Abstract
Long-term depression (LTD) at cerebellar parallel fiber (PF)-Purkinje cell synapses must be balanced by long-term potentiation (LTP) to prevent saturation and allow reversal of motor learning. The only previously analyzed form of cerebellar LTP is induced by 4–8 Hz PF stimulation and requires cAMP but not nitric oxide. It is a poor candidate to reverse LTD because it is presynaptically expressed whereas LTD is postsynaptic. We now characterize a new form of LTP induced by 1 Hz PF stimulation for at least 300 s. This LTP is postsynaptically expressed, enhanced by chelating postsynaptic Ca2+, and depends on nitric oxide but not cAMP or cGMP, making it a plausible anti-Hebbian counterpart to Hebbian LTD.
The cerebellar cortex plays a crucial role in a wide variety of behavioral modifications, of which Pavlovian conditioning of eyelid blinking and gain modulation of the vestibuloocular reflex have been the most extensively studied (see refs. 1–4 for reviews). In the cerebellar cortex, the most extensively investigated form of synaptic plasticity has been long-term depression (LTD) of the glutamatergic synapses from parallel fibers (PFs) onto Purkinje cells (PCs). PF-PC LTD is associative (requires concurrent pre- and postsynaptic activity) and fairly synapse-specific (5), requires postsynaptic Ca2+ elevation, and is expressed postsynaptically. Coincidence of the postsynaptic Ca2+ with nitric oxide (NO), normally generated presynaptically and diffusing anterogradely, is necessary and sufficient for a major form of LTD in young adult cerebellar slices; NO acts via cGMP and protein kinase G (6–9). However, the PF-PC synapse also needs long-term potentiation (LTP), driven by presynaptic activity without postsynaptic depolarization, to prevent saturation of LTD, allow extinction of learned associations, and refine the temporal precision of learned responses (10–12). Although a few examples of LTP at the PF-PC synapse had been anecdotally reported in articles focusing on LTD (8, 13–16), the first systematic characterization of LTP was by Salin et al. (17), who stimulated the PFs 120 times at 4–8 Hz and observed a subsequent 40–50% long-term enhancement of excitatory postsynaptic potentials. This work and several subsequent studies have shown that such LTP is induced and expressed presynaptically by means of cAMP signaling (18–21).
Although cAMP-mediated presynaptic LTP clearly exists, it remains an unattractive solution to the problem of resetting postsynaptically expressed LTD, because the two processes are expressed in different locations and cannot truly reverse each other (10, 11). Also, presynaptic LTP is independent of the presence or absence of PC activity (17, 19). During our studies of associative LTD evoked in young adult slices by coincident increases in Ca2+, NO, and cGMP during 1-Hz stimulation for 30 s, we sometimes observed small LTP when LTD was blocked by chelation of postsynaptic Ca2+ or inhibition of protein kinase G (6, 7). We now show that such LTP is greatly enhanced by longer stimulation periods at 1 Hz. Because it is triggered by NO without a rise in postsynaptic Ca2+ and is expressed postsynaptically, it is a plausible counterpart for associative LTD.
Experimental Procedures
Thin (200–300 μm) sagittal slices were cut from the cerebellar vermis of rats aged 17–21 days. Synaptic currents in PCs were recorded in the whole-cell patch-clamp configuration (22). The cells were directly visualized through a ×40 water immersion objective on an upright microscope (Axioskop, Zeiss). Tight-seal whole-cell recordings (seal resistance >10 GΩ) were made with patch pipettes with 3–4 MΩ resistance and an Axopatch 200A (Axon Instruments, Foster City, CA) amplifier (holding potential −70 mV). A 10-ms, 10-mV test depolarization preceded each PF stimulus to monitor the input resistance of the PC throughout the experiment, which was discarded if the resistance changed by more than 20%. The intracellular solution contained (in mM) potassium gluconate 130, KCl 10, potassium Hepes 10, MgCl2 1, NaATP 4, NaGTP 1, sucrose 16 (pH 7.2). The osmolarity was 300 mOsm. Five millimolar 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) tetrapotassium (Molecular Probes) was added to the intracellular solution in all experiments except where explicitly noted. The external Ringer's solution contained (in mM) NaCl 125, KCl 2.5, CaCl2 2, MgCl2 1, NaH2PO4 1.25, NaHCO3 26, glucose 25 (pH 7.4), and 10 μM (−)-bicuculline methiodide (Research Biochemicals, Natick, MA) to inhibit GABAergic synapses. Although these conditions are adequate for measuring excitatory postsynaptic currents (EPSCs), the voltage clamp is incapable of maintaining space clamp conditions in the dendrites and axon when spikes are generated. Slices were continually perfused with Ringer's solution saturated with 95% O2/5% CO2 during recording. All experiments were performed at room temperature (near 22°C).
PFs were stimulated with 50-μsec pulses delivered by an isolation unit (ISO-Flex, A.M.P.I., Jerusalem) through a bipolar electrode (Haer, Bowdoinham, ME) at the pial surface above the recorded PC. Voltage and current data were digitized and stored with a labview (National Instruments, Portsmouth, NH) program developed by V.L.-R. EPSCs evoked by PF stimulation were recorded from Purkinje neurons in whole-cell patch configuration. Normalized EPSC amplitudes were calculated every 50 sec by averaging 5 successive EPSC amplitudes collected every 10 s, dividing by the average EPSC amplitude obtained before any induction protocol, and averaging over multiple Purkinje neurons subjected to the identical procedure. Error bars indicate standard errors between the averaged cells.
1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was from Tocris Cookson (Ellisville, MO); KT5720, forskolin, N,N′-dicarboxymethyl-N,N′-dinitroso-p-phenylenediamine, 2Na (BNN5Na), and horse skeletal muscle myoglobin were from Calbiochem; and Nω-nitro-l-arginine (NNLA) was from Sigma. CNO-4 was synthesized in our laboratory by L. Makings (23). Oxymyoglobin was prepared as described (6). Mice with homozygous double deletion of adenylyl cyclases 1 and 8 were generated as described (24).
Results
LTP Induction with 300 PF Stimuli at 1 Hz.
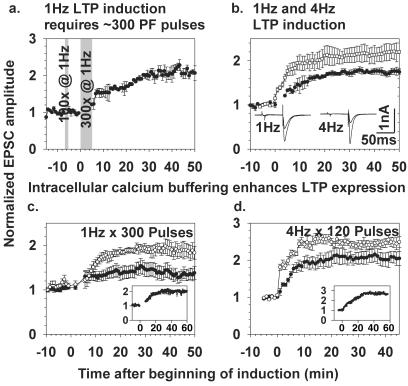
To establish LTP with the same 1-Hz stimulus frequency as used to elicit LTD, we first obtained a stable baseline of synaptic EPSC amplitudes by measuring for 5–10 min at 0.1 Hz to avoid any plasticity. We then increased the stimulus frequency to 1 Hz for 5 min (300 stimuli), then returned to 0.1 Hz to monitor the EPSC amplitude. In 80% of the tested PCs, this protocol produced LTP as defined by at least 30% increase of EPSC amplitude over baseline, lasting at least 50 min (Fig. 1a). Fifty, 100, and 200 spikes at 1 Hz were generally insufficient to cause LTP, as shown in Fig. 1a for the case of 100 stimuli. A superficially similar but slightly larger LTP (Fig. 1b) resulted from 120 PF stimuli at 4 Hz, a protocol based on Salin et al. (17) and Linden and Ahn (19). Because buffering of postsynaptic Ca2+ is well known to prevent LTD, we tested the effects of such buffering on 1 vs. 4 Hz LTP. Fig. 1 c and d show respectively that 1 Hz and 4 Hz LTP were both diminished but still detectable when the Ca2+ buffer BAPTA was omitted from the pipette solution. The effect of buffering postsynaptic Ca2+ is probably to prevent residual LTD induction and thereby to unmask the full amplitude of both 1 and 4 Hz LTP. However, it remains possible that buffering postsynaptic Ca2+ directly enhances 1 Hz LTP in addition to inhibiting LTD.
Figure 1.
Basic features of 1 Hz and 4 Hz LTP. (a) Induction of 1 Hz LTP required about 300 PF pulses. One hundred PF stimuli were not sufficient to potentiate PF-PC synaptic input (n = 3). (b) Comparison of the effect of 300 PF pulses at 1 Hz (●, n = 6) vs. 120 PF pulses at 4 Hz (□, n = 6). Insets show representative individual EPSC traces before and after 1 Hz (left) and 4 Hz LTP (right); in each case the trace after LTP induction has the larger amplitude. (c and d) Enhancement of both 1 Hz (c) and 4 Hz LTP (d) by inclusion of 5 mM BAPTA in the intracellular solution (●, control intracellular solution without BAPTA, n = 7 for each stimulation frequency; □, BAPTA in the intracellular solution, n = 5 for each stimulation frequency). Insets show representative single experiments with 5 mM BAPTA in the pipette solution but without averaging between cells.
NO Is Necessary and Sufficient for 1 Hz but Not 4 Hz LTP.
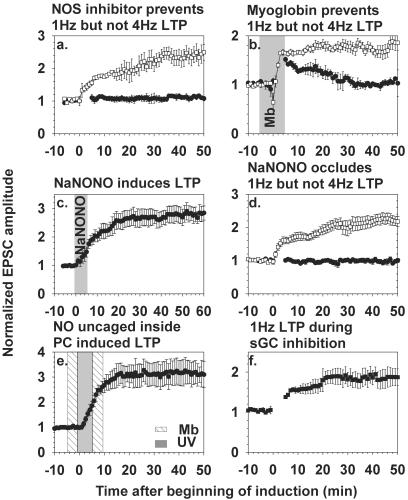
Stimulation of the PF produces NO, which is necessary for LTD under our stimulation conditions (6, 7). To test whether 1 Hz LTP also depends on NO, slices were incubated with the NO synthase inhibitor Nω-nitro-l-arginine (NNLA) (100 μM) for at least 1 h before recording EPSCs. The cells were then stimulated at either 1 or 4 Hz in the continued presence of NNLA (Fig. 2a). Four Hz LTP was normal, confirming Salin et al. (17), whereas induction of 1 Hz LTP was blocked. Thus, 1 Hz LTP, unlike 4 Hz LTP, requires NO synthase activity.
Figure 2.
NO is necessary for, mimics, and occludes 1 Hz but not 4 Hz LTP, without requiring sGC. (a) Application of the NO synthase inhibitor Nω-nitro-l-arginine (100 μM) starting at least 1 h before recording and continuing throughout the experiment prevents 1 Hz (●, n = 6) but not 4 Hz LTP (□, n = 7). (b) Oxymyoglobin (10 μM) as an NO scavenger was added to the perfusion solution for 10 min (shaded region) starting 5 min before LTP induction. The 1-Hz stimulation protocol gave only transient potentiation (●, n = 3), whereas 4 Hz LTP was normal (□, n = 4). (c) Exposure of the slice to the NO donor NaNONO (10 μM) for 5 min (shaded region) causes substantial LTP (n = 5). (d) NO-induced LTP occludes 1 Hz but not 4 Hz LTP. Slices were preincubated with 10 μM NaNONO for 5 min then left for 30–60 min to allow the NO enhancement to reach a stable maximum. We delayed beginning the whole-cell recording until after this period, because prolonged intracellular dialysis would itself prevent further initiation of synaptic modulation. Subsequent 1 Hz LTP (●, n = 5) was prevented, whereas 4 Hz LTP (□, n = 6) was still inducible. (e) Uncaged NO induces LTP. Either N,N′-dicarboxymethyl-N,N′-dinitroso-p-phenylenediamine, 2Na (200 μM) or CNO-4 (200 μM) was introduced through the patch pipette and photolyzed with 300 UV flashes of 5-ms duration at 1 Hz, applied during the gray shaded zone. In the experiments shown (n = 4), 10 μM oxymyoglobin was present in the extracellular superfusate during the crosshatched zone. Similar results (not shown) were obtained without oxymyoglobin (n = 4). In all experiments except f, the pipette solution contained 5 mM BAPTA. (f) Inhibition of guanylyl cyclase with ODQ (1 μM, n = 4), present throughout the experiment, did not prevent 1 Hz LTP induction.
An independent test of the necessity and intercellular nature of the NO signal is to apply extracellular oxymyoglobin as an NO trap. If NO has to cross the intercellular space from its origin to its target, then the presence of oxymyoglobin in this space will block the NO effect. After a whole-cell patch and 5 min of stable EPSC baseline were established, the slice was perfused with 10 μM oxymyoglobin for 5 min, then either 1 or 4 Hz PF stimulation was applied in the continued presence of the protein. Four Hz LTP was unaffected by extracellular NO trapping, whereas 1 Hz stimulation gave only a transient increase of the EPSC amplitude, which declined back to baseline (Fig. 2b). Thus, oxymyoglobin prevented 1 Hz but not 4 Hz LTP expression. The ability to induce 4 Hz LTP in the presence of an NO scavenger confirms that 4 Hz LTP does not require NO, whereas 1 Hz LTP depends on NO crossing an intercellular space.
Because NO is essential for 1 Hz LTP, it was logical to test whether direct delivery of NO via an NO donor was sufficient to induce LTP. We chose NaNONO, the sodium salt of 1,1-diethyl-2-hydroxy-2-nitrosohydrazine (23, 25), as the NO donor because it smoothly decomposes at physiological pH to give two moles of NO radical, whereas other NO donors produce toxic byproducts or release nitrosonium ions rather than NO radical (26). As shown in Fig. 2c, superfusion with 10 μM NaNONO for 5 min, without any synaptic activation except for the 0.1-Hz monitoring pulses, caused substantial potentiation of EPSCs for the remaining 1 h of recording. To test whether NO-induced potentiation shares a common mechanism with PF-driven LTP, NaNONO was applied as above to slices 30–60 min before beginning whole-cell recording. Such prior potentiation from NO occluded 1 Hz but not 4 Hz LTP (Fig. 2d).
To confirm in the most direct way that the crucial site of NO action is inside the PC, we perfused PCs with BAPTA plus either of two membrane-impermeant dianionic forms of caged NO, CNO-4 (23) or N,N′-dicarboxymethyl-N,N′-dinitroso-p-phenylenediamine, 2Na (27), then applied 300 flashes of UV at 1 Hz. Although the two molecules are chemically unrelated except for their ability to release NO upon illumination, both induced LTP. Such potentiation was obtained even when diffusion of NO from the PC to adjacent cells was prevented by extracellular oxymyoglobin (Fig. 2e), proving that the target of the NO is within the PC. Thus, 1 Hz LTP, like LTD, requires NO as an anterograde messenger generated by PF activity and sensed within the PC. The crucial difference between LTD and LTP is the presence vs. absence, respectively, of an increase in postsynaptic Ca2+.
NO activates soluble guanylate cyclase (sGC) to produce cGMP, which is essential for LTD, because inhibition of sGC with ODQ (1 μM) prevents LTD (7). However, if anything, ODQ enhanced the amplitude of LTP obtained without BAPTA to buffer Ca2+ (Fig. 2f), as if the only effect were to inhibit some underlying LTD in the same way that chelation of postsynaptic Ca2+ amplifies LTP by removing concurrent LTD. Thus, sGC and by implication cGMP are not required for 1 Hz LTP, whereas they are essential for LTD. Some confirmation that cGMP could antagonize LTP comes from reexamination of the effects of protein kinase G inhibitors, e.g., figure 6 of V.L.-R. et al. (7), which not only prevented LTD but tended to give potentiation by themselves.
1 Hz LTP, Unlike 4 Hz LTP, Is Expressed Postsynaptically.
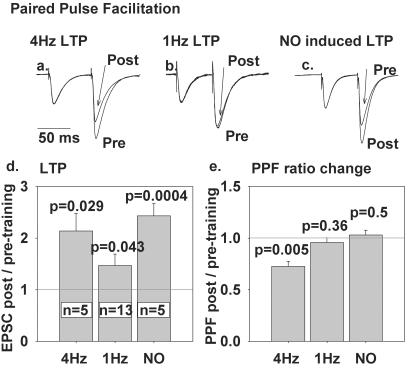
A classic criterion to distinguish presynaptic from postsynaptic modulation is the measurement of paired-pulse facilitation (PPF), a very short-term enhancement in synaptic efficacy usually attributed to residual presynaptic Ca2+ facilitating more transmitter release (17). Changes in PPF during long-term plasticity suggest that the latter is also presynaptic, whereas unchanged PPF argues that the long-term plasticity is postsynaptically expressed. We recorded PPF with 70-ms interpulse intervals as shown in Fig. 3 a–c. In each image, two pairs of EPSC traces have been normalized with respect to the amplitude of the first EPSC in each pair to demonstrate how PPF, the ratio of the second EPSC amplitude to the first, decreases after 4 Hz LTP but not after 1 Hz or NO-induced LTP. Fig. 3 d and e summarizes the changes in EPSC amplitude and PPF from all cells measured. Four Hz LTP reduced PPF by a statistically significant factor of 0.73, in agreement with Salin et al. (17), whereas the corresponding factors for 1 Hz LTP (0.96) and NaNONO-induced LTP (1.03) were insignificantly different from 1. These results argue that 1 Hz and NO-induced LTP show no significant presynaptic component, whereas the expression of 4 Hz LTP is substantially presynaptic.
Figure 3.
PPF is significantly altered by 4 Hz LTP but not 1 Hz LTP. (a–c) Typical waveforms from tests of PPF before and 20 min after LTP. Each trace is an average of 5 pairs of EPSC responses to two PF stimuli 70 ms apart, scaled so that the amplitudes of the first EPSC are matched, to show how the increased amplitude of the second EPSC, i.e., PPF, varies from pretraining control as a result of (a) 4 Hz, (b) 1 Hz, or (c) NO-induced LTP. (d) Mean amplitude and standard errors of 4 Hz, 1 Hz, and NO-induced LTP of the first EPSC of each pair. The number of cells (n) and probability that the deviation from unity is because of chance (P, assessed by two-tailed t test) is given. (e) Mean PPF after 4 Hz, 1 Hz, and NO-induced LTP relative to pretraining PPF for the same cells as in d.
cAMP Is Necessary and Sufficient for 4 Hz but Not 1 Hz LTP.
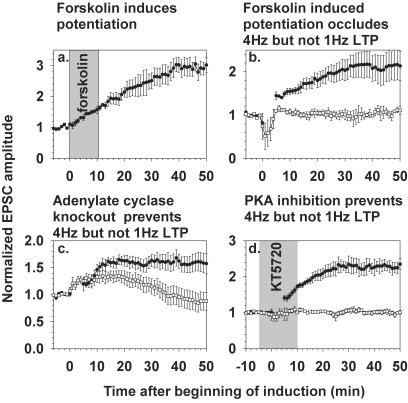
To test whether 1 Hz LTP involves a cAMP-dependent pathway, we asked whether cAMP-induced potentiation could occlude 1 Hz LTP. Application of 50 μM forskolin for 10 min (17, 18) reliably increased EPSC amplitudes (Fig. 4a). In analogy to the NaNONO experiments of Fig. 2d, cerebellar slices were perfused for 5 min with 50 μM forskolin 1–3 h before establishing whole-cell patch clamp. The potentiation presumably induced by the forskolin-evoked increase in cAMP occluded 4 Hz but not 1 Hz LTP, showing that 1 Hz LTP proceeds through a cAMP-independent pathway (Fig. 4b).
Figure 4.
cAMP-induced potentiation mimics, occludes, and is necessary for 4 Hz but not 1 Hz LTP. (a) Application of forskolin (50 μM) for 10 min (shaded region) induced LTP (n = 5). (b) Preexposure of slices to 50 μM forskolin for 10 min 1–2 h before the onset of recording occluded 4 Hz LTP induction (□, n = 6), whereas 1 Hz LTP was still inducible (●, n = 4). Whole-cell recording during the development of the forskolin effect was avoided for the same reasons as in Fig. 2d. (c) 1 Hz and 4 Hz LTP induction protocols were applied to cerebellar slices from mice lacking adenylyl cyclase types 1 and 8. One Hz LTP was induced normally (●, n = 6), whereas 4 Hz stimulation gave only a transient increase in EPSCs (□, n = 6). (d) Application of the protein kinase A inhibitor KT5720 (5 μM, shaded region) 5 min before and during LTP induction prevented 4 Hz LTP (□, n = 7) but not 1 Hz LTP (●, n = 11).
Complementary experiments used cerebella from transgenic mice homozygously deficient in adenylyl cyclase types 1 and 8 (24). One Hz LTP in cells from these knockout mice was normal, whereas 4 Hz LTP failed because the increase in EPSC amplitude was only transient (Fig. 4c). Loss of 4 Hz LTP agrees with results in culture (20). The role of cAMP-dependent protein kinase (PKA) was tested pharmacologically by adding the membrane-permeant inhibitor KT5720 (5 μM) for 5 min before and during LTP induction. KT5720 prevented 4 Hz but not 1 Hz LTP (Fig. 4d). Cumulatively, these results confirm that cAMP elevation is sufficient and necessary for 4 Hz LTP, whereas 1 Hz LTP occurs through cAMP-independent processes.
Discussion
The main experimental finding of this study is that the parallel fiber-PC synapse can undergo postsynaptically expressed, NO-dependent LTP, which is clearly distinct from the previously recognized, presynaptically expressed, cAMP-dependent mechanism of LTP. To prove that differences between the two forms of LTP are real, not just inter-laboratory variations or differences in recording method, we reproduced all of the major features of cAMP-dependent presynaptic LTP in whole-cell voltage-clamp mode while contrasting them with the new NO-dependent postsynaptic mechanism (Table 1). Very recently, Jacoby et al. (28) have suggested that cAMP-dependent presynaptic potentiation elicited by 15 s of 8 Hz stimulation is mediated by NO, because they found such potentiation to be prevented by NO synthase inhibitors and spatially restricted by a small molecule scavenger of NO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide. However, we found 4 Hz LTP to be unaffected by NO synthase inhibition [Fig. 2a, which agrees with the original report of Salin et al. (17)] or myoglobin (Fig. 2b), whereas these NO-interfering agents do prevent 1 Hz LTP. Jacoby et al. also reported that 100 μM spermine NONOate for 10 min caused a 1.5× potentiation of EPSCs, which lasted only 20–30 min after removal of the NO donor. The potentiation was blocked by a protein kinase A inhibitor and had a significant presynaptic component because PPF was somewhat reduced and miniature EPSC frequency increased. In preliminary experiments we too had observed that 100 μM NaNONO applied for 5 min caused a small and transient potentiation, but this response was followed by cell rundown (results not shown). We feared that this was an overdose of NO leading to additional toxicity, because 10 μM NaNONO for 5 min induced a 2.5× potentiation, which remained at full strength an hour later (Fig. 2c), occluded 1 Hz but not 4 Hz LTP (Fig. 2d), and had no significant effect on PPF (Fig. 3e). Thus, our results indicate a simpler and cleaner separation between NO and cAMP-mediated pathways than that proposed by Jacoby et al. (28), although more detailed experimental comparisons would be desirable to resolve the discrepancies in results. One important point of agreement is that NO-mediated potentiation is not affected by blockage of cGMP signaling.
Table 1.
Comparison of mechanisms underlying 1 Hz LTP, 4 Hz LTP, and LTD of PF-PC synapses
| Characteristic | 1 Hz LTP | 4 Hz LTP | LTD | Relevant Fig. |
|---|---|---|---|---|
| Needs postsynaptic Ca2+? | No | No | Yes | 1 c and d |
| Needs NO? | Yes | No | Yes | 2 a and b |
| Mimicked/occluded by NO? | Yes | No | Yes† | 2 c–e |
| Needs cGMP? | No | No* | Yes† | 2 f |
| Site of expression? | Postsynaptic | Presynaptic | Postsynaptic | 3 |
| Needs cAMP? | No | Yes | No* | 4 c and d |
| Mimicked/occluded by cAMP? | No | Yes | No* | 4 a and b |
Not explicitly tested yet, although unlikely.
When combined with postsynaptic Ca2+.
At a PF-PC synapse stimulated at 1 Hz, Hebbian coincidence detection between presynaptic activity and postsynaptic depolarization-induced Ca2+ influx produces LTD, whereas the absence of Ca2+ elevation causes the anti-Hebbian response, LTP. The role of Ca2+ in selecting Hebbian vs. anti-Hebbian responses is reminiscent of hippocampal CA1 synapses (29, 30), although the Hebbian response in CA1 is LTP. Because PF-PC LTD and 1 Hz LTP are both NO-dependent and postsynaptically expressed, 1 Hz LTP is the first plausible extinguishing mechanism for LTD. However, experimental confirmation must await an experiment using completely different recording protocols, because synaptic plasticity cannot be induced more than about 15–20 min after establishment of whole-cell perfusion.
The biochemical mechanism for 1 Hz LTP remains largely obscure. The best understood mechanism for NO-mediated signaling is activation of sGC, but the persistence of 1 Hz LTP in the presence of ODQ, a potent inhibitor of sGC, argues against a requirement for cGMP elevation. The same dose of ODQ prevents LTD (7), providing a positive control for the efficacy of this inhibitor. The next most common signaling pathway for NO is S-nitrosylation, the covalent attachment of NO to cysteines on target proteins such as enzymes, G proteins, transcription factors, transporters, transmitter receptors (31), and ion channels (32–34). For example, L-type calcium channels can be modulated by NO resulting in either inhibition in a cGMP-dependent manner or stimulation of the ion channel by S-nitrosylation (35). Other conceivable mechanisms include modification of tyrosines and stimulation of ADP-ribosylation (36, 37).
The mature PF-PC synapse is unusual in displaying so many distinct mechanisms of long-term plasticity: a Hebbian LTD requiring coincidence of presynaptically generated NO and postsynaptic Ca2+ (6–9), another Hebbian LTD requiring coincidence of postsynaptic inositol-1,4,5-trisphosphate and Ca2+ (38–42), an LTP involving presynaptic cAMP with no obvious postsynaptic involvement (17–20), and now an anti-Hebbian LTP requiring NO without Ca2+ but expressed postsynaptically. In addition there are various short-term forms of plasticity such as PPF and postsynaptic-depolarization-induced inhibition of presynaptic Ca2+ influx and transmitter release, mediated by retrograde cannabinoids (43). Also, crosstalk between cAMP and NO has been proposed (28). It is even more remarkable that the two forms of LTP are fairly specifically induced by stimulation frequencies separated by only a factor of 4. The mechanisms for such frequency specificity remain to be defined. Perhaps 1 Hz is not a high enough stimulation frequency to activate presynaptic adenylate cyclase, whereas the NO-dependent postsynaptic mechanism demands more than 120 stimuli or 30 sec, consistent with Fig. 1a. Nevertheless, it would seem that long-term Hebbian depression and anti-Hebbian potentiation of this synapse are so important for reliable motor learning that at least two independent mechanisms have evolved for each direction of modulation.
Acknowledgments
This work was supported by National Institutes of Health Grant NS27177 (to R.Y.T.) and by the Howard Hughes Medical Institute.
Abbreviations
- LTD
long-term depression
- PF
parallel fiber
- PC
Purkinje cell
- LTP
long-term potentiation
- EPSCs
excitatory postsynaptic currents
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- NaNONO
1,1-diethyl-2-hydroxy-2-nitrosohydrazine Na salt
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluble guanylate cyclase
- PPF
paired-pulse facilitation
References
- 1.Mauk M D, Garcia K S, Medina J F, Steele P M. Neuron. 1998;20:359–362. doi: 10.1016/s0896-6273(00)80978-2. [DOI] [PubMed] [Google Scholar]
- 2.Lisberger S G. Cell. 1998;92:701–704. doi: 10.1016/s0092-8674(00)81397-5. [DOI] [PubMed] [Google Scholar]
- 3.Raymond J L, Lisberger S G, Mauk M D. Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 4.Ito M. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 5.Wang S S H, Khiroug L, Augustine G J. Proc Natl Acad Sci USA. 2000;97:8635–8640. doi: 10.1073/pnas.130414597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lev-Ram V, Makings L R, Keitz P F, Kao J P Y, Tsien R Y. Neuron. 1995;15:407–415. doi: 10.1016/0896-6273(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 7.Lev-Ram V, Jiang T, Wood J, Lawrence D S, Tsien R Y. Neuron. 1997;18:1025–1038. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- 8.Hartell N A. J Neurosci. 1996;16:2881–2890. doi: 10.1523/JNEUROSCI.16-09-02881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel H, Hémart N, Jaillard D, Crepel F. Eur J Neurosci. 1993;5:1079–1082. doi: 10.1111/j.1460-9568.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 10.Medina J F, Nores W L, Ohyama T, Mauk M D. Curr Opin Neurobiol. 2000;10:717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 11.Han V Z, Grant K, Bell C C. Neuron. 2000;27:611–622. doi: 10.1016/s0896-6273(00)00070-2. [DOI] [PubMed] [Google Scholar]
- 12.Medina J F, Garcia K S, Nores W, Taylor N, Mauk M. J Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai M. J Physiol (London) 1987;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakurai M. Proc Natl Acad Sci USA. 1990;87:3383–3385. doi: 10.1073/pnas.87.9.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crepel F, Jaillard D. J Physiol (London) 1991;432:123–141. doi: 10.1113/jphysiol.1991.sp018380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartell N A. Neuron. 2001;16:601–610. doi: 10.1016/s0896-6273(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 17.Salin P A, Malenka R C, Nicoll R A. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Regehr W G. J Neurosci. 1997;17:8687–8694. doi: 10.1523/JNEUROSCI.17-22-08687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linden D J, Ahn S. J Neurosci. 1999;19:10221–10227. doi: 10.1523/JNEUROSCI.19-23-10221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storm D, Hansel C, Hacker B, Parent A, Linden D. Neuron. 1998;20:1199–1210. doi: 10.1016/s0896-6273(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 21.Linden D J. J Neurophysiol. 1998;79:3151–3156. doi: 10.1152/jn.1998.79.6.3151. [DOI] [PubMed] [Google Scholar]
- 22.Edwards F A, Konnerth A, Sakmann B, Takahashi T. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 23.Makings L R, Tsien R Y. J Biol Chem. 1994;269:6282–6285. [PubMed] [Google Scholar]
- 24.Wong S T, Athos J, Figueroa X A, Pineda V V, Schaefer M L, Chavkin C C, Muglia L J, Storm D R. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 25.Maragos C M, Morley D, Wink D A, Dunams T M, Saavedra J E, Hoffman A, Bove A A, Issac L, Hrabie J A, Keefer L K. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 26.Lipton S A, Choi Y B, Pan Z H, Lei S Z, Chen H S, Sucher N J, Loscalzo J, Singel D J, Stamler J S. Nature (London) 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 27.Namiki S, Arai T, Fujimori K. J Am Chem Soc. 1997;119:3840–3841. [Google Scholar]
- 28.Jacoby S, Sims R E, Hartell N A. J Physiol (London) 2001;535:825–839. doi: 10.1111/j.1469-7793.2001.t01-1-00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bear M F, Abraham W C. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 30.Yang S-N, Tang Y-G, Zucker R S. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- 31.Lei S Z, Pan Z H, Aggarwal S K, Chen H S, Hartman J, Sucher N J, Lipton S A. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- 32.Stamler J S. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 33.Stamler J S, Toone E J, Lipton S A, Sucher N J. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 34.Yao J A, Jiang M, Tseng G N. Am J Physiol. 1997;273:H208–H219. doi: 10.1152/ajpheart.1997.273.1.H208. [DOI] [PubMed] [Google Scholar]
- 35.Campbell D, Stampler D, Strauss C. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuman E M, Meffert M K, Schulman H, Madison D V. Proc Natl Acad Sci USA. 1996;91:11958–11962. doi: 10.1073/pnas.91.25.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleppisch T, Pfeifer A, Klatt P, Ruth P, Montkowski A, Fassler R, Hofmann F. J Neurosci. 1999;19:48–55. doi: 10.1523/JNEUROSCI.19-01-00048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finch E A, Augustine G J. Nature (London) 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 39.Miyata M, Finch E A, Khiroug L, Hashimoto K, Hayasaka S, Oda S-I, Inouye M, Takagishi Y, Augustine G J, Kano M. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 40.Linden D J, Connor J A. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 41.Inoue T, Kato K, Kohda K, Mikoshiba K. J Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khodakhah K, Armstrong C M. Proc Natl Acad Sci USA. 1997;94:14009–14014. doi: 10.1073/pnas.94.25.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreitzer A C, Regehr W G. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]