Abstract
Meroterpenoids are a subclass of terpenes that consist of a nonterpene part, that is, in many cases, an aryl residue, linked to a terpene component. Their biosynthesis involves the attachment of the aryl group to the tail end of the acyclic terpene part, followed by terpene cyclization. Here we explore an alternative method for accessing meroterpenoids. The terpene cyclization is performed in the presence of arenes that trap key cationic intermediates in an intermolecular fashion. Our study demonstrates the feasibility of this approach that, to our knowledge, has not been reported before. It enables direct access to the novel meroterpenoid chemical space.
Keywords: Resorcin[4]arene catalysis, carbocation trapping, meroterpenoid synthesis, supramolecular catalysis

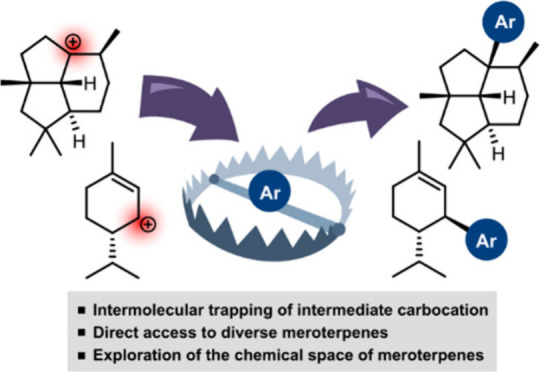
Terpenes represent the largest class of natural products, distinguished by their highly diverse and complex structural architectures. − Remarkably, this diversity arises from a limited set of highly conserved precursor molecules such as farnesyl pyrophosphate (FPP, Figure a). One diversification strategy employed by nature is the incorporation of nonterpene moieties, resulting in the formation of so-called meroterpenoids. − Their biosynthesis involves the attachment of the nonterpene part, which is in many cases an aryl residue, to the tail end of the acyclic terpene part. Only afterward, terpene cyclization, in a head-to-tail fashion, is initiated to form the final cyclized meroterpenoid (Figure a). The cationic intermediate formed during the cyclization is thus quenched by the aryl part in an intramolecular fashion. Intermolecular trapping would (i) enable more direct access to meroterpenes and (ii) enable access to unexplored chemical space. However, there are no examples of natural terpene cyclizations that are terminated by an intermolecular trapping with an aryl group to the best of our knowledge. Such a methodology would further help expand the non-natural terpene space. − Even in the broader context of chemical terpene-like cyclizations, intermolecular trapping is rarely observed. Such examples include the linkage of the menthane framework with aryl groups to form menthane meroterpenes without a preceding cyclization reaction. , Furthermore, Prins-type cyclizations were combined with aryl trapping. −
1.
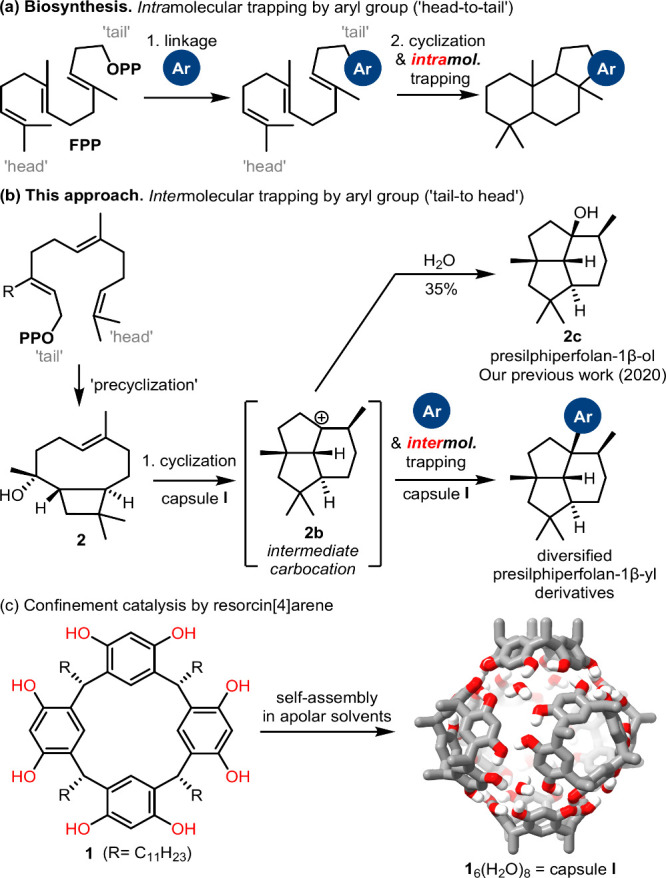
(a) Typical biosynthetic pathway of meroterpene; (b) cyclization of caryophyllene alcohol 2 in capsule I in the presence of an external nucleophile; (c) self-assembly of resorcin[4]arene into a hexameric capsule.
Our group reported the four-step synthesis of presilphiperfolan-1β-ol 2c by confinement catalysis, , utilizing the resorcin[4]arene capsule I (Figure c). − The modification of the precursor also permitted intramolecular trapping of the carbocation, distantly related to meroterpene biosynthesis. The Chappell group also reported intramolecular trapping of a non-natural arene-containing substrate, albeit via enzymatic catalysis. In this work, we explore the possibility of trapping the tail-to-head cyclization with an aryl residue in an intermolecular fashion.
While intermolecular Friedel–Crafts alkylation and acylation have been reported in the resorcin[4]arene capsule, , to our knowledge, the combination with terpene cyclizations has been observed in neither biosynthesis nor a chemical laboratory.
We started the investigation with the sesquiterpene caryophyllene alcohol 2, which was the direct precursor for our capsule-catalyzed cyclization to presilphiperfolan-1β-ol 2c via the intermediary carbocation 2b (Figure b). A first nucleophile screening led to a hit for the reaction with 2-methylfuran 4, producing the furan derivative 10 (Table , see Supporting Information chapter S2 for details). The reaction conditions were optimized regarding temperature, equivalents of nucleophile, and amount of HCl cocatalyst (see chapter S3). For further reactions, the following optimized conditions were used; 10 mol % capsule I, 4 mol % HCl, and 10 equiv of nucleophile at 60 °C. Under these conditions, an isolated yield of 29% of 10 (GC yield of 55%) was obtained. Additional product (18% 10) was collected in separate fractions as a mixture with its C19 epimer, named 10b (ratio of 10/10b approximately 1.8/1). A range of additional nucleophiles, 3-methylfuran 5, 2,3-dimethylfuran 6, 1,3-dimethoxybenzene 7, resorcinol 8, and N-methylpyrrole 9, were tested. Besides 2-methylfuran 4, 2,3-dimethylfuran 6 also delivered the desired product. To our knowledge, these examples represent the first meroterpenoids formed by an intermolecular quench of a terpene cyclization.
1. Scope of the Substrates and Nucleophiles in the Cyclization Reactions .
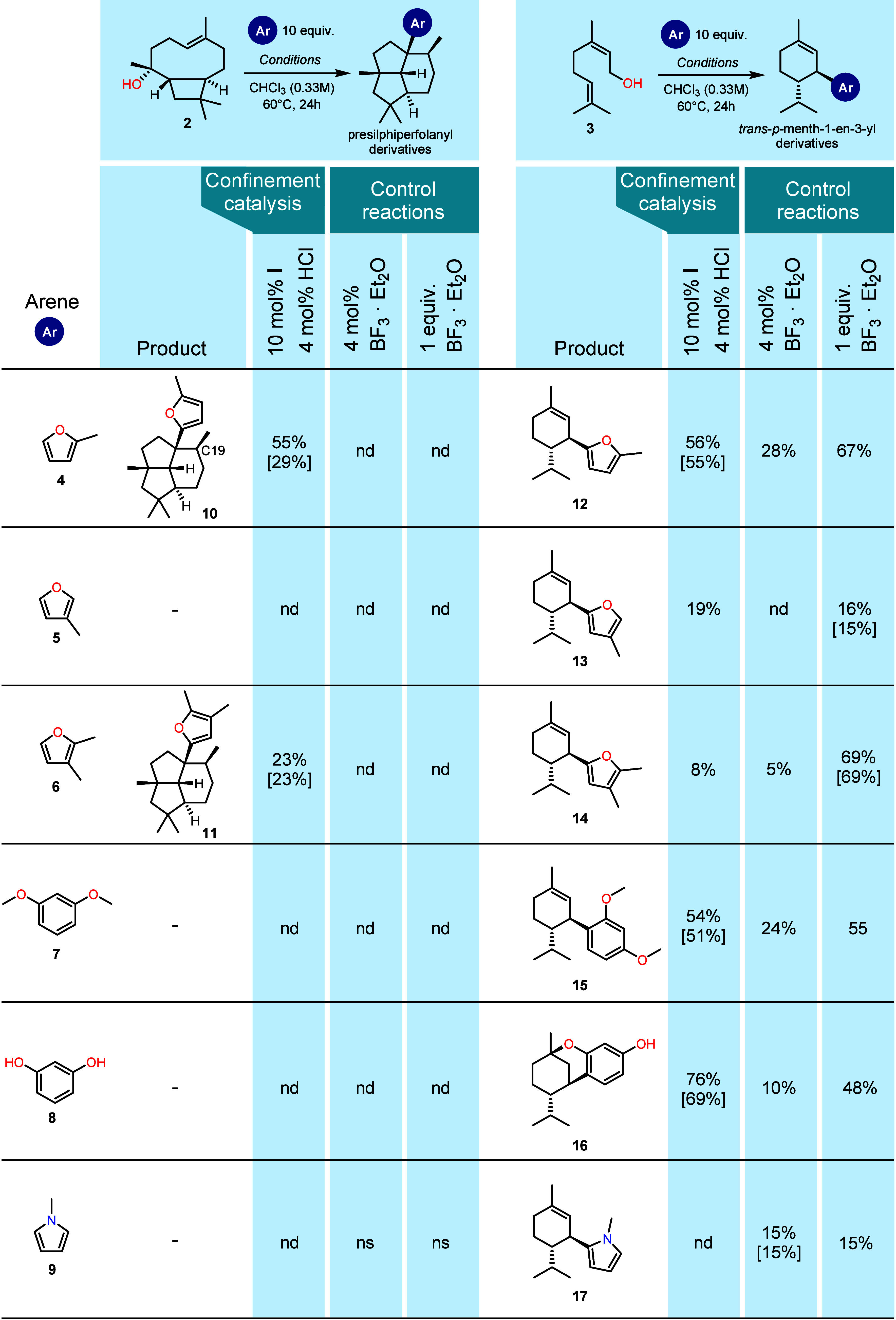
Control reactions with boron trifluoride diethyl etherate in catalytic and stoichiometric amounts are shown. GC yields were corrected using calculated relative response factors. Isolated yields are indicated in [brackets]. nd = not detected, ns = not selective. Reaction conditions: 0.033M substrate in CHCl3 at 60°C; catalyst details and amounts; see table.
The closely related 3-methylfuran 5 did not lead to a detectable product formation. Unsubstituted furan, which is less nucleophilic, , only led to very low yields of product mixtures which did not allow for their isolation (see chapter S9.1). Thus, the 2-methyl substituent on the furan might be required to stabilize the dearomatized intermediate (see chapter S6.3). Related to this observation, 2-methylthiophene also displayed low reactivity (see chapter S9.2), most likely again owing to its lower nucleophilicity. Furthermore, none of the six-membered aromatic nucleophiles explored (such as 1,3-dimethoxybenzene 7 and resorcinol 8) yielded products in isolatable yield. It seems that intermediate carbocation 2b (Figure b) is sterically too encumbered for an attack by benzene derivatives. N-methylpyrrole 9, on the other hand, displayed poor selectivity, and no defined product was isolable.
We then investigated the cyclization of nerol 3, a commercially available monoterpene, that shows tail-to-head cyclization reactivity also with Lewis and Brønsted acids. Its cyclization inside capsule I has been previously studied by our group and led to the formation of a mixture of products. In this work, the reaction demonstrated a higher selectivity. A trans-substituted p-menth-1-en-3-yl carbon skeleton was formed as the major product. To the best of our knowledge, this is the first time that the formation of this structure has been observed from the cyclization of a linear precursor. In contrast to substrate 2, nerol 3 afforded products for all three furans tested (12-14, Table ). Moreover, it was compatible with benzene derivatives (products 15-16). In the case of resorcinol 8, a cyclic ether was formed, most likely owing to the protonation of the endocyclic double bond after the formation of the p-menth-1-en-3-yl scaffold. With the idea of extending the scope to a less nucleophilic benzene derivative, m-xylene was explored as a solvent. However, the reaction was unselective and led to multiple product peaks on the GC trace (see chapter S9.3). Nitrogen heterocycles such as pyrrole 9 led to poor results with resorcin[4]arene-catalysis. Despite their high nucleophilicity, they may act as a base and neutralize the required cocatalyst HCl. Thus, the exploration of amine nucleophiles has not been further pursued.
For all reactions, the main product was isolated from the reaction mixture using either silica gel flash chromatography or recycling GPC (see chapter S1.1 for details). The product structures were elucidated via 1H, 13C, DEPT135, COSY, NOESY, HSQC, and HMBC NMR measurement. In two cases, for products 10 and 19 (Figure ), additionally, a 1,1-ADEQUAT NMR experiment was required to assign the structure since the 1H NMR spectra showed significant signal overlap. Moreover, 13C chemical shifts were calculated for all the structures and were used to exclude other potential isomers (see chapter S14).
2.

Derivatization options for furan 10.
Next, we sought to determine whether the observed reactions could also proceed outside the supramolecular capsule. In our previous work on the cyclization of caryophyllene alcohol 2, we screened 12 different reaction conditions but did not identify any Lewis or Brønsted acid capable of promoting the cyclization to the presilphiperfolane framework. Thus, a screening (see chapter S8) of different acids was performed with the alternative substrate nerol 3 (see also Table , right part). Only boron trifluoride diethyl etherate, in either catalytic or stoichiometric amounts, was identified as a suitable promoter for the cyclization/intermolecular quench reaction of 3 and thus was used as promoter instead of capsule I and HCl in control reactions for all entries. For the substrate nerol 3, in some cases, even higher yields were obtained using boron trifluoride diethyl etherate in catalytic or stoichiometric amounts. Lewis acid-catalyzed cyclization in solution is thus an alternative to produce monoterpene-based meroterpenoids via this strategy. In fact, it was the only successful reaction condition to produce compound 17. Interestingly, when the nucleophile was omitted from the boron trifluoride reactions, no defined product was formed, and the GC trace remained largely silent. Instead, a black suspension was obtained. Based on this observation, it seems that the nucleophile quench decreases the undesirable formation of undefined oligomeric products.
However, for the caryophyllene alcohol substrate 2, all control experiments with either catalytic or stoichiometric amounts of boron trifluoride diethyl etherate failed to deliver any traces of product, confirming our earlier finding that the presilphiperfolane framework was not accessible from 2 without the capsule catalyst. However, the question remained if, after successful capsule-catalyzed cyclization to the presilphiperfolanyl cation 2b (Figure b), the electrophilic aromatic substitution with aryl residues is possible without the capsule. Thus, two additional control experiments were run. (1) Subjecting presilphiperfolan-1β-ol 2c to HCl (4 mol %) and 2-methylfuran 4 (10 equiv) did not lead to the formation of the furan derivative 10. In the presence of resorcin[4]arene 1 and HCl, the derivative 10 was formed in 65% yield. (2) Subjecting presilphiperfolan-1β-ol 2c to the control reaction conditions of Table (boron trifluoride diethyl etherate in catalytic or stoichiometric amount) also did not lead to the formation of the derivative 10. Thus, both steps of the caryophyllene substrate conversion, cyclization, and carbocation trapping require the presence of the capsule. It seems that the capsule is required to stabilize the intermediate carbocation as in its absence alkene formation via elimination was observed.
After having explored the scope and limitations of intermolecular meroterpenoid formation, we tried to expand the chemical space of the meroterpenoids formed. This seemed especially of interest for the presilphiperfolane meroterpenoids, as the aryl nucleophile scope turned out to be limited to 2-substituted furans. Thus, we explored the ring opening of the furan in compound 10 (Figure ). Standard reaction conditions (HCl, EtOH, 100 °C) for the ring-opening step afforded 31% diketone 18. Although incomplete conversion of the starting material was observed, it could be recovered easily (50% recovered sm). The crystal structure of diketone 18 was obtained and unambiguously confirmed the assignment of the presilphiperfolanyl carbon skeleton. Acid-catalyzed Paal-Knorr formation of pyrrole 19 was unsuccessful and led to the formation of the parent furan 10. Moreover, it was found that traces of formed pyrrole 19 decomposed under acidic conditions. Thus, neutral conditions using ammonium acetate were explored, making the pyrrole derivative 19 that was inaccessible directly, attainable via this two-step procedure. Alternatively, furan 10 can be opened oxidatively with mCPBA to the unsaturated diketone 20 in high yield, enabling additional derivatization options.
The study successfully demonstrates a novel approach to meroterpenoids by intermolecular trapping of carbocations formed during terpene cyclizations. It enables direct access to novel meroterpenoid chemical space. For the caryophyllene alcohol substrate 2, the resorcinarene capsule I plays a crucial role in catalyzing the initial terpene cyclization as well as the final intermolecular electrophilic aromatic substitution. These results further illustrate the benefits of conducting reactions within supramolecular containers. − For nerol 3, however, the Lewis acid boron trifluoride also turned out to be a suitable catalyst and/or promoter. The reaction is sensitive to the structure of the nucleophile, with 2-substituted furans showing the best results for the sterically encumbered cationic intermediate formed from the caryophyllene alcohol. Nerol displays a much broader arene scope, reacting with furans, benzene derivatives, and pyrrole derivatives. Chemical derivatization of the furan-containing meroterpenoids allows for the further expansion of the chemical space. The study demonstrates the potential of intermolecular trapping as a valuable tool in the synthesis of structurally novel meroterpenes, providing a foundation for future exploration and development.
Supplementary Material
Acknowledgments
We thank Dr. Michael Pfeffer for the high-resolution mass spectrometry analysis.
Raw data for the table and figures have been deposited at https://zenodo.org/records/15754845.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.5c00492.
General information, additional reaction screenings, reaction optimization, experimental procedures, characterization data of compounds, crystallographic details, 13C NMR shift calculations and NMR spectra (PDF)
CCDC 2441112 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
K.T. conceived and supervised the project. I.C. planned, performed, and analyzed the experimental work. D.H. implemented the 1,1-ADEQUATE NMR experiment and performed and interpreted the measurement of compound 12. A.P. solved and refined the X-ray crystal structure. K.T. and I.C. compiled the first draft of the manuscript. All authors contributed to the final version of the manuscript.
We thank the National Centre of Competence in Research (NCCR) Molecular Systems Engineering for financial support.
The authors declare no competing financial interest.
References
- Cane D. E.. Enzymic Formation of Sesquiterpenes. Chem. Rev. 1990;90(7):1089–1103. doi: 10.1021/cr00105a002. [DOI] [Google Scholar]
- Miller D. J., Allemann R. K.. Sesquiterpene Synthases: Passive Catalysts or Active Players? Nat. Prod Rep. 2012;29(1):60–71. doi: 10.1039/C1NP00060H. [DOI] [PubMed] [Google Scholar]
- Dickschat J. S.. Isoprenoids in Three-Dimensional Space: The Stereochemistry of Terpene Biosynthesis. Nat. Prod. Rep. 2011;28(12):1917–1936. doi: 10.1039/c1np00063b. [DOI] [PubMed] [Google Scholar]
- Dickschat J. S.. Bacterial Terpene Cyclases. Nat. Prod. Rep. 2016;33(1):87–110. doi: 10.1039/C5NP00102A. [DOI] [PubMed] [Google Scholar]
- Christianson D. W.. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017;117(17):11570–11648. doi: 10.1021/acs.chemrev.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geris R., Simpson T. J.. Meroterpenoids Produced by Fungi. Nat. Prod. Rep. 2009;26(8):1063. doi: 10.1039/b820413f. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Abe I.. Biosynthesis of Fungal Meroterpenoids. Nat. Prod. Rep. 2016;33(1):26–53. doi: 10.1039/C5NP00090D. [DOI] [PubMed] [Google Scholar]
- Zhao M., Tang Y., Xie J., Zhao Z., Cui H.. Meroterpenoids Produced by Fungi: Occurrence, Structural Diversity, Biological Activities, and Their Molecular Targets. Eur. J. Med. Chem. 2021;209:112860. doi: 10.1016/j.ejmech.2020.112860. [DOI] [PubMed] [Google Scholar]
- Harms V., Kirschning A., Dickschat J. S.. Nature-Driven Approaches to Non-Natural Terpene Analogues. Nat. Prod. Rep. 2020;37(8):1080–1097. doi: 10.1039/C9NP00055K. [DOI] [PubMed] [Google Scholar]
- Harms V., Ravkina V., Kirschning A.. Mechanistic Similarities of Sesquiterpene Cyclases PenA, Omp6/7, and BcBOT2 Are Unraveled by an Unnatural “FPP-Ether” Derivative. Org. Lett. 2021;23(8):3162–3166. doi: 10.1021/acs.orglett.1c00882. [DOI] [PubMed] [Google Scholar]
- Tran C. D., Dräger G., Struwe H. F., Siedenberg L., Vasisth S., Grunenberg J., Kirschning A.. Cyclopropylmethyldiphosphates Are Substrates for Sesquiterpene Synthases: Experimental and Theoretical Results. Org. Biomol. Chem. 2022;20(39):7833–7839. doi: 10.1039/D2OB01279K. [DOI] [PubMed] [Google Scholar]
- Weigel B., Ludwig J., Weber R. A., Ludwig S., Lennicke C., Schrank P., Davari M. D., Nagia M., Wessjohann L. A.. Heterocyclic and Alkyne Terpenoids by Terpene Synthase-Mediated Biotransformation of Non-Natural Prenyl Diphosphates: Access to New Fragrances and Probes. ChemBioChem. 2022;23(21):e202200211. doi: 10.1002/cbic.202200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller M., Dhar D., Dräger G., Özbasi M., Struwe H., Wildhagen M., Davari M. D., Beutel S., Kirschning A.. Sesquiterpene Cyclase BcBOT2 Promotes the Unprecedented Wagner-Meerwein Rearrangement of the Methoxy Group. J. Am. Chem. Soc. 2024;146(26):17838–17846. doi: 10.1021/jacs.4c03386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucznik T., Syntrivanis L.-D., Baś S., Mikulak-Klucznik B., Moskal M., Szymkuć S., Mlynarski J., Gadina L., Beker W., Burke M. D., Tiefenbacher K., Grzybowski B. A.. Computational Prediction of Complex Cationic Rearrangement Outcomes. Nature. 2023;625(7995):508–515. doi: 10.1038/s41586-023-06854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struwe H., Droste J., Dhar D., Davari M. D., Kirschning A.. Chemoenzymatic Synthesis of a New Germacrene Derivative Named Germacrene F. ChemBioChem. 2024;25(1):e202300599. doi: 10.1002/cbic.202300599. [DOI] [PubMed] [Google Scholar]
- Budde J. L., Çay M.-Y., Dräger G., Droste J., Hassanin A., Davari M. D., Kirschning A.. Reprogramming the Cyclization of the Sesquiterpene Synthase BcBOT2 Using 2,3-Z-Configured FPP Derivatives and by Means of “Methyl Mapping. ACS Catal. 2025;15(10):8125–8139. doi: 10.1021/acscatal.5c01224. [DOI] [Google Scholar]
- Wilkinson S. M., Price J., Kassiou M.. Improved Accessibility to the Desoxy Analogues of Δ9-Tetrahydrocannabinol and Cannabidiol. Tetrahedron Lett. 2013;54(1):52–54. doi: 10.1016/j.tetlet.2012.10.080. [DOI] [Google Scholar]
- Anand R., Anand L. K., Rashid N., Painuli R., Malik F., Singh P. P.. Synthesis and Evaluation of Natural and Unnatural Tetrahydrocannabiorcol for Its Potential Use in Neuropathologies. J. Nat. Prod. 2024;87(2):167–175. doi: 10.1021/acs.jnatprod.3c00172. [DOI] [PubMed] [Google Scholar]
- Kun Lau C., Mintz M., Bernstein M. A., Dufresne C.. Dichlorophenylborane a New Reagent for the Preparation of 2-Phenyl-4H-1,3,2-Benzodioxaborins. Tetrahedron Lett. 1993;34(35):5527–5530. doi: 10.1016/S0040-4039(00)73872-X. [DOI] [Google Scholar]
- Quílez Del Moral J. F., Ruiz Martínez C., Pérez Del Pulgar H., Martín González J. E., Fernández I., López-Pérez J. L., Fernández-Arteaga A., Barrero A. F.. Synthesis of Cannabinoids: “In Water” and “On Water” Approaches: Influence of SDS Micelles. J. Org. Chem. 2021;86(4):3344–3355. doi: 10.1021/acs.joc.0c02698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse R., Gruner K. K., Kataeva O., Schmidt A. W., Knölker H.. Efficient Construction of Pyrano[3,2- a ]Carbazoles: Application to a Biomimetic Total Synthesis of Cyclized Monoterpenoid Pyrano[3,2- a ]Carbazole Alkaloids. Chem. - Eur. J. 2013;19(42):14098–14111. doi: 10.1002/chem.201301792. [DOI] [PubMed] [Google Scholar]
- Appendino G., Cravotto G., Tagliapietra S., Nano G. M., Palmisano G.. The Chemistry of Coumarin Derivatives, Part 2. Reaction of 4-hydroxycoumarin with α,B-unsaturated Aldehydes. Helv. Chim. Acta. 1990;73(7):1865–1878. doi: 10.1002/hlca.19900730709. [DOI] [Google Scholar]
- Zeng H., Duan D., Tang B.. Biomimetic Total Synthesis of 6a,7,8,9,10,10a-Hexahydro-3,6,9-Trimethyl-6-(4-Methylpent-3-En-1-Yl)-1,9-Epoxy-6H-Dibenzo-[b,d]Pyran and Its Diastereoisomer. Synlett. 2015;26(07):927–930. doi: 10.1055/s-0034-1380122. [DOI] [Google Scholar]
- Goossens R., Lhomeau G., Forier B., Toppet S., Meervelt L. V., Dehaen W.. Acid-Catalyzed Condensation of Citronellal and Electron Rich Phenols: Mechanism and Functionalization of the Adducts. Tetrahedron. 2000;56(47):9297–9303. doi: 10.1016/S0040-4020(00)00822-X. [DOI] [Google Scholar]
- Syntrivanis L.-D., Némethová I., Schmid D., Levi S., Prescimone A., Bissegger F., Major D. T., Tiefenbacher K.. Four-Step Access to the Sesquiterpene Natural Product Presilphiperfolan-1β-Ol and Unnatural Derivatives via Supramolecular Catalysis. J. Am. Chem. Soc. 2020;142(12):5894–5900. doi: 10.1021/jacs.0c01464. [DOI] [PubMed] [Google Scholar]
- Cornu I., Syntrivanis L.-D., Tiefenbacher K.. Biomimetic Tail-to-Head Terpene Cyclizations Using the Resorcin[4]Arene Capsule Catalyst. Nat. Protoc. 2024;19(2):313–339. doi: 10.1038/s41596-023-00919-3. [DOI] [PubMed] [Google Scholar]
- MacGillivray L. R., Atwood J. L.. A Chiral Spherical Molecular Assembly Held Together by 60 Hydrogen Bonds. Nature. 1997;389(6650):469–472. doi: 10.1038/38985. [DOI] [Google Scholar]
- Shivanyuk A., Rebek J.. Reversible Encapsulation by Self-Assembling Resorcinarene Subunits. Proc. Natl. Acad. Sci. U. S. A. 2001;98(14):7662–7665. doi: 10.1073/pnas.141226898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram L., Cohen Y.. Spontaneous Formation of Hexameric Resorcinarene Capsule in Chloroform Solution as Detected by Diffusion NMR. J. Am. Chem. Soc. 2002;124(51):15148–15149. doi: 10.1021/ja0272686. [DOI] [PubMed] [Google Scholar]
- Avram L., Cohen Y., Rebek J. Jr.. Recent Advances in Hydrogen-Bonded Hexameric Encapsulation Complexes. Chem. Commun. 2011;47(19):5368–5375. doi: 10.1039/C1CC10150A. [DOI] [PubMed] [Google Scholar]
- Gaeta C., Talotta C., De Rosa M., La Manna P., Soriente A., Neri P.. The Hexameric Resorcinarene Capsule at Work: Supramolecular Catalysis in Confined Spaces. Chem. - Eur. J. 2019;25(19):4899–4913. doi: 10.1002/chem.201805206. [DOI] [PubMed] [Google Scholar]
- Syntrivanis L.-D., Tiefenbacher K.. Reactivity Inside Molecular Flasks: Acceleration Modes and Types of Selectivity Obtainable. Angew. Chem., Int. Ed. 2024;63(49):e202412622. doi: 10.1002/anie.202412622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Némethová I., Schmid D., Tiefenbacher K.. Supramolecular Capsule Catalysis Enables the Exploration of Terpenoid Chemical Space Untapped by Nature. Angew. Chem., Int. Ed. 2023;62(14):e202218625. doi: 10.1002/anie.202218625. [DOI] [PubMed] [Google Scholar]
- Rising K. A., Crenshaw C. M., Koo H. J., Subramanian T., Chehade K. A. H., Starks C., Allen K. D., Andres D. A., Spielmann H. P., Noel J. P., Chappell J.. Formation of a Novel Macrocyclic Alkaloid from the Unnatural Farnesyl Diphosphate Analogue Anilinogeranyl Diphosphate by 5-Epi-Aristolochene Synthase. ACS Chem. Biol. 2015;10(7):1729–1736. doi: 10.1021/acschembio.5b00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin S. V., Shenvi R. A.. Synthesis of Highly Strained Terpenes by Non-Stop Tail-to-Head Polycyclization. Nat. Chem. 2012;4(11):915–920. doi: 10.1038/nchem.1458. [DOI] [PubMed] [Google Scholar]
- La Manna P., Talotta C., Floresta G., De Rosa M., Soriente A., Rescifina A., Gaeta C., Neri P.. Mild Friedel–Crafts Reactions inside a Hexameric Resorcinarene Capsule: C–Cl Bond Activation through Hydrogen Bonding to Bridging Water Molecules. Angew. Chem., Int. Ed. 2018;57(19):5423–5428. doi: 10.1002/anie.201801642. [DOI] [PubMed] [Google Scholar]
- Iuliano V., Talotta C., De Rosa M., Soriente A., Neri P., Rescifina A., Floresta G., Gaeta C.. Insights into the Friedel–Crafts Benzoylation of N -Methylpyrrole inside the Confined Space of the Self-Assembled Resorcinarene Capsule. Org. Lett. 2023;25(35):6464–6468. doi: 10.1021/acs.orglett.3c01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammer J., Nolte C., Mayr H.. Free Energy Relationships for Reactions of Substituted Benzhydrylium Ions: From Enthalpy over Entropy to Diffusion Control. J. Am. Chem. Soc. 2012;134(33):13902–13911. doi: 10.1021/ja306522b. [DOI] [PubMed] [Google Scholar]
- Mayr H., Bug T., Gotta M. F., Hering N., Irrgang B., Janker B., Kempf B., Loos R., Ofial A. R., Remennikov G., Schimmel H.. Reference Scales for the Characterization of Cationic Electrophiles and Neutral Nucleophiles. J. Am. Chem. Soc. 2001;123(39):9500–9512. doi: 10.1021/ja010890y. [DOI] [PubMed] [Google Scholar]
- Croteau R.. Biosynthesis and Catabolism of Monoterpenoids. Chem. Rev. 1987;87(5):929–954. doi: 10.1021/cr00081a004. [DOI] [Google Scholar]
- Zhang Q., Catti L., Pleiss J., Tiefenbacher K.. Terpene Cyclizations inside a Supramolecular Catalyst: Leaving-Group-Controlled Product Selectivity and Mechanistic Studies. J. Am. Chem. Soc. 2017;139(33):11482–11492. doi: 10.1021/jacs.7b04480. [DOI] [PubMed] [Google Scholar]
- Scanlon J. T., Willis D. E.. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985;23(8):333–340. doi: 10.1093/chromsci/23.8.333. [DOI] [Google Scholar]
- Butin A. V., Smirnov S. K., Trushkov I. V.. The Effect of an N-Substituent on the Recyclization of (2-Aminoaryl)Bis(5-Tert-Butyl-2-Furyl)Methanes: Synthesis of 3-Furylindoles and Triketoindoles. Tetrahedron Lett. 2008;49(1):20–24. doi: 10.1016/j.tetlet.2007.11.015. [DOI] [Google Scholar]
- Hadj-Abo F., Bienz S., Hesse M.. Synthesis of [10]Heterophanes Using a Ring Enlargement Reaction. Tetrahedron. 1994;50(29):8665–8672. doi: 10.1016/S0040-4020(01)85341-2. [DOI] [Google Scholar]
- G Kruse C., P Bouw J., van Hes R., van de Kuilen A., A J den Hartog J.. New Methods for the Synthesis of 2-Arylpyrroles. HETEROCYCLES. 1987;26(12):3141. doi: 10.3987/R-1987-12-3141. [DOI] [Google Scholar]
- Tanis S. P., Chuang Y. H., Head D. B.. Furans in Synthesis. 8. Formal Total Syntheses of (.+-.)- and (+)-Aphidicolin. J. Org. Chem. 1988;53(21):4929–4938. doi: 10.1021/jo00256a007. [DOI] [Google Scholar]
- Mouarrawis V., Plessius R., van der Vlugt J. I., Reek J. N. H.. Confinement Effects in Catalysis Using Well-Defined Materials and Cages. Front Chem. 2018;6:623. doi: 10.3389/fchem.2018.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. D., Hunter C. A., Williams N. H.. Coordination Cages Based on Bis(Pyrazolylpyridine) Ligands: Structures, Dynamic Behavior, Guest Binding, and Catalysis. Acc. Chem. Res. 2018;51(9):2073–2082. doi: 10.1021/acs.accounts.8b00261. [DOI] [PubMed] [Google Scholar]
- Hong C. M., Bergman R. G., Raymond K. N., Toste F. D.. Self-Assembled Tetrahedral Hosts as Supramolecular Catalysts. Acc. Chem. Res. 2018;51(10):2447–2455. doi: 10.1021/acs.accounts.8b00328. [DOI] [PubMed] [Google Scholar]
- Percástegui E. G., Ronson T. K., Nitschke J. R.. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020;120(24):13480–13544. doi: 10.1021/acs.chemrev.0c00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M., Bierschenk S. M., Xia K. T., Bergman R. G., Raymond K. N., Toste F. D.. Advances in Supramolecular Host-Mediated Reactivity. Nat. Catal. 2020;3(12):969–984. doi: 10.1038/s41929-020-00528-3. [DOI] [Google Scholar]
- Grommet A. B., Feller M., Klajn R.. Chemical Reactivity under Nanoconfinement. Nat. Nanotechnol. 2020;15(4):256–271. doi: 10.1038/s41565-020-0652-2. [DOI] [PubMed] [Google Scholar]
- Wang K., Jordan J. H., Hu X.-Y., Wang L.. Supramolecular Strategies for Controlling Reactivity within Confined Nanospaces. Angew. Chem., Int. Ed. 2020;59(33):13712–13721. doi: 10.1002/anie.202000045. [DOI] [PubMed] [Google Scholar]
- Hooley R. J.. No, Not That Way, the Other Way: Creating Active Sites in Self-Assembled Host Molecules. Synlett. 2020;31(15):1448–1463. doi: 10.1055/s-0040-1707125. [DOI] [Google Scholar]
- Yu Y., Yang J.-M., Rebek J.. Molecules in Confined Spaces: Reactivities and Possibilities in Cavitands. Chem. 2020;6(6):1265–1274. doi: 10.1016/j.chempr.2020.04.014. [DOI] [Google Scholar]
- Mitschke B., Turberg M., List B.. Confinement as a Unifying Element in Selective Catalysis. Chem. 2020;6(10):2515–2532. doi: 10.1016/j.chempr.2020.09.007. [DOI] [Google Scholar]
- Ashbaugh H. S., Gibb B. C., Suating P.. Cavitand Complexes in Aqueous Solution: Collaborative Experimental and Computational Studies of the Wetting, Assembly, and Function of Nanoscopic Bowls in Water. J. Phys. Chem. B. 2021;125(13):3253–3268. doi: 10.1021/acs.jpcb.0c11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan T. J., Gunawardana V. W. L., Badjić J. D.. Molecular Recognition of Nerve Agents and Their Organophosphorus Surrogates: Toward Supramolecular Scavengers and Catalysts. Chem. - Eur. J. 2021;27(53):13280–13305. doi: 10.1002/chem.202101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa H., Fujita M.. Molecular Confinement Effects by Self-Assembled Coordination Cages. Bull. Chem. Soc. Jpn. 2021;94(10):2351–2369. doi: 10.1246/bcsj.20210273. [DOI] [Google Scholar]
- Olivo G., Capocasa G., Del Giudice D., Lanzalunga O., Di Stefano S.. New Horizons for Catalysis Disclosed by Supramolecular Chemistry. Chem. Soc. Rev. 2021;50(13):7681–7724. doi: 10.1039/D1CS00175B. [DOI] [PubMed] [Google Scholar]
- Wang R., Rebek J., Yu Y.. Organic Radical Reactions Confined to Containers in Supramolecular Systems. Chem. Commun. 2022;58(12):1828–1833. doi: 10.1039/D1CC06851B. [DOI] [PubMed] [Google Scholar]
- Piskorz T. K., Martí-Centelles V., Spicer R. L., Duarte F., Lusby P. J.. Picking the Lock of Coordination Cage Catalysis. Chem. Sci. 2023;14(41):11300–11331. doi: 10.1039/D3SC02586A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Lu Y.-L., Jiao Z., Su C.-Y.. Photocatalysis Meets Confinement: An Emerging Opportunity for Photoinduced Organic Transformations. Angew. Chem., Int. Ed. 2024;63(15):e202317808. doi: 10.1002/anie.202317808. [DOI] [PubMed] [Google Scholar]
- Andrews K. G.. Beyond Symmetric Self-Assembly and Effective Molarity: Unlocking Functional Enzyme Mimics with Robust Organic Cages. Beilstein J. Org. Chem. 2025;21:421–443. doi: 10.3762/bjoc.21.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data for the table and figures have been deposited at https://zenodo.org/records/15754845.


