Abstract
Purpose
Dopamine transporter (DAT) SPECT is a powerful tool for early diagnosis of Parkinson’s disease (PD), while digital phantoms and Monte Carlo (MC) simulations can serve as important research tools. This study aims to develop a novel digital brain phantom population for 99mTc-TRODAT-1 (99mTc) and 123I-ioflupane (123I) brain SPECT, and to assess attenuation correction (AC) and scatter correction (SC) in DAT SPECT.
Methods
Striatum, brain background (gray and white matter), and cold regions (skull and cerebrospinal fluid) were segmented from 200 T1 MRI brain images from the PPMI dataset. Striatal binding ratio (SBR) values were retrospectively collected from 200 123I and 100 99mTc DAT SPECT patients with suspected PD symptoms from PPMI and a local hospital, respectively. Various activity values were assigned to the randomly paired segmented regions according to a range of SBR values based on the SPECT Visual Interpretation (VI) assessment scheme. The new phantom population was combined with MC simulation tool SIMIND to generate realistic noisy projections. Quantitative accuracy of reconstructed images with attenuation correction (AC) and scatter correction (SC) was assessed.
Results
A population of 1000 normal and abnormal PD phantoms was generated for both tracers. Visual comparisons and quantitative analyses demonstrated that simulated data exhibited high similarity to clinical data. Reconstructed images with AC + SC achieved the best quantitative results, followed by AC only, without AC and SC, and SC only.
Conclusion
The developed digital DAT SPECT phantom population can be served for a wide range of PD applications. Attenuation impacts image quality the most in DAT SPECT, while AC + SC is effective to enhance image quality and quantitative accuracy of DAT SPECT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40658-025-00787-8.
Keywords: Parkinson’s disease, DAT SPECT, Phantom population, Monte Carlo simulation, Attenuation correction, Scatter correction.
Introduction
With the global increase of aging population, neurodegenerative disorders are imposing an increasingly heavy burden on both families and societies. Parkinson’s Disease (PD), the second most prevalent neurodegenerative disorder, affected approximately 6.1 million individuals in 2016 [1]. Furthermore, PD is exhibiting the fastest growth rate among neurodegenerative disorders, evident in its age-standardized rates of prevalence, disability, and mortality. Currently, there are several approved therapies for PD [2, 3] and Levodopa [4] stands as the most potent medication for managing the disease. Nonetheless, as there is still no definitive cure for PD so far, early detection and intervention are paramount for better disease management. PD is primarily characterized by the massive cell death of the neuromelanin-containing dopaminergic neurons in the substantia nigra. The degeneration of dopaminergic neurons in the nigrostriatal pathway leads to a significant reduction in dopamine transporter (DAT) levels [5]. Thus, radionuclide tracers which target DAT are commonly used for imaging PD in PET and SPECT. Typically, PET isotopes are short-lived and require onsite cyclotron or more demanding transportation logistics. On the other hand, SPECT with its longer half-life isotopes, offers greater accessibility and lower cost for PD imaging. Two most commonly used radiotracers for PD SPECT are 99mTc-TRODAT-1 (99mTc) [6] and 123I-ioflupane (123I) [7] currently, both tracers target DAT in the striatum region. Although the biodistributions of these two tracers exhibit similarities, 99mTc demonstrates a lower striatal uptake along with reduced photon scatter and penetration while 123I has higher striatal uptake with more contamination of high energy photons [8]. On the other hand, the SPECT image formation process is degraded by both photon attenuation and scatter, where the former is shown to be a more prominent effect than the latter [9]. Therefore, corrections of both effects should lead to a better image quality and quantitation as compared to no corrections and with correcting one factor [10]. A comprehensive analysis of the influence of different physical factors for image quality and quantitative accuracy of these two tracers will be of clinical interest.
Monte Carlo (MC) simulation models the interaction between particles and matters for nuclear imaging research and its results are validated with experiments [11]. While digital phantoms [12], coupled with MC simulations, can serve as important research tools for nuclear imaging physics research and be used as a gold standard for evaluation [13]. They provide a practical and economical alternative for designing and evaluating instrumentation, calculating absorbed dose, optimizing acquisition and reconstruction methods, without actual physical experiments or manufacturing the prototypes [14]. The introduction of a realistic phantom population [15] further enhances the feasibility of a virtual clinical trial [16], which would be particularly valuable for artificial intelligence research where ground truth and labeled data are sometimes difficult to obtain. There are existing digital brain phantoms for radionuclide imaging such as the 3D Hoffman brain phantom [17], XCAT phantom [12] and Zubal phantom [18], all share a common limitation in lacking anatomical variability for brain structure. A realistic PD digital phantom population is desirable to address such limitation. Shao et al. [19]. previously introduced a deep learning method to create SPECT PD phantoms from PET PD imaging. However, their results are restricted to generating 2D image slices. All current available digital brain phantoms also lack the capacity to represent different PD stages. In this work, we propose a method to generate a digital brain phantom population that encompasses variations in DAT tracer uptake, anatomical features, and PD stages. We then employ a MC simulation tool to assess the fidelity of our phantom population as compared to clinical SPECT data. We demonstrate an application of our tool to analyze the effects of scatter correction (SC) and attenuation correction (AC) for 123I-ioflupane and 99mTc-TRODAT-1 in DAT SPECT.
Materials and methods
Anatomy variations
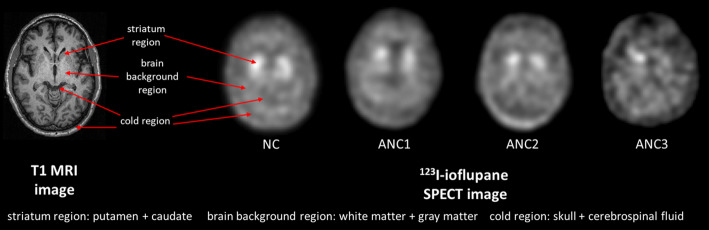
First, patients with both T1-weighted MRI and DAT SPECT data were identified from the Parkinson’s Progression Markers Initiative (PPMI) [20] database. A visual assessment was then performed on their MRI and SPECT images to exclude cases with truncation artifacts. After this screening process, a total of 200 patient datasets (age: 32–84 years, 119 males) were collected for further analysis. Based on the features from clinical DAT SPECT images, three primary regions, i.e., the high-uptake striatum region, the medium-uptake brain background region including gray and white matter, and the no-uptake cold region including skull and cerebrospinal fluid, were modeled in our phantoms (Fig. 1). The striatum region comprises 4 compartments, i.e., the left and right putamen and caudate. According to Owens-Walton et al. [21], there is only a slight volume difference (< 10%) in the striatum region across various PD stages. Vasconcellos et al. [22] also suggested that there is no significant difference in terms of volume of the caudate and putamen between PD and control groups. Thus, in this study, while we modeled the anatomical variations of the striatum, we did not account for the volume difference of the striatum between different PD stages.
Fig. 1.
Brain regions contribute to image contrast on DAT SPECT in different PD stages according to SPECT VI assessment scheme are indicated from sample MRI images. NC: Normal category; ANC1: Abnormal category 1; ANC2: Abnormal category 2; ANC3: Abnormal category 3
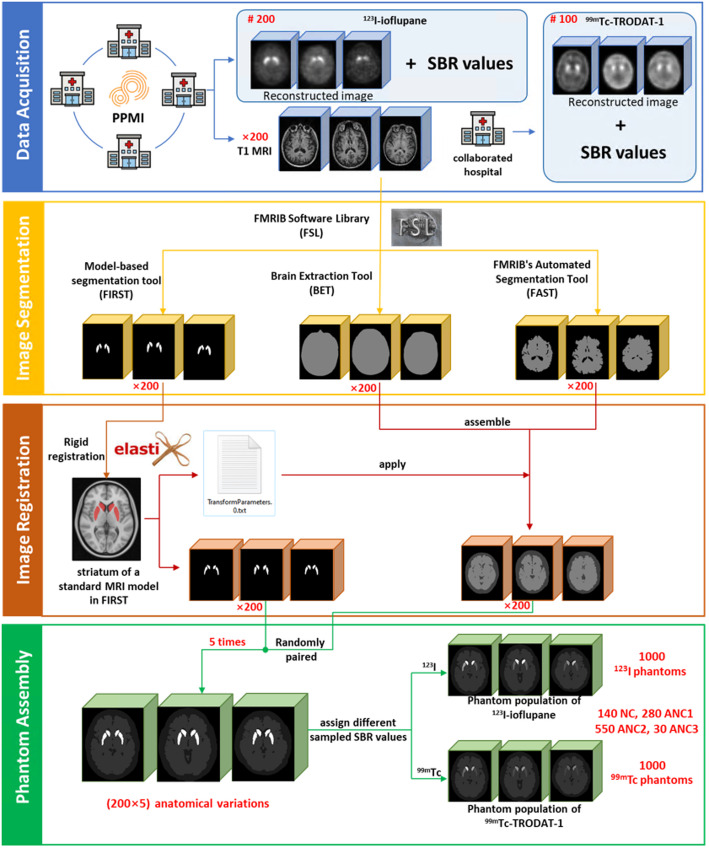
We used FMRIB Software Library (FSL) [23] Version v6 (2018) to segment the MRI images. Within FSL, the model-based segmentation tool FIRST [24] was firstly used to segment left and right putamen and caudate from different T1 MRI data. The Functional MRI of the Brain’s Automated Segmentation Tool was utilized to segment gray matter, white matter and cerebrospinal fluid [25]. The outer skull surface was delineated by using Brain Extraction Tool [26]. To generate more anatomical variations, we randomly paired the striatum region and the combined background region (including the brain background and cold regions) from different patients to form a new brain phantom. To ensure the unity of the coordinates of the paired structures, we first aligned the three-segmented regions from the same MRI images by rigidly registering the striatum region to the reference striatum model of FIRST, and the resultant transformation matrix was applied to the corresponding brain and cold region (Fig. 2) using Elastix [27].
Fig. 2.
Phantom population generation workflow
Activity distribution
In clinical practice, DAT SPECT images for the preliminary diagnosis of PD are commonly evaluated using the SPECT Visual Interpretation (VI) assessment scheme (Fig. 1) [28]:
Normal category (NC): two symmetric comma- or crescent-shaped focal regions of activity.
Abnormal category 1 (ANC1): uptake in putamen of one hemisphere absent or greatly reduced.
Abnormal category 2 (ANC2): uptake absent in the putamen of both hemispheres.
Abnormal category 3 (ANC3): uptake absent in the putamen of both hemispheres and greatly reduced in one or both caudate nuclei.
We then collected corresponding 123I DAT SPECT images and their striatal binding ratios (SBR = mean count in target striatum region/mean count in reference brain background region-1) for both left and right putamen and caudate, based on the previously selected 200 MRI data from the PPMI database. 99mTc DAT SPECT data of 100 patients with suspected PD symptoms (aged 35–87 years, 55 males) were also retrospectively collected in 2016 from Show Chwan Memorial Hospital under the local ethics approval (SCMH_IRB Number, 1311110704). All DAT SPECT images were categorized into different VI categories by a nuclear medicine physician with 11-year experience. For 123I, there were 20% NC, 43% ANC1, 24% ANC2, and 13% ANC3 patients, while there were 31% NC, 40% ANC1, 16% ANC2, and 13% ANC3 patients for 99mTc. For 123I, the SBR of the caudate ranged from 1.53 to 4.24 (2.66 ± 0.48), 0.63–3.76 (2.16 ± 0.60), 0.86–3.17 (1.89 ± 0.53) and 0.38–2.72 (1.48 ± 0.61) for NC, ANC1, ANC2 and ANC3 respectively, while for the putamen, SBR ranged from 0.73 to 3.04 (1.83 ± 0.43), 0.31–3.06 (1.08 ± 0.57), 0.25–2.70 (0.75 ± 0.36), and 0.12–1.58 (0.64 ± 0.30) respectively. Additionally, for the non-symmetric uptake pattern in ANC1 and ANC3, the uptake of one putamen or caudate was set to be ≤ 0.7 times of the counterpart. The uptake in the caudate was consistently higher than that in the putamen for the same phantom, as observed in our selected patient data.
Since the caudate and putamen cannot be discriminated for 99mTc data, the left and right striatum region was segmented by the same nuclear medicine physician with 11-year experience using ITK-SNAP [29]. First a thresholding-based method with a threshold set at 60% of the maximum intensity of the SPECT images [30]. The physician further manually refined each VOI for each patient. The entire segmentation procedure was repeated three times to assess the reproducibility of the segmentation. The mean SBR value of the whole striatum in 99mTc (0.70) was found to be nearly half of that in 123I (1.42), regardless of the PD categories. Thus, we regarded the ranges of SBR values of separated caudate and putamen for 99mTc as half of those for 123I.
To compensate for discrepancies between the estimated SBR values in reconstructed images and the true SBR values in patients, we established SBR recovery curves of a Siemens SPECT scanner for two tracers based on simulations. We selected a sample anatomy in Sect. 2.A and set the brain background region to 1, while the SBRs of caudate and putamen were modelled with values of 0.1, 0.2, 0.5, 1, 1.5, 2, 4, 8, 12, and 20, respectively. We used a MC simulation tool SIMIND V7.0.1 [31] to generate realistic projections. The details of simulations can be found in the .res file in the supplementary data 2, while the reconstructions process is described in Sect. 2.D. The SBR recovery curve and corresponding regression equations were acquired using linear regressions between the phantom and reconstructed images for both caudate and putamen in 123I and 99mTc, respectively (supplementary Fig. 2). We assumed the SBR values follow a truncated Gaussian distribution, characterized by the aforementioned mean, standard deviation, minimum and maximum SBR values, before and after applying the SBR recovery corrections. Then, we assigned the randomly sampled SBR values from these distributions to the 4 striatum compartments and brain background region, according to different VI categories. The cold region activity was set to be half of the brain background region for each phantom for both tracers.
The phantom voxel size was set to 1 × 1 × 1 mm3 and can be resampled based on the specific applications. The corresponding attenuation coefficient maps were obtained by non-rigidly registering an attenuation map generated by the XCAT program [12] to the background region of each individual phantom. Figure 2 shows the flowchart of the phantom population generation. A total of 1000 phantoms (200 NC, 400 ANC1, 250 ANC2, 150 ANC3) for 99mTc and 123I were generated respectively.
Attenuation and scatter correction in PD brain SPECT
We selected 200 phantoms (40 NC, 80 ANC1, 50 ANC2, 30 ANC3) out of the 1000 phantoms to investigate the AC and SC for both 123I and 99mTc DAT SPECT.
A SPECT system, i.e., a low-energy high-resolution (LEHR) parallel-hole collimator mounted on a Siemens SPECT system with a 9.6 mm (3/8 inch) thick NaI crystal was modelled in SIMIND based on the clinical data. The double energy window (DEW) [32] method was implemented for SC for 99mTc while the triple energy window (TEW) [33] method was implemented for 123I. The primary and scatter windows for DEW were set at 126–154 keV and 114–126 keV for 99mTc. For TEW in 123I, the primary and two scatter windows were set at 143–175 keV, 138–143 keV and 175–180 keV respectively. One hundred and twenty projections were simulated with 1 mm bin size and then collapsed from 256 × 200 × 120 to 128 × 100 × 120 with 2 mm bin size for 123I, and to 95 × 74 × 120 with 2.8 mm bin size for 99mTc, to be consistent with our available clinical data and to model continuous-to-discrete activity sampling as in the clinical data acquisition. Poisson noise was then added to the scaled noise-free projections, following a truncated Gaussian distribution of the clinical count levels (2.22 ± 0.52 M, 1.49–4.40 M) for 123I and (2.49 ± 0.73 M, 1.25–4.88 M) for 99mTc. The noisy projections were then reconstructed (i) with CT-based AC and SC (AC + SC), (ii) with AC only, (iii) with SC only, and (iv) without SC and AC (NOC), using the ordered subset expectation maximization (OS-EM) algorithm. An ideal situation without scatter and attenuation modelling was also simulated in SIMIND and their reconstructed images were used as the gold standard (GS) in this study. The maximum scatter order in MC simulation was set to 3, while further scatter accounted to be < 1% of total scatter photons. For 123I, 10 iterations and 10 subsets were used, following the European Association of Nuclear Medicine guidelines [34]. For 99mTc, we used 8 iterations and 4 subsets to be consistent with our clinical data. The voxel size of the reconstructed images was the same as their corresponding projection bin size. Collimator detector response was modeled during the reconstruction, and a Gaussian filter with 4.8 mm full width at half maximum (FWHM) was then applied to smooth the reconstructed images.
Data analysis
For each subject, the reproducibility of segmented striatal volumes (RPV%) was calculated as the standard deviation of the three measured volumes divided by their mean (RPV% = STDV/MeanV×100%) for the same subject. Visual comparisons were made between the MC simulated and clinical DAT SPECT images by a nuclear medicine physician with 11-year clinical experience. Coefficient of variance (COV) (Eq. 1) was measured over a uniform region of interest (ROI, 16 × 16 for 123I, 10 × 10 for 99mTc) on one selected 123I and 99mTc projection from clinical and simulation data, respectively. COV and noise power spectrum (NPS) (Eq. 2) were also evaluated over a 3D uniform cerebellum volume-of-interest (VOI, 16 × 16 × 5 for 123I, 10 × 10 × 5 for 99mTc, Fig. 5) to evaluate the image noise for both clinical and simulated reconstructed images of 123I and 99mTc with AC + SC.
Fig. 5.
Noise power spectrum (NPS) of (a) 123I and (b) 99mTc reconstructed images for simulation and clinical data
 |
1 |
 |
2 |
For COV calculation, n is the number of pixels (n=196 for 123I-, n = 100 for 99mTc- projections)/voxels (n=980 for 123I-, n=500 for 99mTc- reconstructed images),  is the mean pixel/voxel value of the ROI/VOI,
is the mean pixel/voxel value of the ROI/VOI,  is the pixel/voxel value, and
is the pixel/voxel value, and  is the pixel/voxel index. For NPS calculation,
is the pixel/voxel index. For NPS calculation,  are the spatial frequencies corresponding to the spatial coordinates
are the spatial frequencies corresponding to the spatial coordinates  , respectively, FFT denotes the 3D Fourier transform operator, and
, respectively, FFT denotes the 3D Fourier transform operator, and  represents the voxel intensity in the 3D VOI at position
represents the voxel intensity in the 3D VOI at position  ,
,  are the voxel sizes (mm),
are the voxel sizes (mm),  are the number of voxels along the x, y and z direction of the selected VOI. For a simplified 1D representation,
are the number of voxels along the x, y and z direction of the selected VOI. For a simplified 1D representation,  can be generated by binning the averaged
can be generated by binning the averaged  value according to the radial distance
value according to the radial distance  .
.
We evaluated the normalized mean square error (NMSE) (Eq. 3) and structural similarity index 1 (SSIM1) (Eq. 4) of each reconstructed simulation data against all clinical data in the same VI category. We also evaluated each clinical data against other clinical data in the same VI category. The disease prevalence and ratios of different VI categories were the same for both simulation and clinical data for comparison.
 |
3 |
 |
4 |
where  is the total number of voxels,
is the total number of voxels,  is the voxel count value in the clinical images.
is the voxel count value in the clinical images.  and
and  are the mean and standard deviations of the clinical reconstructed images while
are the mean and standard deviations of the clinical reconstructed images while  and
and  are the mean and standard deviations of the simulation reconstructed images or other clinical data,
are the mean and standard deviations of the simulation reconstructed images or other clinical data, is the cross-covariance between the two images. The constants
is the cross-covariance between the two images. The constants  and
and  are used to stabilize the division when the denominator is very small.
are used to stabilize the division when the denominator is very small.  represents the maximum voxel value between the 2 compared images. Typically,
represents the maximum voxel value between the 2 compared images. Typically,  and
and  are empirically set to 0.01 and 0.03 [35].
are empirically set to 0.01 and 0.03 [35].
For the AC and SC study, we evaluated the reconstructed images with different correction methods for the 200 phantoms using SSIM2 (Eq. 5), mean absolute error (MAE) (Eq. 6), SBR error (SBRE) (Eq. 7) and image profiles across the striatum region.
 |
5 |
 |
6 |
 |
7 |
Where  and
and  are the mean and standard deviations of the GS while
are the mean and standard deviations of the GS while  and
and  are the mean and standard deviations of the reconstructed images with different correction methods,
are the mean and standard deviations of the reconstructed images with different correction methods, is the cross-covariance between the two images.
is the cross-covariance between the two images.  and
and  are voxel count values in GS and reconstructed images with different correction methods, respectively.
are voxel count values in GS and reconstructed images with different correction methods, respectively.  is the SBR value of GS and
is the SBR value of GS and  is the SBR value of reconstructed images with different correction. The same cerebellum region used for COV/NPS analysis was used as the reference region for SBR calculation (Fig. 4). The Mann-Whitney U test was performed (SPSS Statistics v.27, IBM) between results of different groups to assess the statistical significance.
is the SBR value of reconstructed images with different correction. The same cerebellum region used for COV/NPS analysis was used as the reference region for SBR calculation (Fig. 4). The Mann-Whitney U test was performed (SPSS Statistics v.27, IBM) between results of different groups to assess the statistical significance.
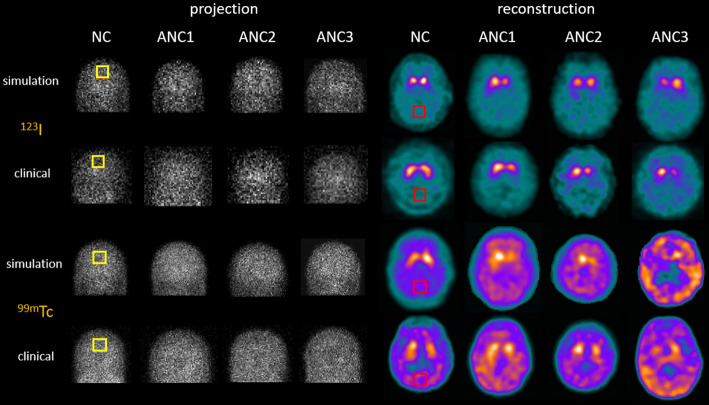
Fig. 4.
Sample simulation and clinical projections, as well as corresponding reconstructed images with AC + SC for 99mTc and 123I in different VI categories. A uniform region marked by the yellow squares is used for the COV analysis in projections. For reconstruction images, a uniform cerebellum region marked by the red squares is employed for COV/NPS evaluation and served as the reference region in the SBR calculation
Results
No significant differences are observed among the 3 segmented striatal volumes across 3 independent segmentation processes. The mean RPV% is 5.58% for 100 patients, consistent with the findings of Erdi et al. (5%) [36]. The box plot and scatter plot of 3 segmented volumes for each subject are presented in supplementary Fig. 1.
Sample phantom images of different VI categories are shown in Fig. 3. Figure 4 shows sample MC simulated projections and reconstructed images with AC + SC along with the clinical data for both tracers, showing high similarity. The physician evaluated the anatomical plausibility of the simulated images by examining overall head contours, positioning, and visibility of major brain structures. Special attention was given to the morphology of the striatum, particularly the caudate nucleus and putamen, ensuring that their shape, size, and orientation were consistent with those observed in clinical SPECT images. The distribution of DAT tracer uptake was assessed by comparing the intensity, symmetry, and contrast in the striatal regions, as well as the background signal in non-target areas and the noise texture. Based on this comprehensive assessment, the simulated images were judged to be highly realistic and visually comparable to actual patient scans.
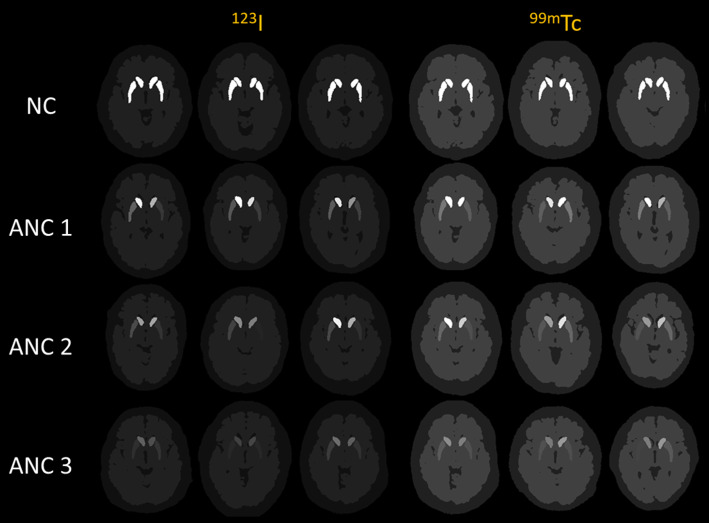
Fig. 3.
Sample 123I and 99mTc phantoms with different VI categories in SPECT
The COV results for both clinical and simulation images are presented in Table 1. These results show that the noise level of projections and reconstructed images generated from our phantom population show no significant difference as compared to the clinical data for the same tracer.
Table 1.
COV values for simulated and clinical projections as well as reconstructions of 123I and 99mTc
| COV | projection | reconstruction | |||
|---|---|---|---|---|---|
| 123I | Simulation | 1.005 ± 0.050 | p = 0.614 | 0.141 ± 0.043 | p = 0.479 |
| Clinical data | 1.009 ± 0.078 | 0.144 ± 0.053 | |||
| 99mTc | Simulation | 0.486 ± 0.066 | p = 0.264 | 0.087 ± 0.022 | p = 0.411 |
| Clinical data | 0.494 ± 0.067 | 0.090 ± 0.029 | |||
The NPS(r) curves for both simulation and clinical reconstructed images are shown in Fig. 5, showing high consistency across the whole spatial frequency range.
The scatter plots of NMSE and SSIM1 results between reconstructed simulation and clinical data, as well as among clinical data for different VI categories for both 123I and 99mTc, are presented in Fig. 6a and b, respectively. The unpaired Mann-Whitney U test indicates that there is no significant difference when comparing NMSE and SSIM1 results among clinical data or clinical data versus simulation data within the same VI category.
Fig. 6 .
NMSE and SSIM1 results between reconstructed simulation and clinical data, as well as among clinical data for different VI categories for the DAT images of (a) 123I and (b) 99mTc. Mann-Whitney U test was evaluated and found no significant difference between two groups
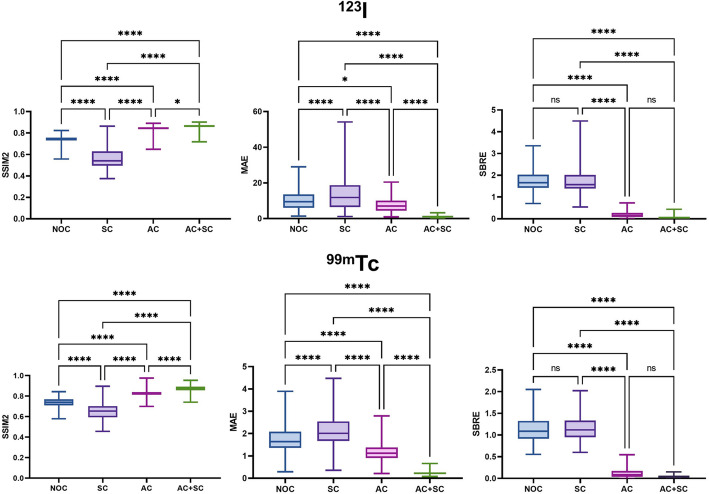
Sample reconstructed images with different correction methods from a sample phantom from both tracers are shown in Fig. 7a. The image profiles across the striatum region of different images are shown in Fig. 7b. The reconstructed image with AC + SC is closest to the GS, followed by AC only, NOC, and SC only for both tracers. The reconstructed images without AC exhibit a noticeable decrease in the uptake value especially in the center. The performance of SC only is even worse than that of no correction. Box plots for SSIM2, MAE, and SBRE are presented in Fig. 8, also indicating AC + SC performs the best, followed by AC only, NOC, and SC only for both tracers.
Fig. 7.
(a) Sample simulated reconstruction images with AC + SC, AC only, SC only, NOC, and GS for 123I and 99mTc distributions. (b) Profiles across the striatum location (yellow line in (a)) for different correction methods for 2 tracers
Fig. 8.
Box plots of SSIM2, MAE, and SBRE results between GS and AC + SC, AC only, SC only, and NOC for the two tracers in the simulated reconstructed DAT images. ns: p > 0.05, *: p < 0.05, ****: p < 0.0001
Discussions
Introducing anatomical and activity variations is a critical aspect to create a diverse set of digital brain phantoms. Though it is possible to generate patient-specific phantoms by segmenting regions from MRI images and setting the activity according to the patient specific SPECT/PET images, this approach requires segmentations of extensive MRI/SPECT/PET data, which may not be clinically feasible to generate a large phantom population. On the other hand, deep learning-based methods require sufficient amount of training data in different VI categories to generate reliable phantoms. To address these constraints, we employ a random pairing approach for the segmented striatum, brain background, and cold region. This method introduces greater anatomical variation while preserving real anatomical information, eliminating the need for segmentation in new patient data.
In this study, the striatum regions may overlap slightly with the ventricles in some phantoms, but the overlapped region was considered negligible (< 5% of the striatum volume) and does not affect the reconstructed results. We further evaluated the differences in 99mTc reconstructed images when modelling the overlapping regions as either striatum or ventricle for 44 phantoms among different VI categories. The violin plots of SBR values are shown in supplementary Fig. 3, showing no significant difference in SBR values between the two scenarios.
Since the actual activity uptake concentration is not available from the clinical data, the SBR recovery curves can be estimated from the simulation data. With the SBR values measured from the reconstructed images, the actual SBR values in the phantoms can then be estimated from the SBR recovery curves. In our study, the SBR curves were generated based on a Siemens SPECT system, the same scanner used for collecting the clinical data. From supplementary Fig. 2, the SBR curves are similar for 123I and 99mTc, for their similar photon energies (159 keV for 123I and 140 keV for 99mTc). After calibrating the SBR value for the two tracers using SBR curves from a specific SPECT system, the SBR setting in the phantom population becomes independent of the SPECT system.
Combined with realistic MC simulations, the noisy projections generated by our phantom yield comparable results in terms of noise and appearance for both tracers. Following the reconstruction process, the simulated images also demonstrate a high degree of similarity in noise levels and activity distributions when compared to clinical data (Figs. 4 and 5; Table 1). The quantitative analysis results (Fig. 6) show that our phantom population could reach a high-level of similarity as compared to the clinical data in different VI categories, further confirming the authenticity of our phantom population.
We evaluated AC and SC on image reconstruction for DAT SPECT as an example of application of our developed phantom population. Figures 7 and 8 both indicated that AC + SC produced the highest image quality, followed by AC only. The image quality and quantitative accuracy were better for NOC as compared to SC only, as scatter may partially offset attenuation effects in the NOC condition. The results also demonstrated that attenuation has the most significant impact on image quality in DAT SPECT, followed by scatter. Both 99mTc and 123I are similarly affected by these image degradation factors. CT-based AC and DEW/TEW-SC are effective corrections for DAT SPECT to compensate for attenuation effects and reduce scatter photons which carry incorrect positional information for the radionuclide decay. These findings are consistent with a previous study [37]. On the other hand, AI-based methods have been applied to compensate for image degradation in SPECT imaging. However, current AI-based correction performance is bounded by the training labels which are standard AC and SC images [38, 39].
Other applications of the phantom population include the evaluation of other image degradation factors such as partial volume effect, motion and the assessment of different correction methods to address these factors. Additionally, our phantom population can serve as an alternative for data augmentation [40], image segmentation [41] and transfer learning [42] in deep learning studies. Our methodology can also be potentially translated to PET studies when the SBR values for PET DAT tracers are available.
The limitations of this study include the absence of modelling intermediate cases between different VI categories, which was also mentioned in the SNM practice guideline for dopamine transporter imaging [28]. Further clinical insights to establish classification criteria for intermediate cases are needed, while they are beyond the scope of this study. Another limitation is the relatively limited sample size used for validating phantom similarity with clinical data, particularly regarding the regional diversity. However, our datasets cover a wide range of age and also a good balance of male and female patients in different VI categories. Moreover, the SBR set in the phantoms can be further validated by SBR curves from data acquired by other systems, yet it is beyond the scope of this study. It is also challenging to verify our proposed method can reflect the actual clinical patterns, yet our comparison with the actual clinical data shows promising results.
Conclusions
In this study, we proposed a novel method to develop a new digital PD brain phantom population, which represents realistic anatomical structures and contrast for PD DAT SPECT in different VI categories. Moreover, realistic simulated projection and reconstruction images can be obtained for both 99mTc and 123I DAT SPECT. With the use of the phantom population, we show that AC + SC produces the highest image quality, followed by AC only, NOC and SC only for both 99mTc and 123I DAT SPECT. The proposed phantom population shows promise for a range of radionuclide imaging applications, and is available online at https://big.fst.um.edu.mo/research/big-tools/big-brain/.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The computing was performed in part at SICC which is supported by SKL-IOTSC, University of Macau.
Abbreviations
- 123I
123I-ioflupane
- 99mTc
99mTc-TRODAT-1
- AC
Attenuation correction
- ANC
Abnormal category
- COV
Coefficient of variance
- DAT
Dopamine transporter
- DEW
Double energy window
- FWHM
Full width at half maximum
- GS
Gold standard
- LEHR
Low-energy high-resolution
- MAE
Mean absolute error
- MC
Monte Carlo
- NC
Normal category
- NMSE
Normalized mean square error
- NOC
Without scatter and attenuation
- NPS
Noise power spectrum
- PD
Parkinson’s Disease
- PPMI
Parkinson’s Progression Markers Initiative
- SBR
Striatal binding ratio
- SBRE
Striatal binding ratio error
- SC
Scatter correction
- SSIM
Structural similarity index
- TEW
Triple energy window
- VI
Visual Interpretation
- VOI
Volume-of-interest
Author contributions
WB.H contributed to the study design and implementation, data preprocess and analysis, drafting the manuscript, and preparing the tables and figures. H.J contributed to the manuscript revision, data curation and clinical interpretation. GU.H, contributed to data acquisition and clinical interpretation. YH.Z and RB.W contributed to the manuscript editing and revision. G.M. contributed to the study design, data analysis, curation, interpretation, supervision, manuscript writing and editing. All authors critically read and provided feedback on previous versions of the manuscript. All authors discussed the results and implications and commented on the manuscript.
Funding
This work is supported by a FDCT Research Grant (0178/2024/AMJ) and a Collaborative Research Grant (MYRG-GRG2024-00061-FST-UMDF) from University of Macau and University of Macau Development Foundation.
Data availability
Authors will share data upon request to the corresponding author.
Declarations
Ethics approval and consent to participate
The patient data used in this study are under local ethics approval (SCMH_IRB No: 1110704).
Consent for publication
All authors are consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL, et al. Global, regional, and National burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopade P, et al. Alzheimer’s and parkinson’s disease therapies in the clinic. Bioeng Translational Med. 2023;8(1):e10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limousin P, Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Reviews Neurol. 2019;15(4):234–42. [DOI] [PubMed] [Google Scholar]
- 4.Group PS. Levodopa and the progression of parkinson’s disease. N Engl J Med. 2004;351(24):2498–508. [DOI] [PubMed] [Google Scholar]
- 5.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in parkinson’s disease. Trends Neurosci. 2007;30(5):244–50. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, et al. SPECT imaging of dopamine transporters with 99mTc-TRODAT-1 in major depression and parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2011;23(1):63–7. [DOI] [PubMed] [Google Scholar]
- 7.Bega D, et al. Clinical utility of DaTscan in patients with suspected parkinsonian syndrome: a systematic review and meta-analysis. Npj Parkinson’s Disease. 2021;7(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hapdey S, Soret M, Buvat I. Quantification in simultaneous 99mTc/123I brain SPECT using generalized spectral factor analysis: a Monte Carlo study. Phys Med Biol. 2006;51(23):6157. [DOI] [PubMed] [Google Scholar]
- 9.Tsui BM, et al. Quantitative single-photon emission computed tomography: basic and clinical considerations. In Seminars In nuclear medicine. Elsevier; 1994. [DOI] [PubMed]
- 10.King MA et al. Attenuation, scatter, and spatial resolution compensation in SPECT. Emission tomography: the fundamentals of PET and SPECT, 2004: pp. 473–498.
- 11.Buvat I, Castiglioni I. Monte Carlo simulations in SPET and PET. QJ Nucl Med. 2002;46(1):48–61. [PubMed] [Google Scholar]
- 12.Segars WP, et al. 4D XCAT Phantom for multimodality imaging research. Med Phys. 2010;37(9):4902–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok GS, et al. Development and validation of a Monte Carlo simulation tool for multi-pinhole SPECT. Mol Imaging Biology. 2010;12:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W, Mok GS. Multi-pinhole collimator design in different numbers of projections for brain SPECT. Front Med, 2023. 10(1211726). [DOI] [PMC free article] [PubMed]
- 15.Segars W, et al. Population of anatomically variable 4D XCAT adult phantoms for imaging research and optimization. Med Phys. 2013;40(4):043701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abadi E, et al. Virtual clinical trials in medical imaging: a review. J Med Imaging. 2020;7(4):042805–042805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman E, et al. 3-D Phantom to simulate cerebral blood flow and metabolic images for PET. IEEE Trans Nucl Sci. 1990;37(2):616–20. [Google Scholar]
- 18.Zubal IG, et al. Computerized three-dimensional segmented human anatomy. Med Phys. 1994;21(2):299–302. [DOI] [PubMed] [Google Scholar]
- 19.Shao W et al. Generation of digital brain phantom for machine learning application of dopamine transporter radionuclide imaging. Diagnostics. 2022;12(8):1945. [DOI] [PMC free article] [PubMed]
- 20.Marek K, et al. The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95(4):629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens-Walton C, et al. Striatal changes in Parkinson disease: an investigation of morphology, functional connectivity and their relationship to clinical symptoms. Psychiatry Research: Neuroimaging. 2018;275:5–13. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcellos LF, et al. Volumetric brain analysis as a predictor of a worse cognitive outcome in parkinson’s disease. J Psychiatr Res. 2018;102:254–60. [DOI] [PubMed] [Google Scholar]
- 23.Woolrich MW, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1):S173–86. [DOI] [PubMed] [Google Scholar]
- 24.Patenaude B, et al. A bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M. BET2: MR-based estimation of brain, skull and scalp surfaces. In: Eleventh annual meeting of the organization for human brain mapping. 2005.
- 27.Klein S, et al. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2009;29(1):196–205. [DOI] [PubMed] [Google Scholar]
- 28.Djang DS, et al. SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J Nucl Med. 2012;53(1):154–63. [DOI] [PubMed] [Google Scholar]
- 29.Yushkevich PA, Gao Y, Gerig G. ITK-SNAP: An interactive tool for semi-automatic segmentation of multi-modality biomedical images. in 2016 38th annual international conference of the IEEE engineering in medicine and biology society (EMBC). 2016. IEEE. [DOI] [PMC free article] [PubMed]
- 30.Fang Y-HD, et al. Fully automated quantification of the striatal uptake ratio of [99mTc]-TRODAT with SPECT imaging: evaluation of the diagnostic performance in parkinson’s disease and the Temporal regression of striatal tracer uptake. Biomed Res Int. 2015;2015(1):461625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljungberg M, Strand S-E. A Monte Carlo program for the simulation of scintillation camera characteristics. Comput Methods Programs Biomed. 1989;29(4):257–72. [DOI] [PubMed] [Google Scholar]
- 32.de Vries DJ, King M. Window selection for dual photopeak window scatter correction in Tc-99m imaging. IEEE Trans Nucl Sci. 1994;41(6):2771–8. [Google Scholar]
- 33.Ichihara T, et al. Compton scatter compensation using the triple-energy window method for single-and dual-isotope SPECT. J Nucl Med. 1993;34(12):2216–21. [PubMed] [Google Scholar]
- 34.Morbelli S, et al. EANM practice guideline/snmmi procedure standard for dopaminergic imaging in parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47:1885–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, et al. Image quality assessment: from error visibility to structural similarity. IEEE Trans Image Process. 2004;13(4):600–12. [DOI] [PubMed] [Google Scholar]
- 36.Erdi YE, et al. Threshold Estimation in single photon emission computed tomography and planar imaging for clinical radioimmunotherapy. Cancer Res. 1995;55(23Supplement):s5823–6. [PubMed] [Google Scholar]
- 37.Kim KM, et al. Contribution of scatter and Attenuation compensation to SPECT images of nonuniformly distributed brain activities. J Nucl Med. 2003;44(4):512–9. [PubMed] [Google Scholar]
- 38.Sun H, et al. Artificial intelligence-based joint Attenuation and scatter correction strategies for multi-tracer total-body PET. EJNMMI Phys. 2024;11(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Y et al. Generative adversarial network-based Attenuation correction for 99mTc-TRODAT-1 brain SPECT. Front Med, 2023. 10(1171118). [DOI] [PMC free article] [PubMed]
- 40.Garcea F, et al. Data augmentation for medical imaging: A systematic literature review. Comput Biol Med. 2023;152:106391. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, et al. Deep-learning-based cross-modality striatum segmentation for dopamine transporter SPECT in parkinson’s disease. IEEE Transactions on Radiation and Plasma Medical Sciences; 2024.
- 42.Sun H, et al. Cross-Tracer and Cross-Scanner transfer Learning-Based Attenuation correction for brain SPECT. IEEE Transactions on Radiation and Plasma Medical Sciences; 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Authors will share data upon request to the corresponding author.