Abstract
Background:
The present study was carried out to investigate the prevalence of intestinal microsporidia in patients with intellectual disabilities in the rehabilitation centers of Hormozgan Province in south of Iran, using microscopic and molecular methods.
Methods:
The current cross-sectional study was conducted on 119 stool samples obtained from patients with intellectual disabilities. Acid-fast trichrome staining was used to detect parasites. Moreover, all of the stool samples were assessed for the presence of microsporidia DNA by the PCR method.
Results:
The mean age of the subjects was 29 (±12.3) years. All stool samples were examined for microsporidia infection, and only one case of Enterocytozoon was detected in one of the samples. The results of the nested-PCR test on 119 stool samples showed one case of infection with Enterocytozoon bieneusi, corresponding to a prevalence of 0.84%. The nested-PCR test for Encephalitozoon intestinalis detected no infections in any of the stool samples.
Conclusion:
The overall prevalence of microsporidia among patients with intellectual disabilities in Hormozgan rehabilitation centers is low and limited to E. bieneusi.
Keywords: Prevalence, Microsporidiosis, Enterocytozoon bieneusi, Intellectual disabilities, Iran, Nested-PCR
Introduction
Microsporidiosis is an emerging opportunistic infection caused by microsporidia, a group of obligate intracellular parasites (1). These pathogens are known to infect a wide range of hosts, including humans, and are particularly problematic in immunocompromised individuals and patients with intellectual disabilities (2–5). The disease manifests in various forms, primarily affecting the gastrointestinal tract, but can also involve other organ systems, leading to significant morbidity and mortality. Immunocompromised patients, such as those with HIV/AIDS, organ transplant recipients, and individuals undergoing chemotherapy, are at a heightened risk for microsporidiosis. The compromised immune system in these patients provides an ideal environment for microsporidia to thrive and cause infection. The most common species affecting humans include Enterocytozoon bieneusi and E. intestinalis, which primarily cause intestinal infections leading to chronic diarrhea, malabsorption, and wasting.
In HIV/AIDS patients, microsporidiosis is a well-documented opportunistic infection (6). The prevalence of microsporidiosis in this population varies geographically, with higher rates reported in regions with limited access to antiretroviral therapy (7). The clinical presentation in these patients often includes severe, persistent diarrhea, which can lead to significant weight loss and dehydration. Diagnosis of microsporidiosis is typically made through stool examination using special staining techniques or molecular methods such as PCR (8).
Organ transplant recipients are another group at high risk for microsporidiosis. The use of immunosuppressive drugs to prevent graft rejection increases their susceptibility to opportunistic infections. In these patients, microsporidiosis can present not only as gastrointestinal disease but also as disseminated infection, affecting multiple organ systems. Early diagnosis and treatment are crucial to prevent severe complications and improve outcomes.
Patients with intellectual disabilities are also at an increased risk for microsporidiosis, although the reasons for this are multifactorial (9). These individuals often reside in institutional settings where hygiene practices may be suboptimal, increasing their exposure to infectious agents. Additionally, behavioral factors such as pica, nail-biting, and poor personal hygiene can facilitate the ingestion of microsporidia spores. The clinical presentation of microsporidiosis in patients with intellectual disabilities can be similar to that in immunocompromised individuals, with gastrointestinal symptoms being the most common (10). However, the diagnosis of microsporidiosis in this population can be challenging due to communication barriers and the presence of other comorbid conditions that may mask the symptoms of infection. Healthcare providers need to maintain a high index of suspicion and consider microsporidiosis in the differential diagnosis when these patients present with unexplained gastrointestinal symptoms.
The diagnosis of microsporidiosis involves the detection of microsporidia spores in clinical specimens such as stool, urine, or tissue biopsies. Traditional methods include light microscopy with special stains like modified trichrome or calcofluor white. However, these techniques have limited sensitivity and specificity. Molecular methods, particularly PCR, have improved the accuracy of diagnosis and are increasingly being used in clinical practice (11).
Parasitic infections are a significant health issue in care centers for patients with intellectual disabilities. These patients are prone to infectious diseases due to their inability to learn personal hygiene practices, such as bathing and sanitary defecation, as well as habits like nail-biting, finger-licking, and walking barefoot (12, 13).
Physical, mental, and nutritional weaknesses in individuals with intellectual disabilities put them at risk for parasitic infections, including microsporidiosis. Therefore, timely diagnosis and periodic treatment can protect these patients from the severe complications of microsporidiosis, such as severe diarrhea (13, 14).
Given the aforementioned points and the lack of sufficient information on the prevalence of microsporidial infections in patients with intellectual disabilities, the present study was conducted to investigate the prevalence of intestinal microsporidiosis in patients with intellectual disabilities in rehabilitation centers in Bandar Abbas in 2021 using microscopic and molecular methods.
Materials and Methods
Area of the study
Bandar Abbas County in Hormozgan Province borders Fars, Kerman, Sistan and Baluchestan, and Bushehr provinces. The city’s elevation is 9 meters above sea level, and the climate is desert-like, hot, and humid. Temperature fluctuations range from 5 to 49 °C, and the average annual rainfall is about 171 mm.
Sampling
In this cross-sectional study, 119 stool samples were collected from 140 individuals residing in a center for intellectually disabled patients in Bandar Abbas city in Hormozgan Province. After obtaining the necessary permits and coordinating with the center’s management, the stool samples were collected by the health workers employed at the center. Before sampling, a questionnaire containing personal information such as age, gender, severity of mental retardation, etc., was completed for each individual.
Each stool sample was divided into two parts based on the required tests: one part was transferred into sodium acetate-acetic acid-formalin (SAF) for preservation, and the other part was placed in 70% ethanol for molecular testing. The collected samples were transferred to the parasitology laboratory of the Hormozgan University of Medical Sciences for initial testing. The study was approved by Ethical Committee of Shiraz University of Medical Sciences (Ref. No. IR.SUMS.MED.REC.1401.005).
Acid-Fast Trichrome Staining
Acid-Fast Trichrome Staining was performed on 119 stool samples collected in SAF (15 g sodium acetate, 20 ml acetic acid, and 40 ml formalin in 925 ml distilled water). Briefly, after passing the sample through four layers of gauze, 200 μl of sediment was placed on a glass slide and allowed to air dry. Slides were quickly immersed in methanol for 5 minutes. Fixed samples were placed in a carbol-fuchsin solution (comprising 25 g of phenol, 500 ml of distilled water, and 25 ml of a saturated alcoholic fuchsin solution with 2 g of basic fuchsin in 25 ml of 96% ethanol) for 10 minutes at room temperature.
The slides were subsequently rinsed with tap water, subjected to decolorization with 0.5% HCl-alcohol, and washed again with tap water. Trichrome solution was utilized to stain the slides for 30 minutes at 37 °C. This solution was prepared by dissolving 6.0 g of Chromotrope 2R, 0.5 g of aniline blue, and 0.7 g of phosphotungstic acid in 3 ml of acetic acid at room temperature for 30 minutes, followed by the addition of 100 ml of distilled water and pH adjustment to 2.5 using HCl. Afterward, the slides were washed (10 seconds with acid alcohol, 30 seconds with 95% ethanol) and were then examined, using oil immersion at a magnification of 1000×.
Molecular evaluation
DNA was extracted from the stool samples using a commercial (Viragen, Iran) kit. The stool samples were mixed with PBS before the extraction process and passed through a four-layer gauze. The filtered suspension was centrifuged at 500g for 2 minutes, the supernatant was separated, and the remaining sediment was used for DNA extraction. Before the extraction process, the sample was placed alternatively in a nitrogen tank and then in a water bath at 65 °C twice, and then subjected to sonication, three times to break down the parasite spores (15, 16).
Nested PCR was used for the detection of microsporidia SSU rRNA gene. The PCR method and the primers used in this study were based on the study by Mirjalali et al. (7). PMic primers were used as the first stage to detect the SSU rRNA gene of microsporidia spp., Enb primers for the detection of E. bieneusi, and Encep primers for the detection of Encephalitozoon species in the second stage of the PCR (Table 1). In the first step of the PCR program, the DNA was denatured at 95 °C for 5 minutes. This was followed by 35 cycles of annealing and extension: 94 °C for 40 seconds, 55 °C for 45 seconds, and 72 °C for 45 seconds, with a final extension at 72 °C for 4 minutes. The DNA product from the first step was then used as a template for the second PCR step, which included an initial denaturation at 95 °C for 5 minutes, followed by 40 cycles of annealing and extension: 94 °C for 35 seconds, 57 °C for 45 seconds, and 72 °C for 45 seconds, concluding with a final extension at 72 °C for 4 minutes.
Table 1:
Target gene and sequence of primers used in Nested PCR to identify microsporidia spp.
| Gene | Primer | Sequence | Product’s size |
|---|---|---|---|
| PMic (Forward) | 5′- GGTTGATTCTGCCTGACG - 3′ | 779 bp | |
| PMic (Revers) | 5′ - CTTGCGAGC(G/A)TACTATCC - 3′ | ||
| SSU rRNA | Enb (Forward) | 5′- GGTAATTTGGTCTCTGTGTG - 3′ | 440 bp |
| Enb (Revers) | 5′- CTACACTCCCTATCCGTTC -3′ | ||
| Encep (Forward) | 5′- AGTACGATGATTTGGTTG- 3′ | 630 bp | |
| Encep (Revers) | 5′- ACAACACTATATAGTCCCGTC- 3′ |
The purified PCR product was sequenced, and a phylogenetic analysis of the isolate was conducted using the Maximum Likelihood method with the Kimura 2-parameter model (17). The results were compared with those of available microsporidia sequences, using BLAST software.
Statistical analysis
After entering the data of the study subjects in SPSS software (version 20), descriptive statistics were used to determine the frequency of parasites in the study subjects. Also, the chi-square test was used to examine potential associations between variables and microsporidia infection occurrence.
Results
Stool samples were collected from 119 intellectually disabled patients. Of these subjects, 55 (46.2%) were men, and 64 (53.8%) were women. The mean age of the participants was 27.6 (±2.24), ranging from 4 to 60 years old. The study participants were divided into several age groups, with the largest group being 31 to 40 years old (33 individuals). Additionally, the participants were categorized based on their length of stay at the rehabilitation center, with the largest group (44 individuals) having stayed for less than 5 years. Table 2 shows the demographic characteristics of the study participants.
Table 2:
Demographic characteristics of studied intellectually disabled individuals
| Variable | Characteristics | No. | Percent |
|---|---|---|---|
|
| |||
| Gender | Female | 64 | 53 .8 |
| Male | 55 | 46.2 | |
|
| |||
| Age groups(yr) | 10 ≥ | 9 | 7.6 |
| 11–20 | 30 | 25.2 | |
| 21–30 | 31 | 26.1 | |
| 31–40 | 33 | 27.7 | |
| > 40 | 16 | 13.4 | |
|
| |||
| Duration of residency in the rehabilitation center | 5 ≥ | 44 | 37 |
| 3–10 | 23 | 19.3 | |
| 11–15 | 25 | 21 | |
| 16–20 | 10 | 8.4 | |
| > 20 | 17 | 14.3 | |
|
| |||
| Type of retardation | Severe | 76 | 63.9 |
| Profound | 43 | 36.1 | |
The results of parasitological methods to detect microsporidia spp.
In this study, all samples were examined for the presence of intestinal microsporidia, using acid-fast trichrome staining. Microsporidia infection was detected in only one of the 119 stool samples (0.84%) examined. Fig. 1 shows the microscopic view of the acid-fast trichrome staining of suspected sample to microsporidia.
Fig. 1:
Acid-fast trichrome staining of suspected sample to microsporidia spp. 1000 X
The results of molecular investigations to detect microsporidia spp.
Using the molecular Nested PCR method, infection with E. bieneusi was identified in only one of the 119 examined subjects. The positive case matched the sample that tested positive in the microscopic examination. It belonged to a 26-year-old female with a severe intellectual disability who had been living at the rehabilitation center for seven years.
In molecular studies using Nested PCR, none of the 119 examined samples tested positive for E. intestinalis. Fig. 2 shows the 440 bp band of the SSU rRNA gene of E. bieneusi detected in the stool sample of the patient.
Fig. 2:

Nested-PCR on the stool sample of a patient infected with E. bieneusi. Lane 1: 100 bp molecular marker, Lane 2: positive control of E. bieneusi. Lane 3: patient’s sample. Lane 4: negative control.
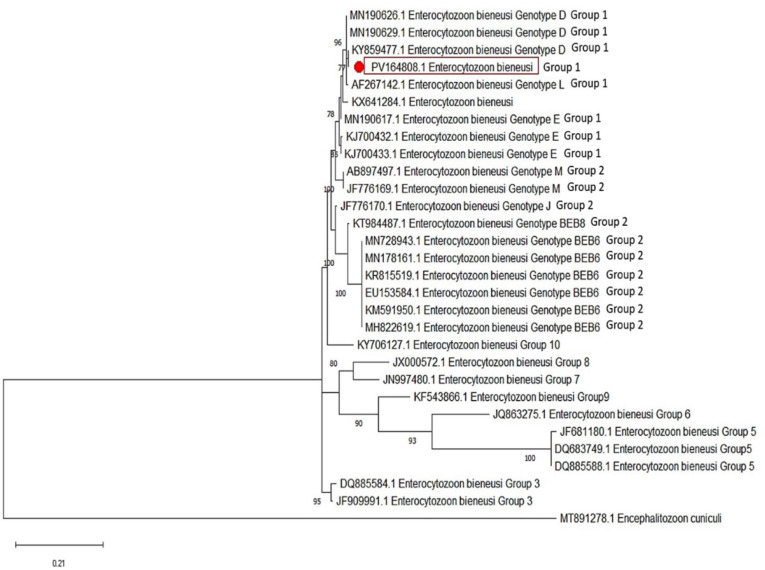
Upon evaluation of the positive case and comparing its sequences with those in Gen-Bank, we identified that our E. bieneusi sample is corresponded to genotype D, zoonotic group 1, and exhibiting 99–100% similarity with other genotypes D documented from Iran (7, 18). The sequenced sample was recorded in GenBank under accession number PV164808. Fig. 3 illustrates the phylogenetic tree of the sequenced sample.
Fig. 3:
Phylogenetic tree, using the Maximum Likelihood method, showing the E. bieneusi infecting intellectually disabled patient.
Discussion
The current study assessed the prevalence of intestinal microsporidia among patients with intellectual disabilities in rehabilitation centers in Hormozgan Province, located in southern Iran, utilizing both microscopic and molecular methods. The findings revealed a prevalence rate of 0.84% with both techniques. Previous studies have indicated that contaminated water and food are potential sources of microsporidia infection. Additionally, demographic factors such as age have been shown to influence susceptibility to microsporidia infection, with very young and elderly individuals being at higher risk.
A study conducted in Urmia, located in the West Azerbaijan Province of Iran, examined 225 intellectually disabled individuals and reported an overall intestinal parasitic infection rate of 20.4%. (19) However, microsporidia were not among the identified parasites, which consisted of various protozoa and worms.
Several studies in Iran have examined the prevalence of microsporidia in populations of immunocompromised patients. A systematic review and meta-analysis determined that the overall prevalence of microsporidia infection among immunocompromised individuals in Iran is 8.18% (9) A study conducted in Kerman Province, located in southeastern Iran, reported a microsporidia infection prevalence of 10.03% among immunocompromised patients (20).
Studies in Iran examining populations without immune system deficiencies have reported a lower prevalence of microsporidial infection, aligning more closely with the findings of the present study. Based on these results, the prevalence observed in patients with intellectual disabilities—at least in this study—does not appear to differ significantly from that of other populations without immune system deficiencies assessed in previous research.
Molecular analysis revealed that the positive microsporidia sample in the current study is genotype D of E. bieneusi, showing high homology (99–100%) with isolates from immunocompromised individuals in Tehran, Mazandaran, and Ahvaz in Iran (7, 18, 21). Genotype D of E. bieneusi is a well-documented zoonotic genotype with potential for cross-species transmission. It has been identified in various hosts, including humans and animals, and is classified within Group 1, which consists of genotypes with zoonotic potential. Studies have shown its presence in captive golden snub-nosed monkeys, suggesting that these primates may serve as reservoir hosts for human microsporidiosis (22).
In the present study, the detection of only one microsporidia-positive sample prevented statistical analysis of the relationship between stool appearance and microsporidial infection. This limitation underscores the need for future research to further investigate this potential association.
In the current study, no cases of infection with E. intestinalis were observed, and the only positive case was related to E. bieneusi. Other studies have also reported E. bieneusi as the most common microsporidia species in humans, causing conditions such as diarrhea associated with human immunodeficiency virus (HIV) (23).
The low prevalence of microsporidia among patients with intellectual disabilities in Hormozgan rehabilitation centers in Iran could be attributed to several factors. Rehabilitation centers in Hormozgan may have implemented strict hygiene and sanitation protocols, along with regular screening and treatment of parasitic infections, which help reduce the risk and prevalence of microsporidia. Additionally, the specific environmental conditions, dietary habits in these centers contribute to a lower risk of microsporidia transmission. Furthermore, population characteristics such as genetic factors might also influence susceptibility to infection.
The present study faced several limitations. The absence of similar research on populations with intellectual disabilities made comparative analysis challenging. Additionally, the microscopic and molecular examination was limited to only two species of microsporidia, leaving other species unexamined. Furthermore, the small sample size introduced additional constraints to the study.
Conclusion
The overall prevalence of microsporidia among patients with intellectual disabilities residing in rehabilitation centers in Hormozgan Province, located in southern Iran, was low and limited to the E. bieneusi species, compared to previous studies conducted in other populations.
Acknowledgements
The study was financially supported by the office of the Vice-Chancellor for Research, Shiraz University of Medical Sciences (grant No. 23697).
The study was the subject of Dr. Fatima Kamrani’s MD dissertation.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interests.
References
- 1.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19(5):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantar C, Herrera F, Didier E, et al. Diarrhea due to microsporidia in a patient with AIDS. Medicina (B Aires). 1995;55(6):685–688. [PubMed] [Google Scholar]
- 3.Calik S, Karaman U, Colak C. Prevalence of and other intestinal parasites in children from Malatya, Turkey. Indian J Microbiol. 2011;51:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo M, Carazo M, Arias ML, et al. Prevalence of Cyclospora sp., Cryptosporidium sp, microsporidia and fecal coliform determination in fresh fruit and vegetables consumed in Costa Rica. Arch Latinoam Nutr. 2004;54(4):428–432. [PubMed] [Google Scholar]
- 5.Seatamanoch N, Kongdachalert S, Sunantaraporn S, et al. Microsporidia, a highly Adaptive organism and its host expansion to humans. Front Cell Infect Microbiol. 2022;12:924007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agholi M, Hatam GR, Motazedian MH. HIV/AIDS-associated opportunistic protozoal diarrhea. AIDS Res Hum Retroviruses. 2013;29(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirjalali H, Mohebali M, Mirhendi H, et al. Emerging intestinal microsporidia infection in HIV+/AIDS patients in Iran: microscopic and molecular detection. Iran J Parasitol. 2014;9(2):149–54. [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Feng Y, Xiao L. Diagnosis and molecular typing of Enterocytozoon bieneusi: the significant role of domestic animals in transmission of human microsporidiosis. Res Vet Sci. 2020;133:251–261. [DOI] [PubMed] [Google Scholar]
- 9.Ghoyounchi R, Ahmadpour E, Spotin A, et al. Microsporidiosis in Iran: A systematic review and meta-analysis. Asian Pac J Trop Med. 2017;10(4):341–350. [DOI] [PubMed] [Google Scholar]
- 10.Hassan N-A, Lim YA, Mahmud R, et al. Molecular diagnosis of microsporidia among immunocompromised patients in Kuala Lumpur, Malaysia. Am J Trop Med Hyg. 2018;99(6):1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia LS. Laboratory identification of the microsporidia. J Clin Microbiol. 2002;40(6):1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti S, Lopes R, Cevini C, et al. Intestinal parasitic infections in an institution for the mentally retarded. Ann Trop Med Parasitol. 2000;94(5):453–460. [DOI] [PubMed] [Google Scholar]
- 13.Agmas A, Alemu G, Hailu T. Prevalence of intestinal parasites and associated factors among psychiatric patients attending Felege Hiwot Comprehensive Specialized Referral Hospital, Northwest Ethiopia. Res Rep Trop Med. 2021; 12:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbaszadeh Afshar MJ, Mohebali M, Mohtasebi S, et al. Intestinal parasites among intellectually disabled individuals in Iran: a systematic review and meta-analysis. Gut Pathog. 2021;13(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier S, Dubrou S, Liguory O, et al. Detection of microsporidia, cryptosporidia and Giardia in swimming pools: a one-year prospective study. FEMS Immunol Med Microbiol. 2002;33(3):209–213. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadpour I, Bozorg-Ghalati F, Motazedian MH. Molecular characterization and phylogenetic analysis of microsporidia and Cryptosporidium spp. in patients with multiple bowel biopsies from Fars Province, Iran. Ann Parasitol. 2016; 62(4):321–330. [DOI] [PubMed] [Google Scholar]
- 17.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi B, Sarvi S, Keihanian S, et al. Microscopic and molecular investigation of intestinal microsporidia in HIV+/AIDS and cancer patients undergoing chemotherapy in Mazandaran province, north of Iran. Acta Parasitol. 2023;68(3):690–698. [DOI] [PubMed] [Google Scholar]
- 19.Tappeh Kh H, Mohammadzadeh H, Rahim RN, et al. Prevalence of intestinal parasitic infections among mentally disabled children and adults of Urmia, Iran. Iran J Parasitol. 2010;5(2):60–64. [PMC free article] [PubMed] [Google Scholar]
- 20.Nooshadokht M, Sharifi I, Mohammadi MA, et al. Intestinal microsporidiosis in Iran: infection in immune-compromised and immunocompetent patients. Curr Med Mycol. 2017;3(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavalla M, Mardani-Kateki M, Abdizadeh R, et al. Molecular identification of Enterocytozoon bieneusi and Encephalitozoon spp. in immunodeficient patients in Ahvaz, Southwest of Iran. Acta Trop. 2017;172:107–112. [DOI] [PubMed] [Google Scholar]
- 22.Yu F, Wu Y, Li T, et al. High prevalence of Enterocytozoon bieneusi zoonotic genotype D in captive golden snub-nosed monkey (Rhinopithecus roxellanae) in zoos in China. BMC Vet Res. 2017;13(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryan RT. Microsporidiosis as an AIDS-related opportunistic infection. Clin Infect Dis. 1995; 21 Suppl 1:S62–5. [DOI] [PubMed] [Google Scholar]