Abstract
The high-level colonization of vancomycin-resistant Enterococci (VRE) in the gastrointestinal tract could lead to systemic infections such as bacteremia, endocarditis, and urinary tract infections, particularly in hospitalized patients. Given the potent bactericidal activity and host specificity of bacteriophages, phage therapy represents a promising alternative strategy for controlling VRE infections. In this study, we isolated and characterized phage A155, which targets vancomycin-resistant Enterococcus faecalis (VR-Efs) V583. Genomic analyses revealed that it is a member of the Kochikohdavirus genus, while functional characterization defined its optimal multiplicity of infection (MOI), one-step growth kinetics, and stability under varying thermal (20–50 °C) and pH (3.0–11.0) conditions. The phage demonstrated a broad lytic spectrum and effective in vitro antibacterial activity. Furthermore, phage A155 could significantly reduce the VRE intestinal colonization loads by 1.13 orders of magnitude in a mouse model. These findings exhibit the characterization and genome analysis of a novel VR-Efs phage A155, highlighting its therapeutic potential against E. faecalis colonization.
Keywords: Phages, Vancomycin-resistant Enterococcus faecalis, Genome analysis, Characterization, Colonization
Introduction
Enterococcus faecalis (E.faecalis) is a Gram-positive bacterium belonging to Enterococcus that widely exists in nature and the human intestinal tract [1]. However, it is one of the common pathogens in nosocomial infections, which can cause a variety of diseases such as urinary tract infections, sepsis, and endocarditis [2, 3]. Due to the overuse of antibiotics, the emergence and spread of multidrug-resistant Enterococci have become an important global public health challenge [4]. In particular, the emergence of VRE has greatly limited the clinical treatment options [5].
While E. faecalis remains susceptible to β-lactam antibiotics (e.g., penicillin, ampicillin) and glycopeptides (e.g., vancomycin), these agents predominantly exert bacteriostatic rather than bactericidal activity against enterococcal infections [6]. Amidst the global crisis of antimicrobial resistance, phage therapy has emerged as a promising alternative due to its high host specificity [7]. Studies have demonstrated that bacteriophages can specifically target cytolytic E. faecalis associated with liver disease severity and mortality in patients with alcoholic hepatitis [8]. Beyond direct bacteriolysis, phage-mediated selection pressure induces evolutionary trade-offs in bacterial populations. Phage-resistant E. faecalis variants frequently exhibit impaired intestinal colonization capacity, a phenomenon attributed to mutations in enterococcal polysaccharide antigen (Epa) biosynthesis pathways that reduce epithelial adherence [9]. Compared to traditional antibiotics, phage-based therapeutics offer distinct advantages, including ease of isolation from environmental reservoirs, self-amplifying capabilities, and broad host specificity [10, 11]. Bacteriophage can effectively lyse E. faecalis in vitro and significantly reduce E. faecalis colonization in murine intestinal tracts through intraperitoneal injection or oral gavage [8, 12]. Nevertheless, the current paucity of characterized E. faecalis phages presents a critical knowledge gap. As of May 19, 2025, only 212 Enterococcus phages (0.03% of bacteriophages in the database) have been characterized and deposited, severely constraining our understanding of their therapeutic potential [13]. This limitation underscores the urgent need for systematic isolation of novel E. faecalis phages to expand phylogenetic diversity, elucidate functional genomics, and facilitate clinical translation. In this study, we isolated a novel E. faecalis phage, designated A155, from sewage and analysed its characteristics. After examining its complete genome sequence and evaluating its antibacterial activity in vitro and in vivo using a mouse model, we observed that phage A155 does not contain antibiotic resistance genes or virulence factors, and can effectively inhibit the growth of V583 within 11 h. More importantly, the colonization of V583 in the mouse gut was significantly reduced following gavage with phage A155.
Materials and methods
Bacteria strains
The 59 strains of E. faecalis, 5 strains of E. faecium, 2 strains of Staphylococcus aureus, 2 strains of Listeria monocytogenes, and 2 strains of Escherichia coli used in this study (for details, see Table 2) were conserved by Institute of Microbe & Host Health, Linyi University (Linyi, Shandong province, China). VR-Efs V583 was provided by the Utrecht University (Netherlands) and stored by the Institute of Microbe & Host Health (Linyi, Shandong province, China).
Table 2.
The efficiency of plating (EOP) of phage A155
| Bacteria | Strain | EOP | Lytic effect | Bacteria | Strain | EOP | Lytic effect |
|---|---|---|---|---|---|---|---|
| E.faecalis | V583a | 1.000a | +++ | E. faecalis | 24E33 | 0.000 | - |
| EfsDS01 | 0.409 | ++ | 24E39 | 0.209 | ++ | ||
| EfsDS02 | 0.000 | - | 24E44 | 0.000 | - | ||
| EfsDS03 | 0.022 | + | 24E49 | 1.545 | +++ | ||
| EfsDS04 | 1.000 | +++ | 23E04 | 0.000 | - | ||
| EfsDS05 | 0.000 | - | 23E08 | 0.000 | - | ||
| EfsDS06 | 0.145 | ++ | 23E10 | 0.909 | +++ | ||
| EfsDS07 | 0.002 | + | 23E17 | 0.000 | - | ||
| DSF02 | 0.436 | ++ | 23E35 | 0.000 | - | ||
| DSF04 | 0.000 | - | 23E37 | 0.100 | ++ | ||
| DSF12 | 0.000 | - | 23E44 | 0.191 | ++ | ||
| DSF16 | 0.255 | ++ | 23E47 | 0.000 | - | ||
| DSF17 | 0.145 | ++ | HF02 | 0.022 | + | ||
| DSF18 | 0.004 | - | HF03 | 0.001 | + | ||
| DSF19 | 0.000 | - | HF05 | 0.127 | ++ | ||
| DSF24 | 0.209 | ++ | HF07 | 0.013 | + | ||
| DSF25 | 0.014 | + | HF09 | 0.014 | + | ||
| DSF34 | 0.000 | - | HF18 | 0.000 | - | ||
| DSF35 | 0.000 | - | HF19 | 0.000 | - | ||
| DSF37 | 0.145 | ++ | HF20 | 0.000 | - | ||
| DSF42 | 0.145 | ++ | HF22 | 1.727 | +++ | ||
| DSF43 | 0.017 | + | HF24 | 0.173 | ++ | ||
| DSF44 | 0.191 | ++ | HZ02 | 0.000 | - | ||
| DSF47 | 0.073 | + | HZ03 | 0.000 | - | ||
| DSF51 | 0.000 | - | E. faecium | E1071 | 0.000 | - | |
| DSF53 | 0.000 | - | E1133 | 0.000 | - | ||
| DSF54 | 0.001 | + | E1644 | 0.000 | - | ||
| DSF64 | 0.000 | - | E1679 | 0.000 | - | ||
| DSF66 | 0.000 | - | E745 | 0.000 | - | ||
| DSF68 | 0.000 | - | S. aureus | ATCC29213 | 0.000 | - | |
| DSF69 | 0.004 | + | ATCC27660 | 0.000 | - | ||
| DSF73 | 0.018 | + | L. monocytogenes | ATCC19112 | 0.000 | - | |
| 24E04 | 0.000 | - | ATCC19114 | 0.000 | - | ||
| 24E14 | 0.000 | - | E. coli | ATCC43895 | 0.000 | - | |
| 24E25 | 0.000 | - | ATCC25922 | 0.000 | - |
Note: a: the original strain of isolation. EOP value of 0.5 or higher was classified as “high production”, representing that at least 50% of PFUs were produced in the target bacteria compared to host bacteria. EOP value between 0.1 and 0.5 was classified as “Medium production”. EOP value between 0.001 and 0.1 was considered “low production”, and EOP values less than 0.001 were referred to as inefficient. High production:“+++”, medium production: “++”, low production: “+”, inefficient: “-”
Isolation and purification of phage
Phage A155 was isolated from a sewage sample from a farm (Minggang Farm, Linyi) using VR-Efs V583 through the modified enrichment technique [14]. In brief, 15 mL of pre-settled sewage supernatant was centrifuged at 2876 ×g for 5 min (Pingke-165–6N). Subsequently, 2.5 mL of clarified supernatant was mixed with 2.5 mL of 2× Brain Heart Infusion (BHI) broth. The mixtures were incubated overnight at 37 °C with shaking(200 r/min). Then, 1.0 mL of the culture was centrifuged at 11,586 ×g for 5 min (ALLSheng-mini-15k), and the supernatant was filtered with a 0.22 μm membrane filter(Jinteng, China), using the double-layer plate method [15] to screen phage presence.
For purification, individual plaques were excised using sterile pipette tips, soaked in 200 µL SM buffer overnight, and subjected to serial dilution and repeated with the double-layer plate method. The purification cycle was iterated until homogeneous plaque morphology was achieved. The phage A155 was cultured and stored at 4℃ and − 80℃ in 25% glycerol.
Phage morphology
The purified phage was amplified using PEG-8000. After overnight treatment at 4 °C, the supernatant was discarded by centrifugation at 6236 ×g for 10 min (Eppendorf-5810R-SL142). The precipitate was resuspended in 2.0 mL of SM buffer (NaCl 5.8 g/L, MgSO4 1.5 g/L, Tris 6.06 g/L, 1 M HCl, gum Aladdin 0.1 g/L, pH 7.5), followed by extraction with an equal volume of chloroform to obtain the phage concentrate. The concentrate was mixed with 1.5 g CsCl in a 15-mL centrifuge tube and centrifuged at 6236 ×g for 20 min at 4℃ using a centrifuge machine (Eppendorf-5810R-SL142). The phage band was collected with a syringe and dialysed overnight in phosphate-buffered saline (PBS) to obtain purified phage particles. The samples were stained using the phosphotungstic acid negative staining method [16] and observed under a transmission electron microscope at 80 kV after 15 min.
Optimal MOI
The multiplicity of infection (MOI) is the ratio of phage to host bacteria used for phage amplification. VR-Efs V583 was cultured to the logarithmic growth phase (OD6₀₀≈0.7), and the bacterial concentration was adjusted to 1 × 10⁹CFU/mL. The phage was serially diluted and mixed with VR-Efs V583 and BHI broth at MOIs of 10, 1, 0.1, 0.01, and 0.001. The mixtures were incubated at 37 °C with shaking at 200 r/min for 6 h. Phage potency in each mixture was determined using the double-layer plate method, and the mixture with the highest potency was identified as the optimal MOI for the phage.
One-step growth curve
VR-Efs V583 was cultured to the log-phase growth (OD6₀₀≈0.7), and 1.0 mL of the culture was centrifuged at 2200 ×g for 5 min at 4 °C (Eppendorf-5418R). Following centrifugation, the collected precipitate was subjected to two rounds of PBS resuspension and centrifugation under the same conditions to remove residual impurities. The supernatant was discarded, and the pellet was resuspended in 1.0 mL of sterile BHI broth. Phage was added at the optimal MOI (MOI = 0.001) and incubated at 37 °C for 15 min. The mixture was then centrifuged at 4827 ×g for 5 min (AllSheng-mini-15k), the supernatant was discarded, and the pellet was resuspended in 10 mL of sterile BHI broth. The suspension was incubated at 37 °C with shaking at 200 r/min. Samples (50 µL) were collected every 5 min for the first 30 min and every 10 min for the next 90 min (total 2 h) for phage titer determination. Three replicates were performed at each time point, and the average values were used. The one-step growth curve was plotted with sampling time on the x-axis and the logarithm of phage titer on the y-axis.
Temperature and pH stability
Difference from previous assay [13], phage A155 (10⁹ PFU/mL) was incubated in a thermostatic water bath at 20 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C for 30 min and 60 min. After incubation, 100 µL of the phage solution was serially diluted 10-fold, and the phage titer was determined using the double-layer plate method. Three replicates were performed at each temperature, and the average values were used to analyze the changes in phage titer.
Phage A155 (10⁹ PFU/mL) was incubated at pH levels ranging from 2.0 to 14.0 (in increments of 1.0) for 60 min at 37 °C. After incubation, 100 µL of the phage solution was serially diluted 10-fold, and the phage titer was determined using the double-layer plate method. Three replicates were performed, and the average values were used to analyze the changes in phage titer under different pH conditions.
Sequencing and bioinformatics analysis of phage A155 genome
Following the instructions of the TIANamp Virus DNA/RNA Kit (Tiangen Bio-Tek Inc., Beijing, China), phage A155 was amplified, and concentrated, and its nucleic acid was extracted. Whole genome sequencing was conducted by Shanghai Personal Biotechnology Co., Ltd., following the method described by Han [17]. Briefly, using the Illumina TruSeq Nano DNA LT protocol (Illumina TruSeq DNA Sample Preparation Guide) to custom 2 × 250 bp paired-end DNA library, the average fragment length was 400 bp. Raw sequencing data quality was assessed by FastQC v0.11.7, followed by adapter trimming. The genome sequence was assembled using A5-MiSeq [18] and SPAdes [19]. Viral genome identification was performed by extracting high-depth sequences and conducting BLASTn alignment against the NCBI NT database [20]. Synteny analysis and gap closure were conducted by MUMmer [21], with subsequent error correction performed via Pilon v1.24 [22]. Protein-coding genes were predicted via GeneMarkS [23]. The genome data were uploaded to the National Center for Biotechnology Information (NCBI) database, GenBank accession number: PQ093903, and a comparative gene circle map was made by BRIG.
Phylogenetic tree construction
Based on the whole sequences, phylogenetic tree analysis was performed comparing phage A155 with multiple Enterococcus faecalis phages. Sequence alignment and phylogenetic tree construction were conducted using VICTOR [24].
Efficiency of plating (EOP)
Following a previously described method with certain modifications [25], the phage host range was quantitatively assessed through the efficiency of plating (EOP). Briefly, phage A155 solution was serially diluted 10-fold. Phage titers were determined in triplicate for each bacterial strain using the double-layer agar method. EOP values were calculated as (mean PFU on target bacteria / mean PFU on host bacteria). An EOP value of 0.5 or higher was classified as “high production”, indicating that at least 50% of PFUs were produced in target bacteria compared to host bacteria. An EOP value between 0.1 and 0.5 was classified as “Medium production.” An EOP value between 0.001 and 0.1 was considered “low production,” and an EOP less than 0.001 was referred to as inefficient.
Inhibitory effect of phage A155 on VR-Efs V583 in vitro
VR-Efs V583 was cultured to the stationary phase, harvested, and resuspended in fresh BHI broth. The suspension was distributed into 12-well plates, adjusted to a final bacterial concentration of 10⁷ CFU/mL(1/100 of the original bacterial liquid volume), and supplemented with BHI broth. Phage A155 was added at MOI of 0.001, 0.01, 0.1, 1, or 10, with a final volume of 2.5 mL per well. Bacterial growth without phage served as the control. All experiments were performed in triplicate. The plates were incubated in a Bacterial Growth Curve Instrument (Scientz MGC-200, Ningbo, China), and OD6₀₀ was measured every 30 min for 16 h to assess the lytic efficiency of phage A155 in vitro.
Phage therapy in the murine bacteremia model
Female BALB/c mice aged 6–8 weeks were purchased from a commercial supplier (Pengyue, Shandong, China). They were provided with water and standard mouse chow ad libitum and monitored daily.
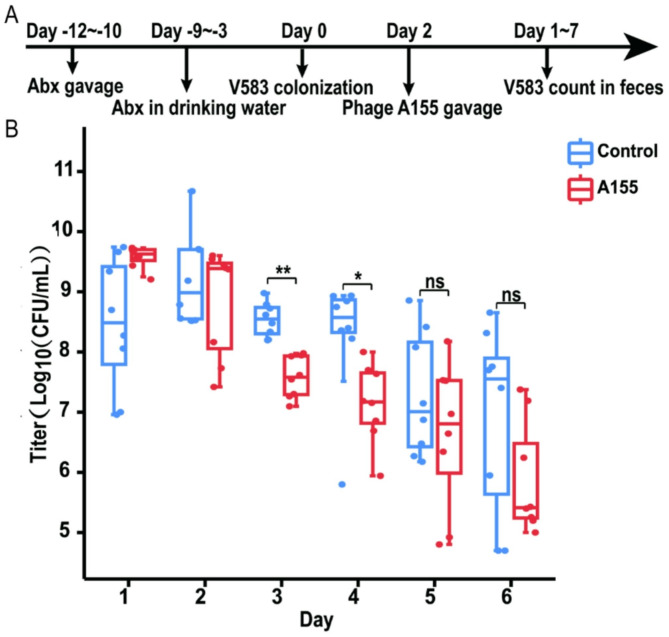
An intestinal model of E. faecalis infection in mice was established as previously described [26] (Fig. 7a). Sixteen SPF BALB/c mice (6–8 weeks old) were randomly divided into two groups (n = 8). After one week of acclimatisation, antibiotics were administered orally to deplete the intestinal flora and create more ecological niches for E. faecalis colonization [27]. Mice received a mixture of antibiotics (vancomycin 10 mg, neomycin 10 mg, ampicillin 10 mg, metronidazole 10 mg) by gavage for 3 days, followed by the same antibiotics in drinking water (vancomycin 500 mg/L, neomycin 500 mg/L, ampicillin 500 mg/L, metronidazole 500 mg/L) for 7 days. Faecal bacterial counts, particularly Enterococci, were monitored using the plate-counting method during this period. After 2 days, 100 µL of VR-Efs V583 suspension (1 × 10⁹ CFU/mL) was administered to both groups to simulate enterococcal proliferation. Faecal Enterococci levels were monitored using PSE agar.
Fig. 7.
The therapeutic evaluation of phage A155 in a mouse intestinal VRE infection model. (A) Therapeutic flow chart of phage A155 in a mouse intestinal VRE infection model; Abx: Antibiotics; (B) Enterococcus count in feces after phage A155 treatment. *: p<0.05; **: p<0.01; ns: no significant
To assess the effect of phage treatment, a single dose of phage A155 (2.4 × 10⁸ PFU/mouse) was administered orally 4 days post-bacterial challenge. The control group received an equivalent volume of BHI broth. Faecal samples were collected daily, and Enterococci counts on selective media were used to evaluate the efficacy of phage treatment in reducing E. faecalis colonization.
Data analysis
GraphPad Prism 8.3.0 was used to analyse the experimental data using statistics. Data were analyzed using one-way ANOVA. Results are expressed as standard deviation (SD). Error bars indicate the standard deviation of the mean, and the p-value was used to indicate the statistical significance of the data.
Result
Isolation and characterization of phage A155
A potent strain of E. faecalis phage was isolated from a farm sewage sample using VR-Efs V583 as the host. After purification, phage A155 produced distinct circular plaques on BHI agar plates overlaid with a bacterial lawn of VR-Efs V583, as shown in Fig. 1A. Electron microscopy revealed that phage A155 possesses a typical icosahedral head and a retractable tail, with a head diameter of approximately 100.1 nm and a tail length of about 156.9 nm. Based on morphological characteristics, phage A155 was classified under the family Myoviridae (Fig. 1B).
Fig. 1.
The morphology of bacteriophage A155. (A) Plaque formed by phage A155 cultured on double agar plates for 12 h. (B) Transmission electron micrograph of phage A155 (TEM, 10 000×)
When the multiplicity of infection (MOI) was set to 0.001, the titer of phage A155 reached its highest value of 9.2 × 1010PFU/mL (Table 1). The one-step growth curve experiment (MOI = 0.001) revealed a latent period of approximately 15 min, followed by a rapid increase in phage titer between 15 and 50 min. After 50 min, the phage titer entered a plateau phase. The burst size was estimated to be approximately 458 PFU per infected cell, as illustrated in Fig. 2.
Table 1.
Determination of optimal multiplicity of infection (MOI) of the phage A155
| No. | Bacteria (CFU) | Phage (PFU) | MOI | 6 h titer (PFU/mL) |
|---|---|---|---|---|
| 1 | 1 × 108 | 1 × 109 | 10 | 2.2 × 108 |
| 2 | 1 × 108 | 1 × 108 | 1 | 2.9 × 108 |
| 3 | 1 × 108 | 1 × 107 | 0.1 | 1.3 × 109 |
| 4 | 1 × 108 | 1 × 106 | 0.01 | 3.6 × 109 |
| 5 | 1 × 108 | 1 × 105 | 0.001 | 9.2 × 1010 |
Fig. 2.
One-step growth curve of the phage A155. A155 was added at MOI of 0.001, error bars represent the standard deviation of three independent experiments
Phage host range
As shown in Table 2, the EOP showed a productive infection rate of 51.7% (30/58) for phage A155, high and medium productive infection rate for phage A155 is 31.0% (18/58). However, no lytic activity was observed against E. faecium, S. aureus, L. monocytogenes, or E. coli.
Temperature and pH sensitivity of phage A155
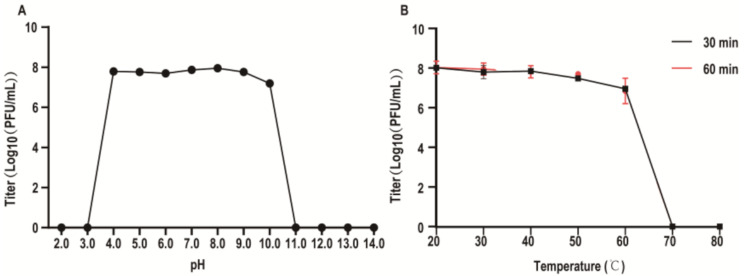
For the pH stability assessment, phage A155 demonstrated stable titers within a pH range of 4.0 to 9.0 In contrast, the titer decreased at pH 10.0 and was inactivated at pH 3.0 or pH 11.0 (Fig. 3A). For the temperature stability assessment, phage A155 exhibited stable titers within the range of 20–50 °C. However, the titer decreased significantly at 60 °C and was completely inactivated at 70 °C (Fig. 3B).
Fig. 3.
The stability of phage A155. (A) pH stability of A155, A155 was added in BHI medium of different pH levels for 1 h. (B) Temperature stability of A155, A155 was added in BHI medium at different temperatures for 30 min and 60 min. Error bars represent the standard deviation of three independent experiments
Genome description
The complete genome sequence of phage A155 has been deposited in GenBank under the accession number PQ093903. The genome consists of a 143,202 bp cicular, double-stranded DNA molecule with an overall G + C content of 35.76%. BLAST analysis revealed that the phage A155 genome encodes 216 putative open reading frames (ORFs), of which 63 (29.2%) were functionally annotated, while the remaining 153 ORFs were classified as hypothetical proteins. The coding sequences (CDS) ranged in length from 135 to 5,478 bp, collectively spanning 127,803 bp, resulting in a gene density of approximately 89.25% across the genome.
Taxonomic analysis based on genome sequencing indicated that phage A155 belongs to the following classification: Viruses; Duplodnaviria; Heunggongvirae; Uroviricota; Caudoviricetes; Herelleviridae: Brockvirinae; Kochikohdavirus. At the genome level, phage A155 exhibited high identity with several other E. faecalis phages, including EFLK1 (98.01%), EF17H (97.88%), phiM1EF22 (98.56%), and Ef2.3 (98.66%). As the genomic differences were all within 5%, these phages, along with A155, can be classified as members of the same Enterococcus phage group (Fig. 4).
Fig. 4.
Whole genome analysis of phage A155. The innermost black circle represents the size and identity of the genome; The black circle with a peak indicates GC content: The outward (inward) peak indicates that the GC content in this region is higher (low) than the average GC content in the whole genome; The higher the peak is, the larger the difference from the average GC content would be; A violet-green circle with a peak indicates GC-skew (GC skew=(G‒C)/(G + C)), green indicates that GC-skew is greater than 0, and purple indicates that GC-skew is less than 0, which could be used to determine the origin of replication of cyclic DNA; The red circle represents the genomic sequence of A155; The three circles of orange, green and light blue respectively represent the genomic sequences of five similar Enterococcus bacteriophage EFLK1, EF17H, phiM1EF22 and Ef2.3 (the color shades indicate the degree of gene identity); The outermost circle is the gene annotation (red for annotated proteins and green for hypothetical proteins)
BLASTp analysis of the 216 ORFs revealed that the gene products are associated with phage structure and assembly, DNA replication and regulation, and perforin and lytic enzymes. Notably, no antibiotic resistance genes or virulence factors were identified in the A155 genome, indicating a low risk of cross-species gene transfer. These findings suggest that phage A155 is a promising candidate for E. faecalis phage therapy.
Phylogenetic tree analysis of phage A155
The phylogenetic tree was constructed based on whole genome sequences of phages, as shown in Fig. 5. Phage A155 exhibited closer phylogenetic relationships to members of the Kochikohdavirus genus, including ECP3, Ef2.3, and phiEF24C, while showing clear divergence from phages belonging to the genus of Schiekvirus, Phifelvirus, Efquatrovirus, and Copernicusvirus.
Fig. 5.
Phylogenetic tree based on the whole genome sequences of phages. The five clusters of green, purple, light orange, light yellow, and blue respectively represent the genomic sequences of Copernicusvirus, Efquatrovirus, Phifelvirus, Schiekvirus, and Kochikohdavirus. Sequence number: The sequence number of the phage genome; The number on the branch indicates the credibility, and the closer the value is to 100, the stronger the credibility. Tree scale: The genetic distance
The in vitro bactericidal effect of the phage A155
As shown in Fig. 6, the bacterial load of VR-Efs V583 increased and stabilized after 8 h in the control group. In contrast, the growth of VR-Efs V583 was significantly inhibited within 11 h when co-cultured with phage A155. The duration of inhibition by the phage at different MOI did not significantly differ. These results demonstrate that phage A155 exhibits strong inhibitory activity against VR-Efs V583 growth in vitro.
Fig. 6.
Inhibition curves of phage A155 under different MOI in vitro. The VR-Efs V583 strain was lysed by phage A155 in BHI medium at 37 ℃. Absorbance (OD600) of the uninfected control culture and the parallel cultures that were infected with the phage at different MOI were measured over time. No phage was added to the negative control. Error bars represent the standard deviation of three independent experiments
Phage therapeutic evaluation in a mouse intestinal VRE infection model
The treatment of VR-Efs V583 colonization in the mouse intestine by phage A155 was conducted following the experimental procedure outlined in Fig. 7A. To evaluate the therapeutic efficacy of phage A155, Enterococci in the feces of treated mice were quantified, and the results are presented in Fig. 7B. After combined antibiotic treatment and VR-Efs V583 infection, VR-Efs V583 established stable colonization levels (approximately 109 CFU/g) in the mouse intestine within a short period (Days 1, 2). Following phage A155 treatment, a significant reduction in VR-Efs V583 colonization was observed in the treatment group compared to the control group (Day 3–4), with a decrease of approximately 1.13 log units by the second day. These results demonstrate that phage A155 effectively reduces VR-Efs V583 colonization in the mouse intestine. However, VR-Efs V583 in the control group failed to maintain stable colonization at high levels, and a significant decline in bacterial load was observed on Day 5–6 post-infection. No significant difference between the treatment and control groups was observed during this period (Day 5–6).
Discussion
Given the observed increase in the prevalence of multidrug-resistant strains of E. faecalis, the development of novel anti-VR-Efm therapies has become a significant focus in both academic and industrial research [28, 29]. In this study, we describe the isolation of a novel E. faecalis phage, designated A155. Phage A155 exhibits a highly efficient capacity to infect the VR-Efs V583 strain, attributed to its low MOI, short latency period, and rapid release phase. The growth characteristics of phage A155 surpass those of previously reported Enterococcus mycoides-tailed phages, such as PEf771 and vB_EfKS5 [30, 31]. Whole-genome sequencing analysis of A155 did not reveal any putative virulence factors or antibiotic resistance genes, suggesting that it fulfills essential criteria for consideration as a candidate therapeutic phage and warrants further investigation into its potential clinical applications [32, 33].
Additionally, the EOP of A155 showed the high and medium productive rate was 31.0% (18/58). No lytic activity was observed against E. faecium (0/5), L. monocytogenes (0/2), S. aureus (0/2), or E. coli (0/2), indicating a high level of species specificity.
VRE colonization in the intestinal tract of patients is a primary source of nosocomial infections. To simulate the proliferation of VRE in hospitalized patients following antibiotic treatment. An antibiotic-pretreated mouse model was used to evaluate the efficacy of phage A155 in eliminating VR-Efs V583 from the intestinal tract. Remarkably, one day after oral administration of the phage, the VR-Efs V583 titer in the mouse intestines was significantly reduced by 1.13 orders of magnitude, suggesting that phage A155 can effectively inhibit VR-Efs V583 colonization. However, complete eradication of VR-Efs V583 was not achieved. By the third day post-treatment, the bacterial load began to rebound. This result is consistent with the lysis assay in vitro. This decline in bactericidal efficacy may be associated with the emergence of phage-resistant bacteria, as experimental evidence suggests that the high selectivity of lytic phages can contribute to bacterial resistance [34]. However, the emergence of phage-resistant strains may not compromise therapeutic efficacy. Phage-resistant Enterococcus faecalis has been shown to have reduced intestinal colonization capacity [9].
Phage-resistant strains are a significant obstacle to the development of phage therapeutics [35]. To address the challenge of phage resistance and enhance antimicrobial efficacy, a phage cocktail therapy that combines phages with different receptor specificities has been proposed [36–38]. This approach has demonstrated greater effectiveness in reducing bacterial populations [39, 40]. Moving forward, we plan to focus on screening additional E. faecalis phages and developing efficient, broad-spectrum phage cocktail therapies. These efforts aim to provide new strategies for the prevention and control of drug-resistant E. faecalis infections.
Author contributions
Zeng Huiran, Ma Junfei, and Zhang XingLin: conception and design of experiments. Zeng Huiran, Ma Junfei, Xu Linan, and Yang Xiangpeng: performance of experiments. Zeng Huiran, Ma Junfei, and Zhang Xinglin: data analysis. Zeng Huiran, Tian Yachen, Wang Yuan, Li Yixuan, Yao Xiaoting, Ma Junfei, and Zhang Xinglin: writing- reviewing and editing. Zhang Xinglin: supervision of the whole project.
Funding
The work was supported by the Project of High-Tech SMES Innovation Improvement of Shandong Province (2023TSGC0419); the Project of High-Tech SMES Innovation Improvement of Shandong Province (2022TSGC2524); the Natural Science Foundation of Shandong Province, China (ZR2019ZD21); the National Key Research and Development Program of China (2019YFE0103900) and Shandong Provincial Natural Science Foundation(Z250223031).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics statement and consent to participate
The study was approved by the Ethics Committee of Linyi University. All procedures were conducted following according guidelines.
Consent for publication
All listed authors have significantly contributed to the study’s conception, data analysis, writing, or critical revision. Each author approves the final manuscript and its submission to Virology Journal.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinglin Zhang, Email: zhangxinglin@lyu.edu.cn.
Junfei Ma, Email: majunfei@lyu.edu.cn.
References
- 1.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. [DOI] [PubMed] [Google Scholar]
- 2.Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect. 2010;16:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancuso G, Midiri A, Gerace E, Marra M, Zummo S, Biondo C. Urinary tract infections: the current scenario and future prospects. Pathogens. 2023;12:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courvalin P. Vancomycin resistance in gram-positive Cocci. Clin Infect Dis. 2006;42(Suppl 1):S25–34. [DOI] [PubMed] [Google Scholar]
- 5.Rice LB. Antimicrobial resistance in gram-positive bacteria. Am J Infect Control. 2006;34:S11–19. discussion S64-73. [DOI] [PubMed] [Google Scholar]
- 6.Kristich CJ, Rice LB, Arias CA. Enterococcal Infection—Treatment and Antibiotic Resistance. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Boston: Massachusetts Eye and Ear Infirmary; 2014 [cited 2024 Oct 21]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK190420/
- 7.Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiki J, Nakamura T, Kreimeyer H, Llorente C, Fouts DE, Schnabl B. Insertion sequence-mediated phage resistance contributes to attenuated colonization of cytolytic Enterococcus faecalis variants in the gut. Microbiol Spectr 13:e03303–24. [DOI] [PMC free article] [PubMed]
- 10.Gordillo Altamirano FL, Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev. 2019;32:e00066–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soleimani-Delfan A, Bouzari M, Wang R. vB_EfaS-DELF1, a novel siphoviridae bacteriophage with highly effective lytic activity against vancomycin-resistant Enterococcus faecalis. Virus Res. 2021;298:198391. [DOI] [PubMed] [Google Scholar]
- 13.Topka-Bielecka G, Bloch S, Nejman-Faleńczyk B, Grabski M, Jurczak-Kurek A, Górniak M, et al. Characterization of the bacteriophage vB_EfaS-271 infecting Enterococcus faecalis. Int J Mol Sci. 2020;21:6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehia FAA, Yahya G, Elsayed EM, Serrania J, Becker A, Gomaa SE. From isolation to application: utilising Phage-Antibiotic synergy in murine bacteremia model to combat Multidrug‐Resistant Enterococcus faecalis. Microb Biotechnol. 2025;18:e70075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enumeration of Bacteriophages by Double Agar. Overlay Plaque Assay| SpringerLink. [cited 2025 May 12]. Available from: https://link.springer.com/protocol/10.1007/978-1-60327-164-6_7 [DOI] [PubMed]
- 16.Wintachai P, Naknaen A, Pomwised R, Voravuthikunchai SP, Smith DR. Isolation and characterization of siphoviridae phage infecting extensively drug-resistant Acinetobacter baumannii and evaluation of therapeutic efficacy in vitro and in vivo. J Med Microbiol. 2019;68:1096–108. [DOI] [PubMed] [Google Scholar]
- 17.Han G, Zhang J, Luo Z, Lu B, Zhang P, Yong K, et al. Characteristics of a novel temperate bacteriophage against Staphylococcus arlettae (vB_SarS_BM31). Int Microbiol. 2023;26:327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coil D, Jospin G, Darling AE. A5-miseq: an updated pipeline to assemble microbial genomes from illumina miseq data. Bioinformatics. 2015;31:587–9. [DOI] [PubMed] [Google Scholar]
- 19.SPAdes. a new genome assembly algorithm and its applications to single-cell sequencing - PubMed. [cited 2025 May 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/22506599/ [DOI] [PMC free article] [PubMed]
- 20.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besemer J, Lomsadze A, Borodovsky M. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001;29:2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karatzas E, Gkonta M, Hotova J, Baltoumas FA, Kontou PI, Bobotsis CJ, et al. VICTOR: A visual analytics web application for comparing cluster sets. Comput Biol Med. 2021;135:104557. [DOI] [PubMed] [Google Scholar]
- 25.Khan Mirzaei M, Nilsson AS. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE. 2015;10:e0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Wu Y, Yang X, Pang X, Wu Y, Li X, et al. The Fe-S cluster biosynthesis in Enterococcus faecium is essential for anaerobic growth and Gastrointestinal colonization. Gut Microbes. 2024;16:2359665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krueger WA, Krueger-Rameck S, Koch S, Carey V, Pier GB, Huebner J. Assessment of the role of antibiotics and enterococcal virulence factors in a mouse model of extraintestinal translocation. Crit Care Med. 2004;32:467. [DOI] [PubMed] [Google Scholar]
- 28.Moelling K, Broecker F, Willy C. A Wake-Up call: we need phage therapy now. Viruses. 2018;10:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Palacios Araya D, Palmer KL. Enterococcus faecium: evolution, adaptation, pathogenesis and emerging therapeutics. Nat Rev Microbiol. 2024;22:705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Telbany M, Lin C-Y, Abdelaziz MN, Maung AT, El-Shibiny A, Mohammadi TN, et al. Potential application of phage vB_EfKS5 to control Enterococcus faecalis and its biofilm in food. AMB Express. 2023;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Y, Yang R, Li X, Huang H, Duan K, Song F. Phage PEf771 for the treatment of periapical periodontitis induced by Enterococcus faecalis YN771. Crit Rev Immunol. 2024;44:41–53. [DOI] [PubMed] [Google Scholar]
- 32.Uchiyama J, Rashel M, Takemura I, Wakiguchi H, Matsuzaki S. In Silico and in vivo evaluation of bacteriophage phiEF24C, a candidate for treatment of Enterococcus faecalis infections. Appl Environ Microbiol. 2008;74:4149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu YD, Stanford K, Kropinski AM, Ackermann H-W, Johnson RP, She Y-M, et al. Genomic, proteomic and physiological characterization of a T5-like bacteriophage for control of Shiga toxin-producing Escherichia coli O157:H7. PLoS ONE. 2012;7:e34585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duerkop BA, Huo W, Bhardwaj P, Palmer KL, Hooper LV. Molecular basis for lytic bacteriophage resistance in enterococci. mBio. 2016;7:e01304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blasco L, López-Hernández I, Rodríguez-Fernández M, Pérez-Florido J, Casimiro-Soriguer CS, Djebara S, et al. Case report: analysis of phage therapy failure in a patient with a Pseudomonas aeruginosa prosthetic vascular graft infection. Front Med (Lausanne). 2023;10:1199657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J, Du F, Long M, Li P. Limitations of phage therapy and corresponding optimization strategies: A review. Molecules. 2022;27:1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henein A. What are the limitations on the wider therapeutic use of phage? Bacteriophage. 2013;3:e24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujiki J, Schnabl B. Phage therapy: targeting intestinal bacterial microbiota for the treatment of liver diseases. JHEP Rep. 2023;5:100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Shi T, Sun Y, Zhang Y. A novel method to create efficient phage cocktails via use of phage-Resistant Bacteria. Appl Environ Microbiol. 2022;88:e0232321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao D, Ji H, Wang L, Li X, Hu D, Zhao J, et al. Fitness Trade-Offs in phage Cocktail-Resistant Salmonella enterica serovar enteritidis results in increased antibiotic susceptibility and reduced virulence. Microbiol Spectr. 2022;10:e0291422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.