Abstract
Humans with lesions to the orbital/medial prefrontal cortex and interconnected areas display impulsive aggressive behavior. To examine further the relationship between impulsive aggression and orbital/medial prefrontal dysfunction, we measured the behavioral performance of psychiatric patients with a disorder characterized by impulsive aggression, Intermittent Explosive Disorder (IED). Presently, no evidence exists for a localized brain lesion in IED subjects. However, on the basis of the location of brain lesions that produce acquired impulsive aggression, we hypothesized that IED subjects would exhibit test performance similar to patients with lesions to the orbital/medial prefrontal cortex. Subjects with IED and controls were administered three tests sensitive to lesions of the orbital/medial prefrontal circuit: the Iowa Gambling Task, facial emotion recognition, and odor identification, and two control tests of working memory. On the gambling task, IED subjects continued to make disadvantageous decisions throughout the 100 trials, whereas controls learned to avoid disadvantageous decisions. On the facial recognition test, IED subjects were impaired at recognizing “anger,” “disgust,” and “surprise,” and they were biased to label neutral faces with “disgust” and “fear.” On odor identification, IED subjects were mildly anosmic and were impaired relative to controls. However, on the working memory control tests, both groups performed similarly. Across tests, the performance of IED subjects resembles the performance of patients with orbital/medial prefrontal lesions in previous studies. These results extend the link between dysfunction of the orbital/medial prefrontal circuit and impulsive aggressive behavior.
A major concern in our society is the prevalence of violence, which stems from various forms of human aggression (1, 2). Aggressive behavior can be divided into two broad categories: premeditated and impulsive. These categories can be defined by the degree of cognitive control over the behavior. Premeditated aggression involves an attempt to control one's environment (e.g., a mugger who steals a wallet at gunpoint), whereas impulsive aggression results from the inability to control one's impulses (e.g., a driver with “road rage”). Here we focus on a well defined psychiatric disorder characterized by impulsive aggression, Intermittent Explosive Disorder (IED; ref. 3). Patients with IED show a chronic pattern of impulsive aggression that is out of proportion to the provocation. Treatment of IED relies on a better understanding of the neurobiology of impulsive aggression (4).
Clues to the neurobiology of impulsive aggression come from studies of patients with specific brain lesions. Lesions to the orbital and medial prefrontal cortex and anatomically connected areas, including the amygdala (5), cause patients to develop impulsive and aggressive behavior, show little control over their emotions, and be unaware of the implications of their actions (2, 6, 7). Thus, a link exists between impulsive aggression and the integrity of the orbital/medial prefrontal cortex circuit (OMPCC).
Presently, no evidence exists for a localized brain lesion in psychiatric patients with IED. However, on the basis of the lesion localization in neurological patients with acquired impulsive aggression, one hypothesis is that psychiatric patients have a functional impairment of the OMPCC. In the present study, we tested IED patients with measures sensitive to dysfunction of the OMPCC. Performance was compared with control tests related to other areas of the prefrontal cortex. It was hypothesized that patients with IED would show a pattern of behavior similar to that of patients with actual OMPCC lesions.
Materials and Methods
Twenty-four IED patients (4 female, 20 male) and 22 control subjects (4 female, 18 male) were recruited in the Clinical Neurosciences Research Unit at the Eastern Pennsylvania Psychiatric Institute (Philadelphia, PA). Subjects were recruited from a larger study, advertisements, and community referrals. Subjects received either treatment or $40 for participation in the study. IED subject race was as follows: European descent (13 subjects), African American (9 subjects), Latino (1 subject), other (1 subject); control subject race was as follows: European descent (11 subjects), African American (10 subjects), Asian (1 subject).
Axis I diagnoses were made by using a screener for the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV. The IED diagnosis was made according to published research criteria (8), through a best-estimate procedure made by a committee composed of two to three psychiatrists and up to two clinical psychologists. Only IED-negative subjects were included in the control group. Exclusion criteria were past diagnosis of a psychotic disorder, current diagnosis of a major depressive disorder or obsessive-compulsive disorder, past neurological illness or traumatic brain injury with loss of consciousness for more than 15 min, hospital stay, or coma. Subjects in the control group were also excluded if they were diagnosed with Borderline Personality Disorder, Histrionic Personality Disorder, or Antisocial Personality Disorder.
Subjects were administered the Buss–Durkee Hostility Index and the Barrett Impulsivity Scale (11-item version). Group definitions were confirmed by the significantly greater levels of impulsivity and aggression in the IED group (Table 1). An abbreviated form of the Wechsler Adult Intelligence Scale-Revised was administered that included two subtests: vocabulary and block design. An estimated IQ was calculated by using normative tables (9). The behavioral battery was composed of tasks that putatively reflect functioning of the orbital/medial prefrontal cortex circuit (Iowa Gambling Task, Facial Emotion Recognition Task, University of Pennsylvania Smell Identification Test) and the dorsolateral prefrontal cortex circuit (Self-Ordered Pointing; Two-back test). Unless stated otherwise, performance is reported as mean ± 1 SEM.
Table 1.
Subject characteristics
| Measure | Group
|
|
|---|---|---|
| IED | Control | |
| Age, years | 36.3 (9.1) | 33.3 (9.9) |
| Education, years | 13.2 (2.0) | 14.2 (1.6) |
| Estimated IQ, standard score | 94.4 (10.6) | 99.6 (9.7) |
| BDHI aggression subscale | 28.0 (9.0)** | 13.3 (3.4) |
| BIS | 60.0 (16.3)* | 51.0 (9.5) |
Mean performance is shown with SD in parentheses. For the population, IQ mean = 100, SD = 15. Abbreviations: BDHI, Buss–Durkee Hostility Inventory; BIS, Barrett Impulsivity Scale.
, P < 0.05;
, P < 0.01.
Tests of Frontal Lobe Functioning.
In the Iowa Gambling Task, subjects are given four decks of cards (A–D) and a “loan” of $2,000 in fake bills and asked to play so that they “lose the least amount of money and win the most.” Turning each card carries an immediate reward ($100 in A and B, and $50 in C and D; see ref. 10 for details). Unpredictably, some cards also carry a penalty, which is large in A and B ($250 to $1,250) and small in C and D ($25 to $250). Playing mostly from the disadvantageous decks (A and B) leads to an overall loss. Playing from the advantageous decks (C and D) leads to an overall gain. Each subject picks 100 cards.
For the test of facial emotion recognition, subjects were shown 44 black-and-white photos of adult men and women expressing either happiness (four), surprise (eight), fear (eight), disgust (eight), anger (eight), or a neutral emotion (eight). Photographs were taken from the Ekman and Friesen series (11). The expression category of each face is by consensus of a large sample (see ref. 11). Subjects were shown the photographs for 5 s and asked to choose the label that best described the expression.
The University of Pennsylvania Smell Identification Test (UPSIT) consists of four envelope-size booklets each containing ten “scratch-and-sniff” odorants (12). Subjects sampled each odorant and selected a label from four choices. A standardized score was calculated from the UPSIT manual. Test–retest reliability of the measure is >0.90 and validity has been established by correlational analysis with similar tests. All subjects were given a clinical interview regarding their ability to perceive odors. Only one individual (not included) was aware of any olfactory problem.
For the Self-Ordered Pointing test (13), subjects were presented with 12 abstract designs on a card. Twelve cards displayed the designs mounted in a different spatial order. For the first card, subjects were asked to pick one of the designs on the page. For subsequent cards, they were asked to pick a design they had not picked before and were asked to pick again if they selected a choice they had made previously. Subjects were not allowed to pick simply from the same location each time. As a control for basic perceptual abilities, a short matching test was conducted. Subjects were shown the same 12 abstract designs on one page and asked to match them with designs in a different order on another page.
For the Two-back test (14), subjects were presented a continuous stream of 101 single letters chosen at random, programmed by using PsyScope on a Macintosh computer. Each letter was presented in the middle of the screen for 500 ms, with a 2,500-ms interval between successive letters. Subjects were instructed to press a button when a letter appeared that had been presented two trials ago. One-back and three-back distracters occurred. The number of hits and false alarms was recorded. As a control for sustained attention, subjects were presented a similar stream of letters and asked to respond when they saw the letter “t”.
Results
Subject Characteristics.
On average, subjects in the IED group (n = 24) and control group (n = 22) were in their mid-thirties, were educated past high school, and had IQs in the normal range (Table 1). The two groups were not significantly different in terms of age [t(44) = 1.06], years of education [t(44) = −1.76], or estimated IQ [t(44) = −1.74]. On self-report measures of aggression (Buss–Durkee Hostility Inventory; ref. 15) and impulsivity (Barrett Impulsivity Scale), subjects with IED scored significantly higher than controls [Buss–Durkee Hostility Index, t(44) = 6.26, P < 0.001; Barrett Impulsivity Scale, [t(44) = 2.18, P < 0.05].
Cognitive Tests Related to the OMPCC.
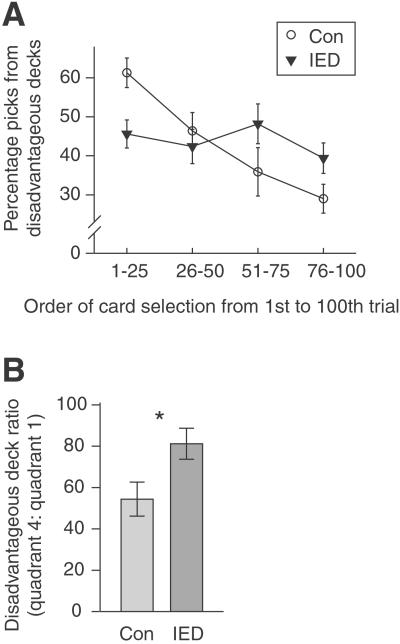
We examined performance on two cognitive tasks sensitive to lesions of the OMPCC and that relate to symptoms of IED. On the Iowa Gambling Task (10, 16) picks from disadvantageous decks were measured in four quadrants of 25 trials each. A two-way ANOVA between group and quadrant showed no effect of group [F(1, 44) = 0.04], an effect of quadrant [F(3, 132) = 6.80, P < 0.001], and a significant interaction [F(3, 132) = 4.65, P < 0.005]. The interaction arose because the subjects with IED picked approximately the same number of cards from the disadvantageous decks across the four quadrants, whereas the control subjects picked incrementally less from the disadvantageous decks across the quadrants (Fig. 1A).
Figure 1.
Subjects with IED did not learn to avoid disadvantageous decks on the Iowa Gambling Task. (A) Percentage of cards selected from disadvantageous decks across time. IED subjects and controls (Con) showed a different pattern of responding across the four blocks of trials. (In this and all subsequent figures, error bars indicate ± 1 SEM.) (B) Ratio of disadvantageous picks from last 25 trials (quadrant 4) to first 25 trials (quadrant 1). IED subject ratio was significantly greater, which indicates that they were impaired at learning to avoid the disadvantageous decks over time. *, P < 0.015.
To quantify how the two groups learned from their experience, a ratio was calculated from the number of disadvantageous picks in the final quadrant (percentage picks, trials 75–100) relative to first quadrant (percentage picks, trials 1–25). By the final quadrant, subjects with IED picked from the disadvantageous decks about 80% as often as they had in the first quadrant, whereas controls picked from these decks only about 55% as often as they had in the first quadrant (two outliers removed). This ratio was significantly greater for subjects with IED than for controls [t(42), = 2.40, P < 0.02] (Fig. 1B).
One reason why the IED group failed to learn to avoid disadvantageous decks may be that they were initially less attracted to these decks in the first quadrant, and thus they had less experience with the larger penalties associated with these decks. However, by the third quadrant (75 picks) the two groups had a nearly identical number of picks from disadvantageous decks [control, 48 ± 2.2%; IED, 45 ± 2.9%; t(44) = −0.61, not significant]. Despite this equal exposure, by the fourth quadrant, IED subjects made significantly more picks from disadvantageous decks [control, 29 ± 3.8%; IED, 39 ± 3.9%; t(44) = 1.80, P < 0.05]. We calculated a ratio for the number of disadvantageous picks in the final quadrant relative to the first three quadrants (percentage picks, trials 1–75). By the final quadrant, subjects with IED picked from the disadvantageous decks 89 ± 9.5% as often as they had in the first three quadrants, whereas controls picked from these decks only 60 ± 7.6% as often as they had in the first three quadrants. This ratio was significantly greater for subjects with IED than for controls [t(44) = 2.30, P < 0.02].
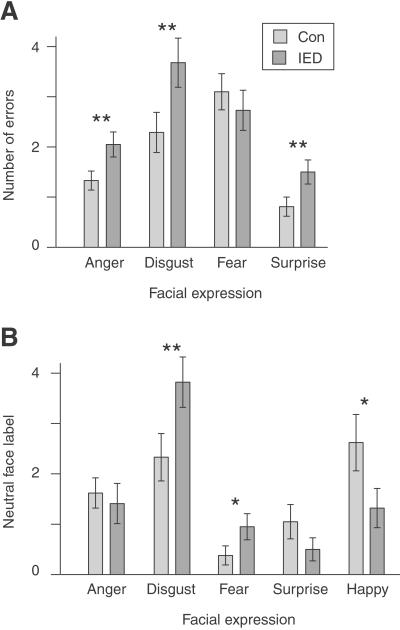
On a test of facial emotion recognition (11) both groups performed nearly perfectly for happy faces (two errors in each group), suggesting that basic perceptual abilities were intact. On the remaining categories, there was an effect of facial expression [F(3, 123) = 17.6, P < 0.001] and IED subjects had more errors overall [F(1, 41) = 4.5, P < 0.05]. A significant interaction also occurred between expression and group [F(3, 123) = 2.8, P < 0.05] (Fig. 2A). The IED group made significantly more errors in judging expressions of anger [t(41) = 2.26, P < 0.025], disgust [t(41) = 2.19, P < 0.025], and surprise [t(41) = 2.22, P < 0.025]. The groups did not differ significantly when judging an expression of fear [t(41) = −0.68].
Figure 2.
Subjects with IED made more errors and were biased to perceive negative facial expressions. (A) Number of errors made by each group for four facial expressions. Subjects with IED made significantly more errors for anger, disgust, and surprise. (B) Number of times subjects labeled neutral faces with each of the five expressions. Subjects with IED were more likely than controls (Con) to label neutral faces with disgust and fear. **, P < 0.025; *, P < 0 05.
Subjects also categorized faces that had neutral expressions to test whether the two groups were differentially biased to perceive certain expressions (Fig. 2B). There was a significant effect of group (χ2 analysis of independence; χ2(4) = 29.0, P < 0.005). Follow-up t tests were run to test the hypothesis that IED subjects would be biased to perceive negative emotions. The IED group, relative to controls, was biased to label neutral expressions with “disgust” [t(41) = 2.16, P < 0.025] and “fear” [t(41) = 1.78, P < 0.05]. The control group, relative to the IED group, was biased to label neutral expressions with “happy” [t(41) = −1.90, P < 0.05] and “surprise” [t(41) = −1.35, P < 0.10].
One surprising finding was that IED subjects were most biased to label neutral expression as “disgusted” (Fig. 2B), and yet these subjects made the most errors in labeling facial expressions of disgust (Fig. 2A). To examine this seemingly paradoxical pattern further, we performed an error analysis on the labeling of expressions. When judging expressions of disgust, the most common mislabel was “anger” (control, 95%; IED, 90%). For expressions of fear, the most common mislabel was “surprise” (control, 83%; IED, 70%); for surprise, the most common mislabel was “fear” (control, 82%; IED, 64%); for anger, mislabels were spread between “fear,” “disgust,” and “surprise,” with no mislabel alone receiving more than 50% for either group. In all cases, the groups did not differ in their pattern of mislabels (χ2 analysis of independence for each expression; all χ2(3) <4.5, not significant). We conclude that IED subjects are biased, relative to controls, to label neutral expressions as “disgusted,” and disgusted expressions as “angry.”
Sensory Test Related to the OMPCC.
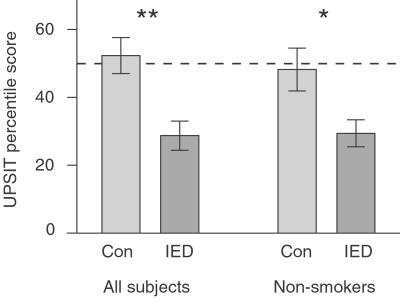
We performed an olfactory control task that has been linked to OMPCC functioning (17, 18) but does not relate specifically to symptoms of IED. Subjects were given the UPSIT (12). A one-way analysis of covariance was conducted to test the hypothesis that subjects with IED would be impaired on smell identification. The amount of lifetime smoking was used as a covariate (19). Subjects with IED were significantly impaired relative to controls [F(2, 43) = 11.18, P < 0.002] (Fig. 3A). A t test was also run which included only the 72% of subjects across both groups who were nonsmokers for at least 10 years. Again, subjects with IED were significantly impaired relative to controls [t(31) = −2.49, P < 0.01] (Fig. 3B).
Figure 3.
Subjects with IED were impaired at olfactory identification. Bars indicate percentile scores on the UPSIT for all subjects or the subset of subjects who were nonsmokers for 10 years [control (Con), n = 17; IED, n = 16]. IED group scores were significantly lower. **, P < 0.002; *, P < 0.01.
Cognitive Tests Related to Dorsolateral Prefrontal Cortex.
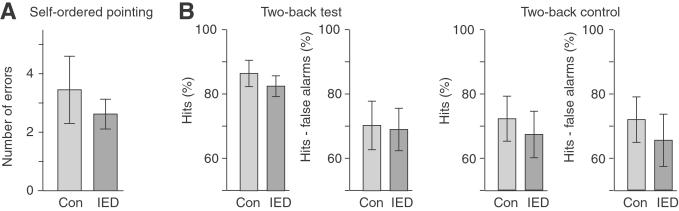
We performed two control tasks of working memory sensitive to the integrity of the dorsolateral prefrontal cortex and related areas of posterior association cortex (20). On the Self-Ordered Pointing test (21) both groups made, on average, about three errors (out of 12), with no difference between groups [t(38) = 0.04, one outlier removed] (Fig. 4A). The groups were also administered a perceptual control task in which a subject was asked simply to match the patterns. No errors were made on this control task by either group.
Figure 4.
Subjects with IED showed normal performance on working-memory tests. (A) Performance on the self-ordered pointing test was not significantly different between groups. (B) Performance on the Two-back test and the Two-back control test was not significantly different between groups. Con, controls.
On the Two-back test (14), no group differences were found for hits [t(41) = −0.77] or hits minus false alarms [t(41) = −0.13]. For both groups, hits minus false alarms was ≈70%, suggesting that the task was challenging and that a ‘ceiling effect’ did not exist (Fig. 4B). On a control test of sustained attention in which subjects responded every time the letter “t” appeared, differences also did not occur for either hits [t(34) = −0.48] or hits minus false alarms [t(34) = −0.59].
Discussion
Cognitive Impairments Related to Impulsive Aggression.
The Iowa Gambling Task requires subjects to learn the optimal choices in a game that combines variable gain and variable loss (10, 16). In the present study, subjects with an impulsive aggressive disorder (IED) continued to pick cards from the disadvantageous decks at about the same rate throughout the task, whereas control subjects learned from experience and chose fewer cards from the disadvantageous decks over time. The control group performance represents the optimal strategy, which is to switch to the advantageous decks over time. However, the subjects with impulsive aggression seemed to be unable to resist the occasional high payout from the disadvantageous decks regardless of the negative consequences.
Psychiatric patients with impulsive aggression performed similarly to patients with orbital frontal and amygdala lesions in previous studies (10, 16, 22–23). In a previous study that contained subjects with orbital/medial prefrontal lesions, subjects did not learn to avoid the disadvantageous decks compared with control subjects (24). The performance of the psychiatric patients in the present study was not as impaired as that of neurological patients in previous studies (10, 16, 22–24). Although patients in both sets of studies showed a similar inability to avoid the disadvantageous decks over time, in the present study psychiatric patients picked fewer cards (≈45%) from the disadvantageous decks than patients with either an orbital/medial or amygdala lesion (≈55–60%; ref. 24), which indicates that the impairment associated with IED may be milder than after an OMPCC lesion.
Previous studies showed that controls developed anticipatory skin conductance responses (SCRs) before choosing from disadvantageous decks, even before they could articulate the nature of the decks (16, 24). However, patients with lesions to the orbital/medial prefrontal cortex or amygdala lacked anticipatory SCRs, even in some cases where the patient could articulate the nature of the decks (16, 24). In the present study, our goal was to investigate the pattern of behavioral response in IED, similar to the initial investigation of patients with lesions (10), and establish the similarity to patients with lesions. Given the behavioral similarity between the groups, the next step would be to determine whether IED subjects also lack anticipatory SCRs. The lack of SCRs in IED subjects would suggest a breakdown, similar to patients with lesions, in the autonomic encoding of punishment, whereas the presence of normal SCRs would suggest that the breakdown is in the ability to make use of these autonomic cues.
Performance on the Gambling Task reveals underlying deficits that could contribute to the symptoms of IED. Patients had difficulty learning to consistently avoid making choices that were associated with high levels of punishment. In everyday life, these patients continually use problem-solving strategies that involve aggression, even though it is socially inappropriate and frequently leads to injury or incarceration. In that sense, patients show an inability to learn from social cues provided by the environment. Another possible hypothesis is that punishment is not a salient enough deterrent to inhibit negative behavior.
On a test of facial-emotion recognition, subjects with IED made more errors than control subjects for expressions of “surprise,” “anger,” and “disgust.” The increased number of errors for “anger” and “disgust” suggests that subjects with IED may have an impairment in accurately perceiving negative emotions. Neutral faces were also included to probe possible emotional perception biases in the absence of strong cues. Subjects with IED were more likely than controls to label neutral faces with a negatively valenced expression (“disgust,” “fear”). Control subjects showed the opposite bias; they were more likely to label neutral faces with a positive expression (“happy”). This finding supports previous evidence that aggressive individuals have a negative attribution bias that affects their ability to interpret social situations correctly such that they consistently label neutral situations negatively (25, 26).
The deficit in negative-emotion recognition in the IED group is similar to patients with orbital frontal and amygdala lesions. A patient with an orbital frontal or amygdala lesion can show both impulsive aggression and a deficit in the recognition of “anger” and “disgust” (27). Patients with amygdala lesions are also impaired at the recognition of “fear” and “approachability” (28, 29). In addition, imaging studies suggest that fearful expressions cause activation of the amygdala (30, 31). Single-unit recording in human orbital frontal cortex showed increased firing rates to aversive stimuli including facial expressions of fear (32). Similar increases in orbital frontal activity were measured with functional imaging to angry expressions (33), to aversive stimuli (34), and to the actual induction of anger (35, 36). Disruption of the medial prefrontal cortex by transcranial magnetic stimulation caused a delay in the recognition of angry faces but not happy faces (37).
IED subjects' deficit in facial emotion recognition could contribute to IED symptoms. Expressions such as anger or disgust can be warning signals of impending physical or psychological danger, but subjects with IED may miss these signals until a dangerous situation has escalated. In addition, subjects with IED are primed to perceive negative emotion in neutral situations, which might explain why individuals with IED seem to be easily provoked into negative interactions and conflicts with others.
Olfactory Impairment Related to Impulsive Aggression.
Although average control group performance was similar to the population mean (50%), average performance of subjects with IED was about two standard deviations below the population mean (12). This performance was not as significantly impaired as patients with documented orbital frontal lesions (17). The scores of subjects with IED, although significantly lower than controls, did not indicate a gross deficit in smell identification (anosmia).
The olfactory deficit is significant for several reasons. The neural pathways involved in smell detection and identification are well understood, and include orbital/medial frontal cortex (18). This test involves relatively simple cognitive operations such as perception and naming. Therefore, conclusions derived from this test are not confounded by potential deficits in more complex cognitive operations. In addition, this finding suggests that rather than a cognitive impairment in a unique domain, IED is related to dysfunction of the OMPCC, which leads to a cluster of cognitive and sensory deficits.
Working-Memory Control Tasks.
The intact performance of IED subjects on two working-memory tasks suggests that their cognition is not globally impaired. Similar working-memory tasks have been related to the integrity of the dorsolateral prefrontal cortex and related areas of posterior association cortex (14, 21, 38). Working memory performance was impaired in patients with dorsolateral lesions but intact in patients with orbital frontal lesions; the opposite finding was found for the Iowa Gambling Task (23). Thus, the pattern of performance was again similar between IED subjects and patients with orbital/medial prefrontal lesions.
Related Psychiatric Conditions.
Several psychiatric conditions related to IED are characterized by the inability to inhibit aggressive or impulsive behavior. In these groups, anatomical evidence exists for a general deficit of prefrontal cortex. Murderers showed a general reduction in glucose metabolism within prefrontal cortex (39). Patients with antisocial personality disorder who displayed impulsive aggression showed an 11% reduction in volume of gray matter in the prefrontal cortex (40).
Several samples of patients with clinical features related to IED provide behavioral evidence for a specific impairment on tasks relating to the integrity of the OMPCC. Prisoners with high scores on a measure of psychopathy showed selective deficits on two tasks related to OMPCC functioning: smell identification and a measure of response inhibition (41). Subjects with antisocial personality disorder were impaired at inhibiting responses to previously rewarded stimuli in a task similar to the Iowa Gambling Task (42). Subjects with obsessive-compulsive disorder also showed deficits on tasks related to the integrity of the OMPCC (43) including the Iowa Gambling Task (44). Thus, other psychiatric conditions, with similar or related behaviors could also involve an underlying deficit in the OMPCC.
Neurobiological Model.
Subjects with IED and patients with orbital/medial prefrontal lesions seem to share a similar locus of neuroanatomical disruption, but the extent and nature of the pathology is probably quite different. One possible explanation for the difference is that subjects with IED simply have a milder form of brain insult because of a genetic and/or developmental abnormality. Studies of twins have already documented a substantial genetic component to impulsive aggression (45, 46). A genetic defect could be mediated by a defect in a gene that codes for serotonin-related function (e.g., a specific polymorphism for the gene for tryptophan hydroxylase, the rate-limiting enzyme for serotonin synthesis, has been shown to correlate with self-report measures of aggression; ref. 47). The functional difference between a developmental abnormality and an acquired lesion can be profound. A developmental abnormality would allow for an increase in time for neural reorganization, leading to less severe symptoms, whereas an acquired lesion to preexisting circuits would lead to focal behavioral deficits that would be more striking.
Impulsive aggression may also be related to a dysfunction of inhibitory projections from the orbital/medial prefrontal cortex to the amygdala (2). This dysfunction might involve the well established neurochemical abnormality in IED related to reduced action of serotonin (48). In some cases of IED, the symptoms are managed by drug treatment with serotonin reuptake inhibitors (4). Subjects exhibiting impulsive aggression have evidence of reduced presynaptic and/or postsynaptic serotonergic activity (49–51). In a recent 18fluorodeoxyglucose positron emission tomography, brain areas in the region of the orbital/frontal cortex of a few IED subjects were shown to be less responsive to serotonergic stimulation (52). These studies suggest the possibility that the fundamental circuitry between the orbital/medial prefrontal cortex and the amygdala is intact but is not being properly modulated by serotonin.
Acknowledgments
We thank Drs. Jonathan Demb and Sharon Thompson-Schill for carefully reading and commenting on this manuscript. This work was supported by National Institutes of Health Grant R01-MH46948 (to E.F.C.).
Abbreviations
- IED
Intermittent Explosive Disorder
- OMPCC
orbital/medial prefrontal cortex circuit
- UPSIT
University of Pennsylvania Smell Identification Test
- SCRs
skin conductance responses
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Coccaro E F. Sci Am Sci Med. 1995;2:38–47. [Google Scholar]
- 2.Davidson R J, Putnam K M, Larson C L. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 3.Coccaro E F. Harvard Rev Psychiatry. 1998;5:336–339. doi: 10.3109/10673229809003583. [DOI] [PubMed] [Google Scholar]
- 4.Coccaro E F, Kavoussi R J. Arch Gen Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 5.Fuster J M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of Frontal Lobe. Philadelphia: Lippincott; 1998. [Google Scholar]
- 6.Grafman J, Schwab K, Warden D, Pridgen A, Brown H R, Salazar A M. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- 7.Anderson S W, Bechara A, Damasio H, Tranel D, Damasio A R. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 8.Coccaro E F, Kavoussi R J, Berman M E, Lish J. Compr Psychiatry. 1998;39:368–376. doi: 10.1016/s0010-440x(98)90050-5. [DOI] [PubMed] [Google Scholar]
- 9.Booker B, Cyr J. J Clin Psychol. 1986;42:982–986. [Google Scholar]
- 10.Bechara A, Damasio A R, Damasio H, Anderson S W. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 11.Ekman P. Pictures of Facial Affect. University of California Medical Center, San Francisco: Human Interaction Laboratory; 1976. [Google Scholar]
- 12.Doty R L. The Smell Identification Test Administration Manual. 3rd Ed. Haddon Heights, NJ: Sensonics; 1995. [Google Scholar]
- 13.Rich J B, Bylsma F W, Brandt J. Neuropsychiatry Neuropsychol Behav Neurol. 1996;9:99–106. [Google Scholar]
- 14.Cohen J D, Forman S, Braver T S, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 15.Buss A, Durkee A. J Consult Psychol. 1957;21:343–348. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 16.Bechara A, Damasio H, Tranel D, Damasio A R. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 17.Jones-Gotman M, Zatorre R. Neuropsychologia. 1988;26:387–400. doi: 10.1016/0028-3932(88)90093-0. [DOI] [PubMed] [Google Scholar]
- 18.Sobel N, Prabhakaran V, Desmond J E, Glover G H, Goode R L, Sullivan E V, Gabrieli J D E. Nature (London) 1998;392:282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- 19.Frye R E, Schwartz B S, Doty R L. J Am Med Assoc. 1990;263:1233–1236. [PubMed] [Google Scholar]
- 20.Wilson F A, Scalaidhe S P, Goldman-Rakic P S. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 21.Petrides M. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechara A, Tranel D, Damasio H, Damasio A R. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 23.Bechara A, Damasio H, Tranel D, Anderson S. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bechara A, Damasio H, Damasio A R, Lee G P. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodge K A, Lochman J E, Harnish J D, Bates J E, Petit G S. J Abnorm Psychol. 1997;106:37–51. doi: 10.1037//0021-843x.106.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Walz N C, Benson B. Am J Ment Retard. 1996;101:282–291. [PubMed] [Google Scholar]
- 27.Blair R J, Cipolotti L. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 28.Adolphs R, Tranel D, Damasio H, Damasio A. Nature (London) 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 29.Adolphs R, Tranel D, Damasio A R. Nature (London) 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 30.Morris J S, Frith C D, Perrett D I, Rowland D, Young A W, Calder A J, Dolan R J. Nature (London) 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 31.Whalen P J, Rauch S L, Etcoff N L, McInerney S C, Lee M B, Jenike M A. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki H, Kaufman O, Damasio H, Damasio A R, Granner M, Bakken H, Hori T, Howard M A, III, Adolphs R. Nat Neurosci. 2001;4:15–16. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- 33.Blair R J, Morris J S, Frith C D, Perrett D I, Dolan R J. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 34.Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, Kaulisch T, Kotter R, Stephan K E, Leschinger A, et al. Cereb Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Dougherty D D, Shin L M, Alpert N M, Pitman R K, Orr S P, Lasko M, Macklin M L, Fischman A J, Rauch S L. Biol Psychiatry. 1999;46:466–472. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 36.Kimbrell T A, George M S, Parekh P I, Ketter T A, Podell D M, Danielson A L, Repella J D, Benson B E, Willis M W, Herscovitch P, Post R M. Biol Psychiatry. 1999;46:454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 37.Harmer C J, Thilo K V, Rothwell J C, Goodwin G M. Nat Neurosci. 2001;4:17–18. doi: 10.1038/82854. [DOI] [PubMed] [Google Scholar]
- 38.Petrides M, Alivisatos B, Evans A C, Meyer E. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raine A, Buchsbaum M S, Stanley J, Lottenberg S, Abel L, Stoddard J. Biol Psychiatry. 1994;36:365–373. doi: 10.1016/0006-3223(94)91211-4. [DOI] [PubMed] [Google Scholar]
- 40.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Arch Gen Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- 41.LaPierre D, Braun C M, Hodgins S. Neuropsychologia. 1995;33:139–151. doi: 10.1016/0028-3932(94)00110-b. [DOI] [PubMed] [Google Scholar]
- 42.Blackburn R. In: Physiological Correlates of Human Behavior. Gale A, Edwards J E, editors. Vol. 3. New York: Academic; 1983. pp. 187–203. [Google Scholar]
- 43.Abbruzzese M, Ferri S, Scarone S. Neuropsychologia. 1997;35:907–912. doi: 10.1016/s0028-3932(96)00095-4. [DOI] [PubMed] [Google Scholar]
- 44.Cavedini P, Riboldi G, D'Annucci A, Belotti P, Cisima M, Bellodi L. Neuropsychologia. 2002;40:205–211. doi: 10.1016/s0028-3932(01)00077-x. [DOI] [PubMed] [Google Scholar]
- 45.Coccaro E F, Bergeman C S, McClearn G E. Psychiatry Res. 1993;48:229–242. doi: 10.1016/0165-1781(93)90074-q. [DOI] [PubMed] [Google Scholar]
- 46.Coccaro E F, Bergeman C S, Kavoussi R J, Seroczynski A D. Biol Psychiatry. 1997;41:273–284. doi: 10.1016/s0006-3223(96)00257-0. [DOI] [PubMed] [Google Scholar]
- 47.Manuck S B, Flory J D, Ferrell R E, Dent K M, Mann J J, Muldoon M F. Biol Psychiatry. 1999;45:603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- 48.Coccaro E F, Kavoussi R J. In: Aggression and Violence: Genetic, Neurobiological, and Biosocial Perspectives. Stoff D M, Cairns R B, editors. Mahwah, NJ: Erlbaum; 1996. pp. 67–85. [Google Scholar]
- 49.Coccaro E F, Siever L J, Klar H M, Maurer G, Cochrane K, Cooper T B, Mohs R C, Davis K L. Arch Gen Psychiatry. 1989;46:587–599. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- 50.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin F K. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 51.Virkkunen M, Rawlings R, Tokola R, Poland R E, Guidotti A, Nemeroff C, Bissette G, Kalogeras K, Karonen S L, Linnoila M. Arch Gen Psychiatry. 1994;51:20–27. doi: 10.1001/archpsyc.1994.03950010020003. [DOI] [PubMed] [Google Scholar]
- 52.Siever L J, Buchsbaum M S, New A S, Spiegel-Cohen J, Wei T, Hazlett E A, Sevin E, Nunn M, Mitropoulou V. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]