Abstract
Adoptive cell therapy (ACT), particularly chimeric antigen receptor T cell (CAR T) therapy, has emerged as a promising approach in cancer treatment, demonstrating efficacy in hematological malignancies but facing challenges in brain tumors. The combination of ACT with radiation therapy (RT) offers a potential strategy to enhance therapeutic outcomes, as RT can stimulate immune responses by promoting antigen presentation and T cell recruitment. However, a major hurdle is the radiosensitivity of immune cells, leading to their rapid depletion within the radiation field, which undermines the benefits of this combination. This review explores strategies to increase the radioresistance of immune cells, highlighting the need for innovative radioprotective approaches. We discuss the potential of extremophile-derived molecules, such as the Damage Suppressor protein from tardigrades, as novel radioprotectants that could be integrated into ACT protocols. Furthermore, we address key considerations for clinical trial design, including the sequencing of RT and ACT, dosing parameters, and safety considerations. By bridging insights from extremophile biology and immuno-oncology, this work aims to optimize the efficacy of ACT in the challenging context of brain tumors, paving the way for enhanced treatment strategies in neuro-oncology.
Subject terms: CNS cancer, Tumour immunology, Tumour immunology, Radiotherapy
Introduction
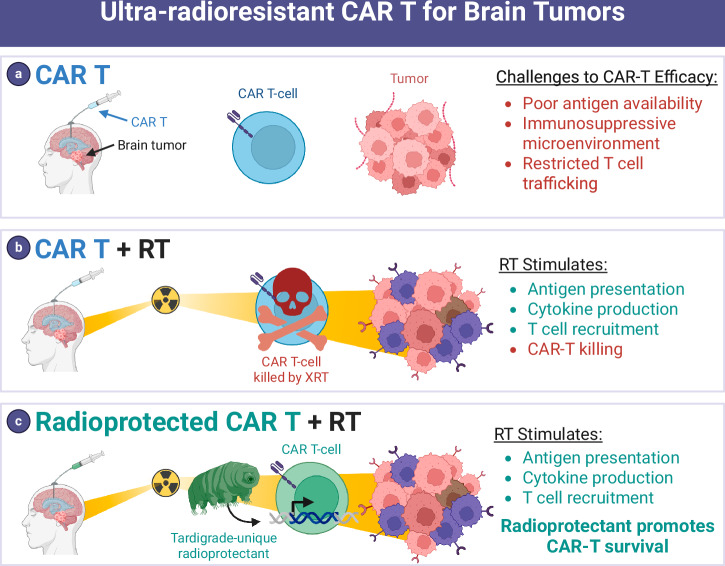
Adoptive cell therapy (ACT) including chimeric antigen receptor (CAR) T-cell therapy has shown promising early signals of activity in treating select patients with brain tumors1. ACT is a type of immunotherapy that uses a patient’s own immune cells to help fight disease, such as cancer. CAR T cells are patient-isolated T cells genetically engineered ex vivo to regain cancer-fighting properties. The efficacy of CAR T for solid tumor types, including brain tumors, remains elusive. However, the approach holds promise as evidenced by (i) responses in small series of brain tumor patients2,3; (ii) success of CAR T in B cell neoplasm settings in which six therapies have been FDA approved4; and (iii) recent FDA approval of a similar cell therapy approach for advanced melanoma5. The autologous in vitro- expanded tumor-infiltrating lymphocyte (TIL) lifileucel was approved by the FDA in 2024 as the first TIL therapy to treat cancer confirming the future use of TILs in mainstream practice. There are also promising TIL early efficacy data in lung cancer6, but these findings are yet to be replicated in immunologically “cold” tumors such as glioma5. Existing ACT modalities have shown limited efficacy against many brain tumors7. This challenge may stem from various mechanistic hurdles, including the scarcity of the brain tumor microenvironment (TME), impaired T cell trafficking to the tumor, downregulated checkpoint molecule expression, tumor heterogeneity, immunosuppressive TME, and lack of tumor antigen presentation4 (Fig. 1a).
Fig. 1. Using an ultra-radioresistant extremophile gene to radioprotect T cells and potentiate ACT.
a Poor immunogenicity is a key barrier to efficacy for CAR T cells in brain tumors. b RT added to CAR T therapy has immunostimulatory effects that may potentiate CAR T therapy. But RT also kills T cells that enter the radiation field which has a counterproductive effect. c Radioprotectants such as a gene from the ultra-radioresistant extremophile Tardigrade are expressed in CAR T cells to protect them from RT, allowing immune stimulation from RT to synergize with CAR T.
Several ACT modalities are currently in trial for central nervous system (CNS) tumors, mainly recurrent glioblastoma, with mixed results. Given the heterogeneity of glioblastoma and CNS tumors, most successful trials are targeting multiple tumor antigens with CARs. For example, one clinical study saw no clinical efficacy in recurrent glioblastoma patients cotreated with CAR T-cell therapy targeting epidermal growth factor receptor (EGFR) III and the anti-PD1 antibody pembrolizumab8. However, an early phase I clinical trial for recurrent glioblastoma saw success in CAR T cells engineered to target both EGFR III and wildtype EGFR9. All patients saw tumor regression, though two of the three patients eventually showed reoccurrence9. Similarly, a study using CAR T-cells targeting both EGFR and IL13Rα2 saw regression in 8 of 13 patients with measurable disease at the time of infusion10. One confirmed partial response by Modified Response Assessment in Neuro-Oncology criteria was observed10. However, patients in both studies exhibited grade 3 adverse events, including neurotoxicity, encephalopathy, and fatigue9,10. Further trials of CAR T treatments for brain tumors show limited efficacy and are summarized in review11. These inconsistent results and small sample sizes highlight the need for further investigation in CAR therapies for brain tumors.
Radiation therapy (RT) is the standard treatment of brain tumors, offering an opportunity to combine with ACT. The majority of brain tumor patients are candidates for RT with curative or palliative intent12. However, the effects of RT on the tumor immune microenvironment are complex. Many of these processes occur acutely after each fraction of RT in a time-limited fashion. These include stimulation of antigen presentation, induction of cytokine release by neoplastic cells and resident immune cells, downregulation of checkpoint protein expression on tumor and immune cells, and attraction of lymphocytes to the tumor site13–15. On the other hand, a major obstacle is that many immune cells are radiosensitive and undergo cell death upon entering the RT field16–19, hampering the ability of RT to stimulate engineered immune cells or other adaptive immune responses13,14 (Fig. 1b). It stands to reason that maximal immune stimulation occurs as tumors are first exposed to RT during the initial fractions of definitive, fractionated RT. Yet this is the precise time when RT is likely killing any CAR T cells or lymphocytes that may enter the radiation field to sample antigens and elicit adaptive immune responses.
Approaches to increase radioresistance of immune cells may hold promise for enhancing ACT efficacy. Increasing radioresistance could overcome the major challenge to combining ACT with RT, which is that many immune cells are highly radiosensitive and die upon entering the radiation field. Engineering radioprotected immune cells could address a critical barrier to the effective combination of ACT with RT (Fig. 1c). The concept of “radioprotecting” T cells for cell therapy has seen limited exploration. Notably, a recent study reported the overexpression of superoxide dismutase 2 (SOD2) in CAR T cells, which yielded promising results in a preclinical model involving head and neck cancer20. However, SOD2’s radioprotective effects may be modest21,22. HeLa cells with SOD2 overexpression still showed signs of DNA damage and reactive oxygen species (ROS) buildup after treatment with 5 Gy21, emphasizing the need for a broader investigation into various candidate genes and the potential of potent radioprotectants derived from extremophiles. Thus, there is an unmet need to identify methods to protect immune cells from RT-induced death to optimize the immune-stimulatory benefits of RT. There is also a pressing need for a deeper understanding of the molecular pathways involved in radioresistance, which could significantly enhance the efficacy of ACT when combined with RT.
Extremophiles, organisms that thrive in extreme environments like high radiation, temperature, or desiccation, possess unique molecular mechanisms to safeguard cellular integrity, making their proteins attractive for radioprotective applications in cell therapy. For instance, the tardigrade-derived Damage Suppressor (Dsup) protein binds to nucleosomes, reducing hydroxyl radical-induced DNA damage and double-strand breaks (DSBs), significantly enhancing cell survival under ionizing radiation without impairing normal functions23,24. Notably, local Dsup mRNA nanoparticle delivery has been shown to effectively radioprotect nearby healthy tissue in mice undergoing RT for orthotopic oral cancer25. Other promising proteins include PprI from Deinococcus radiodurans, which activates DNA repair and reduces apoptosis26; TRID1 from tardigrades, which promotes DNA repair through phase separation and repair machinery recruitment27; and SASP from Bacillus subtilis, which binds to DNA to provide a robust protective shield28.
Here we review the literature that could provide insights on approaches to radioprotect immune cells to stimulate ACT for brain tumors. We delineate knowledge gaps and opportunities to advance immune-cell radioprotectors and rationally combine ACT with RT. We highlight extremophile organisms as an intriguing source of radioprotective molecules that could be applied to human immune cells. A variety of approaches to screen for ideal radioprotectors are considered. Safety and ACT manufacturing considerations are evaluated. Finally, we consider different clinical situations in which clinical trials for radioprotected ACT and RT combination treatments could be deployed. Along with this, we review considerations for clinical trial design, ACT administration, rational sequencing of ACT and RT delivery, and selection of RT fractionation schemes.
Immune-cell types used for ACT and their radiation sensitivity
Several immune-cell types have been explored for ACT, including T cells, natural killer (NK) cells, macrophages, and more recently, unconventional lymphocytes such as γδ T cells and invariant natural killer T (iNKT) cells (Fig. 2)29–31. Each immune-cell type offers unique advantages in terms of tumor recognition, cytotoxicity, and persistence. Understanding their role within the tumor microenvironment and their radiosensitivity is crucial for optimizing radioprotected ACT in combination with radiotherapy (RT), a standard cancer treatment for brain tumors.
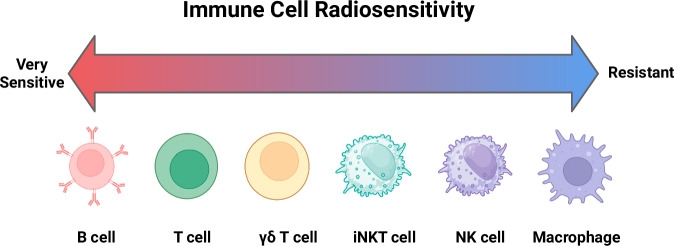
Fig. 2. Baseline radiosensitivity of cell types used for ACT.
This figure summarizes the inherent radiosensitivity and other effects of irradiation on various immune-cell types commonly used in adoptive cell therapy (ACT). While radiosensitivity is likely highly context-dependent, in general B cells are highly sensitive to radiation and undergo apoptosis at even low doses. T cells are sensitive, with sublethal doses (<2 Gy) causing activation, while higher doses (>2 Gy) induce apoptosis. γδ T cells are relatively resistant to radiation and maintain their cytotoxic functions. Invariant natural killer T cells (iNKT cells) exhibit moderate resistance to radiation. Natural killer (NK) cells and macrophages are relatively radioresistant.
A general theme for all immune-cell types is that RT may stimulate responses at low doses but leads to cell death or decreased responsiveness at higher doses32. Patients undergoing standard-of-care RT for glioblastoma, the most aggressive and malignant primary brain tumor, experience worsening lymphopenia (lymphocyte depletion) during RT17. The risk of lymphopenia is related to the volume of tissue being irradiated18,19. While concurrent chemotherapy is a risk factor, RT alone likely induces lymphopenia via distinct mechanisms18,19. Therefore, methods to modulate the radiosensitivity of lymphocytes may be of interest to decrease the risk of lymphopenia and maximize the efficacy of ACT. Understanding the exact radiosensitivity of lymphocytes, however, is imperfect as many experimental methods and results differ, as shown in review33. Additionally, some research suggests that sex and age can affect the radiosensitivity of lymphocytes33.
T lymphocytes
T cells, particularly T cell receptor (TCR)-transgenic T cells and chimeric antigen receptor (CAR) T cells, have been at the forefront of ACT for cancer. CAR T cells, genetically engineered to express receptors targeting tumor antigens, have shown remarkable efficacy in hematologic malignancies34. Essentially all FDA approvals for ACT anticancer therapies utilized T cells, including six approvals for CAR T cell infusions and an approval for tumor-infiltrating lymphocytes for advanced melanoma patients5,34.
T cells are sensitive to ionizing radiation, which can impair their proliferation and function at high doses. In particular, the D10 (dose required to reduce surviving fraction to 10%) for CD4+ and CD8+T cells is ~3 Gray (Gy)16, 20-fold lower than a standard RT dose of 60 Gy. T cells are killed by direct and indirect DNA damage in radiation fields16. Fluorescence tracking demonstrates T cell recirculation is transiently impaired by radiation therapy to the tumor35. Prolonged or high-dose radiation exposure can induce T cell exhaustion, reduce cytokine production (e.g., interferon-gamma), and increase the expression of inhibitory receptors such as PD-136–38.
Of note, sub-lethal doses of radiation (typically <2 Gy) can enhance T cell activation and recruitment to tumors39. Radiation induces the release of neoantigens and pro-inflammatory cytokines, improving T cell recognition of tumor cells40,41. Additionally, RT can upregulate major histocompatibility complex (MHC) molecules and death receptors on cancer cells, enhancing T cell-mediated cytotoxicity42. Some studies also suggest that low-dose RT enhances the trafficking of T cells into the tumor microenvironment (TME) by modulating the expression of chemokines like CXCL9 and CXCL1043.
Natural killer (NK) cells
NK cells are innate immune effectors that recognize and kill tumor cells independently of antigen presentation, through mechanisms such as recognition of downregulated MHC class I molecules or through activating receptors like NKG2D. NK cells offer an advantage in brain tumors that evade T cell immunity by downregulating MHC molecules. NK cells do not induce graft versus host disease (GvHD) and can therefore be collected from allogenic donor sources or cell lines, positing the potential development of universal, radioprotected CAR-NK cells targeting common tumor antigens44.
NK cells are generally more resistant to radiation than T cells45,46. Studies show that NK cell cytotoxicity remains functional at moderate doses of radiation, making them ideal candidates for combination with RT45. Some research suggests that fractionation, in comparison to single, large-dose RT, may improve the cytotoxicity and expansion of NK cells47. In cancers such as prostate cancer48, non-small cell lung cancer49, and hepatocellular carcinoma50, increased levels of NK cells in the blood have been observed following RT.
Radiation can induce the upregulation of stress ligands (e.g., MICA/B, ULBP1-6) on tumor cells, enhancing NK cell recognition and killing51. However, high doses of radiation (>8 Gy) can impair NK cell proliferation and effector functions, including degranulation and cytokine production46. Additionally, radiation can alter the expression of NK cell ligands on tumor cells, either enhancing or diminishing NK cell cytotoxicity depending on the radiation dose and tumor type51. Interestingly, pre-treatment of the tumor site with low-dose radiation has been shown to prime the tumor for NK cell-mediated lysis, suggesting a synergistic effect when combined with NK-based ACT52. This makes NK cells a promising candidate for combinatory therapies involving radiation.
Macrophages
Macrophages play a dual role in cancer, either promoting tumor progression (M2-like macrophages) or mediating tumor destruction (M1-like macrophages). Adoptive transfer of macrophages reprogrammed toward an M1 phenotype is an emerging strategy in ACT53. These macrophages can be engineered to enhance their phagocytic activity against cancer cells or to produce pro-inflammatory cytokines within the TME.
Macrophages are relatively radioresistant compared to T and NK cells54. Low to moderate doses of radiation (≤2 Gy) can induce polarization of macrophages toward an M1 phenotype, promoting anti-tumor activity55,56. Radiation enhances macrophage-mediated phagocytosis by upregulating “eat me” signals (e.g., calreticulin) on tumor cells and increasing the production of inflammatory cytokines such as TNF-α and IL-1257,58. However, macrophages exposed to high-dose radiation (>2 Gy) may undergo apoptosis or shift toward an M2-like immunosuppressive phenotype, supporting tumor growth and immune evasion58. Radiation also affects the recruitment of macrophages into the TME by inducing the expression of macrophage-attracting chemokines, such as CCL259. This can lead to the infiltration of both pro-tumorigenic and anti-tumor macrophages59, highlighting the complexity of their role in radiation-enhanced immune responses.
γδ T cells
γδ T cells represent a small subset of T cells that recognize non-peptide antigens and exhibit MHC-independent tumor recognition60. They have garnered interest for ACT due to their broad tumor specificity and cytotoxic potential30. γδ T cells may be useful for pediatric brain tumors, which have lower mutational loads61.
γδ T cells are relatively radioresistant and maintain their cytotoxic functions even after moderate doses of radiation62,63. Radiation-induced stress ligands on tumor cells, such as NKG2D ligands, enhance the recognition and killing of tumor cells by γδ T cells51. Moreover, γδ T cells can proliferate and produce cytokines such as IFN-γ in irradiated tumors, further promoting anti-tumor immunity64. The combination of γδ T cell-based ACT with low-dose RT has shown promise in preclinical studies, as radiation not only primes tumors for γδ T cell recognition but also enhances the local recruitment of these cells51,59,65.
Invariant natural killer T (iNKT) cells
Invariant natural killer T (iNKT) cells are a subset of T cells that bridge innate and adaptive immunity by recognizing glycolipid antigens presented by CD1d molecules66. Their ability to produce large amounts of cytokines, such as IFN-γ and IL-4, makes them potent activators of anti-tumor immune responses66.
iNKT cells show moderate sensitivity to radiation67. Relatively little is known about the impact of radiation therapy on iNKT activity with inconsistent findings in the literature, with some proposing that radiation decreases iNKT anti-tumor activity67,68. However, iNKT remain an intriguing target for ACT because they do not induce GvHD, similar to NK cells69. iNKT cells also have two target ligands: the natural CD1d ligand and CAR-targeted antigen69. CD1d is expressed in several brain tumors, including glioblastoma70 and medulloblastoma71. Radiation also upregulates the expression of intercellular adhesion molecule-1 (ICAM-1), which is expressed in some gliomas and binds to LFA-1 on the surface of iNKT cells72–74. However, like other immune cells, high-dose radiation can reduce iNKT cell viability and function67, emphasizing the need for dose optimization when combining iNKT cell ACT with RT.
Each immune-cell type utilized in ACT for cancer presents distinct advantages and limitations regarding radiation sensitivity. T cells, NK cells, macrophages, γδ T cells, and iNKT cells all exhibit varied responses to radiation, which can be leveraged to enhance their anti-tumor efficacy in combination with radiotherapy. Figure 3 shows each unique CAR cell type and how radiation may increase the anti-tumor response. A strategic combination of ACT with radiotherapy holds significant promise for improving clinical outcomes in cancer treatment, but careful consideration of radiation dosing is critical to maximize synergistic effects while minimizing damage to immune effector cells.
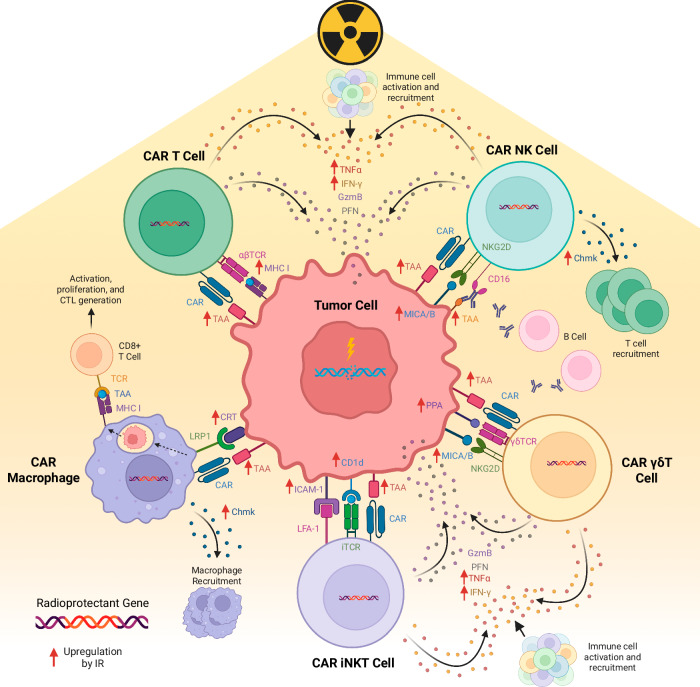
Fig. 3. Anti-tumor effects of radiation on CAR immune cells.
This figure summarizes the anti-tumor impacts of radiation on CAR T, NK, γδ, iNKT, and macrophage cells. Radiation may illicit an immune response via a variety of mechanisms, including upregulation of tumor antigen presentation and increased cytokine release. TAA: tumor associated antigen; CAR: chimeric antigen receptor; GzmB: granzyme B; PFN: perforin; PPA: phosphoantigen; LRP1: low-density lipoprotein receptor 1; MHC I: major histone compatibility complex I; IFNγ: interferon gamma; TNFα: tumor necrosis factor alpha; Chmk: chemokines; CRT: calreticulin; CTL: cytotoxicity T lymphocyte; CD: cluster of differentiation; MICA/B: MHC class I chain-related protein A and B; TCR: T cell receptor; iTCR: invariant T cell receptor.
ACT and chemotherapy
While we propose radioprotected ACT in combination with standard of care RT, many oncologic treatment plans also involve chemotherapies. Radioprotecting gene candidates that modulate DNA damage responses and cell cycle arrest may also decrease the chemosensitivity of the immune-cell75. However, chemotherapeutics that preferentially target immune-cell subtypes may negatively affect CAR T efficacy75. If ACT must be administered with chemotherapy, researchers may consider engineered expression of chemo-resistance proteins75. Modifying cell cycle and DNA damage proteins should be viewed with caution and suicide genes should be added in case of unexpected proliferation (See “Radioprotector safety considerations”)76. Of note, some chemotherapeutics may have an immuo-stimulatory effect on the TME that may improve CAR T cell trafficking to the tumor. For example, CAR T cell trafficking to the TME improved in mice preconditioned with temozolomide, a standard chemotherapeutic for glioblastoma, which was linked to decreased regulatory T cell populations in the TME77.
Mechanisms of radioprotection
Figure 4 outlines various mechanisms of radioprotection, each with unique implications for efficacy and safety. One mechanism, physically protecting DNA from direct damage by radiation, is likely to be safe, as this simply preserves the integrity of the genetic material without altering cellular processes. Similarly, the reduction of indirect DNA damage caused by reactive oxygen species (ROS) appears to be a generally safe approach. ROS scavenging neutralizes harmful free radicals without disrupting normal cellular functions and may be more universally applicable across species due to its fundamental nature in biology. These strategies aim to protect cells from radiation-induced damage without interfering with critical regulatory processes, making them promising candidates for clinical application.
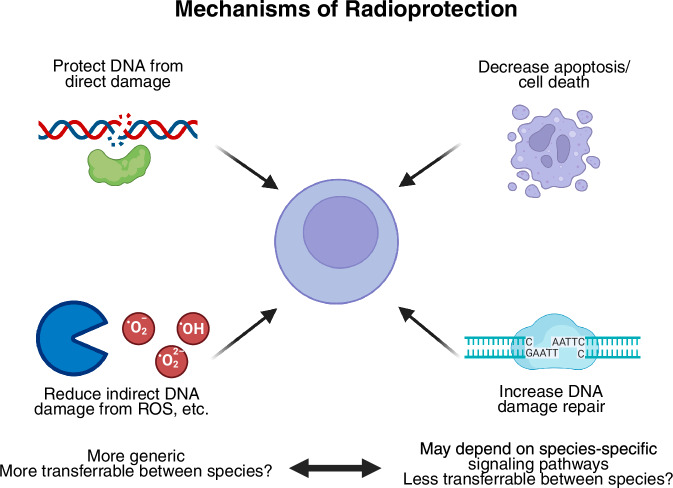
Fig. 4. Mechanisms of radioprotection.
Radioprotective agents mitigate radiation-induced damage through multiple mechanisms. These include protection of DNA from direct damage, reduction of indirect DNA damage by scavenging reactive oxygen species (ROS), and promotion of DNA damage repair. Additionally, radioprotective strategies may involve decreasing apoptosis and cell death. While reducing ROS is a broadly applicable and potentially safer strategy, inhibiting apoptosis could carry risks, such as increasing the potential for carcinogenesis. Mechanisms related to ROS scavenging are likely to be more transferable between species, while apoptosis modulation may depend on species-specific pathways, raising concerns about their safety and efficacy across different models.
However, other mechanisms may raise safety concerns. For example, reducing apoptosis could have unintended consequences, such as increasing the risk of carcinogenesis. Apoptosis is a natural defense mechanism that eliminates damaged or potentially cancerous cells. Dampening this process might allow cells with genomic damage to survive and proliferate, potentially leading to tumorigenesis. Similarly, interventions that enhance DNA repair might inadvertently preserve cells with incomplete or improper repair of damaged DNA, increasing the potential for mutations that could lead to cancer. These mechanisms would require careful evaluation in clinical trials to assess the risks and benefits before being considered for non-oncologic applications, such as protecting stem cell allografts during transplantation. It is crucial that any potential radioprotective strategy be scrutinized not only for efficacy but also for long-term safety to prevent unintended consequences like carcinogenesis.
Extremophiles as a source of radioprotectors
Bridging extremophile biology and immuno-oncology may provide an avenue to radioprotect immune cells for ACT. Extremophiles are organisms that are ultra-tolerant to temperature, pressure, and irradiation extremes. Indeed, a number of bacteria, archaea, fungi, and animal extremophiles exist that can tolerate enormous doses of irradiation (>1000 Gy or 1000 times the human lethal dose, Fig. 5). The genetic basis for this protection is being uncovered by genomic and functional studies, providing a unique opportunity for radioprotection applications. The genetic basis for extremophile radioresistance involves molecules that (i) physically protect DNA from direct damage; (ii) mitigate indirect DNA damage via metabolic activities such as scavenging of reactive oxygen species; (iii) provide improved or redundant DNA damage repair machinery; (iv) alter apoptosis pathways; or (v) modulate cell signal transduction23,24,28,78–80. This raises the opportunity to apply emerging extremotolerant technology to T cells to enable therapeutic approaches.
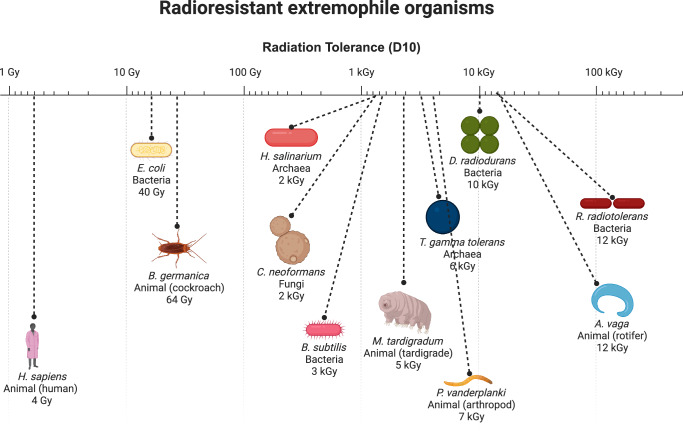
Fig. 5. Radioresistant extremophile organisms.
Select radioresistant extremophile organisms are shown, compared to humans. Radiation dose in Gray (Gy) for D10 (dose required to kill 90% of organism sample) when available or LD50 (dose required to kill 50% of organism sample) is shown. Note log scale indicating >1000X radioresistance compared to humans for many organisms.
Among the candidates, the Damage Suppressor (Dsup) protein from tardigrades stands out. Tardigrades are water-dwelling, eight-legged micro-animals that can be found everywhere from high-altitude mountaintops to the deep sea. Tardigrades have an extraordinary ability to tolerate immense doses of radiation (>5000 Gy) that would be lethal to most other life forms23,24. In the most stress-tolerant Tardigrade species, Ramazzottius varieornatus, the Tardigrade-unique damage suppressor (Dsup) protein colocalizes with DNA (nucleosomes in particular) and protects from hydroxyl radicals, protecting cells from radiation-induced DNA damage and cell death23,24. Transfection of Dsup into immortalized human cells enabled expression of the Dsup protein with no reduction in cell proliferation23. In addition to Dsup, additional radiosensitizers in tardigrades have been explored, including the DOPA (dihydroxyphenylalanine) dioxygenase gene (DODA1), tardigrade-specific radiation-induced disordered protein (TRID1), ubiquinol–cytochrome c reductase (bc1) synthesis protein (BCS1), and NADH dehydrogenase (ubiquinone) 1 beta subcomplex subunit 8 protein (NDUFB8)27. DODA1 leads to the production of betalins, a plant pigment with radical-scavenging properties27. TRID1 assists with liquid-liquid phase separation and enhances the recruitment of DNA repair protein to the double strand break (DSB) sites27. NDUFB8 and BCS1 are non-tardigrade specific proteins part of the mitochondrial respiratory chain complex assembly that are upregulated in tardigrades27. These proteins accelerate NAD+ regeneration for PARP1-mediated DNA repair27.
Other candidate radioprotectors include D. radiodurans PprI, which stimulates DNA repair and radioprotects human cells and mice26 and B. subtilis small acid soluble protein (SASP) which binds and potently protects DNA28. Sulfiredoxin from C. neoformans is strongly induced post-irradiation and radioprotects fungi80. Also, specific heat shock proteins have been linked to maximal irradiation survival response in the rotifer R. vega81. Molecules that mitigate direct or indirect DNA damage may be most likely to function in human cells, while molecules that have more complicated functions (such as in damage repair complexes and signaling pathways) may be less likely to do so.
Expression of foreign proteins, or “xenoproteins,” may present its own challenges in the pathway to engineering radioprotected immune cells. Extremophile-derived proteins may cause unexpected effects when expressed in human T cells. Future work could engineer these proteins to mitigate these issues by rationally combining key domains from these proteins with structurally similar human proteins.
Approaches to identify radioprotectors
Identifying effective radioprotectors requires comprehensive screening strategies that take into account the unique characteristics of human immune cells. One approach is to utilize in vitro genetic screens, which can provide insights into how various candidate genes may confer radioprotection. Both pooled and arrayed screens could be adapted from existing methodologies used to identify costimulatory molecules for CAR T cells82, thus prioritizing radioprotective genes for further study. Executing in vivo screens in animal models may be able to capture aspects of therapeutic efficacy in a more complex biological environment. Computational modeling and in silico analyses can also aid in predicting interactions and outcomes, streamlining the discovery process. Considerations for screens are summarized in Fig. 6.
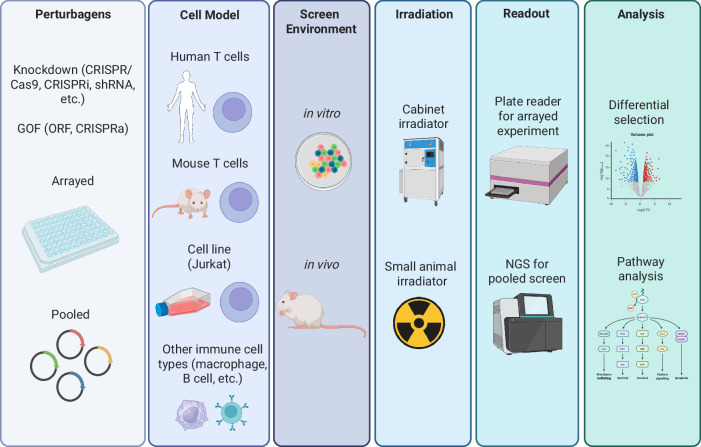
Fig. 6. Approaches to screen for immune-cell specific radioprotectors.
Various strategies can be employed to identify radioprotectors that specifically target immune cells. High-throughput screening of chemical libraries, genetic screens, and functional assays offer ways to discover agents that selectively shield immune cells from radiation damage. Depending on the type of ACT being studied, screens can be conducted in a range of immune-cell types, from easily manipulated cell lines to more clinically relevant, human-derived cells. Screening can be performed in vitro, which allows for more practical, controlled experiments, or in vivo, where complex biological interactions are better represented. Radiation is applied as needed, using tools like cabinet irradiators for in vitro cultures or small animal irradiators for in vivo studies. Readouts can vary depending on the specific goals of the screen, ranging from simple measurements of cell survival or function post-irradiation to more sophisticated next-generation sequencing (NGS) approaches that assess the selection of different perturbations after exposure. Analytical methods can involve straightforward ranking of top perturbations that improve immune-cell survival or function, as well as deeper molecular pathway analyses to gain mechanistic insight. Radioprotectors are also assessed for their ability to maintain immune-cell functionality, prevent apoptosis, or preserve immune-cell subsets during or after radiation. Validation of hits is critical to confirm radioprotective efficacy and ensure that identified candidates not only protect immune cells but also preserve their therapeutic potential. These methods hold promise for identifying radioprotectors that maintain immune competence in ACT, while ensuring therapeutic safety and efficacy.
Logistics of incorporating radioprotectors in cell therapy manufacture
Integrating radioprotectors into ACT manufacturing processes presents logistical challenges and opportunities. Strategies may involve utilizing the same viral vector for gene delivery or opting for separate vectors (Fig. 7), thereby allowing for a modular design that enables the co-expression of radioprotectors alongside therapeutic genes. This flexibility in vector design will facilitate the tailoring of cell therapies to maximize both therapeutic efficacy and safety.
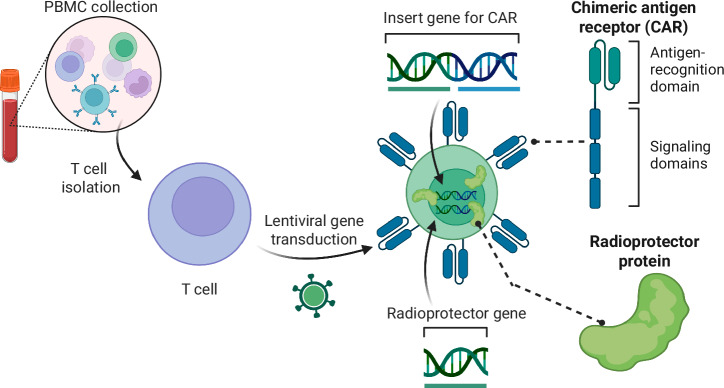
Fig. 7. Formulation of radioresistant CAR T cells.
CAR T cells are developed from peripheral blood mononuclear cells (PBMCs) which are harvested, expanded, sorted into T cells, and then tranduced with lentiviral vectors to deliver the CAR gene playload. Our proposal will identify the most potent radioprotector genes to deliver in a similar fashion during the CAR T manufacturing process, producing radioresistant CAR T cells.
RT dose, fractionation, volume considerations, and administration method for combination with ACT
Combining ACT with radiotherapy offers a potent approach to cancer treatment, but the success of this combination may rely heavily on the careful selection of radiation dose, fractionation, treatment volumes, and administration method to ensure synergy while minimizing damage to immune cells, particularly the infused radioprotected ACT cells. Here, we discuss how different RT regimens and anatomic considerations influence ACT-radiotherapy combinations, and the potential role of radioprotection strategies to preserve ACT cells (Fig. 8).
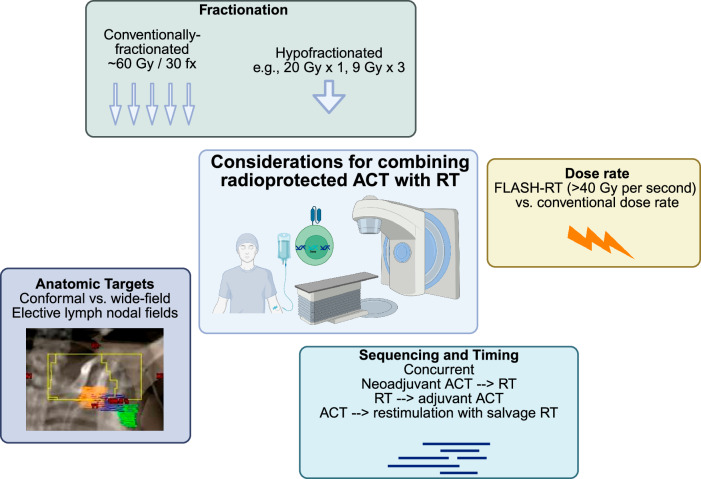
Fig. 8. Considerations for RT delivery in combination with ACT.
Key factors in optimizing the combination of radiation therapy (RT) with adoptive cell therapy (ACT) are presented. Fractionation options include conventionally-fractionated RT (e.g., 60 Gy in 30 fractions) and hypofractionated RT (e.g., 20 Gy in 1 fraction or 9 Gy in 3 fractions). Dose-rate considerations compare the use of conventional dose rates with FLASH-RT, where doses greater than 40 Gy per second are delivered. Anatomic targets are categorized into conformal vs. wide-field approaches, focusing on elective lymph node fields. Sequencing and timing of treatment strategies include concurrent, neoadjuvant ACT followed by RT, RT followed by adjuvant ACT, and using ACT with salvage RT for restimulation of immune responses.
Fractionated RT
Fractionated RT, where a total radiation dose is divided into smaller daily doses, presents an attractive opportunity to combine with radioprotected ACT. Standard fractionation schemes often use daily doses of 1.8–2.0 Gy, which fall near the lethal dose for 50% of T cells (LC50) at ~3 Gy16. Fractionated delivery allows immune cells time to recover between doses, reducing the risk of overwhelming damage to ACT cells.
This approach may progressively enhance the recruitment of ACT cells to the irradiated tumor over time83. Repeated low-dose exposure can upregulate the expression of stress ligands and increase antigen presentation by tumor cells, thus improving ACT cell recognition and tumor infiltration84. Additionally, fractionated RT spares normal tissues, reducing systemic toxicities that could otherwise impair immune responses.
From a logistical perspective, fractionated RT is commonly used in clinical settings for treating brain tumors. Therefore, its widespread clinical use facilitates the integration of ACT without substantial alterations to current RT protocols. Optimizing the timing and sequencing of ACT infusion within a fractionated RT regimen could maximize the beneficial effects of radiation while minimizing immune-cell depletion.
Hypofractionated RT
Hypofractionated RT, which delivers higher doses of radiation per fraction (e.g., >8 Gy), poses a different set of challenges and opportunities for ACT combinations. High-dose treatments, such as 20 Gy delivered in a single fraction in stereotactic radiosurgery (SRS) for brain metastases, may result in greater tumor cell death and release of tumor antigens85,86. However, the steep increase in radiation dose may overwhelm even radioprotected ACT cells, particularly those sensitive to radiation, such as T cells.
To circumvent the potential for immune-cell depletion, hypofractionated RT may be more effective when administered immediately before ACT infusion rather than concurrently. Pre-irradiation could prime the tumor for ACT by increasing tumor antigenicity and altering the tumor microenvironment to favor immune infiltration without subjecting the infused cells to excessive radiation85,86. Careful scheduling of hypofractionated RT prior to ACT infusion may amplify anti-tumor immune responses by using radiation as a priming tool.
Anatomic RT field considerations
One critical consideration when combining ACT with RT is the anatomic location and size of the radiation field. While modern RT technologies such as intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) allow for precise, conformal delivery of radiation, larger or less targeted radiation fields can pose a risk to immune cells within the lymphatic system and circulating immune cells.
Radiation fields that include lymph node basins or areas of high immune-cell trafficking may reduce the availability of functional immune cells for ACT. Lymph node irradiation may deplete T cells and other immune effectors required for ACT efficacy, which could hinder overall treatment outcomes87. Therefore, minimizing the irradiation of at-risk lymphoid structures or employing radioprotective strategies, such as shielding88, may help preserve immune functionality in combination therapy.
FLASH-RT
An emerging technique in the RT field, FLASH radiotherapy (FLASH-RT), involves ultra-high dose-rate radiation delivery, typically delivered at greater than 40 Gy/s89. Studies suggest that FLASH-RT offers the potential to spare normal tissues from the toxicities associated with conventional radiation, while maintaining potent anti-tumor effects90,91. The unique biological mechanisms behind FLASH-RT, such as differential oxygen depletion and modulation of the tumor microenvironment, could also offer a new paradigm for combination with ACT91. Some research suggests that FLASH-RT can overcome hypoxia-mediated tumor resistance, a hallmark of many brain cancers92,93. In glioblastoma, FLASH-RT has also been demonstrated to spare the normal brain from radiation-induced toxicities94.
Although still in experimental stages, FLASH-RT may stimulate CAR T cells and other ACT-based therapies in ways that conventional RT cannot. Furthermore, FLASH-RT may alter the TME in a manner that enhances CAR T cell trafficking and persistence, creating a synergistic effect that could amplify anti-tumor efficacy. In murine models of diffuse midline glioma, FLASH-RT led to the upregulation CD4+ T cells and genes involved in T-cell activation and trafficking on day 10 following treatment in comparison to conventional radiotherapy95. This warrants further investigation into the specific interactions between FLASH-RT and ACT, with an emphasis on understanding the immunological mechanisms involved.
Administration
The blood brain barrier and TME pose crucial considerations for the location of ACT administrations. Though ACTs can reach the brain via intravenous injection, other administration methods may increase ACT population within the CNS, possibly decreasing side effects96. Intrathecal delivery involves direct injection into the cerebral spinal fluid (CSF), bypassing the blood-brain barrier96. Injection can occur into the spine or the ventricles of the brain. While spinal intrathecal delivery, or lumbar punctures, may be useful for single-dose ACT, intraventricular injections, commonly via Ommaya catheters, can be programmed with a pump to deliver repeated doses96. Implantable devices may also offer an easier way to obtain CSF samples to confirm immune activation96. Intra-tumoral delivery of ACT is also possible via convection enhanced delivery (CED) systems. CED systems form a pressure gradient via a microinjection pump, possibly reaching a larger brain volume97. However, the pressure gradient may cause worsening neurotoxicity symptoms, a common side effect in CAR T patients96. Further research on the optimal administration method may maximize the response of radioprotected ACT.
Sequencing of radioprotected ACT and RT
Optimal sequencing of radioprotected ACT and RT is essential to fully leverage the therapeutic potential of these combined modalities. While both ACT and RT are potent treatments on their own, their integration requires careful planning to avoid negative interactions such as radiation-induced immune-cell damage. Incorporating radioprotection strategies into ACT opens new possibilities for safely combining these treatments, potentially allowing for higher radiation doses or more aggressive fractionation schedules. The number of doses should also be considered, as radioprotection may impact the longevity of modified immune cells. Preclinical models and clinical trials are necessary to establish the most effective sequencing protocols.
Preclinical studies to inform sequencing
Preclinical studies are particularly crucial for evaluating how the addition of radioprotective strategies influences the interaction between ACT and RT. In these models, the timing of ACT administration relative to RT can be explored, particularly in the context of radioprotection. For instance, studies can assess whether radioprotected ACT cells retain functionality and viability when delivered before, during, or after RT. Additionally, preclinical research can help define the thresholds of RT dose and fractionation at which radioprotected ACT cells are most effective, without suffering significant damage from radiation. By incorporating radioprotective agents into ACT protocols, it may be possible to use higher radiation doses that would otherwise impair T-cell function. This opens new avenues for combination therapies, but the precise sequencing of these treatments will need to be fine-tuned through preclinical work before transitioning into clinical practice.
Integrating radioprotected ACT into upfront, fractionated RT for curative-intent treatment
For brain cancers where upfront fractionated RT is the standard of care, such as glioblastoma, integrating radioprotected ACT into curative-intent treatment regimens offers an exciting therapeutic strategy. Fractionated RT typically involves daily radiation doses of around 1.8–2.0 Gy delivered over several weeks, creating a potentially favorable environment for ACT. Radioprotected ACT could be administered early in the course of fractionated RT, allowing T cells to persist and accumulate within the tumor over time.
Because radioprotective strategies could enhance the resilience of ACT cells to low daily doses of radiation, this approach may increase the likelihood of immune cells infiltrating the tumor microenvironment and maintaining functionality throughout the RT course. This might improve the overall therapeutic outcome, especially in brain tumors that are traditionally difficult to treat. In this context, radioprotected ACT cells could be infused early in the RT course and allowed to interact with radiation-induced tumor stress signals and antigen presentation over time. Fractionated RT may also create opportunities for selection of radioprotected ACT cells. While normal immune cells will die throughout fractionated RT, radioprotected ACT cells may not, providing a sustained anti-tumor immune response and reducing the risk of T-cell depletion.
Combining salvage RT with radioprotected ACT
For patients undergoing salvage RT, particularly those with recurrent or metastatic cancer, combining radioprotected ACT with higher RT doses or hypofractionated schedules may improve outcomes. In salvage settings, higher RT doses (e.g., 8 Gy per fraction or more) are often required to address treatment-resistant tumors, which can compromise immune-cell viability. The inclusion of radioprotection in ACT protocols allows the possibility of combining these more aggressive RT regimens without overwhelming the immune system.
In these cases, radioprotected ACT could be administered either before or after salvage RT, depending on the patient’s condition and treatment goals. Post-RT administration of radioprotected ACT could allow the immune system to “clean up” any residual tumor cells that survive the high-dose radiation. Alternatively with pre-RT administration of radioprotected ACT, RT could be used to debulk the tumor and prime the tumor microenvironment for subsequent immune attack by radioprotected cells. This combination might offer significant benefits, but the optimal sequencing needs to be explored through preclinical and clinical trials.
Re-stimulation of radioprotected ACT with RT
Radiation has the potential to re-stimulate adoptive immune cells that may have become less effective over time, and radioprotected ACT cells could be especially well-suited for this approach. As tumors progress, ACT cells may experience functional exhaustion, particularly in cases of CAR T-cell therapy. RT can induce tumor cell death85,86 releasing antigens that could “re-prime” the radioprotected ACT cells, thereby restoring or boosting their anti-tumor activity. In this scenario, radioprotected ACT cells would be better able to withstand the re-stimulation process, maintaining their functionality and viability despite the immunosuppressive effects of radiation. This strategy could be particularly useful in treating brain tumors that exhibit immunoediting, where the immune system drives the selection of tumor cells that evade immune detection98. Immunoediting is especially relevant in glioblastoma, which shows poor response to immunotherapies due to tumor herterogeneity99. By re-exposing the tumor to immune surveillance following RT, the radioprotected ACT cells could help target these previously elusive tumor cell populations.
Non-oncologic opportunities
Radioprotective ACT holds promising potential for non-oncologic purposes, such as protecting stem cell allografts during transplantation. One key application could be to reduce the duration and severity of the post-transplant nadir, a period of vulnerability when the patient’s immune system is severely compromised. Typically, patients undergoing stem cell transplantation will receive total body irradiation (TBI) to prep the immune system prior to the transplant100. Patients generally receive 12–15 Gy over 3–4 days100. TBI acts both cytotoxic and immunosuppressive, creating space in the marrow for new cells and decreasing the likelihood of stem cell rejection100. Administering radioprotected ACT prior to radiation could enhance the success and recovery of hematopoietic stem cell transplants, potentially leading to faster immune reconstitution and fewer complications.
Additionally, radioprotected ACT could be explored for specific non-cancerous indications where radiation is necessary but poses risks to healthy cells, such as in certain autoimmune disorders or organ transplants. However, the use of radioprotected ACT for non-oncologic applications would require careful study, as clinical experience builds in oncology. With more research and validation, these innovative approaches could eventually make their way into clinical practice, expanding the therapeutic benefits of radioprotective strategies beyond cancer treatment.
Alternative radioprotection approaches
Other radioprotective strategies include endogenous pathway modulation, pharmacological agents, physical shields, and cellular engineering, each with distinct limitations. Overexpressing human antioxidants like SOD2 reduces oxidative stress but offers limited protection against high radiation doses, while modulating the p53 pathway can prevent apoptosis but risks preserving genetically unstable cells. Pharmacological options like FDA-approved amifostine provide nonspecific ROS scavenging unsuitable for immune cells, and synthetic molecules such as Mn porphyrins lack DNA repair capabilities. Physical approaches, such as nanoparticle shields, can protect cells but add manufacturing complexity and may hinder cell trafficking. Cellular engineering through CRISPR offers precise genetic modifications for radiation resistance, though extensive screening is needed to avoid adverse effects, while leveraging radiation-resistant stem cell-derived immune cells faces scalability challenges.
In vivo delivery of CARs and radioprotector genes
The workflow for manufacturing CAR T cells ex vivo for specific patients is long and costly. In vivo delivery proposes the administration of CAR gene or protein payloads enveloped in viral vectors or nanoparticles101. Relevant immune-cell-targeting ligands are fused to the viral envelope protein to ensure the proper cells receive the CAR101. Lentivirus, retrovirus, and adeno-associated virus have been used in mice to deliver CAR payloads with equivalent efficacy in controlling tumor growth to ex vivo CAR delivery101. Nanocarriers delivering mRNA, plasmid, and protein have also been used101. In vivo delivery methods may also be helpful for radioprotector gene candidates. For example, delivery of viral vectors encoding a CAR and radioprotector gene could eliminate the need for ex vivo processing. In vivo delivery is still developing and requires careful control to limit off-target effects while maximizing immune-cell transduction efficiency101.
Future clinical trial designs
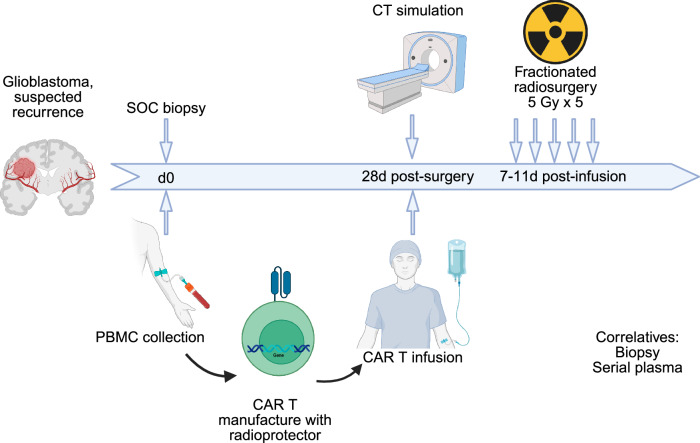
Clinical trial design for combining radiation therapy (RT) and radioprotected ACT must thoughtfully consider the optimal sequencing of these treatments to maximize therapeutic efficacy. This involves determining the timing of RT relative to ACT, as well as establishing parameters such as the appropriate ACT dosage, RT fractionation schedules, and dosing to ensure synergistic effects while minimizing toxicity. Additionally, the use of steroids in conjunction with these therapies should be evaluated, as they can impact immune function and treatment outcomes102. Incorporating correlative studies into trial designs will enable researchers to explore the underlying biological mechanisms at play, facilitating a better understanding of how these therapies interact. Moreover, identifying and validating biomarkers of response could provide critical insights, allowing for early outcome readouts that guide subsequent treatment decisions and adjustments, ultimately enhancing the overall effectiveness of the combined approach. A schematic for a potential clinical trial of radioprotected CAR T in combination with conventionally-fractionated RT for newly-diagnosed glioblastoma brain tumor patients is outlined in Fig. 9.
Fig. 9. Concept for clinical trial of radioprotected CAR T and RT.
Patients with suspected glioma are recruited to the trial. Patients undergo standard-of-care surgical resection followed by radiation therapy. PBMCs are collected and manufactured into CAR T cells including the radioprotector(s) identified here. CAR T cells are re-infused by day 56, providing time for CAR manufacturing but delivering CAR T early in the RT course when immunogenicity may be highest. Concurrent standard-of-care temozolomide may be given or omitted depending on rapid tumor molecular analysis.
Radioprotector safety considerations
Safety is a paramount consideration when integrating radioprotectors into ACT. First and foremost, the potential for radioprotectors to influence apoptosis or cell cycle pathways raises concerns regarding carcinogenesis. Alterations in these critical regulatory mechanisms may inadvertently promote tumorigenesis, underscoring the necessity for thorough preclinical evaluation and long-term monitoring of patients receiving such therapies. Also, any approach involving the expression of foreign radioprotective proteins must be scrutinized for unforeseen immunogenic effects. The introduction of xenoproteins may elicit immune responses that could compromise the safety and effectiveness of the therapy. Another concern is that CAR T cells expressing genes that modulate the cell cycle or cell senescence could become immortal, potentially leading to rapid cell division, autoimmunity, and/or T cell malignancies. For this reason, researchers should consider integrating suicide genes, such as caspase 9103. Suicide genes may also be helpful in case of severe side-effects following CAR T cell infusions, such as cytokine release syndrome103. Ideal suicide genes could be activated by a biologically inert, bioavailable antibody103.
CAR T trials should also be planned with especially close monitoring and planning for patient safety. Moreover, CAR T cell therapy is known to elicit severe immune reactions, the most notable being neurotoxicity, which can necessitate hospitalization. The introduction of RT may exacerbate these adverse effects, complicating the clinical picture and heightening the need for vigilant monitoring and trial designs that prioritize patient safety. Establishing clear protocols for assessing and managing toxicities will be essential to mitigate these risks effectively. Additionally, the concurrent use of steroids and IL-1R agonists, such as anakinra, should be carefully planned104. While these agents can modulate inflammatory responses, their immunosuppressive effects could potentially undermine the therapeutic efficacy of CAR T cells. Thus, balancing the need for symptom management with the preservation of immune functionality will be crucial. Comprehensive safety evaluations and preclinical studies are necessary to identify and mitigate these risks before advancing to clinical trials. In summary, while the potential benefits of radioprotectors in enhancing ACT are compelling, the associated safety concerns must be addressed through rigorous research, careful clinical trial design, and ongoing patient monitoring to ensure a favorable risk-benefit profile.
Conclusion
The potential to enhance ACT for brain tumors through the radioprotection of immune cells represents a groundbreaking frontier in cancer treatment. Immune-cell types used in CAR therapies exhibit distinct anti-tumor properties that must be carefully leveraged to maximize therapeutic impact. Insights from extremophile biology, coupled with advancements in radioprotective technologies and robust screening methodologies, offer a unique opportunity to overcome the challenges posed by the immunosuppressive tumor microenvironment in brain cancers. However, introducing novel proteins carries inherent risks, underscoring the necessity for extensive preclinical research to optimize the sequencing of ACT and RT for improved efficacy. Collaborative research efforts will be pivotal in translating these innovations into clinical practice, ultimately advancing outcomes for brain cancer patients. Future work should prioritize high-throughput screening to identify additional extremophile-derived proteins with complementary radioprotective functions, alongside the development of sophisticated delivery systems that ensure stable, efficient expression of these genes in immune cells without impairing function. Finally, rigorous safety and immunogenicity testing, including long-term preclinical evaluation of these proteins in CAR T cells, will be essential to validate therapeutic efficacy while minimizing potential adverse effects.
Acknowledgements
This work was supported with developmental funds of the Duke Cancer Institute as part of the P30 Cancer Center Support Grant (Grant ID: NIH CA014236) to Z.J.R. Figures were created using BioRender.
Author contributions
Z.J.R. conceived the idea and A.J.G. and Z.J.R. wrote the manuscript. A.J.G. and Z.J.R. designed and created all figures. M.K. provided immunotherapy expertise and revised and edited the manuscript. J.D.B. provided extremophile and radioprotection expertise and revised and edited the manuscript. All authors have read and agree with the final version of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Competing interests
Z.J.R. is listed as an inventor on intellectual property related to brain tumor diagnostics that is managed by Duke and has been licensed to Genetron Health. The Authors declare no competing Non-Financial Interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gallus, M. et al. Chimeric antigen receptor T-cell therapy in patients with malignant glioma—from neuroimmunology to clinical trial design considerations. Neuro Oncol. 10.1093/neuonc/noae203 (2024). [DOI] [PMC free article] [PubMed]
- 2.Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med.375, 2561–2569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature603, 934–941 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol.21, 47–66 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Mullard, A. FDA approves first tumour-infiltrating lymphocyte (TIL) therapy, bolstering hopes for cell therapies in solid cancers. Nat. Rev. Drug Discov.23, 238 (2024). [DOI] [PubMed] [Google Scholar]
- 6.Creelan, B. C. et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat. Med.27, 1410–1418 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhavan, D. et al. CAR T cells for brain tumors: lessons learned and road ahead. Immunol. Rev.290, 60–84 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagley, S. J. et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: a phase 1 trial. Nat. Cancer5, 517–531 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med.390, 1290–1298 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagley, S. J. et al. Intracerebroventricular bivalent CAR T cells targeting EGFR and IL-13Ralpha2 in recurrent glioblastoma: a phase 1 trial. Nat. Med.10.1038/s41591-025-03745-0 (2025). [DOI] [PubMed]
- 11.Lin, Y. J., Mashouf, L. A. & Lim, M. CAR T cell therapy in primary brain tumors: current investigations and the future. Front. Immunol.13, 817296 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunert, M. et al. Radiation and brain tumors: an overview. Crit. Rev. Oncog.23, 119–138 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Hovhannisyan, L., Riether, C., Aebersold, D. M., Medova, M. & Zimmer, Y. CAR T cell-based immunotherapy and radiation therapy: potential, promises and risks. Mol. Cancer22, 82 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin, V. M., Haynes, N. M., D’Souza, C., Neeson, P. J. & Zhu, J. J. CAR-T plus radiotherapy: a promising combination for immunosuppressive tumors. Front. Immunol.12, 813832 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gameiro, S. R. et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget5, 403–416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura, N., Kusunoki, Y. & Akiyama, M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat. Res.123, 224–227 (1990). [PubMed] [Google Scholar]
- 17.Ghosh, S. et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci. Transl. Med.15, eabn6758 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terrones-Campos, C. et al. Hematological toxicity in patients with solid malignant tumors treated with radiation - Temporal analysis, dose response and impact on survival. Radiother. Oncol.158, 175–183 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Takeda, K. et al. Acute hematologic toxicity of radiation therapy—a comprehensive analysis and predictive nomogram. J. Radiat. Res.64, 954–961 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andruska, N. & DeSelm, C. C. C.J. Development of radioresistant CAR T cells for solid tumor therapeutics. Int. J. Radiat. Oncol. Biol. Phys.111, S57–S58 (2021). [Google Scholar]
- 21.Hosoki, A. et al. Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa cells. J. Radiat. Res.53, 58–71 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Zhang, Z. et al. Radiation-induced SOD2 overexpression sensitizes colorectal cancer to radiation while protecting normal tissue. Oncotarget8, 7791–7800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto, T. et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun.7, 12808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikawa, D. D. et al. Radiation tolerance in the tardigrade Milnesium tardigradum. Int. J. Radiat. Biol.82, 843–848 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Kirtane, A. R. et al. Radioprotection of healthy tissue via nanoparticle-delivered mRNA encoding for a damage-suppressor protein found in tardigrades. Nat. Biomed. Eng.10.1038/s41551-025-01360-5 (2025). [DOI] [PubMed]
- 26.Shi, Y. et al. The protein PprI provides protection against radiation injury in human and mouse cells. Sci. Rep.6, 26664 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, L. et al. Multi-omics landscape and molecular basis of radiation tolerance in a tardigrade. Science386, eadl0799 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Setlow, P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol.101, 514–525 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Pan, K. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res.41, 119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganapathy, T., Radhakrishnan, R., Sakshi, S. & Martin, S. C. A. R. gammadelta T cells for cancer immunotherapy. Is the field more yellow than green?. Cancer Immunol. Immunother.72, 277–286 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y. et al. iNKT: A new avenue for CAR-based cancer immunotherapy. Transl. Oncol.17, 101342 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hekim, N., Cetin, Z., Nikitaki, Z., Cort, A. & Saygili, E. I. Radiation triggering immune response and inflammation. Cancer Lett.368, 156–163 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Heylmann, D., Rodel, F., Kindler, T. & Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta1846, 121–129 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Mitra, A. et al. From bench to bedside: the history and progress of CAR T cell therapy. Front. Immunol.14, 1188049 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer, G. et al. Fluorescence tracking demonstrates T cell recirculation is transiently impaired by radiation therapy to the tumor. Sci. Rep.14, 11909 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, C. F. et al. Local interleukin-12 treatment enhances the efficacy of radiation therapy by overcoming radiation-induced immune suppression. Int. J. Mol. Sci.22. 10.3390/ijms221810053 (2021). [DOI] [PMC free article] [PubMed]
- 37.Han, S. K., Song, J. Y., Yun, Y. S. & Yi, S. Y. Effect of gamma radiation on cytokine expression and cytokine-receptor mediated STAT activation. Int. J. Radiat. Biol.82, 686–697 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Sato, H., Okonogi, N. & Nakano, T. Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int. J. Clin. Oncol.25, 801–809 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochoa de Olza, M., Bourhis, J., Irving, M., Coukos, G. & Herrera, F. G. High versus low dose irradiation for tumor immune reprogramming. Curr. Opin. Biotechnol.65, 268–283 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Lhuillier, C. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Investig.131. 10.1172/JCI138740 (2021). [DOI] [PMC free article] [PubMed]
- 41.Gandhi, S. & Chandna, S. Radiation-induced inflammatory cascade and its reverberating crosstalks as potential cause of post-radiotherapy second malignancies. Cancer Metastasis Rev.36, 375–393 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med.203, 1259–1271 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portella, L. & Scala, S. Ionizing radiation effects on the tumor microenvironment. Semin. Oncol.46, 254–260 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Kloess, S. et al. Preclinical assessment of suitable natural killer cell sources for chimeric antigen receptor natural killer-based “Off-the-Shelf” acute myeloid leukemia immunotherapies. Hum. Gene Ther.30, 381–401 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Baude, J., Limagne, E., Ladjohounlou, R. & Mirjolet, C. Combining radiotherapy and NK cell-based therapies: the time has come. Int. Rev. Cell Mol. Biol.378, 31–60 (2023). [DOI] [PubMed] [Google Scholar]
- 46.Park, B., Yee, C. & Lee, K. M. The effect of radiation on the immune response to cancers. Int. J. Mol. Sci.15, 927–943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hietanen, T., Pitkanen, M., Kapanen, M. & Kellokumpu-Lehtinen, P. L. Effects of single and fractionated irradiation on natural killer cell populations: radiobiological characteristics of viability and cytotoxicity in vitro. Anticancer Res.35, 5193–5200 (2015). [PubMed] [Google Scholar]
- 48.Eckert, F. et al. Impact of curative radiotherapy on the immune status of patients with localized prostate cancer. Oncoimmunology7, e1496881 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navarro-Martin, A. et al. Preliminary study of the effect of stereotactic body radiotherapy (SBRT) on the immune system in lung cancer patients unfit for surgery: immunophenotyping analysis. Int. J. Mol. Sci.19, 10.3390/ijms19123963 (2018). [DOI] [PMC free article] [PubMed]
- 50.Li, T. T. et al. The effects of stereotactic body radiotherapy on peripheral natural killer and CD3(+)CD56(+) NKT-like cells in patients with hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int.20, 240–250 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Kim, J. Y. et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp. Mol. Med.38, 474–484 (2006). [DOI] [PubMed] [Google Scholar]
- 52.He, J. et al. Synergistic treatment strategy: combining CAR-NK cell therapy and radiotherapy to combat solid tumors. Front. Immunol.14, 1298683 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noonepalle, S. K. R. et al. Cell therapy using ex vivo reprogrammed macrophages enhances antitumor immune responses in melanoma. J. Exp. Clin. Cancer Res.43, 263 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heylmann, D., Ponath, V., Kindler, T. & Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep.11, 2478 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell24, 589–602 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Prakash, H. et al. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis37, 301–313 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Gough, M. J., Young, K. & Crittenden, M. The impact of the myeloid response to radiation therapy. Clin. Dev. Immunol.2013, 281958 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Genard, G., Lucas, S. & Michiels, C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front. Immunol.8, 828 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, L., Jiang, J., Chen, Y., Jia, Q. & Chu, Q. The roles of CC chemokines in response to radiation. Radiat. Oncol.17, 63 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakimi, K., Matsushita, H., Murakawa, T. & Nakajima, J. gammadelta T cell therapy for the treatment of non-small cell lung cancer. Transl. Lung Cancer Res.3, 23–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abedalthagafi, M., Mobark, N., Al-Rashed, M. & AlHarbi, M. Epigenomics and immunotherapeutic advances in pediatric brain tumors. npj Precis. Oncol.5, 34 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaue, D. & McBride, W. H. T lymphocytes and normal tissue responses to radiation. Front. Oncol.2, 119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seifert, L. et al. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology150, 1659–1672.e1655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, X. et al. Exosomes derived from gammadelta-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in an immunosuppressive microenvironment. J. Immunother. Cancer10. 10.1136/jitc-2021-003832 (2022). [DOI] [PMC free article] [PubMed]
- 65.Paul, S. & Lal, G. Regulatory and effector functions of gamma-delta (gammadelta) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int. J. Cancer139, 976–985 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Takami, M., Ihara, F. & Motohashi, S. Clinical application of iNKT cell-mediated anti-tumor activity against lung cancer and head and neck cancer. Front. Immunol.9, 2021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melo, A. M., Maher, S. G., O’Leary, S. M., Doherty, D. G. & Lysaght, J. Selective effects of radiotherapy on viability and function of invariant natural killer T cells in vitro. Radiother. Oncol.145, 128–136 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Pilones, K. A. et al. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin. Cancer Res.15, 597–606 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simonetta, F. et al. Allogeneic CAR invariant natural killer T cells exert potent antitumor effects through host CD8 T-cell cross-priming. Clin. Cancer Res.27, 6054–6064 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara, A. et al. CD1d expression in glioblastoma is a promising target for NKT cell-based cancer immunotherapy. Cancer Immunol. Immunother.70, 1239–1254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teo, W. Y. et al. Therapeutic implications of CD1d expression and tumor-infiltrating macrophages in pediatric medulloblastomas. J. Neurooncol.120, 293–301 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Jeong, J. U. et al. Effect of irradiation-induced intercellular adhesion molecule-1 expression on natural killer cell-mediated cytotoxicity toward human cancer cells. Cytotherapy20, 715–727 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Olschowka, J. A. et al. ICAM-1 induction in the mouse CNS following irradiation. Brain Behav. Immun.11, 273–285 (1997). [DOI] [PubMed] [Google Scholar]
- 74.Maenpaa, A., Kovanen, P. E., Paetau, A., Jaaskelainen, J. & Timonen, T. Lymphocyte adhesion molecule ligands and extracellular matrix proteins in gliomas and normal brain: expression of VCAM-1 in gliomas. Acta Neuropathol.94, 216–225 (1997). [DOI] [PubMed] [Google Scholar]
- 75.Wang, A. X., Ong, X. J., D’Souza, C., Neeson, P. J. & Zhu, J. J. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front. Immunol.14, 1140541 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amatya, C. et al. Development of CAR T cells expressing a suicide gene plus a chimeric antigen receptor targeting signaling lymphocytic-activation molecule F7. Mol. Ther.29, 702–717 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suryadevara, C. M. et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology7, e1434464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veling, M. T. et al. Natural and designed proteins inspired by extremotolerant organisms can form condensates and attenuate apoptosis in human cells. ACS Synth. Biol.11, 1292–1302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonsson, K. I. & Schill, R. O. Induction of Hsp70 by desiccation, ionising radiation and heat-shock in the eutardigrade Richtersius coronifer. Comp. Biochem. Physiol. B Biochem. Mol. Biol.146, 456–460 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Jung, K. W. et al. Unraveling fungal radiation resistance regulatory networks through the genome-wide transcriptome and genetic analyses of cryptococcus neoformans. mBio7. 10.1128/mBio.01483-16 (2016). [DOI] [PMC free article] [PubMed]
- 81.Moris, V. C. et al. Ionizing radiation responses appear incidental to desiccation responses in the bdelloid rotifer Adineta vaga. BMC Biol.22, 11 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goodman, D. B. et al. Pooled screening of CAR T cells identifies diverse immune signaling domains for next-generation immunotherapies. Sci. Transl. Med.14, eabm1463 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao, L. & Zhang, A. Low-dose radiotherapy effects the progression of anti-tumor response. Transl. Oncol.35, 101710 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol.174, 7516–7523 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Herskind, C., Wenz, F. & Giordano, F. A. Immunotherapy combined with large fractions of radiotherapy: stereotactic radiosurgery for brain metastases-implications for intraoperative radiotherapy after resection. Front. Oncol.7, 147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ene, C. I. et al. Response of treatment-naive brain metastases to stereotactic radiosurgery. Nat. Commun.15, 3728 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koukourakis, M. I. & Giatromanolaki, A. Tumor draining lymph nodes, immune response, and radiotherapy: Towards a revisal of therapeutic principles. Biochim. Biophys. Acta Rev. Cancer1877, 188704 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Wong, J. Y. C., Filippi, A. R., Dabaja, B. S., Yahalom, J. & Specht, L. Total body irradiation: guidelines from the international lymphoma radiation oncology group (ILROG). Int. J. Radiat. Oncol. Biol. Phys.101, 521–529 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Lin, B. et al. FLASH radiotherapy: history and future. Front. Oncol.11, 644400 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Favaudon, V. et al. Ultrahigh dose-rate FLASH irradiation inc.reases the differential response between normal and tumor tissue in mice. Sci. Transl. Med.6, 245ra293 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Vozenin, M. C. et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin. Cancer Res.25, 35–42 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Leavitt, R. J. et al. Acute hypoxia does not alter tumor sensitivity to FLASH radiation therapy. Int. J. Radiat. Oncol. Biol. Phys.119, 1493–1505 (2024). [DOI] [PubMed] [Google Scholar]
- 93.Park, J. H. & Lee, H. K. The role of hypoxia in brain tumor immune responses. Brain Tumor Res. Treat.11, 39–46 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montay-Gruel, P. et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin. Cancer Res.27, 775–784 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Padilla, O. et al. Immune response following FLASH and conventional radiation in diffuse midline glioma. Int. J. Radiat. Oncol. Biol. Phys.119, 1248–1260 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Del Baldo, G. et al. The peculiar challenge of bringing CAR-T cells into the brain: Perspectives in the clinical application to the treatment of pediatric central nervous system tumors. Front. Immunol.14, 1142597 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barua, N. U., Gill, S. S. & Love, S. Convection-enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathol.24, 117–127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pellegatta, S., Cuppini, L. & Finocchiaro, G. Brain cancer immunoediting: novel examples provided by immunotherapy of malignant gliomas. Expert Rev. Anticancer Ther.11, 1759–1774 (2011). [DOI] [PubMed] [Google Scholar]
- 99.Amin, T. et al. Immunoediting dynamics in glioblastoma: implications for immunotherapy approaches. Cancer Control31, 10732748241290067 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sabloff, M., Tisseverasinghe, S., Babadagli, M. E. & Samant, R. Total body irradiation for hematopoietic stem cell transplantation: What can we agree on?. Curr. Oncol.28, 903–917 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bui, T. A., Mei, H., Sang, R., Ortega, D. G. & Deng, W. Advancements and challenges in developing in vivo CAR T cell therapies for cancer treatment. EBioMedicine106, 105266 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karachi, A., Dastmalchi, F., Mitchell, D. A. & Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol.20, 1566–1572 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bouquet, L. et al. Correction: RapaCaspase-9-based suicide gene applied to the safety of IL-1RAP CAR-T cells. Gene Ther.32, 299 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brudno, J. N. & Kochenderfer, J. N. Current understanding and management of CAR T cell-associated toxicities. Nat. Rev. Clin. Oncol.21, 501–521 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.