Abstract
Monocercomonoides exilis is a model species of the amitochondrial eukaryotic group Oxymonadida, which makes it a suitable organism for studying the consequences of mitochondrial loss. Although M. exilis has an endobiotic lifestyle, it can be cultured in vitro in polyxenic conditions alongside an uncharacterized prokaryotic community, while attempts to create axenic cultures have not been successful. In this study, we used metagenomic sequencing, transcriptomics, and metabolomics to characterize the microbial consortium that supports the growth of M. exilis. We assembled genomes for 24 bacterial species and identified at least 30 species in total. M. exilis accounted for less than 1.5% of the DNA reads, while bacterial species dominated the sequence data and shifted in abundance over time. Our metabolic reconstruction and differential gene expression analyses show that the bacterial community relies on organic carbon oxidation, fermentation, and hydrogen production, but does not engage in methanogenesis. We observed rapid depletion of amino acids, nucleotides, glyceraldehyde, lactate, fatty acids, and alcohols in the medium, indicating a reliance on external nutrient recycling. The nitrogen cycle in this community is incomplete, with limited nitrogen fixation and no ammonia oxidation. Despite detailed metabolic profiling, we did not find any direct biochemical connections between M. exilis and the prokaryotes. Several bacterial species produce siderophores to assist themselves and others in the community in acquiring iron. However, M. exilis does not appear to benefit directly from siderophore-mediated iron transport and lacks known iron uptake pathways. This indicates that M. exilis may rely indirectly on the iron metabolism of other bacteria through phagocytosis. Additionally, some bacteria synthesize polyamines like spermidine and phosphatidylcholine, which M. exilis may need but cannot produce on its own. As the culture ages, M. exilis shows changes in gene expression consistent with starvation responses, including the upregulation of carbohydrate storage pathways and processes related to exocytosis. These findings provide new insights into microbial interactions within xenic cultures and emphasize the complex nature of maintaining amitochondriate eukaryotes in vitro.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40793-025-00758-7.
Introduction
The gut microbiota refers to the community of microorganisms inhabiting the gastrointestinal system. Several studies have demonstrated the influence of this community on human health [1–3], but these have mainly focused on the prokaryotic component since this represents the majority of cells and biomass. However, the animal gut microbiota also includes viruses and eukaryotes, such as unicellular protists. The impact of protists on the function and processes in the intestine, as well as their metabolic interactions with prokaryotes, is essentially unknown, except for some medically and veterinary significant parasites, e.g., Entamoeba histolytica, Giardia intestinalis, Cryptosporidium spp., Spironucleus salmonicida, Blastocystis spp. [4, 5]. However, these only represent a small portion of the diversity of intestinal protists, and their presence often results in pathological states that significantly differ from the conditions observed in a healthy gut.
Many intestinal protists can be successfully maintained in xenic cultures using media such as TYSGM-9 [6, 7]. For many of these protists, this is the only viable method for in vitro maintenance, as attempts to axenize them, i.e., remove unwanted organisms, have not been successful. Xenic cultivation depends on establishing a stable community in culture, typically derived from the original sample, i.e., gut content or stool, which can be maintained for years through regular transfers. Although the community may originate from the environment in which it was sampled, its final composition is likely to narrow down during the adaptation to the culture conditions (e.g., nutrient sources and oxygen concentration). The culture is expected to only vaguely mimic the conditions found in the host gut. Nevertheless, it serves as a straightforward and manageable model system that can provide insights into these complex communities. To our knowledge, such attempts are rare [8].
In this study, we aim to characterize the culture community derived from the stool of Chinchilla lanigera. It contains a single eukaryote, the commensal bacterivorous flagellate Monocercomonoides exilis, for which a high-quality genome draft is available and functionally annotated [9–11]. This species belongs to Oxymonadida (Preaxostyla, Metamonada), known as inhabitants of animal intestines [12, 13]. The entire Oxymonadida, including M. exilis, has lost mitochondria, which is very rare among eukaryotes and raises interest in their biochemistry and physiology [11, 14]. Many oxymonad species are known to harbor symbionts [13], and some specific roles and interactions have been partially understood. For example, Streblomastix strix is a protist that plays a role in the complex community found in the hindgut of termites and is involved in cellulose digestion [15]. However, there is no symbiotic interaction known for M. exilis. Light and electron microscopy have demonstrated that species of Monocercomonoides actively feed on prokaryotes [12], acting as predators within the intestinal microbiota. The effect and impact of this grazing on the prokaryotic community, as well as prey selectivity, remain unknown. In vitro, M. exilis is maintained polyxenically in TYSGM-9 medium. Attempts to axenise it or grow it monoxenically using Citrobacter portucalensis as the food source have been unsuccessful.
We employed a multi-omics approach to gain initial insights into the processes occurring within this multipartite culture community over five days of growth. Specifically, we identified the species composition and tracked changes in species abundances over time. Additionally, we predicted metabolic pathways, monitored gene expression changes of the most abundant members as the culture aged, and measured the composition and variations of selected metabolites in the media. With this data, we aimed to reconstruct the main biochemical processes of the community and explore potential interactions among its members.
Methods
Cell culturing
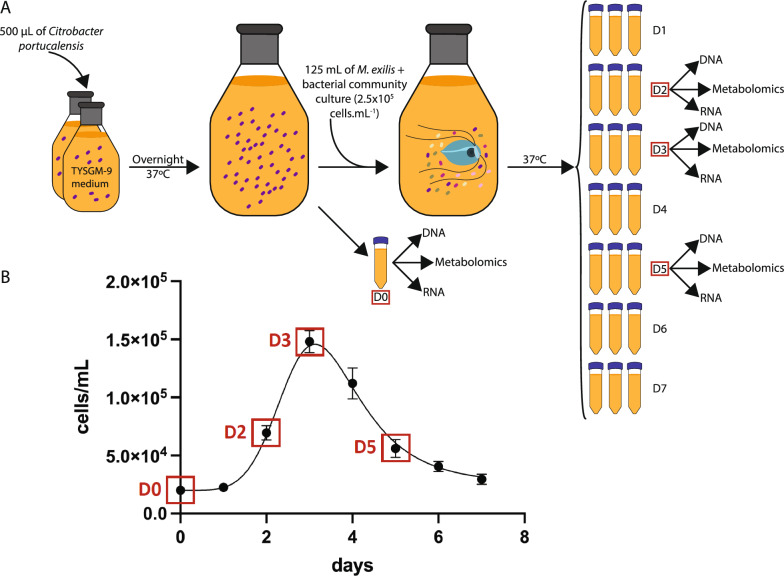
A total of 700 mL of TYSGM-9 medium was distributed into two 1 L glass bottles, which were each inoculated with 500 µL of Citrobacter portucalensis, previously isolated from a culture of M. exilis. After inoculation, the bottles were sealed and allowed to incubate for one day at 37 °C without shaking. This preconditioning of media enhances protist growth, and it is referred to as the bacterization step. The following day, designated as day 0, the contents of the two flasks were combined in a 2 L bottle and mixed thoroughly. A 45 mL aliquot was divided for the isolation of DNA, RNA, and for metabolomic analyses (see Fig. 1). The remaining medium was inoculated with approximately 125 mL of culture containing M. exilis (2.5 × 105 cells/mL) together with the whole prokaryotic community. After inoculation, the media were thoroughly mixed and divided into 50-mL tubes, with 45 mL in each tube. The tubes were then closed and incubated at 37 °C without shaking. Each day, three tubes were removed, one for each replicate (Fig. 1). From each tube, 20 mL of culture was used for DNA isolation, 20 mL for RNA isolation, and a 5 mL aliquot was frozen for metabolomics. Before each nucleic acid extraction, M. exilis cells were counted using a Neubauer cytometer.
Fig. 1.
Methods workflow of the cell culturing experiment and growth curve of Monocercomonoides exilis with sampling points. A Summary of the experiment workflow. One day before the start of the experiment, 700 mL of growth medium divided into two flasks was inoculated with Citrobacter portucalensis. This initial step is referred to as the bacterization step. On day 0, the contents of the two flasks were combined, and a 45 mL aliquot was taken for the isolation of DNA, RNA, and metabolomic analyses. The remaining medium was inoculated with M. exilis along with the entire prokaryotic community. The mixture was then divided into 50-mL tubes and allowed to incubate at 37 °C without shaking. Each day, three tubes were removed to isolate DNA and RNA, and to freeze the sample for metabolomic analysis. Data for this study were collected from days 0, 2, 3, and 5. B Growth curve of M. exilis (cells/mL) grown in TYSGM-9 medium for seven days. Samples for thorough analysis were taken on days 0, 2, 3, and 5, as highlighted by red squares. Error bars provide standard deviations of the values from three measurements
DNA isolation
All DNA isolations were performed using the DNeasy Blood and Tissue Kit (Qiagen). Cells were centrifuged at 1500 xrcf for 10 min at 4 °C. The supernatant was transferred into a fresh 50 mL tube and centrifuged again at 6000 xrcf for 10 min at 4 °C. The pellet from the first centrifugation, enriched with protists, was resuspended in 200 µL of PBS, and DNA was isolated using the cultured cells protocol of the DNeasy Blood and Tissue Kit (Qiagen). The second pellet, enriched with prokaryotes, was resuspended in 200 µL of PBS, and DNA was isolated using the gram-positive protocol of the DNeasy Blood and Tissue Kit (Qiagen). For each isolation, the DNA was eluted in 100 µL of elution buffer. The DNA from both pellets was combined into a single sample. The quality of the DNA was estimated using Nanodrop, and the concentration was measured using the QuantiFluor ONE dsDNA System (Promega).
RNA isolation
Cells were centrifuged at 1500 xrcf for 10 min at 4 °C. The supernatant was transferred to a fresh 50 mL tube and centrifuged again at 6000 xrcf for 10 min at 4 °C. Both pellets were combined and resuspended in 1 mL Tri-Reagent (Sigma-Aldrich), and the total RNA was isolated according to the manufacturer’s protocol. The obtained RNA pellet was resuspended in 60 µL of RNA-grade H2O and heated at 37 °C for 10 min to ensure full resuspension.
The total RNA was further DNAse-treated. For this purpose, we added 6 µL of rDNAse buffer and 0.6 µL rDNAse (Macherey–Nagel) per sample and incubated at 37 °C for 10 min. Samples were subsequently re-isolated with Tri-Reagent (Sigma-Aldrich) as before and finally resuspended in 30 µL of RNA-grade H2O. RNA concentration was measured using Nanodrop.
Identification of compounds within the growth medium—metabolomics
For metabolomics, cells were pelleted as described for DNA and RNA isolation (see above). The supernatant obtained from the second centrifugation was filtered through a Whatman polycarbonate filter with a 3 µm pore size, sterilized and stored at -80 °C. Metabolites present in the media were determined using a non-targeted metabolomics approach. To capture both volatiles and non-volatiles, a combination of two techniques was employed: Liquid Chromatography analysis with an Orbitrap Fusion mass spectrometer (LC–MS/MS; Orbitrap Fusion, Q-OT-qIT, Thermo Scientific) and two-dimensional comprehensive gas chromatography with a mass spectrometer (GCxGCTOF-MS; Pegasus 4D, Leco Corp.).
For LC–MS/MS, media samples harvested in triplicate were precipitated with acetonitrile at a 4:1 ratio (ACN:media) and centrifuged for 20 min at 16,000 xrcf at 5 °C. 100 µL of each supernatant was collected, fully evaporated and dissolved in 50 µL 10% (v/v) ACN. 15 µL were injected into a ProntoSIL column, 150 × 3.0 mm, 3 µm (Bischoff Chromatography). Compounds were eluted under the following conditions: 0–3 min 100% A, 3–18 min linear gradient to 100% B, 18–20 min 100% B using mobile phases. Mobile phase A: 2% (v/v) ACN, 10 mM HCOONH4, 0.1% (v/v) FA; Mobile phase B: 99% (v/v) ACN, 0.1% (v/v) FA with a flow rate of 0.4 mL.min−1. The separated compounds were ionized in an electrospray ion source in positive polarity mode. Master scans of precursors from 80 to 1000 m/z were performed in Orbitrap at 120 K resolution with an intensity threshold filter of 2.0 × 104. Tandem MS was performed by isolation in the quadrupole, HCD fragmentation with stepped collision energies of 15, 30, and 45% and an isolation window of 1.6 m/z. The MS2 ions were analyzed and detected in Orbitrap with a set resolution of 30 k and a maximum injection time of 300 ms. The dynamic exclusion duration occurred every 20 s with a 10 ppm tolerance around the selected precursor. Data processing, including chromatographic peak alignments, was performed using Compound Discoverer 3.3 (Thermo Scientific). Quality control samples were employed for the data correction. The mzCloud libraries were used for fragmentation spectra comparison and compound structure assignment.
Volatiles were analyzed using GCxGC-MS and headspace solid-phase microextraction (HS-SPME) on fiber (DVB/CAR/PDMS_grey; Supelco, USA). 1 mL of media samples was placed in a 20 mL glass vial and 10 µL of an internal standard (2,2,2-trifluoro ethanol, Fluka, 0.11 mg.mL−1) was added. Samples were incubated for 10 min at 40 °C before extraction. The extraction was carried out for 5 min. The volatiles were analyzed using a combination of polar and mid-polar separation columns: a primary column of Stabilwax-DA (30 m × 0.25 mm, Restek, USA) and a secondary column of BPX-50 (1.38 m × 0.1 mm, SGE, Australia). Other parameters were set as follows: inlet temperature 220 °C, split 5 modes, constant He flow 1 mL.min−1, modulation time 3 s (hot pulse 0.7 s), modulation temperature offset with respect to the secondary oven 20 °C. The primary oven temperature program began at 30 °C (hold 1.5 min), then increased to 110 °C (8 °C.min−1), followed by an increase to 250 °C (25 °C.min−1) and held at 250 °C for 5 min. The temperature offset applied to the secondary column was + 5 °C. Transferline temperature was held at 280 °C. The mass spectrometer was equipped with an Electron Ionization ion source (energy of 70 eV was applied), and a Time-of-Flight analyser enabling a united mass resolution. The scanned mass range was 29–400 m/z. The ion source chamber was maintained at 250 °C.
To expand the range of compounds that can be analyzed by gas chromatography to polar non-volatiles, the media samples underwent two consecutive derivatization processes: oximation (25 mg.mL−1 of methoxyamine hydrochloride in anhydrous pyridine hydrochloride, Sigma-Aldrich) and silylation. Two different agents were used for silylation: N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane (BSTFA + 1% (v/v)TMSC) and N-tert-Butyldimethylsilyl-N-methyl trifluoroacetamide (MTBSTFA), both from Sigma-Aldrich.
For silylation using BSTFA + 1% (v/v) TMSC, 50 µL were transferred to a 2 mL glass vial and 2 μL of internal standard were added (Adonitol, 424 μL.mL−1). The liquid was dried in a vacuum concentrator and reconstituted by adding 30 μL of anhydrous pyridine and 30 μL of an oximation agent. The samples were then incubated in a Thermomixer (Eppendorf) at 40 °C for 2 h with constant shaking (1500 rpm). After incubation, 40 μL of neat solution of BSTFA + 1% (v/v) TMSC mixture was added to each sample and they were further incubated at 70 °C for 30 min with constant shaking (1500 rpm). Before analysis, 400 μL of hexane was added to each sample. For silylation using MTBSTFA, the derivatization process was essentially the same as with BSTFA + TMSC, except that 30 μL of media sample was used, plus 5 μL of nor-Valine (550 μL.mL−1) as an internal standard and 20 μL of oximation agent.
Metabolites were analyzed as oximated trimethylsilylated or tert-butyl silylated derivatives. A combination of nonpolar and mid-polar separation columns was used for the separation: a primary Rxi-5SIL MS column (30 m × 0.25 mm, Restek, USA) and a secondary BPX-50 column (1.39 m × 0.1 mm, SGE, Australia). Other parameters were set as follows: inlet temperature 290 °C, splitless mode, constant He flow 1 mL.min−1, modulation time 3 s (hot pulse 0.7 s), modulation temperature offset with respect to the secondary oven 15 °C. The temperature program for the primary oven was 50 °C (hold 1 min), then increased (8 °C.min−1) to 320 °C (hold 2 min). The temperature offset applied to the secondary column was + 5 °C. Transfer line temperature was held at 280 °C. The scanned mass range was 85–800 m/z. The ion source chamber was maintained at 280 °C. Data were processed using ChromaTOF v4.5 software. Detected analytes were relatively quantified after normalization to the internal standards. Metabolites detected by all the methods used were identified by comparing their mass spectra with those available in the main NIST mass library, Fiehn's mass library of silylated compounds, and in-house-built libraries. The retention index was determined using linear hydrocarbons. In cases where the same compounds were detected in both derivatizations, the highest signal was included in the final table.
Library preparation and sequencing
For the DNA samples, sequencing libraries were prepared from 1 µg of gDNA using the Illumina TruSeq DNA PCR free (Illumina) library preparation kit according to the manufacturer’s protocol. The prepared libraries were sequenced on an Illumina HiSeq 4000 with 2 × 150 bp reads.
For RNA, 1 µg of total RNA per sample was used for library preparation. Ribosomal RNA depletion was performed using the NEBNext rRNA Depletion Kit for Bacteria (New England Biolabs), spiked with a custom oligo pool. The oligo pool was designed based on bacterial and eukaryotic ribosomal sequences identified from previous sequencing projects of M. exilis [9, 11]. After rRNA depletion, libraries were prepared using the NEBNext® Ultra™ Directional RNA Library Prep Kit (New England Biolabs) according to the manufacturer’s protocol. The prepared libraries were sequenced using Illumina NextSeq 500 with 2 × 75 bp reads.
Metagenome assembly and binning
The metagenomics raw reads were quality-checked with FASTQC v0.11.5 and trimmed using Trimmomatic v0.39 [16] (ILLUMINACLIP:TruSeq3-PE-2.fa:2:30:10, LEADING:20, TRAILING:20, SLIDINGWINDOW:5:20 MINLEN:50). To reduce complexity during assembly, the eukaryotic reads were removed by mapping the reads against the M. exilis genome [9] using BBMap (minidentity = 0.98, idfilter = 0.98) included in BBTools v38.90 (https://sourceforge.net/projects/bbmap/) and taking only unmapped reads.
The unmapped reads were assembled independently for each sample using metaSPAdes v3.14.0 [17] with k-mers 21,33,55,77,99,127. A reiterative process was used for binning metagenome-assembled genomes (MAGs) within the samples. First, contigs above 2kbp were binned using MaxBin v2.2.7 [18] (minimum probability 0.9, marker set 107) and tetraESOM [19]. Then, bins were manually checked based on the markers identified by MaxBin using Blastp against nr database (April 2021). For each potential MAG, rRNA genes were identified using Prokka v1.14.6 automatic annotation pipeline [20] and used as controls together with the marker genes. Lastly, reads corresponding to trustworthy bins, i.e. markers and rRNA indicating the same species, were removed from the dataset by mapping the reads back to the identified bins using BBSplit (minidentity = 0.98, idfilter = 0.98) included in BBTools v38.90. The assembly process was repeated with the remaining reads. This process was iterated until no new clean MAG could be assembled. In total, 24 prokaryotic MAGs were assembled. The contribution of each prokaryotic MAG and M. exilis to the community per sample was calculated as the percentage of reads mapped to each genome assembly.
Identification of prokaryotic MAGs
The taxonomic classification of the 24 prokaryotic MAGs was determined using classify_wf, included in GTDB-Tk [21–30] with standard parameters. The completeness and contamination of every MAG were estimated based on the presence/absence and number of copies of the 107 marker genes used by MaxBin (see above).
Annotation and metabolic capacities of prokaryotic MAGs
The annotation of the 24 prokaryotic MAGs was performed using Prokka v1.14.6. The functional annotation of each MAG was made using EggNOG-mapper v1.0.3–35 on emapper DB 2.0 [31, 32] (standard parameters except minimum % of query coverage: 50 and minimum % of subject coverage: 50). EggNOG-mapper predictions and Prokka annotations were combined using emapper2gbk, included in the metage2metabo pipeline [33], to generate a compatible input for Pathway-Tools v24.5 [34].
A metabolic database was created for each MAG using Pathologic [35], included in Pathway-Tools (using standard parameters except name matching, which was turned off). All databases were manually curated using the Assign Probable Enzymes, Transport Inference Parser, and Rescore Pathways tools included in Pathologic. Predicted pathways were manually reviewed to remove those that were highly incomplete.
Identification of possible viral particles and plasmids
To identify possible viral particles and plasmids in the culture, the filtered DNA reads were assembled independently for each sample using metaviralSPAdes [36] and metaplasmidSPAdes [37], respectively, with k-mers 21,33,55,77,99,127. Candidate contigs were verified using viralVerify tool (github.com/ablab/viralVerify.git) and Prokka v1.14.6.
RNA reads were assembled independently for each sample using rnaSPAdes [38] with default parameters. Each sample was analyzed also using VirSorter2 [39] (–high-confidence-only, –keep-original-seq and –provirus-off). Candidate contigs were verified using Blastn against the nt database and Blastx (June 2025) against the nr_clustered database.
Identification of metabolic capacities of Monocercomonoides exilis
The genome of M. exilis was downloaded from NCBI. The metabolic capabilities of this species were predicted using Pathologic, included in Pathway-Tools, with standard parameters. The resulting database was manually curated using the Assign Probable Enzymes, Transport Inference Parser, and Rescore Pathways tools included in Pathologic. Information from previous curations [9–11] and EggNOG-mapper was included at this step. Carbohydrate-metabolizing enzymes were identified by conducting searches against the CAZy database using METABOLIC-G with default parameters [40].
Transcriptome processing and identification of differentially expressed genes
Meta-transcriptomic raw reads were quality-checked with FASTQC v0.11.5 and trimmed using Trimmomatic v0.39 (ILLUMINACLIP:TruSeq3-PE-2.fa:2:30:15, LEADING:15, TRAILING:15, SLIDINGWINDOW:4:15 MINLEN:50), resulting in 47 million read pairs per sample on average (except for sample D5R3, which contained 77 million read pairs). Reads were mapped, and the expression of transcripts from prokaryotes and M. exilis was quantified, using Salmon [41] with default parameters.
Due to the low mapping rate and a low number of reads, differential expression was assessed only for M. exilis and the seven most abundant prokaryotic genomes: Bacteroides fragilis, Bacteroides thetaiotaomicron, Bacteroides intestinalis, Parabacteroides sp., Kerstersia gyiorum, Fusobacterium varium, and Citrobacter portucalensis. Raw reads were re-mapped to these eight genomes using Bowtie2 [42]. Reads mapping to protein-coding sequences were counted with the FeatureCounts program of the subread package v2.0.3 [43]. Read counts were normalized using the DESeq2 package v1.32.0 [44] in R v4.1.1 [45] and normalization was based on taxon-specific scaling [46, 47].
Identification of the major biochemical cycles in the community
The metabolic capacities of the prokaryotic community were also analyzed using METABOLIC-C [40] with default parameters, except tax genus, to understand their roles within the community's major biochemical cycles. We manually verified the results concerning the carbon, nitrogen, sulfur, and iron cycles by integrating the results from METABOLIC-C and EggNOG-mapper from previous analyses.
Identification of the metabolic relationships between M. exilis and the prokaryotic community
All reactions identified in M. exilis and the 24 prokaryotic MAGs metabolic database were exported as SBML Pathway-Tools format. The Metacom tool implemented in the metage2metabo (m2m) pipeline [33] was used to identify metabolic interactions between M. exilis and the rest of the community. In this analysis, the sampling days were compared pairwise, i.e., day 0 vs. day 2, day 2 vs. day 3, and day 3 vs. day 5, with M. exilis defined as the host. In each pairwise comparison, compounds detected on the first day were classified as seeds. Meanwhile, those whose concentration increased between days were classified as targets. Metacom identified essential bacterial members (species occurring in all of the minimal community models defined by the pipeline) and key bacterial members (species occurring in at least one of the minimal community models defined by the pipeline) responsible for most of the compound changes observed in the media during the pairwise comparison.
Results
Growth of the culture and sampling of time points
The development of the culture was monitored over seven days in an experiment conducted in triplicate (Fig. 1). An aliquot of the bacterized medium, which is medium preconditioned by overnight growth of Citrobacter portucalensis, representing day 0, along with aliquots from days 2, 3 and 5, corresponding to the exponential, stationary, and decline phases of M. exilis growth, were selected to sample metagenomic, metatranscriptomic, and metabolomic data.
Community composition
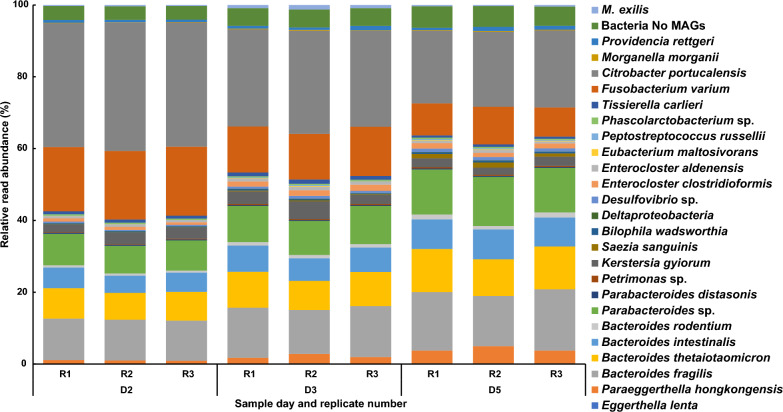
Using metagenomic reads generated from triplicates of each sampled time point, we assembled 24 metagenome-assembled genomes (MAGs) corresponding to the prokaryotic members of the culture community (Fig. 2, Supplementary Table 1). In addition to the assembled MAGs, we identified six additional bacterial species based on the presence of 16S rRNA genes. However, their read abundances were too low to assemble into clean MAGs (Supplementary Table 1). No viral genomes or plasmid DNAs were identified in the multi-omics data. We identified several genes of viral origin. However, they are most likely part of the expected mobilome present in a bacterial community. This suggests that the community consists of one eukaryote and at least 30 bacterial species, representing seven phyla, without any archaeal or viral components.
Fig. 2.
Relative read abundance of the members of the community. Each member of the community is represented as relative read abundance (%) corresponding to its genome on each day and replicate
The quality of the 24 assembled MAGs varied, with the lowest completeness around 83%, while most had genome completeness above 95% (Supplementary Table 1). Estimated contamination levels were below 5%, except for Deltaproteobacteria sp. MAG, which had an estimated contamination of 11.8% (Supplementary Table 1). This MAG was the least abundant during the whole experiment. We calculated the relative DNA read abundance of each bacterium and M. exilis for days 2, 3, and 5 of culture. While M. exilis represented only 0.25% to 1.23% of the DNA reads, the seven most abundant bacterial species comprised 80 – 90% of the reads, and the remaining bacterial species never exceeded 1% of the reads per species (Fig. 2 and Supplementary Table 1). We observed a switch in the dominant groups throughout the culture's growth. Not considering Citrobacter portucalensis (Gammaproteobacteria), which is used for the bacterization (preconditioning) of the media (see Materials and Methods), the most abundant bacterium on day 2 was Fusobacterium varium (Fusobacteriota). On days 3 and 5, the abundance of this bacterium decreased, and members of the phylum Bacteroidota became the dominant species. M. exilis reached its peak at 1.23% on day 3 (Fig. 2).
Monocercomonoides exilis metabolic capacities
Our analysis of M. exilis metabolism identified 933 enzymatic reactions, 31 transport reactions, and 148 enzymatic pathways (Supplementary Fig. 1). As previously published [9–11], M. exilis encodes a limited repertoire for energy metabolism relying on glycolysis to produce pyruvate, which is converted into acetyl-coenzyme A and further into acetate and ethanol. Our analysis showed that M. exilis can obtain glucose from the degradation of glycogen, maltose, or starch. It can also digest chitin and (1,3)-α-D-glucans present in fungal cell walls, as well as peptidoglycan present in bacterial cell walls, which corroborates its bacterivorous feeding mode (Supplementary Table 2). The biosynthesis of amino acids and nucleotides is limited, relying on conversion and salvage pathways from nutrients obtained through the phagocytosis of prey or via transporters from the environment. However, M. exilis is capable of synthesizing dNTPs from NTPs using ribonucleoside-triphosphate reductase. [10, 14].
Bacterial community metabolic capacities
To better understand the metabolic potential of the 24 metagenome-assembled bacterial genomes, we reconstructed the metabolism of each MAG using EggNOG-mapper and Pathway-Tools. Our results showed a higher number of pathways and reactions compared to M. exilis, with significant variation in the number of enzymatic reactions, transporters, and pathways, from 443 in Citrobacter portucalensis to 218 in Peptostreptococcus russellii (Supplementary Table 1). The metabolic maps for the seven most abundant MAGs are provided in Supplementary Figs. 2–8. The number of reactions and pathways identified in closely related MAGs was very similar despite differences in assembly quality. This indicates that the quality of the MAG assemblies did not significantly impact our metabolic analyses.
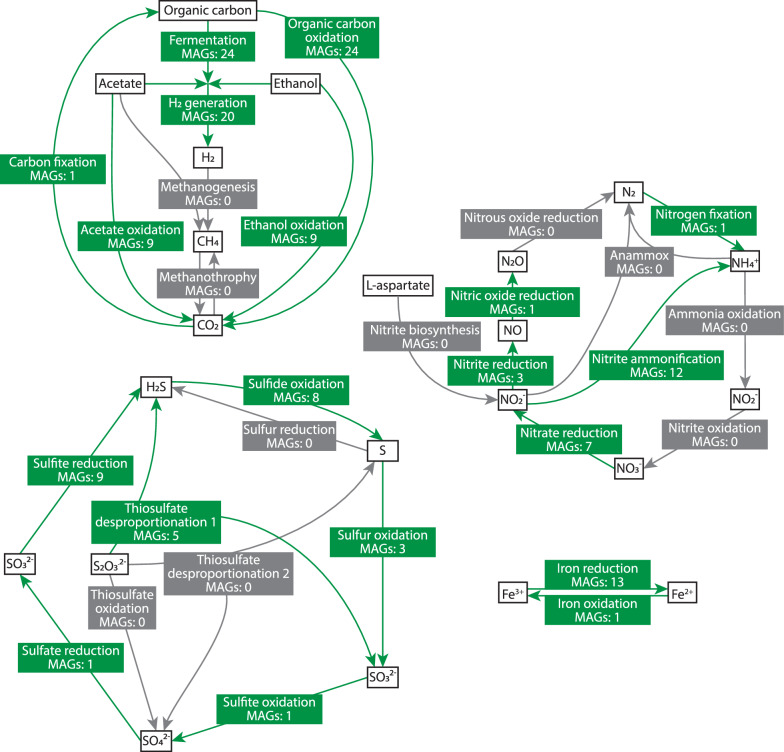
We analyzed the roles of community members within the carbon, nitrogen, sulfur, and iron cycles. The results showed that the community likely depends on the oxidation of organic carbon compounds (mainly amino acids and complex carbon compounds), fermentation, and H2 generation (Fig. 3). However, we did not identify methanogenesis (Fig. 3). Our analyses identified Phascolarctobacterium sp. as the only member capable of fixing N2 to ammonium. However, the abundance of this species is low (Fig. 2 and Supplementary Table 1) suggesting that N2 fixation is most likely not the main source of ammonium in the media. Another potential source of ammonium could be the Dissimilatory Nitrate Reduction to Ammonium (DNRA) pathway [48]. Components of this pathway, such as periplasmic nitrate reductase (NapAB), the nitrate reductase A (NarGHI) and cytochrome c552 nitrite reductase (NrfAH and NrfABCDEF) are encoded by several members of the community (Fig. 3, Supplementary Table 3, Supplementary Figs. 2, 4–7). However, we were unable to identify the sources of nitrates and nitrites. These anions are not added to the cultivation medium, and we did not identify enzymes that produce nitrate or nitrite, either by the oxidation of ammonia derived from amino acid and nucleotide decay or by the biosynthesis of nitrite from aspartate [49]. Consistent with this observation, the expression of the DNRA pathway in most bacteria, for which the transcriptomic data are available, is extremely low. In the two species that express it (B. thetaiotaomicron and Parabacteroides sp.), expression levels are down-regulated in later days (Supplementary Table 3, Supplementary Figs. 2, 4–7). Therefore, we conclude that the community does not operate a complete nitrogen cycle but relies on ammonium derived from amino acids and nucleotides supplemented to the medium or originating from dead cells with a minor contribution from nitrogen gas fixation.
Fig. 3.
Potential contribution of the bacterial community to the biochemical cycling processes of carbon, nitrogen, iron, and sulfur. Labels represent the main steps of the process. Steps supported by the genomic data are indicated in green. Steps, for which the enzymes were not identified are indicated in light grey. MAGs: number of MAGs responsible for a step
Unlike the carbon and nitrogen cycles, the sulfur cycle is complete and is primarily maintained by Desulphovibrio sp., the only member able to reduce sulfate and oxidize sulfite. We identified eight species that encode the sulfide:quinone oxidoreductase, suggesting that they accumulate sulfur in globules within the cytoplasm [50]. Additionally, the reduction of iron is another important activity within this community, involving 13 species in the process (Fig. 3). In contrast, iron oxidation activity was identified only for Saezia sanguinis (Fig. 3).
The consumption and production of compounds by the community
At each time point, triplicate samples of the cell-free medium were collected, and their composition was analyzed through non-targeted metabolomics. Using LC–MS/MS and GCxGC-MS methods, a wide variety of metabolite structural classes were covered, resulting in the detection of a total of 270 unique compounds (see Supplementary Table 4). Among these, 171 participate in biochemical reactions listed in the MetaCyc database [51]; therefore, they were the only ones utilized for the subsequent analyses (Supplementary Table 4).
On the day of inoculation (day 0), the medium was already affected by an overnight growth of C. portucalensis. However, it was relatively rich in nucleotides and their derivates, glyceraldehyde, lactate, fatty acids, alcohols and amino acids. Notably asparagine, aspartate, cysteine, glutamine, and serine were below the detection limit at this time point (Supplementary Table 4). By day 2, a significant depletion of several compounds was observed, specifically nucleotides and their derivates, most amino acids, glyceraldehyde, glyceric acid, malate, citric acid, and citrulline. This indicates that the microbial community had consumed these substances. On the contrary, a spectrum of short-chain fatty acids and aromatic compounds putatively derived from degradation of amino acids, such as tyrosine, phenylalanine and tryptophan, was detected in the medium on this day (Supplementary Table 4). Measurements displayed little change between days 2, 3, and 5, suggesting that the composition of the cell-free media stabilized during this period.
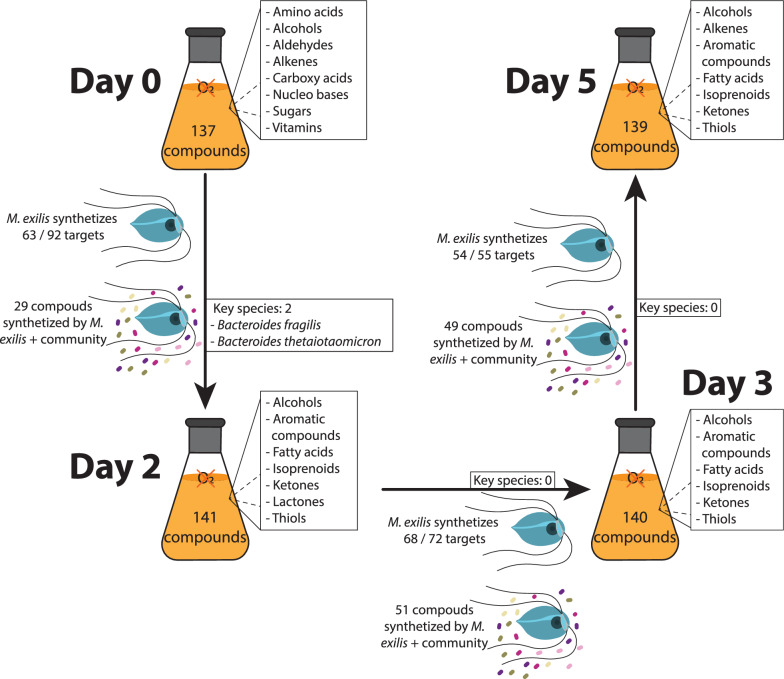
We integrated the synthesis and consumption of compounds into an in silico prediction of metabolic capacity for both M. exilis and the bacterial community, utilizing the metage2metabo (m2m) pipeline [33]. In these predictions, we compared the culture days pairwise: day 0 vs. day 2, day 2 vs. day 3 and day 3 vs. day 5. Compounds were classified into two categories: “seeds” which were present in the media on the first day of the comparison, and “targets”, which were detected only on the second sampling day or their concentration increased on the second sampling day. These results are summarized in Fig. 4. Given that M. exilis represents less than 1.5% of the community metagenomic reads, we infer that its metabolic power is limited and that the prokaryotic component converts most of the metabolites. Nevertheless, we aimed to highlight the metabolic capabilities of this protozoan, which was our main focus. Between day 0 and day 2, 137 compounds were classified as seeds and 92 as targets, of which M. exilis can independently synthesize 63. However, its metabolic capacities extend beyond these target compounds, as it can also synthesize other important compounds like pyruvate, amino acids, medium-chain fatty acids, and vitamins. An additional 29 target compounds can be synthesized through metabolic interactions between M. exilis and the bacterial community (Fig. 4). Among these compounds are several amino acids. In this pairwise comparison, m2m did not identify any bacteria as essential members of the community. However, two Bacteroidota bacteria were classified as key members (occurring in at least one of the minimal community models; Fig. 4). Between days 2 and 3, we identified 141 compounds as seeds and 72 as targets. According to the m2m pipeline, M. exilis can independently synthesize 68 targets and 51 compounds through metabolic interaction with the community (Fig. 4). In this comparison, no bacterial species were considered essential or key members of the community. The final comparison, day 3 vs. day 5, showed a similar result. M. exilis could synthesize 54 of the 55 compounds identified as targets using 140 compounds as seeds. The interaction between M. exilis and the bacterial community allows for the synthesis of additional 49 more compounds, with no bacteria considered essential or key members in this analysis (Fig. 4).
Fig. 4.
Schematic summary of changes in the culture medium. The flasks display numbers and broad chemical classifications of significant compounds identified in the cell-free medium for each day (refer to Supplementary Table 4 for details). Arrows indicate the number of compounds that show an increase in concentration the following day, referred to as “targets.” It also includes the number of targets that M. exilis can synthesize independently and those that can be synthesized through the interaction between M. exilis and the bacterial community. Additionally, the bacterial species identified by the m2m pipeline as “key species” are shown for each consecutive day of comparison
Monocercomonoides exilis differential expression analysis
To investigate how the metabolism of M. exilis changes during the growth curve, we compared the expression of transcripts between days 2, 3 and 5 in pairwise analyses. The most significant changes in gene expression were observed between days 2 and 5, while the comparisons between consecutive days showed less pronounced changes (i.e., day 3 vs. day 2 and day 5 vs. day 3). A total of 2,236 genes were up-regulated and 2,431 genes were down-regulated between days 2 and 5 (Supplementary Table 1). Notably, the majority of these genes—1703 up-regulated and 1,381 down-regulated—are annotated as hypothetical, meaning that their functions are currently unknown.
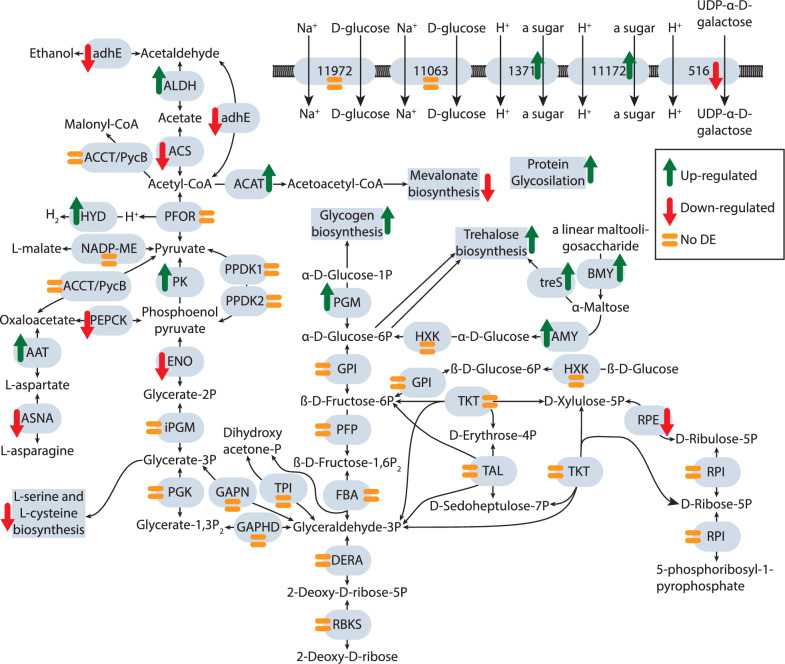
The changes in the expression of all functionally annotated metabolic enzymes and pathways are shown in Supplementary Fig. 1, with additional details provided in Figs. 5 and 6 for those discussed below. We observed an increase in the expression of sugar importers such as α-D-xylose and sn-glycerol-3-phosphate by day 5. This was accompanied by a rise in the expression of enzymes involved in the degradation of starch, (1- > 4)-α-glucan, chitin and other polysaccharides (Fig. 5 and Supplementary Table 2). Additionally, the interconversion between glucose-1-P and glucose-6-P was up-regulated, which may be related to increased trehalose biosynthesis from glucose-6-P. Trehalose serves as a carbohydrate storage molecule and a key protector of cellular components [52]. All three pathways for trehalose synthesis were up-regulated on day 5, including the synthesis directly from α-maltose, facilitated by both α- and β-amylases. The synthesis of glycogen, another storage polysaccharide, was also up-regulated. Most genes involved in carbohydrate metabolism and glycolysis maintained stable expression levels. However, some genes, such as phosphoenolpyruvate carboxykinase (PEPCK), were down-regulated, indicating a regulatory adjustment in glycolysis to produce pyruvate through the upregulated pyruvate kinase (PK). Subsequently, pyruvate was converted to acetyl-CoA by pyruvate-ferredoxin oxidoreductase (PFOR) while the up-regulated hydrogenases (HYD) re-oxidized ferredoxins, allowing the cycle to continue. On day 5, the expression of enzymes of extended glycolysis and ethanol fermentation, such as acetate synthase (ACS), which produces acetate, and aldehyde and alcohol dehydrogenases (ALDH, Adh), responsible for ethanol production, decreased significantly, with the exception of one paralogue of ALDH (Supplementary Table 5). ACS1 was consistently the most expressed enzyme among these across all three time points and replicates. Overall, these expression data suggest that acetate and hydrogen were the preferred metabolic end products throughout all three time points. In contrast to glycolysis, the pentose phosphate pathway may be down-regulated due to the repression of the ribulose-phosphate 3-epimerase (RPE) (Fig. 5).
Fig. 5.
Differential expression of genes related to carbohydrate metabolism and transport in Monocercomonoides exilis between day 5 and day 2. The identified sugar transporters, glycolytic pathway, pentose phosphate pathway, and related reactions (adapted from Karnkowska et al., 2019) are illustrated with the enzyme abbreviations enclosed in gray ovals. Up-regulated genes and processes are indicated by a green arrow pointing upwards, while down-regulated genes and processes are marked with a red arrow pointing downwards. Processes that show no significant change in expression are represented by an orange equal sign. adhE: Bifunctional acetaldehyde-CoA/alcohol dehydrogenase; ALDH: Aldehyde dehydrogenase; ACS: Acetyl-CoA synthetase (ADP-forming); ACCT/PycB: putative malonyl-CoA:pyruvate transcarboxylase; ACAT: Acetyl-CoA acetyltransferase; PFOR: Pyruvate-ferredoxin oxidoreductase; HYD: [FeFe]-hydrogenase; NADP-ME: NADP-dependent malic enzyme; PK: Pyruvate kinase; PPDK: Pyruvate phosphate dikinase; PEPCK: Phosphoenolpyruvate carboxykinase; AAT: Aspartate aminotransferase; ASNA: Asparagine synthetase; ENO: Enolase; iPGM: 2,3-bisphosphoglycerate independent phosphoglycerate mutase; PGK: Phosphoglycerate kinase; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GAPN: NAD(P)-dependent glyceraldehyde-3-phosphate dehydrogenase; TPI: Triose phosphate isomerase; DERA: Deoxyribose-phosphate aldolase; RBKS: Ribokinase; FBA: Fructose-bisphosphate aldolase; PFP: Phosphofructokinase (pyrophosphate-based); GPI: Glucose-6-phosphate isomerase; PGM: Phosphoglucomutase; BMY: β-amylase; AMY: α-amylase; treS: Maltose alpha-D-glucosyltransferase/ alpha-amylase; HXK: Hexokinase; RPDK; ribose-phosphate diphosphokinase; RPI: Ribose-5-phosphate isomerase; RPE: Ribulose-phosphate 3-epimerase; TKT: Transketolase; TAL: Transaldolase
Fig. 6.
Differential expression of genes involved in amino acid metabolism and transport in Monocercomonoides exilis between day 5 and day 2. Identified amino acid importers, amino acid biosynthesis pathways, and the arginine catabolism pathway are illustrated with the enzyme abbreviations enclosed in gray ovals. Up-regulated genes and processes are indicated by a green arrow pointing upwards, while down-regulated genes and processes are marked with a red arrow pointing downwards. Processes that show no significant change in expression are represented by an orange equal sign. Spontaneous reactions are shown in blue. PGDH: Phosphoglycerate dehydrogenase; PSAT: Phosphoserine transaminase; PSPH: putative phosphoserine phosphatase; SAT: Serine O-acetyltransferase; SEPHS: Selenophosphate synthetase; SEPSECS: O-phospho-L-seryl-tRNA[Sec]:L-selenocysteinyl-tRNA synthase; THRC: Threonine synthase: ASNA: Asparagine synthetase; ALAT: Alanine aminotransferase; ACCT/PycB: putative malonyl-CoA:pyruvate transcarboxylase; AAT: Aspartate aminotransferase; ASNA: Asparagine synthetase; DHE: Glutamate dehydrogenase; GLNA: Glutamine synthetase: ADI: Arginine deiminase: OTC: Ornithine transcarbamylase: CK: Carbamate kinase; OAT: Lysine/Ornithine aminotransferase
On day 5, genes responsible for the phosphorylation of (d)NDP, DNA and RNA polymerization, and protein ubiquitination were up-regulated (Supplementary Fig. 1). In contrast, the import of nucleosides and the salvage pathway of adenosine were down-regulated. Additionaly, the mevalonate pathway, which synthesizes isoprenoids from acetoacetyl-CoA was also down-regulated (Fig. 5 and Supplementary Fig. 1). Most amino acid transporters showed reduced expression or no significant change (Fig. 6). This correlates with the substantial decrease in amino acid content in the media from day 2. Moreover, the biosynthesis of asparagine, serine, and cysteine was down-regulated, along with the ATP-producing arginine deaminase pathway (Fig. 6).
A notable expression change observed during cultivation was in the genes for the endomembrane system. Among the proteins regulating endomembrane transport, several showed significant changes in expression (Supplementary Table 6). While proteins involved in the early secretory pathway exhibited little change, several proteins related to the late steps [53–55] were significantly up-regulated on day 5 (Supplementary Table 6). These up-regulated proteins include those involved in trafficking from the trans-Golgi-network (TGN) to the plasma membrane (DSCR3A and exocyst complex components Exo84, Sec5 and Sec15, DSCR), multivesicular body formation and sorting (Vsp2, 4a-c, 23, 24a and 60b, charged multivesicular body protein 7), plasma membrane uptake (AP-2a, TSET complex protein TPLATE subunit B), endocytic/digestive pathway (Vps34bc, Vps41b) and endosomes to TGN transport (Vps51b, TRAPP component Bet3 paralogue A, B, syntaxin 6D and 16). On the contrary, four paralogues of Rab7 were consistently down-regulated, suggesting a decrease in lysosome biogenesis on day 5 [56].
At the end of the growth curve, we observed a decline in the number of M. exilis cells. Our data indicated that most enzymes involved in the apoptosis pathway [57] did not show significant changes in expression (Supplementary Table 7). This suggests that the decrease in cell density during the later days was not due to programmed cell death. Instead, it was likely caused by uncontrolled decay combined with slow growth resulting from nutrient deficiencies.
Bacterial community differential expression analysis
Our data only allowed to perform differential expression analysis on the seven most abundant bacteria: B. fragilis, B. thetaiotaomicron, B. intestinalis, Parabacteroides sp., K. gyiorum, Fusobacterium varium and C. portucalensis. These species account for 80–90% of the community DNA reads, serving as a proxy measure of their abundance. We observed a variable number of upregulated and downregulated genes among these bacteria (see Supplementary Table 1). For example, only 9% of the transcripts in B. intestinalis showed changed in expression between day 2 and day 5, whereas Parabacteroides sp. exhibited a 51% change. The alterations in the expression of metabolic enzymes and pathways in these bacteria are illustrated in Supplementary Figs. 2–8.
We specifically examined metabolic pathways that are absent in C. portucalensis but present in other members of the community. These pathways may explain why we were unable to grow M. exilis using only C. portucalensis as a food source. First, F. varium and Tissierella sp. can ferment lysine into acetate, butanoate and ammonia, while in C. portucalensis, this pathway is incomplete. Furthermore, B. intestinalis, S. sanguinis, K. gyiorum, Phascolarctobacterium sp. and Tissierella sp. encode genes for the synthesis of the essential polyamine spermidine and the phospholipid phosphatidylcholine. While phosphatidylcholine is one of the major components of cell membranes [58], spermidine plays a crucial role in various biological processes, including maintaining membrane potential, controlling intracellular pH, lipid metabolism, and regulating cell growth, proliferation, and death [59]. Our metabolic analysis did not identify a pathway in M. exilis capable of synthesizing these compounds de novo. This suggests that M. exilis may rely on the uptake of these compounds from these prokaryotes.
Iron uptake is another essential function for which no pathway is known in M. exilis. Several bacteria within the community encode transporters for Fe2⁺, including the anaerobic Feo system, the aerobic Efe system, and the Sit system [60] (Supplementary Table 8). Among these systems, only the Sit system in Kerstersia gyiorum shows upregulation between day 2 and day 5, indicating an increase in iron uptake during the later stages of the culture. In contrast, Fe2⁺ transporters in other bacteria are either downregulated or exhibit no significant differential expression (Supplementary Figs. 2–8).
Another important type of molecules used for iron uptake in bacteria is known as siderophores. These iron-chelating molecules are secreted into the environment, and their iron-bound forms are selectively imported [61]. Siderophore production is limited to a few species within the community. Citrobacter portucalensis and Saezia sanguinis synthesize enterobactin, while Fusobacterium varium produces staphylopine, and K. gyiorum encodes a periplasmic iron-binding protein. Interestingly, although only a subset of bacteria produce siderophores, nearly all bacteria encode transport systems for siderophore uptake or transferrin binding. The presence of the Ton uptake system (TonB, ExbB, ExbD) [60] in most gram negative bacteria, along with enterobactin transporters (FepA, FbpABC) and transferrin receptor TbpA [60], suggests that iron acquisition is a shared resource within the community (Supplementary Table 8). Notably, no gene for siderophore and transferrin uptake was detected in M. exilis.
Expression data indicate that the uptake of siderophores is upregulated in F. varium, K. gyiorum, and members of Bacteroidota during the later days of the culture (Supplementary Figs. 3–5, 7, 8). This pattern suggests a dynamic strategy for iron acquisition, in which some species produce siderophores while others act as “cheaters,” utilizing uptake systems to acquire iron-bound molecules without producing their own siderophores [62, 63]. These interactions may influence the stability and composition of the community over time.
Once iron is imported, it must be coordinated within porphyrins to prevent toxicity [64–66]. Two main porphyrins, siroheme and heme b, were identified in the community. C. portucalensis can synthesize both, but under anaerobic conditions, the synthesis of siroheme is upregulated while the synthesis of heme b is downregulated (Supplementary Fig. 2). Conversely, Bacteroides fragilis upregulates oxygen-independent heme b synthesis while downregulating siroheme production (Supplementary Fig. 4). Some bacteria can convert siroheme to heme b, but due to their low abundance, transcriptomic data were unavailable for assessment of expression [64, 66].
Altogether the data indicate that siderophore producers are key players in the prokaryotic community, facilitating iron acquisition for other bacteria. Importantly, their presence offers no direct benefit to M. exilis, which does not have a siderophore uptake mechanism or any other known uptake pathway for soluble iron. This suggests that M. exilis relies entirely on obtaining iron from its bacterial prey through phagocytosis.
Discussion
In this study, we aimed to characterize the species composition of the microbial community associated with the amitochondriate flagellate Monocercomonoides exilis. Our goal was to understand the metabolic interactions between this protist and prokaryotes, as well as the factors that may prevent its axenic growth. Using a multi-omics approach, we analyzed the composition, dynamics, and biochemical processes of this complex system over time. This research provides initial insights into the biochemical cycles maintained within the test tube community and how the community composition and metabolic processes change as the culture ages.
Because this community was isolated from the chinchilla gut, these findings may have some limited relevance to the situation in this host. The only existing study of the gut microbiota composition of chinchillas [67] reports that Betaproteobacteria are the dominant phylum, with Parabacteroides and Barnesiella as the dominant genera. In the culture, we identified 30 bacterial species, with Fusobacterium varium being the most abundant at the beginning of the experiment. However, by day 5, it was replaced by the Bacteroidota group, represented by Bacteroides fragilis, Parabacteroides sp. and B. thetaiotaomicron. On day 5, Parabacteroides sp. became the second most abundant bacterium, but we did not detect any Barnesiella species. We identified only two species of Betaproteobacteria, which were not among the most abundant species. At the phylum level, Bacteroidota and Bacillota were the most abundant in our culture, resembling on a broader scale the gut microbiota composition of guinea pigs [68]. These observations suggest that the prokaryotic community compositions of caviomorph rodents (the group in which chinchilla is classified) and our M. exilis culture are similar; however, the culture community has changed due to long-term cultivation.
The aim of our study was not to characterize the natural gut microbiota of chinchilla; however, it does provide insights into how microbial communities adapt to artificial environments and how M. exilis interacts with bacteria under controlled conditions. M. exilis accounts for approximately 1% of the total reads, indicating that the biochemical processes in the community are primarily driven by prokaryotes. Among the three main biogeochemical cycles, only the sulfur cycle seems to be complete and contains reactions converting all key inorganic sulfur compounds (Fig. 3). Our analyses suggest that the community primarily oxidizes organic carbon substrates, performs fermentation, and generates hydrogen (Fig. 3). The ability to fix carbon from CO2 is only present in Eubacterium maltosivorans, the least abundant species in the community. Therefore, this community must rely on the external supply of organic carbon. This observation aligns with our findings that organic carbon sources such as glyceraldehyde, lactate, fatty acids, alcohols and most amino acids are rapidly consumed within the first two days.
The community does not maintain a complete nitrogen cycle. Although a small amount of nitrogen fixation may occur due to the presence of Phascolarctobacterium sp., this bacterium is among the less abundant members of the community. As a result, its nitrogen fixation is unlikely to meet the ammonium demands. Another pathway for ammonium synthesis is the DNRA, which utilizes nitrate as an electron acceptor and is facilitated by certain bacterial species through periplasmic nitrate reductase, nitrate reductase A, or membrane-bound nitrate reductase (nrf) and allows building the membrane potential [48, 69]. However, transcriptomic data from all bacteria indicate that DNRA is either not expressed or downregulated in the later stages of growth. Consequently, the nitrogen sources available for building biomass primarily consist of amino acids and nucleobases, which are components of the growth medium. These nutrients can be measured on day 0, along with a small supplement of ammonia synthesized by Phascolarctobacterium sp.
The metabolomic analyses of the soluble media revealed that by day 2, when the population of M. exilis only began to grow, almost all soluble carbon sources, nucleotides, and most amino acids had already been consumed (Supplementary Table 4). The active growth of M. exilis after day 2 strongly suggests that the protist primarily derives its carbon, nitrogen, and energy from its bacterial prey in order to build its biomass. A potential exception could be arginine, which was detected in later days and may serve as a potential substrate for energy metabolism [70, 71]. Grazing on bacteria by M. exilis has been previously documented by electron microscopy [12]. Furthermore, the presence of lysozymes among a diverse array of glycosyl hydrolases (see Supplementary Table 2) provides the protist with molecular tools for feeding on bacteria. However, there is no consistent trend indicating the up- or down-regulation of lysozymes as the culture ages. Notably, on day 5, the protist exhibits a significant up-regulation of several genes associated with the late pathways of the endomembrane system. During this period, the expression levels of many genes that encode proteins involved in regulating these processes remained stable. Notably, four paralogs of the Rab7 small GTPase, which is known for its role in lysosome activation [56], were significantly down-regulated by day 5 (Supplementary Table 6). We interpret this pattern as evidence of increased exocytosis and secretion rather than bacterial feeding, which is also consistent with the observed decrease in cell counts of M. exilis at this stage of the culture.
Most genes of M. exilis that show differential expression lack functional annotations, resulting in a significant amount of what we call “transcriptomic dark matter,” comprising approximately three thousand genes. While these genes may possess functional and adaptive significance for the protist, we currently have no information about them. Among the known metabolic genes, we observed an up-regulation of the sugar importers, α-D-xylose and sn-glycerol-3-phosphate. This likely reflects the cell’s response to the absence of these substrates. On day 5, another category of genes that showed increased expression includes enzymes responsible for starch degradation, as well as the synthesis of trehalose and glycogen, indicating the onset of starvation. We also noted an up-regulation in the degradation of nucleosides and nucleotides and the biosynthesis of 5-phospho-α-D-ribose 1-diphosphate (PRPP), suggesting an increase in the storage of nucleotides and phosphate donors. Conversely, pathways such as glycolysis, extended glycolysis, fermentation, the pentose phosphate pathway, amino acids biosynthesis, the arginine deaminase pathway and the mevalonate pathway, which is involved in isoprenoid biosynthesis, are generally down-regulated on day 5, with few exceptions. This trend also applies to the synthesis of very long and ultra-long fatty acids and the aminoacyl-tRNA synthetases, indicating a decline in protein synthesis.
Conclusions
Our investigation into the interactions within the test tube community, which includes approximately 30 bacterial species and the eukaryote M. exilis, found no clear biochemical connections between the flagellate and the bacteria. However, it indicated that M. exilis relies on bacterial prey as a source of a spectrum of essential nutrients, including organic carbon, nitrogen, iron, polyamines like spermidine, and phosphatidylcholine. This dependency on bacterial interactions may explain why M. exilis cannot be cultivated axenically yet. As the culture aged, gene expression analyses in M. exilis showed metabolic adaptations to starvation. This included an upregulation of starch degradation, as well as the synthesis of trehalose and glycogen, along with processes related to exocytosis. Conversely, core metabolic pathways such as glycolysis, amino acid biosynthesis, and protein synthesis were downregulated, indicating a decline in cellular activity. Notably, a significant portion of the genes that were differentially expressed between time points lacked functional annotations, highlighting the extent of the unrecognized adaptive responses. Future research should focus on identifying specific bacterial interactions that support the growth of M. exilis to intelligently explore the strategies for axenic cultivation. Of particular interest is elucidating the mechanism by which M. exilis takes up iron, as this is essential for its unusual iron-sulfur cluster assembly occurring in the cytosol of this amitochondriate organism. Finally, we should aim to clarify the functions of genes in M. exilis that lack functional annotations, as this may uncover novel insights into eukaryotic cell biology and metabolism.
Supplementary Information
Acknowledgements
We thank Professor Joel Dacks for his advice on the analysis of the differentially expressed protein related to the endomembrane system.
Abbreviations
- ACS
Acetate synthase
- Adh
Bifunctional acetaldehyde-CoA/alcohol dehydrogenase
- ALDH
Aldehyde dehydrogenase
- DNRA
Dissimilatory Nitrate Reduction to Ammonium
- HYD
Hydrogenases
- m2m
Metage2metabo pipeline
- MAGs
Metagenome-assembled genomes
- NapAB
Periplasmic nitrate reductase
- NarGHI
Nitrate reductase A
- NrfAH/NrfABCDEF
Cytochrome c552 nitrite reductase
- PEPCK
Phosphoenolpyruvate carboxykinase
- PFOR
Pyruvate-ferredoxin oxidoreductase
- PK
Pyruvate kinase
- PRPP
5-Phospho-α-D-ribose 1-diphosphate
- RPE
Ribulose-phosphate 3-epimerase
- TGN
Trans-Golgi-network
Author contributions
SCT and PPD performed the cell culturing and DNA and RNA isolation; VB prepared the sequencing libraries; AJ and PŽ performed the metabolic identification; AJG assembled and annotated the metagenomic genomes and performed the metatranscriptomic and metabolic analyses. AJG and VH analyzed the data and wrote the first draft; AJG, SCT, PPD, VB, AJ, PŽ and VH edited the manuscript. All authors approved the submitted final version.
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 771592 to VH) and the Centre for Research of Pathogenicity and Virulence of Parasites (registration no. CZ.02.1.01/0.0/0.0/16_019/0000759).
Data availability
DNA reads, RNA reads and the complete 24 MAGs are available under a BioProject with accession number PRJEB70623. MAGs are available with accession numbers from ERS17699982 to ERS17699999. The script used for the metatranscriptomics differential expression analysis is available on https://github.com/alejimgon/Gene-Analysis-Tools (taxon_scaling folder).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 4.Partida-Rodriguez O, Nieves-Ramirez M, Laforest-Lapointe I, Brown EM, Parfrey L, Valadez-Salazar A, et al. Exposure to parasitic protists and helminths changes the intestinal community structure of bacterial communities in a cohort of mother-child binomials from a semirural setting in Mexico. mSphere. 2021. 10.1128/mSphere.00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Huth S, Thingholm LB, Kofoed P-E, Bang C, Rühlemann MC, Franke A, et al. Intestinal protozoan infections shape fecal bacterial microbiota in children from Guinea-Bissau. PLoS Negl Trop Dis. 2021;15: e0009232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond LS. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. J Parasitol. 1982;68:958–9. [PubMed] [Google Scholar]
- 7.Hamann E, Gruber-Vodicka H, Kleiner M, Tegetmeyer HE, Riedel D, Littmann S, et al. Environmental breviatea harbour mutualistic Arcobacter epibionts. Nature. 2016;534:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann E, Tegetmeyer HE, Di R, Littmann S, Ahmerkamp S, Chen J, et al. Syntrophic linkage between predatory Carpediemonas and specific prokaryotic populations. ISME J. 2017;11:1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treitli SC, Peña-Diaz P, Hałakuc P, Karnkowska A, Hampl V. High quality genome assembly of the amitochondriate eukaryote Monocercomonoides exilis. Microb Genom. 2021;7. [DOI] [PMC free article] [PubMed]
- 10.Karnkowska A, Treitli SC, Brzoň O, Novák L, Vacek V, Soukal P, et al. The oxymonad genome displays canonical eukaryotic complexity in the absence of a mitochondrion. Mol Biol Evol. 2019;36:2292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnkowska A, Vacek V, Zubáčová Z, Treitli SC, Petrželková R, Eme L, et al. A eukaryote without a mitochondrial organelle. Curr Biol. 2016;26:1274–84. [DOI] [PubMed] [Google Scholar]
- 12.Treitli SC, Kotyk M, Yubuki N, Jirounková E, Vlasáková J, Smejkalová P, et al. Molecular and morphological diversity of the oxymonad genera Monocercomonoides and Blattamonas gen. nov. Protist. 2018;169:744–83. [DOI] [PubMed] [Google Scholar]
- 13.Preaxostyla HV. In: Archibald JM, Simpson AGB, Slamovits CH, editors. Handbook of the Protists. Cham: Springer International Publishing; 2017. p. 1139–74. [Google Scholar]
- 14.Novák LVF, Treitli SC, Pyrih J, Hałakuc P, Pipaliya SV, Vacek V, et al. Genomics of preaxostyla flagellates illuminates the path towards the loss of mitochondria. PLoS Genet. 2023;19:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treitli SC, Kolisko M, Husník F, Keeling PJ, Hampl V. Revealing the metabolic capacity of Streblomastix strix and its bacterial symbionts using single-cell metagenomics. Proc Natl Acad Sci. 2019;116:19675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. Metaspades: a new versatile metagenomic assembler. Genome Res. 2017;27:824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–7. [DOI] [PubMed] [Google Scholar]
- 19.Dick GJ, Andersson AF, Baker BJ, Simmons SL, Thomas BC, Yelton AP, et al. Community-wide analysis of microbial genome sequence signatures. Genome Biol. 2009. 10.1186/gb-2009-10-8-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–9. [DOI] [PubMed] [Google Scholar]
- 21.Chaumeil PA, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk v2: memory friendly classification with the genome taxonomy database. Bioinformatics. 2022;38:5315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks DH, Chuvochina M, Chaumeil PA, Rinke C, Mussig AJ, Hugenholtz P. A complete domain-to-species taxonomy for bacteria and archaea. Nat Biotechnol. 2020;38:1079–86. [DOI] [PubMed] [Google Scholar]
- 23.Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11. [DOI] [PMC free article] [PubMed]
- 24.Eddy SR. Accelerated profile HMM searches. PLoS Comput Biol. 2011;7 [DOI] [PMC free article] [PubMed]
- 25.Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw J, Yu YW. Fast and robust metagenomic sequence comparison through sparse chaining with skani. Nat Methods. 2023;20:1661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010. 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S, et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukumaran J, Holder MT. DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010;26:1569–71. [DOI] [PubMed] [Google Scholar]
- 30.Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with NumPy. Nature. 2020;585:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, Cook H, et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47:D309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J. EggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol Biol Evol. 2021;38:5825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belcour A, Frioux C, Aite M, Bretaudeau A, Hildebrand F, Siegel A. Metage2metabo, microbiota-scale metabolic complementarity for the identification of key species. Elife. 2020;9:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karp PD, Paley SM, Midford PE, Krummenacker M, Billington R, Kothari A, et al. Pathway Tools version 24.0: Integrated Software for Pathway/Genome Informatics and Systems Biology. arXiv e-prints. 2020.
- 35.Karp PD, Latendresse M, Caspi R. The pathway tools pathway prediction algorithm. Stand Genomic Sci. 2011;5:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antipov D, Raiko M, Lapidus A, Pevzner PA. Metaviral SPAdes: assembly of viruses from metagenomic data. Bioinformatics. 2020;36:4126–9. [DOI] [PubMed] [Google Scholar]
- 37.Antipov D, Raiko M, Lapidus A, Pevzner PA. Plasmid detection and assembly in genomic and metagenomic data sets. Genome Res. 2019;29:961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bushmanova E, Antipov D, Lapidus A, Prjibelski AD. rnaSPAdes: a de novo transcriptome assembler and its application to RNA-Seq data. Gigascience. 2019;8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J, Bolduc B, Zayed AA, Varsani A, Dominguez-Huerta G, Delmont TO, et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome. 2021;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Tran PQ, Breister AM, Liu Y, Kieft K, Cowley ES, et al. METABOLIC: high-throughput profiling of microbial genomes for functional traits, metabolism, biogeochemistry, and community-scale functional networks. Microbiome. 2022. 10.1186/s40168-021-01213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Y, Smyth GK, Shi W. Featurecounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. [DOI] [PubMed] [Google Scholar]
- 44.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. R: A language and environment for statistical computing. 2021.
- 46.Klingenberg H, Meinicke P. How to normalize metatranscriptomic count data for differential expression analysis. PeerJ. 2017;2017. [DOI] [PMC free article] [PubMed]
- 47.Christel S, Herold M, Bellenberg S, Buetti-Dinh A, El Hajjami M, Pivkin IV, et al. Weak iron oxidation by Sulfobacillus thermosulfidooxidans maintains a favorable redox potential for chalcopyrite bioleaching. Front Microbiol. 2018;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaviraj M, Kumar U, Snigdha A, Chatterjee S. Nitrate reduction to ammonium: a phylogenetic, physiological, and genetic aspects in prokaryotes and eukaryotes. Arch Microbiol. 2024;206: 297. [DOI] [PubMed] [Google Scholar]
- 49.Sugai Y, Katsuyama Y, Ohnishi Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat Chem Biol. 2016;12:73–5. [DOI] [PubMed] [Google Scholar]
- 50.Arieli B, Shahak Y, Taglicht D, Hauska G, Padan E. Purification and characterization of sulfide-quinone reductase, a novel enzyme driving anoxygenic photosynthesis in Oscillatoria limnetica. J Biol Chem. 1994;269:5705–11. [PubMed] [Google Scholar]
- 51.Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2014;42:459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iturriaga G, Suárez R, Nova-Franco B. Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci. 2009;10:3793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Law KC, Chung KK, Zhuang X. An update on coat protein complexes for vesicle formation in plant post-Golgi trafficking. Front Plant Sci. 2022;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu Y, Uemura T. The sorting of cargo proteins in the plant trans-Golgi network. Front Plant Sci. 2022;13 August:1–10. [DOI] [PMC free article] [PubMed]
- 55.Tanaka T, Goto K, Iino M. Diverse functions and signal transduction of the exocyst complex in tumor cells. J Cell Physiol. 2017;232:939–57. [DOI] [PubMed] [Google Scholar]
- 56.Bucci C, Thomsen P, Nicoziani P, McCarthy J, Van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teulière J, Bernard G, Bapteste E. The distribution of genes associated with regulated cell death is decoupled from the mitochondrial phenotypes within unicellular eukaryotic hosts. Front Cell Dev Biol. 2020;8 September:1–8. [DOI] [PMC free article] [PubMed]
- 58.Zhukov A, Popov V. Eukaryotic Cell Membranes: Structure, Composition, Research Methods and Computational Modelling. Int J Mol Sci. 2023;24. [DOI] [PMC free article] [PubMed]
- 59.Michael AJ. Polyamines in eukaryotes, bacteria, and archaea. J Biol Chem. 2016;291:14896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braun V, Hantke K. The tricky ways bacteria cope with iron limitation. In: Chakraborty R, Braun V, Hantke K, Cornelis P, editors. Iron Uptake in Bacteria with Emphasis on E. coli and Pseudomonas. Dordrecht: Springer Netherlands; 2013. p. 31–66.
- 61.Kramer J, Özkaya Ö, Kümmerli R. Bacterial siderophores in community and host interactions. Nat Rev Microbiol. 2020;18:152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leventhal GE, Ackermann M, Schiessl KT. Why microbes secrete molecules to modify their environment: The case of iron-chelating siderophores. J R Soc Interface. 2019;16. [DOI] [PMC free article] [PubMed]
- 63.Butaite E, Baumgartner M, Wyder S, Kümmerli R. Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nat Commun. 2017;8. [DOI] [PMC free article] [PubMed]
- 64.Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, et al. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev. 2017;81:1–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryant DA, Hunter CN, Warren MJ. Biosynthesis of the modified tetrapyrroles—the pigments of life. J Biol Chem. 2020;295:6888–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Layer G. Heme biosynthesis in prokaryotes. Biochimica et Biophysica Acta (BBA)—Mol Cell Res. 2021;1868: 118861. [DOI] [PubMed] [Google Scholar]
- 67.O’ Donnell MM, Harris HMB, Ross RP, O’Toole PW. Core fecal microbiota of domesticated herbivorous ruminant, hindgut fermenters, and monogastric animals. Microbiologyopen. 2017;6. [DOI] [PMC free article] [PubMed]
- 68.Crowley EJ, King JM, Wilkinson T, Worgan HJ, Huson KM, Rose MT, et al. Comparison of the microbial population in rabbits and guinea pigs by next generation sequencing. PLoS ONE. 2017. 10.1371/journal.pone.0165779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cabello P, Luque-Almagro VM, Roldán MD, Moreno-Vivián C. Nitrogen cycle. Encyclopedia of Microbiology. 2019;:301–10.
- 70.Yarlett N, Martinez MP, Moharrami MA, Tachezy J. The contribution of the arginine dihydrolase pathway to energy metabolism by Trichomonas vaginalis. Mol Biochem Parasitol. 1996;78:117–25. [DOI] [PubMed] [Google Scholar]
- 71.Schofield PJ, Edwards MR, Matthews J, Wilson JR. The pathway of arginine catabolism in Giardia intestinalis. Mol Biochem Parasitol. 1992;51:29–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA reads, RNA reads and the complete 24 MAGs are available under a BioProject with accession number PRJEB70623. MAGs are available with accession numbers from ERS17699982 to ERS17699999. The script used for the metatranscriptomics differential expression analysis is available on https://github.com/alejimgon/Gene-Analysis-Tools (taxon_scaling folder).