Abstract
The untreated disposal of industrial effluents that contain hazardous toxic metal elements such as cadmium (Cd), chromium (Cr), lead (Pb), nickel (Ni), manganese (Mn) and iron (Fe), causes major threats to the ecosystem. The present study aims to focus on the health of water bodies and riparian vegetation in the Sargodha district. Industrial effluents from sugar mill industries drain the Jhelum River and eventually pose a major threat to the riparian vegetation of the area as it does in other parts of the world. The results suggested the presence of a high level of pollution in drains carrying the industrial effluents and inside the river in the form of increased salinity, metal toxicity, and the acidic and alkaline nature of industrial drainage. Additionally, a few acclimated species such as Eclipta alba, Desmostachya bipinnata, Cynodon dactylon, Paspalum distichum, and Cyperus rotundus were identified and examined for their physiological, proximate, and morpho-anatomical attributes. Most of the species exhibit a high degrees of anatomical modification, such as sclerification and aerenchyma formation which support their survival in such a polluted environment. These plants presented increased production of crude fibers, carbohydrates, and proteins, as well as enhanced photosynthetic pigments, allowing them to cope with stress conditions. This study highlights the need for urgent measures to mitigate the impact of industrial pollution on the environment and ecosystem, and underscores the importance of identifying and conserving plant species that can tolerate and adapt to polluted conditions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-07034-3.
Keywords: Chloroplastidic pigments, Crude fibers, Industrial effluents, Primary metabolites
Introduction
Water is a necessary resource for the existence and growth of living organisms, and reducing environmental pollution and maintaining pollutant-free water are critical [1]. The movement of materials and energy between terrestrial and aquatic ecosystems is facilitated by riparian zones, which are crucial transition sites. Through a variety of ecological services, such as nutrient filtering, sediment trapping, and erosion control, they significantly improve the quality of the water. To preserve and manage the nearby lakes, streams, and other surface water systems, riparian zones serve as natural buffers between waterways and the surrounding landscape [2].
Buffer zones also act as filters for nutrients reduce the leakage of N, P, and pesticides, provide habitats to animals and plants, minimize erosion issues, protect against floods, improve biodiversity and ecological connectivity, and create recreational areas [2], mainly to reduce the transportation of contaminants through subsurface and overland flow [3]. Despite their ecological value, riparian zones are becoming more sensitive to environmental stressors, particularly those caused by human activity [4].
Lakes, streams, and other bodies of water have experienced declines in water quality due to agricultural development, urbanization, and industrialization [4, 5]. The main source of pollutants that reach farmlands is agricultural and residential sewage. These pollutants are further divided into organic pollutants, pesticides, human diseases, drug residues, environmental hormones, and inorganic contaminants (heavy metals, sediments, N, and P). Because they travel through surface runoff, these pollutants have detrimental effects on lakes, rivers, and aquatic ecosystems in addition to the soil [6].
Industrial contamination is one of the greatest dangers to riparian habitats. In riparian zones, industrial operations such as mining, manufacturing, and chemical production are the main source of pollution [7]. Industrial effluents commonly release heavy metals like Cd, Pb, Zn, and Cu into water bodies, endangering both aquatic and terrestrial ecosystems [8, 9]. Because plants in riparian zones are immediately exposed to contaminants through soil absorption, water uptake, and air deposition, these areas are more vulnerable to pollution because of their close proximity to water. Significant physiological, ecological, and morphological changes in riparian plant communities may result from this exposure over time [10].
One of the primary ways in which industrial pollution affects riparian vegetation is by inducing oxidative stress. Industrial pollutants, such as heavy metals and ionic compounds, lead to increased ROS levels in riparian plants, causing cellular damage and impaired physiological functions [11, 12]. Elevated ROS levels are linked to stress responses, including leaf senescence and reduced photosynthetic efficiency [13]. However, the intensity and impact of oxidative stress vary among plant species, depending on their antioxidant capacity and ability to activate stress response mechanisms [14, 15].
To counteract this damage, plants activate their antioxidant defense systems, which include enzymes and non-enzymatic antioxidants such as glutathione and ascorbic acid. In addition to antioxidant production, many plant species also develop anatomical and physiological adaptations such as aerenchyma formation and iron plaque deposition in roots, which help limit metal uptake and enhance oxygenation under stress conditions. Research on riparian vegetation to pollution indicates that species such as Calotropis procera exhibit strong resistance mechanisms, including elevated antioxidant enzyme activity and morphological adaptations, making them particularly effective in polluted environments [12, 16].
Industrial pollution can drastically change the diversity and composition of riparian plant communities in addition to causing physiological stress. These habitats may be dominated by pollution-tolerant species, which would outcompete sensitive species and lower biodiversity overall [17]. This shift in community structure can have cascading effects on ecosystem functioning, as the loss of sensitive species may disrupt ecological interactions and reduce the resilience of riparian zones to environmental changes. Furthermore, the reduction in plant diversity can impact associated fauna, leading to a decline in the overall health of the ecosystem [18].
Morphological changes in riparian vegetation are another notable consequence of industrial pollution. Plants exposed to pollutants often exhibit visible signs of stress, such as reduced growth, chlorosis (yellowing of leaves), and abnormalities in root development [19]. These morphological alterations not only affect the individual plants but also have broader implications for ecosystem stability. For instance, stunted growth and reduced biomass can diminish the ability of riparian vegetation to perform essential functions such as soil stabilization and water filtration. Over time, these changes can compromise the structural integrity of riparian zones, increasing their susceptibility to erosion and habitat degradation. The cumulative impact of industrial pollution on riparian vegetation poses a significant challenge for ecosystem conservation and management. As riparian zones are critical for maintaining water quality, supporting biodiversity, and providing ecosystem services, their degradation can have far-reaching consequences for both natural ecosystems and human communities [20].
This study aims to address this gap by investigating the impact of industrial pollution on riparian vegetation, with a focus on three key areas: antioxidant defense mechanisms, community diversity, and morphological traits. By analyzing these aspects in tandem, this research seeks to provide a detailed assessment of how pollution stress affects riparian ecosystems at multiple levels. The findings of this study will not only contribute to the scientific understanding of pollution impacts but also offer practical insights for the conservation and restoration of riparian zones. Ultimately, this research underscores the importance of protecting these vital ecosystems from the growing threat of industrial pollution, ensuring their continued role in supporting biodiversity and ecosystem services.
Materials and methods
Sampling seasons and sampling sites
The data were recorded from the selected sites in the summer (June) and winter (December) seasons. The present study was carried out to evaluate riparian vegetation adaptation to pollution in the District of Sargodha Punjab, Pakistan. After the diversity of the area was explored and the floristic composition was determined, plants such as Eclipta alba, Desmostachya bipinnata, Cynodon dactylon, Paspalum distichum, and Cyperus rotundus were found to be present at all sites throughout the year. It was hypothesized that these plants have acclimatized to industrial pollution and were selected for further studies. Seven sites (3 mild polluted and one non-polluted/control site) at the river and 3 sites (polluted sites from drains) were selected for the collection of plant, water, and soil samples (Fig. 1 A-C).
Fig. 1.

Paspalum distichum (A) Cyperus rotundus, growing along drain (B), Desmostachya bipinnata (C), Anatomical dissection (D)
In summer, the climate is hot and humid, whereas in winter, it is cold. June is the hottest month of the year, with the maximum temperature soaring above 40 ºC. During frequent rainfall in the monsoon season, rainwater flows into streams through surface runoff and causes flooding which usually has devastating effects on crops and human settlements. Most of the floods result in the deposition of new alluvial soil in the catchment area. The experiment was conducted in a randomized design with five replicates.
Preliminary water and soil sampling
Water and soil samples were taken from potential sites to determine the presence and concentration of heavy metals and other contaminants. A site separate from the industrial polluted area with no visible contamination and low heavy metal levels in preliminary testing was designed as non-polluted.
Water analysis
Water was sampled from three drain sites and four different sites in the Jhelum River. Water samples were collected in polypropylene bottles. Samples were taken to a research center for analysis. The samples were digested for heavy metal determination, and COD and BOD were recorded. Ten metals were analyzed via an atomic absorption spectrometer (AAS). Metal toxicity was checked. Various parameters were measured using a conductivity meter and the evaporation method.
Soil analysis
Soil samples from the river and drain sites were analyzed for their physico-chemical characteristics. The samples were dried, digested, and analyzed for heavy metals. pH, electrical conductivity (EC), organic matter (OM), Phosphorus (P), Potassium (K), Saturation, and various heavy metals were measured. The plant samples were digested and used to measure the metal concentrations. The precision was expressed as relative standard deviation. The recovery rates of the metals were within 90 ± 10%. The results were analyzed using principal component analysis (PCA).
Plant material collection
The roots, stems, and leaves of five selected plants, Eclipta alba, Desmostachya bipinnate, Cynodon dactylon, Paspalum distichum, and Cyperus rotundus, were collected from seven different sites and carefully transported to the laboratory for analysis. Water and soil samples were also taken at the seven selected sites in two seasons as per the guidance and permission from the University of Sargodha, Sargodha, Pakistan and the Higher Education of Pakistan (HEC).
Study of the morphological parameters of the selected plants
Morphological parameters were measured in terms of shoot length (cm), root length (cm), shoot fresh weight (g), root fresh weight (g), shoot dry weight (g), and root dry weight (g) by following the methodology of Ayub et al. [21].
Shoot and root length
The shoot length and root length were recorded with a measuring scale in centimeters (cm).
Shoot and root fresh weights
The roots were removed from the soil and wash off any loose soil was removed. The plants were gently blotted with soft paper towel to remove any free surface moisture and weighed immediately (plants have a high composition of water, so waiting to weigh them may lead to some drying and therefore produce inaccurate data) in grams by using an electronic balance CX-600.
Determination of chlorophyll and carotenoid contents
The chlorophyll content of the leaves was measured using the method of Bruinsma et al. [20]. Fully ground leaf samples (2 g) were extracted in 80% (v/v) acetone (10 mL). The mixture was filtered through Whatman No. 1 filter paper. Absorbance was measured using a spectrophotometer at wavelengths of 645, 652, and 663 nm (Model U-2900 Sr. No 26E82-018) for chlorophyll a, b, and total chlorophyll, respectively. The carotenoid content was determined using the same extract, and the absorbance was read at 480 nm.
Proximate analysis
The protocols designed by the Association of Official Analytical Chemists (AOAC) [23], were followed for the accomplishment of proximate analysis of the plant material. Proximate analysis provides the details about the dry matter, moisture content, crude protein, crude fibers, and ash.
Dry matter
The quantity of dry matter was determined via the following formula:
 |
Moisture content
Weighed samples of shade-dried plants were kept in an incubator at 105 oC for 24 h. The samples were then weighed again, and moisture content was calculated as follows:
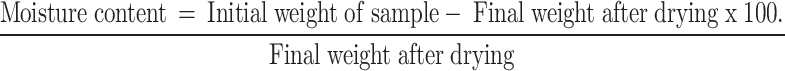 |
Crude protein detection
Crude proteins are quantified by boiling 1 g of plant sample with concentrated H2SO4 and K2SO4. Digestion was carried out until a clear solution formed. The solution was diluted with distilled water. This sample was digested of this sample was brought about with 50 mg of Zn and NaOH. The digested solution was then titrated against conc. H2SO4 using methyl red indicator. The light pink color was taken as the endpoint. The volume of acid used indicates the value of protein in a given sample.
Crude fibers
The determination of crude fibers is based on acid-base digestion. 1 g of fat-free sample was boiled for 30 min with 200 ml of 1.25% H2SO4 and subsequently with 1.25% NaOH. The sample was then strained, washed with distilled water, and kept at 100 oC until drying. The weight of the dry sample was expressed as the concentration of crude fibers.
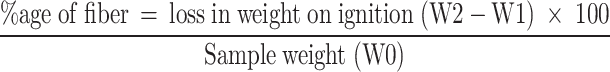 |
Ash content
To calculate the ash content, 1 g of the sample was placed in a crucible and charred on a burner at 600 oC to remove the organic matter. The burnt sample was kept in the muffle furnace at 550 oC for 6 h. After being removed from the furnace, the samples were cooled and weighed.
The ash content was calculated by applying the following formula:
 |
Fat content
The percentage of fat was estimated via the AOAC [23] official method No. 945.16. In a Soxhlet extractor, a known weight of the sample was thoroughly extracted in diethyl ether (boiling point, 55 °C), and the ether was evaporated from the extraction flask to estimate the crude fat content, which was calculated as follows:
 |
Carbohydrate contents
The carbohydrate content was estimated by subtracting the total mass from the total mass of moisture, ash, fat, and crude protein (AOAC) [23]. All the tests were performed in triplicate, and the results were expressed as a percentage of the sample’s total dry matter.
Anatomical attributes
The analysis was performed according to the method of Ruzin [24]. The selected plants were used for morpho-anatomical study. The plant material (fresh stem 1–2 cm long middle portion of fresh organ) was fixed with formalin acetic alcohol (FAA70) for 48 h and stored in acetic alcohol (v/v acetic acid 25%, and ethanol 75%). Freehand sectioning technique was performed to obtain radial sections of stems, roots, and leaves (Fig. 1D). Slides were prepared after serial dehydrations in ethanol and staining by a standard double-stain technique involving the use of safranin and fast green. The slides were observed under a compound light microscope (Nikon Eclipse E200) equipped with an ocular micrometer calibrated via a stage micrometer.
Fixation
Healthy stem Sects. (1–2 mm) were fixed in 4% formaldehyde in 50 mM phosphate buffer (pH 7.2) at 4 °C for 48 h. Then, the same buffer was used to wash the sections for 5 min each. Three replicates were taken (3 different plants for each species per site) and three transverse sections were prepared per organ. From each section, at least three photomicrographs were taken from each section, and five measurements per anatomical parameter were noted.
Statistical analysis
All the collected data were subjected to statistical analysis using R Studio version 3.4.6 version. Analysis of variance and multiple mean comparison analysis were performed using the package “Agricolae”. Grouped and stacked bar plots were drawn using package “ggplot2”. Corrplots and heatmaps were drawn using packages and functions like ggplot2, cormap, and heatmap. CCA plots were drawn via the factoextra and varplot packages.
Results
Physico-chemical characteristics of the water samples
Analysis of the variance of water samples collected from seven different sites (non-polluted, polluted rivers, and polluted drains) revealed that the concentrations of all physicochemical parameters were highly significant with respect to site and season. Moreover, the effect of the site-to-season interaction had a significant effect on the concentrations of all physicochemical parameters of the water samples. The results represent that, the non-contaminated site had the lowest values of pH, electrical conductivity, calcium, magnesium, sodium, bicarbonate, chloride, and sodium absorption ratio, whereas the polluted drain site had the highest values. Furthermore, the non-polluted location had the lowest COD and BOD levels, whereas the polluted drain site had the highest values. The electrical conductivity (EC) of the effluents in the water samples indicated that the variation was highly significant with respect to site and season. The concentration of the chlorides in the water samples highly significantly varied with respect to site, season and their combined interaction. The concentration of Ca + Mg in the water samples collected from the different sites highly significantly varied with respect to site and season (Table 1).
Table 1.
Analysis of variance for physicochemical characteristics of water samples collected from seven different studied sites
| SOV | Df | pH | EC (µS/cm) | Ca + Mg (mEq/L) |
Na (mEq/L) |
Bicarbonates (mEq/L) |
||
|---|---|---|---|---|---|---|---|---|
| Site | 6 | 8.9805*** | 1411.47*** | 35.259*** | 544,285*** | 652.99*** | ||
| Season | 1 | 0.005 | 892.86*** | 70.000*** | 41,286*** | 115.71*** | ||
| Site * Season | 6 | 0.448* | 37.02*** | 0.001 | 5786*** | 6.55*** | ||
| Error | 56 | 1.458 | 0.40 | 0.003 | 4 | 0.002 | ||
| SOV | DF |
Chlorides
(mEq/L) |
SAR
(mEq/L) |
COD
(mg/L) |
BOD
(mg/L) |
|||
|---|---|---|---|---|---|---|---|---|
| Site | 6 | 884.54*** | 102,825*** | 54,188*** | 31,897*** | |||
| Season | 1 | 142.86*** | 7530*** | 68,516*** | 104,838*** | |||
| Site * Season | 6 | 3.69*** | 3307*** | 3499*** | 4042*** | |||
| Error | 56 | 0.01 | 1 | 3 | 13 | |||
Degree of freedom (df) of sources of variation (SOV) is site: 7, season: 2, whereas *, **, *** significant at < 0.05, < 0.01, and < 0.001 alpha respectively
Analysis of variance revealed significant variations in the sodium (Na) and bicarbonate concentrations among the water samples, which were influenced by site, season, and their interaction. The highest Na concentrations were detected at the Al Arabia mill drain (583 mEq/L) and SW mill drain (485 mEq/L), whereas the lowest was detected at the Control site (36.2 mEq/L). Similarly, the bicarbonate concentrations were highest at the SW mill drain (24.2 mEq/L) and the Al Arabia mill drain (20.2 mEq/L), and lowest at the non-polluted sites (2.07 mEq/L) (Table 2).
Table 2.
Season based mean comparison of physicochemical analysis of water samples from seven different studied sites
| Site | pH | EC (µS/cm) | Ca + Mg (meq/L) |
Na (meq/L) |
Bicarbonates (meq/L) |
||
|---|---|---|---|---|---|---|---|
| Non-Polluted | 7.540 ± 0.23d | 19.500 ± 2.17d | 6.280 ± 1.06c | 36.230 ± 5.27d | 2.070 ± 1.054e | ||
| Shakarganj River | 8.394 ± 0.09c | 32.516 ± 5.27c | 9.660 ± 1.054b | 40.238 ± 5.24d | 8.058 ± 1.054d | ||
| Shakarganj Drain | 9.330 ± 0.17b | 52.328 ± 5.27a | 8.728 ± 1.054b | 52.816 ± 5.27d | 14.730 ± 1.054c | ||
| SW River | 8.280 ± 0.10c | 37.420 ± 5.27b | 9.530 ± 1.054b | 43.238 ± 5.27d | 7.966 ± 1.054d | ||
| SW Drain | 10.350 ± 0.17a | 50.030 ± 5.28a | 11.336 ± 1.054a | 485.400 ± 52.89b | 24.240 ± 3.16a | ||
| Alarabia River | 8.278 ± 0.15c | 33.660 ± 3.16bc | 9.630 ± 1.054b | 202.922 ± 52.70c | 19.770 ± 1.58b | ||
| Alarabia Drain | 9.440 ± 0.22c | 48.720 ± 0.52a | 12.120 ± 1.054a | 583.150 ± 52.72a | 20.260 ± 0.52b | ||
| Site |
Chlorides
(meq/L) |
SAR
(me/L) |
COD
(mg/L) |
BOD
(mg/L) |
|||
|---|---|---|---|---|---|---|---|
| Non-Polluted | 13.300 ± 1.06f | 23.222 ± 1.49c | 152.0 ± 5.42d | 177.0 ± 1.13d | |||
| Shakarganj River | 14.700 ± 1.06f | 19.370 ± 1.05c | 283.0 ± 5.88c | 227.0 ± 5.19c | |||
| Shakarganj Drain | 22.242 ± 2.10e | 36.728 ± 1.58c | 363.0 ± 2.72a | 284.6 ± 2.74b | |||
| SW River | 25.660 ± 2.63d | 20.970 ± 2.34c | 294.0 ± 2.72c | 323.1.0 ± 2.72ab | |||
| SW Drain | 37.630 ± 1.05a | 241.100 ± 15.86a | 327.8 ± 2.40b | 313.9 ± 2.37ab | |||
| Alarabia River | 28.270 ± 1.58c | 75.018 ± 1.58b | 288.0 ± 5.47c | 236.0 ± 2.72c | |||
| Alarabia Drain | 35.500 ± 1.06b | 237.200 ± 2.73a | 373.8 ± 5.73a | 338.0 ± 0.79a | |||
Mean followed by same letter is not significant in a column
A comparative analysis of water samples from different sites revealed significant variations in pH, electrical conductivity (EC), calcium + magnesium (Ca + Mg), sodium (Na), bicarbonate, chloride, sodium absorption ratio (SAR), chemical oxygen demand (COD), and biological oxygen demand (BOD). The highest values of these parameters were generally observed at polluted sites (SW mill drain, Al Arabia mill drain, and Shakarganj mill drain), whereas the lowest values were found at the Control site. Seasonal variations were also observed, with higher values of EC, Ca + Mg, Na, bicarbonates, chlorides, SAR, COD, and BOD in winter than in summer (Table 3).
Table 3.
Season based mean comparison of physicochemical analysis of water samples from seven different studied sites
| Site | pH | EC (µS/cm) | Ca + Mg (mEq/L) |
Na (mEq/L) |
Bicarbonates (mEq/L) |
||
|---|---|---|---|---|---|---|---|
| Summer | 8.81 ± 0.2a | 35.59 ± 1.17b | 8.62 ± 1.0b | 181.9 ± 5.27b | 12.58 ± 1.054b | ||
| Winter | 8.79 ± 0.25a | 42.73 ± 1.52a | 10.62 ± 1.05a | 230.5 ± 2.72a | 15.15 ± 0.52a | ||
| Site |
Chlorides
(mEq/L) |
SAR
(me/L) |
COD
(mg/L) |
BOD
(mg/L) |
|||
|---|---|---|---|---|---|---|---|
| Summer | 23.90 ± 1.06b | 82.03 ± 1.49b | 266.0 ± 5.42b | 219.0 ± 1.13b | |||
| Winter | 26.75 ± 1.06a | 103.7 ± 2.73a | 328.6 ± 5.73a | 296.4 ± 0.79a | |||
Mean followed by same letter is not statistically significant in a column (P > 0.05)
Physico-chemical characteristics of the soil samples
Soil samples were taken from seven distinct locations, including polluted drains, polluted rivers, and non-polluted areas. With respect to site and season, there was a highly significant variation in all physicochemical parameters. The SW drain site had the highest pH value (9.59) whereas the non-polluted site had the lowest pH value (7.58). Only a small number of sites had pH values in the lower range, with a minimum of 7.58 noted. The increased accumulation of acidic compounds is indicated by the low pH of the soil (Table 4).
Table 4.
Site based mean comparison of physico-chemical analysis of soil samples from seven different studied sites
| Site | pH | EC (µS/cm) | Organic Matter (%) |
P
(mg/Kg) |
|
|---|---|---|---|---|---|
| Non-Polluted | 7.58 ± 0.23d | 14.73 ± 0.17ab | 1.94 ± 1.06b | 8.16 ± 1.27d | |
| Shakarganj River | 8.51 ± 0.09c | 15.76 ± 0.27a | 1.85 ± 1.054b | 9.93 ± 1.24 cd | |
| Shakarganj Drain | 9.15 ± 0.17ab | 11.5 ± 0.27 cd | 2.63 ± 1.054a | 26.8 ± 1.27b | |
| SW River | 9.59 ± 0.10a | 12.43 ± 1.27c | 2.13 ± 1.054b | 11.17 ± 1.27c | |
| SW Drain | 9.00 ± 0.17ab | 13.98 ± 0.28b | 2.67 ± 0.054a | 32.60 ± 2.89a | |
| Alarabia River | 8.55 ± 0.15c | 12.19 ± 3.16c | 1.94 ± 1.054b | 7.19 ± 2.70e | |
| Alarabia Drain | 9.55 ± 0.22a | 11.50 ± 0.52d | 2.65 ± 0.054a | 28.60 ± 0.72b | |
| Site |
K
(mg/Kg) |
Saturation
(%) |
||
|---|---|---|---|---|
| Non-Polluted | 163.21 ± 1.06e | 33.10 ± 1.49c | ||
| Shakarganj River | 243.00 ± 1.06bcd | 39.20 ± 1.05b | ||
| Shakarganj Drain | 283.00 ± 2.10ab | 43.47 ± 1.58a | ||
| SW River | 207.1 ± 2.63d | 38.70 ± 0.34b | ||
| SW Drain | 233.00 ± 1.05 cd | 43.72 ± 1.86a | ||
| Alarabia River | 257.00 ± 1.58abc | 39.80 ± 1.58b | ||
| Alarabia Drain | 291.66 ± 5.06c | 40.50 ± 2.73b | ||
Analysis of variance of the soil samples collected from seven different sites (non-polluted, polluted rivers and polluted drains) revealed that all physicochemical parameters were highly significantly varied with respect to site and season. Moreover, the effect of site-to-season interaction had a significant effect on all the physicochemical parameters of the soil samples (Table 5).
Table 5.
Analysis of variance for physico-chemical characteristics of soil samples from seven different studied sites
| SOV | Df | pH | EC (µS/cm) | Organic Matter (%) |
|
|---|---|---|---|---|---|
| Site | 6 | 4.94*** | 36.35*** | 1.41*** | |
| Season | 1 | 0.76*** | 157.5*** | 17.50*** | |
| Site * Season | 6 | 0.81* | 5.02*** | 0.001 | |
| Error | 56 | 0.037 | 0.004 | 0.002 | |
| SOV | DF |
K
(mg/Kg) |
Saturation
(%) |
||
|---|---|---|---|---|---|
| Site | 6 | 19,741*** | 126.38*** | ||
| Season | 1 | 128,571*** | 442.51*** | ||
| Site * Season | 6 | 3571*** | 17.21*** | ||
| Error | 56 | 2 | 1.64 | ||
Degree of freedom (df) of sources of variation (SOV) is plant: 5, site: 7, season: 2, *, **, *** significant at < 0.05, < 0.01 and < 0.001 alpha respectively
A seasonal comparison of physico-chemical parameters of the soil samples revealed that the pH values remained relatively consistent between summer and winter, whereas the electrical conductivity (EC) values were higher in winter (14.49 µS/cm) compared to summer (11.49 µS/cm). Additionally, the organic matter content, available phosphorus, and available potassium were greater in winter (2.75%, respectively) than in summer (1.75%, respectively), and the soil saturation levels were also greater in winter than in summer, indicating more pronounced effects of winter on the soil physico-chemical parameters (Table 6).
Table 6.
Season based mean comparison of physico-chemical analysis of soil from seven different studied sites
| Site | pH | EC (µS/cm) | Organic Matter (%) |
P
(mg/Kg) |
|
|---|---|---|---|---|---|
| Summer | 8.74 ± 0.21b | 11.49 ± 1.17b | 1.75 ± 1.06b | 15.72 ± 5.27b | |
| Winter | 8.95 ± 0.25a | 14.49 ± 1.52a | 2.72 ± 1.05a | 19.83 ± 2.72a | |
| Site |
K
(mg/Kg) |
Saturation
(%) |
||
|---|---|---|---|---|
| Summer | 196.84 ± 1.06b | 37.27 ± 1.49b | ||
| Winter | 282.55 ± 1.06a | 42.29 ± 2.73a | ||
Comparison of means among three areas (non-polluted, drain and river sites) and for the morphological characteristics of selected plants
A study on the growth of five plant species (Desmostachya bipinnata, Paspalm. distichum, Eclipta alba, Cynodon dactylon, and Cyprus rotundus) at polluted and non-polluted sites during summer and winter seasons revealed varying effects on shoot length and leaf number. In summer, D. bipinnata grew best at river sites, whereas P. distichum and C. rotundus grew best at non-polluted sites. In winter, D. bipinnata grew best at drain sites, while E. alba and C. dactylon grew best at river sites. The leaf number varied among species and sites, with some species showing no significant differences between sites or seasons. The leaf area of all the selected plants significantly varied at all the selected sites, as the leaf area of D. bipinnata was greater among all the plants at the drain sites than at the river and non-polluted sites in the summer season. P. distichum, C. rotundus, and E. alba had almost equal leaf area at the selected sites, with no significant differences in the recorded data (Fig. 2 A-D).
Fig. 2.
A-D Comparison of means among three areas (non-polluted, drains and river site) for morphological characteristics of five selected plants
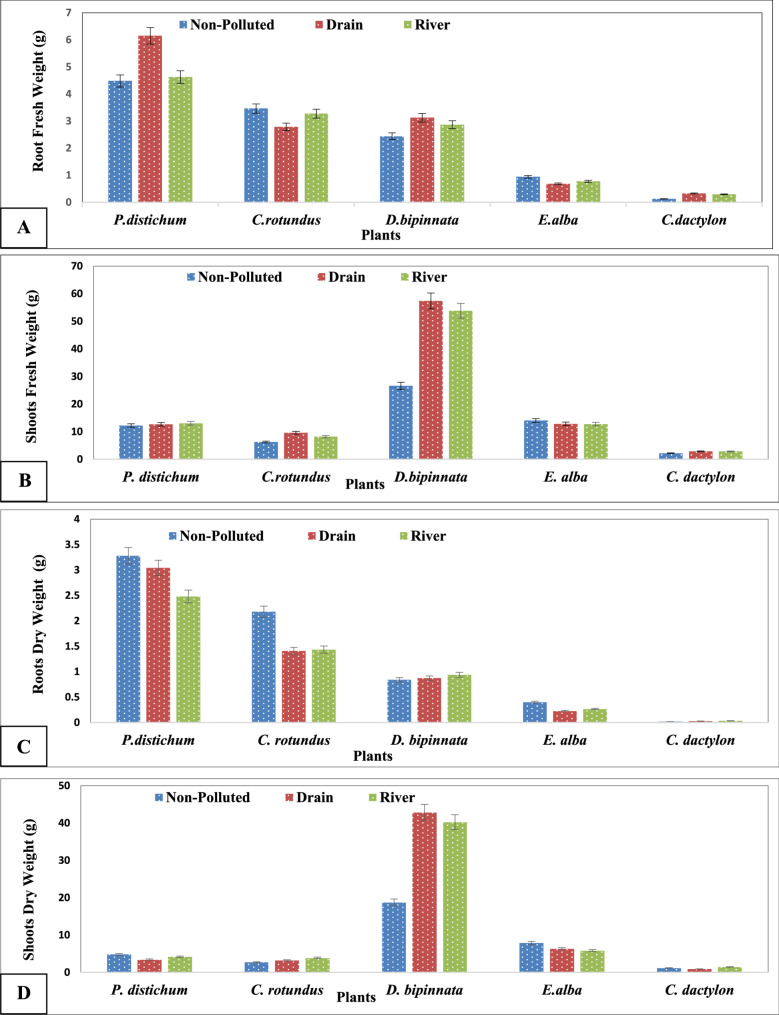
A comparison of the root fresh weights of the roots of five plant species at polluted and non-polluted sites during the summer and winter revealed varying effects. In summer, P. distichum and D. bipinnata presented greater fresh root weights at drain sites, whereas C. rotundus and E. alba presented greater root fresh weights at non-polluted sites. In winter, D. bipinnata presented greater root fresh weights at drain sites, whereas C. rotundus presented greater root fresh weights at river sites. The fresh weight of the roots of E. alba was greater at the non-polluted sites in the winter.
Significant changes were found when the fresh and dry weights of the shoots and roots of five different plant species were compared at polluted and non-polluted areas in the summer and winter. In the summer, P. distichum presented relatively high shoot fresh weights at non-polluted sites, whereas D. bipinnata and C. rotundus presented relatively high shoot fresh weights at drain sites. During the winter, C. rotundus had higher root dry weight at the river sites, and D. bipinnata had a greater root dry weight at the non-polluted sites. The shoot fresh weights of E. alba and C. dactylon varied little between sites, whereas the root dry weights of C. dactylon did not significantly differ between sites during either of the two seasons. D. bipinnata had the highest shoot dry weight at drain sites in both seasons, whereas P. distichum had high shoot dry weight at non-polluted sites in summer but no significant differences in winter. C. rotundus had high shoot dry weights at drain sites in summer but equal weights at all sites in winter, and C. dactylon showed no significant differences in shoot dry weight among sites in either season (Fig. 3 A-D).
Fig. 3.
A-D Comparison of means among three areas (non-polluted, drains and River site) for five different morphological traits of studied plants
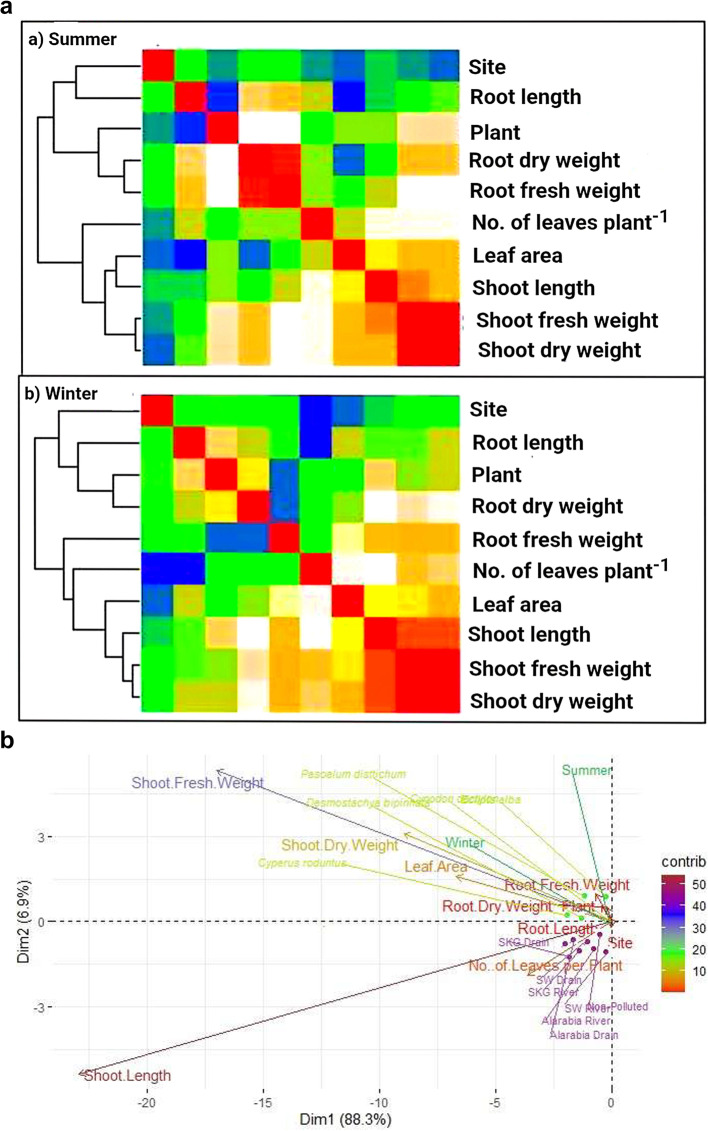
A heatmap analysis of the morphological traits of five plants from seven sites revealed two main groups: one comprising site, plant, and root traits (root length, fresh weight, and dry weight) and the other comprising shoot traits (length, fresh weight, and dry weight) and leaf traits (number of leaves and leaf area) with close resemblance within each group (Fig. 4A). On the other hand, a similar pattern was observed in the heatmap drawn for the data collected in winter, except that shoot length was slightly different from shoot biomass (Fig. 4B).
Fig. 4.
A-B Distance square matrix based heatmaps of morphological traits of five ecotypes collected from seven different sites in summer (a) and winter (b), (A). PCA Biplot of morphological traits of five plants collected from seven different studied sites in summer and winter (B)
Correlations among the morphological, physiological, and proximate traits of the plants
According to a correlation matrix study, root length was positively correlated with carotenoids and morphological growth features throughout the winter but negatively correlated with chlorophyll content. Conversely, with the exception of total chlorophyll, shoot length was highly correlated with the majority of morphological growth characteristics and pigments. Furthermore, the relationships between pigments and the fresh and dry biomass of roots and shoots were comparable to those found for root and shoot length (Fig. 5A).
Fig. 5.
A-B Correlation among morphological and proximate (A) in summer (a) winter (b), morphological and physiological (B) traits of five plants collected from seven different sites in summer (a) winter (b)
In the summer, morphological growth features and pigments were positively correlated with ecotype root and shoot length. However, there were positive correlations with morphological growth features and non-significant negative correlations with pigments in both fresh and dried biomass. In contrast to locations, which presented negative correlations with morphological and pigment traits in both seasons, plants notably presented favourable associations with both traits in both summer and winter (Fig. 5B).
Plants were highly associated with both morphological and proximate traits in summer and winter whereas different sites were negatively related to traits in both summer and winter (Fig. 5 A & B). In winter, the root length and shoot length of studied plants were negatively associated with ash content and total energy. However, fresh and dry biomasses were not significantly negatively associated with proximate contents but were highly associated morphological growth traits (Fig. 5B).
Physiological parameter analysis
Analysis of variance revealed that polluted water and soil significantly affected the physiological growth of the five selected plants in both the summer and the winter seasons at seven different sites. The results revealed that polluted water and soil increased chlorophyll a and affected chlorophyll b, total chlorophyll, and the chlorophyll a/b ratio, with significant variations observed among plants, seasons, and sites. The highest variation was observed for chlorophyll b with respect to season, and the interactions between plants, seasons, and sites were also significant (Table 7).
Table 7.
Analysis of variance (mean squares with significance level) for various pigments of five ecotypes collected from different sites in two seasons
| Source of variation | Df | Mean squares | ||||
|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Total Chlorophyll | Chl a/Chl b | Carotenoids | ||
| Plant | 4 | 0.091*** | 0.0466** | 0.1383*** | 1.4865*** | 0.0114 |
| Site | 6 | 0.077*** | 0.015 | 0.0977*** | 0.8215* | 0.0200 |
| Season | 1 | 0.00002 | 0.5842*** | 0.5910*** | 9.3374*** | 0.0729 |
| Plant x Site | 24 | 0.149*** | 0.0218* | 0.1864*** | 0.6608** | 0.0107 |
| Plant x Season | 4 | 0.0371** | 0.0681*** | 0.1908*** | 1.1742** | 0.0028 |
| Site x Season | 6 | 0.020 | 0.0312* | 0.020 | 0.9911** | 0.0028 |
| Plant x Site x Season | 24 | 0.0253 | 0.0186 | 0.0421* | 0.4963* | 0.0041 |
| Error | 280 | 0.0106 | 0.0132 | 0.0245 | 0.3051 | 0.0084 |
| Total | 349 | |||||
Degree of freedom (df) of sources of variation (SOV) is plant: 5, site: 7, season: 2, *, **, *** significant at < 0.05, < 0.01, < 0.001 alpha respectively
Comparison of the physiological traits of plants
Five plant species (D. bipinnata, P. distichum, C. rotundus, E. alba, and C. dactylon) in polluted and non-polluted areas in the summer and winter presented notable differences in chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents, according to the data. Some plants had relatively high levels of chlorophyll a and b in non-polluted areas, whereas others had relatively high at polluted sites. While C. dactylon and C. rotundus presented high carotenoid contents at river sites in the summer and P. distichum presented relatively high carotenoid contents at non-polluted sites in the winter, D. bipinnata presented the highest total chlorophyll content at river sites in the summer and drain sites in the winter (Fig. 6 A-D).
Fig. 6.
A-D Comparison of means among three areas (non-polluted, drains and river site) for physiological characteristics of selected plant
Relatedness and dispersion among Chloroplast pigments
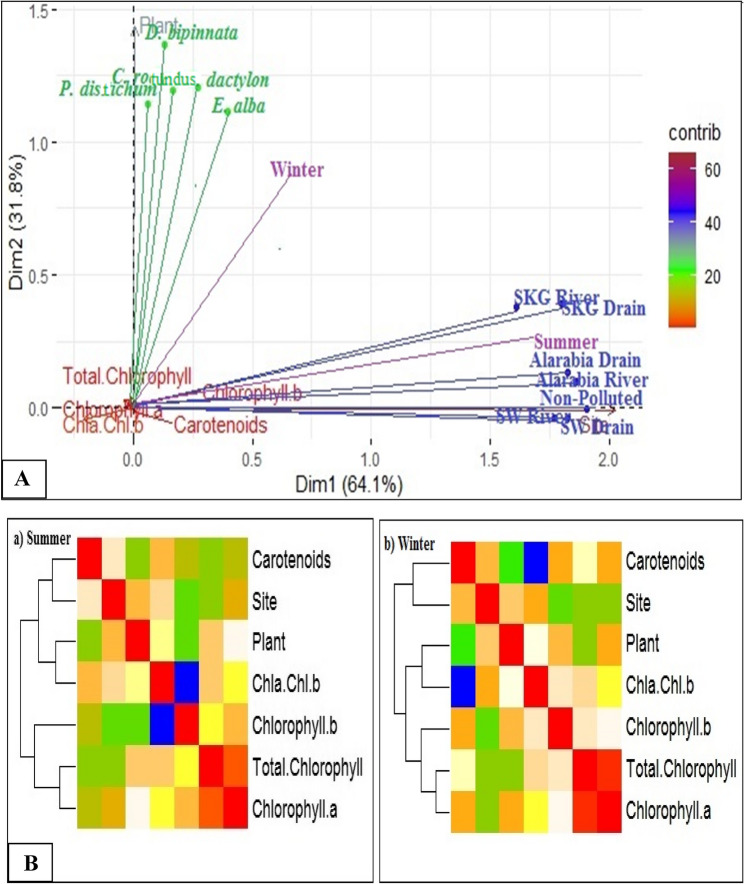
The relatedness and dispersion among the physiological traits of five selected plants collected from seven different sites are shown in heatmaps which were calculated via a distance square matrix. The heatmap drawn for data collected in the summer season shows that the overall data were spread in two groups with various subgroups on the basis of relatedness and dispersion. Sites, plants, and carotenoids were sorted into one group whereas, total chlorophyll, chlorophyll a, and chlorophyll b were sorted in close resemblance were closely related. On the other hand, a similar pattern was observed in the heatmap drawn for the data collected in winter, except that the variation in the winter data was greater than that in the summer data (Fig. 7A).
Fig. 7.
A-B PCA biplots of chloroplast pigments of five plants from different studied sites in summer and winter (A), distance square matrix based heatmaps of physiological traits of five ecotypes from different studied Sites in summer (a) and Winter (b)
Furthermore, variation in the physiological dataset was calculated via principal component analysis as shown in the Biplot. The total variation covered by PC1 was 64.1%, and that covered by PC2 was 31.8% for the physiological traits of five plants collected from seven different sites. A significant positive association was found among the pigment contents which were closely associated with all the plants and sites. Sites were placed in a positive quadrate along the x-axis while ecotypes were along the y-axis on the basis of variation in physiological traits (Fig. 7B).
Comparison of proximate characteristics of selected plants
A study on the biochemical composition of five plant species (E. alba, C. dactylon, D. bipinnata, P. distichum, and C. rotundus) at multiple sites in the summer and winter seasons revealed varying trends. E. alba presented a high total energy content at drain sites in summer and non-polluted sites in winter, whereas C. dactylon and D. bipinnata exhibited no significant differences at all studied sites in either season. Additionally, C. rotundus had a high fiber content at non-polluted sites, followed by drain and river sites in both seasons, and D. bipinnata had a high carbohydrate content at non-polluted sites, followed by drain and river sites, in both seasons. Overall, the study highlights the variations in biochemical composition among plant species and sites, which could be influenced by environmental factors (Fig. 8 A-D).
Fig. 8.
A-D Comparison of mean among three areas and seasons for the proximate analysis of selected plants
Anatomical variations in the stem parameters of the selected plants
Stem radius
A graphical analysis of the stem radius at multiple sites in the summer and winter seasons revealed that the stem radius was greater in winter than in summer. The highest stem radius was observed at the Al Arabia River point source, whereas the minimum was at the SW River point source in both seasons. Among all the selected plants D. bipinnata presented the maximum stem radius among all selected plants, while P. distichum exhibited the highest stem radius at the Al Arabia River point source in both seasons, and the other plants showed varying stem radius patterns across sites and seasons (Figs. 9A & 10).
Fig. 9.
A-F Stacked clustered bar graph of anatomical variation in stem parameters of selected ecotypes in summer and winter
Fig. 10.
Comparison of non-polluted (left) and polluted (right) transverse sections of stem of five plants at seven different study sites
Stem epidermis
Stem epidermal thickness varied more in winter than in summer, with P. distichum showing similar thicknesses at most sites in winter, whereas C. rotundus and D. bipinnata presented maximum thicknesses at different sites in winter and summer. E. alba and C. dactylon also exhibited varying thickness patterns across sites and seasons (Figs. 9B, 10 and 11).
Fig. 11.
Comparison of non-polluted (left) and polluted (right) transverse sections of leaf of five plants at seven different study sites
Stem cortical thickness
D. bipinnata presented the maximum cortical thickness, whereas E. alba presented the minimum. P. distichum and C. rotundus exhibited maximum thickness at different sites in winter and summer, whereas D. bipinnata, E. alba, and C. dactylon presented varying thickness patterns across sites and seasons (Figs. 9C, 10 & 11).
Stem sclerenchyma thickness
E. alba presented the maximum sclerenchyma thickness in winter, whereas C. rotundus presented the minimum thickness. Each plant species exhibited unique patterns of maximum and minimum sclerenchyma thickness at different sites in winter and summer, highlighting the complexity of anatomical adaptations among these species (Figs. 9D, 10 and 11).
Vascular bundle thickness
The maximum vascular bundle thickness was observed in winter and the minimum was observed in summer. Each plant species presented unique patterns of maximum and minimum vascular bundle thickness at different sites in winter and summer, highlighting the complexity of anatomical adaptations among these species (Figs. 9E, 10 and 11).
Stem metaxylem area
The highest metaxylem area was observed for P. distichum in winter, whereas D. bipinnata presented the maximum metaxylem area in summer. Each plant species exhibited unique patterns of maximum and minimum metaxylem areas at different sites in winter and summer, highlighting the complexity of anatomical adaptations among these species (Figs. 9F, 10 & 11).
Discussion
Study of water and soil
Analysis of the variance of water samples collected from seven different sites (non-polluted, polluted rivers, and polluted drains) revealed that the concentrations of all physicochemical parameters were highly significant with respect to site and season. Moreover, the effect of the site-to-season interaction had a significant effect on the concentrations of all the physicochemical parameters of the water samples.
The current study revealed that the EC of the soil and water samples was higher than the FAO standard values, which could seriously harm the nearby vegetation and the crops that are irrigated with these water resources. Kakade [25] also reported the harmful effects of Sugar mill effluents which are responsible for the deterioration of water quality, as the physical and chemical characteristics of river water change and making it unfit for human consumption.
Turck et al. [26]. reported that the water and soil samples contained higher levels of EC, total dissolved solids, sodium absorption ratio, chloride content, and sodium content than did the FAO’s established limits. The high values of these parameters confirmed the salinity stress at the selected sites. The non-polluted site had the lowest COD and BOD values, whereas the Al Arabia drain site had the highest values. Apart from these deductions, it was observed that the levels of iron, manganese, nickel, and chromium exceeded the FAO’s recommended threshold limits [27]. The levels of biochemical oxygen demand (BOD), chemical oxygen demand (COD), and oil and grease were also reported in the sugar mill effluent collected from the mill drains more than their permissible limits [25]. Additionally, soil samples were taken from seven distinct locations, including polluted drains, polluted rivers, and non-polluted areas. With respect to site and season, there was a highly significant variation in all physicochemical parameters.
Study of morphological growth attributes
The exposure of plants to industrial effluents induces different responses, which depend on the intensity and duration of the stress. Understanding the morphological and anatomical changes caused by this stress is crucial for obtaining a comprehensive understanding of how plants develop resistance mechanisms to conditions of metal excess metal [28]. Due to the sessile nature of plants, they are unable to move from one place to another, so they are constantly exposed to a variety of harmful environmental factors, both living and non-living. To survive under these conditions, plants can adapt by changing their morphological features and anatomical structures to better endure these stresses [29].
The morphological characteristics of the stems, roots, and leaves at seven distinct sites in the summer and winter, the contaminated soil and water were significantly affected significant effect on the morphological growth traits of the five selected plants. A thorough statistical analysis of a number of morphological growth traits revealed significant variation in terms of both season and site. These traits included shoot length, root length, leaf area per plant, number of leaves per plant, and root fresh and dry weights. The morphology and anatomy of the stems and leaves of particular plants showed the greatest alterations in growth traits in response to stress. There were notable differences in the cortical thickness, sclerenchymatous tissue thickness, stem radius, vascular bundle thickness, and stem epidermis between the plants and sites [30].
The responses observed are consistent with those of a few other recent studies. For instance, drought stress leads to changes in stem anatomy, including variations in the epidermis, cortex, xylem, and phloem thickness [31–33]. All other morphological growth parameters showed the same pattern and were closely clustered together. Furthermore, every ecotype showed a negative but weak association with sites and a close positive association with all morphological growth attributes in the summer and winter. Furthermore, it was discovered that the morphological growth characteristics and the amounts of carotenoids and photosynthetic pigments were highly correlated with all the plants. A similar trend was observed for all other traits under consideration in winter. These findings are in line with the findings of Gao et al. [34]. and Siqueira-Silva et al. [35].
The lengths of the roots and shoots of the plants were highly correlated with their morphological growth traits and pigments during the summer. However, there was a non-significant negative correlation with pigments and a non-significant positive correlation with morphological growth traits for both fresh and dry biomasses. The plants were highly associated with both morphology and pigments in both seasons, whereas site type was negatively related to the characteristics under consideration in both the summer and the winter. Nauer and colleagues published similar research in 2022 [36].
Numerous studies by Godoy et al. [37]. and Hussain et al. [38]. have demonstrated that plants exhibit significant declines in certain characteristics in response to biotic and abiotic stresses, particularly osmotic and oxidative stresses, which supports the findings of the current study. An increase in the epidermal thickness of plant cells indicates how plants respond to salinity, heavy metal toxicity, and polluted soil and water bodies. Several studies have reported similar increases in additional depositions in plant epidermal layers when plants are exposed to different stress conditions [39–41].
Study of physiological attributes
The oxidative stress caused by a high concentrations of toxic elements in the soil and water negatively impacts the physiological growth of five distinct plant ecotypes that were collected from various carefully chosen sites (both polluted and non-polluted) in the summer and winter. Seasons had a negligible effect on chlorophyll a, whereas plant-to-site and plant-to-season interactions had highly significant effects. The mean square values of chlorophyll a in all the plants presented the greatest variation. The high pH and EC of the soil and water bodies at the polluted sites, due to the high accumulation of alkaline and acidic cations and anions as well as heavy metals were the most detrimental factors that drastically influenced plant growth and metabolism. The findings are in line with similar previous studies on physiological characteristics of plants and grasses [42–44]. Nasircilar et al. [45]. reported that both exposure to heavy metals and salinity stress both cause plants to produce a variety of reactive oxygen species (ROS) in plants, leading to oxidative damage and cellular stress. Salinity stress promotes the accumulation of ROS due to ionic imbalances and reactive oxygen species in cells, ultimately leading to oxidative stress and reduced crop yield [46, 47]. This leads to a high rate of lipid peroxidation, which disturbs the stability and permeability of the cell membrane, ultimately impairing the osmotic balance of the cellular environment. These cellular events work in a cascade and lead to the dysfunction of various metabolic processes [48].
The photosynthetic pigments were increased in the drains and drainage basin areas as compared with those in the other areas. The highest concentration of chlorophyll b was reported at drain sites as compared with the river sites as plant growth flourished along the nutrient rich areas of the drains. Overall, relatively high chlorophyll and carotenoid contents were detected in the plants in this study. The highest chlorophyll a, chlorophyll b, and carotenoid contents were reported in E. alba in the summer season. Two plants, E. alba and D. bipinnata have shown the ability to adapt themselves to polluted sites and survive under high degrees of pollution.
Photosynthetic and other accessory pigments are also damaged by exposure to induced osmotic and oxidative stresses which directly stop photosynthesis [49]. To quantify the degree of oxidative damage that is induced, Del Rio et al. [50]. assessed the rate of lipid peroxidation as well as the production and activity of malondialdehyde, an enzyme that reacts to stress, to quantify the oxidative damage that is induced. Furthermore, under various biotic and abiotic stress conditions, carotenoid pigments play a very important role in the plant defense mechanisms [51].
Study of proximate parameters
The present study reported that contaminated soil and water had a significant effect on the dry matter content in terms of plants and sites, however the effect was not significant in terms of season. While the season to site had no discernible effect and the moisture percentage showed a similar pattern, the effects of the plant-to-site and plant-to-season interactions on the dry matter were highly significant. In terms of plants, sites, and seasons, the mean square values for ash and crude fibers were highly significant.
Crude fats were important in terms of location, but not in terms of plant or season. There were also notable interactions observed between the plants and various combinations, sites, and seasons of these elements. Donhouedé et al. [52] reported that the moisture, fiber, and ash contents of plant and vegetable species are critical to soil health and human well-being. Proximate analysis offers useful information and helps assess sample quality in plants. Ash is an indicator of the total amount of minerals present because it is the inorganic residue left over after heating water and organic matter to remove them [53].
The results revealed increased production of crude fibers in D. bipinnata in response to stress conditions and the production of large amounts of carbohydrates by E. alba under nutrient-rich conditions. Among the plants under study, P. distichum presented a significantly greater concentration of fat accumulation in response to polluted conditions. Similarly, D. bipinnata and E. alba accumulated the highest quantity of protein at the drains, which suggests their greater adaptability to the industrially polluted environment in the study area.
Study of anatomical attributes
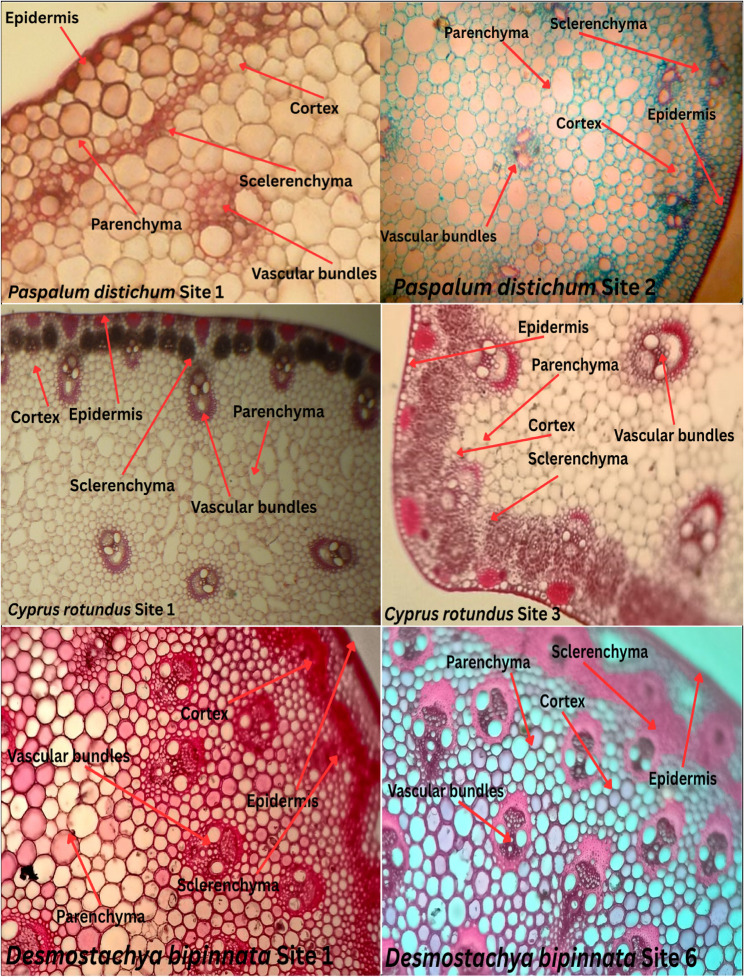
A recent study revealed that among all the plants, both C. dactylon and P. distichum presented the thickest vascular bundles, suggesting their ability to adapt to their environment. This finding is consistent with that of Soumya et al. [28], who highlighted the morpho-anatomical responses of plants to heavy metal stress.
The main areas of anatomical changes occur in the vasculature and surrounding tissues when heavy metals enter the stem from the root via vascular tissue, primarily the xylem [54]. According to Liza et al. [55], stem diameter decreased in plants exposed to Cd, primarily as a result of smaller cells and vascular components. Comparing with those of the control plants, the stem epidermal cells of the Control site plants were thicker and had more multicellular glandular trichomes. There were fewer small-sized vessels with phenolic compounds inside them at higher concentrations of Cd because of to the thin cambium ring.
The stem cortex width, cortical cell row number, and vascular bundle number all decrease with increasing Zn concentration [56]. As concentrated stems presented deformed epidermis, blackening of cortex cell walls, large pith, diminished and damaged vascular bundles, and reduced intercellular spaces, Gautam [57], reported.
Conclusion
This study comprehensively assessed the toxic effects of industrial effluent discharge on the riparian vegetation along the Jhelum River in the Sargodha district of Pakistan. The results revealed that polluted water and soil, particularly from sugar mill drains, exhibit significantly altered physico-chemical properties, including elevated salinity, pH, electrical conductivity, and heavy metal concentrations, which collectively impair plant health and soil fertility. However, certain native riparian species, notably D. bipinnata, E. alba, and P. distichum, presented important physiological, biochemical, and anatomical adaptations under these stress conditions. These adaptations included increased production of chlorophyll and carotenoids, increased accumulation of crude proteins and fibers, and morpho-anatomical modifications such as stem sclerification and vascular thickening. The correlation analyses further indicated that despite adverse environmental conditions, some studied plants maintained positive associations between physiological traits, biomass accumulation, and biochemical resilience. Such species can serve as ecological indicators and potential candidates for phytoremediation and riparian restoration efforts. The findings underscore the urgent need for implementing stricter environmental regulations and pollution mitigation strategies in industrial regions. Additionally, preserving and utilizing tolerant native plant species could play a pivotal role in maintaining riparian ecosystem integrity and resilience under escalating anthropogenic stress.
Supplementary Information
Acknowledgements
The authors extended their appreciation to the Dean ship of Research and Graduate Studies at King Khalid University for the funding this work through Large Research Project under grant number RGP2/204/46.
Voucher specimens
Voucher specimens of all five collected plant species (Eclipta alba, Desmostachya bipinnata, Cynodon dactylon, Paspalum distichum, and Cyperus rotundus) were prepared and authenticated. The specimens were identified by Dr. Abdul Ghani (Associate Professor, University of Sargodha) and Dr. Mansoor Hameed (Professor, University of Agriculture, Faisalabad). Herbarium sheets (Voucher numbers: R2-D123 to R2-D134) are deposited at the Department of Botany Herbarium, University of Sargodha, Sargodha, Pakistan. Contact person: Mr. Muhammad Ali (+ 92 303 6200205).
Authors’ contributions
A.S., collected and analyzed the samples; K.A., and Z.I.K., supervised the study; A.K, revised the original draft and design the graphs; I.A., worked on methodology and reviewed the article. All authors contributed in the manuscript.
Funding
Researchers supporting the project under grant number RGP2/204/46 at King Khalid University, Saudi Arabia.
Data availability
Data are provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The plants were collected from drains and Jhelum River sites at Sargodha, Pakistan (public land) and governing body Higher Education Commission, Pakistan and University of Sargodha, Sargodha, Pakistan gave its approval for the collection of samples as per Ethics and Guideline Committee of the University of Sargodha (Approval No. 778-D103/2017 UOS). All the experimental methods of this study followed all the appropriate guidance and regulations. The manuscript is part of PhD thesis of Anum Sajid and all the protocols and experimental procedure used in this investigation were approved by Board of Studies of University of Sargodha, Pakistan and Higher Education Commission of Pakistan.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xia Y, Zhang M, Tsang DC, Geng N, Lu D, Zhu L, Igalavithana AD, Dissanayake PD, Rinklebe J, Yang X, Ok YS. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: current practices and future prospects. Appl Biol Chem. 2020;63:1–13. [Google Scholar]
- 2.Lind L, Hasselquist EM, Laudon H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J Environ Manage. 2019;249:109391. [DOI] [PubMed] [Google Scholar]
- 3.Hill AR. Groundwater nitrate removal in riparian buffer zones: a review of research progress in the past 20 years. Biogeochemistry. 2019;143:347–69. [Google Scholar]
- 4.Wang M, Duan L, Wang J, Peng J, Zheng B. Determining the width of lake riparian buffer zones for improving water quality base on adjustment of land use structure. Ecol Eng. 2020;158:106001. [Google Scholar]
- 5.Sarker B, Keya KN, Mahir FI, Nahiun KM, Shahida S, Khan RA. Surface and ground water pollution: causes and effects of urbanization and industrialization in South Asia. Sci Rev. 2021;7(3):32–41. [Google Scholar]
- 6.Yi X, Lin D, Li J, Zeng J, Wang D, Yang F. Ecological treatment technology for agricultural non-point source pollution in remote rural areas of China. Environ Sci Pollut Res. 2021;28:40075–87. [DOI] [PubMed] [Google Scholar]
- 7.Singh BP, Samanta P, Choudhury M, Gupta P, Chadha U, Eticha TK. Physicochemical and heavy metal pollution level in hindon river ecosystem: an implication to public health risk assessment. Environ Qual Manage. 2024;34(2):e22263. [Google Scholar]
- 8.Nasiruddin M, Islam ARMT, Siddique MAB, Hasanuzaman M, Hassan MM, Akbor MA, Hasan M, Islam MS, Khan R, Al Amin M, Pal SC. Distribution, sources, and pollution levels of toxic metal (loid) s in an urban river (Ichamati), Bangladesh using SOM and PMF modeling with GIS tool. Environ Sci Pollut Res. 2023;30(8):20934–58. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Arora R, Mehra N. Persistence of heavy metals in river Sirsa around industrial hub baddi, India. Curr World Environ. 2023;18(1):289. [Google Scholar]
- 10.Dong W, Niu B, Li H, Yan D, Li J, Xu Z, Wang D, Yang X, Zhang Y, Chen Y, Wang H. Sources, contamination and risk assessment of heavy metals in riparian soils of the Weihe river based on a receptor model and Monte Carlo simulation. Sustainability. 2024;16(23):10779. [Google Scholar]
- 11.Fanton H, Affre L, Franquet E, Bertrand C, Cavalli L, Dumas E, Guiller C, Kaldonski N, Meineri E, Mutillod C, Oursel B. Heavy ionic pollution disrupts assemblages of algae, macroinvertebrates and riparian vegetation. Environ Pollut. 2023;331:121791. [DOI] [PubMed] [Google Scholar]
- 12.Abbas T, Ahmad I, Khan ZI, Shah AA, Casini R, Elansary HO. Stress mitigation by riparian flora in industrial contaminated area of river Chenab punjab, Pakistan. PeerJ. 2023;11:e15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabir MA, Nawaz MF, Khan TH, Zulfiqar U, Naseer J, Hussain S, Gul S, Maqsood MF, Iqbal R, Ali B, Roy R. Impact of dust load and lead (Pb) stress on leaf functioning of urban vegetation. Turkish J Agric Forestry. 2023;47(5):713–26. [Google Scholar]
- 14.Neves NR, Oliva MA, da Cruz Centeno D, Costa AC, Ribas RF, Pereira EG. Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Sci Total Environ. 2009;407(12):3740–5. [DOI] [PubMed] [Google Scholar]
- 15.Heckmann M, Stadlbauer V, Drotarova I, Gramatte T, Feichtinger M, Arnaut V, Atzmüller S, Schwarzinger B, Röhrl C, Blank-Landeshammer B, Weghuber J. Identification of oxidative-stress-reducing plant extracts from a novel extract library, comparative analysis of cell-free and cell-based in vitro assays to quantitate antioxidant activity. Antioxidants. 2024;13(3):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Araújo TO, de Freitas-Silva L, Santana BVN, Kuki KN, Pereira EG, Azevedo AA, da Silva LC. Morphoanatomical responses induced by excess iron in roots of two tolerant grass species. Environ Sci Pollut Res. 2015;22:2187–95. [DOI] [PubMed] [Google Scholar]
- 17.Ejaz U, Khan SM, Khalid N, Jehangir S, Shah SFA, Svenning JC. Elucidating the phytoremediation potentials and ecophysiological mechanisms of indicator plants in the industrial polluted region. J Environ Manage. 2024;366:121821. [DOI] [PubMed] [Google Scholar]
- 18.Janssen P, Couloigner C, Piégay H, Evette A. The accumulation of anthropogenic stressors induces a progressive shift in the ecological preferences and morphological traits shared by riparian plant communities. Freshw Biol. 2023;68(11):1981–94. [Google Scholar]
- 19.Dubey R, Gupta DK, Sharma GK. 2020. Chemical stress on plants. New frontiers in stress management for durable agriculture, pp.101–128.
- 20.Graziano MP, Deguire AK, Surasinghe TD. Riparian buffers as a critical landscape feature: Insights for riverscape conservation and policy renovations. Diversity. 2022;14(3):172. [Google Scholar]
- 21.Ayub F, Hanan Legharisk, Ahmed A, Baloch S, Ismail A, T. and, Ponya Sga. 2022. Evaluation of saffron (Crocus sativus L.) cultivars grown under agro Climatic conditions of Quetta valley, balochistan, Pakistan. Plant Cell Biotechnol Mol Biology, pp.17–30.
- 22.Bruinsma J. Absorption of light by chlorophyll a and b in plant extracts. Photochem Photobiol. 1963;2:241–9. [Google Scholar]
- 23.Association of official analytical chemists (AOAC). (2006). Official Methods of Analysis. 18th Edition, Gaithersburgs, MD: p. 1.
- 24.Ruzin SE. Plant microtechnique and microscopy. Volume 198. New York: Oxford University Press; 1999. p. 322. [Google Scholar]
- 25.Kakade P. Study of Physico- chemical parameters of sugar industry effluent. Int J Environ Agric Biotechnol. 2022;7(4):172–7. [Google Scholar]
- 26.Turck D, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Kearney J, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Pelaez C. EFSA panel on nutrition, novel foods and food allergens (NDA), dietary reference values for sodium. EFSA J. 2019;17(9):e05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latif A, Bilal M, Asghar W, Azeem M, Ahmad MI, Abbas A, Ahmad MZ, Shahzad T. Heavy metal accumulation in vegetables and assessment of their potential health risk. J Environ Anal Chem. 2018;5(234):2380–91. [Google Scholar]
- 28.Soumya V, Kiranmayi P, Kumar KS. Morpho-anatomical responses of Catharanthus roseus due to combined heavy metal stress observed under scanning Electron microscope. Plant Sci Today. 2022;9(3):623–31. [Google Scholar]
- 29.Shehzadi A, Akram NA, Ali A, Ashraf M. Exogenously applied Glycinebetaine induced alteration in some key physio-biochemical attributes and plant anatomical features in water stressed oat (Avena sativa L.) plants. J Arid Land. 2019;11(2):292–305. [Google Scholar]
- 30.Martin RA, da Silva CR, Moore MP, Diamond SE. When will a changing climate outpace adaptive evolution? Wiley Interdisciplinary Reviews: Clim Change. 2023;14(6):e852. [Google Scholar]
- 31.Pasaribu SA, Basyuni M, Purba E, Tistama R. 2022. Growth and leaf anatomy of rubber clones (Hevea brasiliensis Muell. Arg.) IRR 400 series toward drought stress. In IOP Conference Series: Earth and Environmental Science, 977(1),012042. IOP Publishing.
- 32.Hemon AF, Dewi SM, Susilowati LE, Gunawan BW. 2022. Changes in the anatomical characters of root and stem of three large-seeded soybean (Glycine max (L.) Merrill) under drought stress. In IOP Conference Series: Earth and Environmental Science (Vol. 1107, No. 1, p. 012031). IOP Publishing.
- 33.Yao XC, Meng LF, Zhao WL, Mao GL. Changes in the morphology traits, anatomical structure of the leaves and transcriptome in Lycium barbarum L. under salt stress. Front Plant Sci. 2023;14:1090366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao T, Wang H, Li C, Zuo M, Wang X, Liu Y, Yang Y, Xu D, Liu Y, Fang X. Effects of heavy metal stress on physiology, hydraulics, and anatomy of three desert plants in the Jinchang mining area, China. Int J Environ Res Public Health. 2022;19(23):15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siqueira-Silva AI, Pereira EG, Modolo LV, Lemos-Filho JP, Paiva EAS. Impact of cement dust pollution on Cedrela fissilis Vell.(Meliaceae): A potential bioindicator species. Chemosphere. 2016;158:56–65. [DOI] [PubMed] [Google Scholar]
- 36.Nauer F, Cabral Oliveira M, Plastino EM, Yokoya S, N., Toyota Fujii M. Thermal tolerance of Hypnea pseudomusciformis ecotypes (Cystocloniaceae, Rhodophyta) related to different floristic provinces along the Brazilian Coast. Phycological Res. 2022;70(2):108–17. [Google Scholar]
- 37.Godoy-Gallardo M, Eckhard U, Delgado LM, de Roo Puente YJ, Hoyos-Nogués M, Gil FJ, Perez RA. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioactive Mater. 2021;6(12):4470–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain A, Abbas N, Arshad F, Akram M, Khan Z, Ahmad K, Mansha M, Mirzaei F. Effects of diverse doses of lead (Pb) on different growth attributes of Zea-Mays L. Agricultural Sci. 2013;4:262–5. [Google Scholar]
- 39.Haddadi BS, Hassanpour H, Niknam V. Effect of salinity and waterlogging on growth, anatomical and antioxidative responses in Mentha aquatica L. Acta Physiol Plant. 2016;38:1–11. [Google Scholar]
- 40.Abbas T, Ahmad I, Khan ZI, Okla MK, Saleh IA, AbdElgawad H. Comparative physiological adaptations to industrial pollution stress mediated by melatonin in riparian vegetation and Phyla nodiflora an ornamental plant. Sci Hort. 2023;321:112367. [Google Scholar]
- 41.Gatasheh MK, Abbas T, Shaffique S, Kang SM, Lee IJ, Shah AA. Comparative analysis of biodiversity, physiology, and anatomical adaptations in riparian flora exposed to industrial pollution stress. Scientif Rep. 2025;15(1):3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rios CO, Siqueira-Silva AI, Pereira EG. How does drought affect native grasses’ photosynthesis on the revegetation of iron ore tailings? Environ Sci Pollut Res. 2021;28:14797–811. [DOI] [PubMed] [Google Scholar]
- 43.Alnaimy MA, Elrys AS, Zelenakova M, Pietrucha-Urbanik K, Merwad ARM. The vital roles of parent material in driving soil substrates and heavy metals availability in arid alkaline regions: A case study from Egypt. Water. 2023;15(13):2481. [Google Scholar]
- 44.Abbas T, Ahmad I, Khan ZI, Ahmad K. Micromorphological and anatomical responses of native dicots to industrial effluents released from contaminated region of the Chenab river in Pakistan. SABRAO J Breed Genet. 2023;55(4):1222–44. [Google Scholar]
- 45.Nasircilar AG, Ulukapi K, Topcuoglu B, Kurubas S, Erkan M. Salt and heavy metal stress responses and metal uptake potentials of some leafy vegetables. Agrosystems Geosci Environ. 2024;7(1):e20487. [Google Scholar]
- 46.Anwar A, Zhang S, Lilong HE, Jianwei GAO. Understanding the physiological and molecular mechanism of salinity stress tolerance in plants. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2022;50(4):12959–12959. [Google Scholar]
- 47.Pereira EG, Oliva MA, Kuki KN, Cambraia J. Photosynthetic changes and oxidative stress caused by iron ore dust deposition in the tropical CAM tree Clusia hilariana. Trees. 2009;23:277–85. [Google Scholar]
- 48.Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21(4):204–24. [DOI] [PubMed] [Google Scholar]
- 49.Moharramnejad S, Sofalian O, Valizadeh M, Asgari A, Shiri M. Proline, glycine betaine, total phenolics and pigment contents in response to osmotic stress in maize seedlings. J Bioscience Biotechnol. 2015;4(3):313-319.
- 50.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metabolism Cardiovasc Dis. 2005;15(4):316–28. [DOI] [PubMed] [Google Scholar]
- 51.Kaur S, Tiwari V, Kumari A, Chaudhary E, Sharma A, Ali U, Garg M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: an emerging application in sustainable agriculture. J Biotechnol. 2023;361:12–29. [DOI] [PubMed] [Google Scholar]
- 52.Donhouedé JC, Salako KV, Assogbadjo AE, Ribeiro-Barros AI, Ribeiro N. The relative role of soil, climate, and genotype in the variation of nutritional value of Annona senegalensis fruits and leaves. Heliyon. 2023;9(8). 10.1016/j.heliyon.2023.e19012. [DOI] [PMC free article] [PubMed]
- 53.Frances E, Enoch N, Johnson O, Ann M, Eziamaka AE. Determination of proximate and phytochemical composition of three species of beans sold in Uli. Asian J Food Res Nutr. 2023;2(4):418–29. [Google Scholar]
- 54.Pita-Barbosa A, Gonçalves EC, Azevedo AA. Morpho-anatomical and growth alterations induced by arsenic in Cajanus cajan (L.) DC (Fabaceae). Environ Sci Pollut Res. 2015;22:11265–74. [DOI] [PubMed] [Google Scholar]
- 55.Liza SJ, Shethi KJ, Rashid P. Effects of cadmium on the anatomical structures of vegetative organs of Chickpea (Cicer arientinum L). Dhaka Univ J Biol Sci. 2020;29(1):45–52. [Google Scholar]
- 56.Pandey AK, Zorić L, Sun T, Karanović D, Fang P, Borišev M, Wu X, Luković J, Xu P. The anatomical basis of heavy metal responses in legumes and their impact on plant–rhizosphere interactions. Plants. 2022;11(19):2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautam PK, Gautam RK, Banerjee S, Chattopadhyaya MC, Pandey JD. Heavy metals in the environment: fate, transport, toxicity and remediation technologies. Nova Sci Publishers. 2016;60:101–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided within the manuscript or supplementary information files.