Abstract
Group A streptococci (GAS) produce several exoproteins that are thought to contribute to the pathogenesis of human infection. Two such proteins, streptolysin O (SLO) and NAD+-glycohydrolase (NADase), have been shown to interact functionally as a compound signaling toxin. When GAS are bound to the surface of epithelial cells in vitro, SLO forms pores in the cell membrane and delivers NADase to the epithelial cell cytoplasm. In vitro, intoxication of keratinocytes with NADase is associated with cytotoxic effects and induction of apoptosis; however, the importance of NADase during infection of an animal host has not been established. We employed isogenic GAS mutants to assess the contribution of NADase activity to GAS virulence in vivo using mouse models of invasive soft-tissue infection and septicemia. In both models, mutant GAS that lacked NADase activity were significantly attenuated for virulence compared with the isogenic wild-type parent, confirming an important role for NADase in the infection of a host animal. A double mutant lacking SLO and NADase activity had an intermediate virulence phenotype, consistent with the hypothesis that SLO evokes a protective innate immune response. We conclude that NADase and SLO together enhance GAS virulence in vivo.
Streptococcus pyogenes, group A Streptococcus (GAS), is an exclusively human pathogen responsible for substantial morbidity from pharyngitis (streptococcal sore throat), infections of the skin and underlying soft tissues, and rare but life-threatening invasive infection syndromes. Over the past two decades, heightened interest in GAS pathogenesis has been stimulated by an apparent resurgence of severe invasive disease in North America and Europe and by continued morbidity from the postinfectious sequelae of acute rheumatic fever in developing countries (5, 7, 16).
Among the many known and suspected virulence factors produced by GAS, two secreted proteins appear to interact in a unique way to comprise a compound toxin that has deleterious effects on human epithelial cells in vitro. Streptolysin O (SLO) is a well-characterized member of the cholesterol-dependent cytolysin family of toxins, representatives of which are produced by diverse gram-positive bacteria (2, 13). Like other toxins in this family, SLO creates large pores in eukaryotic cell membranes by binding to cholesterol, oligomerizing, and inserting into the plasma membrane. SLO has been found to act as a vehicle for translocation of a second GAS protein, NAD+-glycohydrolase (NADase), into the cytoplasm of host epithelial cells (11). The GAS NADase not only cleaves NAD to produce nicotinamide and ADP-ribose but also produces cyclic ADP-ribose (cADPR) in a side reaction (8). The latter molecule can act as an effector of intracellular Ca2+ signaling pathways and may contribute to observed cellular effects of SLO and NADase, including enhanced cytotoxicity, reduced bacterial uptake, and induction of apoptosis (4, 11).
The in vitro effects of SLO and NADase have suggested that the combined action of the proteins contributes to GAS virulence. However, epidemiologic studies have reached conflicting conclusions. Stevens et al. noted that the temporal appearance of NADase activity in M1 strains of GAS correlated with emergence of that serotype in invasive GAS infection syndromes (18). On the other hand, a study of GAS infection among aboriginal people in Australia found no relationship between NADase production and severity or outcome of GAS infection (6).
Experimental infection studies have shown modest attenuation of an SLO mutant in mice (10, 17). However, these studies have not distinguished between effects of SLO itself and those of the functionally linked NADase. To explore further the functional role of SLO and NADase in invasive infection, we used two mouse models to investigate the relative virulence of isogenic mutant strains deficient in NADase or both NADase and SLO. The results support a role for NADase in systemic infection and suggest that the virulence function of SLO may be mitigated by its proinflammatory properties.
MATERIALS AND METHODS
Bacterial strains and plasmids and bacterial growth.
GAS strain 950771Sm is a spontaneous streptomycin-resistant derivative of the M-type 3 clinical isolate strain 950771 (3). Strains 771nga+slo+, 771nga−slo+, and 771slo−nga− are derivatives of 950771Sm and were described previously (4). GAS strains were grown in Todd-Hewitt broth (Difco Laboratories) supplemented with yeast extract (Difco Laboratories) to a final concentration of 0.5% (wt/vol; THY) or grown on either trypticase soy blood agar medium (TSA blood; PML Microbiologicals) or THY agar medium supplemented with 5% (vol/vol) defibrinated sheep blood (THY blood; PML Microbiologicals). GAS liquid cultures were grown at 37°C without shaking.
Rederivation of the nga mutant.
An independent rederivation of the strain 771nga−slo+ was accomplished as described previously (4). Briefly, cells of GAS parent strain 950771Sm were made electrocompetent and transformed with the plasmid pngaΔ. Repeated passage of selected transformants resulted in allelic exchange and deletion of most of the nga coding sequence. To control for the possibility of spontaneous nonspecific effects on the expression of other virulence determinants, a revertant nga+ excisant was isolated simultaneously to act as the mutant strain's wild-type control. Both new strains were tested to confirm wild-type levels of hyaluronic acid and M-protein content as described previously (4, 12, 20).
Measurement of SLO activity.
SLO activity of GAS strains was assessed by measuring the capacity of supernatants from early-stationary-phase liquid GAS cultures to lyse sheep erythrocytes as described previously (14).
Measurement of NADase activity.
GAS culture supernatants from early-stationary-phase liquid cultures were assayed for NADase activity as described previously (4).
Soft-tissue infection model.
All animal studies were conducted in accordance with federal and institutional guidelines. Virulence was assessed in a mouse model of invasive soft-tissue infection, essentially as described previously (3). Briefly, GAS cells were grown to early exponential phase in THY broth at 37°C without shaking. Female ICR mice (Harlan), 6 to 8 weeks of age, were anesthetized by pentobarbital injection, and their right flanks were shaved prior to superficial subcutaneous inoculation with 108 CFU of GAS suspended in 50 μl of culture medium. The lesion at the inoculation site was measured daily, and the lesion area was calculated as maximum length multiplied by width. Blood cultures were performed every other day beginning 1 day after inoculation. Surviving mice were euthanized on day 8, and their spleens were removed for culture.
Intraperitoneal challenge model.
GAS cells were grown to early exponential phase in THY broth at 37°C without shaking. Female ICR mice (6 to 8 weeks old; Harlan) were injected intraperitoneally with approximately 900 CFU in 1 ml of THY. Mice were monitored for mortality for 72 h after challenge.
Statistical analysis.
For evaluation of differences in the size of injection site necrotic lesions, the Wilcoxon nonparametric two-sample test was employed. The probability of bacteremia was assessed using repeated measures logistic regression. Survival times were assessed using a log-rank comparison. R software was used for statistical analyses (www.r-project.org) (9).
RESULTS
NADase and SLO in invasive soft-tissue infection.
NADase has been associated with invasive GAS infection, but direct evidence is lacking that NADase enhances clinical invasiveness in vivo. We used a mouse model of invasive soft-tissue infection to compare the relative virulence of an invasive clinical isolate of an M type 3 strain of GAS (771nga+slo+) with that of isogenic mutants deficient in NADase (771nga−slo+) or both NADase and SLO (771nga−slo−). This model mimics several important pathophysiologic features of human necrotizing fasciitis, including progressive tissue necrosis, infarction of overlying epidermis, and development of eventually fatal secondary bacteremia (3, 15). Groups of mice were inoculated subcutaneously with wild-type or mutant GAS. A subcutaneous abscess with or without cutaneous necrosis developed at the injection site, and the area of tissue necrosis reached a maximum approximately 6 days after inoculation. Infection with wild-type 771nga+slo+ resulted in necrotic lesions that were significantly larger than those induced by 771nga−slo+.
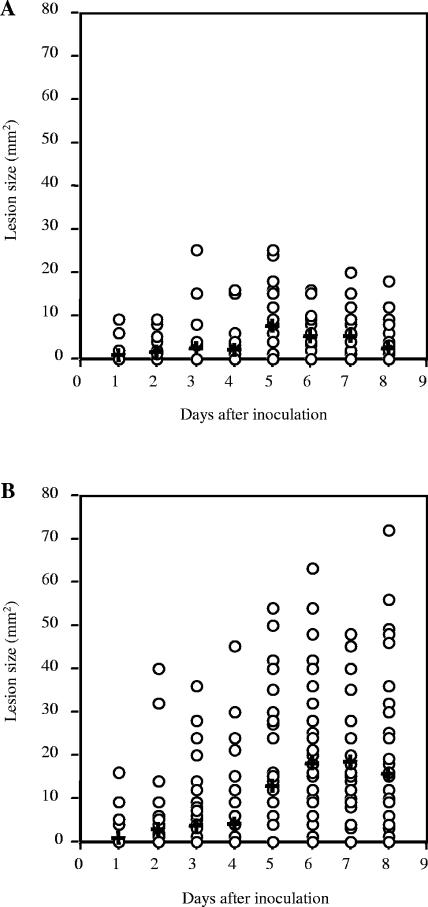
While the observed attenuation of 771nga−slo+ might be due to loss of NADase, it also is possible that the mutant strain had become attenuated through spontaneous loss of other, unknown virulence attributes. To address this possibility, we derived a second, independent nga-deficient slo+ mutant in the same GAS strain background. Results of virulence testing with the independently derived 771nga−slo+/771nga+slo+ strain pair were similar to those obtained with the first mutant strain, suggesting that the reduced virulence of the NADase mutant strains was a direct result of the targeted nga mutation. Data from both groups of experiments are combined in Fig. 1A and B. Mean lesion size at day 6 for 771nga+slo+ was 17.8 mm2 versus 5.0 mm2 for mice infected with 771nga−slo+ (P < 0.001).
FIG. 1.
Effect of inactivating NADase on local tissue necrosis after subcutaneous inoculation with GAS. Each data point represents the area of skin necrosis surrounding the inoculation site on the indicated day after subcutaneous inoculation with GAS strain 771nga−slo+ (A) or GAS wild-type strain 771nga+slo+ (B). Mean lesion size is indicated by a cross. Data are from four independent experiments and a total of 40 mice for each challenge strain. P = 0.001 for comparison between strains.
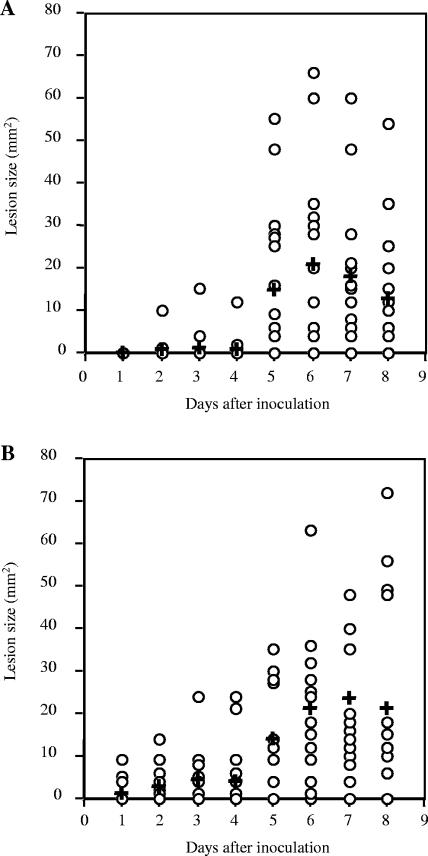
In contrast, experiments that compared groups of mice inoculated with 771nga+slo+ to mice inoculated with 771nga−slo− showed no significant difference in mean lesion size (Fig. 2A and B). This surprising result suggested that loss of NADase alone had a more profound effect of reducing GAS virulence in soft-tissue infection than did loss of both NADase and SLO. The apparently greater degree of attenuation of 771nga−slo+ compared to 771nga−slo− was observed for two independently derived 771nga−slo+ mutants, so it seems unlikely to be due to a coincidental unknown mutation.
FIG. 2.
Effect of inactivating both NADase and SLO on local tissue necrosis after subcutaneous inoculation with GAS. Each data point represents the area of skin necrosis surrounding the inoculation site on the indicated day after subcutaneous inoculation with GAS strain 771nga−slo− (A) or GAS wild-type strain 771nga+slo+ (B). Mean lesion size is indicated by a cross. Data are from two independent experiments and a total of 20 mice for each challenge strain. P values were not significant for comparison between strains.
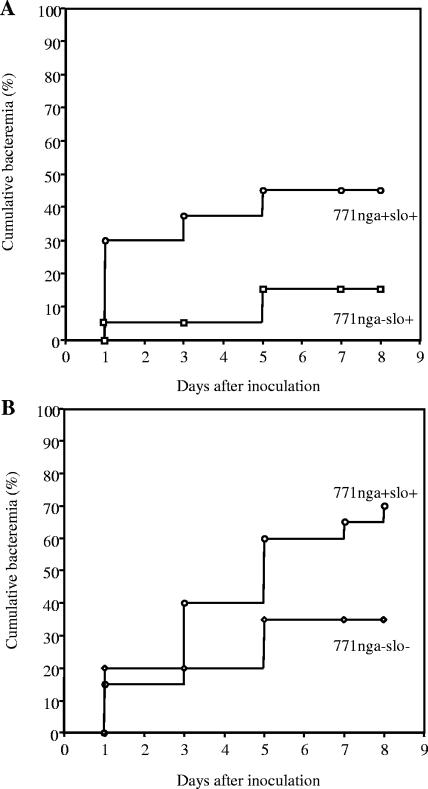
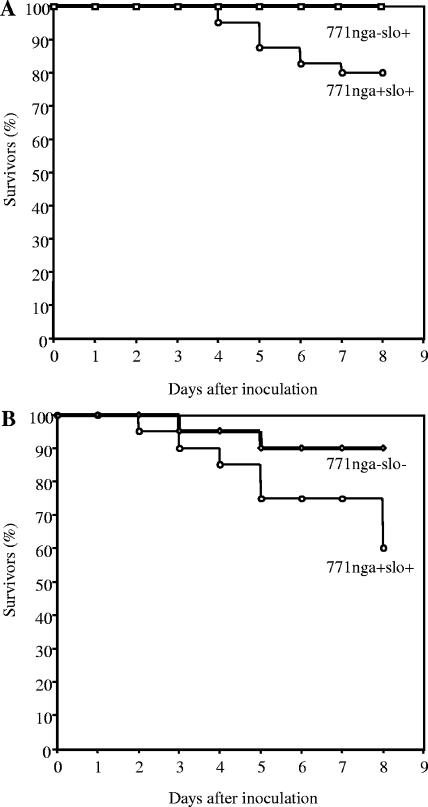
As a second measure of virulence in the soft-tissue infection model, development of secondary bacteremia was assessed. In human necrotizing fasciitis, progressive local infection often is accompanied by spread of GAS into the bloodstream and development of sepsis syndrome. Blood cultures of mice inoculated with wild-type GAS 771nga+slo+ became positive in approximately 45% of infected animals during the 8 days after challenge, whereas only 15% of mice inoculated with 771nga−slo+ developed detectable bacteremia (P = 0.013; Fig. 3A). In contrast, the rate of bacteremia in mice challenged with 771nga−slo− did not differ significantly from that in mice challenged with wild type 771nga+slo+ (P = 0.42; Fig. 3B). These results are consistent with the finding of reduced soft-tissue necrosis by 771nga−slo+ and suggest that the absence of NADase (in the presence of SLO) affects the capacity of GAS to invade the bloodstream from a local site of tissue infection. The mortality of mice infected with either 771nga−slo+ or 771nga−slo− was 0% and 10%, respectively. Both were significantly lower than that of wild-type strain 771nga+slo+ (P = 0.04, Fig. 4A and B).
FIG. 3.
Cumulative bacteremia after subcutaneous inoculation with wild-type or mutant strains of GAS. Each data point represents the percentage of mice with at least one positive blood culture as of the indicated day. (A) Mice were challenged with GAS strain 771nga−slo+ (squares) or wild-type GAS strain 771nga+slo+ (circles); data are from four independent experiments and a total of 40 mice for each challenge strain. P < 0.01 for comparison between strains. (B) Mice were challenged with GAS strain 771nga−slo− (diamonds) or wild-type GAS strain 771nga+slo+ (circles); data are from two independent experiments and a total of 20 mice for each challenge strain. P values were not significant for comparison between strains.
FIG. 4.
Survival after subcutaneous inoculation with wild-type or mutant strains of GAS. (A) Mice challenged with GAS strain 771nga−slo+ (squares) or wild-type GAS strain 771nga+slo+ (circles); data are from four independent experiments and a total of 40 mice for each challenge strain. P = 0.04 for comparison between strains. (B) Mice challenged with GAS strain 771nga−slo− (diamonds) or wild-type GAS strain 771nga+slo+ (circles); data are from two independent experiments and a total of 20 mice for each challenge strain. P = 0.04 for comparison between strains.
NADase as a virulence factor in GAS septicemia.
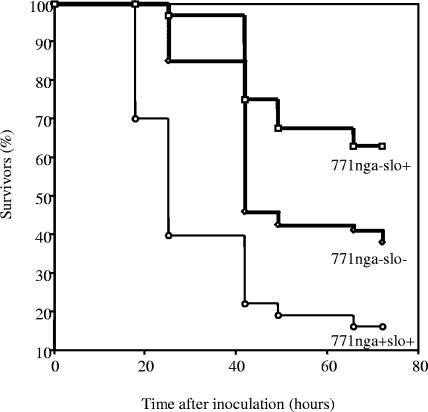
We also investigated the role of NADase in virulence in a second model of invasive GAS infection in mice, systemic infection after intraperitoneal challenge. In this model, infection with wild-type 771nga+slo+ resulted in 95% mortality at 72 h (Fig. 5). The NADase mutant strain 771nga−slo+ was significantly less virulent (37% mortality; P < 0.001), and the double mutant 771nga−slo− showed an intermediate level of virulence (63% mortality; P = 0.003 for comparison with 771nga+slo+). These results are consistent with those in the soft-tissue infection model in that a hierarchy of virulence was observed: 771nga+slo+ > 771nga−slo− > 771nga−slo+.
FIG. 5.
Survival after intraperitoneal inoculation with wild-type or mutant strains of GAS. Mice were challenged with GAS strain 771nga−slo+ (squares), 771nga−slo− (diamonds), or wild-type strain 771nga+slo+ (circles). Data are from 30 to 36 mice for each challenge strain. Survival was significantly greater in animals challenged with 771nga−slo+ (P < 0.001) or 771nga−slo− (P = 0.003) compared to 771nga+slo+.
DISCUSSION
The potential importance and specific mode of action of NADase in GAS pathogenesis remains incompletely understood. While all GAS strains appear to possess the nga gene, only 56% to 92% of clinical isolates have produced demonstrable NADase activity during growth in vitro (1, 6, 18). These results suggest that NADase is not essential for GAS infection or that enzyme expression is regulated differently during infection than during growth in vitro. Epidemiologic arguments have been made for and against a role of NADase in GAS invasive infection. NADase activity was detected in 92% of clinical isolates associated with an outbreak of invasive GAS disease (1). NADase production was rare among M type 1 GAS strains isolated prior to 1988 but subsequently has been almost universal among M1 strains from invasive infections (18). A study of isolates from Australia found no increase in NADase-positive strains in cases of invasive infection compared to uncomplicated infections (6). However, interpretation of all these studies is limited by potential bias in how strains were selected and by possible discrepancies between NADase production in vitro versus in vivo.
In the present study, we sought to investigate directly whether NADase contributes to GAS virulence in murine models of infection. Although the mouse is, in general, not a natural host for GAS, murine models have proven useful in evaluating the role in pathogenesis of other GAS virulence determinants. We found that inactivation of the nga locus reduced the virulence of an M3 strain of GAS originally isolated from a patient with necrotizing fasciitis. In a model of invasive soft-tissue infection, the NADase mutant produced a smaller necrotic lesion at the site of injection, a lower rate of bacteremia, and a lower mortality than the parent wild-type strain. The NADase mutant also was significantly less virulent than the wild type in mice after intraperitoneal challenge. Surprisingly, a mutant that lacked both NADase and SLO had a virulence phenotype intermediate between that of the wild type and the NADase mutant in both models. These results support a role for NADase in virulence and suggest that SLO expression, in the absence of NADase, actually may mitigate GAS virulence.
Previous studies indicated that SLO was required for delivery of NADase into the cytosol of host cells. The combined action of the two proteins resulted in cytotoxic injury to epithelial cells, impaired uptake of bound GAS, and epithelial cell apoptosis (4, 11). In addition, SLO is known to have pore-forming activity for eukaryotic cell membranes that would be expected to contribute to virulence in a manner independent of the effects of NADase. However, consistent with the present results, previous studies of SLO-negative mutants have shown either a modest effect or no effect of SLO on virulence in mice (10, 17).
The apparently greater attenuating effect of inactivation of NADase compared to SLO or both toxins may be a consequence of the strong proinflammatory effects of SLO. Exposure of keratinocytes in vitro to SLO-producing GAS resulted in increased release of interleukin-1β (IL-1β), IL-6, and IL-8 compared to exposure to an SLO mutant strain (14). The proinflammatory effect of SLO required adherence of GAS to the affected cells, so a possible contribution of NADase to induction of cytokine production in that study cannot be excluded (19). IL-8 is chemotactic for neutrophils, so one anticipated consequence of IL-8 secretion is the recruitment of host phagocytes to the site of infection, an event that would be expected to hasten the resolution of local infection. Thus, the presence of SLO in the absence of NADase may provoke a host inflammatory response that results in better control of infection, whereas such a response is not provoked by infection with an SLO-negative strain.
Results of the present investigation extend previous in vitro observations on the importance of NADase in pathogenesis. Animal models of GAS infection must be viewed with caution, since human beings are the only natural hosts for the organism. Nevertheless, the mouse models of infection used in this work have served as useful test systems to study the contribution to pathogenesis of several putative virulence determinants, including M protein, the hyaluronic acid capsular polysaccharide, cysteine protease, and/or streptolysin S (3, 17). The mechanism(s) through which NADase intoxicates host cells is still uncertain. Possible modes of action include depletion of intracellular NAD+, ADP-ribosylation of cellular proteins, and disruption of intracellular signaling pathways by cADPR-stimulated release of intracellular calcium stores. The finding that NADase contributes to virulence in vivo strengthens the view that SLO and NADase play an important role in GAS infection and emphasizes the importance of further investigation to define the molecular basis of NADase toxicity.
Acknowledgments
We thank Colette Cywes for helpful discussions.
This work was supported in part by NIH grant AI29952 and contract AI30040.
Editor: V. J. DiRita
REFERENCES
- 1.Ajdic, D., W. M. McShan, D. J. Savic, D. Gerlach, and J. J. Ferretti. 2000. The NAD-glycohydrolase (nga) gene of Streptococcus pyogenes. FEMS Microbiol. Lett. 191:235-241. [DOI] [PubMed] [Google Scholar]
- 2.Alouf, J. E., and C. Geoffroy. 1991. The family of the antigenically-related cholesterol-binding (“sulphydryl-activated”) cytolytic toxins, p. 147-186. In J. E. Alouf and J. H. Freer (ed.), Sourcebook of bacterial toxins. Academic Press, London, United Kingdom.
- 3.Ashbaugh, C. D., H. B. Warren, V. J. Carey, and M. R. Wessels. 1998. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Investig. 102:550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bricker, A. L., C. Cywes, C. D. Ashbaugh, and M. R. Wessels. 2002. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol. Microbiol. 44:257-269. [DOI] [PubMed] [Google Scholar]
- 5.Davies, H. D., A. McGeer, B. Schwartz, K. Green, D. Cann, A. E. Simor, D. E. Low, and the O. G. A. S. S. Group. 1996. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 335:547-554. [DOI] [PubMed] [Google Scholar]
- 6.DelVecchio, A., M. Maley, B. J. Currie, and K. S. Sriprakash. 2002. NAD-glycohydrolase production and speA and speC distribution in group A streptococcus (GAS) isolates do not correlate with severe GAS diseases in the Australian population. J. Clin. Microbiol. 40:2642-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, D. R., J. T. Wotton, A. Shet, and E. L. Kaplan. 2002. A comparison of group A streptococci from invasive and uncomplicated infections: are virulent clones responsible for serious streptococcal infections? J. Infect. Dis. 185:1586-1595. [DOI] [PubMed] [Google Scholar]
- 8.Karasawa, T., S. Takasawa, K. Yamakawa, H. Yonekura, H. Okamoto, and S. Nakamura. 1995. NAD(+)-glycohydrolase from Streptococcus pyogenes shows cyclic ADP-ribose forming activity. FEMS Microbiol. Lett. 130:201-204. [DOI] [PubMed] [Google Scholar]
- 9.Lawless, J. F. 1985. Statistical models and methods for lifetime data. Wiley, New York, N.Y.
- 10.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 12.Ouchterlony, O. 1958. Diffusion-in-gel methods for immunological analysis. Prog. Allergy 5:1-78. [PubMed] [Google Scholar]
- 13.Palmer, M. 2001. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon 39:1681-1689. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz, N., B. Wang, A. Pentland, and M. Caparon. 1998. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol. Microbiol. 27:337-346. [DOI] [PubMed] [Google Scholar]
- 15.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz, B., A. Schuchat, M. J. Oxtoby, S. L. Cochi, A. Hightower, and C. V. Broome. 1991. Invasive group B streptococcal disease in adults, a population-based study in metropolitan Atlanta. JAMA 266:1112-1114. [PubMed] [Google Scholar]
- 17.Sierig, G., C. Cywes, M. R. Wessels, and C. D. Ashbaugh. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect. Immun. 71:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens, D. L., D. B. Salmi, E. R. McIndoo, and A. E. Bryant. 2000. Molecular epidemiology of nga and NAD glycohydrolase/ADP-ribosyltransferase activity among Streptococcus pyogenes causing streptococcal toxic shock syndrome. J. Infect. Dis. 182:1117-1128. [DOI] [PubMed] [Google Scholar]
- 19.Wang, B., N. Ruiz, A. Pentland, and M. Caparon. 1997. Keratinocyte proinflammatory responses to adherent and nonadherent group A streptococci. Infect. Immun. 65:2119-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wessels, M. R., A. E. Moses, J. B. Goldberg, and T. J. DiCesare. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 88:8317-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]