of tert-butyl group of tBA [82, 83]. The preparation scheme was designed to prevent deactivation of the ATRP catalyst with anionic monomers during polymerization. The block copolymer brush has a basal anionic segment and an upper thermoresponsive segment. The block copolymer brush adheres to endothelial cells and smooth muscle cells at 37 °C owing to the dehydration and shrinking of the upper PNIPAAm segment. By reducing the temperature to 20 °C, the upper PNIPAAm segment gets hydrated and extended, leading to detachment of the adhered endothelial cells. On the contrary, smooth muscle cells remain attached to the copolymer brush even at 20 °C. This is due to the strong adhesive properties of smooth muscle cells from the possible interaction with the bottom anionic polymer segment. Using the properties of thermoresponsive anionic block copolymer brush, the endothelial and smooth muscle cells can be separated by simply changing the temperature.
Effective temperature-modulated cell separation has been achieved using thermoresponsive ionic block copolymer brushes. Mixed polymer brushes comprising two types of polymers have been developed for effective temperature-modulated protein separation [125]. Silica bead-packed column modified with mixed polymer brush PNIPAAm and PDMAPAAm exhibited temperature-modulated acidic protein adsorption. At high temperatures, PNIPAAm shrinks and PDMAPAAm is exposed, leading to effective adhesion of the acidic protein. While at low temperatures, PNIPAAm is extended and hydrated, leading to protein desorption.
Thermoresponsive cationic mixed-polymer brushes have been developed for the effective separation of MSCs (Fig. 2B) [126]. A mixed polymer brush composed of PNIPAAm and PDMAPAAm was developed on a glass substrate by the reversible addition-fragmentation chain transfer (RAFT) polymerization of DMAPAAm and subsequent ARGET-ATRP of NIPAAm. The mixed-polymer brushes exhibited temperature-modulated cationic properties. At 37 °C, PNIPAAm of mixed polymer brush shrinks and exposes PDMAPAAm, leading to increased cationic property. On the contrary, at 20 °C, extension of PNIPAAm conceals PDMAPAAm, leading to decreased cationic properties. Thus, at 37 °C, acidic bone marrow-derived mesenchymal stem cells (BMMSCs) selectively adhere to the mixed polymer brush because of enhanced electrostatic interaction. By reducing temperature to 20 °C, PNIPAAm hydrates and extends, thereby decreasing the interaction between BMMSCs and DMAPAAm. Thus, the adhered BMMSCs are detached from the mixed polymer brushes. In contrast, adipocyte differentiated from BMMSCs do not adhere to mixed-polymer brushes owing to their low adhesive activity. Based on this property, BMMSCs and differentiated adipocytes can be separated using a mixed polymer brush by varying the temperature.
Temperature-modulated cell separation with thermoresponsive cell-affinity polymer brush
Peptide sequences with specific affinities for cells have been discovered and used in various medical applications [127–133]. For example, the Arg-Glu-Asp-Val (REDV) peptide is used to endothelialize the lumen of artificial blood vessels because of its specific interaction with vascular endothelial cells [132, 134]. Since these cell-affinity peptides interact with specific cells, they can be utilized as effective ligands for cell separation.
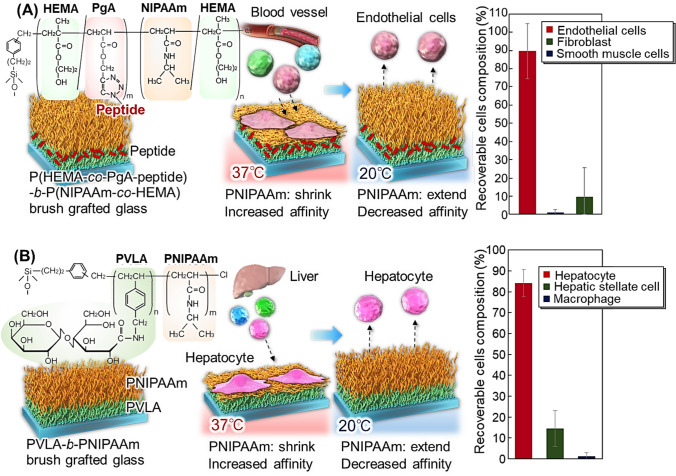
To improve the separation efficiency of endothelial cells, a cell-affinity peptide, REDV, is introduced into the thermoresponsive block copolymer brushes (Fig. 3) [135, 136]. A block copolymer brush composed of a bottom poly(2-hydroxyethyl methacrylate-co-propargyl acrylate)(P(HEMA-co-PgA)) segment and an upper P(NIPAAm-co-HEMA) segment was prepared using two-step ARGET-ATRP (Fig. 3A). Then, an Arg-Glu-Asp-Val (REDV) peptide with affinity for endothelial cells was conjugated to the bottom segment through a click reaction [137, 138]. The copolymer brushes exhibited a temperature-modulated affinity for target cells. At 37 °C, the upper segment shrinks, exposing the bottom segment conjugated with cell-affinity peptide at the outermost layer of the copolymer brush. This exposure leads to the effective adhesion of endothelial cells to the copolymer brush due to the affinity between the peptide and endothelial cells. By reducing the temperature to 20 °C, the upper segment gets extended and the affinity interaction between peptide and cells is reduced, leading to the detachment of endothelial cells. On the contrary, other contaminant cells, such as fibroblasts and smooth muscle cells, do not adhere to the copolymer brush at 37 °C. This occurs because the peptide in the copolymer has no affinity with fibroblasts and smooth muscle cells. Using this property, endothelial cells can be effectively separated from other vascular cells by simply changing their temperature.
Fig. 3.
Temperature-modulated cell separation using thermoresponsive affinity polymer brushes. A Vascular cell separation using peptide-conjugated thermoresponsive block copolymer brush-grafted glass substrate and B hepatocyte separation using thermoresponsive glycopolymer block copolymer brush-grafted glass substrate
A similar approach was used for poly(2-(2-methoxyethoxy)ethyl methacrylate) (PMEO2MA)-based copolymer brushes [136]. PMEO2MA-based copolymers have been investigated as functional materials for bioapplications requiring both thermo-responsiveness and bio-compatibility [139–146]. A P(HEMA-co-PgA)-b-poly(MEO2MA-co-HEMA-co-poly(ethylene glycol)methacrylate (PEGMA)) brush was prepared using two-step ARGET-ATRP. The REDV peptide was conjugated to the copolymer brush via a click reaction. The copolymer brush attaches to endothelial cells at 37 °C, attributed to the shrinkage of the PMEO2MA copolymer segment and exposed REDV peptide. In contrast, contaminant cells and fibroblasts do not adhere to the copolymer brush because the fibroblasts have no affinity for the peptide, and the PMEO2MA copolymer suppresses cell adhesion. By reducing the temperature to 20 °C, adhered endothelial cells are successfully recovered, leading to the separation of endothelial cells from contaminant cells.
Hepatocytes are important in regenerative medicine for the treatment of liver diseases[147–150]. Effective hepatocyte separation was achieved using the thermoresponsive glycopolymer brush (Fig. 3B) [151]. Glycopolymer poly(N-p-vinylbenzyl-O-β-D-galactopyranosyl-(1 → 4)-D-gluconamide) (PVLA) contains galactose moieties that interact with the asialoglycoprotein receptor (ASGPR) of hepatocytes [152–154]. Thus, PVLA can be used as a ligand for the effective capture of hepatocytes [151]. PVLA-b-PNIPAAm brush-grafted glass substrate using two-step ARGET-ATRP. At 37 °C, the upper PNIPAAm segment shrinks, and the bottom PVLA segment gets exposed. Thus, hepatocytes selectively adhere to the copolymer brushes through interactions between galactose and the ASGPR of hepatocytes. In contrast, hepatic stellate cells do not adhere to the copolymer brushes owing to their negligible affinity for the bottom glycopolymer. Other contaminant cells, such as macrophages, can adhere to the copolymer brush because of their strong adhesive properties. By reducing the temperature to 20 °C, the upper PNIPAAm segment extends and the interaction between galactose and ASGPR of hepatocytes is reduced. This leads to the successful recovery of hepatocytes from the copolymer brush. In contrast, adhered macrophages do not detach even at low temperatures, which is attributed to the strong adhesion properties of macrophages. Using the thermoresponsive properties of block-copolymer brushes, hepatocytes were separated from contaminant cells, macrophages, and stellate cells. Additionally, induced pluripotent stem (iPS) cell-derived hepatocytes are purified from undifferentiated iPS cells using a PVLA-b-PNIPAAm brush with a simple temperature change. iPS cell-derived hepatocytes adhered to the PVLA-b-PNIPAAm brush because they have an ASGPR. In contrast, iPS cells do not adhere to PVLA-b-PNIPAAm brush. By reducing the temperature to 20 °C, the upper PNIPAAm segment extends and the interaction between galactose and ASGPR of iPS cell-derived hepatocytes is reduced. This leads to the successful recovery of iPS cell-derived hepatocytes from the copolymer brush. Using the thermoresponsive properties of block-copolymer brushes, iPS cell-derived hepatocytes were separated from iPS cells.
Temperature-modulated cell separation column for effective stem cell separation
The developed PNIPAAm copolymer brush-modified glass could separate cells simply by changing the temperature. However, cell separation is limited by the small surface area of copolymer-modified glass substrates. To improve cell separation performance, cell separation columns using PNIPAAm copolymers have been investigated [155–158].
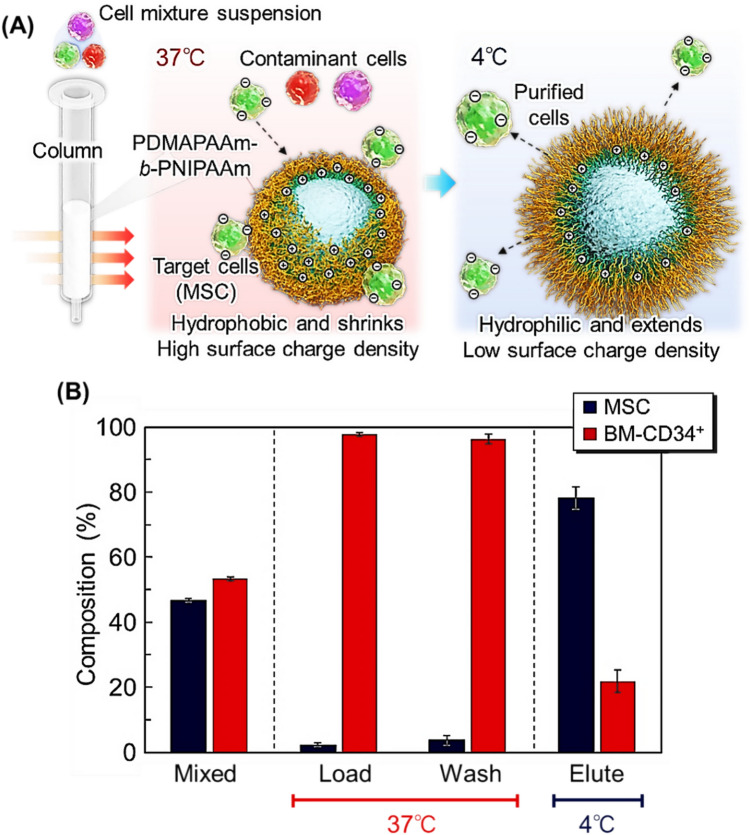
A temperature-responsive cell separation column was developed for bone marrow-derived MSC using PDMAPAAm-b-PNIPAAm brush-modified beads as the packing material (Fig. 4) [157]. Silica beads with a diameter of 150–210 μm were used as base materials because a relatively large bead diameter was required for cells to pass through the bead-packed layer. PDMAPAAm-b-PNIPAAm was modified via two ATRP steps. Because MSCs exhibit strong acidic properties, the cationic polymer PDMAPAAm functions as an effective ligand for capturing MSCs [157]. The copolymer-brush-modified beads are packed in syringe-type columns. At 37 °C, MSCs are introduced into the column. The upper PNIPAAm segment shrinks and gets dehydrated, exposing the bottom PDMAPAAm layer as the outermost layer of the beads. MSCs are negatively charged compared to other contaminant cells [157] and are adsorbed onto copolymer brushes through electrostatic and hydrophobic interactions. In contrast, the contaminant cells, BM-CD34+, are not adsorbed onto the beads owing to weak electrostatic interaction compared to the interaction between MSCs and PDMAPAAm. Thus, BM-CD34+ will pass through the column even at 37 °C. By reducing the temperature to 20 °C, the PNIPAAm segment gets extended and hydrated, reducing the electrostatic and hydrophobic interactions and causing the adsorbed MSC to desorb from the copolymer brush. Using this column, MSCs can be successfully separated from the contaminant BM-CD34+ cells by simply changing the column temperature. In addition, recovered MSCs maintained their proliferative and multilineage differentiation abilities.
Fig. 4.
Temperature-modulated cell separation column. A Illustration of the cell separation column using thermoresponsive cationic block copolymer brush-grafted beads and B cell composition at each fraction
A similar cell separation column was developed for the effective separation of adipose tissue-derived MSCs [158]. Unlike the separation columns for bone marrow-derived MSCs, silica beads with large diameters (212–250 μm) are used as base materials because the adipose tissue-derived MSCs have larger diameters compared to that of bone marrow-derived MSCs [158]. The PDMAPAAm-b-PNIPAAm brush was modified on silica beads through two ATRP steps, and the prepared beads were packed into a syringe-type column. The column retained adipose tissue-derived MSCs at 37 °C due to their adsorption on the PDMAPAAm-b-PNIPAAm brush through electrostatic and hydrophobic interactions. In contrast, contaminant Jurkat cells pass through the column because the electrostatic interactions between Jurkat cells and the copolymer are relatively weak. By changing the column temperature to 4 °C, MSCs are eluted from the column. These get desorbed from the copolymer brush due to reduced electrostatic and hydrophobic interactions. Using this column, adipose tissue-derived MSCs are separated from the contaminant cells by simply changing their temperature. Furthermore, the recovered MSC maintained their viability, proliferation, and differentiation abilities compared to those before cell separation using the column. These results indicated that the cell separation column.
Temperature-modulated separation of viral vectors
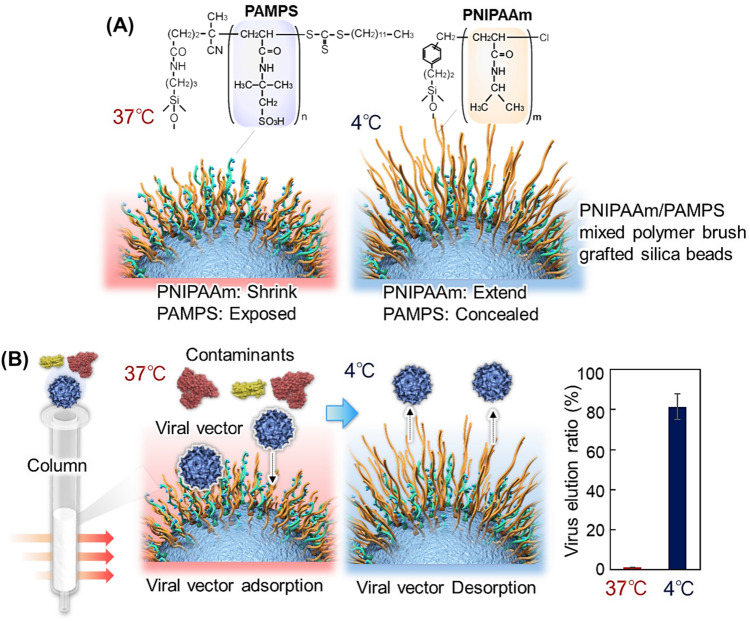
Viral vectors are used for gene therapy because of their ability to deliver nucleic acids to target cells via transfection [159–161]. During the production of viral vectors, viral vector-producing cells are cultured, and the cells produce viral vectors in cell culture medium. Contaminants in the culture medium must be removed from viral vectors. Various purification methods for viral vectors have been developed [162–167]. The purification of viral vectors using cesium chloride density gradient centrifugation and column chromatography has been extensively investigated as effective separation methods [166, 167]. However, to improve the production efficiency of viral vectors further, effective viral vector purification methods using simple procedures are urgently required. Thus, viral vector purification methods using PNIPAAm have been investigated [168]. Adeno-associated virus type 2 (AAV2) vectors are particularly useful because of their stability, versatility, and non-pathogenicity [169–171]. Therefore, an AAV2 vector purification column was developed using PNIPAAm-based mixed-polymer brush-modified beads (Fig. 5) [168]. A mixed polymer brush composed of poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) and PNIPAAm served as an effective ligand for capturing viral vectors. During AAV2 infection, the AAV2 vector recognizes heparan sulfate proteoglycans on the cell surface [172–174]. Thus, PAMPS anionic polymers with sulfonic acid groups can interact with AAV2. Silica beads (40–64 μm) are used as base materials. Two types of initiators, a radical polymerization initiator and an ATRP initiator, are immobilized on the silica beads. PAMPS is grafted onto the silica bead surface via RAFT polymerization, and PNIPAAm is grafted onto the beads via ATRP, resulting in the formation of a mixed polymer brush. The beads are packed into a syringe column. AAV2 is introduced into the column at 40 °C, and AAV2 gets adsorbed onto the mixed polymer brush. At 40 °C, PNIPAAm shrinks, and PAMPS is exposed to the outermost layer of the mixed polymer brush, leading to effective adsorption of AAV2 on the mixed polymer brush. By reducing the temperature to 5 °C, adsorbed AAV2 is desorbed. At 5 °C, PNIPAAm is extended, and PAMPS gets concealed. Thus, the interaction between PAMPS and the viral vector is reduced, leading to the desorption of AAV2 from the mixed polymer brush and the elution of AAV2 from the column. Based on the properties of the mixed polymer brushes, AAV2 is successfully separated from bovine serum by varying the temperature. Furthermore, the recovered AAV2 strain retains its infectivity.
Fig. 5.
Temperature-modulated viral vector purification. A Illustration of packing materials for the purification column and B purification procedure and virus elution at two temperatures
Temperature-modulated separation of exosomes
Recently, exosomes have attracted considerable attention as potential diagnostic markers and therapeutic agents for cancer. Exosomes are small vesicles (40–100 nm) composed of endosomal cell membranes attributed to derived cells. Exosomes contain nucleic acids such as messenger RNA and microRNAs, and various proteins are attributed to their derived cells. Thus, exosomes can be used as diagnostic markers because they contain nucleic acids that are derived from cells [175, 176]. In addition, the administration of exosomes has been shown to have therapeutic effects in a variety of diseases, owing to the presence of nucleic acids and proteins [177–182]. Exosomes are secreted from cells and must be purified from cell culture media, which often contains various foreign substances. Various exosome purification methods have been developed, including ultracentrifugation, size exclusion, and affinity separation [183–187]. However, these separation methods are limited by complicated procedures, low selectivity, and reduced activity. Therefore, effective exosome purification methods that involve simple procedures and preserve exosome activity are urgently required.
Recently, innovative exosome isolation methods have been developed [188, 189]. The T cell immunoglobulin domain and mucin domain-containing protein 4 (Tim4), which specifically bind phosphatidylserine present on the surface of exosomes, were used for exosome purification [188]. In this method, magnetic beads conjugated with Tim4-Fc protein are added to exosome-secreted culture medium. Exosomes adhered to the beads via a Tim4-Fc-phosphatidylserine binding process in the presence of Ca2+ ions. Later, the bound exosomes are released from the beads using an elution buffer containing the chelating agent, ethylenediaminetetraacetic acid.
Further, exosome isolation methods using net-charge invertible curvature-sensing peptides (NIC) have been developed [189]. Curvature-sensing peptides recognize exosome vesicles by binding to lipid-packing defects on the highly curved exosome membranes. Additionally, the NIC design ensures efficient capture and release of the exosomes with pH changes. The NIC is modified on a resin and added to an exosome suspension maintained at pH 6.0 to bind the exosomes to the resin. Later, upon using a buffer having pH 10.0, the bound exosomes can be released.
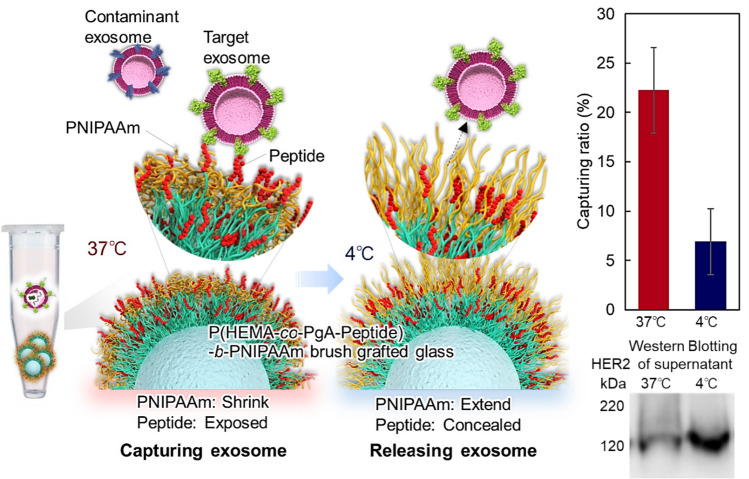
We developed an innovative exosome purification method using PNIPAAm by simply changing the temperature (Fig. 6) [190]. Nonporous silica beads (diameter 4.0 μm) were used as base materials. First, a P(HEMA-co-PgA) brush is grafted onto the silica beads as a peptide conjugation segment during the first ATRP. To these grafted beads, PNIPAAm brush is grafted via a second ATRP step. Peptides having exosome affinity are then conjugated to the propargyl group in the bottom segment through a click chemistry reaction. After incubating the prepared beads and the exosomes derived from SK-BR-3 cells in a microtube at 37 °C, the exosomes are captured on the copolymer brush-modified beads. At 37 °C, the PNIPAAm segment shrinks and exposes the peptide-conjugated bottom segment on the outermost surface of the block copolymer brush. This leads to the effective capture of exosomes on the copolymer brush. Upon decreasing the temperature to 4 °C, the captured exosomes are released from the block copolymer brush. This is attributed to the extension of PNIPAAm, which reduced the peptide-exosome affinity. Hence, target exosomes can be purified by using these beads at different temperatures.
Fig. 6.
Temperature-modulated capture of exosomes using a thermoresponsive block copolymer brush with affinity peptides
Conclusions
This review summarizes innovative separation methods for new therapeutic modalities such as cells, viral vectors, and exosomes. It also highlights the design of thermoresponsive polymers. MSCs and endothelial cells can be effectively separated using thermoresponsive ionic block copolymer brush-modified glass substrates. These substrates regulate ionic properties with temperature changes. A mixed polymer brush composed of PDMAPAAm and PNIPAAm enables MSC separation from adipocytes using temperature modulation. In temperature-modulated cell separation, cell-affinity peptides and glycopolymers are introduced into block copolymer brushes. This enhances the affinity between copolymers and target cells. For effective MSC separation, cell separation columns have been developed using silica beads modified with thermoresponsive cationic block copolymer brush. A temperature-modulated viral vector purification column has also been developed using a mixed polymer brush composed of PAMPS and PNIPAAm. Additionally, functional microbeads incorporating block copolymer brushes and affinity peptides allow for temperature-modulated exosome capture and release. These innovative bioseparation methods hold significant potential for biopharmaceutical production and separation.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant No. JP21 KK0199, JP22 K19899, and JP24 K01181), the Precise Measurement Technology Promotion Foundation (PMTP-F), the CASIO Science Promotion Foundation, and the Mukai Science and Technology Foundation.
Funding
Open Access funding provided by Hiroshima University.
Data availability
Data are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
There are no competing interests to declare.
Contributor Information
Kenichi Nagase, Email: nagase@hiroshima-u.ac.jp.
Hideko Kanazawa, Email: hkanazawa@keio.jp.
References
- 1.M. Heskins, J.E. Guillet, Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. A 2, 1441–1455 (1968). 10.1080/10601326808051910 [Google Scholar]
- 2.E.S. Gil, S.M. Hudson, Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 29, 1173–1222 (2004). 10.1016/j.progpolymsci.2004.08.003 [Google Scholar]
- 3.A. Halperin, M. Kröger, F.M. Winnik, Poly(N-isopropylacrylamide) phase diagrams: fifty years of research. Angew. Chem. Int. Ed. 54, 15342–15367 (2015). 10.1002/anie.201506663 [DOI] [PubMed] [Google Scholar]
- 4.K. Nagase, T. Onuma, M. Yamato, N. Takeda, T. Okano, Enhanced wettability changes by synergistic effect of micro/nanoimprinted substrates and grafted thermoresponsive polymer brushes. Macromol. Rapid Commun. 36, 1965–1970 (2015). 10.1002/marc.201500393 [DOI] [PubMed] [Google Scholar]
- 5.K. Nagase, J. Matsuda, A. Takeuchi, Y. Ikemoto, Hydration and dehydration behaviors of poly(N-isopropylacrylamide)-grafted silica beads. Surf. Interf. 40, 103058 (2023). 10.1016/j.surfin.2023.103058 [Google Scholar]
- 6.K. Nagase, K. Yamaoka, R. Shimane, N. Kojima, N.L. Yamada, H. Seto, Y. Fujii, Temperature-dependent behavior of poly(N-isopropylacrylamide) brushes via neutron reflectometry. Surf. Interf. 54, 105268 (2024). 10.1016/j.surfin.2024.105268 [Google Scholar]
- 7.J. Kobayashi, N. Masamichi, K. Nagase, Molecular design of dynamically thermoresponsive biomaterials. Sci. Technol. Adv. Mater. 26, 2475736 (2025). 10.1080/14686996.2025.2475736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.S. Cammas, K. Suzuki, C. Sone, Y. Sakurai, K. Kataoka, T. Okano, Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 48, 157–164 (1997). 10.1016/S0168-3659(97)00040-0 [Google Scholar]
- 9.M. Nakayama, T. Okano, Multi-targeting cancer chemotherapy using temperature-responsive drug carrier systems. React. Funct. Polym. 71, 235–244 (2011). 10.1016/j.reactfunctpolym.2010.08.006 [Google Scholar]
- 10.J. Akimoto, M. Nakayama, T. Okano, Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J. Control. Release 193, 2–8 (2014). 10.1016/j.jconrel.2014.06.062 [DOI] [PubMed] [Google Scholar]
- 11.M. Nakayama, J. Akimoto, T. Okano, Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting. J. Drug Target. 22, 584–599 (2014). 10.3109/1061186X.2014.936872 [DOI] [PubMed] [Google Scholar]
- 12.J. Wang, E. Ayano, Y. Maitani, H. Kanazawa, Enhanced cellular uptake and gene silencing activity of siRNA using temperature-responsive polymer-modified liposome. Int. J. Pharm. 523, 217–228 (2017). 10.1016/j.ijpharm.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 13.K. Nagase, M. Hasegawa, E. Ayano, Y. Maitani, H. Kanazawa, Effect of polymer phase transition behavior on temperature-responsive polymer-modified liposomes for siRNA transfection. Int. J. Mol. Sci. 20, 430 (2019). 10.3390/ijms20020430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.M. Maekawa-Matsuura, K. Fujieda, Y. Maekawa, T. Nishimura, K. Nagase, H. Kanazawa, LAT1-targeting thermoresponsive liposomes for effective cellular uptake by cancer cells. ACS Omega 4, 6443–6451 (2019). 10.1021/acsomega.9b00216 [Google Scholar]
- 15.R. Nemoto, K. Fujieda, Y. Hiruta, M. Hishida, E. Ayano, Y. Maitani, K. Nagase, H. Kanazawa, Liposomes with temperature-responsive reversible surface properties. Colloids Surf. B 176, 309–316 (2019). 10.1016/j.colsurfb.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 16.T. Mori, M. Maeda, Temperature-responsive formation of colloidal nanoparticles from poly(N-isopropylacrylamide) grafted with single-stranded DNA. Langmuir 20, 313–319 (2004). 10.1021/la0356194 [DOI] [PubMed] [Google Scholar]
- 17.D. Miyamoto, Z. Tang, T. Takarada, M. Maeda, Turbidimetric detection of ATP using polymeric micelles and DNA aptamers. Chem. Commun. (2007). 10.1039/B709775A [DOI] [PubMed] [Google Scholar]
- 18.Z. Tang, T. Takarada, M. Maeda, Non-cross-linking aggregation of DNA-carrying polymer micelles triggered by duplex formation. Langmuir 34, 14899–14910 (2018). 10.1021/acs.langmuir.8b01840 [DOI] [PubMed] [Google Scholar]
- 19.N. Uehara, Y. Numanami, T. Oba, N. Onishi, X. Xie, Thermal-induced immuno-nephelometry using gold nanoparticles conjugated with a thermoresponsive polymer for the detection of avidin. Anal. Sci. 31, 495–501 (2015). 10.2116/analsci.31.495 [DOI] [PubMed] [Google Scholar]
- 20.H. Kobayashi, M. Nishikawa, C. Sakamoto, T. Nishio, H. Kanazawa, T. Okano, Dual temperature- and pH-responsive fluorescence molecular probe for cellular imaging utilizing a PNIPAAm-fluorescein copolymer. Anal. Sci. 25, 1043–1047 (2009). 10.2116/analsci.25.1043 [DOI] [PubMed] [Google Scholar]
- 21.Y. Hiruta, M. Shimamura, M. Matsuura, Y. Maekawa, T. Funatsu, Y. Suzuki, E. Ayano, T. Okano, H. Kanazawa, Temperature-responsive fluorescence polymer probes with accurate thermally controlled cellular uptakes. ACS Macro Lett. 3, 281–285 (2014). 10.1021/mz5000569 [DOI] [PubMed] [Google Scholar]
- 22.M. Matsuura, M. Ohshima, Y. Hiruta, T. Nishimura, K. Nagase, H. Kanazawa, LAT1-targeting thermoresponsive fluorescent polymer probes for cancer cell imaging. Int. J. Mol. Sci. 19, 1646 (2018). 10.3390/ijms19061646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Y. Hiruta, Poly(N-isopropylacrylamide)-based temperature- and pH-responsive polymer materials for application in biomedical fields. Polym. J. 54, 1419–1430 (2022). 10.1038/s41428-022-00687-z [Google Scholar]
- 24.R. Yoshida, Creation of softmaterials based on self-oscillating polymer gels. Polym. J. 54, 827–849 (2022). 10.1038/s41428-022-00638-8 [Google Scholar]
- 25.R. Tamate, A. Mizutani Akimoto, R. Yoshida, Recent advances in self-oscillating polymer material systems. Chem. Record 16, 1852–1867 (2016). 10.1002/tcr.201600009 [DOI] [PubMed] [Google Scholar]
- 26.T. Masuda, A.M. Akimoto, K. Nagase, T. Okano, R. Yoshida, Design of self-oscillating polymer brushes and control of the dynamic behaviors. Chem. Mater. 27, 7395–7402 (2015). 10.1021/acs.chemmater.5b03228 [Google Scholar]
- 27.K. Homma, T. Masuda, A.M. Akimoto, K. Nagase, K. Itoga, T. Okano, R. Yoshida, Fabrication of micropatterned self-oscillating polymer brush for direction control of chemical waves. Small 13, 1700041 (2017). 10.1002/smll.201700041 [DOI] [PubMed] [Google Scholar]
- 28.T. Masuda, A.M. Akimoto, M. Furusawa, R. Tamate, K. Nagase, T. Okano, R. Yoshida, Aspects of the belousov-zhabotinsky reaction inside a self-oscillating polymer brush. Langmuir 34, 1673–1680 (2018). 10.1021/acs.langmuir.7b03929 [DOI] [PubMed] [Google Scholar]
- 29.K. Homma, T. Masuda, A.M. Akimoto, K. Nagase, T. Okano, R. Yoshida, Stable and prolonged autonomous oscillation in a self-oscillating polymer brush prepared on a porous glass substrate. Langmuir 35, 9794–9801 (2019). 10.1021/acs.langmuir.9b00928 [DOI] [PubMed] [Google Scholar]
- 30.K. Homma, Y. Ohta, K. Minami, G. Yoshikawa, K. Nagase, A.M. Akimoto, R. Yoshida, Autonomous nanoscale chemomechanical oscillation on the self-oscillating polymer brush surface by precise control of graft density. Langmuir 37, 4380–4386 (2021). 10.1021/acs.langmuir.1c00459 [DOI] [PubMed] [Google Scholar]
- 31.N.S. Terefe, O. Glagovskaia, K. De Silva, R. Stockmann, Application of stimuli responsive polymers for sustainable ion exchange chromatography. Food Bioprod. Process. 92, 208–225 (2014). 10.1016/j.fbp.2014.02.003 [Google Scholar]
- 32.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermally-modulated on/off-adsorption materials for pharmaceutical protein purification. Biomaterials 32, 619–627 (2011). 10.1016/j.biomaterials.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 33.K. Nagase, S.F. Yuk, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermo-responsive protein adsorbing materials for purifying pharmaceutical protein on exposed charging surface. J. Mater. Chem. 21, 2590–2593 (2011). 10.1039/C0JM03453C [Google Scholar]
- 34.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive anionic block copolymer brushes with a strongly anionic bottom segment for effective interactions with biomolecules. RSC Adv. 6, 93169–93179 (2016). 10.1039/C6RA20944K [Google Scholar]
- 35.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Protein separations via thermally responsive ionic block copolymer brush layers. RSC Adv. 6, 26254–26263 (2016). 10.1039/C6RA01061J [Google Scholar]
- 36.K. Okubo, K. Ikeda, A. Oaku, Y. Hiruta, K. Nagase, H. Kanazawa, Protein purification using solid-phase extraction on temperature-responsive hydrogel-modified silica beads. J. Chromatogr. A 1568, 38–48 (2018). 10.1016/j.chroma.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 37.K. Nagase, S. Ishii, K. Ikeda, S. Yamada, D. Ichikawa, A. Akimoto, Y. Hattori, H. Kanazawa, Antibody drug separation using thermoresponsive anionic polymer brush modified beads with optimised electrostatic and hydrophobic interactions. Sci. Rep. 10, 11896 (2020). 10.1038/s41598-020-68707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.K. Nagase, S. Ishii, A. Takeuchi, H. Kanazawa, Temperature-modulated antibody drug separation using thermoresponsive mixed polymer brush-modified stationary phase. Sep. Purif. Technol. 299, 121750 (2022). 10.1016/j.seppur.2022.121750 [Google Scholar]
- 39.A. Mizutani, K. Nagase, A. Kikuchi, H. Kanazawa, Y. Akiyama, J. Kobayashi, M. Annaka, T. Okano, Effective separation of peptides using highly dense thermo-responsive polymer brush-grafted porous polystyrene beads. J. Chromatogr. B 878, 2191–2198 (2010). 10.1016/j.jchromb.2010.06.026 [DOI] [PubMed] [Google Scholar]
- 40.D. Nomoto, K. Nagase, Y. Nakamura, H. Kanazawa, D. Citterio, Y. Hiruta, Anion species-triggered antibody separation system utilizing a thermo-responsive polymer column under optimized constant temperature. Colloids Surf. B 205, 111890 (2021). 10.1016/j.colsurfb.2021.111890 [DOI] [PubMed] [Google Scholar]
- 41.Y. Maekawa, K. Yamazaki, M. Ihara, K. Nagase, H. Kanazawa, Simultaneous analysis of multiple oligonucleotides by temperature-responsive chromatography using a poly(N-isopropylacrylamide)-based stationary phase. Anal. Bioanal. Chem. 412, 5341–5351 (2020). 10.1007/s00216-020-02749-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.K. Nagase, A. Kimura, T. Shimizu, K. Matsuura, M. Yamato, N. Takeda, T. Okano, Dynamically cell separating thermo-functional biointerfaces with densely packed polymer brushes. J. Mater. Chem. 22, 19514–19522 (2012). 10.1039/C2JM31797D [Google Scholar]
- 43.K. Nagase, Y. Hatakeyama, T. Shimizu, K. Matsuura, M. Yamato, N. Takeda, T. Okano, Hydrophobized thermoresponsive copolymer brushes for cell separation by multistep temperature change. Biomacromol 14, 3423–3433 (2013). 10.1021/bm4006722 [DOI] [PubMed] [Google Scholar]
- 44.K. Nagase, Y. Hatakeyama, T. Shimizu, K. Matsuura, M. Yamato, N. Takeda, T. Okano, Thermoresponsive cationic copolymer brushes for mesenchymal stem cell separation. Biomacromol 16, 532–540 (2015). 10.1021/bm501591s [DOI] [PubMed] [Google Scholar]
- 45.K. Nagase, Y. Sakurada, S. Onizuka, T. Iwata, M. Yamato, N. Takeda, T. Okano, Thermoresponsive polymer-modified microfibers for cell separations. Acta Biomater. 53, 81–92 (2017). 10.1016/j.actbio.2017.02.033 [DOI] [PubMed] [Google Scholar]
- 46.K. Nagase, R. Shukuwa, T. Onuma, M. Yamato, N. Takeda, T. Okano, Micro/nano-imprinted substrates grafted with a thermoresponsive polymer for thermally modulated cell separation. J. Mater. Chem. B 5, 5924–5930 (2017). 10.1039/C7TB01251A [DOI] [PubMed] [Google Scholar]
- 47.T.-C. Sung, H.C. Su, Q.-D. Ling, S.S. Kumar, Y. Chang, S.-T. Hsu, A. Higuchi, Efficient differentiation of human pluripotent stem cells into cardiomyocytes on cell sorting thermoresponsive surface. Biomaterials 253, 120060 (2020). 10.1016/j.biomaterials.2020.120060 [DOI] [PubMed] [Google Scholar]
- 48.K. Nagase, R. Shukuwa, H. Takahashi, N. Takeda, T. Okano, Enhanced mechanical properties and cell separation with thermal control of PIPAAm-brushed polymer-blend microfibers. J. Mater. Chem. B 8, 6017–6026 (2020). 10.1039/D0TB00972E [DOI] [PubMed] [Google Scholar]
- 49.N. Yamada, T. Okano, H. Sakai, F. Karikusa, Y. Sawasaki, Y. Sakurai, Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells, Makromol. Chem. Rapid Commun. 11, 571–576 (1990). 10.1002/marc.1990.030111109 [Google Scholar]
- 50.T. Okano, N. Yamada, M. Okuhara, H. Sakai, Y. Sakurai, Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 16, 297–303 (1995). 10.1016/0142-9612(95)93257-e [DOI] [PubMed] [Google Scholar]
- 51.M. Ebara, M. Yamato, T. Aoyagi, A. Kikuchi, K. Sakai, T. Okano, Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest. Tissue Eng. 10, 1125–1135 (2004). 10.1089/ten.2004.10.1125 [DOI] [PubMed] [Google Scholar]
- 52.Y. Akiyama, A. Kikuchi, M. Yamato, T. Okano, Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 20, 5506–5511 (2004). 10.1021/la036139f [DOI] [PubMed] [Google Scholar]
- 53.A. Mizutani, A. Kikuchi, M. Yamato, H. Kanazawa, T. Okano, Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 29, 2073–2081 (2008). 10.1016/j.biomaterials.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 54.K. Nagase, M. Watanabe, A. Kikuchi, M. Yamato, T. Okano, Thermo-responsive polymer brushes as intelligent biointerfaces: preparation via ATRP and characterization. Macromol. Biosci. 11, 400–409 (2011). 10.1002/mabi.201000312 [DOI] [PubMed] [Google Scholar]
- 55.H. Takahashi, M. Nakayama, M. Yamato, T. Okano, Controlled chain length and graft density of thermoresponsive polymer brushes for optimizing cell sheet harvest. Biomacromol 11, 1991–1999 (2010). 10.1021/bm100342e [DOI] [PubMed] [Google Scholar]
- 56.Y. Arisaka, J. Kobayashi, M. Yamato, Y. Akiyama, T. Okano, Switching of cell growth/detachment on heparin-functionalized thermoresponsive surface for rapid cell sheet fabrication and manipulation. Biomaterials 34, 4214–4222 (2013). 10.1016/j.biomaterials.2013.02.056 [DOI] [PubMed] [Google Scholar]
- 57.A.M. Akimoto, E. Hasuike, H. Tada, K. Nagase, T. Okano, H. Kanazawa, R. Yoshida, Design of Tetra-arm PEG-crosslinked Thermoresponsive Hydrogel for 3D Cell Culture, Anal. Sci., 32 pp.1203–1205, (2016) http://www.jsac.or.jp/analsci/abst.php/32/11/1203/ [DOI] [PubMed]
- 58.V. Capella, R.E. Rivero, A.C. Liaudat, L.E. Ibarra, D.A. Roma, F. Alustiza, F. Mañas, C.A. Barbero, P. Bosch, C.R. Rivarola, N. Rodriguez, Cytotoxicity and bioadhesive properties of poly-N-isopropylacrylamide hydrogel. Heliyon 5, e01474 (2019). 10.1016/j.heliyon.2019.e01474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A. Akimoto, E. Niitsu, K. Nagase, T. Okano, H. Kanazawa, R. Yoshida, Mesenchylmal Stem Cell Culture on Poly(N-isopropylacrylamide) Hydrogel with Repeated Thermo-Stimulation, Int J Mol Sci, 19 pp.1253, (2018) http://www.mdpi.com/1422-0067/19/4/1253 [DOI] [PMC free article] [PubMed]
- 60.M. Nakao, K. Kim, K. Nagase, D.W. Grainger, H. Kanazawa, T. Okano, Phenotypic traits of mesenchymal stem cell sheets fabricated by temperature-responsive cell culture plate: structural characteristics of MSC sheets. Stem Cell Res. Ther. 10, 353 (2019). 10.1186/s13287-019-1431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.M. Nakao, D. Inanaga, K. Nagase, H. Kanazawa, Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins, Regenerative. Therapy 11, 34–40 (2019). 10.1016/j.reth.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.M. Nakao, M. Matsui, K. Kim, N. Nishiyama, D.W. Grainger, T. Okano, H. Kanazawa, K. Nagase, Umbilical cord-derived mesenchymal stem cell sheets transplanted subcutaneously enhance cell retention and survival more than dissociated stem cell injections. Stem Cell Res. Ther. 14, 352 (2023). 10.1186/s13287-023-03593-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M. Nakayama, Y. Toyoshima, A. Kikuchi, T. Okano, Micropatterned smart culture surfaces via multi-step physical coating of functional block copolymers for harvesting cell sheets with controlled sizes and shapes. Macromol. Biosci. 21, 2000330 (2021). 10.1002/mabi.202000330 [DOI] [PubMed] [Google Scholar]
- 64.K. Nagase, M. Nagaoka, Y. Nakano, R. Utoh, bFGF-releasing biodegradable nanoparticles for effectively engrafting transplanted hepatocyte sheet. J. Control. Release 366, 160–169 (2024). 10.1016/j.jconrel.2023.12.040 [DOI] [PubMed] [Google Scholar]
- 65.M. Nakao, K. Nagase, Harvesting methods of umbilical cord-derived mesenchymal stem cells from culture modulate cell properties and functions, regenerative. Therapy 26, 80–88 (2024). 10.1016/j.reth.2024.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.K. Nagase, H. Kuramochi, D.W. Grainger, H. Takahashi, Functional aligned mesenchymal stem cell sheets fabricated using micropatterned thermo-responsive cell culture surfaces, Materials Today Bio, 32 pp.101657, (2025) https://www.sciencedirect.com/science/article/pii/S2590006425002157 [DOI] [PMC free article] [PubMed]
- 67.K. Nagase, M. Nagaoka, J. Matsuda, N. Kojima, Thermoresponsive block-copolymer brush-modified interfaces for effective fabrication of hepatocyte sheets. Mater. Des. 239, 112824 (2024). 10.1016/j.matdes.2024.112824 [Google Scholar]
- 68.H. Kanazawa, K. Yamamoto, Y. Matsushima, N. Takai, A. Kikuchi, Y. Sakurai, T. Okano, Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silica. Anal. Chem. 68, 100–105 (1996). 10.1021/ac950359j [DOI] [PubMed] [Google Scholar]
- 69.T. Yakushiji, K. Sakai, A. Kikuchi, T. Aoyagi, Y. Sakurai, T. Okano, Effects of cross-linked structure on temperature-responsive hydrophobic interaction of poly(N-isopropylacrylamide) hydrogel-modified surfaces with steroids. Anal. Chem. 71, 1125–1130 (1999). 10.1021/ac980677t [Google Scholar]
- 70.K. Nagase, A. Mizutani Akimoto, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Effect of reaction solvent on the preparation of thermo-responsive stationary phase through a surface initiated atom transfer radical polymerization. J. Chromatogr. A 1218, 8617–8628 (2011). 10.1016/j.chroma.2011.09.082 [DOI] [PubMed] [Google Scholar]
- 71.K. Nagase, H. Kanazawa, Temperature-responsive chromatography for bioseparations: a review. Anal. Chim. Acta 1138, 191–212 (2020). 10.1016/j.aca.2020.07.075 [DOI] [PubMed] [Google Scholar]
- 72.A. Mizutani, K. Nagase, A. Kikuchi, H. Kanazawa, Y. Akiyama, J. Kobayashi, M. Annaka, T. Okano, Preparation of thermo-responsive polymer brushes on hydrophilic polymeric beads by surface-initiated atom transfer radical polymerization for a highly resolutive separation of peptides. J. Chromatogr. A 1217, 5978–5985 (2010). 10.1016/j.chroma.2010.07.067 [DOI] [PubMed] [Google Scholar]
- 73.Y. Maekawa, N. Okamoto, Y. Okada, K. Nagase, H. Kanazawa, Green analytical method for the simultaneous analysis of cytochrome P450 probe substrates by poly(N-isopropylacrylamide)-based temperature-responsive chromatography. Sci. Rep. 10, 8828 (2020). 10.1038/s41598-020-65270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive polymer brush on monolithic-silica-rod for the high-speed separation of bioactive compounds. Langmuir 27, 10830–10839 (2011). 10.1021/la201360p [DOI] [PubMed] [Google Scholar]
- 75.H. Kanazawa, Y. Kashiwase, K. Yamamoto, Y. Matsushima, A. Kikuchi, Y. Sakurai, T. Okano, Temperature-responsive liquid chromatography. 2. Effects of hydrophobic groups in N-Isopropylacrylamide copolymer-modified silica. Anal. Chem. 69, 823–830 (1997). 10.1021/ac961024k [DOI] [PubMed] [Google Scholar]
- 76.H. Kanazawa, T. Sunamoto, Y. Matsushima, A. Kikuchi, T. Okano, Temperature-responsive chromatographic separation of amino acid phenylthiohydantoins using aqueous media as the mobile phase. Anal. Chem. 72, 5961–5966 (2000). 10.1021/ac0004658 [DOI] [PubMed] [Google Scholar]
- 77.K. Nagase, M. Kumazaki, H. Kanazawa, J. Kobayashi, A. Kikuchi, Y. Akiyama, M. Annaka, T. Okano, Thermoresponsive polymer brush surfaces with hydrophobic groups for all-aqueous chromatography. ACS Appl. Mater. Interf. 2, 1247–1253 (2010). 10.1021/am100122h [DOI] [PubMed] [Google Scholar]
- 78.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive hydrophobic copolymer brushes modified porous monolithic silica for high-resolution bioseparation. RSC Adv. 5, 66155–66167 (2015). 10.1039/C5RA11038F [Google Scholar]
- 79.K. Nagase, Y. Umemoto, H. Kanazawa, Effect of pore diameter on the elution behavior of analytes from thermoresponsive polymer grafted beads packed columns. Sci. Rep. 11, 9976 (2021). 10.1038/s41598-021-89165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Preparation of thermoresponsive cationic copolymer brush surfaces and application of the surface to separation of biomolecules. Biomacromol 9, 1340–1347 (2008). 10.1021/bm701427m [DOI] [PubMed] [Google Scholar]
- 81.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermally modulated cationic copolymer brush on monolithic silica rods for high-speed separation of acidic biomolecules. ACS Appl. Mater. Interf 5, 1442–1452 (2013). 10.1021/am302889j [DOI] [PubMed] [Google Scholar]
- 82.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, M. Annaka, T. Okano, Preparation of thermoresponsive anionic copolymer brush surfaces for separating basic biomolecules. Biomacromol 11, 215–223 (2010). 10.1021/bm9010744 [DOI] [PubMed] [Google Scholar]
- 83.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Monolithic silica rods grafted with thermoresponsive anionic polymer brushes for high-speed separation of basic biomolecules and peptides. Biomacromol 15, 1204–1215 (2014). 10.1021/bm401779r [DOI] [PubMed] [Google Scholar]
- 84.K. Nagase, M. Watanabe, F. Zen, H. Kanazawa, Temperature-responsive mixed-mode column containing temperature-responsive polymer-modified beads and anionic polymer-modified beads. Anal. Chim. Acta 1079, 220–229 (2019). 10.1016/j.aca.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 85.K. Nagase, K. Matsumoto, H. Kanazawa, Temperature-responsive mixed-mode column for the modulation of multiple interactions. Sci. Rep. 12, 4434 (2022). 10.1038/s41598-022-08475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.K. Nagase, Bioanalytical technologies using temperature-responsive polymers. Anal. Sci. 40, 827–841 (2024). 10.1007/s44211-024-00545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.K. Nagase, T. Nishiyama, M. Inoue, H. Kanazawa, Temperature responsive chromatography for therapeutic drug monitoring with an aqueous mobile phase. Sci. Rep. 11, 23508 (2021). 10.1038/s41598-021-02998-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.K. Nagase, S. Inoue, M. Inoue, H. Kanazawa, Two-dimensional temperature-responsive chromatography using a poly(N-isopropylacrylamide) brush-modified stationary phase for effective therapeutic drug monitoring. Sci. Rep. 12, 2653 (2022). 10.1038/s41598-022-06638-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.K. Nagase, H. Takagi, H. Nakada, H. Ishikawa, Y. Nagata, T. Aomori, H. Kanazawa, Chromatography columns packed with thermoresponsive-cationic-polymer-modified beads for therapeutic drug monitoring. Sci. Rep. 12, 12847 (2022). 10.1038/s41598-022-16928-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.R. Langer, J. Vacanti, Tissue engineering. Science 260, 920–926 (1993). 10.1126/science.8493529 [DOI] [PubMed] [Google Scholar]
- 91.S.V. Murphy, A. Atala, 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773 (2014). 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 92.P. Menasché, A.A. Hagège, M. Scorsin, B. Pouzet, M. Desnos, D. Duboc, K. Schwartz, J.-T. Vilquin, J.-P. Marolleau, Myoblast transplantation for heart failure. The Lancet 357, 279–280 (2001). 10.1016/S0140-6736(00)03617-5 [DOI] [PubMed] [Google Scholar]
- 93.T. Shin’oka, Y. Imai, Y. Ikada, Transplantation of a tissue-engineered pulmonary artery. N. Engl. J. Med. 344, 532–533 (2001). 10.1056/NEJM200102153440717 [DOI] [PubMed] [Google Scholar]
- 94.K. Nishida, M. Yamato, Y. Hayashida, K. Watanabe, K. Yamamoto, E. Adachi, S. Nagai, A. Kikuchi, N. Maeda, H. Watanabe, T. Okano, Y. Tano, Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196 (2004). 10.1056/NEJMoa040455 [DOI] [PubMed] [Google Scholar]
- 95.Y. Sawa, S. Miyagawa, T. Sakaguchi, T. Fujita, A. Matsuyama, A. Saito, T. Shimizu, T. Okano, Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg. Today 42, 181–184 (2012). 10.1007/s00595-011-0106-4 [DOI] [PubMed] [Google Scholar]
- 96.T. Ohki, M. Yamato, M. Ota, R. Takagi, D. Murakami, M. Kondo, R. Sasaki, H. Namiki, T. Okano, M. Yamamoto, Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 143, 582-588.e2 (2012). 10.1053/j.gastro.2012.04.050 [DOI] [PubMed] [Google Scholar]
- 97.K. Nagase, Y. Nagumo, M. Kim, H.-J. Kim, H.-W. Kyung, H.-J. Chung, H. Sekine, T. Shimizu, H. Kanazawa, T. Okano, S.-J. Lee, M. Yamato, Local release of VEGF using fiber mats enables effective transplantation of layered cardiomyocyte sheets. Macromol. Biosci. 17, 1700073 (2017). 10.1002/mabi.201700073 [DOI] [PubMed] [Google Scholar]
- 98.K. Yamamoto, M. Yamato, T. Morino, H. Sugiyama, R. Takagi, Y. Yaguchi, T. Okano, H. Kojima, Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. npj Regen. Med. 2, 6 (2017). 10.1038/s41536-017-0010-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.M. Kanzaki, R. Takagi, K. Washio, M. Kokubo, M. Yamato, Bio-artificial pleura using an autologous dermal fibroblast sheet. npj Regen. Med. 2, 26 (2017). 10.1038/s41536-017-0031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.T. Iwata, M. Yamato, K. Washio, T. Yoshida, Y. Tsumanuma, A. Yamada, S. Onizuka, Y. Izumi, T. Ando, T. Okano, I. Ishikawa, Periodontal regeneration with autologous periodontal ligament-derived cell sheets—a safety and efficacy study in ten patients, Regenerative. Therapy 9, 38–44 (2018). 10.1016/j.reth.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.M. Sato, M. Yamato, G. Mitani, T. Takagaki, K. Hamahashi, Y. Nakamura, M. Ishihara, R. Matoba, H. Kobayashi, T. Okano, J. Mochida, M. Watanabe, Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. npj Regen. Med. 4, 4 (2019). 10.1038/s41536-019-0069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.L.A. Herzenberg, L.A. Herzenberg, Analysis and separation using fluoresence activated cell sorter (FACS), In: D.M. Weir (Ed.), Handbook of Experimental Immunology, Blackwell Scientific Publication, Oxford, pp. 22.1–22.21 (1978)
- 103.S. Miltenyi, W. Müller, W. Weichel, A. Radbruch, High gradient magnetic cell separation with MACS. Cytometry 11, 231–238 (1990). 10.1002/cyto.990110203 [DOI] [PubMed] [Google Scholar]
- 104.J.C. Giddings, N. Barman Bhajendra, M.-K. Liu, Separation of Cells by Field-Flow Fractionation, in Cell Separation Science and Technology. (American Chemical Society Washington, 1991), pp.128–144 [Google Scholar]
- 105.R.K. Kumar, A.W.J. Lykke, Cell separation: a review. Pathology 16, 53–62 (1984). 10.3109/00313028409067911 [DOI] [PubMed] [Google Scholar]
- 106.K. Kataoka, Y. Sakurai, T. Hanai, A. Maruyama, T. Tsuruta, Immunoaffinity chromatography of lymphocyte subpopulations using tert-amine derived matrices with adsorbed antibodies. Biomaterials 9, 218–224 (1988). 10.1016/0142-9612(88)90087-7 [DOI] [PubMed] [Google Scholar]
- 107.M. Kamihira, A. Kumar, Development of Separation Technique for Stem Cells, in Adv Biochem Engin/Biotechnol. ed. by A. Kumar, I. Galaev, B. Mattiasson (Springer, Berlin / Heidelberg, 2007), pp.173–193 [DOI] [PubMed] [Google Scholar]
- 108.A. Mahara, T. Yamaoka, Continuous separation of cells of high osteoblastic differentiation potential from mesenchymal stem cells on an antibody-immobilized column. Biomaterials 31, 4231–4237 (2010). 10.1016/j.biomaterials.2010.01.126 [DOI] [PubMed] [Google Scholar]
- 109.M. Yamada, W. Seko, T. Yanai, K. Ninomiya, M. Seki, Slanted, asymmetric microfluidic lattices as size-selective sieves for continuous particle/cell sorting. Lab Chip 17, 304–314 (2017). 10.1039/C6LC01237J [DOI] [PubMed] [Google Scholar]
- 110.A. Otaka, K. Kitagawa, T. Nakaoki, M. Hirata, K. Fukazawa, K. Ishihara, A. Mahara, T. Yamaoka, Label-free separation of induced pluripotent stem cells with anti-SSEA-1 antibody immobilized microfluidic channel. Langmuir 33, 1576–1582 (2017). 10.1021/acs.langmuir.6b04070 [DOI] [PubMed] [Google Scholar]
- 111.A. Moldavan, Photo-electric technique for the counting of microscopical cells. Science 80, 188–189 (1934). 10.1126/science.80.2069.188 [DOI] [PubMed] [Google Scholar]
- 112.A. Kumar, A. Srivastava, Cell separation using cryogel-based affinity chromatography. Nat. Protocols 5, 1737–1747 (2010). 10.1038/nprot.2010.135 [DOI] [PubMed] [Google Scholar]
- 113.T. Chianéa, N.E. Assidjo, P.J.P. Cardot, Sedimentation field-flow-fractionation: emergence of a new cell separation methodology. Talanta 51, 835–847 (2000). 10.1016/S0039-9140(99)00335-5 [DOI] [PubMed] [Google Scholar]
- 114.L.A. Herzenberg, D. Parks, B. Sahaf, O. Perez, M. Roederer, L.A. Herzenberg, The history and future of the fluorescence activated cell sorter and flow cytometry: a view from stanford. Clin. Chem. 48, 1819–1827 (2002). 10.1093/clinchem/48.10.1819 [PubMed] [Google Scholar]
- 115.A. Otaka, A. Mahara, K. Ishihara, T. Yamaoka, Adhesion of Flk1-expressing cells under shear flow in phospholipid polymer-coated immunoaffinity channels. J. Micromech. Microeng. 31, 045012 (2021). 10.1088/1361-6439/abe52a [Google Scholar]
- 116.T. Okano, N. Yamada, H. Sakai, Y. Sakurai, A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 27, 1243–1251 (1993). 10.1002/jbm.820271005 [DOI] [PubMed] [Google Scholar]
- 117.K. Nagase, M. Watanabe, A. Kikuchi, T. Okano, Effective cell sheet preparation using thermoresponsive polymer brushes with various graft densities and chain lengths. Biomater. Sci. 13, 1657–1670 (2025). 10.1039/D4BM01705F [DOI] [PubMed] [Google Scholar]
- 118.K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive anionic copolymer brushes containing strong acid moieties for effective separation of basic biomolecules and proteins. Biomacromol 15, 3846–3858 (2014). 10.1021/bm5012163 [DOI] [PubMed] [Google Scholar]
- 119.K. Nagase, M. Geven, S. Kimura, J. Kobayashi, A. Kikuchi, Y. Akiyama, D.W. Grijpma, H. Kanazawa, T. Okano, Thermoresponsive copolymer brushes possessing quaternary amine groups for strong anion-exchange chromatographic matrices. Biomacromol 15, 1031–1043 (2014). 10.1021/bm401918a [DOI] [PubMed] [Google Scholar]
- 120.K. Nagase, A. Ota, T. Hirotani, S. Yamada, A.M. Akimoto, H. Kanazawa, Thermoresponsive cationic block copolymer brushes for temperature-modulated stem cell separation. Macromol. Rapid Commun. 41, 2000308 (2020). 10.1002/marc.202000308 [DOI] [PubMed] [Google Scholar]
- 121.K. Matyjaszewski, W. Jakubowski, K. Min, W. Tang, J. Huang, W.A. Braunecker, N.V. Tsarevsky, Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents, Proc. Natl. Acad. Sci. USA, 103 pp.15309, (2006) http://www.pnas.org/content/103/42/15309.abstract [DOI] [PMC free article] [PubMed]
- 122.W. Jakubowski, K. Matyjaszewski, Activators regenerated by electron transfer for atom-transfer radical polymerization of (Meth)acrylates and related block copolymers. Angew. Chem. Int. Ed. 45, 4482–4486 (2006). 10.1002/anie.200600272 [DOI] [PubMed] [Google Scholar]
- 123.T. Hirotani, K. Nagase, Temperature-modulated separation of vascular cells using thermoresponsive-anionic block copolymer-modified glass, Regenerative. Therapy 27, 259–267 (2024). 10.1016/j.reth.2024.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.H. Takahashi, N. Matsuzaka, M. Nakayama, A. Kikuchi, M. Yamato, T. Okano, Terminally functionalized thermoresponsive polymer brushes for simultaneously promoting cell adhesion and cell sheet harvest. Biomacromol 13, 253–260 (2012). 10.1021/bm201545u [DOI] [PubMed] [Google Scholar]
- 125.K. Nagase, S. Kitazawa, S. Yamada, A.M. Akimoto, H. Kanazawa, Mixed polymer brush as a functional ligand of silica beads for temperature-modulated hydrophobic and electrostatic interactions. Anal. Chim. Acta 1095, 1–13 (2020). 10.1016/j.aca.2019.10.058 [DOI] [PubMed] [Google Scholar]
- 126.K. Nagase, H. Wakayama, J. Matsuda, N. Kojima, H. Kanazawa, Thermoresponsive mixed polymer brush to effectively control the adhesion and separation of stem cells by altering temperature. Materials Today Bio 20, 100627 (2023). 10.1016/j.mtbio.2023.100627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.K.M. Yamada, S.K. Akiyama, T. Hasegawa, E. Hasegawa, M.J. Humphries, D.W. Kennedy, K. Nagata, H. Urushihara, K. Olden, W.-T. Chen, Recent advances in research on fibronectin and other cell attachment proteins. J. Cell. Biochem. 28, 79–97 (1985). 10.1002/jcb.240280202 [DOI] [PubMed] [Google Scholar]
- 128.M.J. Humphries, S.K. Akiyama, A. Komoriya, K. Olden, K.M. Yamada, Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J. Cell Biol. 103, 2637–2647 (1986). 10.1083/jcb.103.6.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.J.A. Hubbell, S.P. Massia, N.P. Desai, P.D. Drumheller, Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Nat Biotech 9, 568–572 (1991). 10.1038/nbt0691-568 [DOI] [PubMed] [Google Scholar]
- 130.S. Kakinoki, T. Yamaoka, Single-step immobilization of cell adhesive peptides on a variety of biomaterial substrates via tyrosine oxidation with copper catalyst and hydrogen peroxide. Bioconjug. Chem. 26, 639–644 (2015). 10.1021/acs.bioconjchem.5b00032 [DOI] [PubMed] [Google Scholar]
- 131.W. Wang, L. Guo, Y. Yu, Z. Chen, R. Zhou, Z. Yuan, Peptide REDV-modified polysaccharide hydrogel with endothelial cell selectivity for the promotion of angiogenesis. J. Biomed. Mater. Res. A 103, 1703–1712 (2015). 10.1002/jbm.a.35306 [DOI] [PubMed] [Google Scholar]
- 132.A. Mahara, S. Somekawa, N. Kobayashi, Y. Hirano, Y. Kimura, T. Fujisato, T. Yamaoka, Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials 58, 54–62 (2015). 10.1016/j.biomaterials.2015.04.031 [DOI] [PubMed] [Google Scholar]
- 133.M. Okochi, S. Nomura, C. Kaga, H. Honda, Peptide array-based screening of human mesenchymal stem cell-adhesive peptides derived from fibronectin type III domain, Biochemical and Biophysical Research Communications, 371 pp.85–89, (2008) http://www.sciencedirect.com/science/article/pii/S0006291X08006529 [DOI] [PubMed]
- 134.S. Kakinoki, K. Takasaki, A. Mahara, T. Ehashi, Y. Hirano, T. Yamaoka, Direct surface modification of metallic biomaterials via tyrosine oxidation aiming to accelerate the re-endothelialization of vascular stents. J. Biomed. Mater. Res. A 106, 491–499 (2018). 10.1002/jbm.a.36258 [DOI] [PubMed] [Google Scholar]
- 135.K. Nagase, M. Shimura, R. Shimane, K. Hanaya, S. Yamada, A.M. Akimoto, T. Sugai, H. Kanazawa, Selective capture and non-invasive release of cells using a thermoresponsive polymer brush with affinity peptides. Biomaterials Science 9, 663–674 (2021). 10.1039/D0BM01453B [DOI] [PubMed] [Google Scholar]
- 136.K. Nagase, R. Shimane, Ethylene glycol-based thermoresponsive block copolymer brushes with cell-affinity peptides for thermally controlled interaction with target cells. Mater. Des. 233, 112234 (2023). 10.1016/j.matdes.2023.112234 [Google Scholar]
- 137.H.C. Kolb, M.G. Finn, K.B. Sharpless, Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001). 10.1002/1521-3773(20010601)40:11%3c2004::AID-ANIE2004%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 138.M. Meldal, C.W. Tornøe, Cu-catalyzed azide−alkyne cycloaddition. Chem. Rev. 108, 2952–3015 (2008). 10.1021/cr0783479 [DOI] [PubMed] [Google Scholar]
- 139.L. Tang, Y. Yang, T. Bai, W. Liu, Robust MeO2MA/vinyl-4,6-diamino-1,3,5-triazine copolymer hydrogels-mediated reverse gene transfection and thermo-induced cell detachment. Biomaterials 32, 1943–1949 (2011). 10.1016/j.biomaterials.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 140.Y. Kotsuchibashi, R. Narain, Dual-temperature and pH responsive (ethylene glycol)-based nanogels via structural design. Polym. Chem. 5, 3061–3070 (2014). 10.1039/C3PY01772A [Google Scholar]
- 141.S. Desseaux, H.-A. Klok, Temperature-controlled masking/unmasking of cell-adhesive cues with poly(ethylene glycol) methacrylate based brushes. Biomacromol 15, 3859–3865 (2014). 10.1021/bm501233h [DOI] [PubMed] [Google Scholar]
- 142.S. Jiang, M. Müller, H. Schönherr, Propagation and purification of human induced pluripotent stem cells with selective homopolymer release surfaces. Angew. Chem. Int. Ed. 58, 10563–10566 (2019). 10.1002/anie.201903299 [DOI] [PubMed] [Google Scholar]
- 143.Y. Stetsyshyn, J. Raczkowska, K. Harhay, K. Gajos, Y. Melnyk, P. Dąbczyński, T. Shevtsova, A. Budkowski, Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: synthesis, properties, and applications. Colloid Polym. Sci. 299, 363–383 (2021). 10.1007/s00396-020-04750-0 [Google Scholar]
- 144.O. Lishchynskyi, Y. Stetsyshyn, J. Raczkowska, K. Awsiuk, B. Orzechowska, A. Abalymov, A.G. Skirtach, A. Bernasik, S. Nastyshyn, A. Budkowski, Fabrication and impact of fouling-reducing temperature-responsive POEGMA coatings with embedded CaCO3 nanoparticles on different cell lines. Materials (2021). 10.3390/ma14061417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Y. Shymborska, Y. Stetsyshyn, J. Raczkowska, K. Awsiuk, H. Ohar, A. Budkowski, Impact of the various buffer solutions on the temperature-responsive properties of POEGMA-grafted brush coatings. Colloid Polym. Sci. 300, 487–495 (2022). 10.1007/s00396-022-04959-1 [Google Scholar]
- 146.Y. Shymborska, Y. Stetsyshyn, K. Awsiuk, J. Raczkowska, A. Bernasik, N. Janiszewska, P. Da̧bczyński, A. Kostruba, A. Budkowski, Temperature- and pH-responsive schizophrenic copolymer brush coatings with enhanced temperature response in pure water. ACS Appl. Mater. Interf. 15, 8676–8690 (2023). 10.1021/acsami.2c20395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.K.M. Kulig, J.P. Vacanti, Hepatic tissue engineering. Transpl. Immunol. 12, 303–310 (2004). 10.1016/j.trim.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 148.Y. Nahmias, F. Berthiaume, M.L. Yarmush, Integration of Technologies for Hepatic Tissue Engineering, in Tissue Engineering II: Basics of Tissue Engineering and Tissue Applications. ed. by K. Lee, D. Kaplan (Springer, Berlin Heidelberg, Berlin, Heidelberg, 2007), pp.309–329 [DOI] [PubMed] [Google Scholar]
- 149.K. Matsuura, R. Utoh, K. Nagase, T. Okano, Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release 190, 228–239 (2014). 10.1016/j.jconrel.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 150.T. Takebe, K. Sekine, M. Enomura, H. Koike, M. Kimura, T. Ogaeri, R.-R. Zhang, Y. Ueno, Y.-W. Zheng, N. Koike, S. Aoyama, Y. Adachi, H. Taniguchi, Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013). 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- 151.K. Nagase, N. Kojima, M. Goto, T. Akaike, H. Kanazawa, Thermoresponsive block copolymer brush for temperature-modulated hepatocyte separation. J. Mater. Chem. B 10, 8629–8641 (2022). 10.1039/D2TB01384C [DOI] [PubMed] [Google Scholar]
- 152.A. Kobayashi, T. Akaike, K. Kobayashi, H. Sumitomo, Enhanced adhesion and survival efficiency of liver cells in culture dishes coated with a lactose-carrying styrene homopolymer. Makromol. Chem. Rapid Commun. 7, 645–650 (1986). 10.1002/marc.1986.030071005 [Google Scholar]
- 153.M. Goto, H. Yura, C.-W. Chang, A. Kobayashi, T. Shinoda, A. Maeda, S. Kojima, K. Kobayashi, T. Akaike, Lactose-carrying polystyrene as a drug carrier: investigation of body distributions to parenchymal liver cells using 125I-labelled lactose-carrying polystyrene. J. Control. Release 28, 223–233 (1994). 10.1016/0168-3659(94)90169-4 [Google Scholar]
- 154.S.-H. Kim, T. Hoshiba, T. Akaike, Hepatocyte behavior on synthetic glycopolymer matrix: inhibitory effect of receptor–ligand binding on hepatocyte spreading. Biomaterials 25, 1813–1823 (2004). 10.1016/j.biomaterials.2003.08.035 [DOI] [PubMed] [Google Scholar]
- 155.K. Nagase, N. Mukae, A. Kikuchi, T. Okano, Thermally modulated retention of lymphocytes on polymer-brush-grafted glass beads. Macromol. Biosci. 12, 333–340 (2012). 10.1002/mabi.201100283 [DOI] [PubMed] [Google Scholar]
- 156.K. Nagase, D. Inanaga, D. Ichikawa, A. Mizutani Akimoto, Y. Hattori, H. Kanazawa, Temperature-modulated cell-separation column using temperature-responsive cationic copolymer hydrogel-modified silica beads. Colloids Surf. B 178, 253–262 (2019). 10.1016/j.colsurfb.2019.02.057 [DOI] [PubMed] [Google Scholar]
- 157.K. Nagase, G. Edatsune, Y. Nagata, J. Matsuda, D. Ichikawa, S. Yamada, Y. Hattori, H. Kanazawa, Thermally-modulated cell separation columns using a thermoresponsive block copolymer brush as a packing material for the purification of mesenchymal stem cells. Biomater. Sci. 9, 7054–7064 (2021). 10.1039/D1BM00708D [DOI] [PubMed] [Google Scholar]
- 158.K. Nagase, A. Okada, J. Matsuda, D. Ichikawa, Y. Hattori, H. Kanazawa, A thermoresponsive cationic block copolymer brush-grafted silica bead interface for temperature-modulated separation of adipose-derived stem cells. Colloids Surf. B 220, 112928 (2022). 10.1016/j.colsurfb.2022.112928 [DOI] [PubMed] [Google Scholar]
- 159.P.D. Robbins, S.C. Ghivizzani, Viral vectors for gene therapy. Pharmacol. Ther. 80, 35–47 (1998). 10.1016/S0163-7258(98)00020-5 [PubMed] [Google Scholar]
- 160.K.-W. Peng, S.J. Russell, Viral vector targeting. Curr. Opin. Biotechnol. 10, 454–457 (1999). 10.1016/S0958-1669(99)00009-9 [DOI] [PubMed] [Google Scholar]
- 161.C.E. Thomas, A. Ehrhardt, M.A. Kay, Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4, 346–358 (2003). 10.1038/nrg1066 [DOI] [PubMed] [Google Scholar]
- 162.R. Morenweiser, Downstream processing of viral vectors and vaccines. Gene Ther. 12, S103–S110 (2005). 10.1038/sj.gt.3302624 [DOI] [PubMed] [Google Scholar]
- 163.C.S.M. Fernandes, B. Gonçalves, M. Sousa, D.L. Martins, T. Barroso, A.S. Pina, C. Peixoto, A.A. Ricardo, A.C.A. Roque, Bio-based monoliths for adenovirus purification. ACS Appl. Mater. Interf (2015). 10.1021/am508907b [DOI] [PubMed] [Google Scholar]
- 164.V. Orr, L. Zhong, M. Moo-Young, C.P. Chou, Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 31, 450–465 (2013). 10.1016/j.biotechadv.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 165.M. Potter, B. Lins, M. Mietzsch, R. Heilbronn, K. Van Vliet, P. Chipman, M. Agbandje-McKenna, B.D. Cleaver, N. Clément, B.J. Byrne, S. Zolotukhin, A simplified purification protocol for recombinant adeno-associated virus vectors. Mole. Ther. - Methods Clin. Develop. 1, 14034 (2014). 10.1038/mtm.2014.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.G. Gao, G. Qu, M.S. Burnham, J. Huang, N. Chirmule, B. Joshi, Q.-C. Yu, J.A. Marsh, C.M. Conceicao, J.M. Wilson, Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 11, 2079–2091 (2000). 10.1089/104303400750001390 [DOI] [PubMed] [Google Scholar]
- 167.T. Nasukawa, J. Uchiyama, S. Taharaguchi, S. Ota, T. Ujihara, S. Matsuzaki, H. Murakami, K. Mizukami, M. Sakaguchi, Virus purification by CsCl density gradient using general centrifugation. Adv. Virol. 162, 3523–3528 (2017). 10.1007/s00705-017-3513-z [DOI] [PubMed] [Google Scholar]
- 168.K. Nagase, S. Kitazawa, T. Kogure, S. Yamada, K. Katayama, H. Kanazawa, Viral vector purification with thermoresponsive-anionic mixed polymer brush modified beads-packed column. Sep. Purif. Technol. 286, 120445 (2022). 10.1016/j.seppur.2022.120445 [Google Scholar]
- 169.J. Bennett, M. Ashtari, J. Wellman, K.A. Marshall, L.L. Cyckowski, D.C. Chung, S. McCague, E.A. Pierce, Y. Chen, J.L. Bennicelli, X. Zhu, G.-S. Ying, J. Sun, J.F. Wright, A. Auricchio, F. Simonelli, K.S. Shindler, F. Mingozzi, K.A. High, A.M. Maguire, AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Trans. Med. 4, 15 (2012). 10.1126/scitranslmed.3002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.J. Bennett, J. Wellman, K.A. Marshall, S. McCague, M. Ashtari, J. DiStefano-Pappas, O.U. Elci, D.C. Chung, J. Sun, J.F. Wright, D.R. Cross, P. Aravand, L.L. Cyckowski, J.L. Bennicelli, F. Mingozzi, A. Auricchio, E.A. Pierce, J. Ruggiero, B.P. Leroy, F. Simonelli, K.A. High, A.M. Maguire, Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 388, 661–672 (2016). 10.1016/S0140-6736(16)30371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.W.J. Marks, R.T. Bartus, J. Siffert, C.S. Davis, A. Lozano, N. Boulis, J. Vitek, M. Stacy, D. Turner, L. Verhagen, R. Bakay, R. Watts, B. Guthrie, J. Jankovic, R. Simpson, M. Tagliati, R. Alterman, M. Stern, G. Baltuch, P.A. Starr, P.S. Larson, J.L. Ostrem, J. Nutt, K. Kieburtz, J.H. Kordower, C.W. Olanow, Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 9, 1164–1172 (2010). 10.1016/S1474-4422(10)70254-4 [DOI] [PubMed] [Google Scholar]
- 172.C. Summerford, J. Samulski Richard, Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Viro. 72, 1438–1445 (1998). 10.1128/JVI.72.2.1438-1445.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.M. Cabanes-Creus, A. Westhaus, R.G. Navarro, G. Baltazar, E. Zhu, A.K. Amaya, S.H.Y. Liao, S. Scott, E. Sallard, K.L. Dilworth, A. Rybicki, M. Drouyer, C.V. Hallwirth, A. Bennett, G. Santilli, A.J. Thrasher, M. Agbandje-McKenna, I.E. Alexander, L. Lisowski, Attenuation of heparan sulfate proteoglycan binding enhances in vivo transduction of human primary hepatocytes with AAV2. Mole. Ther.—Methods Clin. Develop. 17, 1139–1154 (2020). 10.1016/j.omtm.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.L. Perabo, D. Goldnau, K. White, J. Endell, J. Boucas, S. Humme, M. Work Lorraine, H. Janicki, M. Hallek, H. Baker Andrew, H. Büning, Heparan sulfate proteoglycan binding properties of adeno-associated virus retargeting mutants and consequences for their in vivo tropism. J. Virol. 80, 7265–7269 (2006). 10.1128/JVI.00076-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.J. Skog, T. Würdinger, S. van Rijn, D.H. Meijer, L. Gainche, W.T. Curry, B.S. Carter, A.M. Krichevsky, X.O. Breakefield, Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008). 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.G. Rabinowits, C. Gerçel-Taylor, J.M. Day, D.D. Taylor, G.H. Kloecker, Exosomal MicroRNA: a diagnostic marker for lung cancer. Clin. Lung Cancer 10, 42–46 (2009). 10.3816/CLC.2009.n.006 [DOI] [PubMed] [Google Scholar]
- 177.J. Chen, M. Chopp, Exosome therapy for stroke. Stroke 49, 1083–1090 (2018). 10.1161/STROKEAHA.117.018292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.K. Popowski, H. Lutz, S. Hu, A. George, P.-U. Dinh, K. Cheng, Exosome therapeutics for lung regenerative medicine. J. Extracell. Vesicles 9, 1785161 (2020). 10.1080/20013078.2020.1785161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.H. Jing, X. He, J. Zheng, Exosomes and regenerative medicine: state of the art and perspectives. Transl. Res. 196, 1–16 (2018). 10.1016/j.trsl.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 180.S. Muthu, A. Bapat, R. Jain, N. Jeyaraman, M. Jeyaraman, Exosomal therapy—a new frontier in regenerative medicine. Stem Cell Investig. (2021). 10.2037/sci-2020-037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.M.D. Hade, C.N. Suire, Z. Suo, Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells (2021). 10.3390/cells10081959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.A. Hassanzadeh, H.S. Rahman, A. Markov, J.J. Endjun, A.O. Zekiy, M.S. Chartrand, N. Beheshtkhoo, M.A.J. Kouhbanani, F. Marofi, M. Nikoo, M. Jarahian, Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 12, 297 (2021). 10.1186/s13287-021-02378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.P. Li, M. Kaslan, S.H. Lee, J. Yao, Z. Gao, Progress in Exosome Isolation Techniques, Theranostics, 7 pp.789–804, (2017) https://www.thno.org/v07p0789.htm [DOI] [PMC free article] [PubMed]
- 184.X. Fang, Y. Wang, S. Wang, B. Liu, Nanomaterials assisted exosomes isolation and analysis towards liquid biopsy. Mater. Today Bio 16, 100371 (2022). 10.1016/j.mtbio.2022.100371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.S.Z. Shirejini, F. Inci, The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol. Adv. 54, 107814 (2022). 10.1016/j.biotechadv.2021.107814 [DOI] [PubMed] [Google Scholar]
- 186.T. Liangsupree, E. Multia, M.-L. Riekkola, Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 1636, 461773 (2021). 10.1016/j.chroma.2020.461773 [DOI] [PubMed] [Google Scholar]
- 187.I. Kimiz-Gebologlu, S.S. Oncel, Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 347, 533–543 (2022). 10.1016/j.jconrel.2022.05.027 [DOI] [PubMed] [Google Scholar]
- 188.W. Nakai, T. Yoshida, D. Diez, Y. Miyatake, T. Nishibu, N. Imawaka, K. Naruse, Y. Sadamura, R. Hanayama, A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 6, 33935 (2016). 10.1038/srep33935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.K. Kawano, Y. Kuzuma, K. Yoshio, K. Hosokawa, Y. Oosugi, T. Fujiwara, F. Yokoyama, K. Matsuzaki, Extracellular-vesicle catch-and-release isolation system using a net-charge invertible curvature-sensing peptide. Anal. Chem. 96, 3754–3762 (2024). 10.1021/acs.analchem.3c03756 [DOI] [PubMed] [Google Scholar]
- 190.K. Nagase, K. Yamazaki, Y. Maekawa, H. Kanazawa, Thermoresponsive bio-affinity interfaces for temperature-modulated selective capture and release of targeted exosomes. Mater. Today Bio 18, 100521 (2023). 10.1016/j.mtbio.2022.100521 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.