Abstract
Efficient photocatalytic tumor therapy relies on deep tissue penetration via near-infrared (NIR) light absorption and potent oxidative stress induced by efficient photogenerated hole (h+) migration. Herein, we report a supramolecular donor–acceptor (D–A) structure fabricated by integrating self-assembled tetra(4-carboxyphenyl) porphyrin (SA-TCPP) with amino-functionalized graphene quantum dots (GQDs-NH2). The D–A configuration significantly narrowed the band gap for extended NIR absorption and amplified the interfacial electric field 6.2 times. This facilitated efficient h+ migration with a 5.3-fold enhancement, generating highly oxidation holes. Under 700 nm irradiation, this system induced potent oxidative cell apoptosis, leading to 100% tumor elimination in vivo. This study presents a dual-promotion strategy involving NIR responsiveness and charge migration, advancing the efficiency of photocatalytic cancer therapy.
Keywords: photocatalytic therapy, near-infrared response, charge migration, interfacial electric field, D−A structure


Introduction
Despite the emergence of various clinical therapies, cancer remains a formidable threat to human health, often complicated by challenges such as drug resistance, low treatment response rate, and significant toxic side effects. , Photocatalytic therapy (PCT) has recently emerged as a promising strategy, offering the potential for efficient, safe, and precise treatment. PCT leverages photocatalysts that absorb light energy, triggering reactions that disrupt the local the oxidation–reduction (REDOX) balance within the tumor microenvironment and inducing irreversible cancer cell apoptosis. Specifically, upon light excitation, photogenerated holes (h+) in the valence band and the electrons (e–) in the conduction band migrate to the catalyst surface, generating reactive oxygen species (ROS) or directly oxidizing biomolecules to kill cancer cells. However, the efficacy of PCT is often hampered because most traditional photocatalysts are activated by ultraviolet–visible (UV–vis) light, which has limited tissue penetration depth (only a few millimeters). Near-infrared (NIR) light (700–1100 nm), conversely, penetrates biological tissues more effectively (up to ca. 1 cm) through a light transparent window, making it ideal for treating deeper tumors. Consequently, there is an urgent need for advanced photocatalysts that exhibit strong NIR absorption and facilitate efficient charge migration under NIR excitation. ,− While some NIR-responsive materials exist, they often rely on strategies like incorporating potentially toxic heavy metals or employing upconversion techniques, the latter of which can suffer from low overall light utilization efficiency. , Therefore, developing biocompatible photocatalysts directly excitable by NIR light is crucial for advancing safe and effective deep-tissue PCT.
Organic semiconductor photocatalysts, including porphyrin, phthalocyanine, and indocyanine green, have garnered significant attention for biomedical photocatalysis due to their tunable spectral responses, good biocompatibility, − and facile structure modification. , Porphyrins, a class of chromophores with π-conjugated electrons, are particularly noteworthy for their broad light absorption, excellent photoelectric conversion capabilities, and high chemical stability. Among them, tetra(4-carboxyphenyl) porphyrin (TCPP) is an attractive precursor owing to its strong light-harvesting ability and good hydrophilicity. , Our previous work demonstrated that self-assembled TCPP (SA-TCPP), formed through hydrogen bonding, π–π stacking, and hydrophobic interaction, adopts a unique supramolecular structure that can selectively accumulate in tumor cells through size regulation. Under 600 nm irradiation, SA-TCPP can generate strong oxidative h+ for rapid elimination of solid tumors. However, it exhibits poor photocatalytic activity under NIR irradiation due to its limited intrinsic NIR absorption and substantial charge carrier recombination. Thus, designing SA-TCPP derivatives with enhanced NIR-responsiveness and improved charge separation and migration efficiency is essential for effective tumor in-depth treatment.
Several strategies have been developed to broaden the light absorption and enhance charge transfer in organic semiconductors, including constructing donor–acceptor (D–A) structure, introducing auxochromic groups, extending π-conjugate systems, and leveraging plasmon resonance. Among these, constructing D–A heterostructures is particularly effective, as it can establish a strong interfacial electric field (IEF), providing an intrinsic driving force for the separation and migration of photogenerated carriers. , Amino-functionalized graphene quantum dots (GQDs-NH2), as auxochrome groups containing lone π-electrons, can effectively enhance light absorption through quantum confinement effects and π-plasmon resonance. , Inspired by the excellent electron transfer properties, which are known to facilitate charge separation, we hypothesized that modifying SA-TCPP with GQDs-NH2 to construct a D–A heterostructure could not only expand light to the NIR region but also improve charge separation efficiency, thereby addressing current challenges in photocatalytic tumor therapy.
Herein, we report the successful fabrication of a D–A supramolecular structure, designated SA-TCPP@GQDs, via electrostatic assembly of SA-TCPP and GQDs-NH2. This integration significantly enhanced intermolecular π–π conjugation and extended the intrinsic absorption band edge to the NIR region (up to 833 nm). Crucially, the D–A interaction established a strong interfacial electric field (IEF) within SA-TCPP@GQDs, markedly promoting the separation and migration efficiency of photogenerated carriers (a 5.3-fold increase for h+ migration). Once internalized by cancer cells and irradiated with near-infrared light (700 nm), SA-TCPP@GQDs generated a substantially enhanced flux of highly oxidizing holes. These holes directly induced potent oxidative stress, damaging vital intracellular components and triggering irreversible tumor cell apoptosis (Scheme ). Notably, the efficient generation of oxidizing h+ under NIR light offers potential advantages for treating hypoxic tumors, which are often resistant to therapies relying solely on oxygen-dependent ROS. Furthermore, SA-TCPP@GQDs demonstrated favorable tumor targeting capabilities, blood circulation stability, and biological metabolism in vivo, making it highly promising for safe and efficient PCT treatment. This study presents a novel approach for NIR-responsive cancer therapy by constructing the D–A system that enhanced light absorption and promoted charge separation simultaneously.
1. Schematic Illustration of Supramolecular SA-TCPP@GQDs Nanoparticles Increased Photogenerated Holes under Near-Infrared (NIR) Light for Efficient Photocatalytic Cancer Therapy.

Results and Discussion
Fabrication and Characterization of Supramolecular SA-TCPP@GQDs with D–A Structure
Self-assembled tetra(4-carboxyphenyl) porphyrin (SA-TCPP) was prepared following the previously reported method. Subsequently, amino-functionalized graphene quantum dots (GQDs-NH2) were integrated into SA-TCPP through coassembly to yield the SA-TCPP@GQDs. Transmission electron microscopy (TEM) images showed that SA-TCPP@GQDs formed nanoparticles with an average size of 34.7 nm and a thickness of about 50 nm (Figure a, ). GQDs-NH2 was uniformly distributed on the surface of SA-TCPP (). High-resolution transmission electron microscopy (HRTEM) images displayed distinct closely adjacent lattice fringes of SA-TCPP and GQDs-NH2, corresponding to a d-spacing of 0.35 and 0.20 nm, respectively (Figure b). The former (0.35 nm) confirms π–π stacking in the porphyrin supramolecular structure, while the latter demonstrates the successful integration of GQDs-NH2. This close interfacial contact between the two components confirms the formation of an intimate nanoheterojunction.
1.
Characterization of D–A supramolecular SA-TCPP@GQDs. (a) TEM images and (b) HRTEM images of SA-TCPP@GQDs (the mass ratio of GQDs-NH2 to SA-TCPP is 5%). (c) XRD pattern of TCPP, SA-TCPP, GQDs-NH2, and SA-TCPP@GQDs. (d) FT-IR spectra of SA-TCPP and SA-TCPP@GQDs. (e) Zeta potential measurements of SA-TCPP, GQDs-NH2, and SA-TCPP@GQDs. (f) Frontier molecular orbital distribution of SA-TCPP@GQDs.
The presence of high crystallinity and a well-defined interface within SA-TCPP@GQDs is expected to be conducive to establishing a strong interfacial electric field. In the X-ray diffraction (XRD) pattern (Figure c), SA-TCPP shows a diffraction peak of about 21° due to π–π stacking of supramolecular structure, while the diffraction peak of SA-TCPP@GQDs shifts to higher angles, indicating that the introduction of GQDs-NH2 enhances the conjugation interaction of the porphyrin assembly, thereby shortening the intermolecular π–π stacking distance. Raman spectra further reveal that the ratio of the two characteristic vibration peaks (I 1/2) of SA-TCPP@GQDs is 0.87, which is slightly greater than that of SA-TCPP (0.83), suggesting that the π–π stacking degree of SA-TCPP@GQDs is higher than that of SA-TCPP ().
A Fourier-transformed infrared (FT-IR) spectrum (Figure d) shows that the vibration frequency of C–O in SA-TCPP@GQDs is lower than that of SA-TCPP, attributed to the electrostatic interaction between −COOH of SA-TCPP and −NH2 of GQDs-NH2. Furthermore, SA-TCPP@GQDs have a zeta potential of +13.6 mV, in contrast to −68.3 mV for SA-TCPP, following electrostatic adsorption by amine-rich GQDs-NH2 (Figure e). This positive zeta potential is beneficial for cell membrane combination, thereby improving intracellular uptake efficiency. Besides, SA-TCPP exhibits good hydrophilicity due to the four carboxyl groups at the end of TCPP, and the hydrophilicity is further enhanced after modification by GQDs-NH2 ().
Moreover, the theoretical calculations of the frontier molecular orbital distribution showed that the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of SA-TCPP@GQDs are located on the GQDs-NH2 moiety and SA-TCPP moiety, respectively (Figure f). This distribution directly confirms the characteristics of the donor–acceptor (D–A) structure. Collectively, the above results show that supramolecular SA-TCPP@GQDs have a D–A structure and enhanced conjugation effect, which is expected to expand the range of light absorption and promote charge transfer transition.
D–A Structure Broadens NIR Absorption and Promotes Charge Migration
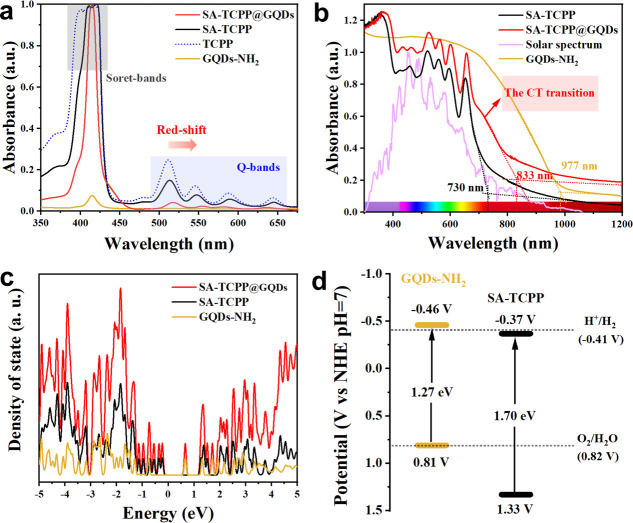
The strong electrostatic interaction between the carboxyl and amino groups promotes the π–π conjugation and decreases the π–π* transition energy, thus contributing to the redshift of SA-TCPP@GQDs. As shown in Figure a, the typical Q-bands (500–700 nm) in SA-TCPP and SA-TCPP@GQDs show a redshift compared with the porphyrin monomer (TCPP), indicating the formation of J-type aggregates in the supramolecular systems. Notably, the absorption redshift of SA-TCPP@GQDs is even larger, and the absorption peak in Q-bands decreases, proving that the electrostatic interaction further promoted the intermolecular conjugated interaction. As a result, the intrinsic absorption band edge of SA-TCPP@GQDs reaches the NIR region of 833 nm (Figure b).
2.
D–A charge transfer transition enhances the NIR absorption. (a) UV–vis absorption spectra of TCPP, SA-TCPP, SA-TCPP@GQDs, and SA-TCPP@GQDs-NH2. (b) Diffuse reflection spectroscopy of samples and solar energy utilization. (c) Calculated electronic density of states (DOS). (d) Energy band structure diagrams for GQDs-NH2 and SA-TCPP.
UV–vis diffuse reflection spectroscopy (DRS) reveals a new absorption peak at ∼700 nm, indicating a charge transfer (CT) transition between SA-TCPP and GQDs-NH2 (). The theoretical model for the calculation of the electronic density of states (DOS) () was further carried out. As shown in Figure c, SA-TCPP has semiconductor properties where the Fermi level is positioned in the middle of the gap without any electron distribution around the Fermi level, and the incorporation of GQDs-NH2 introduces positively correlated DOS near the Fermi level. This suggests the formation of transition energy states during the CT transitions in SA-TCPP@GQDs, which are composed of localized or isolated occupied states. Such states not only narrow the band gap but also facilitate electron diffusion via a polaron-hopping mechanism. ,
Based on the electrochemical Mott–Schottky curve and band gap analysis (), the band structure diagram is obtained and shown in Figure d. The valence band position of GQDs-NH2 is higher than that of SA-TCPP, while the conduction band position is lower than that of SA-TCPP. Their staggered energy level structure would greatly facilitate photogenerated carrier migration between materials. These findings underscore the impact of the D–A structure and electrostatic interactions on enhancing light absorption and promoting charge separation and migration, positioning SA-TCPP@GQDs as a promising candidate for NIR-responsive photocatalysts.
The charge transfer mechanism between GQDs-NH2 and SA-TCPP was further investigated using Kelvin probe force microscopy (KPFM) in a N2 atmosphere to measure the contact potential difference (CPD). The work functions (WFs) of SA-TCPP and GQDs-NH2 are calculated as 4.53 and 4.59 eV, and the relative Fermi levels (FLs) of the two are determined and illustrated in . The FL of SA-TCPP was significantly higher than that of GQDs-NH2 before contact. When the two are in close contact due to electrostatic interaction, as shown in Figure a, the intrinsic electrons spontaneously transfer from the GQDs-NH2 to SA-TCPP until the Fermi levels of the two are in equilibrium and the energy bands upward and downward, respectively. As a result, the charge transfer pathway at the interface was successfully established. In this case, an interfacial electric field (IEF) from the SA-TCPP toward the GQDs-NH2 was generated, which could be a powerful driving force to promote the separation and migration of photogenerated carriers. On that account, the IEF intensity was quantitatively determined by the model developed by Kanata et al. (), and the IEF of SA-TCPP@GQDs is about 6.2 times higher than that of SA-TCPP (Figure b). The internal CT transition behavior of SA-TCPP@GQDs not only increases the NIR absorption but also induces the generation of IEF, which is expected to promote charge separation and migration.
3.
Efficient charge transfer facilitated by the interfacial electric field (IEF) in D–A structure. (a) Schematic of charge transfer route and IEF direction in SA-TCPP@GQDs. (b) IEF intensity for SA-TCPP and SA-TCPP@GQDs. (c) Transient photovoltage (TPV) spectra (excitation pulse at 355 nm and 50 mJ). (d) Electrochemical impedance spectroscopy (EIS) Nyquist plots. (e) Transient absorption spectra of SA-TCPP@GQDs at different time delays and SA-TCPP (femtosecond laser excitation at 370 nm). (f) Time profiles of normalized fitted decay kinetics of transient absorption at 465 nm. (g) Time-resolved fluorescence decay spectra (ex@430 nm). (h) Surface photovoltage spectra (SPV). (i) Photogenerated holes of SA-TCPP and SA-TCPP@GQDs detected with in situ CLSM.
Furthermore, the separation and migration of photogenerated carriers facilitated by the IEF are systematically investigated. First, KPFM was used to determine the surface potential. The surface potential of SA-TCPP@GQDs (ΔE = 48 mV) is about 1.9 times that of SA-TCPP (ΔE = 25 mV) (), which proves the photogenerated carrier separation efficiency of SA-TCPP@GQDs driven by IEF. Photoelectron chemical measurement was used to assess the charge behavior. The photocurrent of SA-TCPP@GQDs was much stronger than that of SA-TCPP under light (), indicating that the IEF can promote carrier separation effectively. Transient photovoltage (TPV) spectroscopy showed that SA-TCPP@GQDs exhibit high first peak photovoltage and long second peak photovoltage (Figure c), demonstrating that IEF can significantly improve the charge separation efficiency.
Electrochemical impedance spectroscopy (EIS) was measured to evaluate the resistance of charge transfer (Figure d). Obviously, SA-TCPP@GQDs has a smaller arc diameter and a lower fitted charge transfer resistance (R 2) than SA-TCPP (), indicating that the IEF is conducive to decreasing the charge transfer resistance and thus promoting the charge transfer kinetics. The femtosecond transient absorption spectra (TAS) were measured at 370 nm excitation. As shown in Figure e, the positive signal of SA-TCPP@GQDs at 430–720 nm is wider and stronger than that of SA-TCPP, which comes from the excited state absorption (ESA) of the singlet excited state. Furthermore, the decay kinetics of photogenerated carriers were revealed by fitting the time curve of the transient absorption detected at 465 nm (Figure f). Compared with SA-TCPP (τ1 = 135.73 ps), the captured electron lifetime (τ1) in SA-TCPP@GQDs decreases significantly to 12.96 ps, which indicates that the interfacial electron transfer is faster in D–A structure. At the same time, due to the longer exciton relaxation process, the decay lifetime of SA-TCPP@GQDs τ2 (330.90 ps) is longer than that of SA-TCPP (68.42 ps), which means that photogenerated carriers have more opportunities to participate in the photocatalytic reaction.
The photoluminescence (PL) spectrum showed that the fluorescence emission peak of SA-TCPP@GQDs at 625–750 nm was significantly reduced compared to that of SA-TCPP (), indicating that IEF effectively inhibits the recombination of h+ and e–, thus improving the utilization rate of photogenerated carriers. Moreover, the time-resolved fluorescence decay (TR-PL) spectra measured at the steady-state emission peak show a longer lifetime (τ) of photogenerated carriers in SA-TCPP@GQDs (Figure g), further implying that the long-range transport of photogenerated carriers is significantly enhanced, driven by IEF. Surface photovoltage (SPV) was used to further evaluate the charge transfer behavior. As shown in Figure h, positive SPV signals of SA-TCPP@GQDs and SA-TCPP are displayed at 500–800 nm, indicating that the photogenerated holes accumulate on the catalyst surface as dominant carriers. It is worth noting that the SPV response of SA-TCPP@GQDs is significantly enhanced, indicating a higher carrier migration efficiency.
To map the surface reaction of photogenerated h+ and e–, the previously reported fluorescence detection method of resorufin was used, , and the h+ formation was observed and quantified by confocal laser fluorescence microscopy (CLSM). As illustrated in Figure i, a weak fluorescence signal of h+ exists in SA-TCPP (inset, left), while a stronger signal appears in SA-TCPP@GQDs (inset, right). To compare the concentration of holes, fluorescence quantity and fluorescence intensity are used to define the number and strength of fluorescence signals per unit area. Specifically, the relative fluorescence quantity and intensity of SA-TCPP@GQDs are 3.1 and 5.3 times stronger than those of SA-TCPP, which proves that the giant IEF greatly promotes effective migration of photogenerated h+.
Enhanced Photogenerated Holes for Efficient Photocatalytic Tumor Therapy in Vitro
Inspired by the above properties of NIR response and efficient charge migration, we investigated the cellular uptake behavior of SA-TCPP@GQDs and its therapeutic potential for PCT. Based on the unique multiwavelength excited self-fluorescence properties of SA-TCPP@GQDs (), the cellular uptake of nanoparticles can be detected and observed without additional fluorescence modifications. Flow cytometry analysis revealed concentration-dependent uptake in HeLa cells with an endocytosis efficiency of 97.6% at 12.5 μg/mL (Figure a). Notably, SA-TCPP@GQDs exhibited higher cellular uptake than SA-TCPP under identical conditions (), attributed to their positively charged surface (Figure e), which enhances electrostatic interaction with the negatively charged cell membrane. In contrast, uptake in normal cells (293T, MCF-10A, and L02) remained below 20% for both SA-TCPP and SA-TCPP@GQDs, as further confirmed by confocal microscopy (). Strikingly, >95% uptake efficiency was observed in various cancer cells (HeLa, MCF-7, and HepG2, Figure b), highlighting their exceptional selectivity for tumor cells and promising potential for targeted therapy.
4.
Tumor cell endocytosis of SA-TCPP and SA-TAPP@GQDs nanoparticles. (a) Cellular uptake rate of HeLa cells treated with different concentrations of SA-TCPP and SA-TCPP@GQDs for 12 h. (b) Comparison of cellular uptake rate of SA-TCPP and SA-TCPP@GQDs (12.5 μg/mL) in cancerous or normal cells. (c) Cellular uptake pathway study of SA-TCPP@GQDs (12.5 μg/mL). (d) CLSM images of HeLa cells incubated with SA-TCPP or SA-TCPP@GQDs. The cell nucleus and lysosome were stained by Hoechst 33258 (10 μg/mL) and 100 nM LysoTracker@Red before CSLM imaging, respectively. Scale bar: 20 μm.
Next, the cellular uptake pathway of the SA-TCPP@GQDs was studied. Prior to incubation with SA-TCPP@GQDs, HeLa cells were pretreated with different endocytosis inhibitors, including chlorpromazine and sucrose (clathrin-mediated endocytosis inhibitor), rottlerin (micropinocytosis inhibitor), nystatin (caveolae-mediated endocytosis inhibitor), and methyl-β-cyclodextrin (Me-β-CD, clathrin-independent endocytosis inhibitor), or incubated at 4 °C. As shown in Figure c, after the above inhibitor treatment, sucrose can slightly inhibit endocytosis, while Me-β-CD is significantly inhibited with the cellular uptake rate reduced to 16.3%. Furthermore, the cellular uptake of SA-TCPP@GQDs was significantly inhibited when the cells were treated at 4 °C. Therefore, clathrin-independent and energy-dependent endocytosis was confirmed for the uptake of SA-TCPP@GQDs.
Furthermore, the intracellular localization of the nanoparticles was monitored by using confocal laser scanning microscopy (CLSM). After treating HeLa cells with SA-TCPP and SA-TCPP@GQDs for 12 h, the accumulation of fluorescent nanoparticles in the cytosol was observed (Figure d). Moreover, it was shown that SA-TCPP@GQDs could more obviously enter the lysosomes by staining of the cell nucleus and endolysosomes before CLSM imaging (it appears as color overlap). Cytotoxicity experiments under dark conditions showed that HeLa cells treated with 400 μg/mL SA-TCPP and SA-TCPP@GQDs still had a survival rate of more than 85% (). This demonstrates the low intrinsic toxicity of both nanomaterials in the absence of light, even after cellular internalization and trafficking, including potential accumulation within the lysosomes. Therefore, the efficient cellular uptake observed for SA-TCPP@GQDs, combined with its excellent biocompatibility under dark conditions, provides a solid foundation for its subsequent use in light-triggered photocatalytic tumor therapy.
To explore the PCT potential of SA-TCPP@GQDs, the intracellular generation of ROS was monitored using the fluorescent probe DCFH-DA. As shown in Figure a, DPBS, SA-TCPP, and SA-TCPP@GQDs treated cells showed no obvious green fluorescence signal under dark conditions, indicating minimal baseline ROS. After irradiation at 700 nm for 15 min, HeLa cells treated with SA-TCPP showed enhanced fluorescence to a certain extent, while SA-TCPP@GQDs showed more obvious ROS green fluorescence. Flow cytometry was employed for the quantitative analysis (Figure b). The results confirmed that the fluorescence intensity of SA-TCPP@GQDs-treated cells was 2.6 times higher than that of SA-TCPP, clearly indicating superior ROS generation by SA-TCPP@GQDs under NIR irradiation. Furthermore, active species capture experiments were performed to identify the primary active species. As shown in Figure c, by comparing the apoptosis rate of HeLa cells treated with SA-TCPP@GQDs and then adding different trapping agents under lighting, it was found that sodium azide (NaN3) did not significantly reduce the killing effect after capture of 1O2, proving that this process is different from the photodynamic therapy (PDT) dependent on O2. In addition, 1O2, •OH, •O2 –, and h+ were captured by sodium azide (NaN3), tert-butanol (t-BuOH), p-benzoquinone (p-BQ), and formic acid (KI), respectively, and the cell viability rate of HeLa cells decreased gradually under light. The above results showed that holes were the main reactive oxygen species, and the contribution sequence of these active species to photocatalytic cell killing was h+ > •O2 – > •OH > 1O2. Furthermore, the above contribution order was also proven in the capture experiments of bisphenol A (BPA) degradation (). Additionally, the signals of the above active species were detected by ESR and showed varying degrees of signal enhancement over time ().
5.
ROS levels enhanced under NIR light for effectively killing cancer cells. (a) Fluorescence images of intracellular ROS assay using 10 μM DCFH-DA after HeLa cells treated with DPBS, SA-TCPP, and SA-TCPP@GQDs for 12 h. Scale bar: 50 μm. The concentration of photocatalyst was 12.5 μg/mL. The illumination conditions were 700 nm, 70 mW/cm2, and 15 min. (b) Normalized fluorescence intensity of HeLa cells with the above treatments. n = 3, ***p < 0.001 (TTEST). (c) Active species capture experiment in the SA-TCPP@GQDs (12.5 μg/mL) photocatalytic killing of tumor cells. (d) Cytotoxicity of HeLa cells was treated by DPBS, SA-TCPP, and SA-TCPP@GQDs with different wavelength irradiation. (e) Comparison of growth inhibition of different cancer cell lines treated with SA-TCPP@GQDs. All cell lines were treated with 12.5 μg/mL SA-TCPP@GQDs for 12 h before viability was assayed.
On this basis, the photocatalytic therapy of tumor cells was further explored. Under 700 nm irradiation (30 min, 70 mW/cm2), HeLa cells treated with SA-TCPP@GQDs showed concentration-dependent apoptosis (), while SA-TCPP treatment had no obvious inhibition of cell growth. To explore the light response window of the two materials, HeLa cells treated with 12.5 μg/mL SA-TCPP@GQDs were irradiated with wavelengths of 600, 650, 700, and 750 nm, respectively, and the cell survival rate was analyzed. As shown in Figure d, SA-TCPP showed certain inhibition of HeLa cell growth at 600 and 650 nm but had no ideal inhibition effect starting from 700 nm. The LC50 value of SA-TCPP-treated HeLa cells (600 nm) was 12.5 μg/mL, while it was 6.25 μg/mL for SA-TCPP@GQD-treated (700 nm) cells, demonstrating the low-dose and NIR-responsive photocatalytic properties. Notably, the cell survival rate of SA-TCPP@GQDs under the above light source conditions was lower than 40%, demonstrating excellent NIR light-responsive cell killing ability. Furthermore, under NIR-light, SA-TCPP@GQDs significantly enhanced photogenerated h+ and achieved strong oxidative killing of a variety of tumor cells (Figure e). In addition, the PCT efficiency of SA-TCPP@GQDs is significantly higher than those of other organic supramolecular materials (), which fully proves its high efficiency and universality, highlighting its great potential for biological applications.
Furthermore, proteomic analyses have been conducted to investigate how photogenerated h+ affects the cellular redox balance. HeLa cells were incubated with DPBS, SA-TCPP, and SA-TCPP@GQDs for 12 h, irradiated for 20 min, and centrifuged for proteomic analysis. A total of 7,011 proteins were quantified in the three groups of samples (, ). Principal component analysis (PCA) revealed significant differences among the three data groups, indicating distinct changes in protein expression under different drug treatments (). Initially, we compared the data of the SA-TCPP group and the SA-TCPP@GQDs group with the DPBS group, individually. Based on fold change greater than 2 and P-values of t test of less than 0.05, 786 proteins were upregulated, and 653 proteins were downregulated in the SA-TCPP group. In the SA-TCPP@GQDs group, 1,203 proteins were upregulated, and 604 proteins were downregulated (, ).
Subsequently, we conducted signal pathway enrichment analysis for differential proteins and found that the upregulated proteins in the SA-TCPP group were enriched in signaling pathways, such as the tricarboxylic acid (TCA) cycle, reactive oxygen species, mitochondrial ATP transmembrane transport, and mitochondrial ATP synthesis (Figure a, ). The DNA repair and DNA damage response pathways were also significantly enriched. These results indicate that cells respond to reactive oxygen species, altering basic metabolic processes, affecting DNA synthesis, and intensifying DNA damage. Downregulated proteins were enriched in the processes of cell division and the cell cycle as well as in the negative regulation of apoptosis and the positive regulation of cell adhesion (Figure a, ).
6.
Proteomic analysis of HeLa cells treated with nanoparticles and photocatalytic therapy. Signaling pathway enrichment analysis of differential proteins in (a) SA-TCPP and (b) SA-TCPP@GQDs. (c) Schematic diagram of the photogenerated hole (h+)-induced apoptosis of tumor cells.
The signaling pathway enrichment results in the SA-TCPP@GQDs group were largely similar to those in the SA-TCPP group. Protein expression was upregulated in the TCA cycle, reactive oxygen species, and DNA damage response pathways and downregulated in the cell-matrix adhesion and negative regulation of apoptosis pathways (Figure b, ). Notably, these consistent pathways had stronger enrichment effects in the SA-TCPP@GQD group. Additionally, fatty acid degradation was upregulated, suggesting that abnormal cell metabolism following SA-TCPP@GQD treatment involves not only glucose metabolism but also lipid metabolism. Meanwhile, regulation of lysosome size was downregulated in the SA-TCPP@GQDs group, suggesting that lysosome activity was abnormal and the apoptosis process may increase, demonstrating the superiority of the enhanced the NIR absorption and charge migration of the D–A structure.
By further analyzing the function of the proteins involved in the signaling pathways (), we summarized the tumor cell effects induced by ROS. As illustrated in Figure c, photogenerated h+ causes irreversible cell damage through a variety of pathways, as described below: (1) The abnormal expression of enzymes in glucose and lipid metabolism disrupts cell metabolism and indirectly affects mitochondrial metabolism. This leads to enhanced respiratory chain and electron transport activities, increasing ATP and NAD+ production. (2) Elevated ROS levels exacerbate endoplasmic reticulum (ER) stress and increase Ca2+ release, potentially inhibiting DFFA’s effect on DNA fragmentation and thereby enhancing the DNA fragmentation process. (3) Some DNA damage response proteins, such as PARP1, were significantly enhanced, suggesting the occurrence of DNA damage. (4) Elevated ROS levels also inhibit certain tumor-regulated signaling pathways, such as the NF-κB pathway, which promotes cell proliferation, thus weakening the processes of cell proliferation and division. (5) Apoptosis is the most significant phenotype observed, with apoptosis-promoting protein AIFM1 being upregulated and antiapoptotic proteins BID, TRIAP1, and AVEN being downregulated. Notably, the antioxidant enzyme SOD1 is also downregulated. In summary, the excessive increase in the intracellular photogenerated h+ level induces a strong intracellular stress response, disrupting the redox balance and leading to irreversible cell apoptosis.
Near-Infrared Light-Driven Complete Elimination of Solid Tumors in Vivo
Encouraged by the high performance of SA-TCPP@GQDs to inactivate cancer cells, we next evaluated their efficacy for photocatalytic solid tumor elimination in vivo. To track the metabolic process of nanoparticles, mice were intravenously injected with DPBS, SA-TCPP, and SA-TCPP@GQDs dispersions, and the real-time distribution was observed by a small animal imaging system. As shown in Figure a, SA-TCPP and SA-TCPP@GQDs exhibit time-dependent fluorescence enhancement in mice, and the fluorescence signals throughout the whole body appear around 9 h and gradually fade with metabolism 12 h later. It is worth mentioning that porphyrin supramolecular nanoparticles can maintain stability in a variety of physiological solutions for days ( and ), which ensures their real-world applicability. Besides, the nanoparticles detected in mouse urine still maintain the original nanoparticle morphology (), demonstrating the blood circulation stability of supramolecular nanoparticles in complex physiological environments.
7.
Targeted enrichment and photocatalytic elimination for solid tumors in vivo. (a) Fluorescence images of metabolic processes at different times (from left to right) after intravenous administration of DPBS, SA-TCPP, and SA-TCPP@GQDs. (b) Distribution of nanoparticles in various organs and tumors after HeLa tumor-bearing mice were treated with DPBS, SA-TCPP, and SA-TCPP@GQDs (0.5 mg/kg) for 6 h. (c) Schematic diagram of photocatalytic therapy process. (d) Images of mice with solid tumor or tumor removal after photocatalytic treatment. (e) Tumor volume of HeLa tumor-bearing mice treated with DPBS, SA-TCPP, and SA-TCPP@GQDs nanoparticles. Scale bar: 1 cm. The results were presented as mean ± SD (n = 6). (f) Mouse body weight changes during the treatment.
To assess the tumor-targeted efficiency of the nanoparticles, a HeLa tumor bearing mouse xenograft model was established by subcutaneously injecting HeLa cells into the armpit of female BALB/C nude mice. Tumor-bearing mice were intravenously administered DPBS, SA-TCPP, or SA-TCPP@GQDs for subsequent fluorescence imaging analysis. Nanoparticles typically accumulate in solid tumors via the enhanced permeability and retention (EPR) effect. In this study, the tumor-targeting ability of SA-TCPP@GQDs primarily arises from passive targeting, driven by the size-dependent accumulation of supramolecular porphyrin-based nanoparticles. The nanoparticles exhibit time-dependent tumor enrichment, with notably enhanced imaging observed at 6 h postinjection (). As shown in Figure b, after injection of SA-TCPP@GQDs and SA-TCPP nanoparticles into mice, significant fluorescence signals were generated at the tumor site. Moreover, relatively enhanced fluorescence signals were also observed in the lungs of mice in the SA-TCPP@GQD group, which can be attributed to the efficiency improvement brought about by the positive charge property. Furthermore, fluorescence monitoring of various organs further confirmed the targeted enrichment of the tumor (). Therefore, SA-TCPP@GQDs is considered as a photocatalytic therapeutic agent with easy biometabolism, mechanical stability, and tumor targeting potential.
Considering the above metabolism and tumor enrichment time, we selected 6 h after intravenous injection as the appropriate time for PCT. Figure c illustrates the process of photocatalytic tumor therapy. To this end, the mice were treated with DPBS, SA-TCPP, and SA-TCPP@GQDs and irradiated under 700 nm NIR light. The changes of the tumor site were observed, as shown in Figure d. The solid tumor was convex with a size of about 100 mm3, and it turned white after treatment with SA-TCPP@GQDs and irradiation. The temperature change curves () and thermal imaging of mice (Figure S27) during the treatment showed that the treatment was mainly focused on photocatalytic therapy rather than photothermal therapy caused by local thermal effects. After 2 days, a clear black scab appeared at the radiation site, and the skin became flat with the elimination of the solid tumor. Moreover, nanoparticles did not spread to other organs after irradiation and continued to stay in the shrunken tumor site ().
The in vivo antitumor efficacy of SA-TCPP@GQDs nanoparticles was subsequently studied by intravenous injection of different nanoparticles to HeLa tumor-bearing mice, including DPBS, SA-TCPP, and SA-TCPP@GQDs at a dosage of 0.5 mg kg–1. Irradiation was performed 6 h after injection (), and tumor changes were monitored every 2 days. As shown in Figure e, in the control group injected with DPBS, the tumor volume continued to grow substantially. Despite a certain inhibition after injection of SA-TCPP, the tumor volume also gradually increased over time due to the repeated growth of cancer cells without complete elimination. Fortunately, after being treated by SA-TCPP@GQDs, the solid tumor no longer showed growth after a significant reduction in volume, and the skin gradually returned to normal as the scab fell off. There was no significant change in the body weight of mice in the three groups (Figure f), which proved that drug treatment and photocatalytic therapy had no obvious interference with the physiological state of mice and had good biocompatibility.
Hematoxylin and eosin (H&E) staining were used for histological analysis of tumor sections, as shown in Figure g, and the cell density of SA-TCPP@GQD treatment was significantly reduced compared with DPBS and SA-TCPP treatment. Furthermore, the use of SA-TCPP and SA-TCPP@GQDs nanoparticles did not cause significant hepatocellular injury, as revealed by liver function analysis (). These results highlight the significant advantages of SA-TCPP@GQDs in terms of their photocatalytic efficiency and biocompatibility. Compared with other porphyrin-based phototherapeutic materials (), SA-TCPP@GQDs demonstrate outstanding performance in NIR light-driven photocatalytic cancer therapy. Their metal-free composition avoids the risk of long-term biological accumulation and toxicity, while the spontaneously generated photogenerated holes serve as the primary active species, effectively overcoming the challenges posed by the deeply hypoxic tumor microenvironment. These combined features enable more efficient tumor ablation and underscore the strong potential of SA-TCPP@GQDs for future applications in photocatalytic cancer treatment.
Conclusion
In summary, we successfully developed a supramolecular nanostructure, SA-TCPP@GQDs, featuring a well-defined D–A structure. Strong electronic coupling between the SA-TCPP and GQDs-NH2 components effectively reduced the band gap and expanded the light absorption to the NIR region. Crucially, the D–A structure in SA-TCPP@GQDs generated a 6.2-fold enhancement in the interfacial electric field, markedly facilitating efficient separation and migration of the photogenerated carriers. Consequently, once taken up by tumor cells, SA-TCPP@GQDs nanoparticles generated an enhanced flux of potent photogenerated holes (h+) under NIR irradiation, inducing severe oxidative stress and leading to rapid cell apoptosis. Furthermore, in vivo studies demonstrated the excellent tumor-targeting capability of SA-TCPP@GQDs and confirmed their high therapeutic efficacy, achieving complete elimination of solid tumors. This work presents a novel and effective supramolecular D–A strategy for engineering photocatalytic materials with both robust NIR responsiveness and superior charge carrier dynamics, holding significant promise for advancing targeted and highly effective photocatalytic cancer therapy.
Experimental Section
Preparation of Self-Assembled TCPP (SA-TCPP)
A 0.35 g portion of TCPP was dissolved with 15 mL of 0.1 M KOH, and the mixture was stirred at 60 °C until completely dissolved. Afterward, 0.1 M HCl was added dropwise until the pH was weakly acidic (pH 6), and there was no significant solid precipitation. The mixture was stirred at room temperature for 8 h, then centrifuged and washed with deionized water twice to remove additional K+ and Cl–. SA-TCCP was collected and dried under a vacuum at 60 °C.
Preparation of SA-TCPP@GQDs
The commercial GQDs-NH2 (1 mg/mL) was added to the SA-TCCP assembly solution under stirring and high-power ultrasound for 3 h. Finally, the dark purple solid was collected by filtration with a 0.22 μM filter membrane and dried at 60 °C to obtain SA-TCPP@GQDs. In this work, the mass ratio of GQDs-NH2 to SA-TCPP of 5% was used due to the optimal photocatalytic performance.
Characterization
Microscopic morphology was observed with a transmission electron microscope (TEM, Hitachi HT 7700) and a high-resolution transmission electron microscope (HRTEM, JEOL JEM-2100F). The atomic force microscopy study was carried out using Cypher VRS with a Kelvin probe (HQ NSC18/Pt). Zeta potential and particle size were measured with a nanoparticle analyzer (Horiba SZ-100). X-ray diffraction (XRD) patterns were measured on a Rigaku (Smart-Lab) X-ray diffractometer with Cu Kα (λ = 1.54 Å). Fourier transform infrared (FT-IR) spectra were carried out using a Bruker V70 FTIR spectrometer. The ultraviolet–visible diffuse reflectance spectrum (DRS) was obtained by a U-3010 spectrometer (Hitachi, Japan). Transient photovoltage (TPV) spectra were obtained on a photovoltage spectrometer (CEL-TPV1000) with an excitation pulse at 355 nm. Femtosecond transient absorption (TAS) spectra were collected by an amplifier laser system (35 fs, 1 kHz, 800 nm, Spitfire Ace, Spectra Physics), two fiber optic spectrometers (AcaSpec-ULS2048CL-EVO, AvaSpec-NIR256-1.7-HSC-EVO), and a mid-infrared spectroscopy instrument (FPAS-0144) with an excitation wavelength of 370 nm. Time-resolved photoluminescence spectra were collected on a fluorescence spectrometer (Edinburgh FLSP920) with an excitation wavelength of 430 nm. Room-temperature photoluminescence (PL) spectra were recorded on a fluorescence spectrometer (Edinburgh F900) with an excitation wavelength of 413 nm. Kelvin probe force microscopy (KPFM, SPM-9700) was utilized to ascertain the surface potential of the materials. The surface photovoltage (SPV) spectra were obtained on a surface photovoltage spectrometer (Zolix RAOB05–1), a home-built instrument as previously reported. An electrochemical workstation (Chenhua CHI660E) was used for photoelectrochemical measurements. Electron spin resonance (ESR) spectra (JEOL FA-200) were used to identify the main active species. Confocal laser scanning microscopy (CLSM) images were obtained from a laser scanning confocal microscope (Zeiss LSM 700). Multiskan SkyHigh (A51119770DPC) was used to analyze the absorbance at 491 nm of the MTT assay in cell experiments. Flow cytometric analysis was conducted using a CytoFLEX flow cytometry system (Beckman Coulter, USA). Fluorescence imaging and distribution study were performed on an IVIS small animal imaging system (PerkinElmer, USA).
Electrochemical Measurements
The photoelectrochemical measurements were performed on an electrochemical workstation (Chenhua CHI660E). A 300 W Xe lamp with cutoff filter (λ ≥ 420 nm) was used as the light source. The Pt plate was used as a counter electrode, saturated Ag/AgCl as a reference electrode, and working electrode as a sample coated on ITO glass, respectively. 0.1 M Na2SO4 was used as the electrolyte solution. A positive bias voltage of 0.2 V was provided in the electrochemical test.
Supplementary Material
Acknowledgments
This work is supported by the National Natural Science Foundation of China (22136002, 22309093, 22202128, 32300974), National Key Research and Development Project of China (2020YFA0710304), Special Fund Project of Jiangsu Province for Scientific and Technological Innovation in Carbon Peaking and Carbon Neutrality (BK20220023), China Postdoctoral Science Foundation (2023M741975).
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.5c00535.
Expanded experimental and computation details, characterization data (TEM, Raman, DRS, etc.) for materials, photoelectrochemical testing analysis, the efficacy of photocatalytic treatment in vitro and in vivo, and biological stability and safety assessment (PDF)
Proteomic analysis methods and data (Table S2–S6) in standalone excel format (XLSX)
W.L. proposed the concept, performed the experiment and the writing. J.J., X.Z., Y.Z. guided the project and revised the manuscript. Z.W. participated in experiments related to cancer treatment. J.X., Y.D., Y.W., J.L. assisted with material characterization. W.S. performed proteomic analysis tests and results processing. H.W. contributed to theoretical calculations and data analysis. All authors have given approval to the final version of the manuscript. CRediT: Wenting Li conceptualization, investigation, methodology, writing - original draft; Jianfang Jing investigation, methodology, writing - review & editing; Ziyu Wei data curation; Wenhao Shi data curation, investigation; Xiaolin Zhu funding acquisition, investigation, methodology, supervision, writing - original draft, writing - review & editing; Yongfa Zhu funding acquisition, project administration, supervision, writing - review & editing.
The authors declare no competing financial interest.
References
- Gao J., Luo T., Wang J.. Gene interfered-ferroptosis therapy for cancers. Nat. Commun. 2021;12:5311. doi: 10.1038/s41467-021-25632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich O., Muiños F., Lolkema M. P., Steeghs N., Gonzalez-Perez A., Lopez-Bigas N.. The mutational footprints of cancer therapies. Nat. Genet. 2019;51:1732–1740. doi: 10.1038/s41588-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Wang Y., Yao X., Chen D., Fan M., Jin Z., He Q.. Photocatalysis-mediated drug-free sustainable cancer therapy using nanocatalyst. Nat. Commun. 2021;12:1345. doi: 10.1038/s41467-021-21618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S., Liu F., Meng M., Xu C., Tian H., Chen X.. Dual reactive oxygen species generator independent of light and oxygen for tumor imaging and catalytic therapy. CCS Chem. 2022;4:2321–2332. doi: 10.31635/ccschem.021.202101103. [DOI] [Google Scholar]
- Nakamura H., Takada K.. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021;112:3945–3952. doi: 10.1111/cas.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Kundu B. K., Liang Y., Sun Y.. Near-infrared light-driven photocatalysis with an emphasis on two-photon excitation: Concepts, materials, and applications. Adv. Mater. 2024;36:2307759. doi: 10.1002/adma.202307759. [DOI] [PubMed] [Google Scholar]
- Smith A. M., Mancini M. C., Nie S.. Second window for in vivo imaging. Nat. Nanotechnol. 2009;4:710–711. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Nakatsuji H., Inada M., Matoba Y., Umeyama T., Tsujimoto M., Isoda S., Hashida M., Imahori H.. Photodynamic and photothermal effects of semiconducting and metallic-enriched single-walled carbon nanotubes. J. Am. Chem. Soc. 2012;134:17862–17865. doi: 10.1021/ja3079972. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan P., Liu C. H., Vankayala R., Chiang C. S., Hwang K. C.. Designing multi-branched gold nanoechinus for NIR light activated dual modal photodynamic and photothermal therapy in the second biological window. Adv. Mater. 2014;26:6689–6695. doi: 10.1002/adma.201400703. [DOI] [PubMed] [Google Scholar]
- Liu C., Zhang S., Li J., Wei J., Müllen K., Yin M.. A water-soluble, NIR-absorbing quaterrylenediimide chromophore for photoacoustic imaging and efficient photothermal cancer therapy. Angew. Chem., Int. Ed. 2019;58:1638–1642. doi: 10.1002/anie.201810541. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhang X., Yang L., Ren L., Wang D., Ye J.. Metal nanoparticles induced photocatalysis. Natl. Sci. Rev. 2017;4:761–780. doi: 10.1093/nsr/nwx019. [DOI] [Google Scholar]
- Liu S., Yan L., Huang J., Zhang Q., Zhou B.. Controlling upconversion in emerging multilayer core-shell nanostructures: From fundamentals to frontier applications. Chem. Soc. Rev. 2022;51:1729–1765. doi: 10.1039/D1CS00753J. [DOI] [PubMed] [Google Scholar]
- Wang D., Niu L., Qiao Z. Y., Cheng D. B., Wang J., Zhong Y., Bai F., Wang H., Fan H.. Synthesis of self-assembled porphyrin nanoparticle photosensitizers. ACS Nano. 2018;12:3796–3803. doi: 10.1021/acsnano.8b01010. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Y., Zhang L., Chen Z., Zheng B. Y., Ke M., Li X., Huang J. D.. Nanostructured phthalocyanine assemblies with efficient synergistic effect of type I photoreaction and photothermal action to overcome tumor hypoxia in photodynamic therapy. J. Am. Chem. Soc. 2021;143:13980–13989. doi: 10.1021/jacs.1c07479. [DOI] [PubMed] [Google Scholar]
- Gao F., Zhu L., Jiang L., Zhang J., Ji S., Gao W., Ma G., Chang Y., Ma X., Guo Y.. Enhanced penetration and retention of CuS-based nanosystem through NIR light and in Situ enzyme response for improved tumor therapy. Adv. Funct. Mater. 2024;34:2312182. doi: 10.1002/adfm.202312182. [DOI] [Google Scholar]
- Ran B., Wang Z., Cai W., Ran L., Xia W., Liu W., Peng X.. Organic photo-antimicrobials: Principles, molecule design, and applications. J. Am. Chem. Soc. 2021;143:17891–17909. doi: 10.1021/jacs.1c08679. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu D., Zhu Y., Zhou S., Guan S.. Supramolecular packing dominant photocatalytic oxidation and anticancer performance of PDI. Appl. Catal., B. 2018;231:251–261. doi: 10.1016/j.apcatb.2018.03.026. [DOI] [Google Scholar]
- Kand D., Liu P., Navarro M. X., Fischer L. J., Rousso-Noori L., Friedmann-Morvinski D., Winter A. H., Miller E. W., Weinstain R.. Water-soluble BODIPY photocages with tunable cellular localization. J. Am. Chem. Soc. 2020;142:4970–4974. doi: 10.1021/jacs.9b13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Wang H., Yang Y., Xu J. F., Zhang X.. A bacteria-responsive porphyrin for adaptable photodynamic/photothermal therapy. Angew. Chem., Int. Ed. 2022;61:e202200799. doi: 10.1002/anie.202200799. [DOI] [PubMed] [Google Scholar]
- Huo J., Jia Q., Huang H., Zhang J., Li P., Dong X., Huang W.. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem. Soc. Rev. 2021;50:8762–8789. doi: 10.1039/D1CS00074H. [DOI] [PubMed] [Google Scholar]
- Jia S., Wang S., Li S., Hu P., Yu S., Shi J., Yuan J.. Specific modification and self-transport of porphyrins and their multi-mechanism cooperative antitumor studies. J. Mater. Chem. B. 2021;9:3180–3191. doi: 10.1039/D0TB02847A. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun J., Zhang H., Zhao Z., Liu W.. Tetra (4-carboxyphenyl) porphyrin for efficient cofactor regeneration under visible light and its immobilization. Catal. Sci. Technol. 2018;8:2578–2587. doi: 10.1039/C8CY00320C. [DOI] [Google Scholar]
- Xie Y., Wang M., Sun Q., Wang D., Li C.. Recent advances in tetrakis (4-Carboxyphenyl) porphyrin-based nanocomposites for tumor therapy. Adv. Nanobiomed Res. 2023;3:2200136. doi: 10.1002/anbr.202200136. [DOI] [Google Scholar]
- Zhang Z., Zhu Y., Chen X., Zhang H., Wang J.. A full-spectrum metal-free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv. Mater. 2019;31:1806626. doi: 10.1002/adma.201806626. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang L., Liu W., Yan Z., Zhu Y., Zhou S., Guan S.. Photogenerated-hole-induced rapid elimination of solid tumors by the supramolecular porphyrin photocatalyst. Natl. Sci. Rev. 2021;8:nwaa155. doi: 10.1093/nsr/nwaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng K. X., Niu L. Y., Yang Q. Z.. Supramolecular photosensitizer enables oxygen-independent generation of hydroxyl radicals for photodynamic therapy. J. Am. Chem. Soc. 2023;145:4081–4087. doi: 10.1021/jacs.2c11868. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wang R., Feng B., Zhong X., Ostrikov K.. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021;12:6856. doi: 10.1038/s41467-021-27071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Yang J., Li M., Zhang F., Bu W., Li H., Wu Q., Yin D., Jiao L., Hao E.. Unique double intramolecular and intermolecular exciton coupling in ethene-bridged aza-BODIPY dimers for high-efficiency near-infrared photothermal conversion and therapy. Angew. Chem., Int. Ed. 2022;61:e202211081. doi: 10.1002/anie.202211081. [DOI] [PubMed] [Google Scholar]
- Chen X., Wang Z., Shen X., Zhang Y., Lou Y., Pan C., Zhu Y., Xu J.. A plasmonic Z-scheme Ag@AgCl/PDI photocatalyst for the efficient elimination of organic pollutants, antibiotic resistant bacteria and antibiotic resistance genes. Appl. Catal. B Environ. 2023;324:122220. doi: 10.1016/j.apcatb.2022.122220. [DOI] [Google Scholar]
- Liu L., Chen X., Chai Y., Zhang W., Liu X., Zhao F., Wang Z., Weng Y., Wu B., Geng H.. et al. Highly efficient photocatalytic hydrogen production via porphyrin-fullerene supramolecular photocatalyst with donor-acceptor structure. Chem. Eng. J. 2022;444:136621. doi: 10.1016/j.cej.2022.136621. [DOI] [Google Scholar]
- Zhu X., Jia Y., Liu Y., Xu J., He H., Wang S., Shao Y., Zhai Y., Zhu Y.. Enhancing built-in electric fields via molecular symmetry modulation in supramolecular photocatalysts for highly efficient photocatalytic hydrogen evolution. Angew. Chem., Int. Ed. 2024;63:e202405962. doi: 10.1002/anie.202405962. [DOI] [PubMed] [Google Scholar]
- Li W., Han B., Liu Y., Xu J., He H., Wang G., Li J., Zhai Y., Zhu X., Zhu Y.. Unsymmetric protonation driven highly efficient H2O2 photosynthesis in supramolecular photocatalysts via one-step two-electron oxygen reduction. Angew. Chem., Int. Ed. 2025;64:e202421356. doi: 10.1002/anie.202421356. [DOI] [PubMed] [Google Scholar]
- Tetsuka H., Asahi R., Nagoya A., Okamoto K., Tajima I., Ohta R., Okamoto A.. Optically tunable amino-functionalized graphene quantum dots. Adv. Mater. 2012;24:5333–5338. doi: 10.1002/adma.201201930. [DOI] [PubMed] [Google Scholar]
- Fernando K. S., Sahu S., Liu Y., Lewis W. K., Guliants E. A., Jafariyan A., Wang P., Bunker C. E., Sun Y. P.. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces. 2015;7:8363–8376. doi: 10.1021/acsami.5b00448. [DOI] [PubMed] [Google Scholar]
- Yang J., Miao H., Jing J., Zhu Y., Choi W.. Photocatalytic activity enhancement of PDI supermolecular via π-π action and energy level adjusting with graphene quantum dots. Appl. Catal. B Environ. 2021;281:119547. doi: 10.1016/j.apcatb.2020.119547. [DOI] [Google Scholar]
- Li W., Zhang H., Huang S., Xu J., Liu L., Li J., Jing J., Zhu Y.. Electron-enriched supramolecular PDI-SiO2 promoting PDS activation for enhanced photocatalytic advanced oxidation. Appl. Catal. B Environ. 2024;340:123262. doi: 10.1016/j.apcatb.2023.123262. [DOI] [Google Scholar]
- Shao X. R., Wei X. Q., Song X., Hao L. Y., Cai X. X., Zhang Z. R., Peng Q., Lin Y. F.. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Proliferat. 2015;48:465–474. doi: 10.1111/cpr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J., Li J., Su Y., Zhu Y.. Non-covalently linked donor-acceptor interaction enhancing photocatalytic hydrogen evolution from porphyrin assembly. Appl. Catal. B Environ. 2023;324:122284. doi: 10.1016/j.apcatb.2022.122284. [DOI] [Google Scholar]
- Liu F., Shi R., Wang Z., Weng Y., Che C. M., Chen Y.. Direct Z-scheme hetero-phase junction of black/red phosphorus for photocatalytic water splitting. Angew. Chem., Int. Ed. Engl. 2019;131:11917–11921. doi: 10.1002/ange.201906416. [DOI] [PubMed] [Google Scholar]
- Zhang G., Ji Q., Wu Z., Wang G., Liu H., Qu J., Li J.. Facile “spot-heating” synthesis of carbon dots/carbon nitride for solar hydrogen evolution synchronously with contaminant decomposition. Adv. Funct. Mater. 2018;28:1706462. doi: 10.1002/adfm.201706462. [DOI] [Google Scholar]
- Zhang Y., Jiang S., Song W., Zhou P., Ji H., Ma W., Hao W., Chen C., Zhao J.. Nonmetal P-doped hematite photoanode with enhanced electron mobility and high water oxidation activity. Energy Environ. Sci. 2015;8:1231–1236. doi: 10.1039/C4EE03803G. [DOI] [Google Scholar]
- Guo Y., Shi W., Zhu Y.. Internal electric field engineering for steering photogenerated charge separation and enhancing photoactivity. EcoMat. 2019;1:e12007. doi: 10.1002/eom2.12007. [DOI] [Google Scholar]
- Kanata-Kito T., Matsunaga M., Takakura H., Hamakawa Y., Nishino T.. Photoreflectance characterization of built-in potential in MBE-produced As-grown GaAs surface. Modul. Spectrosc. 1990;68:5309. doi: 10.1063/1.347023. [DOI] [Google Scholar]
- Wei Z., Wang W., Li W., Bai X., Zhao J., Tse E. C., Phillips D. L., Zhu Y.. Steering electron-hole migration pathways using oxygen vacancies in tungsten oxides to enhance their photocatalytic oxygen evolution performance. Angew. Chem., Int. Ed. Engl. 2021;60:8236–8242. doi: 10.1002/anie.202016170. [DOI] [PubMed] [Google Scholar]
- Nikoloudakis E., Pigiaki M., Polychronaki M. N., Margaritopoulou A., Charalambidis G., Serpetzoglou E., Mitraki A., Loukakos P. A., Coutsolelos A. G.. Self-assembly of porphyrin dipeptide conjugates toward hydrogen production. ACS Sustain. Chem. Eng. 2021;9:7781–7791. doi: 10.1021/acssuschemeng.1c00978. [DOI] [Google Scholar]
- Cordones A. A., Pemmaraju C. D., Lee J. H., Zegkinoglou I., Ragoussi M. E., Himpsel F. J., De La Torre G., Schoenlein R. W.. Excited-state charge distribution of a donor- π-acceptor Zn porphyrin probed by N K-edge transient absorption spectroscopy. J. Phys. Chem. Lett. 2021;12:1182–1188. doi: 10.1021/acs.jpclett.0c03725. [DOI] [PubMed] [Google Scholar]
- Jing J., Yang J., Li W., Wu Z., Zhu Y.. Construction of interfacial electric field via dual-porphyrin heterostructure boosting photocatalytic hydrogen evolution. Adv. Mater. 2022;34:2106807. doi: 10.1002/adma.202106807. [DOI] [PubMed] [Google Scholar]
- Sambur J. B., Chen T. Y., Choudhary E., Chen G., Nissen E. J., Thomas E. M., Zou N., Chen P.. Sub-particle reaction and photocurrent mapping to optimize catalyst-modified photoanodes. Nature. 2016;530:77–80. doi: 10.1038/nature16534. [DOI] [PubMed] [Google Scholar]
- Wang W. K., Chen J. J., Lou Z. Z., Kim S., Fujitsuka M., Yu H. Q., Majima T.. Single-molecule and-particle probing crystal edge/corner as highly efficient photocatalytic sites on a single TiO2 particle. Proc. Natl. Acad. Sci. U.S.A. 2019;116:18827–18833. doi: 10.1073/pnas.1907122116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Liu J., Shao L., Mao L., Wang M.. DNAzyme-catalyzed cellular oxidative stress amplification for pro-protein activation in living cells. ChemBioChem. 2021;22:2608–2613. doi: 10.1002/cbic.202100225. [DOI] [PubMed] [Google Scholar]
- Chen R., Fan F., Dittrich T., Li C.. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem. Soc. Rev. 2018;47:8238–8262. doi: 10.1039/C8CS00320C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.