Abstract
Background
Few studies to date have explored correlations between red blood cell distribution width (RDW) and prognostic outcomes in acute ischemic stroke (AIS) patients, and conclusions based on the available evidence are controversial. This study was designed to better clarify the association between RDW and 3-month prognostic outcomes in AIS patients.
Methods
This was a secondary analysis of a cohort study. The prospective cohort used to conduct these analyses was comprised of 1,901 AIS patients who underwent treatment in South Korea between January 2010 and December 2016. A modified Rankin Scale (mRS) value above 2 was used to define a poor 3-month prognosis. The link between RDW and poor 3-month prognostic outcomes in subjects with AIS was explored with a binary logistic regression model, with further subgroup analyses and interaction tests also being conducted.
Results
Of 1,901 individuals analyzed in this study, 1,167 (61.4%) were male, and 543 (28.6%) met the criteria for poor 3-month outcomes. Following adjustment for confounding factors, RDW was positively correlated with poor 3-month outcomes in these individuals with AIS (OR = 1.12, 95% CI; 1.03–1.23, P = 0.0120). Subgroup analyses demonstrated a consistent positive association (OR > 1.0) between elevated RDW and adverse outcomes across the majority of baseline characteristics. No interactions were observed between RDW and poor 3-month outcomes among AIS patients.
Conclusion
These results demonstrate a positive correlation between RDW and poor 3-month prognosis among AIS patients. The findings suggest RDW may serve as a practical prognostic biomarker, warranting further validation in diverse populations.
Keywords: Ischemic stroke, Red blood cell distribution width, Poor prognosis, Modified Rankin scale
Background
Acute ischemic stroke (AIS) is a leading global cause of mortality and disability, adversely impacting patients and societies as a whole [1, 2]. One of the major barriers to stroke management is the poor prognosis facing most patients, who face a risk of death, long-term disability, and significant reductions in quality of life [3, 4]. The modified Rankin score (mRS) is widely employed to assess evaluate prognostic outcomes in stroke patients, offering a tool that can be used to gauge limitations to the activities of daily life among affected individuals [5, 6]. Higher mRS scores are indicative of more severe disability and functional limitations [7]. Effective risk stratification, patient management, and targeted treatment development efforts hinge on the establishment of prognostic indicators that can be reliably implemented in clinical settings.
Red blood cell distribution width (RDW) can be utilized to measure peripheral red blood cell size variability, often being employed to evaluate patients for anemia [8]. RDW has been demonstrated to be linked to a higher risk of several forms of cardiovascular disease [9–11]. Relatively little is known about the association between RDW and the prognosis of patients with ischemic stroke, and the connection between the two remains somewhat controversial. A univariate analysis of 1,504 acute stroke patients revealed that RDW was associated with NIHSS scores (β = 0.24, P = 0.01) and poor functional outcomes (OR = 10.73, P < 0.001), though multivariate analysis did not confirm its independence [12]. Similarly, a study of 282 tissue plasminogen activator (tPA)-treated stroke patients stratified by RDW levels (< 12.9% vs. ≥13%) found no significant differences in 3-month modified Rankin Scale scores [13]. Conversely, a multicenter study of 847 first-time acute ischemic stroke patients demonstrated that each 1% increase in RDW raised the risk of poor functional outcomes by 22.2% (OR = 1.222, P = 0.006) and mortality by 39.5% (OR = 1.395, P < 0.001) [14]. Another study of 629 acute ischemic stroke patients, grouped by RDW quartiles, showed that higher RDW levels were significantly linked to moderate-to-severe stroke (OR = 2.21), poor 3-month functional outcomes (OR = 1.86), and lower Barthel Index scores (OR = 2.27) [15]. These findings conducted to date have been limited by their small sample sizes and lack of appropriate stratified analyses or sensitivity testing.
Higher RDW values are related to inflammatory activity, oxidative stress, and the impairment of erythropoiesis [16, 17]. As inflammation is closely tied to post-stroke pathology, RDW has the potential to impact stroke patient prognostic outcomes through its responsivity to the overall inflammatory state [18, 19]. We hypothesized that elevated RDW levels at hospital admission would be independently correlated with unfavorable functional outcomes (mRS > 2) at 3 months in patients with AIS. This study was devised to probe the link between RDW and poor 3-month mRS outcomes in individuals affected by AIS. The results of these analyses have the potential to inform the development of personalized therapeutic approaches and to improve patient outcomes.
Methods
Study design
This was a cohort study based on a previously conducted single-center prospective study performed in South Korea between January 2010 and December 2016 [20]. Here, RDW was utilized as an independent variable, while mRS scores for patients with AIS served as the dependent variable.
Data source
Kang et al. [20] kindly made the original data used to conduct this study freely available. The findings were released through an unrestricted access model with a Creative Commons Attribution license, allowing for their unlimited sharing, distribution, and reproduction in any form so long as the initial source is properly acknowledged. We thank all parties who contributed the data used for this research effort.
Study population
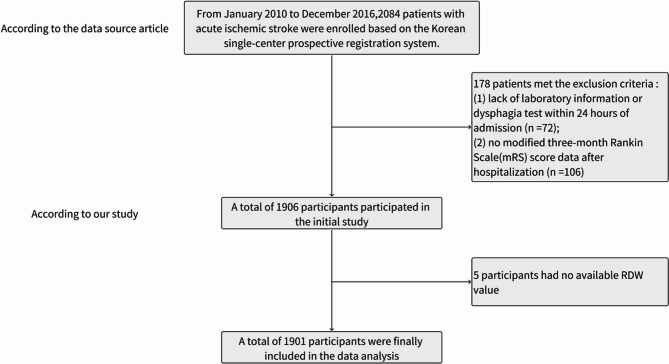
The primary study included 2,084 individuals diagnosed with AIS, of whom 178 were excluded based on exclusion criteria established in the original study [20], including (1) the lack of any evaluation of dysphagia or laboratory testing conducted within 24 h of admission (n = 72), and (2) the lack of mRS scores calculated 3 months following hospital discharge (n = 106). The remaining 1,906 patients were retained in the initial study dataset. For the present secondary analysis, 5 additional patients without available RDW values were excluded. The patient selection process is outlined in Fig. 1.
Fig. 1.
An overview of the selection process
AIS patients admitted to the hospital within 7 days of symptom onset were recruited by the original investigator team [20], using data from a single-center prospective registry [20]. The primary study was approved by Seoul National University Hospital’s institutional review board, which waived any requirement for informed patient consent (IRB No. 1009-062-332) [20]. No additional ethical oversight was deemed necessary for secondary analyses. The original study was performed as per the Declaration of Helsinki in accordance with appropriate laws and guidelines, and the same was true for this secondary analysis.
Variables
RDW, which was measured within 24 h of admission and obtained from electronic medical records [20], was treated as a continuous variable.
Patient 3-month prognostic outcomes
Patient outcomes at 3 months after AIS onset were analyzed based on mRS scores [21]. Follow-up information was collected via outpatient visits or telephone calls [20]. Patients were classified into those with a good prognosis (mRS ≤ 2) and those with a poor prognosis (mRS ≥ 3) [22].
Covariates
All information related to data collection was derived from the original study [20]. Covariate selection was performed as per prior studies and our clinical experience. The covariates included in this study were categorized into continuous and categorical variables. The continuous variables comprised red blood cell (RBC) count, white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), platelet count (PLT), triglycerides (TG), total cholesterol (TC), low-density lipoproteins (LDL), high-density lipoproteins (HDL), creatinine (CR), albumin (ALB), blood urea nitrogen (BUN), high sensitivity c-reactive protein (hs-CRP), body mass index (BMI), activated partial thromboplastin time (APTT), international normalized ratio (INR), glycosylated hemoglobin (HbA1c), and National Institutes of Health Stroke Scale (NIHSS) scores. The categorical variables included age, gender, hypertension, previous stroke or transient ischemic attack (TIA), dyslipidemia, coronary heart disease, diabetes, current smoking status, atrial fibrillation, and stroke etiology.
Statistical analysis
Categorical data are given as frequencies and percentages, while continuous variables are given as the mean (standard deviation) or median (minimum and maximum). Categorical variables were compared with the χ2 test, while skewed and normally distributed continuous variables were compared with Kruskal-Wallis H tests and one-way ANOVAs. Initially, differences between RDW tertiles were analyzed [23], after which the impact of each variable on unfavorable AIS patient outcomes was assessed with a univariate logistic regression approach. Links between RDW and poor 3-month prognostic outcomes in AIS patients were then analyzed with three multivariate binary logistic regression models. Model 1 received no adjustment. Model 2 adjusted for age and gender. Model 3 was further adjusted for age, gender, BMI, current smoking, WBC, RBC, ESR, TC, TG, HDL, LDL, BUN, ALB, hs-CRP, INR, initial NIHSS score, previous stroke/TIA, hypertension, diabetes, hyperlipidemia, atrial fibrillation, and stroke etiology. Confounding factors were assessed by evaluating the impact of covariates on the effect estimate of RDW exceeding 10%, or the regression coefficient of covariates on poor prognosis at 3 months with a significance level of P < 0.1 [23, 24]. Sensitivity analyses were then conducted to assess result reliability. RDW values were converted into tertiles as a categorical variable, and the P-value for trend was calculated to validate results for RDW as a continuous variable, and to explore any possibility of nonlinearity. Subgroup analyses were performed using stratified binary logistic regression models to assess the association between RDW and poor prognosis across prespecified strata defined by age, gender, previous stroke/TIA, hypertension, diabetes, hyperlipidemia, current smoking, atrial fibrillation, coronary heart disease, and stroke etiology. Subgroup interactions were examined with a likelihood ratio test. Data was missing only for WBC (n = 1, 0.05%) and TG (n = 9, 0.47%). Given the negligible missingness, we implemented complete-case analysis without imputation [25]. EmpowerStats (X&Y Solutions, Inc., MA) and R (http://www.r-project.org) were used for statistical analyses, and a two-sided P < 0.05 was regarded as significant.
Results
Participant characteristics
Detailed participant demographic and clinical characteristics are presented in Table 1. This study enrolled 1,901 patients of whom 61.4% were male. This included 543 (28.6%) patients who exhibited a poor 3-month prognosis. The overall patient cohort included 435 (22.88%), 505 (26.56%), 668 (35.14%), and 293 (15.41%) who were respectively < 60, 60–70, 70–80, and > 80 years old. RDW values were used to separate patients into three tertiles, with the groups exhibiting low, medium, and high RDW values respectively being classified as those in the 11.10 − 12.60%, 12.70 − 13.40%, and 13.50 − 22.50% ranges.
Table 1.
The basic traits of participants
| RDW tertiles, % | Low(11.10–12.60) | Middle(12.70–13.40) | High(13.50–22.50) | P-value |
|---|---|---|---|---|
| No. of subjects | 560 | 708 | 633 | |
| Age, n (%) | < 0.001 | |||
| < 60 | 166 (29.64%) | 166 (23.45%) | 103 (16.27%) | |
| 60 to < 70 | 159 (28.39%) | 194 (27.40%) | 152 (24.01%) | |
| 70 to < 80 | 169 (30.18%) | 242 (34.18%) | 257 (40.60%) | |
| ≥ 80 | 66 (11.79%) | 106 (14.97%) | 121 (19.12%) | |
| Gender, n (%) | 0.495 | |||
| Male | 342 (61.07%) | 446 (62.99%) | 379 (59.87%) | |
| Female | 218 (38.93%) | 262 (37.01%) | 254 (40.13%) | |
| BMI, mean (SD), kg/m2 | 23.98 (3.20) | 23.78 (3.19) | 22.78 (3.25) | < 0.001 |
| WBC, mean (SD), 109/L | 8.32 (2.88) | 8.07 (2.64) | 8.05 (3.15) | 0.196 |
| RBC, mean (SD), 1012/L | 4.47 (0.53) | 4.39 (0.58) | 4.12 (0.73) | < 0.001 |
| PLT, mean (SD), 109/L | 229.62 (57.62) | 224.30 (68.46) | 217.34 (83.90) | 0.011 |
| ESR, median (min-max), mm/h | 0.00 (0.00-140.00) | 0.00 (0.00-107.00) | 0.00 (0.00-138.00) | < 0.001 |
| TC, mean (SD), mg/dL | 187.44 (43.35) | 180.04 (41.53) | 171.65 (45.61) | < 0.001 |
| TG, mean (SD), mg/dL | 111.05 (65.75) | 105.10 (57.14) | 100.92 (57.45) | 0.014 |
| HDL, mean (SD), mg/dL | 43.80 (16.44) | 45.77 (16.31) | 42.66 (17.52) | 0.003 |
| LDL, mean (SD), mg/dL | 108.58 (42.59) | 104.84 (41.39) | 99.89 (42.88) | 0.002 |
| BUN, mean (SD), mg/dL | 16.22 (6.93) | 17.06 (7.17) | 19.41 (11.48) | < 0.001 |
| CR, median (min-max), mg/dL | 0.86 (0.37–11.12) | 0.90 (0.36–13.91) | 0.91 (0.37–11.38) | < 0.001 |
| ALB, mean (SD), g/dL | 4.18 (0.35) | 4.06 (0.36) | 3.84 (0.49) | < 0.001 |
| HbA1c, mean (SD), % | 5.00 (2.94) | 5.14 (2.73) | 5.14 (2.61) | 0.596 |
| hs-CRP, median (min-max), mg/dL | 0.10 (0.00-22.61) | 0.12 (0.00-28.06) | 0.19 (0.00-36.80) | < 0.001 |
| INR, median (min-max) | 1.01 (0.24) | 1.04 (0.36) | 1.07 (0.35) | 0.007 |
| APTT, median (min-max), s | 31.11 (6.46) | 30.58 (5.32) | 30.77 (7.66) | 0.353 |
| Initial NIHSS score, median (min-max) | 3.00 (0.00–25.00) | 3.00 (0.00–33.00) | 4.00 (0.00–28.00) | < 0.001 |
| Current smoking, n (%) | 0.547 | |||
| No | 341 (60.89%) | 418 (59.04%) | 392 (61.93%) | |
| Yes | 219 (39.11%) | 290 (40.96%) | 241 (38.07%) | |
| Previous stroke/TIA, n (%) | 0.126 | |||
| No | 456 (81.43%) | 560 (79.10%) | 485 (76.62%) | |
| Yes | 104 (18.57%) | 148 (20.90%) | 148 (23.38%) | |
| Hypertension, n (%) | 0.900 | |||
| No | 208 (37.14%) | 258 (36.44%) | 227 (35.86%) | |
| Yes | 352 (62.86%) | 450 (63.56%) | 406 (64.14%) | |
| Diabetes, n (%) | 0.568 | |||
| No | 380 (67.86%) | 489 (69.07%) | 420 (66.35%) | |
| Yes | 180 (32.14%) | 219 (30.93%) | 213 (33.65%) | |
| Hyperlipidemia, n (%) | 0.091 | |||
| No | 342 (61.07%) | 439 (62.01%) | 422 (66.67%) | |
| Yes | 218 (38.93%) | 269 (37.99%) | 211 (33.33%) | |
| Atrial fibrillation, n (%) | < 0.001 | |||
| No | 468 (83.57%) | 580 (81.92%) | 449 (70.93%) | |
| Yes | 92 (16.43%) | 128 (18.08%) | 184 (29.07%) | |
| Coronary heart disease, n (%) | 0.003 | |||
| No | 511 (91.25%) | 631 (89.12%) | 539 (85.15%) | |
| Yes | 49 (8.75%) | 77 (10.88%) | 94 (14.85%) | |
| mRS score, n (%) | < 0.001 | |||
| 0–2 | 423 (75.54%) | 531 (75.00%) | 404 (63.82%) | |
| 3–6 | 137 (24.46%) | 177 (25.00%) | 229 (36.18%) | |
| Stroke etiology, n (%) | < 0.001 | |||
| SVO | 193 (34.46%) | 240 (33.90%) | 174 (27.49%) | |
| LAA | 118 (21.07%) | 154 (21.75%) | 93 (14.69%) | |
| CE | 123 (21.96%) | 167 (23.59%) | 199 (31.44%) | |
| Other determined | 43 (7.68%) | 45 (6.36%) | 82 (12.95%) | |
| Undetermined | 83 (14.82%) | 102 (14.41%) | 85 (13.43%) |
BMI body mass index, WBC white blood cell, RBC red blood cell, PLT platelet count, ESR erythrocyte Sedimentation Rate, TC total cholesterol, TG triglycerides, HDL high-density lipoproteins, LDL low-density lipoproteins, BUN blood Urea Nitrogen, CR creatinine, ALB albumin, HbA1c glycosylated hemoglobin, hs-CRP high sensitive c-reactive protein, INR international normalized ratio, APTT activated partial thromboplastin time, NIHSS National Institutes of Health Stroke Scale, TIA transient ischemia attack, mRS modified rankin scale, SVO small-vessel occlusion, LAA large-artery atherosclerosis, CE cardioembolism
BMI showed a decreasing trend with increasing RDW (P < 0.001). Laboratory indices indicated that RBC, PLT, TC, TG, HDL, and LDL were significantly lower in the high RDW tertile than in the low RDW tertile (all P < 0.05), while BUN, CR, and hs-CRP were significantly higher (all P < 0.001). Regarding clinical comorbidities, the prevalence of atrial fibrillation (29.07%) and coronary heart disease (14.85%) in the high RDW tertile was significantly higher than that in the low RDW tertile (16.43% and 8.75%, respectively; all P < 0.001). The mRS showed a higher proportion of moderate-to-severe disability (mRS 3–6) in the high RDW tertile (36.18%) compared to the low RDW tertile (24.46%, P < 0.001). Stroke etiologies differed significantly, with a higher proportion of CE (31.44%) and lower proportions of SVO and LAA in the high RDW tertile (all P < 0.001). No significant differences were observed in gender, WBC, HbA1c, APTT, current smoking, previous stroke/TIA, hypertension, diabetes, or hyperlipidemia across the three tertiles (all P > 0.05) (Table 1).
Univariate analysis
As reflected in Table 2, univariate analysis identified distinct associations between clinical variables and 3-month poor prognosis after AIS. Positive correlations were observed in RDW (OR = 1.26, P < 0.001), advanced age (70–80 years: OR = 1.89, ≥ 80 years: OR = 3.93, both P < 0.001), female sex (OR = 1.66, P < 0.001), inflammatory markers [WBC (OR = 1.08), ESR (OR = 1.02), hs-CRP (OR = 1.15), all P < 0.001], renal index (BUN: OR = 1.02, P = 0.004), coagulation marker (INR: OR = 1.43, P = 0.017), and acute stroke severity (NIHSS score: OR = 1.23, P < 0.001). Comorbidities including prior stroke/TIA (OR = 1.81), hypertension (OR = 1.34), diabetes (OR = 1.43), atrial fibrillation (OR = 1.99, all P < 0.01), and stroke etiologies of CE (OR = 1.48) and other determined causes (OR = 2.03, both P < 0.01) also showed positive associations. Conversely, negative correlations were found in TC (OR = 1.00, P = 0.0002), TG (OR = 1.00, P < 0.0001), LDL (OR = 1.00, P = 0.0014), higher BMI (OR = 0.92, P < 0.001), RBC (OR = 0.59, P < 0.001), ALB (OR = 0.28, P < 0.001), HDL (OR = 0.99, P = 0.03), current smoking (OR = 0.61, P < 0.001), hyperlipidemia (OR = 0.79, P = 0.02), and LAA (OR = 0.62, P = 0.003). Variables without significant associations included PLT, CR, HbA1c, APTT, and coronary heart disease (all P > 0.05).
Table 2.
Univariate analysis of poor prognosis at 3 months after acute ischemic stroke
| Statistics | Odds ratio (95% CI) | P-value | |
|---|---|---|---|
| Age, n (%) | |||
| < 60 | 435 (22.88%) | 1.0 | |
| 60 to < 70 | 505 (26.56%) | 1.15 (0.84, 1.58) | 0.3897 |
| 70 to < 80 | 668 (35.14%) | 1.89 (1.42, 2.52) | < 0.0001 |
| ≥ 80 | 293 (15.41%) | 3.93 (2.82, 5.47) | < 0.0001 |
| Gender, n (%) | |||
| Male | 1167 (61.39%) | 1.0 | |
| Female | 734 (38.61%) | 1.66 (1.36, 2.03) | < 0.0001 |
| BMI, mean (SD), kg/m2 | 23.50 ± 3.25 | 0.92 (0.89, 0.95) | < 0.0001 |
| WBC, mean (SD), 109/L | 8.14 ± 2.89 | 1.08 (1.04, 1.12) | < 0.0001 |
| RBC, mean (SD), 1012/L | 4.33 ± 0.64 | 0.59 (0.50, 0.69) | < 0.0001 |
| PLT, mean (SD), 109/L | 223.55 ± 71.31 | 1.00 (1.00, 1.00) | 0.2929 |
| ESR, median (min-max), mm/h | 0.00 (0.00-140.00) | 1.02 (1.01, 1.02) | < 0.0001 |
| TC, mean (SD), mg/dL | 179.43 ± 43.88 | 1.00 (0.99, 1.00) | 0.0002 |
| TG, mean (SD), mg/dL | 105.45 ± 60.00 | 1.00 (0.99, 1.00) | < 0.0001 |
| HDL, mean (SD), mg/dL | 44.15 ± 16.81 | 0.99 (0.99, 1.00) | 0.0295 |
| LDL, mean (SD), mg/dL | 104.29 ± 42.37 | 1.00 (0.99, 1.00) | 0.0014 |
| BUN, mean (SD), mg/dL | 17.59 ± 8.88 | 1.02 (1.01, 1.03) | 0.0037 |
| CR, median (min-max), mg/dL | 0.89 (0.36–13.91) | 1.01 (0.92, 1.11) | 0.7602 |
| ALB, mean (SD), g/dL | 4.02 ± 0.43 | 0.28 (0.22, 0.35) | < 0.0001 |
| HbA1c, mean (SD), % | 5.10 ± 2.75 | 1.01 (0.97, 1.04) | 0.6898 |
| hs-CRP, median (min-max), mg/dL | 0.12 (0.00-36.80) | 1.15 (1.11, 1.20) | < 0.0001 |
| INR, median (min-max) | 1.04 ± 0.33 | 1.43 (1.07, 1.92) | 0.0167 |
| APTT, median (min-max), s | 30.80 ± 6.51 | 0.99 (0.98, 1.01) | 0.2914 |
| Initial NIHSS score, median (min-max) | 3.00 (0.00–33.00) | 1.23 (1.20, 1.26) | < 0.0001 |
| Current smoking, n (%) | |||
| No | 1151 (60.55%) | 1.0 | |
| Yes | 750 (39.45%) | 0.61 (0.50, 0.75) | < 0.0001 |
| Previous stroke/TIA, n (%) | |||
| No | 1501 (78.96%) | 1.0 | |
| Yes | 400 (21.04%) | 1.81 (1.44, 2.29) | < 0.0001 |
| Hypertension, n (%) | |||
| No | 693 (36.45%) | 1.0 | |
| Yes | 1208 (63.55%) | 1.34 (1.09, 1.66) | 0.0063 |
| Diabetes, n (%) | |||
| No | 1289 (67.81%) | 1.0 | |
| Yes | 612 (32.19%) | 1.43 (1.16, 1.77) | 0.0007 |
| Hyperlipidemia, n (%) | |||
| No | 1203 (63.28%) | 1.0 | |
| Yes | 698 (36.72%) | 0.79 (0.64, 0.97) | 0.0245 |
| Atrial fibrillation, n (%) | |||
| No | 1497 (78.75%) | 1.0 | |
| Yes | 404 (21.25%) | 1.99 (1.58, 2.50) | <0.0001 |
| Coronary heart disease, n (%) | |||
| No | 1681 (88.43%) | 1.0 | |
| Yes | 220 (11.57%) | 1.03 (0.76, 1.40) | 0.8540 |
| Stroke etiology, n (%) | |||
| SVO | 607 (31.93%) | 1.0 | |
| LAA | 365 (19.20%) | 0.62 (0.45, 0.85) | 0.0031 |
| CE | 489 (25.72%) | 1.48 (1.14, 1.91) | 0.0029 |
| Other determined | 170 (8.94%) | 2.03 (1.43, 2.89) | < 0.0001 |
| Undetermined | 270 (14.20%) | 0.86 (0.61, 1.19) | 0.3598 |
| RDW, mean (SD), % | 13.36 ± 1.37 | 1.26 (1.17, 1.35) | < 0.0001 |
Associations between RDW and poor 3-month prognostic outcomes in AIS patients
Under Model 1, every 1 unit increase in RDW was linked to a 26% rise in the risk of a poor 3-month prognosis (OR = 1.26, P < 0.0001). In Model 2, the results did not show obvious changes (OR = 1.24, P < 0.0001). Under Model 3, the association showed a slight attenuation while maintaining statistical significance. Specifically, each 1 unit increase in RDW was associated with a 12% elevated risk of poor 3-month prognosis (OR = 1.12, P = 0.0120). Analyses of RDW tertiles showed that compared with the low RDW group, the high RDW group had a 75% higher risk of poor prognosis in the unadjusted model (Model 1: OR = 1.75, 95%CI 1.36–2.25, P < 0.0001) and a 55% higher risk in the model adjusted for age and gender (Model 2: OR = 1.55, 95%CI 1.19–2.01, P = 0.001). However, this association was no longer significant in the fully adjusted model (Model 3: OR = 1.08, 95%CI 0.78–1.50, P = 0.6371). Trend analyses also revealed that under Model 1 (P < 0.0001) and Model 2 (P = 0.0005), increases in RDW levels exhibited a significant linear association with a risk of poor prognostic outcomes, but this trend was no longer significant under Model 3 (P = 0.5782) (Table 3).
Table 3.
Relationship between RDW and poor prognosis at 3 months after AIS in different models
| Exposure | Model 1 OR (95%CI) P value |
Model 2 OR (95%CI) P value |
Model 3 OR (95%CI) P value |
|---|---|---|---|
| RDW (per 1 unit increase) | 1.26 (1.17, 1.35) < 0.0001 | 1.24 (1.15, 1.33) < 0.0001 | 1.12 (1.03, 1.23) 0.0120 |
| RDW tertiles | |||
| Low | Ref | Ref | Ref |
| Middle | 1.03 (0.80, 1.33) 0.8263 | 0.97 (0.75, 1.26) 0.8262 | 0.85 (0.62, 1.16) 0.3142 |
| High | 1.75 (1.36, 2.25) < 0.0001 | 1.55 (1.19, 2.01) 0.0010 | 1.08 (0.78, 1.50) 0.6371 |
| P for trend | 1.35 (1.18, 1.53) < 0.0001 | 1.26 (1.11, 1.44) 0.0005 | 1.05 (0.89, 1.23) 0.5782 |
Model 1: Non-adjusted model
Model 2: Adjusted for age and gender
Model 3: Adjusted for age, gender, BMI, current smoking, WBC, RBC, ESR, TC, TG, HDL, LDL, BUN, ALB, hs-CRP, INR, initial NIHSS score, previous stroke/TIA, hypertension, diabetes, hyperlipidemia, atrial fibrillation, stroke etiology
Subgroup analyses
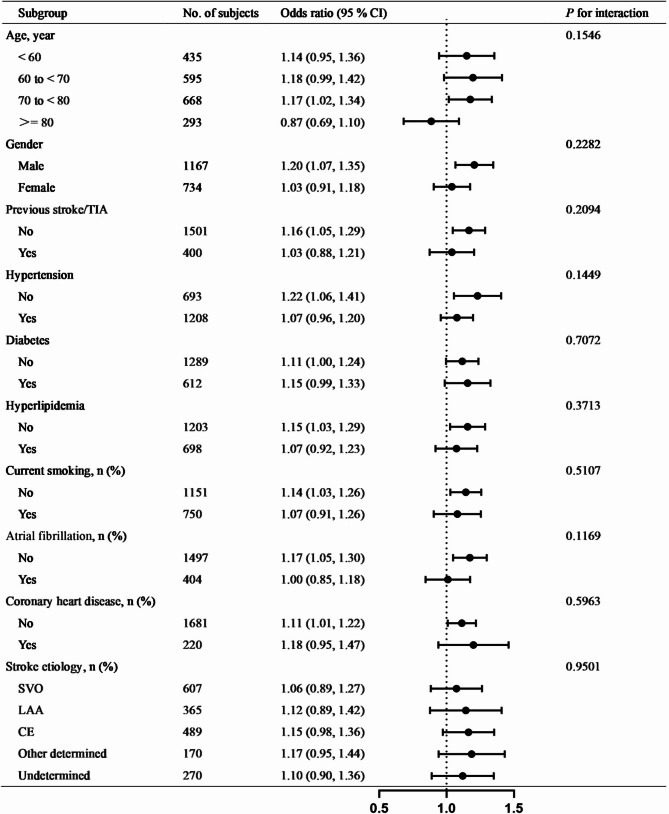
Subgroup analyses revealed a consistently positive association between elevated RDW and poor 3-month outcomes across most predefined strata. Statistically significant associations (P < 0.05) were observed in males (OR 1.20, 95% CI 1.07–1.35), patients without prior stroke/TIA (OR 1.16, 1.05–1.29), non-hypertensive individuals (OR 1.22, 1.06–1.41), those without hyperlipidemia (OR 1.15, 1.03–1.29), non-smokers (OR 1.14, 1.03–1.26), patients without atrial fibrillation (OR 1.17, 1.05–1.30), and those without coronary heart disease (OR 1.11, 1.01–1.22). In contrast, a non-significant inverse trend was noted in the ≥ 80 years subgroup (OR 0.87, 0.69–1.10). No significant interactions were detected across all subgroups (P-interaction > 0.05) (Fig. 2).
Fig. 2.
Relationships between RDW and poor prognosis at 3 months after AIS in various subgroups
Discussion
This study was developed to probe the relationship between RDW and poor 3-month prognostic outcomes among patients with AIS. This is the largest such study conducted to date, and these analyses revealed a positive linear correlation between RDW and poor 3-month prognostic outcomes in AIS patients. This relationship remained intact following adjustment for possible confounding variables (OR = 1.12, 95% CI: 1.03–1.23, P = 0.0120), with a 12% increase in the risk of a poor 3-month prognosis being observed for each additional unit (1%) rise in RDW.
In past studies, the association between RDW and ischemic stroke patient prognosis has been subject to some degree of controversy. A retrospective hospital-based study of 847 consecutive first-episode AIS patients conducted a stepwise logistic regression analysis following adjustment for relevant confounding factors, revealing that higher RDW values were related to poor functional prognostic outcomes at 3 months (OR = 1.222,95% CI 1.059–1.409, P = 0.006) [26]. In a separate cohort study of 629 AIS patients, following multivariate logistic adjustment for confounding factors, a higher RDW was significantly related to higher mRS scores fo 3–6 after 3 months (OR 1.86, 95% CI, 1.02–3.41, P = 0.044) [15]. The meta-analysis additionally indicated that elevated RDW is associated with poor functional outcomes following stroke (OR/RR 1.220 at discharge; OR/RR 1.277 at 90 days) as well as an increased mortality rate (OR/RR 1.278) [27]. These findings have not been universal, however. For instance, in one retrospective analysis of 1,504 consecutive AIS patients, multivariate regression indicated that RDW as assessed on admission was not predictive of functional prognostic outcomes in stroke patients [12]. In a separate retrospective study of 570 AIS patients who underwent intravenous thrombolytic treatment, multivariate analyses suggested that RDW at baseline was not related to 1-year survival function among stroke survivors [28]. A meta-analysis also revealed that RDW is not significantly associated with poor functional outcomes at three months (mRS score ≥ 3) (OR 1.05, 95% CI 0.95–1.16) [29]. Several factors may account for these discrepant results, including sample size, differences in population characteristics (age, ethnicity, gender, underlying comorbidities, stroke severity, etc.), the chosen time points for RDW measurement, and/or differences in the confounding factors adjusted for in these studies. Most of these prior analyses included relatively small sample populations, were retrospective in nature, and did not incorporate sensitivity or subgroup analyses. The present study incorporated 1,901 AIS patients. Following adjustment for relevant confounding factors, baseline RDW in this patient population was positively correlated with a poor 3-month prognosis (OR = 1.12, 95% CI: 1.03–1.23, P = 0.0120).
The specific mechanistic basis underlying the association between RDW and poor prognostic outcomes after AIS remains elusive. Several potential mechanisms have been proposed in previous investigations, which can be categorized into the following aspects:
Inflammatory response: Inflammation is a key contributor to poor stroke outcomes. Elevated RDW values have been consistently associated with heightened systemic inflammation [30, 31]. During the post-stroke inflammatory cascade, cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are released, promoting oxidative stress and increasing erythrocyte membrane permeability [32]. This oxidative damage causes heterogeneous RBC maturation, leading to increased RDW as a marker of RBC size variability [33].
Oxidative stress: RDW elevation may reflect oxidative stress-induced RBC dysregulation. Oxidative stress not only disrupts normal RBC production but also impairs vascular endothelial function, promotes thrombosis, and exacerbates ischemic injury [34–37]. These effects collectively contribute to worse clinical outcomes after AIS, suggesting a bidirectional relationship between RDW, oxidative stress, and stroke prognosis.
Hemorheological alterations: Increased RDW is often accompanied by elevated blood viscosity and reduced RBC deformability, which can impede microcirculatory perfusion [38, 39]. In the context of stroke, these hemorheological changes may further compromise cerebral blood flow, leading to extended ischemic damage and poorer recovery.
Nutritional status: Low-grade malnutrition, particularly deficiencies in vitamins essential for RBC maturation (e.g., folate, vitamin B12), has been linked to higher RDW values [40]. Such nutritional deficiencies can impair neurovascular repair mechanisms, potentially contributing to adverse post-stroke outcomes.
Collectively, RDW likely influences post-stroke neural recovery through interconnected pathways of inflammation, oxidative stress, hemorheological dysfunction, and nutritional status. Longitudinal studies integrating mechanistic analyses are needed to clarify whether RDW represents a modifiable therapeutic target or merely a biomarker of systemic stress.
To assess the robustness of the association between the RDW and 3-month poor functional outcomes and to explore potential effect modification, we conducted pre-specified subgroup analyses across several clinically relevant baseline characteristics. The subgroup analyses demonstrated consistent directionality of the RDW-outcome association (OR > 1.0) across most baseline characteristics, with nonsignificant interaction P-values supporting robustness. Interestingly, the point estimate in the ≥ 80 years subgroup suggested a potential inverse association (OR = 0.87, 95% CI: 0.69–1.10; P-interaction = 0.1546). However, given the non-significant interaction term and the relatively small sample size within this stratum, this finding warrants cautious interpretation and further investigation.
Key strengths of this study include the fact that this is the largest sample size used to probe the link between RDW and poor prognostic outcomes in AIS patients to date. Additionally, the association between RDW and poor prognosis was explored using strict statistical adjustments in an effort to ensure clarity, while sensitivity and subgroup analyses were conducted to ensure the results were reliable.
This study is subject to certain limitations. First, as an observational and retrospective analysis, the study design may introduce selection bias and inherently limits the ability to establish causal relationships between exposures and outcomes. For one, these analyses were performed in Korean patient cohort such that validation in other ethnic groups will be necessary. In addition, age was treated as a categorical rather than a continuous variable in the original study such that the available data may be incomplete. Moreover, RDW was only measured a single time on admission such that it was not possible to assess any relationships between dynamic RDW changes and poor post-stroke outcomes. Lastly, this was a secondary analysis of a published dataset, and the original data did not include details related to potentially relevant confounding factors including a detailed medication history or intravenous thrombolysis.
Conclusion
In this Korean cohort of AIS patients, elevated RDW demonstrates a modest but consistent association with poor functional outcomes. While categorical analyses showed attenuated significance upon comprehensive adjustment, the persistent directional risk pattern supports RDW’s potential clinical relevance in this population. Further validation in larger cohorts is warranted, particularly exploring age-related variations.
Acknowledgements
As a secondary study, the data and explanation of the methodology are mainly taken from: Kim Y, Nam K-W, Jeong H-Y, Kang MK, Kim TJ, Kim SK, et al. (2020) Automated undernutrition screen tool: Geriatric nutritional risk score predicts poor outcomes in individuals with acute ischemic stroke. https://doi.org/https://doi.org/10.1371/journal.pone. 0228738; PLoS ONE 15(2): e0228738. All authors of the study deserve our appreciation.
Authors’ contributions
The research was jointly initiated and designed by QX, DK, and ZGH. The initial manuscript preparation and data analysis were conducted by QX and ZGH. Further analytical input and crucial intellectual contributions were provided by SMX, DPW, XL, WPZ, and JT, who also participated in the manuscript revision process. The final manuscript was reviewed and endorsed by all contributing authors prior to submission.
Funding
No funding.
Data availability
The “PLoS One” database allows for the downloading of data (https://journals.plos.org/plosone/article?id=https://doi.org/10.1371/journal.pone.0228738IF: 2.9 Q1 B3).
Declarations
Ethics approval and consent to participate
The institutional review board of Seoul National University Hospital approved the study protocol and waived the need for patient consent (IRB NO. 1009-062-332).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang G, Zhang Z, Ayala C, Dunet DO, Fang J, George MG. Costs of hospitalization for stroke patients aged 18–64 years in the united States. J Stroke Cerebrovasc. 2014;23(5):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abedi V, Avula V, Razavi SM, Bavishi S, Chaudhary D, Shahjouei S, et al. Predicting short and long-term mortality after acute ischemic stroke using EHR. J Neurol Sci. 2021;427:117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagayoshi M, Everson-Rose SA, Iso H, Mosley TJ, Rose KM, Lutsey PL. Social network, social support, and risk of incident stroke: atherosclerosis risk in communities study. Stroke. 2014;45(10):2868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang S, Yin J, Liu C, Sun M, Lee J, Sun Y, et al. Low pulse pressure after acute ischemic stroke is associated with unfavorable outcomes: the Taiwan stroke registry. J Am Heart Assoc. 2017;6(6):e005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. 2017;48(7):2007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–6. [DOI] [PubMed] [Google Scholar]
- 7.Leppert MH, Poisson SN, Carroll JD, Thaler DE, Kim CH, Orjuela KD, et al. Cost-Effectiveness of patent foramen ovale closure versus medical therapy for secondary stroke prevention. Stroke. 2018;49(6):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbayiyev S, Simsek GK, Ceran B, Akin MS, Kanmaz KH, Canpolat FE. Could red cell distribution width be used for predicting cardiac injury in neonates with COVID-19? J Med Virol. 2022;94(12):5739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amar D, Sinnott-Armstrong N, Ashley EA, Rivas MA. Graphical analysis for phenome-wide causal discovery in genotyped population-scale biobanks. Nat Commun. 2021;12(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng X, Mell B, Alimadadi A, Galla S, Mccarthy CG, Chakraborty S, et al. Genetic predisposition for increased red blood cell distribution width is an early risk factor for cardiovascular and renal comorbidities. Dis Model Mech. 2020;13(5):dmm044081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horne BD, Anderson JL, Muhlestein JB, Ridker PM, Paynter NP. Complete blood count risk score and its components, including RDW, are associated with mortality in the JUPITER trial. Eur J Prev Cardiol. 2015;22(4):519–26. [DOI] [PubMed] [Google Scholar]
- 12.Ntaios G, Gurer O, Faouzi M, Aubert C, Michel P. Red cell distribution width does not predict stroke severity or functional outcome. Int J Stroke. 2012;7(1):2–6. [DOI] [PubMed] [Google Scholar]
- 13.Shahsavarinia K, Ghavam LY, Moharramzadeh P, Pouraghaei M, Sadeghi-Hokmabadi E, Seifar F, et al. The predictive value of red cell distribution width for stroke severity and outcome. Bmc Res Notes. 2020;13(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DY, Hong DY, Kim SY, Park JJ, Kim JW, Park SO, et al. Prognostic value of red blood cell distribution width in predicting 3-month functional outcome of patients undergoing thrombolysis treatment for acute ischemic stroke. Medicine. 2021;100(37):e27255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue J, Zhang D, Zhang XG, Zhu XQ, Xu XS, Yue YH. Red cell distribution width is associated with stroke severity and unfavorable functional outcomes in ischemic stroke. Front Neurol. 2022;13:938515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Zhao B, Wang MY, Chen XY, Li D, Jiang XQ, et al. Associations between the red blood cell distribution width and primary angle-closure glaucoma: a potential for disease prediction. Epma J. 2019;10(2):185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsueh CY, Lau HC, Li S, Tao L, Zhang M, Gong H, et al. Pretreatment level of red cell distribution width as a prognostic Indicator for survival in a large cohort study of male laryngeal squamous carcinoma. Front Oncol. 2019;9:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang YX, Wang WX, Xue Z, Zhu L, Wang SB, Sun ZH. Electrical stimulation of the vagus nerve protects against cerebral ischemic injury through an anti-infammatory mechanism. Neural Regen Res. 2015;10(4):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou P, Xue HP, Mao XE, Li YN, Wu LF, Liu YB. Inflammation markers are associated with frailty in elderly patients with coronary heart disease. Aging. 2018;10(10):2636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang MK, Kim TJ, Kim Y, Nam KW, Jeong HY, Kim SK, et al. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke - Automated undernutrition screen tool. PLoS ONE. 2020;15(2):e228738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lord AS, Langefeld CD, Sekar P, Moomaw CJ, Badjatia N, Vashkevich A, et al. Infection after intracerebral hemorrhage: risk factors and association with outcomes in the ethnic/racial variations of intracerebral hemorrhage study. Stroke. 2014;45(12):3535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv G, Wang GQ, Xia ZX, Wang HX, Liu N, Wei W, et al. Influences of blood lipids on the occurrence and prognosis of hemorrhagic transformation after acute cerebral infarction: a case-control study of 732 patients. Military Med Res. 2019;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ao T, Huang Y, Zhen P, Hu M. Association between red cell distribution width and 30-day mortality in patients with sepsis-associated liver injury: a retrospective cohort study. Front Med-Lausanne. 2024;11:1510997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. New Engl J Med. 2000;343(25):1826–32. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. Bmc Med Res Methodol. 2017;17(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Kim YD, Song TJ, Park JH, Lee HS, Nam CM, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemostasis. 2012;108(2):349–56. [DOI] [PubMed] [Google Scholar]
- 27.Song SY, Hua C, Dornbors DR, Kang RJ, Zhao XX, Du X, et al. Baseline red blood cell distribution width as a predictor of stroke occurrence and outcome: A comprehensive Meta-Analysis of 31 studies. Front Neurol. 2019;10:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye WY, Li J, Li X, Yang XZ, Weng YY, Xiang WW, et al. Predicting the One-Year prognosis and mortality of patients with acute ischemic stroke using red blood cell distribution width before intravenous thrombolysis. Clin Interv Aging. 2020;15:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Wang C, Wu S, Li Y, Guo W, Liu M. Red blood cell distribution width is associated with mortality after acute ischemic stroke: a cohort study and systematic review. Ann Transl Med. 2020;8(4):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marta-Enguita J, Navarro-Oviedo M, Rubio-Baines I, Aymerich N, Herrera M, Zandio B, et al. Association of calprotectin with other inflammatory parameters in the prediction of mortality for ischemic stroke. J Neuroinflamm. 2021;18(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8(7):401–10. [DOI] [PubMed] [Google Scholar]
- 32.Bai YD, Yang YR, Mu XP, Lin G, Wang YP, Jin S, et al. Hydrogen Sulfide Alleviates Acute Myocardial Ischemia Injury by Modulating Autophagy and Inflammation Response under Oxidative Stress. Oxid Med Cell Longev. 2018;2018:3402809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bester J, Swanepoel AC, Windberger U. Editorial: pathological changes in erythrocytes during inflammation and infection. Front Physiol. 2022;13:943114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Liang S, Geng J, Wang Q, Wang P, Cao Y, et al. Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: a retrospective cohort study. J Intensive Care. 2020;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herlitz-Cifuentes H, Vejar C, Flores A, Jara P, Bustos P, Castro I, et al. Plasma from patients with rheumatoid arthritis reduces nitric oxide synthesis and induces reactive oxygen species in A Cell-Based biosensor. Biosensors-Basel. 2019;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–62. [DOI] [PubMed] [Google Scholar]
- 37.Liu YH, Chen YH, Ko CH, Kuo CW, Yen CC, Chen W, et al. SOD3 and IL-18 predict the first kidney disease-Related hospitalization or death during the One-Year Follow-Up period in patients with End-Stage renal disease. Antioxidants-Basel. 2022;11(6):1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korei C, Szabo B, Varga A, Barath B, Deak A, Vanyolos E, et al. Hematological, Micro-Rheological, and metabolic changes modulated by local ischemic Pre- and Post-Conditioning in rat limb Ischemia-Reperfusion. Metabolites. 2021;11(11):776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu W, Huo J, Chen G, Yang K, Huang Z, Peng L, et al. Association between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: A retrospective cohort study. Front Nutr. 2022;9:1019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KM, Lui LY, Cauley JA, Ensrud KE, Orwoll ES, Schousboe JT, et al. Red cell distribution width is a risk factor for hip fracture in elderly men without Anemia. J Bone Min Res. 2020;35(5):869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The “PLoS One” database allows for the downloading of data (https://journals.plos.org/plosone/article?id=https://doi.org/10.1371/journal.pone.0228738IF: 2.9 Q1 B3).