Abstract
Objectives
To analyze the safety, adequacy and accuracy of ultrasound-guided tru-cut biopsy in the diagnosis of ovarian cancer.
Methods
A systematic search of PubMed, Web of Science, and Scopus was conducted through June 2024. Studies meeting predefined criteria were included in the review. The quality of diagnostic accuracy studies was assessed using QUADAS-2. A meta-analysis was performed on studies reporting complete 2 × 2 diagnostic data.
Results
A total of 2,250 articles were initially screened, and after the removal of duplicates, 54 articles were deemed eligible for full-text assessment. Ultimately, 18 studies, comprising 1,867 patients who underwent ultrasound-guided tru-cut biopsy, were included in the systematic review. A total of 16 complications were reported across 1,898 biopsies performed in the included studies, resulting in a mean complication rate of 0.58% (95% CI: 0.187– 0.964%). Adequacy for histological and immunohistochemical examination after one attempt was reported in 16 studies, with a mean adequacy rate of 95.1% (95% CI: 92.69– 97.50%) and a median rate of 95.97%. Diagnostic accuracy was assessed in 13 studies, revealing a mean diagnostic accuracy of 95.54% (95% CI: 93.19– 97.89%) and a median of 97.48%.In the meta-analysis of 10 studies, pooled sensitivity was 98.6%, specificity 41.9%, positive predictive value (PPV) 99.0%, and negative predictive value (NPV) 47.2%, with high heterogeneity observed in specificity and NPV estimates.
Conclusions
Ultrasound guided tru-cut biopsy is a safe and effective diagnostic method, demonstrating a high adequacy rate for histological and immunohistochemical analysis. It shows excellent performance in confirming malignancy and supports preoperative decision making. To further define its role in the diagnostic pathway for ovarian cancer, additional prospective multicenter studies are needed—both to validate its reliability in negative cases and to ensure tissue adequacy for advanced molecular testing in the context of personalized medicine.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-025-01739-7.
Keywords: Ovarian cancer, Ultrasound, Tru-cut biopsy, Core needle biopsy, Systematic review
Introduction
Ovarian cancer is a heterogeneous group of diseases, characterized by histological, anatomical and molecular diversity [1]. It remains the most lethal gynecologic malignancy, largely due to the lack of early detection and its presentation at a late stage [2, 3]. Patients with advanced ovarian cancer benefit most from cytoreductive surgery with optimal cytoreduction, followed by chemotherapy. However, the timing of the surgery - whether upfront or after the application of neoadjuvant chemotherapy remains a subject of ongoing debate [4–8]. In cases where the multidisciplinary team determines that primary cytoreductive surgery is not the best initial approach, obtaining a histopathological diagnosis becomes crucial for making informed clinical decisions [9].
Ultrasound is a reliable imaging modality that is commonly available, non-invasive, inexpensive, and risk-free for the patient [10]. The use of ultrasound by an expert sonographer has been shown to yield excellent results in differentiating benign and malignant ovarian tumors, including the ability to suggest a specific histological subtype [11]. This capability is of significant importance, as the ovary is also a frequent site for secondary malignancies [12]. Tru-cut biopsy is a minimally invasive method for obtaining biopsy samples from suspicious tissue, and it can be performed with or without image guidance [13]. In the gynecological field, the use of ultrasound-guided tru-cut biopsy has been widely documented and is increasingly playing a significant role in the diagnosis and management of gynecological patients [14, 15]. However, to date, no systematic review of its use in the diagnosis of ovarian cancer has been published in the English literature.
With this systematic review, we aim to present relevant data in the literature on the safety, adequacy and accuracy of ultrasound-guided tru-cut biopsy in patients with suspected ovarian cancer and to advocate for its standardized use in the management of this patient group.
Methods
This systematic review was performed using the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies method (PRISMA-DTA Checklist) [16]. The PRISMA checklist is provided as ‘Supplementary material for review’. The protocol for the systematic review was registered with PROSPERO under the number CRD42024589023.
Literature search
Two independent investigators (D.H., M.N.) systematically searched PubMed, Scopus, and Web of Science databases up to June 2024. The search term, adapted for each database was: (“core needle or tru-cut”) and (“biopsy”) and (“ovary or gynecology or pelvis”). No language restriction was applied during the initial search. The complete search strategy is detailed in the Online supplement Appendix 1.
Selection criteria
This review includes retrospective and prospective cohort studies in English that evaluate the adequacy, accuracy when available, and safety of ultrasound-guided tru-cut biopsy performed via transvaginal (TVUS), transabdominal (TAUS), or transrectal (TRUS) approaches in patients with suspected ovarian cancer. Studies with mixed gynecologic or pelvic malignancies were eligible if they included at least one confirmed case of ovarian cancer and reported outcomes relevant to this review. Participants must be ≥ 18 years of age. The exclusion criteria consisted of meeting abstracts, review articles, case reports, meta-analyses, editorials, letters to the editor, books, and studies that did not include at least one confirmed case of ovarian cancer.
Data extraction
The Rayyan app was used to screen all the articles retrieved from the initial literature search [17]. After uploading all the articles to the web application, M.N. removed any duplicates resulting from searches being performed in three databases. Then, two investigators (D.H. and M.N.) screened the remaining articles in a blind mode by reviewing the titles and abstracts, excluding articles that met the exclusion criteria or clearly did not meet the inclusion criteria. In cases of disagreement, M.P. acted as a mediator. Articles deemed potentially relevant to the review were selected for full-text review, which was again conducted in blind mode using the Rayyan app by both investigators (D.H. and M.N.). The articles that met the inclusion criteria, after mutual consensus by both investigators, were included in the systematic review. In cases where consensus could not be reached, M.P. served as a mediator.
The following data were extracted independently by F.M. and P.B. from the included studies into an Excel table: article name, authors, year of publication, center, total population studied, population with ultrasound, population with at least a suspicion of or having ovarian cancer, approach (not specified, TVUS, TAUS, TRUS), adequacy (sufficient sample), reference standard for adequacy (histology, histology and immunohistochemistry), adequacy after one procedure, adequacy after repeated procedures (if reported), accuracy among the adequate samples, reference standard for accuracy (definitive surgical specimen, definitive surgical specimen + clinical signs), true positive, false positive, true negative, false negative, sensitivity, specificity, positive predictive value, negative predictive value, conclusions, complications (yes or no), number of complications, complications categorized according to the Clavien-Dindo scale of surgical complications [18] and conclusions. We extracted data only for ultrasound-guided procedures performed for diagnostic purposes. After data extraction, a double-check was performed to confirm the accuracy of the extracted information. In cases of uncertainty, N.B. intervened to facilitate the decision-making process. The main characteristics of the included studies are presented in the Results section as Table 1.
Table 1.
Main characteristics of included studies
| Author, year | City | Study Type | Overall population | Approach | Included | Pathologist assessment | Adequate after 1st attempt (n) | Repeated attempts | Adequate after 2nd attempt (n) | Adequate after 5th attempt (n) | Reference standard | Accuracy (population tested) | Biopsy accuracy (n) | Biopsies (total n) | Compli-cations (n) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fischerova (2008)[36] | Prague | Prosp | 86 | TVUS, TAUS | 86 | Histo, IHC | 80 | 4 | 84 | NA | 90 | 1 | ||||||
| Griffin (2009)[22] | Cambridge | Retro | 60 | TAUS | 35 | Histo, IHC | 31 | NA | 35 | 0 | ||||||||
| Zikan (2010)[37] | Prague | Retro | 190 | TVUS, TAUS | 184 | Histo, IHC | 170 | 5 | 175 | DSS | 118 | 116 | 189 | 2 | ||||
| Oge (2013)[27] | Eskisehir | Retro | 55 | TAUS | 55 | Histo, IHC | 53 | NA | 55 | 0 | ||||||||
| Thabet (2014)[23] | Boston | Retro | 27 | TVUS + TAUS | 13 | Histo, IHC | 13 | DSS | 8 | 8 | 13 | 0 | ||||||
| TVUS | 7 | Histo, IHC | 7 | 2 | 2 | |||||||||||||
| TAUS | 6 | Histo, IHC | 6 | 6 | 6 | |||||||||||||
| Kong (2016)[29] | Suwon | Retro | 52 | TVUS | 52 | Histo, IHC | 49 | DSS | 49 | 52 | 0 | |||||||
| Epstein (2016)[28] | Stockholm | Prosp | 143 | TVUS, TAUS, TRUS | 143 | Histo, IHC | 126 | 5 | 131 | DSS | 30 | 30 | 148 | 4 | ||||
| Lin (2017)[32] | Guangzhou | Retro | 200 | TVUS | 200 | Histo | 192 | 2 | 193 | DSS, CL | 192 | 190 | 202 | 0 | ||||
| Yousefi (2017)[24] | Mashhad | Prosp | 12 | TVUS | 12 | Histo, IHC | 12 | DSS | 8 | 8 | 12 | 0 | ||||||
| Majid (2018)[34] | Peshawar | Retro | 78 | NS | 2 | NS | 2 | NA | 2 | 0 | ||||||||
| Mascilini (2019)[25] | Rome | Retro | 62 | TVUS | 62 | Histo, IHC | 62 | DSS, CL | 17 | 15 | 62 | 0 | ||||||
| Vlasak (2020)[21] | Pilsen | Retro | 123 | TVUS + TAUS | 123 | Histo, IHC | 118 | DSS | 51 | 46 | 123 | 0 | ||||||
| TVUS | 91 | Histo, IHC | 88 | |||||||||||||||
| TAUS | 32 | Histo, IHC | 30 | |||||||||||||||
| Verschuere (2021)[26] | Leuven | Retro | 176 | TVUS | 100 | Histo, IHC | 83 | 8 | 91 | DSS | 43 | 35 | 108 | 0 | ||||
| Lengyel (2021)[31] | Budapest | Prosp | 303 | TVUS | 303 | Histo, IHC | 299 | DSS | 94 | 82 | 303 | 3 | ||||||
| Buonomo (2022)[35] | Trieste | Retro | 42 | TVUS, TAUS | 42 | Histo, IHC | 40 | 2 | 42 | DSS | 17 | 16 | 42 | 1 | ||||
| Asp (2023)[33] | Lund | Retro | 309 | TVUS, TAUS | 293 | Histo, IHC | NA | NA | NA | 253 | DSS | 159 | 155 | 300 | 4 | |||
| Pelayo (2023)[20] | Madrid, Murcia | Retro | 42 | TVUS | 34 | Histo, IHC | NA | NA | 34 | DSS | 10 | 9 | 34 | 0 | ||||
| Mascilini (2023)[30] | Rome | Prosp | 158 | TVUS + TAUS | 128 | Histo, IHC | 128 | DSS | 102 | 96 | 128 | 1 | ||||||
| TVUS | 121 | 121 | 98 | 92 | ||||||||||||||
| TAUS | 7 | 7 | 4 | 4 | ||||||||||||||
Prosp, prospective; Retro, retrospective; TVUS, transvaginal ultrasound; TAUS, transabdominal US; TRUS transrectal US; Histo, histological; IHC, immunohistochemistry; NA, not applied, DSS, definitive surgical specimen, CL, clinical; NS, not specified
Assessment of the risk of bias
The quality of the studies included in the systematic review of the accuracy of ultrasound-guided tru-cut biopsy was assessed by two investigators (M.S. and D.K.) using the revised Quality Assessment for Studies of Diagnostic Accuracy tool (QUADAS-2) [19]. The QUADAS-2 tool was divided into three phases: (1) We defined the expected patient characteristics, the index test, the reference standard, and the target condition. (2) A virtual flowchart was created for each study, detailing patient selection, adequacy testing, and the number of accuracy tests performed. (3) Judgment and labeling of the patient selection, index test, reference standard, flow, and timing as low risk, high risk, or unclear when sufficient information was not provided in the article.
Statistical analysis
Statistical analysis was conducted using NCSS (version 24.0.3). We calculated means (with 95% confidence intervals), medians, and standard deviations for adequacy, accuracy, and complication rates. Two-sample t-tests were used to compare subgroups (TVUS vs. TAUS, first vs. repeat biopsy, retrospective vs. prospective studies), with significance set at p < 0.05. Heterogeneity was explored descriptively through subgroup comparisons by biopsy approach and study design.
For studies reporting diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated from available 2 × 2 tables. When true negative or false negative values were zero, resulting in undefined denominators, these metrics were labeled as ‘NaN’ (Not a Number), a standard statistical placeholder indicating that the value could not be calculated.
A meta-analysis was conducted on studies reporting complete 2 × 2 data. Sensitivity, specificity, PPV, and NPV with 95% confidence intervals were extracted or calculated. Standard errors were derived using the formula: (Upper CI– Lower CI) / (2 × NORM.S.INV(0.975)) when not reported. Analyses were performed in JASP (version 0.18.1) using the Meta-Analysis– Proportions module. Random-effects models were used throughout, based on the Restricted Maximum Likelihood (REML) method. Forest plots were generated to visualize individual and pooled estimates. Heterogeneity was assessed using the I² statistic, τ², and Q-test. Publication bias was explored using funnel plots. Where substantial heterogeneity was observed, it was considered in the interpretation.
Results
Study selection
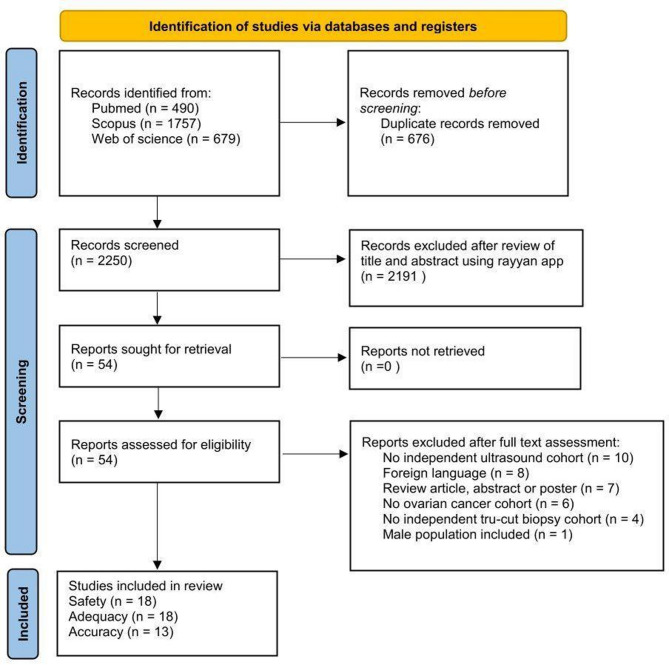
A total of 2,926 articles were retrieved, of which 676 were deleted due to being duplicates. 2,250 articles were screened by reading their titles and abstracts, resulting in the exclusion of 2,196 articles, leaving 54 for full-text evaluation. After full-text evaluation, 36 articles were excluded: 10 for not having independent data for the ultrasound cohort, 8 for being in a foreign language, 7 for being review articles/abstracts/posters, 6 for not including a single ovarian cancer patient, 4 for lacking an independent ultrasound-guided tru-cut biopsy cohort, and 1 for including male populations without separate data. 18 articles were included in the systematic review of the adequacy and safety of ultrasound-guided tru-cut biopsy, of which 13 reported results on accuracy. The flowchart outlining the study selection process is presented below in Fig. 1.
Fig. 1.
Flowchart summarizing inclusion of studies in systematic review
Safety
18 articles were included in this part of the systematic review with a total of 1,898 biopsies performed on 1,867 patients [20–37]. 5 studies were prospective cohort studies [24, 28, 30, 31, 36] with the remaining studies being retrospective in nature. 11 Studies reported the absence of complications [20–27, 29, 32, 34]. The remaining 7 studies reported some form of complication, including 4 out of the 5 prospective studies [28, 30, 31, 36]. The 1 prospective study with no reported complications included only 12 patients [24].
A total of 16 complications were reported after 1,898 biopsies; however, 6 of these complications required surgical intervention. The mean (95% CI) and median rates of complications across all studies were 0.58% (0.187–0.964) and 0%, respectively. The mean (95% CI) and median rates of complications for the prospective studies were 1.12% (0.344–1.890) and 0.99%, respectively, while the mean (95% CI) and median rates for the retrospective studies were 0.37% (-0.026–0.761) and 0%, respectively. All reported complications are presented in Table 2.
Table 2.
All reported complications
| Author | Complication rate | Clavien Dindo classification | Case | Intervention |
|---|---|---|---|---|
| Fischerova (2008)[36] | 1.1% | IIIb | Bleeding from tumor in a patient with mild thrombocytopenia | Laparotomy |
| Zikan (2010)[37] | 1.1% | IIIb | Hemoperitoneum | Laparotomy |
| IIIb | Bleeding into ascitic fluid | Laparoscopy | ||
| Epstein (2016)[28] | 2.7% | I | Mild bleeding (< 100mL) | None |
| I | Mild bleeding (< 100mL) | None | ||
| II | Abdominal wall hematoma | Hospitalization | ||
| II | Abdominal wall hematoma | Hospitalization | ||
| Lengyel (2021)[31] | 1.0% | IIa | Superinfection of large mesothelial tumor | Ultrasound drainage, IV ATB |
| IIIb | Asymptomatic abscess of benign ovarian cystic lesion | Surgical exploration | ||
| IIIb | Asymptomatic abscess of benign ovarian cystic lesion | Surgical exploration | ||
| Buonomo (2022)[35] | 2.4% | I | 8 cm endopelvic clot after the procedure | None |
| Asp (2023)[33] | 1.3% | II | Intestinal perforation | IV ATB |
| II | Superficial puncture infection | IV ATB | ||
| II | Pelvic abscess | IV ATB | ||
| II | Pelvic abscess, pain | IV ATB | ||
| Mascilini (2023)[30] | 0.8% | I | Fever | None |
IV ATB, intravenous antibiotics
Adequacy
A total of 18 articles were included in this part of the systematic review, involving 1,867 patients [20–37]. 5 of these studies were prospective cohort studies [24, 28, 30, 31, 36] while the remaining studies were retrospective in nature. Adequacy after the first attempt was reported in 16 of the 18 studies [21–32, 34–37]. 6 of these 16 studies also reported adequacy after a second attempt in selected patients who had inadequate samples following the first attempt [26, 28, 32, 35–37]. 1 study reported adequacy only after 2 attempts [20] and 1 study reported adequacy after up to 5 attempts [33]. 7 studies combined the TVUS and TAUS approaches [21, 23, 30, 33, 35–37] 3 of which presented separate adequacy reports for each approach [21, 23, 30]. 7 studies used the TVUS approach only [20, 24–26, 29, 31, 32] 2 studies used the TAUS approach only [22, 27] 1 study combined the TAUS, TVUS and TRUS approach [21] and the last study did not specify the approach used [34].
A total of 1,540 biopsies were performed with results of adequacy after the first attempt, of which 1,458 were deemed adequate and 82 inadequate. The mean (95% CI) adequacy after one attempt was 95.1% (92.69–97.50), with a median of 95.98%. The mean (95% CI) and median adequacy after two attempts (including the first attempt) in relation to the whole population increased to 95.98% (93.47–98.49) and 96.5% respectively. Only 26 repeat biopsies of the 82 inadequate biopsies after the first attempt were reported as a second attempt, of which 25 were deemed adequate. The adequacy after up to five attempts reported in a single study was 86.34%. The mean (95% CI) adequacy after one attempt using the TVUS approach was 96.51% (92.38–99.77), with a median of 98.68%. While the mean (95% CI) adequacy after one attempt using the TAUS approach was 95.73% (91.976–99.498), with a median of 96.36%.
Accuracy
14 out of the 18 studies reported the histological accuracy of the adequate sample obtained after ultrasound-guided tru-cut biopsy [20, 21, 23–26, 28–33, 35, 37]. However, 1 study was eventually excluded from the systematic review of accuracy because it did not have at least one true positive case of ovarian cancer [25]. Of the 13 studies included in the systematic review of accuracy, 12 studies used the histological diagnosis of the definitive surgical specimen as the reference standard, while 1 study used both the histological diagnosis of the definitive surgical specimen and clinical diagnosis as the reference standard [32]. 4 studies were prospective in nature [24, 28, 30, 31] with the remaining 9 being retrospective. 10 studies provided data for a 2 × 2 table of true positive (TP), false positive (FP), true negative (TN) and false negative (FN), using only the specific histopathological diagnosis of the definitive surgical specimen as the reference standard [21, 23, 24, 26, 28–31, 35, 37].
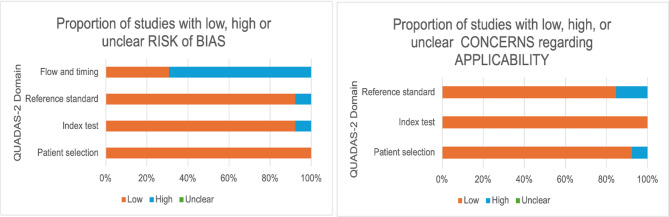
Relevant information required for the QUADAS-2 assessment was available for all studies included in the systematic review evaluating the accuracy of ultrasound-guided tru-cut biopsy. The results are presented in Fig. 2. Overall, the included studies demonstrated a low concern regarding applicability and a low risk of bias in patient selection, the index test, and the reference standard. However, 9 studies were identified as having a high risk of bias in the domain of flow and timing. This was primarily attributed to a significant proportion of patients with adequate samples obtained from the tru-cut biopsy who did not undergo the reference standard procedure (surgical intervention) [20, 21, 26, 28, 31–33, 35, 37].
Fig. 2.
QUADAS-2 graph analysis
The 13 studies included in the systematic review of accuracy had a mean accuracy of 95.5% (95% CI: 93.2–97.9) and median of 97.48% in making the right clinical diagnosis. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the correct histological diagnosis are presented in Table 3 for the 10 studies that provided data for a 2 × 2 table.
Table 3.
Data on histopathological accuracy
| STUDY | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Zikan (2010)[37] | 116 | 1 | 1 | 0 | 0.991 (0.953 to 0.998) | 0 (0 to 0.793) | 0.991 (0.953 to 0.998) | 0 (0 to 0.793) |
| Thabet (2014)[23] | 7 | 0 | 0 | 1 | 1 (0.646 to 1) | 1 (0.207 to 1) | 1 (0.646 to 1) | 1 (0.207 to 1) |
| Kong (2016)[29] | 49 | 0 | 0 | 0 | 1 (0.927 to 1) | (NaN to NaN) | 1 (0.927 to 1) | (NaN to NaN) |
| Epstein (2016)[28] | 30 | 0 | 0 | 0 | 1 (0.886 to 1) | (NaN to NaN) | 1 (0.886 to 1) | (NaN to NaN) |
| Yousefi (2017)[24] | 8 | 0 | 0 | 0 | 1 (0.676 to 1) | (NaN to NaN) | 1 (0.676 to 1) | (NaN to NaN) |
| Vlasak (2020)[21] | 46 | 5 | 0 | 0 | 1 (0.923 to 1) | 0 (0 to 0.434) | 0.902 (0.79 to 0.957) | (NaN to NaN) |
| Verschuere (2021)[26] | 35 | 1 | 3 | 4 | 0.921 (0.792 to 0.973) | 0.8 (0.376 to 0.964) | 0.972 (0.858 to 0.995) | 0.571 (0.25 to 0.842) |
| Lengyel (2021)[31] | 66 | 7 | 4 | 16 | 0.943 (0.862 to 0.978) | 0.696 (0.491 to 0.844) | 0.904 (0.815 to 0.953) | 0.8 (0.584 to 0.919) |
| Buonomo (2022)[35] | 16 | 1 | 0 | 0 | 1 (0.806 to 1) | 0 (0 to 0.793) | 0.941 (0.73 to 0.99) | (NaN to NaN) |
| Mascilini (2023)[30] | 96 | 0 | 6 | 0 | 0.941 (0.878 to 0.973) | (NaN to NaN) | 1 (0.962 to 1) | 0 (0 to 0.39) |
TP, true positive; FP, false positive; TN, true negative; FN, false negative; PPV, positive predictive value; NPV, negative predictive value; NaN, Not estimable due to zero values in denominator
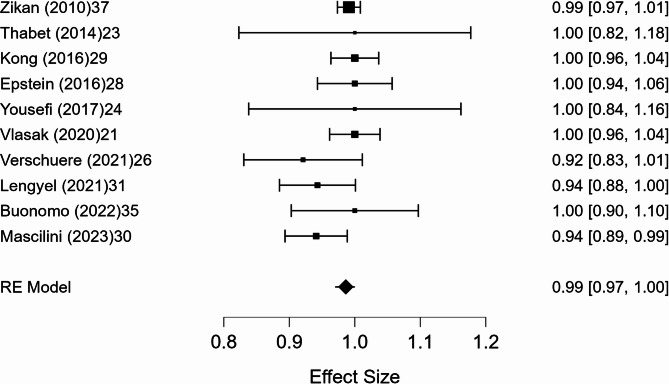
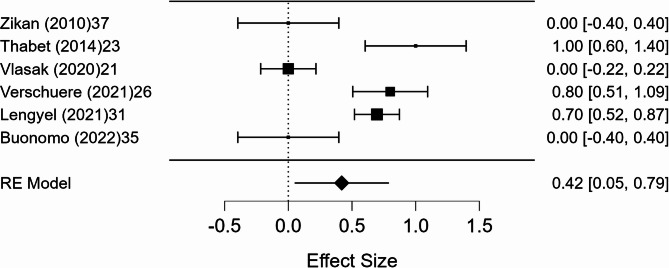
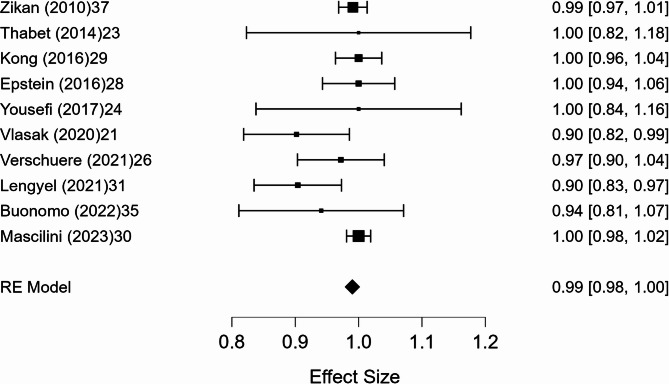
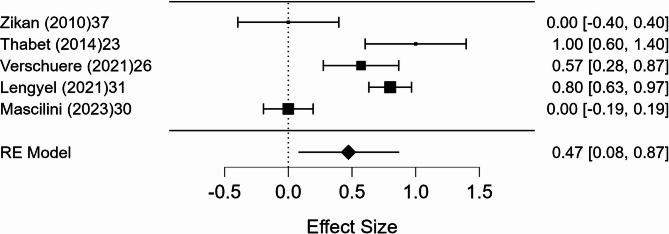
A meta-analysis was conducted on 10 studies that reported full 2 × 2 diagnostic data. The pooled sensitivity was 98.6% (95% CI, p < 0.001), with negligible heterogeneity (I² = 0.8%). The pooled specificity was 41.9% (95% CI: 5.3–78.6%, p = 0.025), with substantial heterogeneity (I² = 89.9%). The pooled positive predictive value (PPV) was 99.0% (95% CI: 97.7–100.3%, p < 0.001), with minimal heterogeneity (I² = 1.8%). The pooled negative predictive value (NPV) was 47.2% (95% CI: 7.6–86.9%, p = 0.020), with marked heterogeneity (I² = 91.4%). Forest plots of these estimates are shown in Figs. 3, 4, 5 and 6.
Fig. 3.
Forest plot of sensitivity
Fig. 4.
Forest plot of specificity
Fig. 5.
Forest plot of positive predictive value
Fig. 6.
Forest plot of negative predictive value
Discussion
This systematic review and meta-analysis provide updated insight into the role of ultrasound-guided tru-cut biopsy in the diagnostic workup of suspected ovarian cancer. By pooling and synthesizing available data, we demonstrate that this technique offers high diagnostic adequacy and sensitivity, with a favorable safety profile and increasing relevance in clinical decision-making.
The studies reviewed report low complication rates, with minor adverse events such as localized pain, infection, or bleeding being the most common. While prospective studies showed a higher rate of complications compared to retrospective studies, the difference was not statistically significant (p < 0.14). Notably, a significant proportion of the reported complications required surgical intervention, particularly due to intraabdominal bleeding and abscess formation. Importantly, no significant cases of needle tract seeding were observed, addressing a critical concern in biopsy procedures for malignancies. This supports the feasibility of ultrasound-guided tru-cut biopsy as a minimally invasive diagnostic tool, particularly in patients who may not be candidates for more invasive procedures due to comorbidities or advanced disease. The ability to perform this procedure under real-time ultrasound guidance allows for precise targeting of ovarian masses while avoiding adjacent structures, reducing the likelihood of complications [14].
Ultrasound-guided tru-cut biopsy demonstrated a high rate of adequacy in the included studies, with most achieving adequate tissue sampling for histopathological and immunohistochemical evaluation. Adequate tissue biopsy also allows for comprehensive molecular testing, including Breast Cancer (BRCA) gene 1 and 2 somatic mutations [33] which can provide critical insights into the cancer’s genetic makeup and help tailor treatments like Poly [ADP-ribose] polymerase (PARP) inhibitors [38, 39]. No statistical difference (p < 0.77) was found in the mean adequacy after the first attempt between the TAUS and TVUS approaches. In patients where a repeat biopsy was indicated after inadequate samples, there was a high rate of success. However, since most inadequate biopsies were not repeated, this did not lead to statistical significance (p < 0.109) in the mean adequacy between the first and second attempt in the studies that reported both. Variability in adequacy and complication rates across studies likely reflects differences in operator expertise and procedural technique. Although ultrasound guidance aids precision, successful biopsy still depends on targeting viable tumor tissue and avoiding necrotic areas. These findings highlight the need for standardized training protocols to ensure consistent biopsy quality and patient safety.
The included studies demonstrated a high level of diagnostic performance, with a mean accuracy of 95.5% (95% CI: 93.2–97.9%). Meta-analysis showed a pooled sensitivity of 98.6%, with negligible heterogeneity (I² = 0.8%), confirming that ultrasound-guided tru-cut biopsy reliably detects ovarian cancer when present. The positive predictive value was also very high (99.0%), with minimal heterogeneity (I² = 1.8%), indicating that a positive biopsy result can be considered highly reliable for confirming malignancy. In contrast, specificity and negative predictive value were lower and more heterogeneous, with pooled estimates of 41.9% and 47.2%, respectively, and substantial heterogeneity (I² = 89.9% and 91.4%). These findings suggest that while a positive tru-cut biopsy strongly supports the presence of ovarian cancer, a negative result does not reliably exclude malignancy and should be interpreted with caution in clinical decision-making. This heterogeneity may also be partly explained by flow and timing bias, as noted in the QUADAS-2 assessment, where several studies included patients who did not undergo the reference standard, potentially affecting the reliability of accuracy estimates.
This finding supports previous knowledge in other tumor types, where the use of image-guided biopsies has become a standard procedure before initiating treatment [40, 41]. In advanced ovarian cancer, timely histological confirmation is critical for initiating neoadjuvant chemotherapy. Given its high sensitivity and PPV, ultrasound-guided tru-cut biopsy is a reliable tool for confirming malignancy in patients who are not surgical candidates. However, the limitations of a negative result underscore the importance of integrating clinical, radiological, and, if needed, repeat histological evaluation into decision-making. Tru-cut biopsy appears to perform well in identifying histological subtypes, which is important given the heterogeneity of ovarian cancer and its impact on treatment planning [42, 43].
Ultrasound is widely available, cost-effective, and when performed by an experienced operator, offers diagnostic performance for ovarian cancer that is comparable to computed tomography in many staging and treatment-planning scenarios, especially for accessible pelvic and abdominal lesions [44]. While CT-guided biopsy provides excellent visualization, particularly for deeply located lesions, it is associated with higher radiation exposure and requires more resources [14]. Laparoscopic biopsy, though highly accurate, is invasive and may not be suitable for all patients. Furthermore, ultrasound-guided tru-cut biopsy has been shown to yield comparable results [30].
This systematic review has several limitations. Most included studies were retrospective and single-center, which may limit generalizability. There was also variability in biopsy techniques, needle sizes, and operator expertise, contributing to methodological heterogeneity. Reported complication rates were low, with no significant difference between retrospective and prospective studies; however, long-term follow-up was often lacking, limiting the detection of rare events such as delayed needle tract seeding. Moreover, the limited number of true negative and false negative cases made it difficult to calculate specificity and NPV reliably in several studies, with zero values for TN and/or FN in some cases. This contributed to substantial heterogeneity in these estimates (I² = 89.9% for specificity, 91.4% for NPV), reflecting inconsistencies in study populations and diagnostic standards. These factors reduce the reliability of negative biopsy results. Clinicians should therefore be cautious in acting on negative biopsy results alone and consider additional diagnostic confirmation in cases with persistent clinical or radiologic suspicion. Future multicenter, prospective studies with standardized protocols and adequate representation of negative cases are needed to validate and refine these findings.
Conclusion
Ultrasound-guided tru-cut biopsy is a safe, effective, and minimally invasive diagnostic tool for suspected ovarian cancer. It offers reliable histological confirmation and is well-suited for outpatient use due to its accessibility and cost-effectiveness. Our meta-analysis confirms strong performance in confirming malignancy. However, the limited reliability of negative results highlights the need for prospective studies with standardized follow-up to improve specificity, NPV, and overall diagnostic confidence.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors have no acknowledgement to declare.
Author contributions
M.N., F.M., D.H., D.P. and N.B. were responsible for the concept and design of the systematic review. M.N., D.H., M.P. and P.B. performed the systematic literature search including the inclusion and exclusion process. F.M. and P.B. extracted all the data from the included articles. M.N. and I.S. were responsible for processing the data, including statistical analysis. M.N., J.M.H. and J.H. performed the meta-analysis. M.S. and D.K. performed the risk of bias assesment. M.N., D.H., D.P. and A.U. prepared the first draft of the paper. D.K. prepared all the figures and tables. M.P., N.B., M.S., J.M.H and J.H. and I.S. substantially revised the paper. I.S. supervised the project. All the authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study); AND to have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This study was supported by the Grant Agency of Charles University in Prague (GAUK), Faculty of Medicine in Hradec Kralove, Project number: 246125. This work was supported by MH CZ–DRO (UHHK, 00179906). Additionally, it was supported by the Cooperation Program of Charles University, research area ONCO and MATC.
Data availability
The data that support the findings of this systematic review are available from the corresponding author upon reasonable request. This includes the list of included studies, data extraction forms, and any additional materials used during the screening and analysis processes.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L. Molecular characterization of epithelial ovarian cancer: implications for diagnosis and treatment. Int J Mol Sci. 2016;17(12):2113. 10.3390/ijms17122113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed]
- 3.Chandra A, Pius C, Nabeel M, et al. Ovarian cancer: current status and strategies for improving therapeutic outcomes. Cancer Med. 2019;8(16):7018–31. 10.1002/cam4.2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–57. 10.1016/s0140-6736(14)62223-6 [DOI] [PubMed] [Google Scholar]
- 5.Fagotti A, Ferrandina MG, Vizzielli G, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer. 2020;30(11):1657–64. 10.1136/ijgc-2020-001640 [DOI] [PubMed] [Google Scholar]
- 6.Coleridge SL, Bryant A, Kehoe S, Morrison J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;2021(7):CD005343. 10.1002/14651858.cd005343.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onda T, Satoh T, Ogawa G, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer. 2020;130:114–25. 10.1016/j.ejca.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 8.Reuss A, Bois A, du, Harter P, et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int J Gynecol Cancer. 2019;29(8):1327. 10.1136/ijgc-2019-000682 [DOI] [PubMed] [Google Scholar]
- 9.Atallah GA, Kampan NC, Chew KT, et al. Predicting prognosis and platinum resistance in ovarian cancer: role of immunohistochemistry biomarkers. Int J Mol Sci. 2023;24(3):1973. 10.3390/ijms24031973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Hossack JA, Klibanov AL. From anatomy to functional and molecular biomarker imaging and therapy: ultrasound is safe, ultrafast, portable, and inexpensive. Investig Radiol. 2020;55(9):559–72. 10.1097/rli.0000000000000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmerman D, Planchamp F, Bourne T, et al. ESGO/ISUOG/IOTA/ESGE consensus statement on pre-operative diagnosis of ovarian tumors. Int J Gynecol Cancer. 2021;31(7):961–82. 10.1136/ijgc-2021-002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Waal YRP, Thomas CMG, Oei ALM, Sweep FCGJ, Massuger LFAG. Secondary ovarian malignancies. Int J Gynecol Cancer. 2009;19(7):1160–5. 10.1111/igc.0b013e3181b33cce [DOI] [PubMed] [Google Scholar]
- 13.Pouedras M, Briand S, Crenn V, Cassagnau E, Gouin F. Non image-guided core needle biopsies can be used safely to improve diagnostic efficiency for soft tissue tumors. Surg Oncol. 2021;37:101518. 10.1016/j.suronc.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Fischerova D, Cibula D. Ultrasound in gynecological cancer: is it time for re-evaluation of its uses? Curr Oncol Rep. 2015;17(6):28. 10.1007/s11912-015-0449-x [DOI] [PubMed] [Google Scholar]
- 15.Arezzo F, Loizzi V, Forgia DL, et al. The role of ultrasound guided sampling procedures in the diagnosis of pelvic masses: a narrative review of the literature. Diagnostics. 2021;11(12):2204. 10.3390/diagnostics11122204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 17.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 20.Pelayo-Delgado I, Sancho J, Pelayo M, et al. Contribution of outpatient ultrasound transvaginal biopsy and puncture in the diagnosis and treatment of pelvic lesions: a bicenter study. Diagnostics. 2023;13(3):380. 10.3390/diagnostics13030380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VLASAK P, BOUDA J, KOSTUN J, et al. Diagnostic reliability, accuracy and safety of ultrasound-guided biopsy and ascites puncture in primarily inoperable ovarian tumours. Anticancer Res. 2020;40(6):3527–34. 10.21873/anticanres.14341 [DOI] [PubMed] [Google Scholar]
- 22.Griffin N, Grant LA, Freeman SJ, et al. Image-guided biopsy in patients with suspected ovarian carcinoma: a safe and effective technique? Eur Radiol. 2009;19(1):230–5. 10.1007/s00330-008-1121-8 [DOI] [PubMed] [Google Scholar]
- 23.Thabet A, Somarouthu B, Oliva E, Gervais DA, Hahn PF, Lee SI. Image-guided ovarian mass biopsy: efficacy and safety. J Vasc Interv Radiol. 2014;25(12):1922–7.e1. 10.1016/j.jvir.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Yousefi Z, Frazestanian M, Davachi B, Saeed S, Azad A, Khorasani ST. Is transvaginal core needle biopsy a safe method in diagnosis of ovarian cancer? Int J Cancer Manag. 2018;11(2). 10.5812/ijcm.8121
- 25.Mascilini F, Quagliozzi L, Moro F, et al. Role of transvaginal ultrasound-guided biopsy in gynecology. Int J Gynecol Cancer. 2020;30(1):128. 10.1136/ijgc-2019-000734 [DOI] [PubMed] [Google Scholar]
- 26.Verschuere H, Froyman W, Bosch TV et al. den,. Safety and efficiency of performing transvaginal ultrasound-guided tru-cut biopsy for pelvic masses. Gynecol Oncol. 2021;161(3):845–851. 10.1016/j.ygyno.2021.03.026 [DOI] [PubMed]
- 27.Oge T, Yalcin OT, Ozalp SS, Kebapci M, Aydin Y, Telli E. Sonographically guided core biopsy: a minimally invasive procedure for managing adnexal masses. J Ultrasound Med. 2013;32(11):2023–7. 10.7863/ultra.32.11.2023 [DOI] [PubMed] [Google Scholar]
- 28.Epstein E, Calster BV, Timmerman D, Nikman S. Subjective ultrasound assessment, the ADNEX model and ultrasound-guided tru-cut biopsy to differentiate disseminated primary ovarian cancer from metastatic non-ovarian cancer. Ultrasound Obstet Gynecol: Off J Int Soc Ultrasound Obstet Gynecol. 2015;47(1):110–6. 10.1002/uog.14892 [DOI] [PubMed] [Google Scholar]
- 29.Kong TW, Chang SJ, Paek J, et al. Transvaginal sonography-guided core biopsy of adnexal masses as a useful diagnostic alternative replacing cytologic examination or laparoscopy in advanced ovarian Cancer patients. Int J Gynecol Cancer. 2016;26(6):1041–7. 10.1097/igc.0000000000000728 [DOI] [PubMed] [Google Scholar]
- 30.Mascilini F, Quagliozzi L, Mirandola M, et al. Transvaginal ultrasound-guided biopsy in patients with suspicious primary advanced tubo-ovarian carcinoma. Int J Gynecol Cancer. 2023;33(2):236–42. 10.1136/ijgc-2022-003890 [DOI] [PubMed] [Google Scholar]
- 31.Lengyel D, Vereczkey I, Kőhalmy K, Bahrehmand K, Novák Z. Transvaginal ultrasound-guided core biopsy—experiences in a comprehensive cancer centre. Cancers. 2021;13(11):2590. 10.3390/cancers13112590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin S, Xiong Y, Yun M, et al. Transvaginal ultrasound-guided core needle biopsy of pelvic masses. J Ultrasound Med. 2018;37(2):453–61. 10.1002/jum.14356 [DOI] [PubMed] [Google Scholar]
- 33.Asp M, Mockute I, Måsbäck A, Liuba K, Kannisto P, Malander S. Tru-cut biopsy in gynecological cancer: adequacy, accuracy, safety and clinical applicability. J Multidiscip Healthc. 2023;16:1367–77. 10.2147/jmdh.s396788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majid A, Mahmood S, Mansoor M. Ultrasound guided biopsy (Targeted biopsy): a useful method of extraction of sample tissues to examine the diagnostic accuracy of malignant and benign lesions. PJMHS. Published online October 1, 2018. https://pjmhsonline.com/2018/oct_dec/pdf/1465.pdf
- 35.Buonomo F, Bussolaro S, de Fiorillo C. Ultrasound-guided tru-cut biopsy in gynecological and non-gynecological pelvic masses: a single-center experience. J Clin Med. 2022;11(9):2534. 10.3390/jcm11092534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FISCHEROVA D, CIBULA D, DUNDR P, et al. Ultrasound-guided tru‐cut biopsy in the management of advanced abdomino‐pelvic tumors. Int J Gynecol Cancer. 2008;18(4):833–7. 10.1111/j.1525-1438.2007.01015.x [DOI] [PubMed] [Google Scholar]
- 37.Zikan M, Fischerova D, Pinkavova I, Dundr P, Cibula D. Ultrasound-guided tru‐cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet Gynecol. 2010;36(6):767–72. 10.1002/uog.8803 [DOI] [PubMed] [Google Scholar]
- 38.Manchanda R, Sun L, Sobocan M, et al. Cost-effectiveness of unselected multigene germline and somatic genetic testing for epithelial ovarian cancer. J Natl Compr Cancer Netw. 2024;22(2D). 10.6004/jnccn.2023.7331 [DOI] [PubMed]
- 39.Lara OD, Krishnan S, Wang Z, et al. Tumor core biopsies adequately represent immune microenvironment of high-grade serous carcinoma. Sci Rep. 2019;9(1):17589. 10.1038/s41598-019-53872-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornford P, Bergh RCN, van den, Briers E, EAU-EANM-ESTRO-ESUR-ISUP-SIOG, et al. Guidelines on prostate cancer—2024 update. Part I: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2024;86(2):148–63. 10.1016/j.eururo.2024.03.027 [DOI] [PubMed]
- 41.Holloway CMB, Al-Riyees L, Saskin R. Utilization of percutaneous needle biopsy for breast diagnosis in a comprehensive breast center: implications for development of quality indicators. World J Surg. 2016;40(7):1590–9. 10.1007/s00268-015-3293-0 [DOI] [PubMed] [Google Scholar]
- 42.Tavares V, Marques IS, de Melo IG, Assis J, Pereira D, Medeiros R. Paradigm shift: a comprehensive review of ovarian cancer management in an era of advancements. Int J Mol Sci. 2024;25(3):1845. 10.3390/ijms25031845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez A, Nagel CI, Haight PJ. Targeted therapies in low-grade serous ovarian cancers. Curr Treat Options Oncol. 2024;25(7):854–68. 10.1007/s11864-024-01205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischerova D, Burgetova A. Imaging techniques for the evaluation of ovarian cancer. Best Pr Res Clin Obstet Gynaecol. 2014;28(5):697–720. 10.1016/j.bpobgyn.2014.04.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this systematic review are available from the corresponding author upon reasonable request. This includes the list of included studies, data extraction forms, and any additional materials used during the screening and analysis processes.