Abstract
Staphylococcus epidermidis is a commensal of human skin and a leading cause of nosocomial bloodstream infections. Limited information is available about S. epidermidis proteins that are expressed upon transition to the bloodstream or those involved in host-pathogen interactions. A cell surface fraction from S. epidermidis 0-47 grown in rabbit serum to mimic environmental signals encountered during a bloodstream infection was separated by two-dimensional (2D) gel electrophoresis. Following 2D separation, the proteins were transferred to nitrocellulose and detected with either pooled sera generated in rabbits immunized with live S. epidermidis 0-47 or with biotin-labeled serum proteins eluted from the surface of bacteria grown in rabbit serum. Twenty-nine immunoreactive or serum binding proteins of S. epidermidis were identified by mass spectrometry. Twenty-seven of the corresponding genes were expressed in Escherichia coli, and the purified recombinant proteins were used to immunize mice. In a preliminary screen, 12 of the 27 recombinant proteins induced a response that reduced the number of bacteria recovered from the spleen or bloodstream of infected mice. In subsequent vaccination studies, 5 of the 12 proteins resulted in a statistically significant reduction in the number of bacteria. The identification of five candidate vaccine antigens from the initial screen of only 29 proteins demonstrates the utility of this approach.
Staphylococcus epidermidis is a major component of the normal human microbial flora on the skin and mucous membranes and was once considered only a contaminant when cultured from an infected patient (16, 40). It is now widely accepted to be an opportunistic pathogen of great importance and a leading cause of nosocomial bloodstream infections (1, 7, 8). S. epidermidis infections are primarily associated with the placement of an indwelling device such as a venous catheter, prosthetic joint, or prosthetic heart valve (16, 40). Infection is thought to result from introduction of S. epidermidis upon insertion of the prosthetic device. Colonization and subsequent biofilm formation can lead to bacteremia with potential for spread to other sites in the body. These infections are often difficult to treat, due to the reduced bactericidal activity of antibiotics within a biofilm and an increase in antibiotic resistance among clinical isolates (7, 8, 20, 27). S. epidermidis with reduced susceptibility to vancomycin has been reported (32, 33). The difficulty of treating these infections places ever more importance on the development of a vaccine capable of preventing infection.
Biofilm formation plays a major role in the pathogenesis of S. epidermidis infections. Consequently, research on S. epidermidis surface components has focused on those involved in biofilm formation. These molecules have been divided into groups based on their involvement or proposed role in the two major steps of biofilm formation: (i) primary attachment, staphylococcal surface protein-1, autolysin E (AtlE), Fbe (SdrG), and GehD; and (ii) bacterial cell accumulation, polysaccharide intercellular adhesin or poly-N-acetylglucosamine, Bap homologous protein, and accumulation-associated protein (AAP) (2, 6, 18, 23, 31, 37, 40, 41). Surface molecules such as those involved in biofilm formation (i.e., poly-N-acetylglucosamine or polysaccharide intercellular adhesin), the recently identified poly-gamma-dl-glutamic acid capsule, and other surface proteins offer potential targets for effective vaccine antigens (19, 26, 28, 36).
In the present work, cell wall-associated proteins were isolated from S. epidermidis after growth in serum. Following separation by two-dimensional (2D) electrophoresis, the proteins were transferred to membranes and analyzed for reactivity with sera from rabbits immunized with live S. epidermidis. Similar 2D gels were used for Western ligand analysis probed with biotin-labeled, immunoglobulin G (IgG)-depleted rabbit serum components to identify putative bacterial receptors that bind host factors. Duplicate 2D gels were used to identify the corresponding spots, and the S. epidermidis proteins were identified by mass spectrometry (MS). Experiments were conducted to determine if the identified antigens elicited an immune response that reduced colonization in a murine challenge model. Five proteins were identified that resulted in a significant reduction of colonization in a mouse model of infection.
MATERIALS AND METHODS
Bacterial strains and chemicals.
Studies were performed with the clinical isolate S. epidermidis 0-47 (14), for which a genomic sequence is available (Incyte, Palo Alto, CA). Bacteria were grown overnight in tryptic soy broth (TSB) or 70% rabbit serum (Life Technologies, Rockville, MD) in TSB with shaking (200 rpm) at 37°C. Bacteria were diluted from an overnight culture to an optical density at 600 nm (OD600) of ∼0.1 and grown for an additional 4 h in the same medium. Cells were then harvested by centrifugation and further processed.
All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise.
Preparation of cell wall fraction for 2D electrophoresis.
Pelleted bacteria were resuspended to an OD600 of ∼40 and washed three times with ice-cold Tris-buffered saline (TBS; 20 mM Tris [pH 8.0], 150 mM NaCl), followed by a wash for 10 min at 4°C with 20 mM Tris, pH 8.0, containing 0.5 M NaCl to remove surface-bound serum proteins from the bacteria grown in serum. Bacteria grown in TSB were treated in the same manner as the bacteria grown in serum. The bacteria were again pelleted by centrifugation and resuspended to OD600 of ∼40 in TBS containing 30% sucrose, 100 μg/ml lysostaphin, 10 μg/ml DNase, 1 μg/ml Pefablock (Boehringer Mannheim, Indianapolis, IN), 10 μg/ml lysozyme, and 100 U/ml mutanolysin and incubated at 37°C for 1 h. The protoplasts were pelleted by centrifugation at 3,000 × g for 10 min and the supernatant was decanted, supplemented with Complete Mini protease inhibitor tablets (Roche Diagnostics, Indianapolis, IN), and dialyzed overnight against water at 4°C using a 10,000 molecular weight cutoff dialysis membrane (Pierce Biotechnology, Inc., Rockford, IL). Following dialysis, the cell wall fractions were frozen at −20°C.
In preparation for 2D gel electrophoresis, the frozen cell wall extracts were thawed and precipitated with 70% acetone on ice for 4 h. The protein precipitate was pelleted, dried in a SpeedVac (Thermo Savant, Holbrook, NY), and solubilized with ReadyPrep (Bio-Rad) sequential extraction reagent 3 (5 M urea, 2 M thiourea, 2% [wt/vol] CHAPS, 2% [wt/vol] SB 3-10, 40 mM Tris, 0.2% Bio-Lyte 3/10). {CHAPS is 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate.}
Two-dimensional gel electrophoresis.
Immobilized pH gradient (IPG; 11 cm) strips (pH 4 to 7; Bio-Rad) were rehydrated overnight at room temperature with 250 μg of solubilized cell wall extract in a total volume of 200 μl. The strips were covered with mineral oil (Bio-Rad) to prevent evaporation. Following rehydration of the strips, the excess mineral oil was removed on blotting paper saturated with water, and the strips were loaded into a pHaser IEF apparatus (Genomic Solutions, Inc., Ann Arbor, MI). The strips were prefocused with a current limit of 50 mA/strip with the voltage gradually increased from 250 V to 2.8 V. Voltage was then held constant at 2,800 kV for a total of 50 kVh. Second-dimension sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using 12.5% Criterion precast gels (Bio-Rad). For analysis by mass spectrometry, gels were stained with Sypro Ruby protein gel stain (Bio-Rad) according to the manufacturer's instructions.
Gels were blotted to nitrocellulose (Bio-Rad) using a semidry blotting apparatus (Owl Separations Systems, Portsmouth, NH) at 12 V for 1 h. The blots were then stained with Sypro Ruby protein blot stain following the manufacturer's instructions and visualized in a FluorS Imager (Bio-Rad). The blot was incubated in blocking buffer (phosphate-buffered saline [PBS] with 0.05% Tween 20 and 5% dry milk) for 10 min at room temperature and then probed overnight with either a 1:2,000 dilution of immune rabbit sera or 40 μg/ml biotinylated serum proteins (see below). Following overnight incubation, blots were washed three times with wash buffer (PBS with 0.5% Tween 20) and incubated with either goat anti-rabbit IgG-alkaline phosphatase conjugate (Biosource International, Camarillo, CA) or streptavidin alkaline phosphatase conjugate (Biosource) for 2 h at room temperature in blocking buffer. Blots were again washed three times in wash buffer and visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium membrane phosphatase substrate system (KPL, Inc., Gaithersburg, MD). Pictures were taken with the FluorS Imager. All analysis of 2D gels was done using Melanie 3.0 software (Bio-Rad).
Protein concentrations were determined using the Bio-Rad protein assay kit.
Challenge of rabbits with S. epidermidis.
Three female New Zealand white rabbits (each, 2.2 kg) with sera that did not exhibit reactivity to S. epidermidis whole-cell lysates upon Western blot analysis were repeatedly immunized with 109 CFU S. epidermidis 0-47 (log-phase cells grown in TSB) by intraperitoneal injection to produce hyperimmune sera, as measured by Western blotting against a whole-cell lysate of S. epidermidis 0-47. The animals were then bled, and the resulting antisera were used in the present studies.
Elution of serum proteins from S. epidermidis.
S. epidermidis 0-47 was grown in 70% rabbit serum at 37°C to OD600 of ∼0.8, and the cells were pelleted. The cells were resuspended at OD600 of ∼40 and washed three times with TBS while being rocked at 4°C for 10 min. The bound serum proteins were eluted sequentially with 20 mM Tris, pH 8.0, containing either 0.5 M NaCl, 1.0 M NaCl, or 4.0 M urea for 1 h with rocking at 4°C. The bacteria were then removed by centrifugation, and the supernatants containing the serum proteins were eluted from the surface of the bacteria collected. Proteins eluted under the different conditions were analyzed by SDS-PAGE with 4 to 20% gradient Tris-glycine gels (Cambrex Biosciences Rockland, Inc., Rockland, ME) and stained with the Owl Separation Systems silver stain kit.
Biotinylation of serum proteins.
Serum proteins eluted with 0.5 M NaCl were dialyzed overnight against PBS at 4°C. The IgG component in the eluted fraction was depleted by overnight incubation with protein G-Sepharose (Amersham-Pharmacia, Piscataway, NJ) at 4°C. Assuming an average protein mass of 50 kDa in the eluted fraction, the proteins were labeled with a 15-fold molar excess of EZ-Link NHS-biotin (Pierce Biotechnology) for 1.5 h at 4°C. The reaction was quenched with excess glycine and dialyzed (10,000 molecular weight cutoff, Pierce) overnight against PBS.
In-gel tryptic digestion of proteins.
Protein spots were removed from the gel and cut into ∼1-mm pieces. The gel pieces were washed three times, dehydrated with acetonitrile, dried in a SpeedVac, and stored frozen at −20°C. The gel pieces were then thawed and subjected to in-gel tryptic digestion overnight at 37°C with sequencing-grade modified trypsin (Promega Corporation, Madison, WI) at 12.5 ng/ml. The trypsin digest solution was then removed, and the remaining peptides were extracted from the gel pieces. This combined solution was then loaded onto a Zip-Tip C18 P10 column (Waters Corporation, Milford, MA), and peptides were eluted in 5 to 10 μl of 50% acetonitrile-0.1% formic acid. Samples were transferred to a 96- by 2-well Teflon-coated stainless steel plate (PerSeptive Biosystems, Framingham, MA) for mass fingerprinting analysis on the matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) instrument (PerSeptive Biosystems) or glass nanospray tips (New Objective, Inc., Woburn, MA) to be sprayed into the ion trap mass spectrometer (LCQ Deca XP; ThermoFinnigan, San Jose, CA).
Peptide mass fingerprinting mass spectrometry.
Sample was applied to the Teflon coated stainless steel plate with the α-cyano-4-hydroxycinnamic acid thin-layer application. Mass spectral data was acquired with a Voyager DE-STR MALDI-TOF mass spectrometer (PerSeptive Biosystems) equipped with delayed extraction technology and a reflector. The settings were as follows: accelerating voltage (20 kV), mode of operation (reflector), extraction mode (delayed), polarity (positive), grid voltage (65%), mirror voltage ratio (1.12), extraction delay time (200 ns), mass range (800 to 3,500 Da), and laser shots per spectrum (200).
Static nanospray ion trap-mass spectrometry.
Mass spectral data were acquired with an LCQ DECA quadrupole ion trap mass spectrometer (ThermoFinnigan) equipped with a nanoelectrospray interface. The static nanospray needle interface (2-μm orifice diameter) delivered a flow of 20 to 80 nl/min.
Peptide analyses were conducted on the LCQ-DECA ion trap mass spectrometer (ThermoFinnigan) operating at a spray voltage setting from 0.8 to 1.2 kV and using a heated capillary temperature of 200°C. Data sets were acquired in an automated tandem MS (MS/MS) mode. The acquisition method included one MS scan (400 to 1,800 m/z), followed by MS/MS scans of the top three most abundant ions in the MS scan.
Database search for protein identification.
Automated analysis of mass fingerprinting data was performed using MSFIT (Protein Prospector) and MASCOT (Matrix Science) software database search engines using Incyte's PathoSeq S. epidermidis 0-47 database. MSFIT and MASCOT database search parameters were set at comparable levels. Protein identifications were determined by MOWSE score, and a 95% confidence score was determined by MSFIT and MASCOT, respectively.
Automated analysis of MS/MS data was performed using SEQUEST (9) incorporated into the Finnigan Bioworks data analysis package (ThermoFinnigan) to query Incyte's PathoSeq S. epidermidis 0-47 database. Primary protein identification was first determined by observing the Xcorr (generally, >2.5), dCN (>0.10), rSP (<10), and SP (generally, >500) values of the uniquely matched peptides as designated by SEQUEST. The peptides' fragmentation spectra were then manually verified. Protein identification was also dependent on the number of unique peptides and total number of matched peptides.
Cloning and expression of recombinant proteins.
Genes were cloned using primers designed based on the sequence of the S. epidermidis 0-47 database. The genes were amplified by PCR using Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA), and adenine overhangs were added with Taq DNA polymerase (Roche Diagnostics). The reaction products were cloned into pCRT7/NT-TOPO or pBAD/TOPOThio (Invitrogen, Carlsbad, CA) following the manufacturer's instructions and transformed into E. coli Top10 (Invitrogen). Positive clones were detected by colony PCR using ReddyMix PCR mastermix (ABgene, Rochester, NY) and sequenced to ensure that there were no spurious mutations. Plasmids from pCRT7 clones were purified and transformed into E. coli BL21(DE3) (Invitrogen) for expression. Proteins were expressed by growth of the positive clones in HySoy broth (1% HySoy [Quest, Int., Stockbridge, GA], 0.5% yeast extract, 100 mM NaCl, 50 mM Na2HPO4 · 7H2O, 40 mM NaH2PO4 · H2O) supplemented with 100 μg/ml ampicillin at 37°C with shaking (200 rpm) until OD600 was ∼1.0. Protein expression was induced with either 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (pCRT7) or 0.2% arabinose (pBAD), and the cultures were grown for an additional 3 h. The cells were then harvested by centrifugation, and expression was assessed by SDS-PAGE of whole-cell lysates.
Purification of recombinant proteins.
Cell pellets were resuspended in 100 ml of TBS (20 mM Tris [pH 8.0], 150 mM NaCl) and lysed by one passage through a French pressure cell (SLM-Aminco, Rochester, NY). Samples were then separated into a soluble fraction or insoluble pellet by centrifugation at 10,000 × g for 10 min. Location of recombinant protein was assessed by SDS-PAGE. If soluble, protein was loaded onto iminodiacetic acid agarose resin charged with Ni2+. The column was washed with 30 mM imidazole in TBS. Bound proteins were eluted with 300 mM imidazole in TBS. If an additional purification step was required, the proteins were dialyzed into 20 mM Tris (pH 8.0), 50 mM NaCl, and 1 mM EDTA and loaded onto a column packed with POROS-Q resin (Applied Biosystems, Foster City, CA). Bound proteins were eluted with a 50 mM to 500 mM NaCl gradient in 20 mM Tris (pH 8.0)-1 mM EDTA. Fractions containing the protein of interest were determined by SDS-PAGE and frozen at −20°C.
Proteins found in the insoluble fraction were treated with 100 ml 1% Triton X-100 in TBS for 4 h at 4°C. The insoluble proteins were pelleted by centrifugation, and the supernatant was discarded. The insoluble pellet was then extracted for 8 h at room temperature with 100 ml of TBS containing 8 M urea. Insoluble debris was pelleted, and the protein was purified as above except that all buffers contained 2 M urea. Following purification, the proteins were brought to 0.1% Triton X-100, then dialyzed into TBS with 0.1% Triton, and stored at −20°C.
All liquid chromatography was done using an AKTA explorer (Amersham-Pharmacia Biotech, Piscataway, NJ). All SDS-PAGE was done using 4 to 20% gradient Tris-glycine gels (Cambrex).
Mouse vaccination and infection.
Groups of 5 or 10 4-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were vaccinated at 0, 3, and 6 weeks with 10 μg of recombinant protein in 20 μg of QS21 (Antigenics, Lexington, MA) by subcutaneous injection. The mice were bled on week 0 prior to the first vaccination and on week 8. Two days following the final bleed, the mice were challenged by intraperitoneal injection of 3 × 108 CFU of S. epidermidis 0-47 grown overnight on Columbia salt agar (1× Columbia agar, 0.1% glucose, 1% yeast extract, 0.5% NaCl). This leads to a transient infection in which multiple organs are infected (i.e., liver, kidney and spleen), and the mice clear the organism by ∼48 h. Twenty-four hours following challenge, the mice were sacrificed and the bacteria were enumerated in the spleen and blood. Typical recoveries in the control groups were 5.5 to 6.4 log10 CFU/spleen and 2.5 to 3.5 log10 CFU/ml of blood.
RESULTS
Growth in serum alters expression of S. epidermidis cell wall-associated proteins.
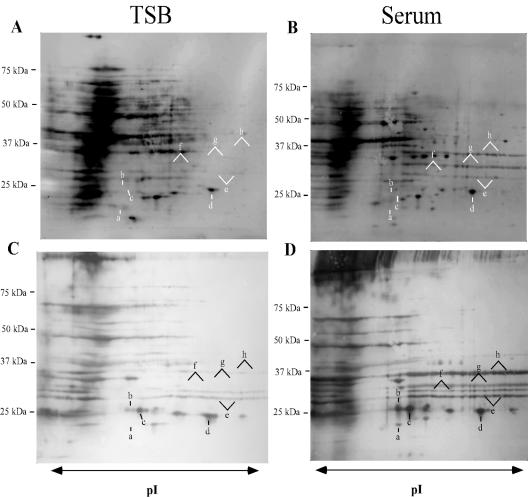
2D gel profiles of cell wall-associated proteins from S. epidermidis grown in TSB or 70% rabbit serum were compared (Fig. 1). Growth in 70% rabbit serum resulted in a change in the expression profile of cell wall-associated proteins from S. epidermidis that was easily detected in fluorescence-stained transfers of 2D gels (Fig. 1A and B). This change was more pronounced when the transfers of the 2D gels were probed with hyperimmune rabbit anti-S. epidermidis 0-47 sera (Fig. 1C and D). Eight proteins detected by fluorescent stain were differentially regulated between S. epidermidis grown in TSB or in the presence of rabbit serum (Fig. 1, a to h). Most notable was an increase in the fluorescent staining of three protein streaks between 25 kDa and 37 kDa in the cells grown in 70% serum (Fig. 1A to D, e, g, and h). These upregulated proteins are also strongly reactive with pooled anti-S. epidermidis antisera (Fig. 1D), suggesting they were expressed upon exposure to the environment within the rabbits. Five other immunoreactive streaks or spots from the serum-grown cells were expressed at either lower or undetectable levels in TSB grown cells (Fig. 1A to D, a to d and f). Bacteria grown in 70% serum were used in subsequent proteomic experiments, working under the assumption that the changes detected during growth in serum more accurately reflect alterations in gene expression made by the bacteria in response to environmental signals seen within the bloodstream of the host.
FIG. 1.
Protein expression profiles of cell wall fractions from S. epidermidis 0-47 grown in TSB (A and C) or 70% rabbit serum (B and D) were compared by 2D gel electrophoresis. Prior to fractionation of the cell wall-associated proteins from the cells grown in serum, the cells were washed with a buffer containing 0.5 M NaCl to remove serum proteins. The TSB cells were treated the same. Proteins were separated on pH 4 to 7 IPG strips followed by SDS-PAGE, transferred to nitrocellulose, and detected by fluorescent stain (A and B). Immunoreactive proteins were visualized with immune sera (C and D) from rabbits immunized with S. epidermidis 0-47. Spots or streaks expressed at different levels in the presence of serum are labeled a to h. Molecular mass markers are shown on the left.
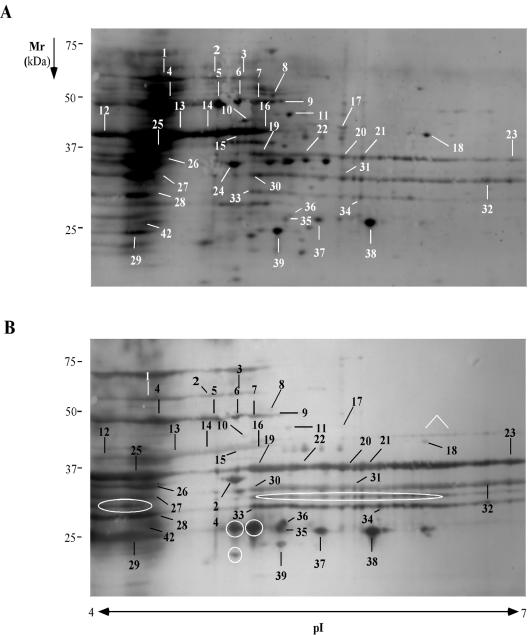
Spots consistently detected on both fluorescent and immunostained transfers from S. epidermidis grown in 70% serum, were located and labeled for identification by mass spectrometry (Fig. 2). A total of 40 immunoreactive spots were cut and subjected to mass spectrometry analysis for identification (Table 1). Six proteins (Fig. 2B) were consistently present in immunostained blots, but no corresponding spots were detected on the fluorescence-stained transfers. Although these proteins were likely expressed in the rabbits and elicited an immune response, they were not expressed at levels that allowed for their detection by fluorescent protein staining under the conditions used in these experiments.
FIG. 2.
Fluorescence-stained blot (A) and immunoblot (B) of a cell surface fraction from S. epidermidis 0-47 grown in 70% rabbit serum separated on pH 4 to 7 IPG strips in the first dimension and SDS-PAGE in the second dimension. The spots were identified by mass spectral analysis and labeled with numbers corresponding to the identified proteins in Table 1. Proteins only present on immunoblot are indicated by white circles and an arrow.
TABLE 1.
List of spots identifieda
| Spot no. | Predicted functionb | ATCC 12228 equivalent ORF (GenBank) | Method of detectiond | Log CFU Reductione
|
|
|---|---|---|---|---|---|
| Spleen | Blood | ||||
| 1, 2 | Dihydrolipoamide dehydrogenase | gi|27467712 | I,S | 0 | 0 |
| 3 | Dihydrolipoamide dehydrogenase | gi|27467712 | I,S | ||
| Glutamate-1-semialdehyde 2,1-aminomutase | gi|27468260 | I,S | 0 | 0 | |
| 4 | Enolase | gi|27467479 | I,S | 1.5 | 0.7 |
| Elongation factor TU | gi|27467230 | I,S | 0 | 0 | |
| 5-7, 9 | Enolase | gi|27467479 | I,S | ||
| 8 | Phosphogluconate dehydrogenase | gi|27468110 | I | 0 | 0 |
| 10 | Enolase | gi|27467479 | I,S | ||
| Elongation factor TU | gi|27467230 | I,S | |||
| Na+/H+ antiporter | gi|27468791 | I,S | 0.9 | 0 | |
| 11 | Alanine dehydrogenase | gi|27468302 | I,S | 0 | 1 |
| 12 | GAPDH | gi|27467475 | I,S | 0 | 0 |
| 13 | GAPDH | gi|27467475 | I,S | ||
| Elongation factor TS | gi|27467851 | I,S | 0 | 0 | |
| 14 | No match | I,S | |||
| 15-16 | GAPDH | gi|27467475 | I,S | ||
| 17 | Acetyl-CoA C-acetyltransferase | gi|27469302 | I | 1.2 | 0.9 |
| 18 | Cystathionine gamma-synthase | gi|27469241 | I | 0 | 0 |
| 19 | Ferrichrome binding lipoprotein | gi|27468686 | I,S | 0 | 0 |
| Oligopeptide permease | gi|27467598 | I,S | NT | NT | |
| 20-23, 25 | Ferrichrome binding lipoprotein | gi|27468686 | I,S | ||
| 24 | Fructose-bisphosphate aldolase | gi|27469074 | I,S | 0 | 0 |
| 26 | Fructose-bisphosphate aldolase | gi|27469074 | I,S | ||
| Hypothetical protein | gi|27469180 | I,S | 0 | 0 | |
| Hypothetical protein | gi|27468551 | I,S | 0.5 | 0 | |
| 27, 30-32 | Lipoprotein (SitC) | gi|27467323 | I,S | 0.8 | 1 |
| 28, 33 | Amino acid-binding lipoprotein | gi|27468911 | I,S | 2 | 0 |
| 29 | Putative hexulose-6-phosphate synthase | gi|27467259 | I,S | 0.5 | 0 |
| 34 | Lipoate ligase | gi|27467278 | I,S | 1.1 | 0 |
| 35 | No match | I | |||
| 36 | No match | I | |||
| 37 | No match | I,S | |||
| 38 | IsaA | gi|27469044 | I,S | 0 | 0 |
| 39 | Embp | gi|27468046 | I,S | NT | NT |
| 40 | Cysteine synthase | gi|27469188 | S | 1 | 0.8 |
| Fructose-bisphosphate aldolase homologue | gi|27468641 | S | 1 | 0 | |
| Thioredoxine reductase | gi|27467465 | S | 0 | 0 | |
| 41 | Cysteine synthase | gi|27469188 | S | ||
| 42 | Putative transaldolase | gi|27468367 | I | 0 | 0 |
| 43 | Embp | gi|27468046 | S | NT | NT |
| Transketolase | gi|27467943 | S | 0 | 0 | |
| Hypothetical protein | gi|27467889 | S | 0 | 0 | |
| 44 | Glutamyl-tRNAGln amidotransferase subunit | gi|27468502 | S | 0 | 0 |
List of spots detected on 2D blots by reactivity with immune sera or binding to serum components. Corresponding ORFs in Incyte's PathoSeq S. epidermidis 0-47 database were identified by analysis of mass spectrometry data using MSFIT (Protein Prospector) and MASCOT (Matrix Science) database search engines.
The predicted function of the proteins was determined by comparison with complete genome homologues from ATCC 12228 (44).
The GenBank accession numbers from ATCC 12228 are listed.
Method by which the spot was detected following transfer to nitrocellulose, reactive immune sera from infected rabbits (I) or binding serum components (S).
Mice (five animals/group) vaccinated with the recombinant proteins were challenged with 3 × 108 CFU of S. epidermidis 0-47 and the bacteria in the spleen and blood were enumerated 24 h postinfection. The data are reported as the log reduction in bacteria compared to data for mice vaccinated with adjuvant control alone, where 5.5 to 6.4 log10 CFU were recovered from spleen and 2.5 to 3.5 log10 CFU were recovered per ml of blood. NT, not tested.
Elution of serum proteins bound to the surface of S. epidermidis.
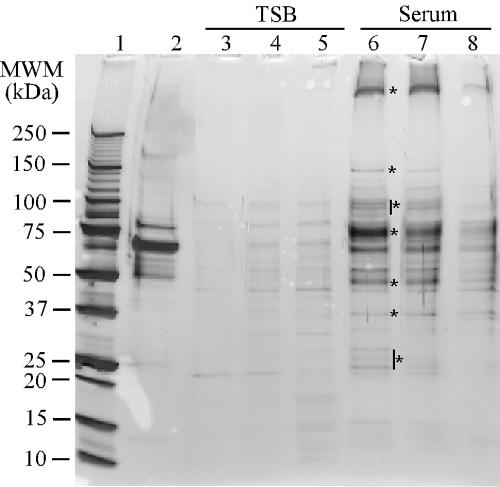
To identify cell wall-associated proteins involved in binding serum components, labeled serum proteins were used to probe 2D transfers. To enrich for serum proteins that bind to S. epidermidis, bacteria were grown in 70% rabbit serum and then washed with an increasing concentration of NaCl or 4 M urea. Serum proteins eluted from the bacteria under these conditions were compared by SDS-PAGE to normal rabbit serum and to the bacterial proteins eluted from the surface of S. epidermidis grown in TSB. Equal amounts of rabbit serum and the proteins eluted from the surface of S. epidermidis (0.75 μg) were loaded onto the gel. No protein was detected by protein assay in the fraction eluted from the surface of S. epidermidis grown in TSB. As a result, 25 μl of these fractions was loaded onto the gel to see if anything could be detected by silver stain. Buffers containing 0.5 M NaCl, 1 M NaCl, or 4 M urea each eluted bound serum proteins from the bacterial cells (Fig. 3). The eluted fractions represent a pool of proteins eluted from the bacterial surface that was enriched for serum proteins. Although some bands eluted from bacteria grown in TSB could be detected by silver stain, these bands did not correspond to the more intensely stained proteins eluted from the surface of bacteria grown in serum (Fig. 3) and were present at levels that could not be detected by a protein assay. Elution with 0.5 M NaCl was used for subsequent elution experiments, since it was the least denaturing condition and eluted the fewest staphylococcal proteins and the most serum proteins.
FIG. 3.
Proteins eluted from the surface S. epidermidis 0-47 (grown in TSB or 70% rabbit serum) with increasing concentrations of NaCl or 4.0 M urea. Bacteria were washed three times with TBS and then sequentially with 0.5 M and 1.0 M NaCl and 4.0 M urea. Protein concentrations were determined for each of the samples, and 0.75 μg was run on a 4 to 20% gradient gel. No protein was detected by protein assay in the samples eluted from the surface of TSB-grown bacteria (lanes 2 to 5). As a result, 25 μl of these samples was run on the gel. Lane 1, rabbit serum; lanes 2 and 5, to 0.5 M NaCl, lanes 3 and 6, to 1.0 M NaCl; lane 4 and 7, to 4.0 M urea. Asterisks indicate enriched proteins eluted from the surface of S. epidermidis grown in the presence of serum.
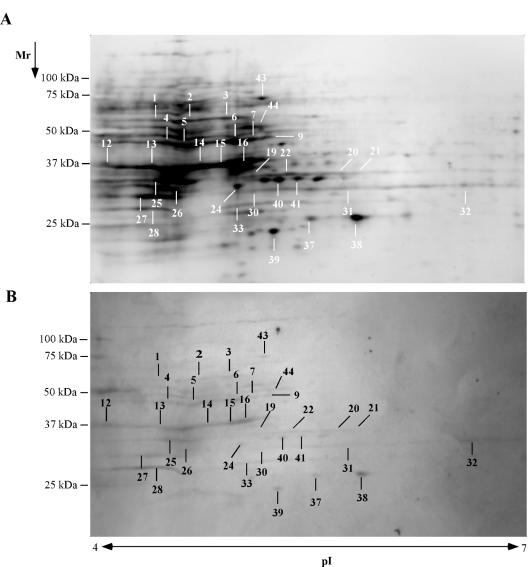
To reduce the naturally occurring anti-staphylococcal IgG in the eluted serum proteins, total IgG was depleted by incubation with protein G-Sepharose. This reduces the likelihood of identifying a protein that is recognized by naturally occurring antibody. Following antibody depletion, the eluted serum proteins were biotin labeled and used to probe a 2D blot of S. epidermidis cell surface proteins (Fig. 4). Thirty-four spots and regions were visualized by this method. All but four of these were also detected using the immune sera from rabbits immunized with S. epidermidis (Fig. 2 and Table 1).
FIG. 4.
A 2D transfer of cell surface proteins from S. epidermidis was fluorescently stained for protein (A) and probed with biotinylated serum proteins (B) eluted from S. epidermidis grown in 70% rabbit serum. The spots were visualized with a streptavidin-alkaline phosphatase conjugate. The spots were identified by mass spectrometry analysis and labeled with numbers corresponding to the identified proteins in Table 1.
Identification of proteins by mass spectrometry.
The proteins detected by each of the methods described were cut out of a gel and identified by mass fingerprint analysis using MALDI-TOF, followed by searching Incyte's PathSeq S. epidermidis 0-47 database for the corresponding genomic coding region (see Materials and Methods). Spots with multiple protein hits or questionable signal were further analyzed with static nanospray. A total of 29 proteins were identified, with some spots containing more than one protein (Table 1). Twenty-three of the proteins identified were immunoreactive, 25 bound to serum components, and 19 of the proteins were both immunoreactive and serum binding. This large overlap was expected, as most proteins on the surface of S. epidermidis involved in binding to serum factors would likely elicit an immune response.
Active immunization of mice with recombinant proteins.
Twenty-seven open reading frames (ORFs) encoding either serum binding or immunoreactive proteins were cloned from S. epidermidis 0-47, and the recombinant proteins were expressed in E. coli with a hexahistidine tag and purified. The genes encoding oligopeptide permease and extracellular matrix binding protein (Embp) were successfully cloned but could not be expressed at levels sufficient for purification. An initial screen of vaccine candidates was done on groups of five mice each to enable for the screening of numerous proteins. Although the resulting data were not statistically significant, these experiments facilitated determination of the vaccine potential for a large number of candidate vaccine antigens. Eleven of the recombinant proteins reduced the bacteria recovered from the spleen or blood by 0.5 log or greater (Table 1). When these 11 antigens were used to vaccinate groups of 10 mice, acetyl-coenzyme A (CoA) acetyltransferase, Na+/H+ antiporter, lipoate ligase, cysteine synthase, and alanine dehydrogenase significantly reduced (P ≤ 0.05) the number of bacteria recovered from the spleen or blood (Table 2).
TABLE 2.
Summary of challenge dataa
| Antigen | Log10 CFU reductiona
|
|
|---|---|---|
| Spleen | Bloodc | |
| Na+/H+ antiporter | 1.2* | NT |
| Alanine dehydrogenase | 0.8 | 1.2** |
| Enolase | 0.9 | NT |
| Fructose bisphosphate aldolase homologue | 0.5 | NT |
| Acetyl-CoA C-acetyltransferase | 1.4* | NT |
| Hypothetical | 0.3 | NT |
| Lipoate ligase | 1.6** | NT |
| Putative hexulose-6-phosphate synthase | 0 | NT |
| SitC | 0.5 | NT |
| Amino acid binding lipoprotein | 0.8 | NT |
| Cysteine synthase | 1.2* | 1.2** |
Groups of 10 female (4-week-old) BALB/c mice were vaccinated by subcutaneous injection with saline or 10 μg of antigen with 20 μg of QS21 as adjuvant: Two weeks following the last vaccination, the mice were challenged with ≈5 × 108 CFU S. epidermidis 0-47 by intraperitoneal injection. Twenty-four hours after challenge, the bacteria in the blood and spleen were enumerated.
Reduction in CFU is determined as compared to a control of QS21 in saline with recoveries of 5.5 to 6.4 log10 CFU/spleen and 2.5 to 3.5 log10 CFU/ml of blood. Data analyzed by Student's t test with resulting P values of 0.05 (*) or 0.01 (**).
The numbers of CFU in the bloodstream were not tested (NT) for most of the antigens based upon initial results (Table 1), which indicated that attaining statistical significance would be unlikely.
DISCUSSION
Upon exposure to the bloodstream, invading bacteria encounter signals specific to the new environment that result in adaptive changes in protein expression. Frequently, upregulated proteins interact with host factors or facilitate nutrient acquisition and there-by contribute to survival of the bacteria and progression of pathogenesis. Growth of bacteria in body fluids (i.e., serum, peritoneal dialysate fluid, and urine) has been used as a model system to mimic some of the signals bacteria encounter within the host (21, 34, 35, 43). One or more of these culture conditions was found to alter gene expression in Enterococcus faecalis, Staphylococcus aureus, and S. epidermidis. In the present study, the expression profile of cell wall-associated proteins from S. epidermidis 0-47 grown in 70% rabbit serum was analyzed by 2D gel electrophoresis. The results indicate that growth in serum induces a different profile than growth in TSB. Three proteins predicted to be involved in nutrient acquisition, SitC (iron or manganese binding lipoprotein), ferrichrome binding lipoprotein, and amino acid-binding lipoprotein, were all significantly increased (Fig. 1) following growth in serum. All three proteins were not well resolved by isoelectric focusing and formed horizontal streaks across the gel. This may be the consequence of multiple charge isomers or related to their predicted lipoprotein composition. Additionally, all three proteins were highly reactive with sera from rabbits immunized with live S. epidermidis 0-47. Since these proteins were not highly expressed on the TSB-grown bacteria, the data suggest these proteins are expressed in vivo following intraperitoneal administration to the rabbits. In total, 23 proteins were identified as reactive with immune sera from infected rabbits. Not only did these proteins elicit an immune response in vivo, but they were also expressed during growth in serum. In addition, immunization with 11 of the proteins resulted in a reduction in colonization in a mouse model of S. epidermidis infection. Taken together, the data suggest that the reactive antigens identified in these experiments are expressed during an infection. In fact, expression of the transcripts from these ORFs within the bloodstream of S. epidermidis-infected mice was confirmed by quantitative reverse transcription-PCR for each of the proteins identified in this work (L. Croy, B. Sellman, and S. Baker, unpublished results).
Since some of the spots contained more than one protein, each recombinant protein was screened by Western blotting for reactivity with the hyperimmune rabbit anti-S. epidermidis serum. Only one of the proteins identified in an immunoreactive spot was not reactive, hypothetical protein (gi 27468551) in spot 26 (data not shown). This suggests the reactivity in spot 26 was directed against both of the other two proteins identified in the spot. Less likely is the possibility that the immunological properties of the recombinant protein were drastically altered from those of the native protein expressed by S. epidermidis. The fact that the immune rabbit sera recognize the vast majority of recombinant proteins also provides confirmatory evidence for the correct identification of the proteins within the spots.
The most common predisposing factor for a S. epidermidis infection is the implantation of a prosthetic device. An implanted prosthetic device becomes coated with plasma and matrix proteins, including fibrinogen, vitronectin, von Willebrand factor, and fibronectin (39). These proteins often act as ligands for staphylococcal surface proteins, thus allowing the bacteria to bind and colonize the prosthetic device. S. epidermidis is known to express proteins that bind to fibrinogen, vitronectin, and fibronectin (6, 15, 23, 42). It is reasonable to expect that S. epidermidis will bind additional serum proteins after making the transition from commensal to pathogen. S. epidermidis grown in serum had serum proteins bound to the bacterial surface that were eluted with 0.5 M and 1 M NaCl (Fig. 3). Undoubtedly, the eluted serum proteins are a heterogeneous mixture, including some that may be nonspecifically associated with the staphylococcal surface. It is possible that some staphylococcal proteins, expressed only in serum, are also eluted by the high-salt treatment. Analysis by mass spectrometry indicates that three staphylococcal proteins, AtlE, immunodominant antigen A, and AAP are present in the eluted serum fraction; however, the majority of proteins are not staphylococcal in origin (A. Severin, B. Sellman, and S. M. Baker, unpublished results). It is possible these staphylococcal proteins contribute to the reactivity seen on the ligand blot; however, most of the reactivity likely comes from interactions with serum components. Twenty-seven staphylococcal proteins were identified that appear to interact with serum components. Work is in progress to determine if the recombinant forms of these proteins bind serum components and to identify the specific ligands.
Several of the staphylococcal proteins found in this study to bind serum components or their homologues in other bacterial species have previously been shown to interact with host proteins. For example, Embp from S. epidermidis NCTC11047 was shown to bind to fibronectin (42), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and enolase from Streptococcus pyogenes bind fibronectin and plasminogen, respectively (24, 25). Also, SitC is a homologue of a family of lipoprotein adhesins termed lipoprotein receptor antigens (LraI) that have been shown to bind host ligands and function as protective vaccine antigens (3, 5, 38). Of note are also those proteins that are absent from this list such as SdrG and autolysin, which bind fibrinogen and vitronectin, respectively (6, 15). There are several reasons why they might not have been detected in these experiments. (i) They are not expressed at detectable levels (several immunoreactive proteins were not expressed at levels that were visually detected). (ii) They remain insoluble following preparation for 2D gel electrophoresis. (iii) The reactive epitope or binding site is lost during the unfolding and refolding process on the nitrocellulose. (iv) The protein is lost during the washing process (AtlE, AAP, and IsaA were identified in the salt wash). Also, the separation of cell wall preparations has historically been difficult for gram-positive organisms due to the intractable nature of these proteins (13). The proteins presented in this study are therefore not a comprehensive representation of surface proteins, but simply a subset of proteins that can be detected under the experimental conditions employed.
A number of the proteins identified in the cell wall-associated extract have a cytosolic function and do not have a signal sequence. This is not completely unexpected as there are numerous examples of “cytosolic” proteins that are actually localized to the surface of bacteria and yeast. Such proteins include fructose bisphosphate aldolase, GAPDH, alanine dehydrogenase, and enolase. Fructose bisphosphate aldolase and alanine dehydrogenase have both been reported to be on the surface of Mycobacterium tuberculosis (29, 30). GAPDH has been identified on the surface of other microbial species in addition to S. pyogenes discussed above, such as Candida albicans (11), Neisseria meningitidis (12), and Streptococcus agalactiae (17). Enolase has been identified on the surface of numerous organisms such as S. agalactiae (17), C. albicans (11), Pneumocystis carinii (10), and S. aureus (22). The mechanisms of secretion and surface attachment of these proteins are not clear, but the proteins are present on the surface and in some cases function on the cell surface as important virulence determinants (4). Evidence for the surface localization of the proteins identified in this study is as follows. (i) Cell walls were hydrolyzed in an osmotically stabilized buffer. (ii) The proteins were recognized by the immune system during an infection with live S. epidermidis. (iii) The bacterial proteins are capable of binding serum components. (iv) Some of the proteins identified function as protective antigens. Although all these lines of evidence suggest that the proteins identified in this work are surface localized, this should be confirmed by additional methods.
The proteins in this study were selected based on their presence in an extract of cell wall-associated proteins, expression in rabbit serum, ability to elicit an antibody response during infection of rabbits, and the ability to bind serum components. Although it is not required that a vaccine candidate have any of these characteristics, they are all attractive attributes for a vaccine candidate. It is essential the antigen be expressed while in the host so the host can recognize the antigen and mount a response. If the antigen is surface localized, a humoral immune response can opsonize the bacteria and target them for phagocytosis and clearance. Of the 27 recombinant candidate vaccine antigens tested, 5 significantly reduced the bacterial numbers recovered from the spleen or blood of infected mice. Protection in an infection model provides support for the surface localization of these antigens, as the humoral immune response would not have access to cytosolic antigens. These in vivo-expressed antigens are promising as candidate vaccine antigens and will be further characterized in regard to their protective efficacy and their role in host-pathogen interactions.
Acknowledgments
We thank Stephen Olmsted and Bruce Green for critical review of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Anonymous. 1999. National Nosocomial Infections Surveillance (NNIS) System report: data summary from January 1990-May 1999, issued June 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 2.Bowden, M. G., L. Visai, C. M. Longshaw, K. T. Holland, P. Speziale, and M. Hook. 2002. Is the GehD Lipase from Staphylococcus epidermidis a collagen binding adhesin? J. Biol. Chem. 277:43017-43023. [DOI] [PubMed] [Google Scholar]
- 3.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 63:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chhatwal, G. S. 2002. Anchorless adhesins and invasins of gram-positive bacteria: a new class of virulence factors. Trends Microbiol. 10:205-208. [DOI] [PubMed] [Google Scholar]
- 5.Cockayne, A., P. J. Hill, N. B. Powell, K. Bishop, C. Sims, and P. Williams. 1998. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect. Immun. 66:3767-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, S. L., S. Gurusiddappa, K. W. McCrea, S. Perkins, and M. Hook. 2001. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bβ chain. J. Biol. Chem. 276:27799-27805. [DOI] [PubMed] [Google Scholar]
- 7.Diekema, D. J., M. A. Pfaller, and R. N. Jones. 2002. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America. SENTRY Antimicrobial Surveillance Program, 1997-2000. Int. J. Antimicrob. Agents 20:412-418. [DOI] [PubMed] [Google Scholar]
- 8.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 9.Eng, J. K., A. L. McCormack, and J. R. Yates. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 10.Fox, D., and A. G. Smulian. 2001. Plasminogen-binding activity of enolase in the opportunistic pathogen Pneumocystis carinii. Med. Mycol. 39:495-507. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Navarro, I., M. L. Gil, M. Casanova, J. E. O'Connor, J. P. Martinez, and D. Gozalbo. 1997. The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is a surface antigen. J. Bacteriol. 179:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J. Berger, G. Ratti, R. Petracca, G. Galli, M. Agnusdei, M. Monica Giuliani, L. Santini, B. Brunelli, H. Tettelin, R. Rappuoli, F. Randazzo, and G. Grandi. 2002. Previously unrecognized vaccine candidates against group B meningococcus identified by DNA microarrays. PG-914-21. Nat. Biotechnol. 20:914-921. [DOI] [PubMed] [Google Scholar]
- 13.Hecker, M., S. Engelmann, and S. J. Cordwell. 2003. Proteomics of Staphylococcus aureus—current state and future challenges. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 787:179-195. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Gotz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilmann, C., M. Hussain, G. Peters, and F. Gotz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 16.Heilmann, C., and G. Peters. 2000. Biology and pathogenicity of Staphylococcus epidermidis, p. 442-449. In V. A. Fischetti (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 17.Hughes, M. J., J. C. Moore, J. D. Lane, R. Wilson, P. K. Pribul, Z. N. Younes, R. J. Dobson, P. Everest, A. J. Reason, J. M. Redfern, F. M. Greer, T. Paxton, M. Panico, H. R. Morris, R. G. Feldman, and J. D. Santangelo. 2002. Identification of major outer surface proteins of Streptococcus agalactiae. Infect. Immun. 70:1254-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain, M., M. Herrmann, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocianova, S., C. Vuong, Y. Yao, J. M. Voyich, E. R. Fischer, F. R. Deleo, and M. Otto. 2005. Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Investig. 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermid, K. P., D. W. Morck, M. E. Olson, M. K. Dasgupta, and J. W. Costerton. 1993. Effect of growth conditions on expression and antigenicity of Staphylococcus epidermidis RP62A cell envelope proteins. Infect. Immun. 61:1743-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molkanen, T., J. Tyynela, J. Helin, N. Kalkkinen, and P. Kuusela. 2002. Enhanced activation of bound plasminogen on Staphylococcus aureus by staphylokinase. FEBS Lett. 517:72-78. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson, M., L. Frykberg, J. I. Flock, L. Pei, M. Lindberg, and B. Guss. 1998. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect. Immun. 66:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pancholi, V., and V. A. Fischetti. 1998. α-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273:14503-14515. [DOI] [PubMed] [Google Scholar]
- 25.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 27.Raad, I., A. Alrahwan, and K. Rolston. 1998. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin. Infect. Dis. 26:1182-1187. [DOI] [PubMed] [Google Scholar]
- 28.Rappuoli, R. 2000. Reverse vaccinology. Curr. Opin. Microbiol. 3:445-450. [DOI] [PubMed] [Google Scholar]
- 29.Raynaud, C., G. Etienne, P. Peyron, M. A. Laneelle, and M. Daffe. 1998. Extracellular enzyme activities potentially involved in the pathogenicity of Mycobacterium tuberculosis. Microbiology 144:577-587. [DOI] [PubMed] [Google Scholar]
- 30.Rosenkrands, I., R. A. Slayden, J. Crawford, C. Aagaard, C. E. Barry III, and P. Andersen. 2002. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J. Bacteriol. 184:3485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Gotz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 32.Sanyal, D., and D. Greenwood. 1993. An electronmicroscope study of glycopeptide antibiotic-resistant strains of Staphylococcus epidermidis. J. Med. Microbiol. 39:204-210. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal, D., A. P. Johnson, R. C. George, B. D. Cookson, and A. J. Williams. 1991. Peritonitis due to vancomycin-resistant Staphylococcus epidermidis. Lancet 337:54. [DOI] [PubMed] [Google Scholar]
- 34.Shepard, B. D., and M. S. Gilmore. 2002. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 70:4344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, D. G., M. H. Wilcox, P. Williams, R. G. Finch, and S. P. Denyer. 1991. Characterization of cell envelope proteins of Staphylococcus epidermidis cultured in human peritoneal dialysate. Infect. Immun. 59:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda, S., G. B. Pier, Y. Kojima, M. Tojo, E. Muller, T. Tosteson, and D. A. Goldmann. 1991. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation 84:2539-2546. [DOI] [PubMed] [Google Scholar]
- 37.Veenstra, G. J., F. F. Cremers, H. van Dijk, and A. Fleer. 1996. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J. Bacteriol. 178:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viscount, H. B., C. L. Munro, D. Burnette-Curley, D. L. Peterson, and F. L. Macrina. 1997. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect. Immun. 65:994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Eiff, C., C. Heilmann, and G. Peters. 1999. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:843-846. [DOI] [PubMed] [Google Scholar]
- 40.von Eiff, C., G. Peters, and C. Heilmann. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677-685. [DOI] [PubMed] [Google Scholar]
- 41.Vuong, C., C. Gerke, G. A. Somerville, E. R. Fischer, and M. Otto. 2003. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect. Dis. 188:706-718. [DOI] [PubMed] [Google Scholar]
- 42.Williams, R. J., B. Henderson, L. J. Sharp, and S. P. Nair. 2002. Identification of a fibronectin-binding protein from Staphylococcus epidermidis. Infect. Immun. 70:6805-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiltshire, M. D., and S. J. Foster. 2001. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect. Immun. 69:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]