Abstract
The hospital sink drain microbiome can harbour opportunistic pathogens and antimicrobial resistance genes (ARGs). Aspects of this habitat, such as exposure to disinfectants, antibiotics, nutrients, and body fluids could exacerbate horizontal gene transfer of ARGs and clinically impactful pathogen resistance. Here, we explore features of the hospital sink drain that may favour ARG acquisition and transmission, highlight studies providing evidence of transfer, and consider strategies to mitigate these risks.
Subject terms: Microbial ecology, Antimicrobials, Environmental microbiology, Pathogens
Introduction: the evolution of resistant opportunistic pathogens
One of the greatest concerns in the rise of antimicrobial resistance (AMR) is the increase of multidrug resistance in opportunistic pathogens: species that are usually non-pathogenic when encountering healthy individuals but can cause severe infections in vulnerable populations. The risks posed by multidrug-resistant opportunistic pathogens is evident in the 2024 World Health Organisation Bacterial Priority Pathogens List, where, of the 15 species or groups named, 10 are generally considered opportunistic pathogens. These include drug-resistant strains of Acinetobacter baumannii and Enterobacterales in the critical group, as well as Enterococcus faecium, Pseudomonas aeruginosa, and Staphylococcus aureus in the high-priority group1. Because opportunistic pathogens exist in large populations outside of an infection context, such as in the environment or as human commensals, evolutionary processes occurring in these natural habitats shape the risks subsequently posed by opportunistic pathogens when they do cause disease. In a hospital setting, where the direct threat of opportunistic infection is already elevated for patients who may be immunocompromised or have other underlying health conditions, microbial evolution in response to complex environmental selection pressures can facilitate the emergence of difficult-to-treat pathogens2.
Antimicrobial resistance can arise in pathogens through de novo mutations, and through horizontal gene transfer (HGT) of antimicrobial resistance genes (ARGs), which is often mediated by mobile genetic elements (MGEs)3. This ability to acquire resistance is crucial for understanding the rapid rise of AMR among opportunistic pathogens, because many of the clinically important ARGs are often found on MGEs. For example, plasmid-disseminated mcr genes confer resistance to colistin4, while carbapenem resistance genes are often carried on plasmids which have been responsible for the rapid global dissemination of carbapenemases, and the rise of infections caused by carbapenemase-producing Enterobacterales (CPEs)5. Importantly, MGEs can often provide resistance to multiple antimicrobials at once because multiple ARGs can be present on a single MGE and transfer together in a single event. Subsequent selection for just one of these traits can result in other resistance traits rising to high frequency without direct selection, thanks to genetic linkage6,7. In other cases, resistances can spread across a population solely through the ability of the MGE to act as an infectious element8. Owing to the activity of MGEs, opportunistic pathogens might gain resistance to multiple antimicrobials in their natural habitats without ever experiencing antibiotic treatment in an infection context. Understanding the ecology of opportunistic pathogens and their cognate MGEs—and identifying how and where organisms acquire clinically-relevant resistance traits—could focus and inform strategies to impede HGT in these habitats to reduce the threat of subsequent difficult-to-treat infections.
The microbiome of the built environment
Microbiomes are microbial ecosystems, with the term ‘microbiota’ describing the microbes present, and the term ‘microbiome’ describing the microbes, DNA, and environmental conditions9. While the human gut microbiome has been widely explored as an extensive reservoir for ARGs and opportunistic pathogens—and is undoubtedly an important location for HGT events10—environmental microbiomes, including the microbiome of the built environment, may also play a significant role in the spread of ARGs and emergence of resistant pathogens. The ‘built environment’ describes human-built areas including buildings and any other manmade structures or systems. The relative inhospitality of these environments to microbes, due to the lack of water and nutrients, means that the microbiomes found in the built environment are distinct to those found in nature. The unique habitats formed by the built environment, both physically and chemically, cause the resident microbes to face selective pressures that few species can survive and thrive in, making them less diverse than some other environmental microbiomes (e.g. those of soil) as a consequence2,11.
The development of next-generation sequencing technologies in recent decades has rapidly advanced the study of microbiomes. Metagenomic sequencing, where all DNA in a sample is extracted and sequenced12, has allowed a comprehensive view of the taxonomic profiles of a sample without the need for culturing, as well as revealing the genes and MGEs present13. Microbiome studies employing these techniques, and others, have indicated that humans are the primary source of microbes in the built environment14. However, the microbial community is influenced by the types of activity occurring. For example, in domestic settings, the presence of pet cats or dogs increases bacterial richness and diversity in a household15, and studies sampling kitchens often find foodborne pathogens like Campylobacter, especially when unhygienic handling of food has occurred16,17. In the hospital built environment, human presence has a profound impact on microbiome composition. A study tracking the microbiomes of surfaces in a hospital found a distinct shift in community composition once the hospital became operational, with the bacterial communities of room surfaces converging to increasingly resemble those of their resident patients over time18. Similarly, the microbiomes of bedrails, computer keyboards, and sinks in an intensive care unit (ICU) shifted to more environmental-associated bacteria such as Bacillaceae and Rhizobiaceae during renovations, and back to more human-associated microbes like Staphylococcus and Cutibacterium after re-opening19.
As well as microbes, the built environment can act as a reservoir of ARGs13. The contribution of hospital ARGs to global environmental ARG load is likely to be relatively small compared with contributions from agricultural and community inputs20,21. However, the presence of patients who are likely to have compromised immunity heightens the risks posed by the microbiome of the hospital built environment, which can act as a source of both ARGs and opportunistic pathogens that can lead to hospital-acquired infections22. Within days of occupying a room, bacteria and ARGs originating from a patient can disseminate into the hospital environment23. Likewise, patients can be colonised by microbes originating from the hospital environment. For example, there is overlap between the preterm infant gut microbiome and the hospital room microbiome, where exchange of strains between the infant and room have been documented in both directions24. Within the hospital built environment there are numerous microbiomes that patients and staff may encounter, such as those present on surfaces like the floor and door handles18,25. Of these, the concealed microbiomes of sink and shower drains contain the greatest non-human-associated microbial biomass in the hospital built environment23,26,27. This ‘grey water’ microbiome located immediately downstream of water outlets therefore has the potential to be an important reservoir for the evolution and dissemination of clinically relevant opportunistic pathogens.
Features of the sink drain microbiome promoting resistance gene exchange
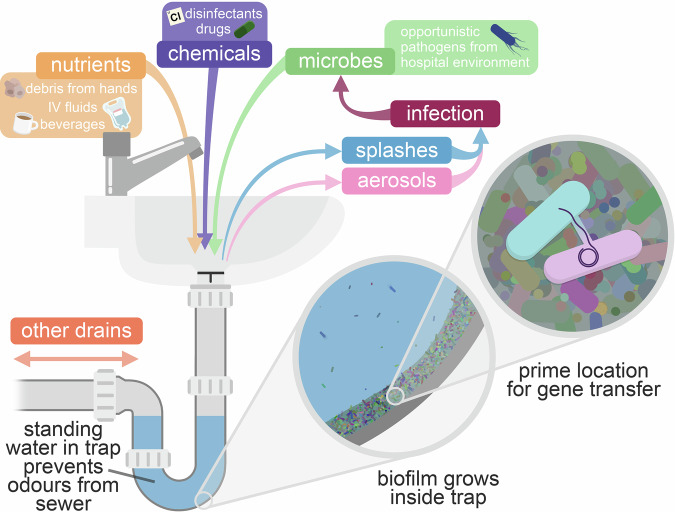
Sink traps are plumbing devices designed to prevent foul odours from emanating from the sewer by placing a pool of standing water immediately downstream of the waste outlet (Fig. 1). Water exiting the sink flushes and re-fills the trap. Sink traps therefore offer a moist and aerated surface in which a microbiome can develop. Whilst all microbiomes in the hospital setting provide potential for the spread of opportunistic pathogens and ARGs, the hospital sink drain microbiome has features that may especially favour acquisition and transmission of ARGs. Firstly, sink traps are significantly more challenging to clean compared to other hospital surfaces, since they are located down a narrow and often curved pipe, usually partially obscured by a strainer installed to prevent larger objects from passing through and blocking the drain further downstream, which prevents physical disruption. Microbes attach and grow inside the drain as biofilms: surface-attached communities which are highly resistant to typical disinfection procedures28 and are considered hotspots for the maintenance, evolution, and transmission of resistance-encoding plasmids29. In a 2020 study by Ledwoch et al., the authors developed an in vitro sink drain biofilm model, and found that widely-used chorine-based disinfectants such as sodium hypochlorite (bleach) only partially reduced viability of bacteria in the model, and biofilms were able to recover just 4 days after disinfection30. Similarly, a study on real-world hospital sink drains found that biofilms could fully recover within 7 days of treatment with hydrogen peroxide or bleach31.
Fig. 1.
The hospital sink drain microbiome as a melting pot for AMR transmission to nosocomial pathogens.
As well as failing to completely inhibit biofilms, disinfectants might actually stimulate emergence of AMR. Chlorine-based disinfectants were shown to promote ARG transmission by HGT both within strains of Escherichia coli and between E. coli and Salmonella when present at subinhibitory concentrations32. Other disinfectants, such as quaternary ammonium compounds (QACs)33 and triclosan34, have been shown to have similar effects, though it should be noted that triclosan is no longer used in hospital detergents in the European Union since 201735. HGT increases after disinfectant exposure due to the production of reactive oxygen species, which triggers the SOS response—promoting both expression of ARGs and mobile genetic elements—and increases cell membrane permeability32,36. Disinfectants can also select for chromosomal mutations that provide direct cross-resistance to antimicrobials37, or promote AMR emergence indirectly by increasing conjugation permissiveness34. While harsh disinfectant stresses might transiently reduce the microbiological load, the survivors may pose an even greater threat.
Besides disinfectants, hospital sink traps can be exposed to diverse types of waste entering the sink, such as debris, oils, and dead cells from hands during washing, left over beverages, IV fluids, and drugs. These can act as sources of nutrients to facilitate bacterial growth, as triggers for HGT, and can impose selective pressures favouring resistance. When assessing different water chemistry dipsticks, Rodger et al. detected both tetracycline and sulphonamide antibiotics in a hospital sink trap38. The same group surveyed 287 sinks across 29 hospitals in the United Kingdom and detected antibiotic residues in 33% of sink trap samples, with the majority of detections being for beta-lactam antibiotics39. Another study detected antimicrobial residues in 31/115 hospital sink samples taken over a 12-week period40. Like disinfectants, exposure to low concentrations of some antibiotics is known to promote HGT41. Although most hospitals have strict rules on sink usage, including patient-facing sinks being designated to handwashing only, a study monitoring activity at four ICU sinks in the United States found that only 4% of total interactions with the sinks were for handwashing42. On top of the risk of increasing selective pressures in the sink drain, the use of patient-facing sinks for activity outside of handwashing has a risk of enriching the sink drain microbiome. Addition of nutrients into sink traps is crucial for the growth of some opportunistic pathogens, particularly CPEs43.
The sink drain microbiome as a persistent reservoir of opportunistic pathogens and ARGs
Many studies have demonstrated that the hospital sink drain is a key, long-term, persistent reservoir for opportunistic pathogens and ARGs, and have suggested that it can act as a source of infection44–63. A systematic review by Kizny Gordon et al. in 2017 identified 32 studies reporting carbapenem-resistant bacteria in hospital water, with drains representing the most common location, and with Pseudomonas aeruginosa being the most frequent species44. Another systematic review by Volling et al. in 2020 identified 51 studies suggesting that sink drainage systems are a reservoir for hospital-acquired Gammaproteobacterial patient colonisation or infection, again implicating P. aeruginosa as the most common organism involved45. More recent reports describe similar patterns. Sink drains were proposed to be a major reservoir when investigating the sources of Serratia marcescens colonisation in a neonatal ICU, with an average of 44% of sink samples being positive for S. marcescens across five periods of sampling55. In a study that collected sink samples over 27 months in two hospitals and sequenced over 800 isolates, species of Pseudomonas and Serratia persisted for the whole sampling duration, as well as several ARG-carrying plasmids56. Genes encoding extended spectrum beta-lactamases (ESBLs), conferring resistance to beta-lactam antibiotics, are commonly found in the hospital sink drain. In a culture-based study sampling hospital surfaces in non-outbreak settings, 26.7% of sink drains contained ESBL-producing bacteria—a higher proportion than any other hospital surface57. During a long-term outbreak of carbapenemase-producing organisms on an ICU in a Belgian hospital, sink drains were a persistent reservoir, even withstanding disinfection attempts and replacement of contaminated equipment58. Using whole-genome sequencing, the authors found that the sink isolates carried the same multidrug resistance plasmid as the patient isolates, suggesting an epidemiological link. Interestingly, the plasmids also contained genes conferring resistance to QACs, which could explain why these strains could withstand disinfection attempts with QAC-based disinfectants58. Sinks were found to be a prolonged and recalcitrant reservoir of Klebsiella pneumoniae carbapenemase (KPC)-producing E. coli at a hospital in the United Kingdom59. The outbreak clone of carbapenem-resistant E. coli was identified in patient colonisation and infection, as well as in the sink drains. Multiple ward closures and replacement of sink traps and other plumbing infrastructure only resulted in temporary decline in incidence, as the sinks were rapidly recolonised59. In another case, sampling of sinks over a year in five different wards of a hospital found opportunistic pathogens to be highly prevalent, with Pseudomonas spp. and Stenotrophomonas being the most predominant, including multidrug-resistant Pseudomonas aeruginosa60. A further study describing an outbreak of extensively drug-resistant P. aeruginosa found the resistant strains persisting in the hospital environment, primarily in sinks and toilets, despite regular disinfection61. Even when a resistance gene has very low prevalence in the patient population, it can persist in the sink drain and potentially pose a risk to future patients. For example, an Acinetobacter pittii strain isolated from a sink drain in a Japanese hospital was found to harbour a plasmid carrying the ESBL gene blaNDM-1, despite no other New Delhi metallo-beta-lactamase (NDM)-producers being detected in any previous environmental or patient samples at the hospital62. In a study comparing sink drain biofilms in a neonatal ICU and an adult ICU in a French hospital, the authors found that the two wards had distinct bacterial communities: Pseudomonas, Stenotrophomonas, and Staphylococcus dominating the adult ICU; and Achromobacter, Serratia, and Acidovorax dominating the neonatal ICU. These differences were likely due to the regular disinfection regime at the neonatal ICU. Despite the differences in the communities, the ARG profiles of the sink communities on both wards showed no significant differences, indicating that the ARG abundance is independent of the taxonomic identities of the microbiota63. Whilst most studies have focused on the bacterial portion of the sink drain microbiota, it should be noted that hospital sink drains also act as a reservoir for eukaryotic opportunistic pathogens64 (though the risk of trans-species ARG HGT is likely smaller for eukaryotes).
In some cases, the causal role of the sink reservoir in driving an outbreak has been inferred by the success of interventions targeting sinks or sink use. A year-long outbreak of blaOXA-48-positive Serratia marcescens at an ICU in 2016 was not contained until the sink traps were identified as the source65. With a total of 34 cases and 3 patient deaths attributed to infection by the outbreak S. marcescens, all environmental samples were negative for the outbreak strains except for the sink outlets and traps. It was found that tap water was sometimes being used to clean the patients or flush gastric tubes, which the authors postulated led to contamination from the sinks and transmission of S. marcescens. Efforts to both replace and disinfect the traps failed to stop the outbreak, but behavioural changes after workshops for the staff to raise awareness of the risks of hospital sinks were successful in outbreak containment65. An outbreak of ESBL-producing Klebsiella oxytoca at a Canadian ICU was attributed to the handwashing sink drains after isolates, deemed identical by pulsed-field gel electrophoresis (PFGE), were found in patient and environmental samples. No new infections followed after replacement of all sink traps and increased sink disinfection regimes66. Another outbreak of NDM-producing K. pneumoniae on a surgical ICU in France was associated with the sinks, as the epidemic strain, consistent with 9 patient isolates based on PFGE, was isolated from a sink. An NDM-producing Enterobacter cloacae was also found in another sink. The outbreak was only halted after installation of new sink traps designed to reduce production of aerosols67. During a 2-year outbreak of K. oxytoca carrying blaIMP-8 at an ICU in Spain, a strain consistent with the outbreak strain via PFGE was detected in the drainpipe and trap of one sink located in a storage room. All other environmental and staff screening samples were negative. Permanent removal of the K. oxytoca-positive sink resulted in reduced cases, but the outbreak continued with new cases clustering in a cubicle adjacent to the storage room. The outbreak was finally eradicated after removal of the horizontal drainage system that had previously connected the sink in the cubicle to the removed sink in the storage room, indicating that connection between waste pipes could allow transmission of bacteria between sinks68. A narrative 5-year review of 19 studies reporting drain-associated outbreaks and interventions indicated that control measures were ultimately successful at stopping the outbreak in all cases where results were reported69.
Many of the studies described above provide circumstantial evidence linking outbreaks to the sink drain microbiome, and their observational design can struggle with proving a causal, directional link between an environmental reservoir and patient infection. Though an increasing number provide genomic data necessary for high-resolution identification of outbreak clones in the environment, most start after an outbreak has begun, introducing the possibility that the environmental reservoir has been contaminated by material from an infected patient, or from a shared third party, rather than acting as a source for infection. However, laboratory studies show that microbes inside sink traps can be readily transmitted out to the surrounding environment. Kotay et al. added green fluorescent protein (GFP)-expressing E. coli to a sink trap. Although running water into the sink did not disperse the bacteria directly from the trap, the authors showed that when a small amount of nutrients was added, the E. coli biofilm could grow upwards in the tailpipe at 1 inch per day, and reach the strainer in a week, causing dispersal when operating the faucet70. Such a design mimics a real hospital environment, where the biofilm inside a sink trap can mature for long periods of time with small amounts of nutrients entering regularly. Another study showed that droplets containing carbapenem-resistant Enterobacteriaceae could travel up to 1 metre from the sink to surrounding areas, and that sink and drain design could affect this droplet dispersal71. Improved drainage rates and avoidance of placing the drain directly under the tap both resulted in reduced dispersion, although it still could not be fully prevented71. These and other studies provide compelling evidence that bacteria in the sink trap microbiome can pose a threat, with opportunistic pathogens able to be dispersed to the surrounding environment, potentially risking patient colonisation and infection.
The sink drain microbiome as a melting pot for resistance gene transmission
HGT of mobile genetic elements in a hospital setting allows clinically important ARGs to rapidly disseminate between opportunistic pathogens, and between persistent environmental microbes and opportunistic pathogens. A landmark study focussing on KPC-positive Enterobacteriaceae isolates in a hospital over 5 years found that blaKPC was being carried by 62 different strains of 13 different species, which included 8 different genera72. The authors showed that blaKPC spread in the hospital at multiple genetic levels, from strains transferring between patients, to transfer of plasmids between species, to transposition of ARG-harbouring transposons between plasmids. Clearly, HGT was important for the transmission of this ARG72, and the results hinted at a large, unsampled network of genetic exchange occurring outside of an infection context. Subsequent work sampling six sinks in the same hospital identified 14 distinct taxonomic clades with blaKPC resident in the sink drain microbiome, with genomic analyses implicating sink-specific plasmid conjugation and transposition of blaKPC from plasmids to chromosomes73. In a 2018 study, Weingarten et al. collected and sequenced isolates from patients and environmental locations over a 2 year period, and showed that hospital sinks were a vast reservoir for opportunistic pathogens74. The authors found isolates of K. oxytoca in the ICU sink that were identical to a patient isolate collected a year earlier, despite thorough cleaning of the pipework during this time, showing persistent colonisation of the sink drains by opportunistic pathogens. They also found that carbapenem resistance was highly prevalent in ICU wastewater pipes, despite low prevalence in the patient population, highlighting sink drains as long-term, persistent reservoirs of ARGs. Importantly, they were able to identify putative HGT events occurring in the hospital plumbing system. For example, the authors identified a strain of E. cloacae from a patient which carried two blaKPC plasmids. The same strain of E. cloacae was isolated from the sink in the patient’s room 11 months later, and sequencing revealed it had gained an additional blaKPC plasmid. This additional plasmid had also been found in a K. pneumoniae strain isolated from a different patient who stayed in the same room 8 months after the first patient. The authors hypothesised that the additional plasmid found in the E. cloacae sink drain isolate had been gained via an HGT event in the sink drain whereby the K. pneumoniae strain that had colonised the second patient transferred the plasmid to the strain that had originally colonised the first patient74. These findings are consistent with the hypothesis that bacteria colonising patients can enter hospital sinks and result in plasmid transfer—a process that is likely enhanced by the multi-species biofilm nature of the sink drain microbiome and its exposure to diverse selective pressures. Tracking such transfer events is not trivial, as observing such events requires extensive sampling, however other studies have also provided evidence of HGT in hospital sinks. Whilst tracking plasmid diversity in CPEs, a 2015 study isolated a strain of K. pneumoniae carrying blaKPC-2 from a patient. Four days later, two strains of Citrobacter freundii and Enterobacter cloacae were isolated from the sink in the patient’s room, both carrying the same blaKPC-2 plasmid as the patient isolate54. Similarly in a different study, during a hospital outbreak of K. pneumoniae, a strain of Enterobacter asburiae was isolated in a sink carrying the same blaKPC-2 plasmid as the K. pneumoniae outbreak strain, suggesting intergenus transfer in the hospital environment52. The high abundance of ARG-carrying conjugative plasmids in the hospital sink drain microbiome is implicated both by metagenomic analyses40,51 and by many studies that employ whole-genome sequencing on sink drain isolates51,56,58,73–76. The contributions of transduction and transformation to ARG exchange in the sink drain microbiome is less clear. Phage particles carrying ARGs can be highly abundant in hospital wastewater77, and organisms harbouring blaOXA-204 on an intact, active prophage were identified in hospital sink drain samples78, but the generally narrower host range of phages compared with plasmids might render the former less effective at transferring ARGs between diverse bacterial taxa. There is evidence that short pieces of exogenous extracellular DNA can be detected in sink drain biofilms for over a week79, and many opportunistic pathogens have some capacity for transformation80. As with tracking microbes, identifying MGE transfer and inferring directionality from observational sequence data is not straightforward—and the smaller size of MGEs compared with the chromosome can result in fewer nucleotide polymorphisms for tracking plasmid lineages during an outbreak81—but criteria have been proposed to infer dynamics of plasmid and MGE transfer using isolate sequencing data73,82.
Taken together, the above sections highlight (1) that the sink drain microbiome is ubiquitous and persistent, (2) that opportunistic pathogens from patients can enter and join the sink drain microbiome, (3) that ARG-encoding MGEs can transfer between pathogens and environmental organisms in greywater plumbing and that physicochemical features of this habitat can act as a stimulus for HGT, and (4) that members of the sink drain microbiome can disseminate to the patient-facing environment. Consequently, the sink drain microbiome could be considered a crucial location where clinically-relevant resistance traits are acquired by opportunistic pathogens with a high potential to cause subsequent infections.
Interventions to reduce the risk of ARG HGT in the sink drain microbiome
Various interventions have been proposed and implemented to mitigate the risks posed by opportunistic pathogens in the sink drain microbiome, including a variety of engineering approaches such as changes to sink design, novel devices to decontaminate traps, or implementation of rigorous chemical disinfection regimes (reviewed by Kearney et al.83). These changes do not always have the outcomes either predicted or desired, with subsequent interventions often necessary84. For example, large-scale replacement of the drainage pipework to control a prolonged outbreak of carbapenem-resistant Enterobacterales resulted in significant increase in Enterobacterales and resistance gene load, as a possible response to the significant ecological disturbance represented by pipe replacement85. As the risks inherent with standing water cannot be fully ameliorated, the most effective protocol appears to be complete removal of sinks—and their associated drains—from patient rooms altogether83. However, such radical changes to hand hygiene conventions are not always viable, particularly when considering the wide healthcare context in which sink drain opportunistic pathogens and ARGs might pose a risk, such as nursing homes or the wider hospital environment.
Alternate approaches might come from a deeper understanding of the evolutionary ecology of the sink drain microbiome, and interventions based on the biotic interactions between its resident organisms86 (Box 1: The invasibility of the sink drain microbiome to opportunistic pathogens), and genetic elements (Box 2: Factors influencing the invasibility of a microbiome to ARG-encoding MGEs). For example, probiotics have been used as a means of controlling opportunistic pathogens in drinking water87, and communities recalcitrant to opportunistic pathogen invasion might ultimately be deployed to displace or impede invasion of pathogens in sink drains too. Probiotic-based surface cleaners have been evaluated in hospital settings, and one study found a significant reduction in total ARG counts in sink surface samples when using probiotic-based cleaning compared to traditional disinfectant26, but such approaches remain speculative in the context of influencing the function of the sink drain microbiome, and further experimental studies are necessary. Bacteriophages—viral parasites of bacteria—can be highly specific to particular hosts and thus could be a powerful tool in manipulating the sink drain microbiome to remove or reduce the risks posed by opportunistic pathogens88,89. An even more potent approach might stem from targeting the elements that are responsible for resistance. An unexpected diversity of phages that specifically target plasmid-harbouring bacteria have recently been catalogued, and these plasmid-dependent phages can target diverse organisms simply by virtue of their harbouring the same antibiotic resistance plasmid90. While bacteria can evolve resistance to phages, such mutations are commonly associated with increased susceptibility to antibiotics, or loss of the ability of the plasmid to transfer91, outcomes that would also ameliorate the threat of multidrug resistance gene transfer to opportunistic pathogens in the sink drain microbiome.
Box 1 The invasibility of the sink drain microbiome to opportunistic pathogens.
Invasibility describes how easily an introduced organism (such as an opportunistic pathogen) can establish in a target community92. Higher dispersal rates—i.e. increased exposure to an opportunistic pathogen—provide more opportunities for invasion, and boost invasion success by increasing invader population size92. There are many routes for opportunistic pathogens to disperse to sink traps (Fig. 1). To persist, an arriving invader must then find a niche. A vacant niche may be available to invaders that arrive early and possess a beneficial trait (such as a metabolic pathway) absent from the target community, but more likely the invader will be competing with one or more residents93. Biological features of invader and target can heighten competitive ability to influence invasion outcome, particularly the diverse arsenals of ‘interference competition’ systems that directly inhibit competitors94: incoming organisms expressing such systems usually make better invaders, while resident microbes with a surfeit of antagonistic interactions tend to more successfully impede invasion95,96. A more diverse community can be more recalcitrant to invasion, as it is more likely to contain traits that diminish any competitive advantages possessed by the invader, and more likely to express antagonistic molecules to inhibit invader growth92. Invasion success thus depends both on the invading organism and the target community, consistent with experimental sink drain models in which different carbapenemase-producing Klebsiella pneumoniae strains varied in their invasion success across biofilm communities97. Moreover, perturbations or disturbances to a community can favour invasions by reducing resident community population size and richness, and releasing resources that might be used by an invader92. Inconsistent use of sinks and irregular introduction of nutrients, biocides, pharmaceuticals, or bodily fluids42 could be considered perturbations that may offer potential pathogens an opportunity to become (re-)established, as has been suggested by experimental sink drain models43,98.
Box 2 Factors influencing the invasibility of a microbiome to ARG-encoding MGEs.
While most theory on microbial invasions has been developed to describe strains and species of microorganisms, analogous concepts might be applied to understand and influence the invasion of ARG-encoding MGEs in a microbiome. As with microorganisms, plasmid invasion is enhanced by dispersal: occasional disturbances to a biofilm can spread plasmid carriers across a population, increasing the chances of a plasmid accessing a new host99. It is conceptually straightforward to appreciate how selection for the beneficial traits harboured by MGEs, such as those encoded by ARGs, can facilitate invasion of that MGE into a novel population. Less obvious is the fact that other features of MGEs such as low fitness costs and high transmission rates can allow resistance-encoding elements to find a prolonged niche in microbiomes in the absence of regular direct selection for resistance genes8,100,101, a process that can be enhanced by perturbations such as nutrient availability and chemical stresses33,99. Similarly, it is increasingly appreciated that MGEs can have antagonistic interactions with other MGEs in a population, encoding elaborate systems to directly interfere with one another, which may impede or enhance invasion of high-impact ARG-encoding MGEs into the sink drain microbiomes102.
Conclusions and perspective
Tracking and risk assessing drug resistance in the environment is not straightforward—environmental microbiomes are diverse, unobserved (and in some cases unobservable), and multitudinous. However, it is clear from genomic surveillance to date that opportunistic pathogens disseminating from the sink drain microbiome pose a threat to patients in hospital settings, and that interactions in this ‘non-infection’ habitat offer unique opportunities for such pathogens to evolve and acquire resistance traits prior to encountering chemotherapy during infection. The ability for genes to readily transfer from non-pathogenic organisms necessarily broadens our perspective on the evolution of multidrug resistance in opportunistic pathogens, highlighting the roles that MGEs can play and heightening the need to survey high-consequence MGEs, as well as pathogens per se, in the clinical environment. Finally, interventions to mitigate the threat posed by the sink drain microbiome will inevitably interact with the evolutionary ecology of pathogens, non-pathogens, and their resident MGEs in this microbiome. Studies to understand the evolutionary ecology of the sink drain microbiome—how it responds to disturbances, the principal drivers of AMR, and factors promoting gene transfer in this habitat—are necessary to improve predictions of how such interventions would play out in a real-world setting.
Acknowledgements
We would like to thank Ginny Moore for fascinating discussions on this subject and comments on an earlier draft. G.E.M. and J.P.J.H. are supported by a Medical Research Council Career Development Award to J.P.J.H. (MR/W02666X/1). We apologise to all authors of relevant works whom we were unable to cite for reasons of space.
Author contributions
Conceptualisation: G.E.M. and J.P.J.H. Writing—original draft: G.E.M. and J.P.J.H. Visualisation: G.E.M. Writing—review & editing: G.E.M. and J.P.J.H. Both G.E.M. and J.P.J.H. read and approved the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gregory E. McCallum, Email: G.E.McCallum@liverpool.ac.uk
James P. J. Hall, Email: j.p.j.hall@liverpool.ac.uk
References
- 1.Sati, H. et al. The WHO Bacterial Priority Pathogens List 2024: a prioritisation study to guide research, development, and public health strategies against antimicrobial resistance. Lancet Infect. Dis.0, 10.1016/S1473-3099(25)00118-5 (2025). [DOI] [PubMed]
- 2.Gilbert, J. A. & Stephens, B. Microbiology of the built environment. Nat. Rev. Microbiol.16, 661–670 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Partridge, S. R., Kwong, S. M., Firth, N. & Jensen, S. O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev.31, e00088–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, R. et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun.9, 1179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopotsa, K., Osei Sekyere, J. & Mbelle, N. M. Plasmid evolution in carbapenemase-producing Enterobacteriaceae: a review. Ann. N. Y. Acad. Sci.1457, 61–91 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Pal, C., Bengtsson-Palme, J., Kristiansson, E. & Larsson, D. G. J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genomics16, 964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray, A. K., Zhang, L., Snape, J. & Gaze, W. H. Comparing the selective and co-selective effects of different antimicrobials in bacterial communities. Int. J. Antimicrob. Agents53, 767–773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopatkin, A. J. et al. Persistence and reversal of plasmid-mediated antibiotic resistance. Nat. Commun.8, 1689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchesi, J. R. & Ravel, J. The vocabulary of microbiome research: a proposal. Microbiome3, 1–3 (2015). [DOI] [PMC free article] [PubMed]
- 10.McInnes, R. S., McCallum, G. E., Lamberte, L. E. & van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol.53, 35–43 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Chase, J. et al. Geography and location are the primary drivers of office microbiome composition. mSystems1, 10.1128/msystems.00022-16 (2016). [DOI] [PMC free article] [PubMed]
- 12.Quince, C., Walker, A. W., Simpson, J. T., Loman, N. J. & Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol.35, 833–844 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Danko, D. et al. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell184, 3376–3393.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai, S., Singh, D. K. & Kumar, A. Microbial, environmental and anthropogenic factors influencing the indoor microbiome of the built environment. J. Basic Microbiol.61, 267–292 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Fujimura, K. E. et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J. Allergy Clin. Immunol.126, 410–412.e3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores, G. E. et al. Diversity, distribution and sources of bacteria in residential kitchens. Environ. Microbiol.15, 588–596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrusso, P. A. & Quinlan, J. J. Prevalence of pathogens and indicator organisms in home kitchens and correlation with unsafe food handling practices and conditions. J. Food Prot.80, 590–597 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lax, S. et al. Bacterial colonization and succession in a newly opened hospital. Sci. Transl. Med.10.1126/scitranslmed.aah6500 (2017). [DOI] [PMC free article] [PubMed]
- 19.Chopyk, J. et al. Temporal variations in bacterial community diversity and composition throughout intensive care unit renovations. Microbiome8, 86 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buelow, E. et al. Limited influence of hospital wastewater on the microbiome and resistome of wastewater in a community sewerage system. FEMS Microbiol. Ecol.94, fiy087 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Karkman, A., Pärnänen, K. & Larsson, D. G. J. Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments. Nat. Commun.10, 80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otter, J. A., Yezli, S., Salkeld, J. A. G. & French, G. L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control41, S6–S11 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Klassert, T. E. et al. Bacterial colonization dynamics and antibiotic resistance gene dissemination in the hospital environment after first patient occupancy: a longitudinal metagenetic study. Microbiome9, 169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks, B. et al. Strain-resolved analysis of hospital rooms and infants reveals overlap between the human and room microbiome. Nat. Commun.8, 1814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chng, K. R. et al. Cartography of opportunistic pathogens and antibiotic resistance genes in a tertiary hospital environment. Nat. Med.26, 941–951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klassert, T. E. et al. Comparative analysis of surface sanitization protocols on the bacterial community structures in the hospital environment. Clin. Microbiol. Infect.28, 1105–1112 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Brooks, B. et al. The developing premature infant gut microbiome is a major factor shaping the microbiome of neonatal intensive care unit rooms. Microbiome6, 112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridier, A., Briandet, R., Thomas, V. & Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling27, 1017–1032 (2011). [DOI] [PubMed]
- 29.Stalder, T. et al. Evolving populations in biofilms contain more persistent plasmids. Mol. Biol. Evol.37, 1563–1576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledwoch, K., Robertson, A., Lauran, J., Norville, P. & Maillard, J.-Y. It’s a trap! The development of a versatile drain biofilm model and its susceptibility to disinfection. J. Hosp. Infect.106, 757–764 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Buchan, B. W. et al. Effectiveness of a hydrogen peroxide foam against bleach for the disinfection of sink drains. Infect. Control Hosp. Epidemiol.40, 724–726 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Zhang, Y., Gu, A. Z., He, M., Li, D. & Chen, J. Subinhibitory concentrations of disinfectants promote the horizontal transfer of multidrug resistance genes within and across genera. Environ. Sci. Technol.51, 570–580 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Liu, C. et al. Low concentration quaternary ammonium compounds promoted antibiotic resistance gene transfer via plasmid conjugation. Sci. Total Environ.887, 163781 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, Q. E. et al. Evolution of triclosan resistance modulates bacterial permissiveness to multidrug resistance plasmids and phages. Nat. Commun.15, 3654 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halden, R. U. et al. The florence statement on triclosan and triclocarban. Environ. Health Perspect.125, 064501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, S. et al. Comprehensive analysis of disinfectants on the horizontal transfer of antibiotic resistance genes. J. Hazard. Mater.453, 131428 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Garratt, I., Aranega-Bou, P., Sutton, J. M., Moore, G. & Wand, M. E. Long-term exposure to octenidine in a simulated sink trap environment results in selection of Pseudomonas aeruginosa, Citrobacter, and Enterobacter isolates with mutations in efflux pump regulators. Appl. Environ. Microbiol.87, e00210–e00221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodger, G. et al. A workflow for the detection of antibiotic residues, measurement of water chemistry and preservation of hospital sink drain samples for metagenomic sequencing. J. Hosp. Infect.144, 128–136 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodger, G. et al. Survey of healthcare-associated sink infrastructure, and sink trap antibiotic residues and biochemistry, in 29 UK hospitals. J. Hosp. Infect. 159, 140–147 (2025). [DOI] [PubMed]
- 40.Snell, L. B. et al. The drainome: longitudinal metagenomic characterization of wastewater from hospital ward sinks to characterize the microbiome and resistome and to assess the effects of decontamination interventions. J. Hosp. Infect.153, 55–62 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jutkina, J., Marathe, N. P., Flach, C.-F. & Larsson, D. G. J. Antibiotics and common antibacterial biocides stimulate horizontal transfer of resistance at low concentrations. Sci. Total Environ.616–617, 172–178 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Grabowski, M. et al. Characterizations of handwashing sink activities in a single hospital medical intensive care unit. J. Hosp. Infect.100, e115–e122 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Kotay, S. M. et al. Nutrients influence the dynamics of Klebsiella pneumoniae carbapenemase producing enterobacterales in transplanted hospital sinks. Water Res.176, 115707 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Kizny Gordon, A. E. et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections—a systematic review of the literature. Clin. Infect. Dis.64, 1435–1444 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Volling, C. et al. Are sink drainage systems a reservoir for hospital-acquired gammaproteobacteria colonization and infection? A systematic review. Open Forum Infect. Dis.8, ofaa590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamathewatta, K. et al. Colonization of a hand washing sink in a veterinary hospital by an Enterobacter hormaechei strain carrying multiple resistances to high importance antimicrobials. Antimicrob. Resist. Infect. Control9, 163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wendel, A. F., Ressina, S., Kolbe-Busch, S., Pfeffer, K. & MacKenzie, C. R. Species diversity of environmental GIM-1-producing bacteria collected during a long-term outbreak. Appl. Environ. Microbiol.82, 3605–3610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanafiah, A. et al. Insights into the microbiome and antibiotic resistance genes from hospital environmental surfaces: a prime source of antimicrobial resistance. Antibiotics13, 127 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly, S. A. et al. Large-scale characterization of hospital wastewater system microbiomes and clinical isolates from infected patients: profiling of multi-drug-resistant microbial species. J. Hosp. Infect.141, 152–166 (2023). [DOI] [PubMed] [Google Scholar]
- 50.Aracil-Gisbert, S. et al. The ICU environment contributes to the endemicity of the “Serratia marcescens complex” in the hospital setting. mBio15, e03054-23 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Constantinides, B. et al. Genomic surveillance of Escherichia coli and Klebsiella spp. in hospital sink drains and patients. Microb. Genomics6, e000391 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tofteland, S., Naseer, U., Lislevand, J. H., Sundsfjord, A. & Samuelsen, Ø. A long-term low-frequency hospital outbreak of KPC-producing Klebsiella pneumoniae involving intergenus plasmid diffusion and a persisting environmental reservoir. PLoS ONE8, e59015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franco, L. C. et al. A microbiological survey of handwashing sinks in the hospital built environment reveals differences in patient room and healthcare personnel sinks. Sci. Rep.10, 8234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conlan, S. et al. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci. Transl. Med.6, 254ra126–254ra126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourdin, T. et al. Serratia marcescens colonization in a neonatal intensive care unit has multiple sources, with sink drains as a major reservoir. Appl. Environ. Microbiol.89, e00105–e00123 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diorio-Toth, L. et al. Intensive care unit sinks are persistently colonized with multidrug resistant bacteria and mobilizable, resistance-conferring plasmids. mSystems8, e00206–e00223 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muzslay, M., Moore, G., Alhussaini, N. & Wilson, A. P. R. ESBL-producing gram-negative organisms in the healthcare environment as a source of genetic material for resistance in human infections. J. Hosp. Infect.95, 59–64 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Anantharajah, A. et al. Long-term intensive care unit outbreak of carbapenemase-producing organisms associated with contaminated sink drains. J. Hosp. Infect.143, 38–47 (2024). [DOI] [PubMed] [Google Scholar]
- 59.Decraene, V. et al. A large, refractory nosocomial outbreak of Klebsiella pneumoniae carbapenemase-producing Escherichia coli demonstrates carbapenemase gene outbreaks involving sink sites require novel approaches to infection control. Antimicrob. Agents Chemother. 62, 10.1128/aac.01689-18 (2018). [DOI] [PMC free article] [PubMed]
- 60.Laço, J., Martorell, S., Gallegos, M. del C. & Gomila, M. Yearlong analysis of bacterial diversity in hospital sink drains: culturomics, antibiotic resistance and implications for infection control. Front. Microbiol. 15, 1501170 (2025). [DOI] [PMC free article] [PubMed]
- 61.Buhl, M. et al. Molecular evolution of extensively drug-resistant (XDR) Pseudomonas aeruginosa strains from patients and hospital environment in a prolonged outbreak. Front. Microbiol. 10, 1742 (2019). [DOI] [PMC free article] [PubMed]
- 62.Iimura, M. et al. Detection of Acinetobacter pittii ST220 co-producing NDM-1 and OXA-820 carbapenemases from a hospital sink in a non-endemic country of NDM. J. Glob. Antimicrob. Resist.21, 353–356 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Hennebique, A. et al. The hospital sink drain biofilm resistome is independent of the corresponding microbiota, the environment and disinfection measures. Water Res.284, 123902 (2025). [DOI] [PubMed] [Google Scholar]
- 64.Olm, M. R. et al. Genome-resolved metagenomics of eukaryotic populations during early colonization of premature infants and in hospital rooms. Microbiome7, 26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regev-Yochay, G. et al. Sink traps as the source of transmission of OXA-48–producing Serratia marcescens in an intensive care unit. Infect. Control Hosp. Epidemiol.39, 1307–1315 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Lowe, C. et al. Outbreak of extended-spectrum β-lactamase–producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg. Infect. Dis.18, 1242–1247 (2012). [DOI] [PMC free article] [PubMed]
- 67.Bourigault, C. et al. Hospital outbreak of NDM-producing Klebsiella pneumoniae in a surgical intensive care unit: sink traps as the causing source of epidemic strain resurgence. Am. J. Infect. Control10.1016/j.ajic.2025.01.003 (2025). [DOI] [PubMed]
- 68.Vergara-López, S., Domínguez, M. C., Conejo, M. C., Pascual, Á & Rodríguez-Baño, J. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin. Microbiol. Infect.19, E490–E498 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Inkster, T. A narrative review and update on drain-related outbreaks. J. Hosp. Infect.151, 33–44 (2024). [DOI] [PubMed] [Google Scholar]
- 70.Kotay, S., Chai, W., Guilford, W., Barry, K. & Mathers, A. J. Spread from the sink to the patient: in situ study using green fluorescent protein (GFP)-expressing Escherichia coli to model bacterial dispersion from hand-washing sink-trap reservoirs. Appl. Environ. Microbiol. 83, e03327–16 (2017). [DOI] [PMC free article] [PubMed]
- 71.Aranega-Bou, P. et al. Carbapenem-resistant Enterobacteriaceae dispersal from sinks is linked to drain position and drainage rates in a laboratory model system. J. Hosp. Infect.102, 63–69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheppard, A. E. et al. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob. Agents Chemother.60, 3767–3778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathers, A. J. et al. Developing a framework for tracking antimicrobial resistance gene movement in a persistent environmental reservoir. Npj Antimicrob. Resist.2, 50 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weingarten, R. A. et al. Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. mBio9, 10.1128/mbio.02011-17 (2018). [DOI] [PMC free article] [PubMed]
- 75.Regev-Yochay, G. et al. Sink-traps are a major source for carbapenemase-producing Enterobacteriaceae transmission. Infect. Control Hosp. Epidemiol.45, 284–291 (2024). [DOI] [PubMed] [Google Scholar]
- 76.Mathers, A. J. et al. Klebsiella quasipneumoniae provides a window into carbapenemase gene transfer, plasmid rearrangements, and patient interactions with the hospital environment. Antimicrob. Agents Chemother.63, e02513–e02518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pires, J., Santos, R. & Monteiro, S. Antibiotic resistance genes in bacteriophages from wastewater treatment plant and hospital wastewaters. Sci. Total Environ.892, 164708 (2023). [DOI] [PubMed] [Google Scholar]
- 78.Gobeille Paré, S. et al. Arrival of the rare carbapenemase OXA-204 in Canada causing a multispecies outbreak over 3 years. J. Antimicrob. Chemother.75, 2787–2796 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Winder, E. M. & Bonheyo, G. T. DNA persistence in a sink drain environment. PLoS ONE10, e0134798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lerminiaux, N. A. & Cameron, A. D. S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol.65, 34–44 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Li, S. et al. Implications of mobile genetic elements for salmonella enterica single-nucleotide polymorphism subtyping and source tracking investigations. Appl. Environ. Microbiol.85, e01985–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans, D. et al. Empirically derived sequence similarity thresholds to study the genomic epidemiology of plasmids shared among healthcare-associated bacterial pathogens. eBioMedicine93, 104681 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kearney, A., Boyle, M. A., Curley, G. F. & Humphreys, H. Preventing infections caused by carbapenemase-producing bacteria in the intensive care unit—think about the sink. J. Crit. Care66, 52–59 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Carling, P. C. Wastewater drains: epidemiology and interventions in 23 carbapenem-resistant organism outbreaks. Infect. Control Hosp. Epidemiol.39, 972–979 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Kotay, S. M. et al. The unintended consequences of a surge in antibiotic resistance when removing wastewater drain biofilm. Preprint at 10.1101/2020.11.06.372326 (2020).
- 86.Sheth, R. U., Cabral, V., Chen, S. P. & Wang, H. H. Manipulating bacterial communities by in situ microbiome engineering. Trends Genet.32, 189–200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang, H., Edwards, M. A., Falkinham, J. O. I. & Pruden, A. Probiotic approach to pathogen control in premise plumbing systems? A review. Environ. Sci. Technol.47, 10117–10128 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Stachler, E., Kull, A. & Julian, T. R. Bacteriophage treatment before chemical disinfection can enhance removal of plastic-surface-associated Pseudomonas aeruginosa. Appl. Environ. Microbiol.87, e00980–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santiago, A. J., Burgos-Garay, M. L., Kartforosh, L., Mazher, M. & Donlan, R. M. Bacteriophage treatment of carbapenemase-producing Klebsiella pneumoniae in a multispecies biofilm: a potential biocontrol strategy for healthcare facilities. AIMS Microbiol.6, 43–63 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quinones-Olvera, N. et al. Diverse and abundant phages exploit conjugative plasmids. Nat. Commun.15, 3197 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ojala, V., Laitalainen, J. & Jalasvuori, M. Fight evolution with evolution: plasmid-dependent phages with a wide host range prevent the spread of antibiotic resistance. Evol. Appl.6, 925–932 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinnunen, M. et al. A conceptual framework for invasion in microbial communities. ISME J.10, 2773–2779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Debray, R. et al. Priority effects in microbiome assembly. Nat. Rev. Microbiol.20, 109–121 (2022). [DOI] [PubMed] [Google Scholar]
- 94.Granato, E. T., Meiller-Legrand, T. A. & Foster, K. R. The evolution and ecology of bacterial warfare. Curr. Biol.29, R521–R537 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Brown, S. P., Chat, L. L., De Paepe, M. & Taddei, F. Ecology of microbial invasions: amplification allows virus carriers to invade more rapidly when rare. Curr. Biol.16, 2048–2052 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Li, S., Tan, J., Yang, X., Ma, C. & Jiang, L. Niche and fitness differences determine invasion success and impact in laboratory bacterial communities. ISME J.13, 402–412 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burgos-Garay, M. et al. Colonization of carbapenem-resistant Klebsiella pneumoniae in a sink-drain model biofilm system. Infect. Control Hosp. Epidemiol.42, 722–730 (2021). [DOI] [PubMed] [Google Scholar]
- 98.Burgos-Garay, M. L., Santiago, A. J., Kartforosh, L., Kotay, S. & Donlan, R. M. Supplemental nutrients stimulate the amplification of carbapenemase-producing Klebsiella pneumoniae (CPKP) in a sink drain in vitro biofilm reactor model. Biofouling37, 465–480 (2021). [DOI] [PubMed]
- 99.Fox, R. E., Zhong, X., Krone, S. M. & Top, E. M. Spatial structure and nutrients promote invasion of IncP-1 plasmids in bacterial populations. ISME J.2, 1024–1039 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Metzger, G. A. et al. Biofilms preserve the transmissibility of a multi-drug resistance plasmid. Npj Biofilms Microbiomes8, 95 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Røder, H. L. et al. Biofilms can act as plasmid reserves in the absence of plasmid specific selection. Npj Biofilms Microbiomes7, 78 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rocha, E. P. C. & Bikard, D. Microbial defenses against mobile genetic elements and viruses: who defends whom from what?. PLoS Biol.20, e3001514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.