Abstract
Nile tilapia, is a crucial fish for warmwater aquaculture globally and plays a key role in food security. The experiment’s goal was to ascertain the actual effects of lemongrass and/or Arthrospira platensis (A. platensis) on Oreochromis niloticus (O. niloticus) growth. Along with liver and kidney functioning, some organ histopathology, and the expression of genes linked to immunity in liver tissue both before and after an infection with Aeromonas hydrophila (A. hydrophila). There were four diets created: with the first serving as a control group and being additive-free, while the second included 7.5 g kg− 1 of diet of A. platensis, the third included 200 mg kg− 1 of diet of lemongrass (Cymbopogon citratus), and the fourth group included lemongrass and A. platensis. The results of the growth trail were significantly higher in the treatment groups, especially with A. platensis treatment. However, the results of the biochemistry and immunological assay were pronounced with improved intestinal morphometry in the combination groups without pathological lesions. Moreover, the antioxidant and proinflammatory cytokine gene expressions improved with the treatments used. After experimental challenges, the results of the treatment groups showed the immunomodulatory effects of A. platensis and lemongrass oil against A. hydrophila infection. Thus, A. platensis alone or with lemongrass improved growth performance before experimental infection. After experimental infection, they decreased mortality rate and pathological lesions in liver and spleen and improved biochemical parameters and antioxidant and proinflammatory cytokine gene expression.
Keywords: Nile tilapia, Lemongrass, Arthrospira platensis, Growth performance, Gene expression
Subject terms: Biochemistry, Physiology
Introduction
In addition to providing jobs and revenue, aquaculture is a significant economic activity on a global scale, currently accounting for 50% of fish consumption1. However, disease-related issues have caused economic losses to the world’s aquaculture output that are estimated to be between 1.05 and 9.58 US billion dollars per year2. Nowadays, the plants and algae are used to improve aquaculture through increasing growth and immunity3.
lemongrass essential oil (LEO) is a volatile oil that can be extracted straight from fresh lemongrass (Cymbopogon citratus). The grass has an essential oil content of 0.035%4. The primary ingredient in LEO, citral, is well-known for its fungistatic, anti-inflammatory, antiseptic, immunomodulatory, and antibacterial qualities5. Moreover, other phytoconstituents such as menthone, geranial, trans-caryophyllene, and delta-3-carene are present in LEO4. Because of its antibacterial properties, LEO can be used in fish aquaculture in place of antibiotics6,7. Lemongrass oil has antioxidant activity and a high vitamin C content8. Although LEO had no effect on fish survival following an infection with A. hydrophila, 0.50 ml/kg diet might enhance growth and haematological parameters in tambaqui9. Moreover, lemongrass is widely utilized in the cosmetics, perfume, and food processing industries as a food flavoring10. Alasgah et al.11 found that LEO might prevent the formation of V. parahaemolyticus biofilms and improve the safety of fish that are eaten.
Arthrospira platensis (A. platensis) is the most popular cultivated microalga being produced commercially12. Arthrospira platensis is a type of algae that is blue-green in color and grows in oceans and salty lakes in subtropical climates. It is a type of Cyanobacteria that has an unusually high protein content (55–70% by dry weight); it contains all of the essential amino acids and matches the profile of a well-balanced reference protein provided by the Food and Agriculture Organization/World Health Organization13. With its many uses in aquatic systems and ecosystem management, the adaptable microalga A. platensis has become a potent instrument for advancing environmental sustainability14. Arthrospira platensis is a superfood due to its richness in plant pigments and its ability to regulate photosynthesis, which has made it a popular ingredient in nutritional supplements. Arthrospira platensis has been used as a partial or complete replacement for protein in fish feed for a variety of fish species, including Nile tilapia15, great sturgeon16, rainbow trout17, olive flounder18, parrot fish19, catfish20, goldfish21, mrigal carp22, and guppy23. However, A. platensis is a major contributor to sustainability in the food and health industries due to its dual nature as an economical and environmentally benign solution for carbon neutrality and bioproduct creation24.
Despite all this research, it still lacks focus on the effect of A. platensis on the most important and economically important species of fish. Arthrospira platensis has drawn more and more attention lately as a possible option for treating wastewater, especially fish culture effluent25. Arthrospira platensis appears to be a very good integrated strategy for fish farming when used as a nutritional supplement and to treat effluent for water quality control.
While reducing the use of traditional synthetic chemotherapeutics—many of which are prohibited in various countries owing to the dangers of toxicity to fish and handlers as well as environmental contamination—it is imperative to pursue technical advancements in order to produce fish products of higher quality1. Thus, in order to improve the activity’s overall sustainability, contemporary research has concentrated on natural, non-toxic, and eco-friendly therapies. So, the A. platensis and lemongrass alone or in combination were used to improve performance and immunity against experimental infection.
Materials and methods
Feed additives
Lemongrass was used as oil, contributed by the Faculty of Agriculture, Aswan University, Aswan, Egypt. Meanwhile, dried Arthrospira platensis (A. platensis) granules from the Cyanobacteria Research Lab at the Sakha Agriculture Research Station in Kafrelsheikh, Egypt, were utilized.
Utilizing fish and designing an experiment
Mono sex O. niloticus (49.25 ± 0.5 g) fish (45 fish/group, each in three glass aquaria (80 х 30 х 45 cm)) were randomly assigned to perform this study (n = 180). Fish were introduced from a local farm owned by High Dam Lake Development Authority in Aswan Governorate to the Faculty of Fish and Fisheries Technology, Aswan University, Aswan, Egypt, which approved (2/2023) this study and acclimatized for 14 days. During acclimatization, fish fed on control experimental diet 30% protein. Four diets with similar quantities of calories and 30% protein each were created (Table 1). The first serving as a control group and being additive-free, while the second (SP) included 7.5 g kg− 1 of diet of A. platensis26, the third (LG) included 200 mg kg− 1 of diet of lemongrass (Cymbopogon citratus)27, and the fourth (LG + SP) group included lemongrass at 200 mg kg− 1 pulse 7.5 g kg− 1 of A. platensis. For two months, fish feed twice a day at a rate of 3%. The dechlorinated water was used to replace 30% of the aquarium water each day. The water oxygen, salinity, and temperature were adjusted at 5.8–6.1 ppm, 1.1-2‰, pH 7.4–8.1, and 24 °C, respectively. All methods were performed in accordance with the relevant guidelines and regulations.
Table 1.
Physical and chemical composition of basal diet.
| Formulation | Basal diet |
|---|---|
| g | |
| Fish meal (60% cp.) | 178.5 |
| Soybean meal (44% cp.) | 378.9 |
| Meat meal (55%) | 13.1 |
| Wheat middling | 195 |
| Rice polishing | 177.3 |
| Fat Soyabean oil | 25.7 |
| Lime stone | 8.7 |
| Vitamin and mineral mix | 9.3 |
| Di-calcium phosphate | 2.2 |
| NaCl | 4.3 |
| Antimycotoxin | 5.9 |
| Vitamin premix E | 1.1 |
| Crude protein | 33.67 |
| Lipid | 8.4 |
| Fibers | 6.3 |
| Ash | 11.8 |
| Gross energy (kJ/g) | 19.06 |
CP crude protein.
The growth performance of the experimented fish was calculated every 2 weeks. A full day prior to the sampling, fish was fasted. Fish was anaesthetized with tricaine methane sulphonate (MS-222) at a dose of 25 mg/L prior to sampling. On days 60 (end of the growth trial) and 78 (7 days after A. hydrophila infection) of the experiment, blood was collected from the caudal vein with anticoagulant (for hemogram and leukogram) and without anticoagulant (for liver and kidney functions) to separate serum (9 fish/group, three/replicate). The phagocytic activity and index were determined according to the following equations:
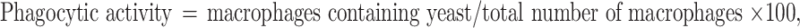 |
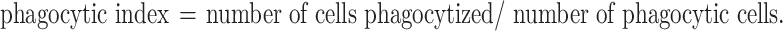 |
It was there that the number of phagocytic cells was counted.
When gathering data, the researchers were not blinded. Analysis was done using blinding.
An analysis of intestinal morphometry and histopathology
Liver, intestine, spleen, and gills sections were prepared for pathological assessment using the standard paraffin embedding method after being promptly fixed in 10% formalin. For light microscopic analysis, sections with a thickness of 5 μm were cut and stained with hematoxylin and eosin (HE)28. Villi length and width and intravilli space were measured as part of the intestinal morphometry.
Gene expression
Tissue samples (liver) were taken from five fish per replicate (15 fish/group) after euthanizing in a bath of clove oil. The samples were gathered in liquid nitrogen and stored at − 80 °C until used. The total RNA was extracted from each sample using Trizol (Applied Biotechnology, Egypt) according to the accompanied Kit instructions. The integrity of the extracted RNA was inspected using ethidium bromide-stained agarose gel electrophoresis. The mRNA was reverse transcribed backward to the cDNA using a specific kit (Applied Biotechnology, Egypt).
Real-time PCR
The gene thermal amplification was done by means of the Stratagene MX3000P real-time PCR system using the Low-Rox-SYBR green kits (Bioline, UK). The amplification conditions were done according to Esam et al.29, and the specific annealing temperatures for each gene are listed in Table 2.
Table 2.
The primers used in this study.
| Gene | Primer | Accession no | References |
|---|---|---|---|
| B actin |
F: CAGCAAGCAGGAGTACGATGAG R: TGTGTGGTGTGTGGTTGTTTTG |
XM_003455949.2 | El-Kassas et al. (2020)98 |
| il-8 |
F: CTGTGAAGGCATGGGTGTGGAG R: TCGCAGTGGGAGTTGGGAAGAA |
NM_001279704.1 | Abdo et al. (2022)99 |
| il-1β |
F: TCAGTTCACCAGCAGGGATG R: GACAGATAGAGGTTTGTGCC |
XM_019365842.1 | |
| nfkb2 |
F: GAACATCAGACCGACGACCA R: TCTCCGCCAGTTTCTTCCA |
XM_003457469.4 | |
| tnf-α |
F: AAGCCAAGGCAGCCATCCAT R: TTGACCATTCCTCCACTCCAGA |
NM_001279533.1 | Esam et al. (2022)29 |
| Cat |
F: CCCAGCTCTTCATCCAGAAAC R: GCCTCCGCATTGTACTTCTT |
JF801726.1 | Abdo et al. (2021)100 |
| Gpx |
F: CCAAGAGAACTGCAAGAACGA R: CAGGACACGTCATTCCTACAC |
DQ355022 | El-Kassas et al. (2022)101 |
β-actin housekeeping gene, nf-κb2 Nuclear factor kappa B, il-1β Interleukin 1β, il-8 Interleukin 8, cat catalase, gpx glutathione peroxidase.
The gene expression levels in pre challenged and post challenged groups were calculated as a fold change (2−ΔΔCT) according to Livak and Schmittgen30. Where the data were normalized against the cycle threshold of the control group (fed a basal diet and not infected). The β-actin was chosen as a control to normalize the expression levels of the genes.
Aeromonas hydrophila infection
After the end of the growth trial, 20, 15, 15, and 15 fish from the control, A. platensis, lemongrass, and mixed groups were challenged (0.2 ml dose of 24 h.) intraperitoneally (IP) with the pathogenic strain of Aeromonas hydrophila. The pathogenic strain was acquired from Kafrelsheikh University in Egypt, Department of Fish Diseases, Faculty of Veterinary Medicine. A. hydrophila concentration (1 × 108 cells/ml) was adjusted to the density of McFarland Standard 131. According to Amos32, fish were observed for 7 days to record the clinical signs and mortality rate.
The blood and tissue samples were collected after 60 days to ensure that the treatment affects growth performance, where the minimum period for a growth trial is 60 days. Moreover, the samples were collected after 7 days from experimental infection to avoid the effect of stress as a result of the handling of fish during experimental infection and to ensure the results were produced due to experimental infection and didn’t increase the period to avoid the death of fish as a result of infection.
Statistical analysis
The minimum sample size required for a research study was ascertained by power analysis prior to the experiment’s commencement. Additionally, in order to demonstrate homoscedasticity and normality, the data were subjected to the Shapiro-Wilk and Levene tests for normal distribution. Next, using SPSS version 20, a one-way analysis of variance was used to statistically analyze the data.
Results
Clinical signs
The mortality of fish following an experimental A. hydrophila infection continued for five days, then stopped (Table 3). Compared to the SP platensis, LG, and LG + SP groups, the control group’s mortality rate increased significantly (P ≤ 0.05). Furthermore, the SP, LG, and LG + SP groups’ survival rates were considerably (P ≤ 0.05) higher than those of the control group. The fish started dying after days of infection. The clinical symptoms appeared in skin ulcers (Fig. 1A, B), tail erosions (Fig. 1c). Postmortem, there were enlarged liver, spleen, and gall bladder with abdominal edema (Fig. 1D).
Table 3.
Experimental infection of nile tilapia with Aeromonas hydrophila after growth trial.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | SP | LG | LG + SP | |
| Total number | 15 | 10 | 10 | 15 |
| Dead number | 10 | 2 | 2 | 2 |
| Survival rate | 1.67 ± 0.33b | 4 ± 0.58a | 4 ± 0.0a | 4.33 ± 0.33a |
| Mortality rate | 3.33 ± 0.33a | 1 ± 0.57b | 1 ± 0.0b | 0.67 ± 0.33b |
Values are expressed as mean ± standard errors. Means in the same row (a-c) with different letters significantly differ at (P ≤ 0.05).
Fig. 1.
Displayed the postmortem lesion and clinical symptoms of the artificially infected fish.
Growth
The leverage of lemongrass and/or A. platensis on the performance of Nile tilapia is shown in Table 4. The statistical analysis of the collected data on the final body weight, weight gain, and FCR of the SP group significantly (P < 0.05) improved in contrast to the other groupings. However, as compared to the control group, the LG and LG + SP groups showed improvements in the same parameters.
Table 4.
Impact of A. platensis and Lemongrass on nile tilapia growth performance after two months (n = 45/group).
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | SP | LG | LG + SP | |
| Initial body weight (g/fish) | 49.80 ± 0.49 | 50.20 ± 0.37 | 49.6 ± 0.51 | 49.4 ± 0.68 |
| Final body weight (g/fish) | 87.60 ± 0.81c | 98.80 ± 0.80a | 95.6 ± 0.51b | 94.6 ± 0.51b |
| Weight gain (g/fish) | 37.80 ± 0.66c | 48.60 ± 0.68a | 46.0 ± 0.32b | 45.20 ± 0.20b |
| Feed intake (g/fish/2 months) | 98.85 ± 0.52 | 97.14 ± 2.06 | 98.96 ± 1.45 | 99.96 ± 1.51 |
| FCR | 2.62 ± 0.04a | 2.0 ± 0.03c | 2.15 ± 0.02b | 2.21 ± 0.04b |
Values are expressed as mean ± standard errors. Means in the same row (a-c) with different letters significantly differ at (P ≤ 0.05).
FCR feed conversion ratio.
Blood and immunological assays
The leverage of lemongrass and/or A. platensis on the blood and immunological assays of Nile tilapia are shown in Tables 5 and 6 before and after experimental infection. Before infection, the statistical analysis of the collected data on the RBCs, Hb, and PCV significantly (P < 0.05) improved in the all-treatment groups when compared with the control group. These improvements were superior to those in the LG + SP group. After infection, the RBCs of the LG + SP group were not affected by the experimental infection. Meanwhile, PCV and Hb significantly (P < 0.05) decreased in the LG + SP group when compared with the same group before infection. On the other side, the data of the hematological parameters of the A. platensis and LG groups significantly decreased in contrast to the same groups prior to infection (Table 5).
Table 5.
Impact of A. platensis and Lemongrass on nile tilapia hematological parameters experimentally infected with A. hydrophila (n = 9).
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Time | Control | SP | LG | LG + SP | |
| RBCs count (× 106/µl) | Before infection | 1.68 ± 0.04cX | 1.86 ± 0.04bX | 2.16 ± 0.26abX | 2.45 ± 0.04 aX |
| After infection | 1.41 ± 0.05cY | 1.70 ± 0.04bcY | 1.86 ± 0.16bX | 2.34 ± 0.07aX | |
| Hb (g/dl) | Before infection | 6.47 ± 0.34cX | 7.95 ± 0.22bX | 8.14 ± 0.41bX | 9.41 ± 0.25aX |
| After infection | 4.00 ± 0.23bY | 6.43 ± 0.38aY | 6.40 ± 0.29aY | 7.27 ± 0.46aY | |
| PCV% | Before infection | 18.55 ± 0.40cX | 23.50 ± 0.73bX | 24.13 ± 0.23bX | 28.36 ± 0.34aX |
| After infection | 10.34 ± 0.77cY | 18.44 ± 0.11bY | 18.33 ± 0.96bY | 21.22 ± 0.40aY | |
Values are expressed as mean ± standard errors. Means in the same row (a–c) with different letters significantly differ at (P ≤ 0.05) meanwhile means in the same column (X–Y) with different letters significantly differ at (P ≤ 0.05).
Table 6.
Impact of A. platensis and Lemongrass on nile tilapia differential leukocytic count experimentally infected with A. hydrophila (n = 9).
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Time | Control | SP | LG | LG + SP | |
| WBCs count (× 103/µl) | Before infection | 20.9 ± 0.2Y | 20.27 ± 0.1Y | 20.03 ± 0.05Y | 20.48 ± 0.51 Y |
| After infection | 43.53 ± 1.04aX | 28.6 ± 1.2bcX | 31.79 ± 0.92bX | 27 ± 0.92cX | |
| Heterophils (× 103/µl) | Before infection | 6.9 ± 0.4aY | 6.6 ± 0.2abY | 6.11 ± 0.49abY | 5.42 ± 0.33bY |
| After infection | 24.15 ± 0.85aX | 11.5 ± 0.7bX | 13.63 ± 0.62bX | 6.95 ± 0.58cX | |
| Lymphocyte (× 103/µl) | Before infection | 8.2 ± 0.3cX | 9.1 ± 0.1bX | 8.93 ± 0.19bX | 11.27 ± 0.04ay |
| After infection | 6.4 ± 0.26cY | 9.3 ± 0.5bX | 9.9 ± 0.15bX | 14.38 ± 0.27aX | |
| Monocytes (× 103/µl) | Before infection | 5.1 ± 0.0aY | 4.1 ± 0.0bY | 4.46 ± 0.22bY | 3.34 ± 0.11cY |
| After infection | 12.16 ± 0.46aX | 7.1 ± 0.04bX | 7.56 ± 0.17bX | 5.05 ± 0.06cX | |
| Eosinophiles (× 103/µl) | Before infection | 0.60 ± 0.0aY | 0.54 ± 0.0bY | 0.55 ± 0.01bY | 0.46 ± 0.03cY |
| After infection | 0.83 ± 0.01aX | 0.73 ± 0.01bX | 0.71 ± 0.02bX | 0.62 ± 0.01cX | |
Values are expressed as mean ± standard errors. Means in the same row (a–c) with different letters significantly differ at (P ≤ 0.05) meanwhile means in the same column (X–Y) with different letters significantly differ at (P ≤ 0.05).
However, the immunological assay’s findings demonstrated that the WBC count in each group before infection was unaffected. Following infection, the three treatment groups’ counts were lower than those of the control group. The lymphocyte count was higher in the three treatment groups prior to and following infection in contrast to the control group. Prior to and following infection, the three treatment groups’ counts of basophils, monocytes, and heterophils were lower than those of the control group (Table 6).
Serum parameters
Before and after infection, the liver enzymes (ALT, AST, and alkaline phosphatase) were considerably (P < 0.05) lower in the all-treatment groups than in the control group. This decline was better in the LG + SP group. When compared to the same group prior to infection, the control group’s liver enzyme activities significantly increased after infection (Table 7).
Table 7.
Impact of A. platensis and Lemongrass on nile tilapia liver enzymes parameters experimentally infected with A. hydrophila (n = 9).
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Time | Control | SP | LG | LG + SP | |
| AST (U/L) | Before infection | 48.13 ± 0.17ay | 47.36 ± 0.16aby | 46.65 ± 0.84aby | 46.08 ± 0.35by |
| After infection | 110.68 ± 0.75ax | 90.90 ± 0.80bx | 88.87 ± 0.90bx | 79.10 ± 0.42cx | |
| ALT (U/L) | Before infection | 13.22 ± 0.34ay | 12.54 ± 0.12aby | 12.45 ± 0.18by | 12.22 ± 0.12by |
| After infection | 33.62 ± 0.53ax | 19.6 ± 0.44bx | 19.22 ± 0.62bcx | 17.88 ± 0.25cx | |
| ALP (U/L) | Before infection | 34.48 ± 0.70ay | 32.45 ± 0.42by | 28.88 ± 0.68cy | 20.92 ± 0.10dy |
| After infection | 56.4 ± 0.75ax | 44.75 ± 0.89bx | 43.85 ± 0.26bcx | 42.42 ± 0.11cx | |
Values are expressed as mean ± standard errors. Means in the same row (a–d) with different letters significantly differ at (P ≤ 0.05) meanwhile means in the same column (X–Y) with different letters significantly differ at (P ≤ 0.05).
Regarding the results of the serum proteins and kidney function, the combination treatment (LG + SP) higher serum total protein, albumin, and globulin levels before infection. Meanwhile, creatinine and urea decreased in the same group when compared with the other groups before the infection. After infection, compared to the same group prior to infection, all groups’ blood protein and kidney function test levels dropped, except albumin in the SP group (Table 8).
Table 8.
Impact of A. platensis and Lemongrass on nile tilapia liver and kidney functions experimentally infected with A. hydrophila (n = 9).
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Time | Control | SP | LG | LG + SP | |
| Total protein | Before infection | 4.9 ± 0.40bX | 4.9 ± 0.15Bx | 4.93 ± 0.09bX | 6 ± 0.53aX |
| After infection | 3.71 ± 0.05bY | 4.28 ± 0.12aY | 4.44 ± 0.12aY | 4.75 ± 0.23aY | |
| Albumin | Before infection | 1.3 ± 0.06bX | 1.33 ± 0.03bX | 1.3 ± 0.058bX | 1.6 ± 0.06aX |
| After infection | 0.93 ± 0.02bY | 1.4 ± 0.09aX | 1.15 ± 0.07abY | 1.31 ± 0.13aY | |
| Globulin | Before infection | 3.6 ± 0.36bX | 3.57 ± 0.12abX | 3.63 ± 0.13abX | 4.4 ± 0.51aX |
| After infection | 2.78 ± 0.07Y | 2.88 ± 0.10Y | 3.29 ± 0.13Y | 3.44 ± 0.32Y | |
| Urea (mg/L) | Before infection | 7.9 ± 0.40aY | 6.73 ± 0.32bY | 5.93 ± 0.33bY | 4.83 ± 0.22cY |
| After infection | 12.06 ± 0.31aX | 9.26 ± 0.39bX | 11 ± 0.27aX | 8.74 ± 0.58bX | |
| Creatinine (mg/L) | Before infection | 1.23 ± 0.03aY | 1.03 ± 0.07bcY | 1.07 ± 0.03bY | 0.9 ± 0.06cY |
| After infection | 1.63 ± 0.07aX | 1.3 ± 0.06bX | 1.32 ± 0.06bX | 1.27 ± 0.03bX | |
Values are expressed as mean ± standard errors. Means in the same row (a–c) with different letters significantly differ at (P ≤ 0.05) meanwhile means in the same column (X–Y) with different letters significantly differ at (P ≤ 0.05).
Phagocytic activity and index
The leverage of lemongrass and/or A. platensis on the phagocytic activity and index of Nile tilapia are shown in Table 9 before and after the experimental infection. The results of the three treatment groups were significantly (P < 0.05) higher than the results of the control group, with a superior effect to the LG + SP group.
Table 9.
Impact of A. platensis and Lemongrass on nile tilapia phagocytic activity and index experimentally infected with A. hydrophila (n = 9).
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Time | Control | SP | LG | LG + SP | |
| Phagocytic activity % | Before infection | 21.1 ± 0.3cX | 25.8 ± 0.8bX | 24.9 ± 0.3bX | 31.6 ± 0.6aX |
| After infection | 15.0 ± 0.2dY | 22.3 ± 0.8bY | 19.9 ± 0.2cY | 26.4 ± 0.4aY | |
| Phagocytic index % | Before infection | 2.3 ± 0.1cX | 3.0 ± 0.1bX | 3.0 ± 0.0bX | 4.2 ± 0.1aX |
| After infection | 1.2 ± 0.0cY | 2.4 ± 0.1by | 2.2 ± 0.0bY | 3.0 ± 0.0aY | |
Values are expressed as mean ± standard errors. Means in the same row (a–c) with different letters significantly differ at (P ≤ 0.05) meanwhile means in the same column (X–Y) with different letters significantly differ at (P ≤ 0.05).
Histopathological finding and intestinal morphometry
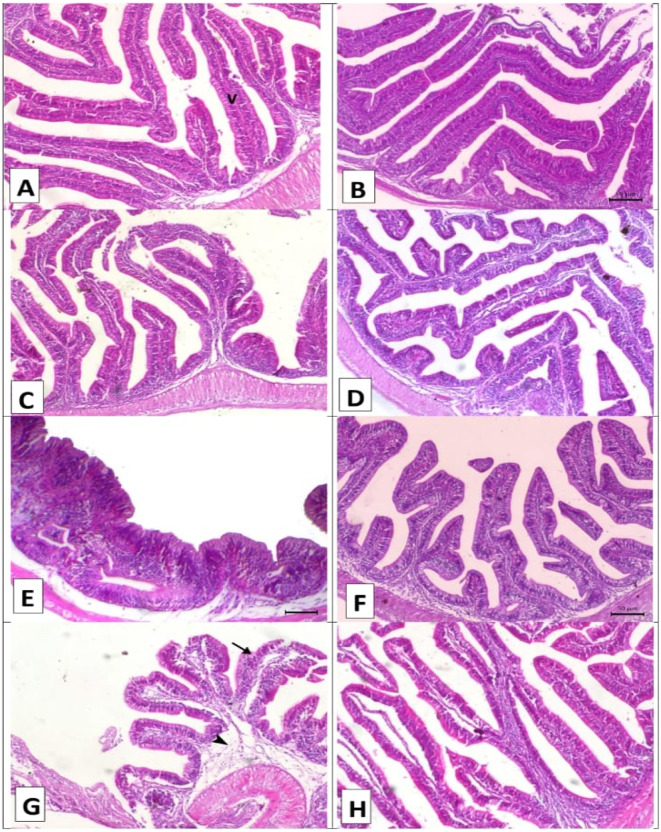
Figures 2, 3 and 4, and 5 shows the impact of A. platensis and lemongrass oil on the intestine, liver, spleen, and gills pathology. Before each group was experimentally infected, the intestinal photomicrograph; (Fig. 2A–D) for the control, SP, LG, and LG + SP groups, respectively, showed normal intestine histo-architecture with normal villous. After experimental infection, there was noticeable submucosal edema, inflammatory cell infiltration, and diffuse villous necrosis in the control infected group (Fig. 2E). Individual villous necrosis was normal in the SP-infected group, and there was modest submucosal edema (Fig. 2F). Significant submucosal edema and necrosis of several villi were observed in the LG-infected group (Fig. 2G). The intestinal histoarchitecture was normal in the LG + SP-infected group (Fig. 2H).
Fig. 2.
Displayed intestinal histopathology stained with H&E prior to and after artificial infection. (A–D) for the control, SP, LG, and LG + SP non infected groups showed normal intestine histology with normal villous (V). (E) The control infected group showed diffuse villous necrosis (arrow) with marked submucosal edema and inflammatory cells infiltration (arrowhead). (F) The A. platensis infected group showed normal necrosis of individual villous (arrow) with mild submucosal edema (arrowhead). (G) The LG infected group showed necrosis of some villi (arrow) with significant submucosal edema (arrowhead), and (H) the LG + SP infected group showed normal intestine histology. x 160.
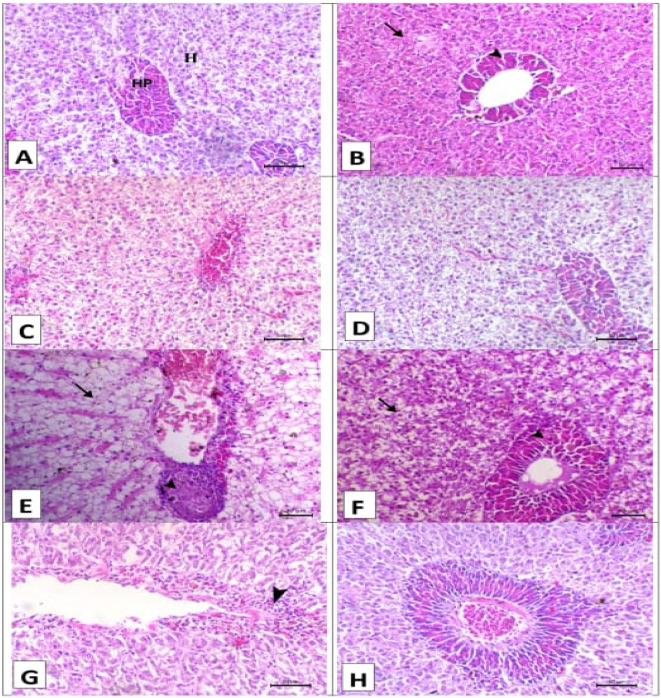
Fig. 3.
Displayed hepatopancreas histopathology stained with H&E prior to and after artificial infection. (A–D) for the control, SP, LG, and LG + SP non infected groups showed normal hepatopancreatic histology where hepatocytes cords (H) and pancreatic acini surrounding central viens (HP). (E) The control infected group showed widespread hepatopancreatic necrosis. (F) The A. platensis group infected with Aeromonas shown hepatic necrosis. (G) The LG infected group showed pancreatic necrosis (arrowhead), and (H) the LG + SP infected group showed normal hepatopancreatic histology. Scale bar = 50 μm.
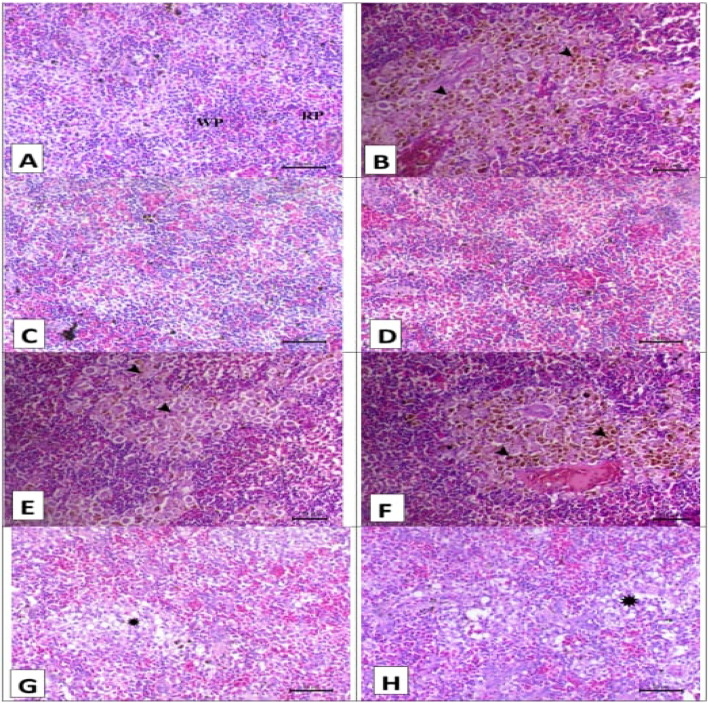
Fig. 4.
Displayed spleen histopathology stained with H&E prior to and after artificial infection. (A–D) for the control, SP, LG, and LG + SP non infected groups showed normal histology consisted mainly of white pulp that mainly contains lymphocytes (WP) and red pulp that mainly contains erythrocytes (RP). (E) The control infected group showed marked depletion of white pulps (asterisks) and activation of melanomacrophage center (arrowhead). (F) The A. platensis infected group showed moderate depletion of white pulps (asterisk) and activation of melanomacrophage center (arrowhead). (G) The LG infected group showed moderate depletion of white pulps (asterisk), and (H) the LG + SP infected group showed mild depletion of white pulp (asterisk). Scale bar = 50 μm.
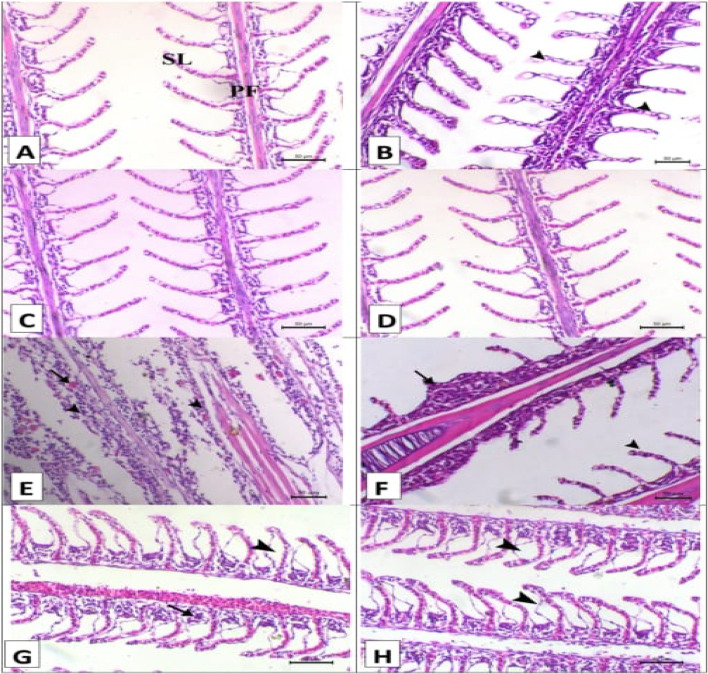
Fig. 5.
Displayed gills histopathology stained with H&E prior to and after artificial infection. (A–D) for the control, SP, LG, and LG + SP non infected groups showed normal histology consisted mainly of primary filament (PF) branch out into tiny secondary lamellae (SL). (E) The control infected group showed widespread diffuse filamentous epithelial necrosis (arrowheads). (F) The A. platensis infected group showed multifocal filamentous epithelial necrosis (arrowheads). (G) The LG infected group showed focal filamentous epithelial necrosis (arrow) and diffuse lifting of the covering epithilium of the secondary lamellae (arrowhead), and (H) The LG + SP infected group showed diffuse lifting of the covering epithilium of the secondary lamellae (arrowheads). Scale bar = 50 μm.
About the hepatopancreas photomicrographs of the various groups prior to experimental infection; (Fig. 3A–D): Normal hepatopancreatic histo-architecture was observed in the control, SP, LG, and LG + SP groups, respectively, with hepatocyte cords and pancreatic acini encircling the central viens. The control group experienced extensive hepatopancreatic necrosis following the experimental infection (Fig. 3E). The SP group showed hepatic necrosis (Fig. 3F) after infection. The LG group showed pancreatic necrosis (Fig. 3G) after infection. There was normal hepatopancreatic histoarchitecture in the LG + SP group (Fig. 3H) after infection.
The spleen photomicrographs of the various groups prior to experimental infection are shown in Fig. 4A–D. Normal histoarchitecture was displayed by the control, SP, LG, and LG + SP groups, respectively. This included red pulp that primarily contained erythrocytes and white pulp that primarily included lymphocytes. The control group displayed significant white pulp loss and melanomacrophage center activation upon infection (Fig. 4E). The melanomacrophage center was activated and white pulps were moderately depleted in the SP group upon infection (Fig. 4F). White pulp depletion was moderate in the LG group upon infection (Fig. 4G). The LG + SP group showed mild depletion of white pulp (Fig. 4H).
The gills photomicrographs of the various groups prior to experimental infection; (Fig. 5A–D): the control, SP, LG, and LG + SP groups, respectively, showed normal histo-architecture consisting mainly of primary filament branching out into tiny secondary lamellae. The control group displayed extensive diffuse filamentous epithelial necrosis following infection (Fig. 5E). Multifocal filamentous epithelial necrosis was seen in the SP group following infection (Fig. 5F). The LG group displayed diffuse lifting of the secondary lamellae’s covering epithelium and localized filamentous epithelial necrosis following infection (Fig. 5G). The secondary lamellae’s covering epithelium was diffusely lifted in the LG + SP group following infection (Fig. 5H).
In terms of intestinal morphometry, the SP, LG, and LG + SP groups outperformed the control group in terms of villi length and width both before and after infection. Meanwhile, inter villi space was higher in the control group when compared with the SP, LG, and LG + SP groups (Table 10).
Table 10.
Impact of A. platensis and Lemongrass on nile tilapia intestinal morphometry experimentally infected with A. hydrophila (n = 5).
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Time | Control | SP | LG | LG + SP group | |
| Villi length (um) | Before | 447 ± 1.6dX | 524 ± 1.8bX | 496.9 ± 1.9cX | 621.5 ± 0.9aX |
| After | 203.5 ± 1.1dY | 375 ± 1.0bY | 349.9 ± 0.6cY | 461.7 ± 1.1aY | |
| Villi width (um) | Before | 58.1 ± 1.2dX | 60 ± 0.4cX | 74.1 ± 3.4bX | 105.5 ± 1.7aX |
| After | 52.5 ± 1.75cY | 56 ± 0.2bY | 55 ± 1.8bY | 83.3 ± 1.8aY | |
| Inter villi space (um) | Before | 68 ± 1.5aY | 42 ± 0.6cY | 57.4 ± 1.7bY | 35.6 ± 0.8dY |
| After | 104.1 ± 0.7aX | 83.4 ± 0.5bX | 79.7 ± 2.6dX | 60.5 ± 0.6cX | |
Values are expressed as mean ± standard errors. Means in the same row (a–c) with different letters significantly differ at (P ≤ 0.05) mean while means in the same column (X–Y) with different letters significantly differ at (P ≤ 0.05).
Gene expression
Significant interactive effects of dietary A. platensis and lemongrass inclusion were observed on the expression of the antioxidant-related genes (gpx and cat) in the liver tissue of O. niloticus prior to and upon infection, as shown in Figure 6. The inclusion of A. platensis and lemongrass alone or in combination in the diet of fish significantly (P ≤ 0.05) upregulated the gpx and cat expression with superior effects to combination inclusion. However, the bacterial infection alone was associated with a slight down regulation of both cat and gpx expression compared to the control non infected group (P ≤ 0.05).
Fig. 6.
Impact of A. platensis and lemongrass in the diet of Nile tilapia on liver GPX and CAT mRNA transcript level prior to and after artificial infection. Values were expressed as Mean ± SE. Columns with different litters indicates statistically significant values with P-values ≤ 0.05.
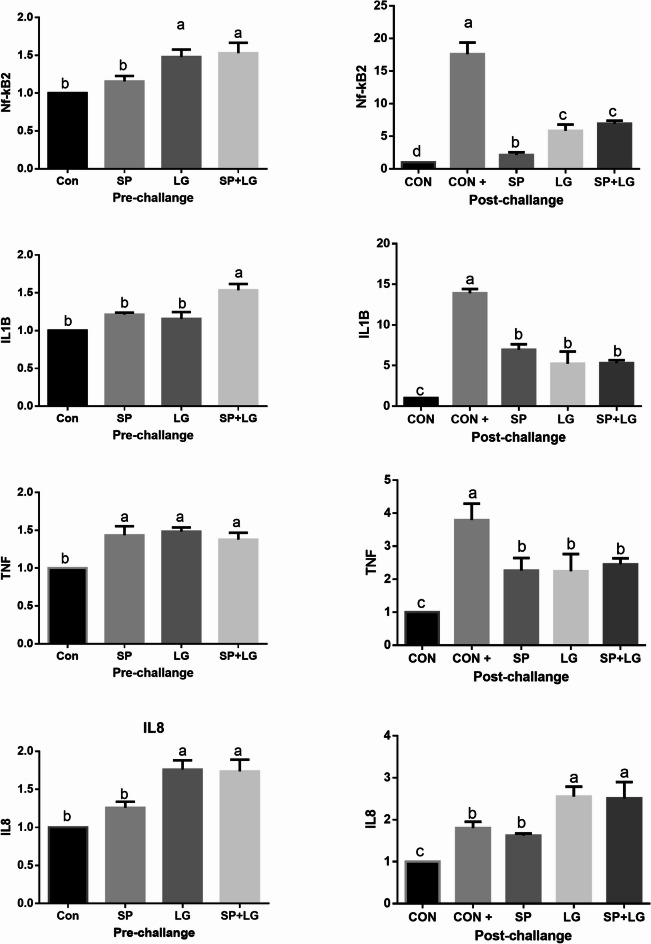
Moreover, there were significant interactive effects of dietary A. platensis and lemongrass inclusion on the expression of proinflammatory-related genes (nfkb2, il1β, tnf, and il8) in the liver tissue of O. niloticus prior to and upon infection, as shown in Figure 7. The inclusion of lemongrass alone or in combination with A. platensis in the diet of fish significantly (P ≤ 0.05) upregulated nlb and ilβ expression. Combination treatment increased the mRNA transcription levels of il1β. Meanwhile, tnf was upregulated in the tree treatment groups. On the other hand, infection increased the mRNA transcription levels of nfkb2, il1β, and tnf. Meanwhile, infection downregulated il8.
Fig. 7.
Impact of A. platensis and lemongrass in the diet of Nile tilapia on liver NKB, IL1B, TNF, and IL8 mRNA transcript level prior to and after artificial infection. Values were expressed as Mean ± SE. Columns with different litters indicates statistically significant values with P-values ≤ 0.05.
Discussion
In aquaculture, the use of phytogenic compounds in fish diets has recently gained a lot of attention33. The results of Okasha et al.34 and Metekia and Ulusoy35 on A. platensis in the O. niloticus against experimental infection are combatable with the results of this trial. Additionally, Mahmoud et al.36 observed supplementation with A. platensis (1%) improved against Pseudomonas fluorescence in Nile tilapia. This effect may be attributed to the carotenoids that are comprised in the A. platensis, which reduce stress and bolster the body’s defenses against infection37. Moreover, Abdo et al.38 demonstrated that bioactive substances found in A. platensis include antibacterial, anti-inflammatory, and antioxidant qualities. On the other hand, the outcomes of the clinical manifestations of the LG group after experimental infection are in harmony with Sukrakanchana et al.39, who found that lemongrass oil decreased mortality in Nile tilapia after Streptococcus agalactiae infection. Moreover, Al-Sagheer et al.27 observed resistance of the diseases by lemongrass oil in the Nile tilapia and ascribed this resistance to the growth promoting effect of lemongrass. There may be a clear correlation between the potent antibacterial properties of lemongrass and their growth-promoting effects40. On the other hand, Oussalah et al.41 attributed this effect to their active constituents, which include 1,8-cineol, α-pinene, and limonene. However, this positive effect was pronounced in the LG + SP group. Similar finding was observed by Abdo et al.38, who reported that remarkably, the Nile tilapias fed with LEO and A. platensis showed the highest immune response, suggesting that their actions were synergistic. Mohammed et al.42 reported the immunomodulatory effect of combination treatment with rosemary and A. platensis in Nile tilapia. Meanwhile, Copatti et al.9 found that lemongrass did not affect the survival of tambaqui after experimental infection. On the other hand, Fadl et al.43 and Yardimci and Aydin44 reported similar results to those of the control group in Nile tilapia after experimental infection with A. hydrophila.
In the current study, the addition of A. platensis and lemongrass to the Nile tilapia fish feed improved growth parameters. Similar findings were obtained by Al-Deriny et al.45 and Al-Sagheer et al.27 in O. niloticus with A. platensis and lemongrass, respectively. The growth parameters include, body weight and gain, specific growth rate, and FCR of O. niloticus improved by dietary supplementation with A. platensis46. Moreover, Amer47 reported that A. platensis had a higher body weight and a lower FCR with Nile tilapia. Similar results were obtained by El-Araby et al.48. These results are confirmed by the results of the intestinal morphometry. By enhancing intestinal morphometry and stimulating the GH/IGF axis, A. platensis has a favorable impact on O. niloticus growth49. Moreover, Sherif et al.50 found that A. platensis increased activity of the Nile tilapia intestinal enzymes. On the other hand, Adeniyi51 reported that lemongrass inclusion in the diet of Clarias gariepinus increased body weight and gain and lowered FCR. Similar findings were reported by Gichana52 in Nile tilapia. Moreover, Orzuna-Orzuna and Granados-Rivera53 reported that O. niloticus growth, antioxidant in the serum, and intestinal morphometry can all be enhanced by adding essential oils to their diet. On the contrary, Mahmoud et al.36 found that supplementation with 1% A. platensis did not improve the growth of Nile tilapia. Also, inclusions of lemongrass leaf at higher levels decreased body weight and weight gain and increased the FCR of African catfish54. This may be attributed to the anti-nutritional factors in lemongrass leaves55. This difference could be attributed to the status of the lemongrass used (leaf or oil) and the species of fish used in the experiment. However, the combination treatment (LG + SP) significantly affected the growth of O. niloticus. Similar finding was observed by Abdo et al.38, who reported that remarkably, the Nile tilapias fed with LEO and A. platensis showed the highest growth performance, suggesting that their actions were synergistic. Mohammed et al.42 reported the growth promoting effect of combination treatment with rosemary and A. platensis in Nile tilapia.
Regarding the results of the hematology and immune response before infection, these results similar to Mahmoud et al.36, who found that supplementation with 1% A. platensis improved hematological parameters and antioxidant enzymes in the serum of Nile tilapia. Moreover, Abdel-Tawwab et al.56 reported that A. platensis improved the growth and hemato-biochemical parameters of the O. niloticus. 7.5 g A. platensis/kg diet significantly increased RBCs, PCV, and hemoglobulin in Nile tilapia fish57. Moreover, Youssef et al.46 and El-Sheekh et al.58 reported that A. platensis inclusion increased the RBCs and haemoglobin of Nile tilapia and hybrid red tilapia, respectively. Similar results were obtained by Sarma et al.59, who attributed this increase to the hemopiotic stimulation effect of A. platensis. On the other hand, Thuong et al.60 found that lemongrass oil positively affected the haematology of the red tilapia. However, these results are confirmed by the results of Mohammady et al.61, who reported the positive effect of the phytogenic extract on the hematology of the O. niloticus. On the contrary, Al-Zayat57 reported that A. platensis inclusion increased WBCs. Sayed and Fawzy62 and Youssef et al.46 have observed comparable outcomes in catfish and O. niloticus, respectively. Lemongrass oil positively affected the WBCs of the red tilapia60. The fish type and the source of the lemongrass and A. platensis utilized in each study could be the cause of this discrepancy.
Upon infection, the findings of the haematological and immunological parameters were similar to the findings of Fadl et al.43, who reported decreased and increased haematological and immunological parameters in the control group, respectively. Similar findings were reported by Talpur et al.63, Hardi et al.64, and Ergena et al.65 in Nile tilapia experimentally infected with A. hydrophila. However, reduced red blood cell numbers signify that the infection is affecting or destroying erythrocytes. Fish that are diseased as a result of infection are likely to exhibit changes in their haematology66. These negative effects were ameliorated by A. platensis and lemongrass inclusion. Similar results were obtained by Fadl et al.43 with microalgae and A. hydrophila. A. platensis has a beneficial effect on the antibacterial activity against A. hydrophila in O. niloticus67. Moreover, Thuong et al.60 found that lemongrass improved hematological parameters of red tilapia after experimental infection with Streptococcus agalactiae.
Liver enzymes such (ALT and AST) are important indicators of liver function. Increased serum activities of liver enzymes indicate liver damage68. The results of the serum liver enzymes in the present trial before infection decreased with different treatments, especially the combination treatment. Similar findings were obtained by Fadl et al.43 with A. platensis and Li et al.69 with lemongrass oil. Meanwhile, Abo El-Ward et al.70 found opposite results. Additionally, A. platensis addition had no influence on serum liver enzyme activity46. However, Gamboa-Delgado et al.71 reported the hepatoprotective effect of A. platensis in O. niloticus.
On the other side, Souza et al.72 reported that lemongrass oil increased serum ALT and AST in Nile tilapia. Following infection, the control infected group’s liver enzyme activity rose. The findings of Fadl et al.43 support these findings. Moreover, Bekele et al.73 and Martins et al.74 reported that liver enzymes increased after experimental infection with vibrio bacteria and Enterococcus, respectively. Meanwhile, the outcomes of A. platensis after infection align with the findings of Fadl et al.43, who found that feeding Nile tilapia A. platensis reduced their liver enzyme activity. To the best of our knowledge, no studies have been conducted on the measures of the liver enzymes that activate in fish that are fed lemongrass oil following an experimental infection.
Dietary inclusion with a combination of lemongrass oil and A. platensis has shown controversial results on O. niloticus serum markers. Serum proteins in the LG + SP group showed significant increases, while serum kidney function tests decreased in all treatment groups when compared with the control group before infection. Acar et al.75 and Baba et al.76 found that diets that include sweet orange and lemon peel essential oils at concentrations of 3 to 80 g/kg of feed have been shown to raise plasma protein levels of O. niloticus. Additionally, Mohammed et al.42 highlighted the beneficial impact of combination treatment with rosemary and A. platensis on serum proteins of Nile tilapia. Meanwhile, Saccol et al.77 discovered that the serum proteins, urea, and creatinine of silver catfish were not affected by lemongrass oil. Similar results were obtained by Rampelotto et al.78 in silver catfish. However, Al-Zayat57 found that a 7.5 g A. platensis /kg diet significantly improved serum total protein and globulin and decreased levels of albumin, urea, and creatinine and the activities of liver enzymes. Meanwhile, Youssef et al.46 reported that dietary inclusion of A. platensis had no effect on the A/G ratio, globulin levels, or blood total protein but decreased urea and creatinine. Also, Ref45. found that A. platensis supplementation does not affect serum proteins. After infection, serum proteins and urea and creatinine decreased and increased, respectively, in contrast to the same group prior to infection. Fadl et al. achieved similar outcomes43.
However, the phagocytic index and activity results, which improved with various treatments both prior to and upon the experimental infection, supported the serum protein results. Similar findings were observed by Mahmoud et al.36, who found that supplementation with A. platensis (1%) increased the bactericidal, phagocytic, and lysozyme activities of Nile tilapia. A. platensis increased phagocytic and lysosomal activity in the blood of Nile tilapia46. Lemongrass oil increased the phagocytic index and activity of broilers79. Lemongrass oil increased the lysosomal activity of O. niloticus27. Similar results were reported by Dawood et al.80 and Sutili et al.81 with essential oil extract and citral in Nile tilapia and silver catfish, respectively. In channel catfish, feeding 0.02–0.05% of essential oils from oregano, lemongrass, and geranium boosted lysozyme and catalase activity82.
Inclusion of A. platensis and lemongrass oil did not affect the histopathology of different organs before infection, and a reduced pathological lesion resulted from the experimental infection. Similar findings were cited by Abdo et al.38, who reported that tissue histology of the Nile tilapias fed with LEO and A. platensis does not affect. Also, Mohammed et al.42 highlighted the beneficial impact of combination treatment with rosemary and A. platensis in Nile tilapia on organs histopathology. Awe et al.83, who reported that lemongrass oil did not impact the liver and gills histology of African mud catfish upon experimental infection with Aeromonas veronii. Also, Dobhal et al.84 highlighted the beneficial impact of lemongrass oil on the liver and pancreas of rats with metabolic syndrome. These results may be attributed to the anti-inflammatory effect of lemongrass oil85 or to its component polyphenols, which have hepatoprotective effects84. Moreover, Huyben et al.86 reported that plant essential oils increase the villi length of rainbow trout. Arthrospira platensis inclusion increased intestinal villi height and width and counts of lymphocytes and goblet cells in the intestine of Nile tilapia46.
However, all the above-mentioned results were confirmed by the results of gene expression before and after infection. The findings of the antioxidant genes are consistent with the results of Mohammady et al.61, who found that a phytogenic mixture improves the expression of the antioxidant-related genes in Nile tilapia. Similar findings were obtained by Teimouri et al.87 with A. platensis in rainbow trout. In contrast, according to research by Chekani et al.88, the expression of immune-associated genes has significantly improved without effect on the antioxidant genes while using dietary herbal feed supplements in O. mykiss.
Proinflammatory cytokine genes were chosen because of their significance as indicators of inflammation and stress89. Monitoring cytokines such as il-1β is important for the immune response alteration90,91. In the current study, the upregulation (about 1.5:2-fold change) of the genes il-1β, il8, and tnf-α in the non-infected group could refer to an active immune system. The il1 family has an important role in the innate immune response under normal conditions92,93. In accordance, it was reported that dietary feed additives such as ferulic acid and Pediocuccus pentosaceus significantly increased the mRNA levels of il-1β, il8, and tnf-α in rainbow trout cultured under normal conditions94. A current investigation supports the notion that, A. platensis causes the tnf-α gene in carp95.
The enhanced expression of proinflammatory cytokines is essential to overcome infection and oxidative stress conditions. The supplementation of A. platensis and/or lemongrass modulated the cytokine expression after the bacterial infection, as the relative expressions of nfkb2, il-1β, and tnf-α were lower than the control infected group. Moreover, SP and/or lemon supplementation significantly enhanced antioxidant genes expression and alleviated tissue necrosis signs in the different organs after infection. In line with our results, A. platensis enhanced tnf-α gene expression in Nile tilapia36. When A. platensis was added to the diet of rainbow trout, the expression of the tnf-α gene increased96. Moreover, Hassaan et al.97 reported the upregulation of il1β and tnf with A. platensis in Nile tilapia..
Conclusion
It is evident that the growth performance, immunological responses, serum proteins, morphometry of the intestine, and antioxidant and proinflammatory cytokine gene expression of tilapia are all positively impacted by A. platensis and lemongrass oil alone or in combination. Moreover, the antibacterial effects of both treatments, especially against A. hydrophila infection. Finally, non-toxic natural remedies like A. platensis and lemongrass oil can be used to improve the activity’s overall sustainability and are considered eco-friendly.
Abbreviations
- A. hydrophila
Aeromonas hydrophila
- ALT
Alanine transaminase
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- CAT
Catalase
- DNA
Deoxyribonucleic acid
- FCR
Feed conversion ratio
- GPx
Glutathione peroxidase
- Hb
Hemoglobin
- HE
Hematoxylin and eosin
- IL-8
Interleukin 8
- IL-1β
Interleukin 1β
- LEO
Lemongrass essential oil
- LG
Lemongrass
- NF-κB
Nuclear factor kappa
- O. niloticus
Oreochromis niloticus
- PCV
Packed cell volume
- PCR
Real-time polymerase chain reaction
- RBCs
Red blood cells
- RNA
Ribonucleic acid
- AP
A. platensis
- WBC
White blood cell
Author contributions
All authors contributed equally to this work whereas they designed, conducted the experiment and wrote the manuscript. Tasneem Ahmed Abdelmohsen and Awatef Hamed Hamouda worked the experiment, SEF done statistical analysis, Tasneem Ahmed Abdelmohsen, Adel Hassan Saad, Rasha A. Al wakeel, Sabreen E. Fadl, and Awatef Hamed Hamouda write original paper, Sabreen E. Fadl revised the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by Faculty of Fish and Fisheries Technology, Aswan University, Aswan, Egypt (2/2023). We obtained informed consent through an email from the High Dam Lake Development Authority to use the fish in our study.
ARRIVE guidelines
The investigation was conducted in accordance with the ARRIVE standards, as confirmed by the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shah, B. R. & Mraz, J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev. Aquac. 1, 1–18. 10.1111/raq.12356 (2019). [Google Scholar]
- 2.Shinn, A. J. et al. Economic impacts of aquatic parasites on global finfish production. Glob Aquacul Advocate 82–84 (2015).
- 3.Vijayaram, S. et al. Use of algae in aquaculture: A review. Fishes9 (2), 63 (2024). [Google Scholar]
- 4.Malee, B., Petchply, D., Chyratch, S. & Sawangwong, C. Lemon grass oil. In Local Herb 1 (Herb Research Institute, Department of Medical Science, 2000).
- 5.Bachiega, T. F. & Sforcin, J. M. Lemongrass and Citral effecton cytokines production by murine macrophages. J. Ethnopharmacol.137, 909–913 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Al-Sagheer, A. A., Mahmoud, H. K., Reda, F. M., Mahgoub, S. A. & Ayyat, M. S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquacult. Nutr.24, 1006–1014. 10.1111/anu.12637 (2018).
- 7.Tiwari, M. R., Jha, P. K., Sah, B., Kunwar, G. & Jha, A. K. Performance of Lemongrass (Cymbopogon citrates) oil as growth promoter in broiler. Bangl J. Anim. Sci.47, 85–91 (2018). [Google Scholar]
- 8.Guimar ~ aes, L. G. et al. Antioxidant and fungitoxic activities of the Lemongrass essential oil and Citral. Rev. Cienc. Agron.42, 464–472 (2011). [Google Scholar]
- 9.Copatti, C. E., Felix e Silva, A., Lorenzo, V. P., da Costa, M. M. & Melo, J. F. Addition of essential oil from Lemongrass to the Tambaqui (Colossoma macropomum) diet: effects on growth, intestinal enzymes, haematological and metabolic variables, and antimicrobial challenge. Aquac. Res.53 (16), 5656–5666 (2022). [Google Scholar]
- 10.Curay, R. E., Dadole, R. C., Sabilla, R. G., Navarro, V. R. & Uba, K. I. N. Anesthetic efficacy of lavender and Lemongrass essential oils in nile Tilapia (Oreochromis niloticus, i-Excel strain) fry. J. Environ. Aquat. Resour.7, 63–76 (2024). [Google Scholar]
- 11.Alasgah, A. A. et al. Antibiofilm potential of Cumin and Lemongrass essential oils against multidrug-resistant Vibrio parahaemolyticus in retailed fish samples. Food Control. 172 (11116), 2 (2025). [Google Scholar]
- 12.Priyadarshani, I. & Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass. 3, 89–100 (2012). [Google Scholar]
- 13.Velasquez, S. F. et al. Dietary Spirulina (Arthrospira platensis) replacement enhances performance of juvenile nile tilapia (Oreochromis niloticus). J. Appl. Phycol.1, 1 (2016). [Google Scholar]
- 14.Shalaby, O. & Elsayed, N. The multifaceted role of spirulina in environmental sustainability: applications in aquatic systems and ecosystem management. Egypt. J. Aquat. Biology Fisheries. 29 (1), 2631–2652 (2025). [Google Scholar]
- 15.Amin, M. et al. Spirulina platensis supplementation remediates microplastics-induced growth Inhibition and stress in nile tilapia, Oreochromis niloticus. J. Hazard. Mater. Adv.1, 100754 (2025). [Google Scholar]
- 16.Adel, M., Yeganeh, S., Dadar, M., Sakai, M. & Dawood, M. A. O. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol.56, 436–444 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Yeganeh, S., Teimouri, M. & Amirkolaie, A. K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci.101, 84–88 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Kim, S. S., Shin, S. J., Han, H. S., Kim, J. D. & Lee, K. J. Effects of dietary Spirulina Pacifica on innate immunity and disease resistance against Edwardsiella tarda in Olive flounder Paralichthys olivaceus. Israeli J. Aquaculture. 67, 9 (2015). [Google Scholar]
- 19.Rahimnejad, S. Partial replacement of fish meal with Spirulina Pacifica in diets for Parrot fish (Oplegnathus fasciatus). Turkish J. Fisheries Aquat. Sci.13 (13), 197–204 (2013). [Google Scholar]
- 20.Dar, B. A., Khaliq, R., Jha, G. N., Kour, P. & Qureshi, T. A. Protective effects of dietary spirulina against cadmium chloride exposed histoarchitectural changes in the liver of freshwater catfish Clarias batrachus (Linnaeus, 1758). Indian J. Fisheries. 61 (3), 83–87 (2014). [Google Scholar]
- 21.Vasudhevan, I. & James, R. Effect of optimum Spirulina along with different levels of vitamin C incorporated diets on growth, reproduction and coloration in goldfish Carassius auratus (Linnaeus, 1758). Indian J. Fisheries. 58 (2), 101–106 (2011). [Google Scholar]
- 22.James, R., Sampath, K., Thangarathinam, R. & Vasudevan, I. Effect of dietary Spirulina level on growth, fertility, coloration and leucocyte count in red swordtail, Xiphophorus helleri. Israeli J. Aquaculture. 58 (2), 97–104 (2006). [Google Scholar]
- 23.Dernekbasi, S., Unal, H., Karayucel, I. & Aral, O. Effect of dietary supplementation of different rates of Spirulina (Spirulina platensis) on growth and feed conversion in Guppy (Poecilia reticulata Peters, 1860). J. Anim. Veterinary Adv.9 (9), 1395–1399 (2010). [Google Scholar]
- 24.Shah, M. A. R. et al. Mechanistic insights into the nutritional and therapeutic potential of Spirulina (Arthrospira) spp.: challenges and opportunities. Trends Food Sci. Technol.1, 104648 (2024). [Google Scholar]
- 25.Kamilya, D., Sarkar, S., Maiti, T. K., Bandyopadhyay, S. & Mal, B. C. Growth and nutrient removal rates of Spirulina platensis and Nostoc muscorum in fish culture effluent: a laboratory-scale study. Aquac. Res.37 (15), 1 (2006). [Google Scholar]
- 26.Ibrahem, M. D., Mohamed, M. F. & Ibrahim, M. A. The role of Spirulina platensis (Arthrospira platensis) in growth and immunity of Nile tilapia (Oreochromis niloticus) and its resistance to bacterial infection (2013).
- 27.Al-Sagheer, A. A., Mahmoud, H. K., Reda, F. M., Mahgoub, S. A. & Ayyat, M. S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from Lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquacult. Nutr.24 (3), 1006–1014 (2018). [Google Scholar]
- 28.Suvarna, K., Layton, C. & Bancroft, J. Bancroft’s Theory and Practice of Histological Techniques, 8 edn, Vol. 672 (Elsevier, 2018).
- 29.Esam, F. et al. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in nile tilapia. Ecotoxicol. Environ. Saf.231, 113187 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Livak, K. J. & Schmittgen, T. D. Schmittgen, analysis of relative gene expression data using realtime quantitative PCR and the 2-∆∆CT method. Methods (Orlando). 25 (2001), 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 31.McFarland, J. Nephelometer: an instrument for media used for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Association. 1907 (14), 1176–1178. 10.1001/jama.1907.25320140022001f (1907). [Google Scholar]
- 32.K Amos, H. Procedures for the Detection and Identification of Certain Fish Pathogens, 3rd edn., 6–21 (Am. Fisheries Soc., 1985).
- 33.Gatlin, D. M. et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res.38 (6), 551–579 (2007). [Google Scholar]
- 34.Okasha, L. A., Abdellatif, J. I., Abd-Elmegeed, O. H. & Sherif, A. H. Overview on the role of dietary Spirulina platensis on immune responses against Edwardsiellosis among Oreochromis Niloticus fish farms. BMC Vet. Res.20 (1), 290 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metekia, W. A. & Ulusoy, B. H. Antimicrobial activity of Spirulina platensis extract on total mesophilic and psychrophilic bacteria of fresh tilapia fillet. Sci. Rep.13 (1), 13081 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud, M. M., El-Lamie, M. M., Kilany, O. E. & Dessouki, A. A. Spirulina (Arthrospira platensis) supplementation improves growth performance, feed utilization, immune response, and relieves oxidative stress in nile tilapia (Oreochromis niloticus) challenged with Pseudomonas fluorescens. Fish Shellfish Immunol.72, 291–300 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Wu, Q. et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of spirulina: an overview. Arch. Toxicol.90 (8), 1817–1840 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Abdo, S. E. et al. Combined dietary spirulina platensis and citrus limon essential oil enhances the growth, immunity, antioxidant capacity and intestinal health of nile Tilapia. Veterinary Sci.11 (10), 474 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sukrakanchana, N., Ruangkhlaii, K. & Choksawasdikorn, P. Effects of dietary essential oils supplementation on the growth performance, blood parameters, non-specific immunity and disease resistance of nile Tilapia (Oreochromis niloticus). Thai Sci. Technol. J.29, 203–215 (2021). [Google Scholar]
- 40.Giannenas, I. et al. Assessment of dietary supplementation with carvacrol or thymol containing feed additives on performance, intestinal microbiota and antioxidant status of rainbow trout (Oncorhynchus mykiss). Aquaculture350, 26–32 (2012). [Google Scholar]
- 41.Oussalah, M., Caillet, S., Saucier, L. & Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control18 (5), 414–420 (2007).
- 42.Mohammed, A. A. B., Saad, A. H., Abdo, S. E., Fadl, S. E. & Hamouda, A. H. Immunomodulatory and growth promotors effects of Rosemary (Rosmarinus officinalis) and/or Spirulina with respect to some gene expression on nile tilapia (Oreochromis niloticus) in Aswan Governorate. Aquaculture596, 741790 (2025). [Google Scholar]
- 43.Fadl, S. E., Barakat, M. E. & Elgohary, M. E. Biochemical studying of Anabaena (Cyanobacteria) on nile tilapia. Alexandria J. Veterinary Sci.39, 91–104 (2013). [Google Scholar]
- 44.Yardimci, B. & AYDIN, Y. Pathological findings of experimental Aeromonas hydrophila infection in nile tilapia (Oreochromis niloticus). Ankara Üniversitesi Veteriner Fakültesi Dergisi. 58 (1), 47–54 (2011). [Google Scholar]
- 45.Al-Deriny, S. H. et al. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of nile tilapia (Oreochromis niloticus). Aquaculture Rep.17, 100390 (2020). [Google Scholar]
- 46.Youssef, I. M. et al. Effect of Spirulina platensis on growth, hematological, biochemical, and immunological parameters of nile tilapia (Oreochromis niloticus). Trop. Anim. Health Prod.55 (4), 275 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amer, S. A. Effect of Spirulina platensis as feed supplement on growth performance, immune response and antioxidant status of mono-sex nile Tilapia (Oreochromis niloticus). Benha Veterinary Med. J.30 (1), 1–10 (2016). [Google Scholar]
- 48.El-Araby, D. A. et al. Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture546, 737413 (2022).
- 49.Islam, M. et al. Probiotics and Spirulina platensis improved growth performance of nile tilapia (Oreochromis niloticus) by upgrading intestinal morphology and activating GH/IGF axis. Aquac. Res.2025, 1839162 (2025). [Google Scholar]
- 50.Sherif, E. M. et al. Growth performance, antioxidative status, and immune response of nile tilapia (Oreochromis niloticus) fed dietary fermented Spirulina platensis. Aquaculture Rep.39, 102324 (2024). [Google Scholar]
- 51.Adeniyi, O. V. Growth performance of Clarias gariepinus (Burchell 1822) fed diets fortified with Lemongrass (Cymbopogon citratus). Acta Vet. Eurasia. 46 (1), 21–29 (2020). [Google Scholar]
- 52.Gichana, Z. Water quality and growth performance of nile tilapia (Oreochromis niloticus), Chia (Salvia hispanica) and lemon grass (Cymbopogon citratus) in a media-based aquaponics system. Asian J. Biology. 20 (5), 12–22 (2024). [Google Scholar]
- 53.Orzuna-Orzuna, J. F. & Granados-Rivera, L. D. Growth performance, antioxidant status, intestinal morphology, and body composition of nile tilapia (Oreochromis niloticus) supplemented with essential oils: A meta-analysis. Res. Vet. Sci.176, 105353 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Adebayo, A., Oke, I. O. & Dada, A. A. Evaluation of lemon grass (Cymbopogon citratus) as phyto-additive in the diet of African catfish (Clarias gariepinus) fingerlings. Appl. Trop. Agric.25 (1), 29–36 (2020). [Google Scholar]
- 55.Francis, G., Makkar, H. P. S. & Becker, K. Anti-nutritional factors present in plant-derived alternative fish feed ingredients and their effects in fish. Aquaculture199 (3–4), 197–228 (2001). [Google Scholar]
- 56.Abdel-Tawwab, M. et al. Dietary spirulina (Arthrospira platenesis) Mitigated the adverse effects of imidacloprid insecticide on the growth performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia. Compar. Biochem. Physiol. C Toxicol. Pharmacol.247, 109067 (2021). [DOI] [PubMed]
- 57.Al-Zayat, A. M. Effect of various levels of Spirulina (Arthrospira platensis) as feed supplement on growth performance, feed utilization, immune response and hematology of the nile tilapia (Oreochromis niloticus) fingerlings. Egypt. J. Aquat. Biology Fisheries. 23 (3), 361–370 (2019). [Google Scholar]
- 58.El-Sheekh, M., El-Shourbagy, I., Shalaby, S. & Hosny, S. Effect of feeding Arthrospira platensis (Spirulina) on growth and carcass composition of hybrid red tilapia (Oreochromis niloticus x Oreochromis mossambicus). Turkish J. Fisheries Aquat. Sci.14 (2), 471–478 (2014). [Google Scholar]
- 59.Sarma, M., Sapcto, D., Sarma, M. & Gohain, A. Herbal growth promoters on hemato- biochemical constituents in broilers. Indian Vet. J.80, 946–948 (2003). [Google Scholar]
- 60.Thuong, H. N., Tran, T. L. N., Le, T. N. T. & Vo, V. T. Hematological parameters of red tilapia (Oreochromis sp.) fed Lemongrass essential oil (Cymbopogon citratus) after challenge with Streptococcus agalactiae. Israeli J. Aquaculture. 1823524, 9 (2022). [Google Scholar]
- 61.Mohammady, E. Y. et al. Can dietary phytogenic mixture improve performance for growth, digestive enzyme activity, blood parameters, and antioxidant and related gene expressions of Nile Tilapia, Oreochromis Niloticus? Anim. Feed Sci. Technol.290, 115369 (2022).
- 62.Sayed, A. E. D. H. & Fawzy, M. A. Effect of dietary supplementation of Spirulina platensis on the growth and haematology of the catfish Clarias gariepinus. J. Adv. Biology. 5 (2), 625–635 (2014). [Google Scholar]
- 63.Talpur, A. D., Ikahwanuddin, M. H. D. & Bolong, A. M. A. Nutritional effect of ginger (Zingiber officinale) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture400, 46–52 (2013). [Google Scholar]
- 64.Hardi, E. H., Kusuma, I. W. & Suwinarti, W. Antibacterial activities of some Borneo plant extracts against pathogenic bacteria of Aeromonas hydrophila and Pseudomonas sp. Aquaculture, aquarium, conservation and legislation international. J. Bioflux Soc.9 (3), 638–646 (2016). [Google Scholar]
- 65.Ergena, A., Natarajan, P. & Bedewi, Z. Determination of some haematological parameters and disease resistance capacity to Aeromonas hydrophila infection in the nile tilapia, Oreochromis niloticus L. fed dietary supplementation of ginger (Zingiber officinale). Egypt. J. Aquat. Biology Fisheries. 27 (3), 551–568 (2023). [Google Scholar]
- 66.Weiss, D. J. & Wardrop, K. J. Schalm’s Veterinary Hematology (Wiley, 2011).
- 67.El-Habashi, N. et al. Effect of using Spirulina and Chlorella as feed additives for elevating immunity status of nile tilapia experimentally infected with Aeromonas hydrophila. Aquac. Res.50 (10), 2769–2781 (2019). [Google Scholar]
- 68.Abdel-Latif, H. M., Abdel-Tawwab, M., Khafaga, A. F. & Dawood, M. A. Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus Carpio L.) fingerlings. Aquaculture526, 735432 (2020). [Google Scholar]
- 69.Li, C. C., Yu, H. F., Chang, C. H., Liu, Y. T. & Yao, H. T. Effects of Lemongrass oil and Citral on hepatic drug-metabolizing enzymes, oxidative stress, and acetaminophen toxicity in rats. J. Food Drug Anal.26 (1), 432–438 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abo El-Ward, A., Eid, A. E., Mohamed, K. A., Abd-elfattah, B. & Hasan, M. A. Growth performance of nile tilapia (Oreochromis niloticus) fingerlings fed diet supplemented with different of Spirulina platensis levels. Egypt. J. Anim. Prod.53 (3), 181–190 (2016). [Google Scholar]
- 71.Gamboa-Delgado, J., Villarreal-Cavazos, D., García-Pérez, O. D. & Cruz-Valdez, J. C. Madagascar cockroach meal and Spirulina as fish meal replacements in diets for nile tilapia: effects on growth, assimilation and hepatoprotection. J. Insects as Food Feed. 1, 1–17 (2025). [Google Scholar]
- 72.Souza, E. M. et al. Cymbopogon flexuosus Essential Oil as an Additive Improves Growth, Biochemical and Physiological Responses and Survival against Aeromonas hydrophila Infection in Nile tilapia 921 (Anais da Academia Brasileira de Ciências, 2020). [DOI] [PubMed]
- 73.Bekele, B., Natarajan, P., Workagegn, K. B. & Devika Pillai, D. Hematological and histopathological changes in artificially infected nile tilapia with vibrio species. J. Aquaculture Res. Dev.12, 1–5 (2021). [Google Scholar]
- 74.Martins, M. L. et al. Haematological changes in nile tilapia experimentally infected with Enterococcus Sp. Brazilian J. Biology. 68, 657–661 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Acar, Ü., Kesbiç, O. S., Yılmaz, S., Gültepe, N. & Türker, A. Evaluation of the effects of essential oil extracted from sweet orange Peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture437 (1), 282–286 (2015). [Google Scholar]
- 76.Baba, E., Acar, Ü., Öntas, C. & Kesbiç, O. S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture465 (1), 13–18 (2016).
- 77.Saccol, E. M. H. et al. Addition of Lippia alba (Mill) N. E. Brown essential oil to the diet of the silver catfish: an analysis of growth, metabolic and blood parameters and the antioxidant response. Aquaculture416–417 (1), 244–524 (2013). [Google Scholar]
- 78.Rampelotto, C. et al. Supplementation with microencapsulated Lemongrass essential oil improves protein deposition and carcass yield in silver catfish (Rhamdia quelen). Acta Scientiarum Anim. Sci.40, 1 (2018). [Google Scholar]
- 79.Ghanima, M. M. A. et al. Impacts of tea tree or Lemongrass essential oils supplementation on growth, immunity, carcass traits, and blood biochemical parameters of broilers reared under different stocking densities. Poult. Sci.100 (11), 101443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dawood, M. A. et al. Antiparasitic and antibacterial functionality of essential oils: an alternative approach for sustainable aquaculture. Pathogens10 (2), 185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sutili, F. J. et al. Effect of dietary supplementation with citral-loaded nanostructured systems on innate immune responses and gut microbiota of silver catfish (Rhamdia quelen). J. Funct. Foods.60, 103454 (2019). [Google Scholar]
- 82.Zheng, Z. L. et al. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture292, 214–218 (2009).
- 83.Awe, F. A., Hammed, A. M. & Olanloye, O. A. Effects of whole lemon grass (Cymbopogon citratus) extract on bacteria (Aeromonas veronii) infected sub-adult Clarias gariepinus. Asian J. Appl. Sci.12 (2), 99–107 (2019). [Google Scholar]
- 84.Dobhal, S., Singh, M. F., Setya, S. & Bisht, S. Comparative assessment of the effect of Lemongrass (Cymbopogon citratus) ethanolic extract, aqueous extract and essential oil in high fat diet and Fructose induced metabolic syndrome in rats. Indian J. Pharm. Educ. Res.56 (2), S281–S293 (2022). [Google Scholar]
- 85.Amujal, M. et al. Hepatoprotective effect of Cymbopogon citratus essential oils against nevirapine-induced hepatic damage in Wistar albino rats. Afr. J. Tradit. Complement. Altern. Med.15 (4), 64–71 (2018). [Google Scholar]
- 86.Huyben, D., Chiasson, M., Lumsden, J. S., Pham, P. H. & Chowdhury, M. A. K. Dietary microencapsulated blend of organic acids and plant essential oils affects intestinal morphology and microbiome of rainbow trout (Oncorhynchus mykiss). Microorganisms9 (10), 2063 (2021). [DOI] [PMC free article] [PubMed]
- 87.Teimouri, M., Yeganeh, S., Mianji, G. R., Najafi, M. & Mahjoub, S. The effect of Spirulina platensis meal on antioxidant gene expression, total antioxidant capacity, and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem.45, 977–986 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Chekani, R., Akrami, R., Ghiasvand, Z., Chitsaz, H. & Jorjani, S. Effect of dietary dehydrated lemon Peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture539, 736597 (2021). [Google Scholar]
- 89.Dawood, M. A. et al. The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture523, 735205 (2020). [Google Scholar]
- 90.Guzmán-Villanueva, L. T. et al. Dietary administration of β-1, 3/1, 6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on Gilthead seabream growth, immune responses and gene expression. Fish Shellfish Immunol.39 (1), 34–41 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Dawood, M. A. et al. Lactobacillus plantarum L-137 and/or β-glucan impacted the histopathological, antioxidant, immune-related genes and resistance of nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Res. Vet. Sci.130, 212–221 (2020). [DOI] [PubMed] [Google Scholar]
- 92.El-Kassas, S., Odemuyiwa, S., Hajishengallis, G., Connell, T. D. & Nashar, T. O. Expression and regulation of cholecystokinin receptor in the chicken’s immune organs and cells. J. Clin. Cell. Immunol.7, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muñoz-Wolf, N. & Lavelle, E. C. A guide to IL-1 family cytokines in adjuvanticity. FEBS J.285, 2377–2401 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Habibnia, M. et al. Growth performance, hematological and immune parameters, and mRNA levels of cytokines and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss) fed on Pediocuccus Pentosaceus and/or ferulic acid. Anim. Feed Sci. Technol.1, 115872 (2024). [Google Scholar]
- 95.Watanuki, H., Ota, K., Tassakka, A. C. M. A., Kato, T. & Sakai, M. Immunostimulant effects of dietary Spirulina platensis on carp, Cyprinus carpio. Aquaculture258 (1–4), 157–163 (2006). [Google Scholar]
- 96.Sheikhzadeh, N. et al. Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture510, 1–8 (2019). [Google Scholar]
- 97.Hassaan, M. S. et al. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile Tilapia, Oreochromis niloticus. Fish Shelfish Immunol.108, 63–72 (2021). [DOI] [PubMed]
- 98.El-Kassas, S. et al. Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex nile tilapia fed Moringa oleifera leaf powder. Aquaculture Rep.18, 100422 (2020). [Google Scholar]
- 99.Abdo, S. E. et al. The synergetic effect of Bacillus species and Yucca Shidigera extract on water quality, histopathology, antioxidant, and innate immunity in response to acute ammonia exposure in nile tilapia. Fish Shellfish Immunol.128, 123–135 (2022). [DOI] [PubMed] [Google Scholar]
- 100.Abdo, S. E. et al. Vitamin C rescues inflammation, immunosuppression, and histopathological alterations induced by Chlorpyrifos in nile tilapia. Environ. Sci. Pollut. Res.28, 28750–28763 (2021). [DOI] [PubMed] [Google Scholar]
- 101.El-Kassas, S. et al. Moringa oleifera leaf powder dietary inclusion differentially modulates the antioxidant, inflammatory, and histopathological responses of normal and Aeromonas hydrophila-infected mono-sex nile Tilapia (Oreochromis niloticus). Front. Veterinary Sci.9, 918933 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.