Abstract
Some cerebellar structures are known to be involved in the memorization of several conditioned responses. The role of the interpositus nucleus (IN) and the vermis (VE) in fear-conditioning consolidation was investigated by means of a combined behavioral and neurophysiological technique. The IN and VE were subjected to fully reversible tetrodotoxin (TTX) inactivation during consolidation in adult male Wistar rats that underwent acoustic conditioned stimulus (CS) and context fear training. TTX was injected in different groups of rats at increasing intervals after the acquisition session. Memory was assessed as conditioned freezing duration measured during retention testing, always performed 72 and 96 h after the stereotaxic TTX administration. This schedule ensures that there is no interference with normal cerebellar function during either the acquisition or the retrieval phase so that any amnesic effect may be due only to consolidation disruption. Our results show that IN functional integrity is necessary for acoustic CS fear response memory formation up to the 96-h after-acquisition delay. VE functional integrity was shown to be necessary for memory formation of both context (up to the 96-h after-acquisition delay) and acoustic CS (up to the 192-h after-acquisition delay) fear responses. The present findings help to elucidate the role of the cerebellum in memory consolidation and better define the neural circuits involved in fear memories.
It has been shown that the cerebellum plays an important role in classic Pavlovian conditioning (1–5), although there are no reports on its involvement in the learning process of fear-conditioned responses. In regard to cerebellar functional organization, it has been shown that damage to the interpositus nucleus (IN) region of the deep cerebellar nuclei almost invariably disrupts the nictitating membrane reflex (2, 3, 6–10), whereas the cerebellar vermis (VE) appears to be involved in conditioned bradycardia (11, 12). Understanding of cerebellar involvement in fear conditioning, besides providing more information on the mnemonic function of the cerebellum, can also clarify the role of neural circuits involved in fear memories. VE appears to be involved in the expression of innate affective and fear-related behaviors such as vocalization, cowering, and freezing in animals (13–16) and anxiety and fear in humans (17, 18).
Freezing is an innate reaction to danger that may become a conditioned response after the experimental subject has undergone appropriate training (19–22). Moreover, a single training session is sufficient to learn the association between conditioned stimulus (CS) [not only an appropriate CS but also the environment in which the unconditioned stimulus (US) is presented, i.e., the context] and US (painful electrical footshocks) for fear conditioning (20–25). These associations are well retained for a long time (19, 20, 23, 26). Furthermore, a paradigm entailing just one learning session makes it possible to know exactly when mnemonic processing starts (23, 26–28). The reversible functional inactivation technique has been profitably used to assess the time course of mnemonic processing in several subcortical and cortical neural sites (3, 7, 9, 27, 28). If the inactivating agents are administered after the acquisition session, and retrieval testing is performed when all pharmacological effects have disappeared, the mnemonic time course in a given neural site may be investigated selectively during consolidation. It is during this phase that the engrams are elaborated from a labile short-term condition to a much more stable long-lasting one (23, 27, 29, 30).
To investigate fear-conditioning consolidation, IN and VE were reversibly inactivated by the stereotaxic administration of tetrodotoxin (TTX) at several postacquisition delays after the single training session. Retrieval testing (measurement of conditioned freezing duration to acoustic CS and to context) was always performed when TTX effects had totally disappeared (72 and 96 h after TTX administration) (27, 31).
Materials and Methods
Animals.
Seventy-day-old male albino Wistar rats (average body weight 290 g) (Stefano Morini S.A.S., S. Polo D'Enza, Italy) were used. The animals were individually housed in stainless steel cages in a room with a natural light/dark cycle and constant temperature of 20 ± 1°C. The rats had free access to food and water throughout the experiment. All animal care and experimental procedures were conducted in accordance with Italian legislation and the official regulations of the European Communities Council (EEC) on the use of laboratory animals (Directive of November 24, 1986; 86/609/EEC).
Behavioral Procedures
Apparatus.
As in previous experiments, a basic Skinner box module (Modular Operant Cage, Coulbourn Instruments, Lehigh Valley, PA) was used to induce fear conditioning (20, 23). Box dimensions were 29 × 31 × 26 cm. The top and two opposite sides were made of aluminium panels; the other two sides were made of transparent plastic. The floor was made of stainless steel rods connected to a shock delivery apparatus (Grid Floor Shocker, Coulbourn Instruments, Model E13–08). There was a loudspeaker to emit acoustic stimuli of known intensity, frequency, and duration. The apparatus was connected to a stimulus programming device (Scatola di comando Arco 2340, Ugo Basile, Varese, Italy) to predetermine number, duration, and rate of CS-US couplings. The apparatus was placed in an acoustically insulated room [3.5 × 1.8 × 2.1 (h) m], kept at a constant temperature of 20 ± 1°C. Illumination inside the room was 60 lux.
Context freezing response was measured in the same apparatus that was used for conditioning. As in previous experiments, the freezing response to acoustic CS was measured in a totally different apparatus from that used for conditioning (20, 23). The apparatus was a modified shuttle box apparatus (Ugo Basile) (20 × 47 × 20 cm). The walls were made of gray opaque plastic with black vertical stripes (width 1 cm, spaced 3 cm apart). The lid was made of transparent plastic and the floor of black opaque plastic. There was a loudspeaker to administer acoustic stimuli to the experimental subjects in the apparatus. The apparatus was connected to a stimulus programming unit (Automatic Reflex Conditioner 7501, Ugo Basile) to predetermine CS (number of stimuli, duration of stimuli, rate of stimulation). The unit could also predetermine intensity and frequency of the acoustic stimulus. The apparatus was placed in an acoustically insulated room [3.5 × 3.6 × 2.1 (h) m] and kept at a constant temperature of 20 ± 1°C. Illumination inside the room was 10 lux.
Conditioning.
On day 1, the rat was gently taken manually from the home cage, placed in a bucket, and carried from the housing room to the appropriate soundproofed room. Once there, it was placed inside the conditioning apparatus. The rat was left undisturbed for 3 min. After this time, CS as an 800-Hz tone from a frequency generator, amplified to 75 dB (20, 23, 32) lasting 6 sec was administered 7 times, at 30-sec intervals. The last 1 sec of each CS was paired with the US as electric footshock. US intensity was 1.0 mA, as in previous experiments (26). The rat was left undisturbed for 2 min after the end of the stimulation pattern. Freezing duration was measured during this period. Rats were brought back to the home cage immediately thereafter.
Conditioned Freezing Measurement.
Freezing duration was measured 72 and 96 h after TTX or saline administration. To measure contextual freezing, the animals were again placed inside the conditioning apparatus and left there for 3 min. While they were there, neither electrical nor acoustic stimuli were administered. After that time, they were brought back to the home cage. The rat's behavior was recorded by means of a closed-circuit TV system. To measure acoustic CS freezing, the animals were placed in the other apparatus to avoid the facilitation of acoustic CS retention because of contextual cues (25, 33). Once inside the apparatus, the animal was left undisturbed for 3 min. After this time, during a subsequent second 3-min period, a series of 7 acoustic stimuli was administered, identical to those used during the conditioning session (frequency, intensity, duration, intervals between stimuli). The rat's behavior was recorded for the entire 6-min period by means of a closed-circuit TV system, after which the animals were brought back to the home cage. Rats of each group were divided in two subgroups (four to five animals). As in previous experiments (20, 23), one subgroup was tested for context freezing on one day (the first) and for CS freezing the day after (the second), whereas the other subgroup underwent the inverse schedule (context, first day; CS, second day). This schedule was used to ensure that exposure of all of the subjects first to context and secondly to CS, or vice versa, would not bias the retention of any of the two responses (34).
Freezing (immobility) was defined as the complete absence of somatic motility except for respiratory movements (35). Measurements were performed by means of a stopwatch by personnel who did not know to which experimental group each animal belonged. Total accumulated freezing time (i.e., total seconds spent freezing during each period) was measured.
Surgery and Drug Administration.
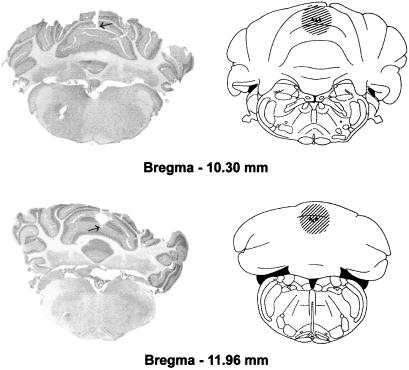
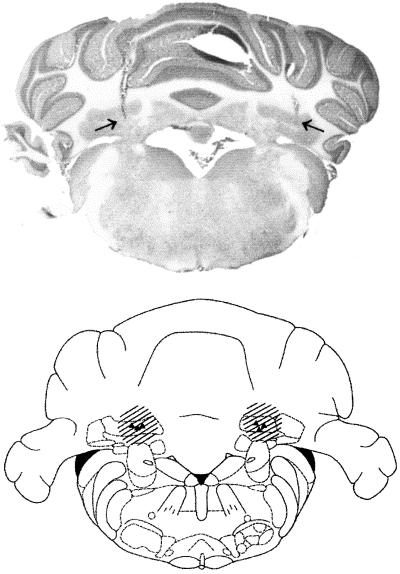
VE functional inactivation was induced by the injection of 10 ng of TTX (Sigma) dissolved in 1.0 μl of saline, into points with the following stereotaxic coordinates: (i) antero-posterior (AP) = −10.3, lateral (L) = 0, and ventral (V) = 2.4 and (ii) AP = −12.0, L = 0 and V = 3.0 according to Paxinos and Watson (36) (see Fig. 1). Bilateral IN functional inactivation was induced by injection of 5 ng of TTX dissolved in 0.5 μl of saline, into points with the following stereotaxic coordinates: AP = −11.35, L = ±2.4, and V = 6.0 according to Paxinos and Watson (36) (see Fig. 2). Mean inactivated nervous tissue radius after TTX administration was estimated at 1 mm for the 10-ng and 0.7–0.8 mm for the 5-ng TTX injection (31, 37) (Figs. 1 and 2). At least 20 min was necessary to reach maximal neural inactivation. Inactivation lasted for no less than 120 min, exponentially decreasing and disappearing completely within 24 h (31). TTX was injected under general anesthesia (ketamine, 100 mg/kg, i.p.) at different postacquisition intervals for each group of animals. Rats were held in the stereotaxic apparatus. The injection needle (outside diameter = 0.3 mm), connected with a short piece of polyethylene tubing to a Hamilton syringe, was fixed in the electrode holder of the stereotaxic apparatus and introduced into the target structure. Either 0.5 μl or 1.0 μl of the solution was injected over a 1- to 2-min period, and the needle was left in place for another 1 min before it was slowly withdrawn.
Figure 1.
Extension of cerebellar VE TTX inactivation estimated on the basis of injection sites (▾, end of needle tracks) and on known characteristics of TTX diffusion (see Materials and Methods). Plates adapted from the atlas of Paxinos and Watson (36). The arrow indicates the end of the needle track in the photomicrographs.
Figure 2.
Extension of TTX inactivation of IN estimated on the basis of injection sites (▾, end of needle tracks) and on known characteristics of TTX diffusion (see Materials and Methods). Plates adapted from the atlas of Paxinos and Watson (36). The arrows indicate the end of the needle track in the photomicrograph.
To obtain postacquisition VE inactivation, different groups of animals were injected at diverse postacquisition delays. A total of 96 rats were randomly divided into 10 groups, and TTX or saline was injected at 5 different postacquisition delays: 0.25, 24, 96, 192, or 384 h. Twelve animals were excluded because of inadequate morphological evidence. The remaining 84 animals made up the following groups of 8 or 9 animals each: T-0.25, S-0.25, T-24, S-24, T-96, S-96, T-192, S-192, T-384, and S-384 (T, TTX; S, saline).
To obtain postacquisition IN inactivation, different groups of animals were injected at diverse postacquisition delays. A total of 80 rats were randomly divided into 8 groups and TTX or saline was injected at 4 different postacquisition delays: 0.25, 24, 96, or 192 h. Ten animals were excluded because of inadequate morphological evidence. The remaining 70 animals made up the following groups, of 8 to 9 animals each: T-0.25, S-0.25, T-24, S-24, T-96, S-96, T-192, or S-192. Control group rats were injected in the same way with saline (S).
Statistical Analysis.
We used one-way ANOVA, mixed ANOVAs, with treatment (TTX and saline) and different postacquisition delays as a between-subject variable and context and CS freezing as a within-subject variable (tested by using F ratios, to determine whether there was a significant quadratic component), and Newman–Keuls multiple comparisons test.
Morphology.
At the end of the experiments, injected sites were histologically verified. Rats were deeply anesthetized and intracardially perfused with saline, followed by 4% formaldehyde. Brains were cut with a freezing microtome, and injection needle tracks were identified in Nissl-stained serial sections (Figs. 1 and 2). Rats with inadequate histological evidence were excluded from data processing.
Results
During the fear-conditioning acquisition training session, spontaneous behavior was homogeneous in rats of all 18 groups. Locomotory and explorative behavior was the same in all groups during the initial free exploration 3-min period. A very long freezing duration was exhibited by the rats of all groups after the last 2-min shock period. The mean freezing duration of the 18 groups ranged between 81.7 and 89.2% of total time (Table 1). One-way ANOVA showed that there were no significant (N.S.) differences between groups [F(17,136) = 0.32, N.S.], i.e., the conditioned freezing response was homogeneous in all groups. As in previous experiments (20, 23), all groups of animals were divided into two subgroups, which were measured for acoustic CS and context freezing in the 2 days of testing (see Materials and Methods). Because there were no statistically significant differences between subgroups, it was possible to statistically analyze the cumulated freezing results (to acoustic CS and to the context) of the first and second days, so that final statistical analysis was performed on groups of rats ranging between eight and nine animals.
Table 1.
Freezing reaction to fear-conditioning training
| Groups | Mean ± SEM |
|---|---|
| VE-S-0.25 | 86.62 ± 2.83 |
| VE-T-0.25 | 84.88 ± 3.10 |
| VE-S-24 | 81.75 ± 4.72 |
| VE-T-24 | 86.55 ± 3.24 |
| VE-S-96 | 86.44 ± 3.61 |
| VE-T-96 | 86.55 ± 3.10 |
| VE-S-192 | 85.12 ± 3.42 |
| VE-T-192 | 82.12 ± 4.51 |
| VE-S-384 | 87.66 ± 2.78 |
| VE-T-384 | 89.25 ± 2.62 |
| IN-S-0.25 | 85.55 ± 2.72 |
| IN-T-0.25 | 87.22 ± 3.15 |
| IN-S-24 | 84.50 ± 2.84 |
| IN-T-24 | 84.22 ± 3.12 |
| IN-S-96 | 84.50 ± 2.09 |
| IN-T-96 | 82.88 ± 2.84 |
| IN-S-192 | 84.87 ± 3.53 |
| IN-T-192 | 85.88 ± 1.89 |
Mean ± SEM freezing as percentage of total time in the 2-min period after last shock during fear-conditioning acquisition training.
In all control saline-injected groups (Figs. 3 and 4), both context and acoustic CS freezing responses were well developed and did not significantly decrease 19–20 days after the conditioning session (VE-S-384 group, Fig. 3). During the first 3-min subperiod of exposure to the new context (without CS, acoustic stimulation) the freezing response was very low in all groups of animals (see below and Tables 2 and 3) and comparable to that of rats allowed to freely explore the conditioning chamber without receiving footshocks (10–15% freezing of total exposure time) (23, 26). These results show the absence of generalization phenomena and the specificity of the freezing response to the acoustic CS in this new context.
Figure 3.
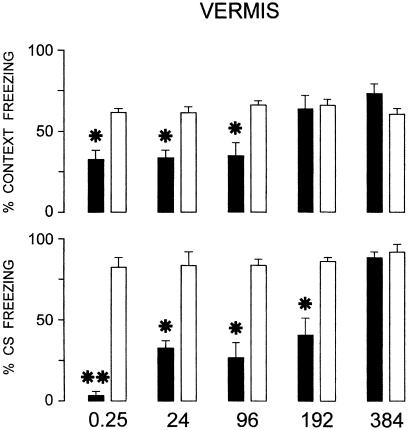
Effects of cerebellar VE TTX inactivation at increasing postacquisition delays (0.25, 24, 96, 192, and 384 h) on fear conditioning to context and acoustic CS. Black columns are TTX-injected groups, white columns are saline-injected groups. Mean ± SEM freezing as percentage of total 3-min period during retention testing (performed 72 and 96 h after TTX or saline administration) in the conditioning apparatus without acoustic stimulation (context) and in the other apparatus with acoustic stimulation (CS). *, P < 0.05; **, P < 0.01, statistically significant differences between treated and respective control groups.
Figure 4.
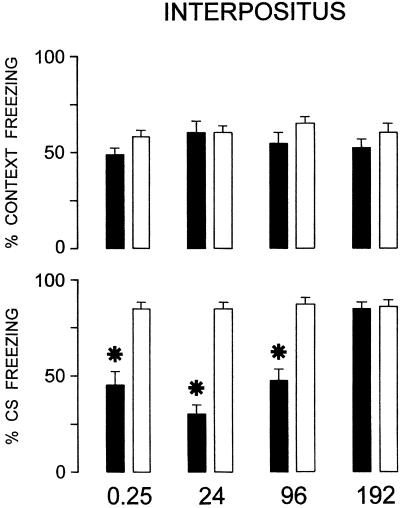
Effects of bilateral IN bilateral TTX inactivation at increasing postacquisition delays (0.25, 24, 96, and 192 h) on fear conditioning to context and acoustic CS. *, P < 0.05 statistically significant differences between treated and respective control groups. For explanation, see Fig. 3.
Table 2.
Absence of generalization in TTX- and saline-injected rats into VE
| Groups | Mean ± SEM |
|---|---|
| VE-S-0.25 | 14.00 ± 1.13 |
| VE-T-0.25 | 13.74 ± 0.82 |
| VE-S-24 | 13.72 ± 0.93 |
| VE-T-24 | 14.37 ± 1.14 |
| VE-S-96 | 14.33 ± 1.29 |
| VE-T-96 | 13.55 ± 1.11 |
| VE-S-192 | 14.62 ± 1.15 |
| VE-T-192 | 15.80 ± 1.16 |
| VE-S-384 | 14.77 ± 1.07 |
| VE-T-384 | 15.12 ± 1.27 |
Mean ± SEM freezing as percentage of total time in the first 3-min period in the new context without acoustic CS presentation during retention testing.
Table 3.
Absence of generalization in TTX- and saline-injected rats into IN
| Groups | Mean ± SEM |
|---|---|
| IN-S-0.25 | 13.25 ± 0.96 |
| IN-T-0.25 | 12.66 ± 1.11 |
| IN-S-24 | 12.00 ± 0.72 |
| IN-T-24 | 12.00 ± 0.74 |
| IN-S-96 | 11.75 ± 0.73 |
| IN-T-96 | 11.75 ± 0.49 |
| IN-S-192 | 11.42 ± 0.82 |
| IN-T-192 | 13.70 ± 0.93 |
For explanation see Table 2.
VE.
Acoustic CS freezing responses were impaired up to the 192-h postacquisition delay after VE reversible inactivation, whereas context freezing ones were impaired up to the 96-h delay (Fig. 3). Mixed ANOVAs (2 × 2 × 5) showed that different responses [F(1,148) = 4.44, P < 0.05], treatments [F(1,148) = 101.7, P < 0.001] and time lapsed from acquisition training [F(4,148) = 10.34, P < 0.001] were significantly different. There were significant interactions between responses and treatments [F(1,148) = 24.94, P < 0.001] and treatments and time lapsed [F(4,148) = 13.61, P < 0.001] and between responses, treatments and time lapsed [F(4,148) = 2.6, P < 0.05]. The posthoc Newman–Keuls test showed that there were significant differences between groups T-0.25, T-24, T-96, and T-192 and the respective control groups (S) for acoustic CS freezing (P < 0.01 at 0.25, P < 0.05 in all other instances) and T-0.25, T-24, T-96 and the respective control (S) groups for context freezing (P < 0.05 in all instances) (Fig. 3). Freezing duration during the first 3 min in the new context without CS presentation ranged between 13.7 and 15.8% of total time (Table 2) in the 10 groups. One-way ANOVA showed that there were no significant differences between groups [F(9, 74) = 0.81, not significant], thus demonstrating that VE inactivation did not cause generalization phenomena.
IN.
After IN reversible inactivation, only acoustic CS freezing responses were impaired up to the 96-h postacquisition delay (Fig. 4). Mixed ANOVAs (2 × 2 × 4) showed that different responses [F(1,126) = 9.95, P < 0.01], treatments [F(1,126) = 59.97, P < 0.001], and time lapsed from acquisition training [F(3,126) = 3.06, P < 0.05] were statistically significant. There were significant interactions between responses and treatments [F(1,126) = 17.98, P < 0.001], responses and treatments [F(3,126) = 4.23, P < 0.01], time lapsed and treatments [F(3,126) = 2.64, P < 0.05], and between responses, treatment, and time lapsed [F(3,126) = 5.88, P < 0.001]. The posthoc Newman–Keuls test showed that there were significant differences between T-0.25, T-24, and T-96 groups and the respective controls (S) for acoustic CS freezing response (P < 0.05 in all instances) (Fig. 4). Freezing duration during the first 3 min in the new context without CS presentation ranged between 11.4 and 13.7% of total time (Table 3) in the 8 groups. One-way ANOVA showed that there were no significant differences between groups [F(7, 62) = 0.74, not significant], thus demonstrating that IN inactivation did not determine generalization phenomena.
Discussion
The present findings show that both IN and VE appear to be necessary for conditioned fear response consolidation. They differ, because IN appears to be involved only in the memory formation of the freezing response to acoustic CS, whereas VE appears to be involved in the memorization of the freezing response both to acoustic CS and to context.
Methodological Considerations
Freezing, as obtained and measured in the present study, is a conditioned (learned) response. Our experimental rats did not exhibit freezing in the conditioning apparatus before the training session (19, 20, 22, 25, 26), and after this session, their freezing response was strictly related to the stimuli (acoustic CS and context) received during training. During retrieval testing when both CS and context were not presented again (rats placed in different surroundings without acoustic stimulation), no conditioned freezing was exhibited (Tables 2 and 3) (19–21, 23, 25).
The freezing behavior of all 18 groups immediately after conditioning (Table 1) shows that all of the experimental subjects exhibited a homogeneous response to shocks. Moreover, during retrieval testing, after the single acquisition session, all S control groups exhibited a very good conditioned freezing response both to acoustic CS and to context (Figs. 3 and 4), quite similar to that measured in previous experiments (20, 23, 32). Therefore, the shorter freezing response of TTX-injected rats must be because of the amnesic effect of the functional inactivation performed during consolidation. As already stated, the single training session acquisition paradigm is a necessary prerequisite for chronological investigation of the involvement of a given neural structure in memory consolidation, because there is a well defined starting time of mnemonic processing in these experimental conditions. In contrast, it is impossible to determine exactly when consolidation begins in multitraining session acquisition paradigms (27–29).
To ascertain consolidation chronology, reversible TTX inactivations were performed during consolidation. In these experiments, as in previous ones, the cannulation of the rats was unnecessary, the active compound being directly administered stereotaxically. This procedure is advantageous, because the surgical trauma inherent to the permanent-cannulating procedure is avoided, thus restricting trauma to a single needle penetration (38). It has been shown that in this phase, general anesthesia does not negatively interfere with memory trace consolidation (38–40). TTX was locally injected at increasing postacquisition delays, and retention testing was always performed 72 and 96 h after TTX administration. Thus the two sites were inactivated only during consolidation, without any interference with acquisition (inactivation was always performed after the acquisition session) or retrieval (retention testing was always performed when there were no residual TTX effects) (31). Because there was no interference with normal function during acquisition and retrieval, not only any state-dependent effect, but also any interference with acoustic CS and US sensory perception or with motor control functions may be excluded. This experimental design is of interest because, when discussing cerebellar involvement in learning and memory, the question has been raised of the possible interference of the experimental manipulations on sensory perception and/or motor performance control (41, 42). The present amnesic findings are specifically related only to effects of the imposed interferences on memory processing. Moreover, TTX local injection effects are region-specific and, above all, TTX inactivation is reversible so that vicarious circuits have no time to intervene and obscure findings. Thus, the present results show that IN and VE play a clear-cut role in memory consolidation. As far as we know, there are few precise data on cerebellar involvement in memory consolidation. There is one report of a nictitating membrane reflex (NMR) consolidation deficit after anisomycin postacquisition administration in the IN.§ In other NMR studies, irreversible neural lesions were used (8), thus making it impossible to know which of the putative mnemonic phases (acquisition, consolidation, or retrieval) was affected. Other researchers performed reversible inactivations but during either acquisition or retrieval (3, 6, 7, 9, 10, 43).
Cerebellar Mnemonic Characterization.
As stated above, the present findings show that there are at least two sites in the cerebellum that are necessary for the consolidation of the memory traces of fear conditioning: one cortical (VE) and one nuclear (IN). The two sites are functionally differentiated. As far as we know, the present results show, for the first time, that some cerebellar regions are necessary to consolidate fear responses to acoustic stimuli and context. We show that cerebellar mnemonic involvement in fear-conditioning consolidation is quite long-lasting (from not less than 96 up to 192 h), and that there are functional differences between the two investigated sites. In fact, IN functional integrity appears to be necessary only to consolidate memory of fear of the acoustic CS, whereas VE functional integrity seems necessary for the consolidation of memory of fear of both acoustic CS and context.
So far it has been shown, by using the functional ablation technique, that quite a number of neural sites, from the parabrachial nuclei (bulbo-pontine) to the neocortex, are necessary for fear inhibitory conditioning (22, 23, 27, 29). After acquisition training, i.e., during consolidation, the functional integrity of these sites is necessary for unequal durations ranging from 1 up to 192 h. Necessary functional integrity durations have been taken as indicative of the relative mnemonic importance of the single site (23, 27, 28). In terms of duration, a useful comparison (involvement in acoustic CS and context fear-conditioning consolidation) can be made between the present results and those from some previously investigated sites, e.g., the basolateral amygdala, the dorsal hippocampus, and the perirhinal cortex (23), all known to be of great importance in memory processing (21). It is worth noting that these involvement durations of cerebellar sites are similar to the longer ones previously reported, e.g., perirhinal cortex: 192 h for both traces. It may be surmised that fear memories are elaborated contemporaneously in many neural sites, and that therefore the activated neural circuits are larger and more complex than so far assessed (21, 22).
With regard to mnemonic characterization, the present findings show that the two cerebellar sites can be differentiated. We show that IN is not necessary for multimodal learning (e.g., context conditioning) and confirm that IN functional integrity is necessary for the consolidation of the acoustic CS trace, i.e., for a single distinct sensorial input (2, 3, 7–10).§ In this connection, we may recall that context learning, which entails the mnemonic processing of many different sensory inputs, has been thought to be an elementary form of spatial learning (19, 21, 44) and therefore requires a far more complex associative elaboration than that sufficient for a single strong sensory stimulus (acoustic CS). On the other hand, VE appears to be necessary for the memorization of fear conditioning both to acoustic CS and to context traces: VE involvement is quite long-lasting for both traces and significantly longer for the fear conditioning to acoustic CS trace. These results in general confirm and expand previous ones showing that VE is involved in classic conditioning using acoustic CS (11, 12, 45, 46) and contextual cues in spatial learning (47–50). On this point, it may be recalled that recently it has been stated that the cerebellum not only controls the execution of an action or movement but also is also necessary to learn to recognize the location where the action is to be performed (18, 51). Incidentally, the finding that VE involvement in fear conditioning to acoustic CS and to context is of unequal duration may further confirm the statement that the two mnemonic traces can be elaborated separately and independently, even within the same neural site (21–25, 52).
The role of the cerebellar cortex in the learning process is still under discussion. Does it mainly modulate cerebellar nuclei activity, or is it itself a plasticity site (2, 4, 9, 53–56)? Given the necessity of VE functional integrity to consolidate both mnemonic traces, and of IN only for fear conditioning to the acoustic CS, and given also that the critical duration of VE functional integrity for fear conditioning to the acoustic CS consolidation is longer than that of IN, the present results support the hypothesis of VE being an independent mnemonic elaboration site. Anatomical and electrophysiological studies show that cortical and nuclear regions of the cerebellum receive converging projections from the pontine nuclei and inferior olive, projections that may carry information about sensory stimuli during classical conditioning (1, 2, 57, 58). Acoustic information can be carried by mossy fibers from the pontine nuclei lateral region directly to both cerebellar cortex and IN (1, 57, 58). On the other hand, context fear memories could be mediated by a VE–fastigium loop. Evidently, it is to be hoped that more exhaustive studies will better define the cerebellar circuits underlying fear memories. More than one type of synaptic plasticity may play a role in these regions, including parallel Purkinje fiber cell long-term depression (59, 60) and long-term potentiation (61), parallel Purkinje fiber cell γ-aminobutyric acid-transmitting rebound potentiation (62), Purkinje cell-deep nuclear neuron long-term depression (63, 64) and long-term potentiation of extracellular responses in the cerebellar deep nuclei (65). Such complex plasticity may support memorization in both the cerebellar cortex and deep nuclei (2, 66, 67). Finally, the long duration of the postacquisition necessary to functional integrity of both sites not only indicates that VE and IN are crucially involved in the consolidation of aversely motivated acoustic CS and context memory traces but also suggests that these sites may be involved in subsequent trace storage (27), as proposed for the amygdala and perirhinal cortex (23).
Acknowledgments
We thank A. Aiazzi, S. Cammarata, M. Dolfi, and A. Vannucchi for technical assistance.
Abbreviations
- CS
conditioned stimulus
- US
unconditioned stimulus
- IN
interpositus nucleus
- VE
vermis
- TTX
tetrodotoxin
- T
TTX
- S
saline
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 7814.
Bracha, V., Webster, M. L., Stachowiak, M. K. & Bloedel, J. R. (1995) Neurosci. Abstr. 21, 1222.
References
- 1.Ito M. The Cerebellum and Neural Control. New York : Raven; 1984. [Google Scholar]
- 2.Kim J J, Thompson R F. Trend Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 3.Krupa D J, Thompson J K, Thompson R F. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 4.Garcia K S, Steele P M, Mauk M D. J Neurosci. 1999;19:10940–10947. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansel C, Linden D J, D'Angelo E. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 6.Bracha V, Webster M L, Winters N K, Irwin K B, Bloedel J R. Exp Brain Res. 1994;100:453–468. doi: 10.1007/BF02738405. [DOI] [PubMed] [Google Scholar]
- 7.Clark R E, Zhang A A, Lavond D G. Behav Neurosci. 1992;106:879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz J E, Lavond D G, Ivkovich D, Logan C G, Thompson R F. J Neurosci. 1992;12:4403–4426. doi: 10.1523/JNEUROSCI.12-11-04403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamounas L A, Thompson R F, Madden J., 4th Proc Natl Acad Sci USA. 1987;84:2101–2105. doi: 10.1073/pnas.84.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordholm A F N, Thompson J K, Dersarkinssian C, Thompson R F. Behav Neurosci. 1993;107:882–886. doi: 10.1037//0735-7044.107.5.882. [DOI] [PubMed] [Google Scholar]
- 11.Supple W F, Jr, Leaton R N. Behav Neurosci. 1990;104:934–947. doi: 10.1037//0735-7044.104.6.934. [DOI] [PubMed] [Google Scholar]
- 12.Supple W F, Jr, Krapp B S. J Neurosci. 1993;13:3705–3711. doi: 10.1523/JNEUROSCI.13-09-03705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert T J, Dempesy C W, Sorenson C A. Biol Psychiatry. 1985;20:1267–1276. doi: 10.1016/0006-3223(85)90111-8. [DOI] [PubMed] [Google Scholar]
- 14.Asdourian D, Frerichs K. Psychonom Sci. 1970;18:261–262. [Google Scholar]
- 15.Bernston G G, Torello M W. Physiol Psychol. 1982;10:2–12. [Google Scholar]
- 16.Bobee S, Mariette E, Tremblay-Leveau H, Caston J. Behav Brain Res. 2000;112:107–117. doi: 10.1016/s0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 17.Nashold B S, Slaughter D G. J Neurosurg. 1969;31:172–186. doi: 10.3171/jns.1969.31.2.0172. [DOI] [PubMed] [Google Scholar]
- 18.Parvizi J, Anderson S W, Martin C O, Damasio H, Damasio A R. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- 19.Fanselow M S. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- 20.Sacchetti B, Ambrogi Lorenzini C, Baldi E, Tassoni G, Bucherelli C. Arch Ital Biol. 1999;137:235–248. [PubMed] [Google Scholar]
- 21.LeDoux J E. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Maren S, Fanselow M S. Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 23.Sacchetti B, Ambrogi Lorenzini C, Baldi E, Tassoni G, Bucherelli C. J Neurosci. 1999;19:9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J J, Fanselow M S. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 25.Corodimas K P, LeDoux J E. Behav Neurosci. 1995;109:613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- 26.Sacchetti B, Ambrogi Lorenzini C, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Eur J Neurosci. 2001;13:2291–2298. doi: 10.1046/j.0953-816x.2001.01609.x. [DOI] [PubMed] [Google Scholar]
- 27.Ambrogi Lorenzini C G, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Neurobiol Learn Mem. 1999;71:1–18. doi: 10.1006/nlme.1998.3865. [DOI] [PubMed] [Google Scholar]
- 28.Bures J, Buresova O. Concepts Neurosci. 1990;1:69–89. [Google Scholar]
- 29.Ambrogi Lorenzini C, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Arch Ital Biol. 1998;136:279–296. [PubMed] [Google Scholar]
- 30.McGaugh J L. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 31.Zhuravin I A, Bures J. Exp Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]
- 32.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 33.Balaz M A, Capra S, Kasprow W J, Miller R R. Anim Learn Behav. 1982;10:242–248. [Google Scholar]
- 34.Winocur G. Behav Brain Res. 1997;88:219–229. doi: 10.1016/s0166-4328(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 35.LeDoux J E, Sakaguchi A, Reis D J. J Neurosci. 1983;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 37.Ambrogi Lorenzini C G, Baldi E, Bucherelli C, Tassoni G. Neurobiol Learn Mem. 1995;63:87–93. doi: 10.1006/nlme.1995.1008. [DOI] [PubMed] [Google Scholar]
- 38.Ambrogi Lorenzini C G, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Brain Res Protoc. 1997;1:391–398. doi: 10.1016/s1385-299x(97)00017-2. [DOI] [PubMed] [Google Scholar]
- 39.Bucherelli C, Tassoni G. Behav Brain Res. 1992;49:175–180. doi: 10.1016/s0166-4328(05)80162-7. [DOI] [PubMed] [Google Scholar]
- 40.Tassoni G, Bucherelli C, Bures J. Behav Neural Biol. 1992;57:116–123. doi: 10.1016/0163-1047(92)90605-4. [DOI] [PubMed] [Google Scholar]
- 41.Welsh J P, Harvey J A. J Neurosci. 1989;9:299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh J P, Harvey J A. J Physiol. 1991;444:459–480. doi: 10.1113/jphysiol.1991.sp018888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomi H, Sun W, Finch C E, Itohara S, Yoshimi K, Thompson R F. J Neurosci. 1999;19:9530–9537. doi: 10.1523/JNEUROSCI.19-21-09530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nadel L, Willner J. Physiol Psychol. 1980;8:218–228. [Google Scholar]
- 45. Lopiano, L., de'Sperati, C. & Montarolo, P. G. (1990) Neuroscience35, 79–84. [DOI] [PubMed]
- 46.Leaton R N, Supple W F., Jr Science. 1986;232:513–515. doi: 10.1126/science.3961494. [DOI] [PubMed] [Google Scholar]
- 47.Caston J, Chianale C, Delhaye-Bouchand N, Mariani J. Brain Res. 1998;808:232–237. doi: 10.1016/s0006-8993(98)00847-6. [DOI] [PubMed] [Google Scholar]
- 48.Gandhi C C, Kelly R M, Wiley R G, Walsh T J. Behav Brain Res. 2000;109:37–47. doi: 10.1016/s0166-4328(99)00160-6. [DOI] [PubMed] [Google Scholar]
- 49.Conquet F, Bashir Z I, Davies C H, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, et al. Nature (London) 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 50.Leggio M G, Molinari M, Neri P, Graziano A, Mandolesi L, Petrosini L. Proc Natl Acad Sci USA. 2000;97:2320–2325. doi: 10.1073/pnas.040554297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis R F, Tamargo R J. Exp Brain Res. 2001;139:263–267. doi: 10.1007/s002210100719. [DOI] [PubMed] [Google Scholar]
- 52.Fanselow M S, LeDoux J E. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 53.Perret S P, Mauk M D. J Neurosci. 1995;15:2074–2080. doi: 10.1523/JNEUROSCI.15-03-02074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCormick D A, Thompson R F. J Neurosci. 1984;4:2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavond D G, Steinmetz J E. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- 56.Bao S, Chen L, Kim J J, Thompson R F. Proc Natl Acad Sci USA. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gould T J, Sears L L, Steinmetz J E. Behav Neural Biol. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- 58.Bao S, Chen L, Thompson R F. Behav Neurosci. 2000;114:254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- 59.Ito M, Kano M. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 60.Linden D J, Connor J A. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 61.Salin P A, Malenka R C, Nicoll R A. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 62.Kano M, Rexhausen U, Dreessen J, Konnerth A. Nature (London) 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- 63.Aizenman C D, Manis P B, Linden D J. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 64.Morishita W, Sastry B R. J Neurophysiol. 1996;76:59–68. doi: 10.1152/jn.1996.76.1.59. [DOI] [PubMed] [Google Scholar]
- 65.Racine R J, Wilson D A, Gingell R, Sutherland D. Exp Brain Res. 1986;63:158–162. doi: 10.1007/BF00235658. [DOI] [PubMed] [Google Scholar]
- 66.Thompson R F, Bao S, Chen L, Cipriano B D, Grethe J S, Kim J J, Thompson J K, Tracy J A, Weninger M S, Krupa D J. Int Rev Neurobiol. 1997;41:151–189. doi: 10.1016/s0074-7742(08)60351-7. [DOI] [PubMed] [Google Scholar]
- 67.Raymond J L, Lisberger S G, Mauk M D. Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]