Abstract
The androgen receptor (AR) usually drives prostate cancer cell growth, yet its role in immune cells such as tumour-associated macrophages (TAMs), remains unclear. We find that macrophages co-expressing AR and triggering receptor expressed on myeloid cells-2 (TREM2) exhibiting potent immunosuppressive and tumour-promoting effects. Genetic ablation of TREM2 combined with pharmacological blockade of AR, significantly reduces tumour progression in prostate cancer mouse models. Mechanistically, apolipoprotein E (APOE) in tumour microenvironment (TME) binds to TREM2 on macrophages and promotes AR expression. AR further upregulates transcription expression of Il10, Tgfb1, Il23a, and Ccl2 in macrophages. AR, TREM2, and APOE expression increases in prostate cancer patients and correlates with poor prognosis. In conclusion, these findings indicate an alternative mechanism of tumour immune evasion, supporting the development of immunomodulatory agents targeting AR and TREM2 in TAMs to delay or reverse endocrine therapy resistance or immune checkpoint therapy resistance in prostate cancer.
Subject terms: Cancer microenvironment, Cancer immunotherapy, Monocytes and macrophages
Androgen receptor (AR) usually drives prostate cancer cell growth, but its role in immune cells like macrophages, remains unclear. The authors here show that AR+TREM2+ macrophages exhibits enhanced immunosuppressive and tumour-promoting effects comping to its counterparts with the mechanism of upregulated APOE/TREM2/AR expression in inducing immunosuppressive gene profile in macrophage.
Introduction
Prostate cancer starts as the most prevalent form of male cancer worldwide and remains the primary cause of cancer-related deaths among men1,2. Androgen deprivation therapy (ADT) and androgen receptor (AR) signalling inhibitors (ARSIs) have shown promising efficacy across various stages of prostate cancer3. However, despite the use of the potent next-generation AR antagonist enzalutamide (ENZA), or abiraterone, an inhibitor of the key enzyme cytochrome P450 family 17 subfamily A member 1 (CYP17A1) in the androgen synthesis pathway, most prostate cancer patients inevitably experience disease recurrence, progressing to castration-resistant prostate cancer (CRPC)3,4. Consequently, there exists an urgent need for novel treatment strategies or innovative combination therapies to complement ADT and ARSIs in addressing advanced prostate cancer.
The AR signalling cascade is essential for maintaining the physiological homeostasis of the prostate. However, AR functions as a central transcription factor and driver in prostate cancer5. The AR binds to androgens in the cytoplasm, leading to the dissociation from heat shock proteins (HSPs)6. Subsequently, upon activation, the AR translocate to the nucleus, where it binds to androgen response elements (AREs) located in target genes, thereby initiating downstream transcriptional regulations6,7. Apart from epithelial cells, several stromal cell types also express AR in prostate cancer and other solid tumours8–10. During the early stages of prostate cancer development, the AR within cancer-associated fibroblasts (CAFs) exhibits pronounced pro-tumoral functionality compared to its role in epithelial cells8. In CD8+ T cells, AR can facilitate T cell exhaustion by modulating epigenetic and transcriptional differentiation programs, thereby promoting sex-biased tumour progression11–13. Additionally, the AR signalling in THP-1, a cell line exhibiting macrophage-like characteristics, enhances triggering receptor expressed on myeloid cells-1 (TREM1) signalling and the subsequent expression of cytokines, including CCL2, CCL3, CCL7, and CCL13, promoting the migratory and invasive capabilities of prostate cancer cells14. In tumour associated macrophages (TAMs), the AR serves as a transcriptional inhibitor of IL1B, leading to the deterioration of the immunosuppressive microenvironment in prostate cancer15. Nevertheless, the functions of immune cell-associated AR signalling pathways related to prostate cancer development remain unelucidated.
Accumulating evidence has shown that cytokines such as IL-6 and IL-23 are essential to the prostate cancer progression. These cytokines induce AR-mediated gene activation through signalling pathways involving signal transducer and activator of transcription 3 (STAT3), Janus kinases (JAKs), mitogen-activated protein kinase (MAPK), and other key mediators16–18. IL-23 secreted by myeloid-derived suppressor cells (MDSCs) activated the AR pathway within prostate cancer cells, fostering the survival and proliferation of tumour cells16. Additionally, apolipoprotein E (APOE) in the tumour microenvironment (TME) of prostate cancer binds to the triggering receptor expressed on myeloid cells-2 (TREM2) on neutrophils, inducing pathogenic senescence-like neutrophils that exhibit enhanced immunosuppressive and tumour-promoting effects19. Furthermore, the retinoic acid receptor-related orphan receptor gamma (ROR-γ) recruits nuclear receptor coactivators 1 and 3 (NCOA1 and NCOA3) to the AR-ROR response elements (ROREs), thereby promoting AR gene transcription and its expression in prostate cancer cells20. Nevertheless, the upstream mechanisms driving the induction of AR expression in tumour-infiltrating immune cells, including TAMs, is yet to be reported. Recently, much attention has been paid to the pivotal role of TAMs expressing the cell surface receptor TREM2 in tumour immunomodulation, which is a member of the Ig superfamily that binds to lipids and transmits intracellular signals via the adaptor protein DAP1221,22. Although the critical role of TREM2+ TAMs has been studied in a variety of tumours, the downstream regulating mechanism of TREM2 in prostate cancer remains unelucidated.
The immune checkpoint therapy (ICT) such as programmed cell death protein 1 (PD1) blockade has been approved for the treatment of various types of cancer, including prostate cancer23. However, ICT is only effective for a limited number of molecular subtypes in prostate cancer24, partly due to the presence of immunosuppressive myeloid cells25, and a lower density of T cells in the TME26. Notably, myeloid cells, particularly TAMs and MDSCs, increased in abundance and mediated immunosuppression of prostate cancer after ADT16,25, potentially contributing to ICT resistance. Consequently, precise blockade of immunosuppressive myeloid cells may represent an effective strategy to enhance the efficacy of ICT.
This study shows that macrophages co-expressing both AR and TREM2 (AR+TREM2+ macrophages) exhibit strong immunosuppressive and tumour-promoting functions, which are mediated through AR-induced transcriptional activation of Il10, Tgfb1, Il23a, and Ccl2. Dual blockade of TREM2 and AR markedly reduces tumour burden in various mouse models of prostate cancer, and enhances the efficacy of anti-PD1 therapy. In conclusion, our findings support the notion that TREM2 serves as a critical modulator of AR in macrophages, and that AR acts as a pro-tumoral transcription factor to induce immunosuppressive macrophages. Therefore, targeting the TREM2-AR axis may represent an innovative therapeutic strategy for prostate cancer.
Results
AR expression is upregulated in monocytes and macrophages and positively correlates with TREM2 in prostate cancer
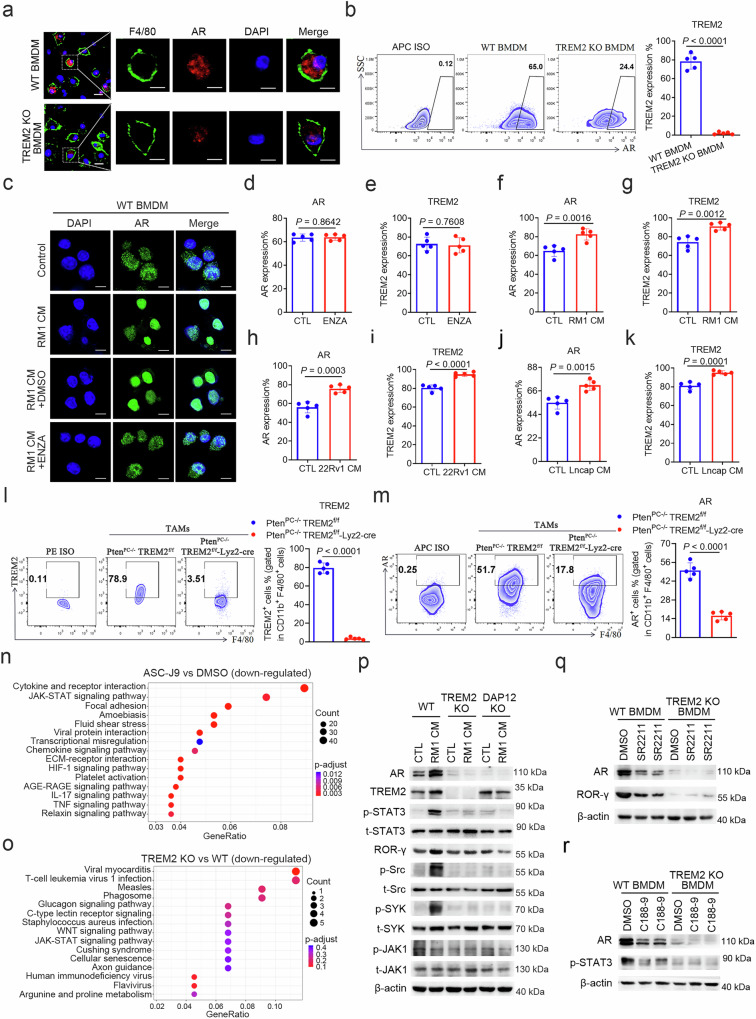
While the AR is primarily found in prostate epithelial cells, it is also present in immune cells and other stromal cells9,13,14. Flow cytometry was first employed to analyse the AR expression profile in peripheral blood mononuclear cells (PBMCs) and tumour-infiltrating immune cells from patients with prostate cancer and healthy individuals. The results demonstrated that AR expression was significantly upregulated in monocytes (Fig. 1a and Supplementary Fig. 1a, b), and macrophages (Fig. 1b and Supplementary Fig. 2a, b) of patients with prostate cancer. To investigate the effect of AR on macrophage gene phenotype, mouse bone marrow-derived macrophages (BMDMs) treated with the AR-degrading agent ASC-J9 in presence of conditioned media (CM) derived from RM1 mouse prostate cancer cells (RM1 CM) was collected for RNA sequencing (RNA-seq). Differential expressed genes (DEGs) analysis showed that compared to DMSO-treated macrophages, ASC-J9-treated macrophages had lower expressions of Trem2 and Trem1, anti-inflammatory M2-like macrophage markers Cd163 and Arg1 expression, pro-migratory factors Ccl2 and Ccl8, but had higher expressions of pro-inflammatory M1-like macrophage markers Cd86 and Tnf (Fig. 1c, d), suggesting that AR may be associated with macrophage phenotypic differentiation. Similarly, in RM1 CM treated TREM2 KO BMDMs, the transcription of Il10 was also down-regulated (Supplementary Fig. 3a), which was primarily secreted by anti-inflammatory M2-like macrophages to mediate T-cell immunosuppression27. Notably, TREM2 was recently reported as an inhibitory regulatory molecule of TAMs28, and TREM1 signalling in macrophages has been reported to promote migration and invasion of prostate cancer cells14. Subsequently, we assessed the expression levels of TREM2 and TREM1 in monocytes and macrophages from patients with prostate cancer. Compared to healthy individuals, TREM2 was significantly upregulated in circulatory monocytes (Fig. 1e and Supplementary Fig. 1a, c), and tumour-infiltrating macrophages (Fig. 1f and Supplementary Fig. 2a, b) of patients with prostate cancer, while TREM1 expression was not significantly altered (Supplementary Fig. 3b). Moreover, there was a positive correlation between AR and TREM2 expression in monocytes of patients with prostate cancer (Fig. 1g). Additionally, monocytes with high TREM2 expression also exhibited higher AR expression (Fig. 1h), and there was an increased proportion of monocytes (Fig. 1i and Supplementary Fig. 3c) and tumour infiltrating macrophages (Fig. 1j and Supplementary Fig. 3d) co-expressing AR and TREM2 (TREM2+AR+) in prostate cancer patients. Western blotting analysis also demonstrated higher protein expression levels of AR and TREM2 in human prostate tumour tissues compared to normal prostate tissues (Supplementary Fig. 3e), as well as in tumour infiltrating macrophages (Fig. 1k and Supplementary Fig. 3f, g). Multiplex immunofluorescence staining for AR, TREM2, and CD206 in prostatectomy specimens from untreated patients with prostate cancer showed higher dual staining for AR and TREM2 in CD206-expressing cells (Fig. 1l). The quantification results identified a median of 40% CD206-positive cells co-expressing AR and TREM2 in the tumour regions, which exhibited a notably higher level compared to that in the distant normal regions (median 10%) (Fig. 1m). Furthermore, we conducted an analysis of the macrophage subtypes within the tumour tissue based on the expression levels of TREM2 and AR. Multiplex fluorescent immunohistochemistry (using TSA technology) revealed that the infiltration of CD68+CD206+ M2-like macrophages was significantly more abundant compared to CD68+CD86+ M1-like macrophages (Fig. 1n). In addition, the CD68+CD206+ M2-like macrophages predominantly displayed TREM2+AR+ cell population (~40%), while the CD68+CD86+ M1-like macrophages primarily displayed TREM2- AR- cell population (~50%), and cells expressing either TREM2 or AR alone, specifically the TREM2+AR- or TREM2-AR+ populations, tend to be a mixed population of M1- and M2-like macrophages (Fig. 1n, o). Moreover, TREM2+AR+ macrophages were predominantly enriched in the tumour stroma (Fig. 1n). These results suggest that the TREM2+AR+ cell population represents the main anti-inflammatory M2-like macrophages, potentially serving as the primary macrophage group that drives tumour progression. Collectively, these results indicate that prostate cancer-associated monocytes and macrophages co-express AR and TREM2 and indicate a positive correlation between AR and TREM2.
Fig. 1. AR expression is upregulated in monocytes and macrophages and positively correlates with TREM2 in prostate cancer.
a Quantification of AR+ cells in CD4+ T cells, CD8+ T cells, monocytes, and neutrophils from the peripheral blood of healthy individuals (n = 31 biologically independent samples) and patients with prostate cancer (n = 53 biologically independent samples) by flow cytometry. b Quantification of AR+ cells in intraprostatic CD4+ T cells, CD8+ T cells, macrophages, and neutrophils was conducted in healthy prostates and prostate cancer tissues by flow cytometry (n = 6 biologically independent samples). c, d The RNA-seq analysis of BMDMs cultured in RM1 CM and treated with ASC-J9 or DMSO. c A heatmap of DEGs in macrophages, where gene counts for the DMSO group have been normalised, and gene expression values are coloured based on upregulation (red) or downregulation (blue). DMSO treatment is represented in black, while ASC-J9 treatment is depicted in red. d A volcano plot displaying the gene expression of selected TREM family members (Trem2 and Trem1), macrophage polarisation markers (Cd163, Arg1, Cd86, and Tnf), and pro-migration factors (Ccl2 and Ccl8), with gene expression values coloured according to upregulation (red) or downregulation (blue). e Quantification of TREM2+ cells in CD4+ T cells, CD8+ T cells, monocytes, and neutrophils from peripheral blood of healthy individuals (n = 31 biologically independent samples) and patients with prostate cancer (n = 53 biologically independent samples) by flow cytometry. f Quantification of TREM2+ cells in intraprostatic CD4+ T cells, CD8+ T cells, macrophages, and neutrophils was conducted in healthy prostates and prostate cancer tissues by flow cytometry (n = 6 biologically independent samples). g Pearson correlation analysis of AR and TREM2 protein levels in peripheral blood monocytes of patients with prostate cancer (n = 53 biologically independent samples). h Representative dot plots of TREM2 expression levels in peripheral blood mononuclear cells classified as TREM2 high (TREM2high), TREM2 low (TREM2low), and TREM2 negative (TREM2neg) (left). Representative dot plots of AR expression in peripheral blood TREM2neg, TREM2low, and TREM2high mononuclear cells (middle). Quantification of AR expression in peripheral blood TREM2neg, TREM2low, and TREM2high mononuclear cells of prostate cancer patients (n = 53 biologically independent samples) (right). i Quantification of co-expression, singular expression, and non-expression of AR and TREM2 in peripheral monocytes of healthy individuals (n = 31 biologically independent samples) and patients with prostate cancer (n = 53 biologically independent samples). j Quantification of co-expression, singular expression, and non-expression of AR and TREM2 in intraprostatic macrophages of healthy prostate and prostate cancer tissues (n = 6 biologically independent samples). k Representative immunoblot analysis of AR and TREM2 in CD68+ macrophages of tumour regions and adjacent normal prostate of prostate cancer patients. Experiment was repeated three times independently with similar results. l Representative multiplex immunofluorescence staining images of AR, TREM2, and CD206 in prostate tumour regions and adjacent normal prostate tissues. Nuclei were stained with DAPI. Scale bar: 10 μm. m Quantification of co-expression, singular expression, and non-expression of AR and TREM2 in CD206-expressing cells in tumour regions and distant normal prostate tissues (n = 5 biologically independent samples) from multiplex immunofluorescence in (Fig. 1l). n Multiplex fluorescent immunohistochemistry (using TSA technology) analysis. Representative tumour regions of FFPE prostatectomy specimens were stained for CD68, CD206, CD86, AR, and TREM2. Each triangle or pentagon represents the CD68+CD206+ cells or CD68+CD86+ cells, respectively. Scale bar: 20 µm. o Percentage of TREM2-AR-, TREM2+AR-, TREM2-AR+, and TREM2+AR+ cells in CD68+CD206+ macrophages or CD68+CD86+ macrophages in multiplex immunofluorescence image of the tumour regions of FFPE prostatectomy specimens, respectively (n = 6 biologically independent samples). For (l, n) experiments were repeated three times independently with similar results. All the data are presented as mean ± SD. The P-values were determined by two-way ANOVA with Sidak’s multiple comparisons for (a−o); by the Wald test under a negative binomial generalized linear model, and adjusted for multiple testing via the Benjamini-Hochberg method for (d); by two-sided Pearson correlation analysis (g); and by one-way ANOVA with Tukey’s multiple comparisons for (h). Source data are provided as a Source Data file.
TREM2 promotes AR expression in macrophages by binding to APOE
Our findings indicate that macrophages invading the prostate cancer-associated stroma co-express AR and TREM2. To investigate the regulatory relationship between AR and TREM2 in macrophages, we utilized TREM2 knockout (TREM2 KO) mice that could not activate downstream TREM2 signalling29. Mouse BMDMs were isolated and stimulated to test AR and TREM2 expression levels. The results showed that both protein (Fig. 2a, b and Supplementary Fig. 4a, b), and mRNA (Supplementary Fig. 4c, d) expression levels of AR and TREM2 were reduced in TREM2 KO BMDMs compared to wild-type (WT) BMDMs, suggesting that TREM2 may have a regulatory effect on AR expression. Subsequently, we treated AR-expressing BMDMs with ENZA, an AR antagonist that blocks AR function and inhibits nuclear translocation of AR30. The results demonstrated that ENZA did not yield a significant effect on protein expression of AR (Fig. 2d), which was consistent with previous studies of ENZA in prostate cancer cells31. We further examined the localization of AR in macrophages, which showed that in the untreated state, AR was diffusely distributed in the cytoplasm and nucleus in macrophages, whereas after RM1 CM stimulation, AR was significantly translocated to the nucleus (Fig. 2c), suggesting that AR signalling was active in macrophages in the tumour state. However, ENZA treatment significantly inhibited RM1 CM-induced nuclear translocation of AR (Fig. 2c). Moreover, functional inhibition of the AR did not affect TREM2 protein expression either (Fig. 2e). These results indicated that TREM2 function has a regulatory effect on AR, while AR function does not affect TREM2. To simulate the TME, we evaluated whether factors secreted by tumour cells affected the expression of AR and TREM2 in macrophages. By culturing BMDMs with RM1 CM, the protein (Fig. 2f, g) and mRNA (Supplementary Fig. 4c, d) expression levels of TREM2 and AR in WT BMDMs were increased, whereas in TREM2 KO BMDMs, RM1 CM didn’t affect the transcription level of TREM2 and AR (Supplementary Fig. 4c, d). Notably, AR levels in TREM2 KO BMDM were extremely low after RM1 CM treatment (Fig. 2p–r), suggesting a strong inhibitory effect of TREM2 on AR expression in the tumour condition. In addition, THP1 cells were cultured using conditioned medium derived from human prostate cancer cell lines 22Rv1 (22Rv1 CM) and Lncap (Lncap CM), and results indicated that both 22Rv1 CM (Fig. 2h, i) and Lncap CM (Fig. 2j, k) promoted protein expression levels of TREM2 and AR in THP1 cells. Furthermore, in the PtenPC-/- mice, which serves as an ideal model for studying spontaneous prostate cancer19, we found that TREM2 and AR expressions were increased in macrophages of PtenPC-/- mice than that in PtenPC+/+ mice (littermate control of PtenPC-/- mice) (Supplementary Fig. 4e, f). Moreover, when comparing PtenPC-/- TREM2f/f mice with PtenPC-/- TREM2f/f-Lyz2-cre mice, there was a decrease in AR expression in the prostate tumor-infiltrating macrophages with TREM2 deficiency (Fig. 2l, m). These results suggest that the expression of TREM2 and AR is upregulated in prostate cancer-associated macrophages, and that TREM2 influences the expression of AR.
Fig. 2. TREM2 promotes AR expression in macrophages through DAP12-Src-Syk-STAT3-ROR-γ pathway.
a Representative immunofluorescence staining images of AR and F4/80 in WT BMDMs and TREM2 KO BMDMs. Scale bar: 5 μm. b Representative flow plots and quantification of AR expression in WT BMDMs and TREM2 KO BMDMs (n = 5 biologically independent samples). c Representative immunofluorescence staining images of AR in WT BMDMs treated with RM1 CM, RM1 CM + DMSO or RM1 CM + ENZA, respectively. Scale bar: 10 μm. d, e Protein expression levels of AR (d) and TREM2 (e) in WT BMDMs treated with DMSO or 10 μM ENZA for 48 h were quantified by flow cytometry (n = 5 biologically independent samples). f, g Quantification of AR (f) and TREM2 (g) expression in WT BMDMs treated with RM1 CM for 48 h (n = 5 biologically independent samples). h, i Relative protein expression of AR (h) and TREM2 (i) in THP1 cells treated with 22Rv1 CM for 48 h (n = 5 biologically independent samples). j, k Protein expression levels of AR (j) and TREM2 (k) in THP1 cells treated with Lncap CM for 48 h (n = 5 biologically independent samples). l, m Representative flow plots and quantification of TREM2 (l) and AR (m) in TAMs of PtenPC-/- TREM2f/f mice and PtenPC-/- TREM2f/f-Lyz2-cre mice (n = 5 biologically independent samples). n The bubble chart of KEGG pathway enrichment analysis illustrated the significantly downregulated signalling pathways following ASC-J9 treatment in WT BMDMs. o The bubble chart of KEGG pathway enrichment analysis illustrated the significantly downregulated signalling pathways in RM1 CM treated TREM2 KO BMDMs compared to WT BMDMs. p Representative immunoblot analysis of AR, TREM2, p-STAT3, ROR-γ, p-Src, p-Syk, and p-JAK1 in WT BMDMs, TREM2 KO BMDMs and DAP12 KO BMDMs treated with RM1 CM for 48 h. Experiment was repeated three times independently with similar results. q Representative immunoblot analysis of AR and ROR-γ in WT BMDMs and TREM2 KO BMDMs treated with DMSO plus RM1 CM or 5 μM SR2211 (ROR-γ inhibitor) plus RM1 CM for 48 h. Experiment was repeated three times independently with similar results. r Representative immunoblot analysis of AR and p-STAT3 in WT BMDMs and TREM2 KO BMDMs treated with DMSO plus RM1 CM or 10 μM C188-9 (p-STAT3 inhibitor) plus RM1 CM for 48 h. Experiment was repeated three times independently with similar results. For a and c, experiments were repeated three times independently with similar results. All the data are presented as mean ± SD. The P-values were determined by two-sided Mann-Whitney U test for (b, d−m); and by Hypergeometric Test for (n, o). Source data are provided as a Source Data file.
To explore the mechanism of TREM2 regulating AR expression in macrophage, we next performed RNA-seq of WT BMDMs cultured in RM1 CM treated with the AR-degrading agent ASC-J9 vs DMSO, or TREM2 KO BMDM vs WT BMDM cultured with RM1 CM only. KEGG pathway enrichment analysis showed that the JAK-STAT signalling pathway was significantly down-regulated in both ASC-J9 treated macrophages (Fig. 2n) and TREM2 KO BMDMs (Fig. 2o), suggesting that the JAK-STAT signalling pathway may play an important role in the regulation of AR by TREM2. Given that AR expression in prostate cancer cells mainly relies on the STAT3-ROR-γ signalling pathway20, and TREM2 exerts its function mainly through the DAP12-Src-Syk signalling pathway21, we hypothesized that TREM2 may induce AR expression through DAP12-Src-Syk-STAT3-RORγ signalling pathway. Upon treating BMDMs with RM1 CM, increased phosphorylation levels of Src and Syk and STAT3, as well as the increased expression of downstream target molecule ROR-γ in WT BMDMs was observed, but there was no significant change in JAK1 phosphorylation levels (Fig. 2p and Supplementary Fig. 4g). However, these protein expression levels in TREM2 KO BMDMs or DAP12 KO BMDMs remained unchanged (Fig. 2p and Supplementary Fig. 4g). Next, macrophages were treated with the STAT3 inhibitor C188-932 or ROR-γ inhibitor SR221120 to validate the regulatory effect of the STAT3-ROR-γ signalling pathway on AR expression in these cells. Western blotting results showed that post-inhibition of ROR-γ (Fig. 2q) or STAT3 (Fig. 2r), the stimulatory effect of RM1 CM on AR expression in WT BMDMs was impaired, while in TREM2 KO BMDMs remained unchanged. Notably, AR levels were extremely low in TREM2 KO BMDM after RM1 CM treatment (Fig. 2p–r), suggesting that there is an important regulatory role of TREM2 on AR expression, especially in tumour status. These data demonstrate that CM from prostate cancer cells can activate the TREM2-dependent DAP12-Src-Syk-STAT3-ROR-γ signalling pathway to promote AR expression in macrophages.
To identify the factors secreted by tumour cells that induce the expression of TREM2 and AR in macrophages, we performed liquid chromatography-mass spectrometry analysis. Through the enrichment of the TREM2 protein in human prostate cancer tissues using an anti-TREM2 antibody, we found that APOE was the most enriched secretory protein (Fig. 3a, b and Supplementary Table 1). Analysis of the prostate cancer dataset from The Cancer Genome Atlas (TCGA) using TIMER2.0 (http://timer.cistrome.org/) revealed a positive correlation between TREM2 and APOE in human prostate cancer tissues (Fig. 3c). Additionally, endogenous Co-IP analysis revealed an interaction between TREM2 and APOE in the TME of human prostate cancer samples (Fig. 3d). Multiplex immunofluorescence staining also confirmed dual staining of TREM2 and APOE in CD68-expressing TAMs in the TME of human prostate cancer tissues, and statistical analysis indicated significantly higher co-staining levels of TREM2 and APOE within the tumour regions compared to the adjacent normal tissues (Fig. 3e, f). Furthermore, by evaluating the expression profile of APOE in prostate cancer, we observed an upregulation of APOE in prostate cancer than in healthy control (Fig. 3g, h). To be specific, APOE protein expression was also upregulated in the serum (Fig. 3g) and tumour tissues (Fig. 3h) of human prostate cancer. Importantly, secretory APOE was increased in RM1 CM (Fig. 3i), indicating the potentially regulatory role of APOE secreted by prostate cancer cells in prostate cancer development.
Fig. 3. TREM2 promotes AR expression in macrophages by binding to APOE.
a Experimental scheme of mass spectrometry. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. Briefly, proteins from human prostate tumour tissues were extracted, followed by immunoprecipitation using anti-TREM2 antibody or IgG and agarose beads, and then the enriched proteins were lysed for peptide identification. b Venn diagram and the table showing the secretory proteins in the anti-TREM2-enriched complex. c Pearson correlation analysis of APOE and TREM2 mRNA levels in TCGA database of prostate cancer (n = 498 biologically independent samples). d Representative immunoblot analysis of TREM2 and APOE in macrophages isolated from human prostate cancer tissues which was immunoprecipitated with anti-TREM2 antibody. Sample processing controls (lysate input, run on the same separate gel) are shown in panel (1 & 2). Experiment was repeated three times independently with similar results. e Representative multiplex immunofluorescence staining images of TREM2, APOE, and CD206 in prostate tumour tissues and adjacent normal prostate tissues. Nuclei were stained with DAPI. Scale bar: 10 μm. f Quantification of co-expression, singular expression, and non-expression of TREM2 and APOE in CD68-expressing cells in tumour regions and distant normal prostate tissues (n = 5 biologically independent samples) from multiplex immunofluorescence of (Fig. 3e). g The concentration of APOE in the serum of both healthy individuals (n = 12 biologically independent samples) and patients (n = 28 biologically independent samples) with prostate cancer was detected using ELISA. h The concentration of APOE in the homogenates of normal prostate and prostate cancer tissues was detected using ELISA (n = 6 biologically independent samples). i The concentration of APOE in normal cell culture media and RM1 CM was measured using ELISA (n = 6 biologically independent samples). j Representative immunoblot analysis of AR, TREM2, p-STAT3, ROR-γ, p-Src, and p-Syk in WT BMDMs, TREM2 KO BMDMs and DAP12 KO BMDMs treated with RM1 CM or 100 nM recombinant APOE protein for 48 h. Experiment was repeated three times independently with similar results. k Representative immunoblot analysis of AR, TREM2, p-STAT3, ROR-γ, p-Src, and p-Syk in WT BMDMs treated with RM1 CM or RM1 CM plus 1 ng/ml anti-APOE for 48 h. Experiment was repeated three times independently with similar results. l, m RT-qPCR analysis of in WT BMDMs and TREM2 KO BMDMs treated with 100 nM recombinant APOE protein (l) or RM1 CM plus 1 ng/ml anti-APOE (m) for 48 h (n = 6 biologically independent samples). Gene expression was normalized to Actb expression. For e, experiments were repeated three times independently with similar results. All the data are presented as mean ± SD. The P-values were determined by two-sided Pearson correlation analysis for (c); by two-way ANOVA with Sidak’s multiple comparisons for (f); by two-sided Mann-Whitney U test for (g−i); and by two-way ANOVA with Tukey’s multiple comparisons for (l, m). Source data are provided as a Source Data file.
As a lipid-binding protein involved in lipid metabolism and transport, APOE binds to multiple receptors, such as TREM233,34. APOE also regulates changes in macrophage polarisation, and plays a role in tumour progression, invasion and metastasis35. TREM2 primarily interacts with the hinge region of APOE through the extracellular immunoglobulin-like domain36. In this study, we primarily aim to explore the regulatory role of soluble APOE on TREM2 within the tumour immune microenvironment. Given that soluble APOE is primarily secreted by macrophages and tumour cells, we additionally assessed the expression levels of APOE in conditional medium from BMDM (BMDM CM) and RM1 (RM1 CM). The results showed that both BMDM and RM1 secreted soluble APOE, and APOE level was higher in the RM1 CM than that in BMDM CM (Supplementary Fig. 4h). Nevertheless, given the possible predominance of macrophage APOE for autocrine regulation of TREM2 in TME, we have not yet been able to demonstrate exactly which cell-derived APOE is more predominant in the regulation of TREM2. To explore the mechanistic influence of APOE on TREM2-AR signalling in macrophages, we pretreated BMDMs with recombinant APOE protein. Results showed that the recombinant APOE protein, similar to RM1 CM, upregulated AR and TREM2 expression, and the phosphorylation levels of downstream signalling molecules Src and Syk and STAT3, as well as the expression of target protein ROR-γ in WT BMDMs (Fig. 3j and Supplementary Fig. 4i). However, in TREM2 KO BMDMs or DAP12 KO BMDMs, the expressions of these proteins were significantly lower and showed no significant changes (Fig. 3j and Supplementary Fig. 4i). Consistent with this result, anti-APOE antibody treatment inhibited the activation of TREM2 downstream signalling pathway and the AR expression in WT BMDMs induced by RM1 CM (Fig. 3k). In addition, APOE recombinant protein activation (Fig. 3l) or APOE antibody blockade (Fig. 3m) promoted or inhibited AR mRNA expression in WT BMDMs, respectively, whereas there was no significant effect on AR mRNA expression in TREM2 KO BMDMs, suggesting that APOE has a regulatory effect on TREM2-induced AR expression. Together, these findings illustrate that APOE binding to TREM2 triggers the downstream DAP12-Src-Syk-STAT3-ROR-γ signalling pathway, leading to the upregulation of AR expression in macrophages.
The TREM2-AR axis promotes anti-inflammatory M2-like macrophage polarisation through transcriptional activation of Il10 and Tgfb1
To delve deeper into the role of the TREM2-AR axis in macrophage polarisation, we induced and stimulated WT BMDMs and TREM2 KO BMDMs to differentiate into TAMs using RM1 CM with or without ENZA (Fig. 4a). Upon stimulation with RM1 CM, the mRNA expression levels of anti-inflammatory M2-like macrophage markers (Il10, Tgfb1, Arg1, and Cd206) were significantly decreased in TREM2 KO BMDMs compared to WT BMDMs (Fig. 4b and Supplementary Fig. 5a). While in WT BMDMs, only Il10 and Tgfb1 were reduced following ENZA treatment (Fig. 4b). Additionally, the mRNA expression levels of M1-like macrophage markers (Cd80, Cd86, Nos2, and Tnf) were increased in RM1 CM-treated TREM2 KO BMDMs compared to WT BMDMs (Supplementary Fig. 5b). However, ENZA treatment showed no effect on these M1-like macrophage markers in BMDMs (Supplementary Fig. 5b). Flow cytometry analyses also revealed that RM1 CM-induced TREM2 KO BMDMs had lower CD206 (Fig. 4d) and higher CD86 (Fig. 4e) protein expression levels compared to those in WT BMDMs, while ENZA did not affect these two proteins in BMDMs (Fig. 4d, e). Subsequently, WT BMDMs and TREM2 KO BMDMs were cultured with RM1 CM, and the TAMs supernatant was collected. ELISA results indicated that the protein concentrations of anti-inflammatory cytokines IL-10 and TGF-β in the supernatant were significantly decreased in TREM2 KO BMDMs compared that in WT BMDMs (Fig. 4c). Moreover, ENZA treatment significantly decreased the levels of these two cytokines in the supernatant (Fig. 4c). These findings suggest that the TREM2-AR axis induces the repolarisation of TAMs into an anti-inflammatory M2-like phenotype and that AR may directly regulate the expression of Tgfb1 and Il10. Since TAMs usually exhibit an inhibitory effect on T cell-mediated antitumour immune responses37, the effects of TREM2-AR in TAMs on the proliferation and secretory function of T cells were subsequently tested (Supplementary Fig. 5c). Compared to WT BMDMs, TREM2 KO BMDMs treated with ENZA significantly enhanced the proliferation (Supplementary Fig. 5d) and secretion of granzyme B and perforin (Supplementary Fig. 5e, f), IFN-γ and TNF (Supplementary Fig. 5g, h) of co-cultured CD8+ T cells. These results suggest that the macrophage TREM2-AR axis not only converts macrophages to an immunosuppressive phenotype, but also inhibits T cell proliferation and secretion of antitumour factors in vitro.
Fig. 4. The TREM2-AR axis promotes M2-like macrophage polarisation through transcriptional activation of Il10 and Tgfb1.
a Schematic representation of TREM2+/+ and TREM2-/- BMDMs induction. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. BMDMs were treated with RM1 CM plus 10 μM ENZA, and gene expression was analysed by RT-qPCR and protein expression by flow cytometry. b RT-qPCR analysis of M2-like macrophage markers Il10 and Tgfb1 in WT BMDMs and TREM2 KO BMDMs treated with RM1 CM plus 10 μM ENZA for 48 h (n = 6 biologically independent samples). Gene expression was normalized to Actb expression. c ELISA analysis of IL-10 and TGF-β in the supernatant of WT BMDMs and TREM2 KO BMDMs treated with RM1 CM plus 10 μM ENZA for 48 h (n = 5 biologically independent samples). d, e Representative flow plots and quantification of CD206 (d) and CD86 (e) in WT BMDMs and TREM2 KO BMDMs treated with RM1 CM plus 10 μM ENZA for 48 h (n = 5 biologically independent samples). f A genomic view of AR enrichment on IL10 and TGFB1 promoters in THP-1 cells was analysed from published ChIP-seq data14. AR peaks under DMSO and 10 nM R1881 conditions are depicted in blue and red, respectively. g Prediction of Ar binding sites on the Il10 (left) and Tgfb1 (right) promoters through the JASPAR database. h The specific binding of Ar to the predicted binding site in Il10 and Tgfb1 promoters in WT BMDMs treated with RM1 CM was detected by ChIP-qPCR (n = 6 biologically independent samples). i The binding of Ar to the Il10 and Tgfb1 promoters in WT BMDMs and TREM2 KO BMDMs treated with RM1 CM was detected by ChIP-qPCR (n = 6 biologically independent samples). Relative fold enrichment was normalised relative to IgG and WT BMDM group, respectively. j The binding of Ar to the Il10 and Tgfb1 promoters in WT BMDMs treated with RM1 CM plus 10 μM ENZA for 48 h was detected by ChIP-qPCR (n = 6 biologically independent samples). Relative fold enrichment was normalised relative to IgG and CTL group, respectively. All the data are presented as mean ± SD. The P-values were determined by two-way ANOVA with Tukey’s multiple comparisons for (b−d); by two-way ANOVA with Sidak’s multiple comparisons for (h); and by two-sided Mann-Whitney U test for (i, j). Source data are provided as a Source Data file.
AR has been reported to function as a transcriptional repressor of Il1B and regulate macrophage function15. Given AR’s potentially regulatory influence on the IL-10 and TGF-β expression, we sought to ascertain whether the core transcription factor AR in macrophage of prostate cancer directly modulates IL-10 and TGF-β expression at the transcriptional level. By analysing AR-enriched ChIP-seq data14, we observed the binding of AR to IL10 and TGFB1 promoters in THP-1 cells, which was enhanced after AR agonist R1881 treatment (Fig. 4f). Subsequently, we predicted the potential Ar-binding sites in Il10 and Tgfb1 promoters using the JASPAR database (https://jaspar.genereg.net/) (Fig. 4g). ChIP-qPCR experiments confirmed the specific binding of Ar to the predicted binding sites of Il10 and Tgfb1 promotors in RM1 CM-treated BMDMs (Fig. 4h). However, the specific binding of Ar to Il10 and Tgfb1 promotors was attenuated in TREM2 KO BMDMs (Fig. 4i), and in ENZA-treated WT BMDMs (Fig. 4j). In conclusion, these results indicate that the TREM2-AR axis significantly induces anti-inflammatory polarisation of macrophages by promoting AR-mediated transcriptional activation of Il10 and Tgfb1 in macrophages, which may result in an immunosuppressive TME.
The TREM2-AR axis promotes proliferation and migration of prostate cancer cells through transcriptional activation of Il23a and Ccl2
Myeloid cells could indirectly promote tumour cell proliferation and migration by secreting cytokines14,16. To investigate the potential impact of the TREM2-AR axis in macrophages on the proliferation and migration of prostate cancer cells, we co-cultured mouse prostate cancer cells RM1 with WT BMDMs and TREM2 KO BMDMs in the presence of ENZA and assessed their proliferation and migration abilities. Remarkably, compared to WT BMDMs, TREM2 KO BMDMs notably suppressed the proliferation (Fig. 5a and Supplementary Fig. 6a) and migration (Fig. 5b and Supplementary Fig. 6b) of RM1 cells. Moreover, after treatment with ENZA, the pro-proliferative (Fig. 5a) and pro-migratory (Fig. 5b) effects of WT BMDMs on RM1 cells were also impaired. Consistent pro-proliferative and pro-migratory results were also observed in human prostate cancer cell lines 22Rv1 (Supplementary Fig. 6c, d) and Lncap (Supplementary Fig. 6e, f), which were co-cultured with THP1 cells and treated with anti-TREM2 antibody or ENZA, suggesting that TREM2-AR axis has a promoting effect on the proliferation and migration of prostate cancer cells. In addition, APOE recombinant protein treatment also markedly promoted the proliferation and migration of RM1 cells co-cultured with WT BMDMs and had little effect on TREM2 KO BMDMs (Fig. 5c, d), whereas anti-APOE treatment significantly inhibited the proliferation and migration of RM1 cells co-cultured with WT BMDMs (Supplementary Fig. 6g, h), suggesting that APOE has a regulatory effect on macrophage TREM2-mediated tumour cell proliferation and migration. In prostate cancer, IL-638 and IL-2316 are considered as key cytokines promoting prostate cancer cell proliferation, while CCL2/7/13 correlates with the migration of prostate cancer cells14. Therefore, we examined the effect of the macrophages TREM2-AR axis on the production of these pro-proliferative and pro-migratory factors. The findings indicated that compared to WT BMDMs, TREM2 KO BMDMs co-cultured with RM1 or treated with RM1 CM had significantly reduced mRNA expression levels of pro-proliferative (Il23a, Il6) and pro-migratory (Ccl2, Ccl7, Ccl13) (Fig. 5e and Supplementary Fig. 6i–k) factors, but ENZA treatment suppressed only the expression levels of Il23a and Ccl2 in WT BMDMs (Fig. 5e). To further investigate whether these cytokines have an impact on macrophage-mediated pro-proliferation and pro-migration of prostate cancer cells, the co-cultured RM1 cells and WT BMDMs were treated with αCCL2/7/13 or αIL-23 antibodies. The treatment resulted in inhibition of the pro-migratory (Fig. 5f) and pro-proliferative (Fig. 5g) effects of WT BMDMs on RM1 cells, indicating that the proliferation and migration of prostate cancer cells depends on these factors. Moreover, the IL-23 and CCL2 protein levels in the supernatant of RM1 CM-cultured TREM2 KO BMDMs was significantly decreased compared to those in WT BMDMs (Fig. 5h). ENZA treatment also significantly reduced the concentrations of these two cytokines in the supernatant of WT BMDMs (Fig. 5h). These results imply that AR may directly regulate the expression of IL-23 and CCL2 in macrophages, and that TREM2-AR axis in macrophages has potent pro-proliferative and pro-migratory abilities.
Fig. 5. The TREM2-AR axis promotes proliferation and migration of prostate cancer cells through transcriptional activation of Il23a and Ccl2.
a, b Assessment of the indirect antitumour effects of BMDMs. WT BMDMs and TREM2 KO BMDMs were co-cultured with RM1 cells at a ratio of 1:1 in Transwell systems with 10 μM ENZA. a RT-qPCR was performed to detect the mRNA levels of Ki67 in RM1 cells after 48 h of co-culture (n = 6 biologically independent samples). b Representative images and quantification of migrated RM1 cells were obtained after 24 h of co-culture (n = 5 biologically independent samples). c, d WT BMDMs and TREM2 KO BMDMs were co-cultured with RM1 cells at a ratio of 1:1 in Transwell systems with 100 nM recombinant APOE protein. c RT-qPCR was performed to detect the mRNA levels of Ki67 in RM1 cells after 48 h of co-culture (n = 6 biologically independent samples). d Representative images and quantification of migrated RM1 cells were obtained after 24 h of co-culture (n = 5 biologically independent samples). e RT-qPCR analysis of pro-migratory cytokines Ccl2 and pro-proliferative cytokine Il23a in WT BMDMs and TREM2 KO BMDMs co-cultured with RM1 cells plus 10 μM ENZA for 48 h (n = 6 biologically independent samples). Gene expression was normalized to Actb expression. f Representative images of Transwell migration assays (with three technical replicates) of RM1 cells cultured in normal medium alone or co-cultured with WT BMDMs at a ratio of 1:1, or co-cultured with WT BMDMs and treated with 1 ug/ml blocking antibodies against CCL2, CCL7, and CCL13. Cells that penetrated the membrane after 24 h of culture were stained using crystal violet. The migration ability of the cells was quantified using the optical density (OD) of the crystal violet-stained cells (n = 3 independent experiments). The schematics in (a−d, f) are created in Biorender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. g WT BMDMs were co-cultured with RM1 cells at a ratio of 1:1 plus blocking antibody against Ki67 in Transwell systems for 48 h, RT-qPCR was performed to detect the mRNA levels of Ki67 in RM1 cells (n = 6 biologically independent samples). h ELISA analysis of CCL2 and IL-23 in the supernatant of WT BMDMs and TREM2 KO BMDMs treated with RM1 CM plus 10 μM ENZA for 48 h (n = 5 biologically independent samples). i A genomic view of AR enrichment on IL23A and CCL2 promoters in THP-1 cells was analysed from published ChIP-seq data14. AR peaks under DMSO and 10 nM R1881 conditions are depicted in blue and red, respectively. j Prediction of AR binding sites on the Ccl2 (left) and Il23a (right) promoters through the JASPAR database. k The specific binding of Ar to the predicted binding site in Ccl2 and Il23a promoters in WT BMDMs treated with RM1 CM was detected by ChIP-qPCR (n = 6 biologically independent samples). l The binding of Ar to the Ccl2 and Il23a promoters in WT BMDMs and TREM2 KO BMDMs treated with RM1 CM was detected by ChIP-qPCR (n = 6 biologically independent samples). Relative fold enrichment was normalised relative to IgG and WT BMDM group, respectively. m The binding of Ar to the Ccl2 and Il23a promoters in WT BMDMs treated with RM1 CM plus 10 μM ENZA for 48 h was detected by ChIP-qPCR (n = 6 biologically independent samples). Relative fold enrichment was normalised relative to IgG and CTL group, respectively. All the data are presented as mean ± SD. The P-values were determined by two-way ANOVA with Tukey’s multiple comparisons for (a−e, h); by two-way ANOVA with Sidak’s multiple comparisons for (k); by one-way ANOVA with Tukey’s multiple comparisons for (f); and by two-sided Mann-Whitney U test for (g−m). Source data are provided as a Source Data file.
Given AR’s potentially regulatory influence on the pro-proliferative and pro-migratory factors IL-23 and CCL2 expression, we investigated whether AR, a crucial transcription factor in prostate cancer, has a direct regulatory effect on IL-23 and CCL2 at the transcriptional level. By analysing AR-enriched ChIP-seq data14, we observed the binding of AR to the IL23A and CCL2 promoters in THP-1 cells, which was enhanced after AR agonist R1881 treatment (Fig. 5i). Subsequently, we predicted the potential Ar-binding sites in Il23a and Ccl2 promoters using the JASPAR database (https://jaspar.genereg.net/) (Fig. 5j). ChIP-qPCR experiments confirmed the specific binding of Ar to the predicted binding site of the Il23a and Ccl2 promotors in RM1 CM-treated BMDMs (Fig. 5k). However, the specific binding of Ar to the Ccl2 and Il23a promotors was attenuated in TREM2 KO BMDMs (Fig. 5l), and in ENZA-treated WT BMDMs (Fig. 5m). In conclusion, these results indicate that the TREM2-AR axis significantly enhances AR-mediated transcription of Il23a and Ccl2 in prostate cancer-associated macrophages, leading to increased proliferation and migration of prostate cancer cells.
The TREM2-AR axis correlates with poor prognosis and promotes cancer progression and blockade of the TREM2-AR axis improves the efficacy of anti-PD1 therapy in prostate cancer
Next, we evaluated the significance of our findings in prostate cancer patients. By analysing publicly available bulk RNA-seq data (including TCGA) on GEPIA database, we observed a significant upregulation of AR, TREM2, and APOE transcription levels in prostate tumour samples (Fig. 6a). Additionally, in patients with prostate cancer, increased expression of AR, TREM2, and APOE was associated with the poorer disease-free survival (Fig. 6b). Furthermore, in prostate cancer patients, the simultaneous elevation of any two or all three of AR, TREM2, and APOE is associated with the poorer disease-free survival rate compared to single elevation, as reflected by a higher hazard ratio (HR) (Supplementary Fig. 7a), suggesting that combination of these indicators demonstrate superior predictive efficacy. However, the three-signature combination was not better than the two-signature combinations, possibly due to the effect caused by other pathways of the three molecules exhibiting cross-reactivity or interference phenomena. Moreover, elevated AR, TREM2 and APOE were also associated with reduced progression-free survival in patients with metastatic prostate cancer (Supplementary Fig. 7b). Additionally, high expression of AR-regulated genes, such as TGFB1, IL10, IL23A, and CCL2, was also correlated with poorer disease-free survival (Supplementary Fig. 7c). These results suggest that AR, TREM2 and APOE are associated with poor prognosis in prostate cancer patients. To further explore the influence of macrophage TREM2-AR axis in prostate cancer progression, we utilized macrophage-specific TREM2 knockout (TREM2f/f-Lyz2-cre) mice, which lack the ability to activate downstream TREM2 signalling in macrophages (Supplementary Fig. 8a). TREM2f/f and TREM2f/f-Lyz2-cre mice were subcutaneously injected with mouse prostate cancer cells RM1 as an ectopic prostate cancer model, followed by surgical castration (CTX) or sham operation on day three and ENZA treatment on day five (Fig. 6c). The results showed that the tumours in TREM2f/f-Lyz2-cre mice were smaller (Fig. 6d, e) and grew more slowly (Fig. 6f) compared to those in TREM2f/f mice. Furthermore, compared to TREM2f/f mice, TREM2f/f-lyz2-cre mice exhibited a greater reduction in tumour weight following either CTX or CTX combined with ENZA treatment, and in TREM2f/f-lyz2-cre mice, CTX combined with ENZA treatment almost completely inhibited tumour growth, which was more effective than CTX alone (Fig. 6e). To investigate the effects of the TREM2-AR axis on immune cells in vivo, the tumour immune microenvironment was analysed by flow cytometry (Supplementary Fig. 8c). Results indicated that AR expression was reduced in TAMs from TREM2f/f-Lyz2-cre mice compared to TREM2f/f mice (Supplementary Fig. 8b). In addition, lower macrophages infiltration (Fig. 6g and Supplementary Fig. 8e), but higher infiltration of CD8+ T cells (Fig. 6j and Supplementary Fig. 8g) and natural killer (NK) cells (Supplementary Fig. 8d, h) were observed in TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment both proportionally and quantitatively. ELISA results indicated that the protein levels of IL-10 and TGF-β were significantly reduced in the tumour grinding supernatant of TREM2f/f-Lyz2-cre mice following treatment with CTX and ENZA compared to those in TREM2f/f mice (Fig. 6i). Furthermore, the anti-inflammatory M2-like macrophages were reduced, while pro-inflammatory M1-like macrophages were increased in TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment (Fig. 6h and Supplementary Fig. 8f). Moreover, the level of the immunosuppressive checkpoint PD1 was decreased on tumour-infiltrating CD8+ T cells of TREM2f/f-Lyz2-cre mice following treatment with CTX and ENZA (Fig. 6k). At the same time, these tumour-infiltrating CD8+ T cells displayed enhanced antitumour activity, as evidenced by the increased secretions of IFN-γ and TNF (Fig. 6l), granzyme and perforin (Fig. 6m). These results provide direct evidence that macrophages expressing higher levels of TREM2 and AR have a crucial influence on promoting the progression of prostate cancer by promoting anti-inflammatory polarisation of macrophages and impairing the antitumour effects of CD8+ T cells.
Fig. 6. The TREM2-AR axis correlates with poor prognosis and promotes cancer progression and blockade of the TREM2-AR axis improves the efficacy of anti-PD1 therapy in prostate cancer.
a Box plot depicting AR (left), TREM2 (medium) and APOE (right) expression in TCGA bulk RNA-seq samples of human normal prostate (n = 152 biologically independent samples) and prostate cancer samples (PRAD, n = 492 biologically independent samples) from GEPIA database. Box plots show the median (centre line), 25th and 75th percentiles (box bounds), and whiskers extend to the 5th and 95th percentiles which represent minima and maxima. b Disease-free survival based on AR, TREM2 and APOE expression in primary prostate tumours (TCGA) from GEPIA database. the High AR (left) /TREM2 (medium) /APOE (right) group (red) (n = 246 biologically independent samples) corresponds to the first 50% of expression, and the Low AR (left) /TREM2 (medium) /APOE (right) group (blue) (n = 246 biologically independent samples) corresponds to the last 50% of expression. c Experimental scheme. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. C57BL/6 TREM2f/f and TREM2f/f-Lyz2-cre mice were subjected to subcutaneous injections of RM1-Luc cells in the inguinal region. Surgical castration (CTX) was performed after 3 days. ENZA treatment commenced on day five. Mice were euthanized on day 15 for analysis and data collection. The sham group for each genotype serves as its own control. RM1-Luc: luciferase-tagged RM1, s.c.: subcutaneous, CTX: Castration, ENZA: Enzalutamide. d−f Representative prostate tumour size (n = 5 mice) (d), tumour weight (n = 6 mice) (e), and tumour growth curve (n = 5 mice) (f) of each group. g The proportion of CD45+CD11b+F4/80+ macrophages in the tumour were quantified by flow cytometry (n = 5 mice). h The proportion of CD11b+F4/80+CD206+ (left) macrophages and CD11b+F4/80+CD86+ (right) macrophages in the tumour were quantified by flow cytometry (n = 5 mice). i ELISA assay was employed to quantify the protein levels of IL-10 and TGF-β in the tumour griding supernatant of tumour-bearing mice (n = 5 mice). j The proportion of CD45+CD3+CD8+ T cells in the tumour was quantified by flow cytometry (n = 5 mice). k The number of PD1+CD8+ T cells mg-1 in the tumour (n = 5 mice). l The number of IFN-γ+CD8+ T cells mg-1 (left) and TNF+CD8+ T cells mg-1 (right) in the tumour (n = 5 mice). m The proportion of granzyme B (left) and perforin (right) in the intraprostatic CD8+ T cells of tumour-bearing mice (n = 5 mice). (n) Experimental scheme. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. C57BL/6 TREM2f/f and TREM2f/f-Lyz2-cre mice were subjected to subcutaneous injections of RM1 cells in the inguinal region. Surgical castration (CTX) was performed after 3 days. ENZA and anti-PD1 treatment commenced on day five. Mice were euthanized on day 15 for analysis and data collection. o, p Representative prostate tumour size (n = 6 mice) (o), and tumour weight (n = 6 mice) (p) of each group. All the data are presented as mean ± SD. The P-values were determined by two-sided Mann-Whitney U test for (a); by Log-rank (Mantel-Cox) test for (b); by two-way ANOVA with Tukey’s multiple comparisons for (f); and by one-way ANOVA with Tukey’s multiple comparisons for (e, g−m, p). Source data are provided as a Source Data file.
The ICT has limited mono-therapeutic efficacy in prostate cancer, partly due to the presence of immunosuppressive myeloid cells in the tumour25. Based on our findings that TREM2-AR signalling promotes the differentiation of macrophages into an anti-inflammatory phenotype and suppresses CD8+ T cell activity both in vitro and in vivo, we further investigated the efficacy of blocking macrophage TREM2-AR signalling in combination with ICT. In the mouse model of prostate cancer treated with castration therapy and ENZA, we incorporated anti-PD1 treatment (Fig. 6n). The results indicated that, in TREM2f/f mice, the anti-PD1 therapy did not demonstrate significant anti-tumour effects and exhibited resistance (Fig. 6o, p), while in TREM2f/f-Lyz2-cre mice, anti-PD1 treatment significantly inhibited tumour progression (Fig. 6o, p), suggesting that the blockade of macrophage TREM2-AR signalling can improve the efficacy or even reverse resistance to anti-PD1 therapy.
Selective inhibition of TREM2-AR axis suppresses the progression and metastasis and recurrence of prostate cancer in vivo
We proceeded to evaluate the impact of the TREM2-AR axis on prostate cancer metastasis and recurrence. In the intraprostatic injection model39, surgical castration or sham operation was performed on day three after (luciferase-tagged RM1) RM1-luc cell injection, followed by ENZA treatment on day five (Fig. 7a). Compared to TREM2f/f mice, AR expression was reduced on TAMs of TREM2f/f-Lyz2-cre mice (Supplementary Fig. 9a). Dynamic monitoring of tumour growth using IVIS showed slower tumour growth in TREM2f/f-Lyz2-cre mice than in TREM2f/f mice; further deceleration of tumour growth was observed in TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment (Fig. 7c). Furthermore, in TREM2f/f-Lyz2-cre mice, the survival period markedly prolonged (Fig. 7b), and the metastases in lymph nodes, lungs, and livers significantly decreased after combination therapy with CTX and ENZA, followed with no significant changes in heart, spleen, and kidney (Fig. 7d and Supplementary Fig. 9b), suggesting that the TREM2-AR axis plays a facilitating role in the metastasis of prostate cancer. Over 40% of intermediate or high-risk patients with prostate cancer experience biochemical recurrence after radical prostatectomy40. To investigate the impact of the macrophage TREM2-AR axis on biochemical recurrence after radical prostatectomy, we established a subcutaneous prostate cancer model in TREM2f/f mice and TREM2f/f-Lyz2-cre mice as previously reported41. Three days after subcutaneous injection of RM1 cells, when the tumour volume reached ~50 mm³, radical prostatectomy was performed, and surgical castration or sham operation commenced 2 days post-resection (Fig. 7e). The results showed that inhibition of the macrophage TREM2-AR axis suppressed the growth of recurrent RM1 tumours, and surgical castration further reduced the tumour size (Fig. 7f, g) and inhibited the growth rate of recurrent tumours (Fig. 7h), suggesting that macrophage TREM2-AR axis inhibition may also attenuate biochemical recurrence of prostate cancer after radical prostatectomy.
Fig. 7. Selective inhibition of TREM2-AR axis suppresses the progression and metastasis and recurrence of prostate cancer.
a Experimental scheme. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. RM1-Luc cells were intra-prostatically injected into C57BL/6 TREM2f/f and TREM2f/f-Lyz2-cre mice. Surgical castration was performed after 3 days. ENZA treatment commenced on day five. IVIS analysis of mice was performed every 3 days starting from day eight. Mice were euthanized on day 17 for analysis and data collection. b Survival curve of mice intra-prostatically injected with RM1-Luc cells (n = 10 mice). c Representative IVIS fluorescence images and ROI quantification of prostate tumours in mice of each group (n = 3 mice). d Representative IVIS fluorescence images and ROI quantification of the lymph node, lung, and liver in tumour-bearing mice (n = 3 mice). e Experimental scheme. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. RM1-Luc cells were subcutaneously injected into C57BL/6 TREM2f/f and TREM2f/f-Lyz2-cre mice. Radical resection was performed to remove the tumour on day three. Surgical castration was performed on day five. IVIS analysis of mice was conducted every 5 days starting from day eight. Mice were euthanized on day 18 for analysis and data collection. f, g Representative recurrent prostate tumour images (f) and tumour weight (g) of each group (n = 5 mice). h Representative IVIS fluorescence images and ROI quantification of recurrent prostate tumours in mice (n = 3 mice). i Experimental scheme. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. BMDMs from C57BL/6 TREM2+/+ CD45.2 mice and C57BL/6 TREM2-/- CD45.2 mice were injected into CD45.1 mice via the tail vein, and RM1-Luc cells were subcutaneously injected into the inguinal region of the mice 24 h later. Surgical castration was performed after 3 days. ENZA treatment commenced on day five. IVIS analysis of mice was performed every 4 days starting from day seven. Mice were euthanized on day 15 for analysis and data collection. j, k Representative prostate tumour images (j) and tumour weight (k) of each group (n = 5 mice). RM1-Luc: luciferase-tagged RM1, s.c.: subcutaneous, CTX: Castration, ENZA: Enzalutamide, IVIS: In vivo imaging system, BMDM: Bone marrow-derived macrophages. All the data are presented as mean ± SD. The P-values were determined by Log-rank (Mantel-Cox) test for (b); by two-way ANOVA with Tukey’s multiple comparisons for (c, h); and by one-way ANOVA with Tukey’s multiple comparisons for (d−k). Source data are provided as a Source Data file.
To further explore the functional contribution of TREM2+AR+ immunosuppressive macrophages to tumour progression, we established a co-injection model by intravenously injecting sorted WT BMDMs or TREM2 KO BMDMs derived from CD45.2 mice into CD45.1 tumour-bearing mice, which were subcutaneously injected with RM1 cells one day before, followed by surgical castration or sham operation and ENZA treatment (Fig. 7i and Supplementary Fig. 9d). In this model, TREM2 KO BMDMs represented a selective inhibition of TREM2 and AR expression compared to WT BMDMs as indicated in (Fig. 2a, b and Supplementary Fig. 4a, b) and served as an ideal model to explore macrophage function42,43. In the BMDMs-transferred mice, adoptive transfer of TREM2 KO BMDMs resulted in reduced prostate tumour size (Fig. 7j, k) and slowed tumour growth (Supplementary Fig. 9c). Importantly, the proportion (Supplementary Fig. 9e, f) and quantity (Supplementary Fig. 9g) of CD45.2+ macrophages infiltrating the tumours was lower in the TREM2 KO BMDMs-transferred mice than in the WT BMDMs-transferred mice. Similarly, the tumour immune microenvironment in mice treated with both CTX and ENZA after TREM2 KO BMDMs injection was improved, as evidenced by a reduction in infiltrating anti-inflammatory M2-like macrophages, but an increase in pro-inflammatory M1 macrophages (Supplementary Fig. 9h), and an increasement in the infiltration of CD8+ T cells and NK cells (Supplementary Fig. 9i). These data indirectly demonstrates that a relatively small proportion of TREM2 KO BMDMs infiltration can exert effective antitumour effects, and the macrophage TREM2-AR axis acts as a promoter of tumour growth. Therefore, selective inhibition of TREM2-AR in macrophages can suppress the progression and metastasis and recurrence of prostate cancer in vivo.
Blockade of TREM2-AR axis enhances the efficacy of standard therapy for prostate cancer in situ
To further assess the therapeutic implications of our findings, PtenPC-/- mice were used, which serves as an ideal model for studying spontaneous prostate cancer19. We generated PtenPC-/- TREM2f/f-Lyz2-cre mice and subsequently evaluated whether blocking the macrophage TREM2-AR axis could reverse castration resistance in vivo. The AR antagonist ENZA serves as the standard of care for CRPC patients following initial ADT but facing treatment resistance3, and we assessed whether macrophage TREM2-AR axis blockade had an effect on alleviating or reversing ENZA resistance (Fig. 8a). Our preclinical studies indicated that genetical mutation and pharmacological blockade of the macrophage TREM2-AR axis enhanced the efficacy of castration therapy and ENZA treatment in prostate cancer as evidenced by reduced tumour volume (Fig. 8b, c). Notably, in PtenPC-/- TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment, we observed normalization of prostate (Fig. 8d, e), and decreased tumour proliferation (Fig. 8d, f), whereas the efficacy of treatment was suboptimal in PtenPC-/- TREM2f/f mice post CTX and ENZA treatment. The immunosuppressive factors IL-10 and TGF-β, and the pro-proliferative factor IL-23 and pro-migratory factor CCL2 were decreased in the tumour grinding supernatant of PtenPC-/- TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment had lower levels of these factors (Fig. 8g and Supplementary Fig. 10a). To investigate the effect of the TREM2-AR axis on immune cells in situ, the tumour immune microenvironment was analysed by flow cytometry (Supplementary Fig. 10b). Results indicated that compared to PtenPC-/- TREM2f/f mice, AR expression was reduced in TAMs of PtenPC-/- TREM2f/f-Lyz2-cre mice (Supplementary Fig. 10c). Further analysis of immune cell composition within tumours revealed reduced macrophage infiltration (Fig. 8h and Supplementary Fig. 10d), but increased CD8+ T cell (Fig. 8k and Supplementary Fig. 10f) and NK cell infiltration (Fig. 8j and Supplementary Fig. 10g) in PtenPC-/- TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment both proportionally and quantitatively. Furthermore, the infiltration of anti-inflammatory M2-like TAMs were decreased, but pro-inflammatory M1-like TAMs were increased significantly in PtenPC-/- TREM2f/f-Lyz2-cre mice post CTX and ENZA treatment (Fig. 8i and Supplementary Fig. 10e). Moreover, the level of PD1 was decreased on tumour-infiltrating CD8+ T cells of PtenPC-/- TREM2f/f-Lyz2-cre mice following treatment with CTX and ENZA (Fig. 8l). At the same time, these tumour-infiltrating CD8+ T cells displayed enhanced antitumour activity, as evidenced by the increased secretions of IFN-γ and TNF (Fig. 8m), granzyme B and perforin (Fig. 8n). In summary, these data suggest that blocking the macrophage TREM2-AR axis can mitigate or even reverse castration resistance in prostate cancer and improve the effectiveness of ENZA.
Fig. 8. TREM2-AR axis blockade enhances the efficacy of standard therapy for prostate cancer by improving the immunosuppressive TME in situ.
a Experimental scheme. Created in BioRender. Qiaohua, W. (2025) https://BioRender.com/ly3agho. PtenPC-/- TREM2f/f and PtenPC-/- TREM2f/f-Lyz2-cre mice were surgically castrated at week eight. ENZA was administrated 12 weeks after surgical castration when castration resistance was observed. Prostate tumour volume was monitored by magnetic resonance imaging (MRI) at weeks 12 and 24. Mice were euthanized at week 24 for analysis and data collection. CTX: Castration, ENZA: Enzalutamide, MRI: Magnetic resonance imaging. b Representative MRI scans of mice in each group at weeks 12 and 24. The red circle highlights the area of prostate carcinoma. c Waterfall plots depicting the proportional change in tumour response at weeks 12 and 24 (n = 3 mice). d Representative haematoxylin and eosin (H&E) and Ki67 staining at the observation endpoint. Scale bar: 40 μm. e Quantitative analysis of adenocarcinoma, prostatic intraepithelial neoplasia (PIN), or normal-like glands at the observation endpoint in each group (n = 3 mice). f Percentage of Ki-67+ cell area relative to total area (n = 3 mice). g ELISA assay was employed to quantify the protein levels of IL-10 (left) and TGF-β (right) in tumour griding supernatant of mice (n = 5 mice). h The proportion of CD45+CD11b+F4/80+ macrophages in the tumour were quantified by flow cytometry (n = 5 mice). i The proportion of CD11b+F4/80+CD206+ (up) macrophages and CD11b+F4/80+CD86+ (down) macrophages in the tumour were quantified by flow cytometry (n = 5 mice). j The proportion of CD45+CD3-NK1.1+ NK cells in the tumour was quantified by flow cytometry (n = 5 mice). k The proportion of CD45+CD3+CD8+ T cells in the tumour was quantified by flow cytometry (n = 5 mice). l The number of PD1+CD8+ T cells mg-1 in the tumour (n = 5 mice). m The number of IFN-γ+CD8+ T cells mg-1 (left) and TNF+CD8+ T cells mg-1 (right) in the tumour (n = 5 mice). n The number of Granzyme B+CD8+ T cells mg-1 (left) and Perforin +CD8+ T cells mg-1 (right) in the tumour (n = 5 mice). For b and d, experiments were repeated three times independently with similar results. All the data are presented as mean ± SD. The P-values were determined by two-way ANOVA with Tukey’s multiple comparisons for (c, e); and by one-way ANOVA with Tukey’s multiple comparisons for (f–n). Source data are provided as a Source Data file.
Discussion
The AR in prostate cancer cells acts as a central transcription factor, playing a crucial role in maintaining tumour cell survival and the production of prostate-specific antigen (PSA)3. The expression of AR in tumour cells is typically regulated through the STAT3-ROR-γ signalling pathway3. The androgen deprivation therapy (ADT) and next-generation ARSIs such as the AR antagonist ENZA serves as a primary treatment for prostate cancer by inhibiting the androgen-AR pathway in tumour cells44,45. However, androgen-deprived condition also leads to changes in the TME16,46. For instance, androgen-deprived condition triggers an increase in IL-23 secretion in MDSCs, thereby promoting resistance to ADT16. In recent years, the role of AR in tumour-infiltrating immune cells has received extensive attention. The AR antagonist ENZA can suppress immune responses by inhibiting activation of initial T cells, and inhibiting IFN-γ production47. Additionally, AR suppresses TCF1/TCF7 transcription, leading to exhaustion of CD8+ T cells48. In TAMs, AR inhibited the transcriptional of IL1B, leading to the deterioration of the immunosuppressive microenvironment15. AR signalling in macrophage-like THP-1 cell line enhances TREM1 signalling and downstream cytokines expression to promote the migration and invasion of prostate cancer cells14. Here, analysis of flow cytometry from human prostate cancer showed that AR is significantly activated in TAMs, which was consistent with previous study15. Nevertheless, the upstream and downstream regulatory mechanisms of AR signalling in TAMs remain largely unknown.
The findings of this research reveal a notable increase in macrophages that co-express AR and TREM2 in prostate cancer, and these macrophages acquire a typical anti-inflammatory M2-like TAMs phenotype in both mouse and human TME. ChIP-seq and ChIP-qPCR analyses confirm that AR acts as a transcriptional activator for genes such as Il10, Tgfb1, Il23a, and Ccl2. Both in vitro and in vivo studies involving gene inactivation or pharmacological blockade of TREM2 or AR consistently indicate that the TREM2-AR axis in macrophages reprograms their secretome by activating the AR-IL-10/TGF-β/IL-23/CCL2 axes, thereby exhibiting profound immunosuppressive and tumour-promoting effects. Specifically, IL-10 and TGF-β serve as immunosuppressive factors which inhibit T cell-mediated antitumour effects49. Additionally, IL-23 can promote tumour cell proliferation while CCL2 can promote tumour cell migration14,16. Findings from liquid chromatography-mass spectrometry, Co-IP experiments, multiplex immunofluorescence, and in vitro gene inactivation or pharmacological blockade of TREM2 and APOE confirmed the role of APOE and TREM2 interaction in inducing AR expression in macrophages via the DAP12-Src-Syk-STAT3-ROR-γ pathway. Collectively, our work reveals several key cytokines that are under direct regulation by the AR pathway in TAMs, providing fresh perspectives on how AR affects TAMs and other immune cells in the TME.
The RNA-seq data revealed that TREM2 was a key regulator of AR. TREM2 is increasingly recognized as a critical receptor in a variety of inflammatory diseases and tumour models21. Recent evidence suggests that activating TREM2 in TAMs leads to tumour immune escape and treatment resistance50. In prostate cancer, APOE secreted by prostate cancer cells binds to TREM2 on neutrophils and promotes their senescence, exhibiting tumour-promoting effects19. In TREM2 KO BMDMs, the expression of AR was downregulated, and we observed a significant upregulation of TREM2 and AR in prostate cancer-associated macrophages. TREM2 lacks intracellular signalling transmitting motifs and primarily transmits signals through the recruitment of the protein tyrosine kinase Syk by the adaptor protein DAP1251,52. Dysregulation of the TREM2-DAP12 signalling is associated with pathogenesis of neurodegenerative diseases and cancer53,54. Accordingly, there was an interaction between TREM2 and APOE, and APOE enriched in TME activates the TREM2-DAP12-Src-Syk-STAT3-ROR-γ pathway, promoting AR expression in macrophages, and inducing immunosuppressive and pro-tumoral phenotypes. Inhibition of TREM2 or AR in macrophages results in the conversion of the immunosuppressive phenotype to an immunostimulatory phenotype. Given that soluble APOE is primarily secreted by macrophages and tumour cells, and that macrophage-derived APOE may predominantly regulate the autocrine modulation of TREM2, we acknowledge that although we observed higher levels of APOE in RM1 CM compared to BMDM CM in vitro, we are unable to definitively demonstrate which cell-derived APOE plays a more dominant role in regulating TREM2 in vivo, which needs further investigation. Furthermore, pharmacological inhibition of STAT3-ROR-γ suppressed AR expression in macrophages, indicating the STAT3-ROR-γ signalling axis is necessary for AR expression in macrophages. Collectively, these data suggest that the binding of APOE to TREM2 promotes the activation of DAP12-Src-Syk-STAT3-ROR-γ pathway, which is subsequently required for inducing AR expression. However, we do not exclude that there may be other important downstream regulators other than AR regulated by TREM2, which have not been identified in this study yet.
Multiplex immunofluorescence (TSA technology) on human prostate tumour samples revealed that TREM2+AR+ macrophages were mainly anti-inflammatory M2-like macrophages and were enriched in the tumour regions. Moreover, TREM2+AR+ macrophages were predominantly enriched in the tumour stroma, where they may interact with tumour cells and other immune cells such as T cells, and be associated with immunosuppression or treatment resistance55. Therefore, this persistent macrophage subpopulation may be a particularly attractive therapeutic target for prostate cancer. This finding further advances the prospects for targeted therapies against immunosuppressive macrophages, suggesting that in tumours, simultaneous inhibition of TREM2 and AR in macrophages should be pursued to improve the efficacy of standard treatments. Using various mouse models of prostate cancer, we observed that genetic blockade of TREM2 in macrophages inhibited the downstream AR signalling, thereby significantly enhancing the efficacy of treatment regimens based on the AR antagonist ENZA. More importantly, AR, TREM2, and APOE expressions increase in prostate cancer and are associated with poor prognosis in TCGA RNA-seq dataset. Although our data identified a correlation between APOE, TREM2, and AR in prostate tumours, direct functional evidence—such as genetic perturbation (e.g., knockout or inhibition of APOE/TREM2) in prostate cancer models—will be essential to confirm their roles. Future studies are needed to explore how APOE-TREM2 interaction modulates tumour progression in vivo.
In recent years, ICT has been approved for the treatment of various types of cancer, including prostate cancer23. However, ICT is only effective for a limited number of molecular subtypes in prostate cancer24, partly due to the presence of immunosuppressive myeloid cells25, and a lower density of T cells in the TME26. In addition, in patients with mCRPC, anti-PD-L1 treatment combined with enzalutamide did not improve overall survival, which is associated with low CD8+ T cell infiltration in tumour tissue, low PD-L1 expression in immune cells, low tumour mutational burden (TMB), and a low incidence of microsatellite instability-high (MSI-H) status56,57. Notably, myeloid cells, particularly TAMs and MDSCs, mediate immunosuppression in prostate cancer through various mechanisms58,59, potentially contributing to ICT resistance. For instance, Lyu A et al25. recently discovered that a population of immunosuppressive macrophages with high expression of SPP1 promotes resistance to ICT in mice by suppressing CD8+ T cell activity. Consequently, precise blockade of immunosuppressive macrophages may represent an effective strategy to enhance the efficacy of ICT. Our findings demonstrate that TREM2 deficiency in macrophages, in combination with ENZA, enhances the efficacy of anti-PD1 therapy and even reverses resistance to anti-PD1 treatment, probably associated with the polarisation of pro-inflammatory macrophages and increased infiltration and anti-tumour activity of CD8+ T cells induced by TREM2-AR axis blockade. These results suggest that more effective immunotherapeutic interventions necessitate simultaneous targeting of other immunosuppressive components within the TME. Therefore, a better understanding of myeloid cells and other immunosuppressive TME elements, as well as their roles in ICI resistance, may reveal additional therapeutic opportunities for prostate cancer.
Regarding the mechanism of TREM2-AR axis-mediated T cell activation, we hypothesize two potential pathways. On the one hand, the blockade of TREM2-AR signalling may promote a shift from anti-inflammatory macrophages to pro-inflammatory macrophages, leading to a reduction in immunosuppressive factors such as IL-10 and TGF-β. These factors are known to inhibit T cell activation27,60, and their reduction could relieve this inhibitory effect. On the other hand, as demonstrated in our previous study61, TREM2 blockade led to an increase in antigen presentation and co-stimulatory signals, ultimately leading to T cell activation. This was characterised by decreased expression of the inhibitory immune checkpoint PD1 and increased secretions of IFN-γ and TNF. However, the precise intracellular signal transduction mechanism underlying T-cell activation in this context remains unexplored and present a promising avenue for future research.
In summary, this study indicates that prostate cancer-associated macrophages represent an attractive AR-responsive cell type, distinct from the previously identified AR-dependent prostate cancer cells. Mechanically, APOE and TREM2 interaction triggers AR expression in macrophages through the DAP12-Src-Syk-STAT3-ROR-γ axis. Our data facilitate a more profound comprehension of the role of immunosuppressive macrophages in cancer, delineating an unexpected characteristic of this immune subset, and propelling the identification of AR in combination with TREM2 as a determinant of macrophage-mediated immunosuppression. The combination therapy introduced in this study, involving macrophage TREM2 blockade along with the AR antagonist ENZA, presents a novel therapeutic approach, broadening the repertoire of myeloid cell targets for prostate cancer and could further enhance the efficacy of standard therapy and immunotherapy for this cancer (Supplementary Fig. 11).
Methods
Ethics statement
Blood samples and tumour tissues were obtained from donors who provided informed consent in accordance with ethical guidelines and regulations as defined by the Medical Ethics Review Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (approval number K161-1). Experiments in mice were performed in accordance with the guidelines of the Animal Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (authorization number 00417).
Human prostate and blood samples
Patients with prostate cancer were recruited to the study team after initial evaluation by their treating physicians from the Department of Urology at the Fifth Affiliated Hospital of Sun Yat-sen University. A total of 31 healthy individuals were recruited to the study team after initial evaluation by their treating physicians from the Health Management Centre. This study was free of any self-selection bias or other bias. A total of 53 prostate cancer blood specimens and 6 prostate cancer tissue samples of radical prostatectomy from patients who visited the Fifth Affiliated Hospital of Sun Yat-sen University from April 2022 to April 2024 were included. These samples were confirmed to have matching histological diagnoses, processed as formalin-fixed paraffin-embedded (FFPE) diagnostic slides, and reviewed and confirmed by two pathologists. Comorbidities affecting outcomes (e.g., prostatitis and prostatic hyperplasia) were excluded. The detailed clinical characteristics of all the subjects are shown in Supplementary Table 2.
Mouse models
All mice were housed under specific pathogen-free (SPF) conditions in individually ventilated cages (IVCs) facilities, and experimental/control animals were co-housed in groups of 5 per cage to standardize microenvironmental factors. Individual identification was achieved by ear punching. The mice were housed in an environment maintained at ~25 °C with 50% relative humidity and subjected to a 12 h light-dark cycle. They had free access to standard laboratory chow and water. C57BL/6 wild-type (WT) mice, CD45.1 mice, TREM2-KO mice, or Lyz2-cre mice were purchased from Jackson Laboratory. C57BL/6 TREM2-flox/flox (TREM2f/f) and DAP12 KO mice were purchased from Shanghai Model Organisms (Shanghai, China), and PtenPC-/- mice were purchased from Jiangsu GemPharmatech LLC. (Jiangsu, China). All mice were on a C57BL/6 background and acclimatized for at least 1 week prior to use (6–8 weeks old). Macrophage-specific TREM2 knockout mice (TREM2f/f-Lyz2-cre) were generated by crossing TREM2-flox/flox mice with Lyz2-cre mice, and their F1 heterozygotes were inter-crossed to yield F2 homozygotes61. PtenPC-/- TREM2f/f-Lyz2-cre mice were generated by crossing PtenPC-/- mice with TREM2f/f-Lyz2-cre mice, and their F1 heterozygous offspring were inter-crossed to generate F2 homozygous progeny. All mice were genotyped by PCR and agarose gel electrophoresis in TBE buffer (Solarbio, T1053-1L) using genomic DNA extracted from tail biopsy tissues.
In the syngeneic transplantation experiments, either 2 × 105 or 5 × 104 RM1 cells or RM1-Luc cells in matrix gel (ABW, 082726) were injected subcutaneously or intra-prostatically into 6-week-old male C57BL/6 TREM2f/f or TREM2f/f-Lyz2-cre mice. Subcutaneous RM1 syngeneic transplants underwent castration surgery or sham operation 3 days post-injection when the tumours reached ~100 mm3 and were randomly assigned to treatment groups. In the intraprostatic RM1 syngeneic transplants, tumour growth was monitored every 2 days using IVIS imaging 8 days post-injection. For the recurrent mouse model, RM1 tumour cells were inoculated subcutaneously in mice. When the tumour volume reached ~100 mm3, the tumour was excised.
For BMDMs transfer experiments, 5 × 105 sorted BMDMs from WT or TREM2-/- mice were intravenously injected via tail vain into 6-week-old male C57BL/6 CD45.1 mice, and after 24 h, a subcutaneous RM1 syngeneic transplant model was established.
In the spontaneous in situ prostate cancer experiments, 8-week-old male C57BL/6 PtenPC-/- mice were castrated or sham-operated at 8 weeks of age, received ENZA treatment at 20 weeks of age, were observed by magnetic resonance imaging (MRI) for tumour growth at 12 and 24 weeks of age, and euthanized at 24 weeks of age.
Surgical castration or sham operation was performed under isoflurane anaesthesia, with post-operative monitoring of anaesthesia recovery and daily checks for 3 days post-surgery. Mice receiving enzalutamide (MedChemExpress, MCE, HY-70002) treatment (30 mg/kg) were orally administrated five times per week, and the untreated group received the control solvent DMSO. Mice receiving anti-PD1 (Clone RMP1-14, BE0146, BioXcell) treatment (200 μg per mouse) were intraperitoneally administrated every 3 days, and the untreated group received the isotype control rat IgG (Clone 2A3, BE0089, BioXcell) treatment (200 μg per mouse).
Tumour-bearing mice were monitored every 3 days for volume and clinical signs at the same time. The formula for calculating tumour volume is: length × width2/2, a strict upper limit of 1500 mm³ was set on tumour volume. For the in situ, metastatic and spontaneous prostate cancer models, no specific maximum tumour size was imposed, provided that animals were euthanized as soon as they reached a predetermined humane endpoint (experiencing pain during treatment or weight loss exceeding 15%), thus prioritizing their welfare. No animal in this study exceeded that limit. Mice were humanely euthanized by CO2 asphyxiation (flow rate 30% chamber volume/min) at the endpoint of each study. All experiments were performed using male mice, as prostate cancer primarily affects males and requires androgen signalling for tumour development. Organisms used in this study are listed in Supplementary Data 1. Chemicals and Recombinant Proteins used in this study are listed in Supplementary Data 2.
Cell culture
Mouse prostate cancer cells RM1 (Cat# CRL-3310; RRID: CVCL_B459), human prostate cancer cells 22Rv1 (Cat# CRL-2505; RRID: CVCL_1045) and LnCap (Cat# CRL-1740; RRID: CVCL_0395), human monocytic-leukemia cells THP1 (Cat# TIB-202; RRID: CVCL_0006), and mouse fibroblasts L929 (Cat# CCL-1; RRID: CVCL_AR58) were purchased from ATCC and cultured in accordance with the instructions provided by the manufacturer. Cells were cultured in DMEM (Procell, PM150210-HR) or RPMI 1640 medium supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at a control temperature of 37 °C and with 5% CO2, and were subsequently employed in experiments at early passaging. Mycoplasma was detected negative in all cell lines. Cell lines used in this study are listed in Supplementary Data 1.
Murine in vitro-derived bone marrow-derived macrophages (BMDMs)
BMDMs were differentiated from tibial and femoral bone marrow precursor cells of WT or TREM2-KO male mice. Bone marrow precursor cells were cultured in DMEM supplemented with 30% v/v L929 conditioned media and 10% FBS and 1% P/S medium. On day 4, fresh complete medium containing 30% v/v L929 conditioned medium was replaced and used for in vitro experiments after 3 days.
Prostate cancer cells and L929 cells conditioned medium (CM) collection
RM1 cells were cultured in DMEM, and 22Rv1 or LnCap cells were cultured in RPMI 1640 supplemented with 10% FBS (heat-inactivated) in cell culture dishes (NEST Biotechnology, 715001). Upon reaching ~40% confluence, fresh complete medium was added. After 3 days, the conditioned medium (CM) was centrifuged at 4 °C at 500 g, 2000g, and 4600 g for 5 min, 10 min, and 20 min, respectively, to eliminate debris and dead cells. Subsequently, the supernatant was transferred into 15 ml centrifuge tubes and preserved at −80 °C. Approximately 4.8 × 105 L929 cells were seeded into T75 flasks (NEST, 709013) and cultured in DMEM containing 10% heat-inactivated FBS and 1% P/S for 7 days. Afterward, the CM was centrifuged at 4 °C at 4000 g for 20 min to eliminate debris and dead cells. Then the supernatant was then aliquoted into 50 ml centrifuge tubes and frozen at −80 °C.
In vitro T cell proliferation and function assays
Primary splenocytes labelled with 5 mM carbyfluorescein succinimidyl ester (CFSE) (Thermo Fisher Scientific, C34750) were cultured in 96-well U-bottom plates (Corning, 7007), followed by in vitro activation using anti-CD3 and anti-CD28 antibodies (BioLegend, 100253/102132). In vitro-induced WT or TREM2 KO BMDMs and RM1 cells were co-cultured with the splenocytes. Three days post-incubation, the proliferation of CFSE-labelled CD8+ T cells was examined utilizing the Attune NxT flow cytometer (Thermo Fisher Scientific). Additionally, primary splenocytes and in vitro-induced WT or TREM2 KO BMDMs and RM1 cells were co-cultured in 96-well U-bottom plates. After 2 days, in vitro activation was performed for 6 h with 120 ng/ml PMA (Sigma-Aldrich, P8139) and 1 µg/ml ionomycin (Merck millipore, 407952). The secretion of granzyme B and perforin by CD8+ T cells was examined by flow cytometry. Details of the antibodies are given in the Supplementary Data 3.
Transwell migration and proliferation assays
Transwell migration and proliferation assays were performed using Falcon cell culture chambers suitable for 24-well plates with 8.0 µm transparent polyester (PET) membranes (Corning, 353097) to measure prostate cancer cell migration and proliferation. In the migration assay, the bottom chamber was seeded with 5 × 104 WT or TREM2 KO BMDMs or THP1 cells in complete medium with the addition of 10 μM ENZA or anti-TREM2 (1 ug/ml) or αCCL2/CCL7/CCL13 (1 ug/ml), while the top chamber was seeded with 5 × 104 RM1 cells or 22Rv1 cells or Lncap cells. The co-culture was maintained for 24 h, following which the upper chamber was extracted, the cells inside were discarded, and the PET membrane was stained with crystal violet. Images of the PET membranes were captured using the OLYMPUS IX71 inverted microscope (Olympus Corporation). Crystal violet was eluted with acetic acid and the OD values were read at 595 nm using the UV spectrophotometer (Thermo Fisher Scientific). In the proliferation assay, 5 × 04 RM1 cells or 22Rv1 cells or Lncap cells were seeded at the bottom chamber in complete medium with the addition of αIL-23 (1 ug/ml) or 10 μM ENZA or anti-TREM2 (1 ug/ml), while the top chamber was seeded with 5 × 104 WT or TREM2 KO BMDMs or THP1 cells. The co-culture was sustained for 48 h, subsequent to which RM1 cells at the bottom were collected for RT-qPCR analysis.
Chromatin Immunoprecipitation (ChIP)
ChIP-seq data were obtained from GEO: GSE131381, encompassing AR-specific antibody-enriched genomics data obtained from DMSO or R1881 treated THP-1 cells14. The Integrative Genomics Viewer (IGV) was used for visualization.
ChIP-qPCR analysis was performed using the SimpleChIP Enzymatic Chromatin IP Kit (Magnetic Beads) (Cell Signalling Technology, 9003S). WT and TRME2 KO BMDMs were treated with RM1 CM or enzalutamide (APExBio, MDV3100) and incubated with 1% formaldehyde (Sigma, 252549) for 10 min at room temperature. The cells were subsequently rinsed three times with ice-cold DPBS (Dakewe, 6062011). Cytoplasmic protein extraction was carried out at 4 °C for 10 min using 1 ml buffer 1 (protease and phosphatase inhibitor cocktail (PIC), 2 mM EDTA pH 8.0, 50 mM Tris-HCl pH 8.0, 10% glycerol, and 0.1% Nonidet P-40). The samples were then centrifuged at 2000g at 4 °C for 5 min. Pellets containing nuclei were lysed with 500 μl buffer 2 (PIC, 5 mM EDTA pH 8.0, 50 mM Tris-HCl pH 8.0, and 1% SDS). The nuclear extracts were sonicated using the Scientz-type ultrasonic cell disruptor to achieve an average fragment size of ~150–900 bp for the cross-linked DNA. The lysates were then incubated with AR antibody (Abcam, 4620, 1:50) overnight at 4 °C, acetyl-histone H3 antibody (Cell Signalling Technology, ab108341, 1:50), or normal rabbit IgG (Cell Signalling Technology, 2729, 1:100) with rotation. The following day, the immune complexes were incubated with Protein G Magnetic Beads (Cell Signalling Technology, 9006) at 4 °C for 2 h with rotation. Then thoroughly washed the beads and centrifuged to elute chromatin, followed by a 2 h incubation at 65 °C for reverse cross-linking. DNA was then detected by qPCR (SYBR green system) with ChIP-qPCR primer sequences targeting the mouse Il10, Tgfb1, Ccl2, or Il23a promoter regions (Supplementary Table 3). Each sample was normalized to its corresponding 2% input sample. The relative abundance of chromatin regions bound to Ar was calculated by normalizing against the IgG control. Details of the antibodies are given in the Supplementary Data 3.
Real-time fluorescent quantitative PCR (RT-qPCR)
Total RNA (TRIzol, Ambion, 15596026) was isolated and reverse transcribed to DNA (miScript Reverse Transcription Kit, QIAGEN, 205313) in accordance with the manufacturer’s guidelines. Quantitative PCR (qPCR) reactions were carried out using GoTaq qPCR Master Mix (Promega, A6002) and specific primers. Specific primers are listed in Supplementary Table 3. Relative gene expression was standardized to Actb, and the fold change was calculated employing the ΔΔCt method.
RNA-seq
This study set up two groups of RNA-seq experiments, namely the ASC-J9 vs DMSO group and the TREM2 KO vs WT group. Both experiments utilised mouse BMDMs cultured with RM1 CM. One group was treated with 10 µM ASC-J9 (APExBIO, A3190) or DMSO for 72 h. The other group involved the analysis of TREM2 KO BMDMs and WT BMDMs. The RNA-seq experimental workflow included sample assessment, library construction, quality control, and sequencing, which was performed by Hangzhou Cosmos Wisdom Biotechnology Co., LTD (Hangzhou, China). RNA-seq was conducted on the Illumina NovaSeq 6000 sequencing platform to sequence all mRNA transcripts expressed in cells during a given time period. Library construction was performed using the Illumina TruSeq™ RNA sample preparation kit. The raw data obtained from sequencing contained a small number of reads with sequencing adapters or low-quality sequences. To ensure the quality and reliability of the data analysis, the raw data was filtered using the fastp software62, with the following criteria: removal of reads containing adapters; removal of reads with ambiguous bases (represented as N); and removal of low-quality reads (where the number of bases with Qphred ≤ 20 constituted 50% or more of the total read length). In this project, FoldChange > 2 and padi < 0.05 were used as differentially expressed genes (DEGs) screening criteria. DESeq2 software was used for differential analysis. DEGs were subjected to KEGG pathway enrichment analysis using clusterProfiler.
Characterization of immune cells in human and mouse prostate cancer tumours and peripheral blood by flow cytometry
Tissues were dissociated and digested with 5 µg/ml type IV collagenase (Sigma-Aldrich, 17104019) and 200 U/ml DNAse I (Roche, 4536282001) at 37 °C for 1 h to acquire single-cell suspensions. Peripheral blood was separated using human lymphocyte separation medium (TBD, LTS1077) to obtain mononuclear cell suspensions. Cell viability was assessed by staining with Fixable Viability Dye eFluor 506 (eBioscience, 65-0866-14, 1: 50). Cells were stimulated with 120 ng/ml PMA and 1 μg/ml ionomycin for 6 h for intracellular cytokine assay. The single-cell suspensions were then stained with fluorescently labelled specific antibodies to identify phenotypes. The antibodies used included anti-CD45, anti-CD14, anti-CD66B, anti-CD15, anti-CD68, anti-CD86, anti-CD206, anti-Ly-6G, anti-Ly6C, anti-NK1.1, anti-CD3, anti-CD4, anti-CD8, anti-CD11b, anti-F4/80, anti-TREM2, anti-AR, anti-granzyme B, and anti-perforin, and all antibodies were diluted 1: 50. Details of the antibodies are given in the Supplementary Data 3. For example, FITC Anti-Mouse CD3 Antibody[17A2] (eBioscience, E-AB-F1013C, 1: 50). Samples were processed through the NxT flow cytometer (Thermo Fisher Scientific) and subsequent data analysis was conducted using FlowJo software (TreeStar, Ashland, OR, USA).
Cell sorting by flow cytometry
Human prostate tissues were dissociated and digested with 5 µg/ml type IV collagenase (Sigma-Aldrich, 17104019) and 200 U/ml DNAse I (Roche, 4536282001) at 37 °C for 1 h to acquire single-cell suspensions. The single-cell suspensions were then stained with fluorescently labelled specific antibodies (PE/Cyanine7-anti-CD45, FITC-anti-CD11b, APC-anti-CD68) to identify phenotypes, and all antibodies were diluted 1: 50. Samples were processed through the Sony MA900 Flow Sorting Instrument (Sony Corporation, Japan) and subsequent data analysis was conducted using FlowJo software (TreeStar, Ashland, OR, USA). For BMDM sorting, cells were stained with fluorescently labelled specific antibodies (Apccy7-anti-CD45, PE-anti-CD11b, APC-anti-F4/80) to identify macrophages, and all antibodies were diluted 1: 50. Details of the antibodies are given in the Supplementary Data 3.
Immunofluorescence and immunohistochemistry
For immunofluorescence (IF) staining: 1 × 105 WT or TREM2 KO BMDMs were seeded in confocal dishes (SAINING, 1051001), and cells were incubated overnight at 4 °C stained with anti-AR antibody (Abcam, ab108341, 1:100), anti-F4/80 antibody (Thermo Fisher Scientific, 14-4801-82, 1:100), or anti-TREM2 antibody (Sino Biological, 50149-R014-100, 1:100). After washing, cells were then stained with Alexa Fluor 488-conjugated anti-mouse IgG (Thermo Fisher Scientific, A-11059, 1:500) and Alexa Fluor 594-conjugated anti-rabbit IgG (Abcam, ab150080, 1:500) secondary antibodies at room temperature for 1 h. The 40, 6-diamidino-20-phenylindole dichalcogenide (DAPI) (Beyotime, C1002) was employed to counterstain the cell nuclei. Images were performed using a Zeiss 880 confocal microscope (Carl Zeiss). Image processing was carried out using ImageJ software. The percentage of AR+TREM2+/TREM2+APOE+ cells was manually counted and assigned in macrophages, analysing 3-5 fields of view from 6 independent prostate samples.
For multiplex IF staining: Formalin-fixed paraffin-embedded (FFPE) sections underwent staining with anti-TREM2 antibody (R&D, AF1828, 1:100), anti-CD206 antibody (Cell Signalling Technology, 24595, 1:100), and either anti-AR antibody (Abcam, ab108341, 1:100) or anti-APOE antibody (Cell Signalling Technology, 13366, 1:100) at 4 °C overnight. After washing, sections were then stained with Alexa Fluor 488-conjugated anti-mouse IgG (Thermo Fisher Scientific, A-11059, 1:500), Alexa Fluor 594-conjugated anti-rabbit IgG (Abcam, ab150080, 1:500), and Alexa Fluor 647-conjugated anti-rat IgG (Abcam, ab150167, 1:500) secondary antibodies at room temperature for 1 h. The DAPI (Beyotime, C1002) was employed to counterstain the cell nuclei. The entire slide images were captured on the P250 FLASH high-resolution digital pathology slide scanning system (3DHISTEC). Spectral separation of images was performed using CaseViewer software (3DHISTEC). The HALO image analysis software (Indica Labs) was used for image analysis. Cell analysis initially involved nucleus identification (DAPI staining) to identify cells, followed by measuring the numbers of cells with fluorescence intensity higher than the average intensity threshold above background. The number of positive cells was subsequently employed to delineate co-localization populations, analysing 3-5 fields of view from 6 independent prostate samples.
For tyramide signal amplification (TSA) technology: Detection was performed using the TSAPLus fluorescent pentamer hexachrome staining kit (Servicebio, G1256-50T). FFPE sections were stained overnight at 4 °C using anti-TREM2 antibody (Merck millipore, MABN755, 1:200), anti-CD206 antibody (Servicebio, GB115273, 1:200), anti-AR antibody (Servicebio, GB11253, 1:200), anti-CD86 antibody (Servicebio, GB115630, 1:200), and anti-CD68 antibody (Servicebio, GB113150, 1:200). After the sections were washed, drops of HRP-labelled secondary antibody of the corresponding species to the primary antibody were added to cover the tissues and incubated at room temperature for 50 min. After the sections were washed, drops of the corresponding TSA were added, and the sections were incubated at room temperature for 10 min. after five rounds of the primary antibody-secondary antibody-TSA reaction were completed, the nuclei of the cells were re-stained with DAPI (Beyotime, C1002). The subsequent analytical steps were consistent with those used in multiplex IF staining.
For immunohistochemistry (IHC), tissues were subjected to fixation in 4% paraformaldehyde (Biosharp, BL539A) and embedded in paraffin. Antigen repair was performed by heating the sections with Pepsin Antigen Repair Solution (Servicebio, G0142-500 ML) for 20 min at 100 °C. Following this, the sections underwent blocking to prevent peroxidase and nonspecific antibody binding. Staining of sections was carried out using anti-Ki67 antibody (Cell Signalling Technology, 12202, 1:100) and HRP-conjugated goat anti-rabbit IgG (MCE, HY-P9001-100ul). Images were acquired using the P250 FLASH high-resolution digital pathology slide scanning system (3DHISTEC). Quantification was performed using ImageJ software, analysing 3-5 fields of view from 6 independent prostate samples. Details of the antibodies are given in the Supplementary Data 3.
Western blot analysis and immunoprecipitation
Tissues and cells treated with Mouse Apolipoprotein E/APOE C-6His (NovoProtein, CJ05-10, 100 nM) or others underwent lysis using RIPA buffer (MCE, HY-K1001-100ml) and protein phosphatase inhibitor mixture (Solarbio, P1260-1ml). Total protein concentration was determined using the BCA protein assay kit (Fdbio Science, FD2001) and then boiled in 5x loading buffer (Dakewe, 8015011). Following SDS-PAGE separation of equal amounts of protein, they were transferred onto 0.45 µm PVDF membranes (Roche, 3010040001). The PVDF membranes were subsequently blocked in TBST containing 5% skimmed milk and 0.1% Tween-20, followed by overnight incubation at 4 °C with approximately diluted primary antibodies. The following primary antibodies were used: TREM2 (D8I4C) Rabbit mAb (Cell Signalling Technology, 91068, 1:1000), Androgen Receptor antibody (HUABIO, ER2001-34, 1:1000), Phospho-STAT3 (Tyr705) (D3A7) mAb (Cell Signalling Technology, 9145 T, 1:1000), Src (36D10) Rabbit mAb (Cell Signalling Technology, 2109, 1:1000), Phospho-Src (Tyr419) Antibody (Affinity Biosciences, AF3162, 1:500), Stat3 (79D7) Rabbit mAb (Cell Signalling Technology, 4904, 1:1000), Anti-Syk Antibody (Cell Signalling Technology, 2712, 1:1000), Phospho-Syk (Tyr525/526) (C87C1) mAb (Cell Signalling Technology, 2710, 1:1000), ROR gamma (t) mAb (AFKJS-9) (Thermo Fisher Scientific, 14-6988-80, 1:1000), Anti-beta-Actin mAb, Clone AC-74 (Sigma-Aldrich, A5316, 1:1000), Anti-Apolipoprotein E [EPR19392] (Abcam, ab183597, 1:1000). In the immunoprecipitation experiment, proteins were incubated at 4 °C overnight with TREM2 (D8I4C) Rabbit mAb (Cell Signalling Technology, 91068, 1:50) and Protein G Plus/Protein A Agarose (Merck Millipore, IP05-1.5 mL). The free proteins were then washed off, and western blot analysis was performed. Protein bands were visualized using FDbio-Femto ECL luminescent solution (Fdbio Science, FD8030) with LAS500 ultrasensitive chemiluminescence imager (GE ImageQuant, Massachusetts, USA). The gray values of the protein band were analysed using Fiji Image J software with subsequent normalization of the relative gray value to that of β-actin. Details of the antibodies are given in the Supplementary Data 3. Uncropped and unprocessed scans of the most important blots are provided in the Source Data file.
Mass spectrometry
Human prostate cancer tissues were finely minced with razor blades on ice. The cells obtained by digestion and filtration were lysed with RIPA buffer (Beyotime, P0013) and immunoprecipitated with TREM2 (D8I4C) Rabbit mAb (Cell Signalling Technology, 91068, 1:50) supplemented with Protein G Plus/Protein A Agarose (Merck Millipore, IP05-1.5 mL) overnight at 4 °C. The free proteins were washed and the agarose beads bound to the antibodies were separated and the binding proteins were collected, followed by liquid chromatography–tandem mass spectrometry analysis using LTQ Orbitrap Velos (ThermoFisher Scientific, Waltham, USA).
Magnetic Resonance Imaging (MRI)
The preclinical MRI scanner (Bruker, BioSpec, 94/30 USR) was employed for MRI scans on PtenPC-/- Trem2WT or PtenPC-/- Trem2f/f-Lyz2-cre mice at week 4 and 16 post-castration. Mice were anesthetized with 1.5-2% isoflurane, maintaining normal respiration (~30 breaths per minute) and body temperature (37 °C). The MRI parameters consisted of rapid acquisition with relaxation enhancement (RARE) high-resolution T2-weighted (T2w) sequences in axial and coronal orientations. Axial sequences had a repletion time (TR) of 3500 ms, echo time (TE) of 40 ms, field of view (FOV) of 25 mm × 16 mm, spatial resolution of 0.095 × 0.088 mm2/pixel, slice thickness of 0.60 mm, 30 slices, and scan time of 5 min. Coronal sequences had a TR of 3000 ms, TE of 35 ms, FOV of 32 mm × 32 mm, spatial resolution of 0.128 × 0.128 mm2/pixel, slice thickness of 0.60 mm, 22 slices, and scan time of 5 min. Analysis of image was conducted utilizing the NIH software Slicer (version 5.6.1). The contour of the entire prostate was delineated on RARE T2w axial slices that contained identifiable prostatic lobes. The number of boundary pixels on each slice was calculated and summed to obtain the prostate volume. The boundaries of the prostate base and apex were accurately identified using T2w coronal images.
In vivo and in vitro bioluminescence imaging
The prostate tumour growth was monitored using in vivo bioluminescence imaging and the organs metastasis of prostate cancer was assessed using in vitro bioluminescence imaging. For in vivo bioluminescence imaging was performed using D-luciferin potassium salt (TargetMol, T4139-1g) at a dosage of 150 mg/kg in the Caliper IVIS Lumina III (Perkin Elmer). For in vitro bioluminescence imaging, mice were injected intraperitoneally with D-luciferin potassium salt, and euthanized with carbon dioxide 10 min later. Subsequently, the heart, liver, spleen, lungs, kidneys, and lymph nodes were removed and imaged with a Caliper IVIS Lumina III (Perkin Elmer). The Region of Interest (ROI) was designated with a radius of 2 covering the prostate region. The average radiance (p/s/cm2/sr) within ROI were analysed using Living Image software (Caliper Life Sciences, USA).
ELISA assay
ELISA tests are performed according to the standard operating procedures provided by the reagent vendor. Mouse ApoE ELISA Kit (# MM-0250M2), Mouse IL-10 ELISA Kit (# MM-0176M2), Mouse MCP1/CCL2 ELISA Kit (# MM-0082M2), Mouse TGF-β1 ELISA Kit (# MM-0135M2), and Mouse IL-23 ELISA Kit (# MM-0018M2) were purchased from Jiangsu Meimian Industrial Co., Ltd (Jiangsu, China). Human ApoE ELISA Kit (# E-EL-H0470c-48T) was purchased from Elabscience Biotechnology Co., Ltd (Wuhan, China). Details of ELISA Kits are given in the Supplementary Data 4.
Statistics
GraphPad Prism (GraphPad Software version 8.0) was employed for statistical analysis, and data presented as Mean ± SD. To compare single group, two-tailed Student’s t-test or Mann–Whitney U test were employed to calculate P-values. For comparisons involving multiple groups, P-values were determined using either one-way or two-way analysis of variance (ANOVA). Survival analysis utilized the log-rank (Mantel-Cox) test to calculate P-values. Correlation analyses were performed using Pearson correlation coefficients. All experiments were repeated at least three times unless otherwise indicated. P < 0.05 was considered statistically significant. Software used in this study are given in the Supplementary Data 5.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We acknowledge all members of the laboratories of Prof. Guanmin Jiang and Prof. Xi Huang for the scientific discussions. We acknowledge all the patients that participated in the study protocols. We also thank all the members of the animal core facility for technical assistance. Illustrations were created with BioRender. This work was supported by grants from the National Natural Science Foundation of China (No. 82272388 (G.J.), 82072365 (G.J.)), Natural Science Foundation of Guangdong Province (No. 2024A1515012898 (G.J.), 2023A1515030065 (Y.W.)), the open research funds from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (No. 202301-102 (Y.W.)).
Author contributions
Conceptualization: Q.W., G.J., Y.W., X.H., Methodology: Q.W., Y.L., R.L., X.L., Y. Zhou., Visualization: Y.S., Y. Zheng., Investigation: Q.W., G.J. Y.W., X.C., Funding acquisition: G.J., Y.W., Project administration: G.J., Supervision: G.J., X.H., Writing—original draft: Q.W., Y.W., Writing—review and editing: G.J., X.H., Q.W., Y.W.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The RNA-seq data of BMDMs treated with ASC-J9 or TREM2 deficiency are deposited in Gene Expression Omnibus (GEO) under the accession number GSE296857. The public ChIP-seq data of AR in THP1 cells used in this paper are available under the accession number GSE131381. All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Source data are provided with this paper. Source data, FCS files and raw images and are also available in Figshare: 10.6084/m9.figshare.28069892 or https://figshare.com/s/cfd94b8f166cef9c7d7f. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qiaohua Wang, Yongjian Wu.
Contributor Information
Xi Huang, Email: huangxi6@mail.sysu.edu.cn.
Guanmin Jiang, Email: jianggm3@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62381-x.
References
- 1.Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA: a cancer J. Clin.73, 17–48 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Teng, M., Zhou, S., Cai, C., Lupien, M. & He, H. H. Pioneer of prostate cancer: past, present and the future of FOXA1. Protein cell12, 29–38 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson, P. A., Arora, V. K. & Sawyers, C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer15, 701–711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol.15, 271–286 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Roubaud, G., Liaw, B. C., Oh, W. K. & Mulholland, D. J. Strategies to avoid treatment-induced lineage crisis in advanced prostate cancer. Nat. Rev. Clin. Oncol.14, 269–283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan, M. H., Li, J., Xu, H. E., Melcher, K. & Yong, E. L. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin.36, 3–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinlein, C. A. & Chang, C. Androgen receptor in prostate cancer. Endocr. Rev.25, 276–308 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Niu, Y. et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc. Natl. Acad. Sci. USA105, 12188–12193 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clocchiatti, A. et al. Androgen receptor functions as transcriptional repressor of cancer-associated fibroblast activation. J. Clin. Investig.128, 5531–5548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai, J. Y. et al. HOTAIR and androgen receptor synergistically increase GLI2 transcription to promote tumor angiogenesis and cancer stemness in renal cell carcinoma. Cancer Lett.498, 70–79 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Yang, C. et al. Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity55, 1268–1283.e1269 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Kwon, H. et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol.7, eabq2630 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature606, 791–796 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cioni, B. et al. Androgen receptor signalling in macrophages promotes TREM-1-mediated prostate cancer cell line migration and invasion. Nat. Commun.11, 4498 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, D. et al. IL-1β is an androgen-responsive target in macrophages for immunotherapy of prostate cancer. Adv. Sci. (Weinh., Baden.-Wurtt., Ger.)10, e2206889 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calcinotto, A. et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature559, 363–369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, T., Wang, L. H. & Farrar, W. L. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res.60, 2132–2135 (2000). [PubMed] [Google Scholar]
- 18.Hobisch, A. et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res.58, 4640–4645 (1998). [PubMed] [Google Scholar]
- 19.Bancaro, N. et al. Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer cell41, 602–619.e611 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Wang, J. et al. ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat. Med.22, 488–496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deczkowska, A., Weiner, A. & Amit, I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell181, 1207–1217 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Katzenelenbogen, Y. et al. Coupled scRNA-seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell182, 872–885.e819 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol.5, 471–478 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyu, A. et al. Evolution of myeloid-mediated immunotherapy resistance in prostate cancer. Nature637, 1207−1217 (2024). [DOI] [PMC free article] [PubMed]
- 26.Subudhi, S. K. et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci. Transs. Med. 12, eaaz3577 2020). [DOI] [PubMed]
- 27.Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov.21, 799–820 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol.23, 580–594 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulland, T. K. et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell170, 649–663.e613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litwin, M. S. & Tan, H. J. The diagnosis and treatment of prostate cancer: a review. JAMA317, 2532–2542 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science324, 787–790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redell, M. S., Ruiz, M. J., Alonzo, T. A., Gerbing, R. B. & Tweardy, D. J. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood117, 5701–5709 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahley, R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science240, 622–630 (1988). [DOI] [PubMed] [Google Scholar]
- 34.Gratuze, M., Leyns, C. E. G. & Holtzman, D. M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegen.13, 66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao, G. et al. From degenerative disease to malignant tumors: Insight to the function of ApoE. Biomed. Pharmacother. Biomed. Pharmacother.158, 114127 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Kober, D. L. et al. Functional insights from biophysical study of TREM2 interactions with apoE and Aβ(1-42). Alzheimer’s Dementia J. Alzheimer’s Assoc. 8, 10.1002/alz.12194 (2020). [DOI] [PMC free article] [PubMed]
- 37.DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol.19, 369–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, P. C., Hobisch, A., Lin, D. L., Culig, Z. & Keller, E. T. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev.12, 33–40 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Hall, S. J., Mutchnik, S. E., Chen, S. H., Woo, S. L. & Thompson, T. C. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int. J. Cancer70, 183–187 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Zaorsky, N. G. et al. Salvage therapy for prostate cancer after radical prostatectomy. Nat. Rev. Urol.18, 643–668 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Liu, F. et al. Targeting integrin αvβ3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin. Transl. Med.11, e548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, Y. et al. Targeting MS4A4A on tumour-associated macrophages restores CD8+ T-cell-mediated antitumour immunity. Gut72, 2307–2320 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, R. et al. TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma. Sci. Adv.9, eade3559 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buck, S. A. J., Koolen, S. L. W., Mathijssen, R. H. J., de Wit, R. & van Soest, R. J. Cross-resistance and drug sequence in prostate cancer. Drug Resist. Updat.56, 100761 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Desai, K., McManus, J. M. & Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev.42, 354–373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Bujanda, Z. A. et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat. Cancer2, 803–818 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pu, Y. et al. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Sci. Transl. Med.8, 333ra347 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Yang, C. et al. Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity55, 1747 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Pathria, P., Louis, T. L. & Varner, J. A. Targeting tumor-associated macrophages in cancer. Trends Immunol.40, 310–327 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Molgora, M. et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell182, 886–900.e817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, S. et al. TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways. Cell185, 4153–4169.e4119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peshoff, M. M. et al. Triggering receptor expressed on myeloid cells 2 (TREM2) regulates phagocytosis in glioblastoma. Neuro Oncol.26, 826−839 (2024). [DOI] [PMC free article] [PubMed]
- 53.Turnbull, I. R. & Colonna, M. Activating and inhibitory functions of DAP12. Nat. Rev. Immunol.7, 155–161 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Yeh, F. L., Wang, Y., Tom, I., Gonzalez, L. C. & Sheng, M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron91, 328–340 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Nalio Ramos, R. et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell185, 1189–1207.e1125 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Powles, T. et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat. Med.28, 144–153 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siddiqui, B. A., Subudhi, S. K. & Sharma, P. Anti-PD-L1 plus enzalutamide does not improve overall survival in prostate cancer. Cell Rep. Med.3, 100613 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martori, C. et al. Macrophages as a therapeutic target in metastatic prostate cancer: a way to overcome immunotherapy resistance Cancers14, 440 (2022). [DOI] [PMC free article] [PubMed]
- 59.Koinis, F. et al. Myeloid-derived suppressor cells in prostate cancer: present knowledge and future perspectives. Cells11, 20 (2021). [DOI] [PMC free article] [PubMed]
- 60.Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol.14, 399–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, Q., Wu, Y., Jiang, G. & Huang, X. Galectin-3 induces pathogenic immunosuppressive macrophages through interaction with TREM2 in lung cancer. J. Exp. Clin. Cancer Res. CR43, 224 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The RNA-seq data of BMDMs treated with ASC-J9 or TREM2 deficiency are deposited in Gene Expression Omnibus (GEO) under the accession number GSE296857. The public ChIP-seq data of AR in THP1 cells used in this paper are available under the accession number GSE131381. All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Source data are provided with this paper. Source data, FCS files and raw images and are also available in Figshare: 10.6084/m9.figshare.28069892 or https://figshare.com/s/cfd94b8f166cef9c7d7f. Source data are provided with this paper.